Abstract
Purpose
Emerging preclinical data suggests that tea possess anticarcinogenic and antimutagenic properties. We therefore hypothesize that white tea extract (WTE) is capable of favorably modulating apoptosis, a mechanism associated with lung tumorigenesis.
Experimental Design
We examined the effects of physiologically relevant doses of WTE on the induction of apoptosis in the nonsmall cell lung cancer (NSCLC) cell lines, A549 (adenocarcinoma) and H520 (squamous cell carcinoma) cells. We further characterized the molecular mechanisms responsible for the WTE-induced apoptosis, including the induction of PPAR-γ and the 15-lipoxygenase (15-LOX) signaling pathway.
Results
We found that WTE was effective in inducing apoptosis in both A549 and H520 cells, and inhibition of PPAR-γ with GW 9662 partially reversed the WTE-induced apoptosis. We further demonstrate that WTE increased PPAR-γ activation and mRNA expression, concomitantly increased 15-HETE release, and up-regulated 15-LOX-1 and 2 mRNA expression by A549 cells. Inhibition of 15-LOX with NGDA, as well as caffeic acid, abrogated the WTE-induced PPAR-γ activation and up-regulation of PPAR-γ mRNA expression in A549 cells. WTE also induced cyclin-dependent kinase inhibitor 1A (CDKN1A) mRNA expression and activated caspase 3. Inhibition of caspase 3 abrogated the WTE-induced apoptosis.
Conclusions
Our findings indicate that WTE is capable of inducing apoptosis in NSCLC cell lines. The induction of apoptosis appears to be mediated, in part, through the up-regulation of the PPAR-γ and 15-LOX signaling pathways, with enhanced activation of caspase 3. Our findings support the future investigation of WTE as an antineoplastic and chemopreventive agent for lung cancer.
Keywords: 15-HETE, Caspase 3, chemoprevention
Introduction
Results from epidemiological studies as well as laboratory experiments suggest that tea consumption confers protection against the development of chronic diseases, including cancer (1-6). Tea contains high levels of flavonoids, such as catechins and other polyphenols. These phytochemicals are thought to play key roles in mechanisms that may provide health benefits (7-9). Through these mechanisms, tea has demonstrated significant chemopreventive effects in animal models of lung, skin, bladder, esophageal and gastrointestinal cancers (10-14). For example, green tea has been shown to inhibit lung tumor development in A/J mice treated with 4-(methylnitrosamino)-1-(3-pyridyl)-l-butanone (NNK), a potent nicotine-derived lung carcinogen found in tobacco (15). Whereas majority of the work evaluating the antineoplastic effects of tea has been done on green tea, the potential health benefits of white tea and its advantage over green tea have becoming increasingly recognized in recent years.
White tea is a rare tea that comes from the same plant (Camellia sinensis) as green and black teas. The descriptive term “white” stems from the high proportion of silvery buds harvested from the plants to produce the tea. White tea is the least processed of all teas, as it is picked then rapidly steamed and dried, without fermentation, roasting or subjected to a prior withering stage. Green tea is produced when freshly harvested leaves are subjected to withering, then panfried/steamed prior to rolling/shaping and drying. Black tea, which represents the largest percentage of the total tea consumption worldwide, is produced by following some of the processing steps used for green tea, with critical differences in which the leaves are bruises, crushed, or broken, thus allowing the polyphenol oxidases in the leaf to convert the endogenous catechins into theaflavins, thearubigins, and other complex polyphenols. The degree of protection against mutagenesis and carcinogenesis appears to be related to the extent of processing, because the catechins are generally considered to be more active based on their antioxidant and other protective properties. As such, the minimal processing of white tea yields a higher concentration of polyphenol antioxidants.
To date, most of the studies evaluating the effects of tea on lung cancer have been focused on green tea, and a review of the literature yielded no published work involving WTE. On the basis that white tea has been shown to possess higher antimutagenic potency than green tea (16), we hypothesize that WTE will be effective in modulating mechanisms associated with lung tumorigenesis. We evaluated the effects of WTE on apoptosis in NSCLC cells, because apoptosis is an essential regulatory process that maintains homeostasis in normal epithelial cells, and resistance to apoptosis is a critical mechanism that confers the malignant phenotype. As such, restoring apoptosis may represent an important anti-carcinogenic mechanism mediated by WTE.
Emerging data continue to link carcinogenesis to inflammatory events involving the eicosanoid metabolic pathways. Arachidonic acid, released from membrane phospholipids upon cell stimulation, is converted to prostanoids by cyclooxygenases (COX), to leukotrienes by 5-lipoxygenase (5-LOX), and to 15(S)-hydroxy-eicosatetraenoic acid (15-HETE) by 15-lipoxygenases (17). Over-expression of COX-2 and prostaglandin E2 (PGE2) are associated with a variety of well-established lung cancer risk factors, including resistance to apoptosis (18). Preclinical data suggests that in addition to the prostaglandins, the LOX signaling pathways may play a significant role in lung cancer tumorigenesis. Shankaranarayanan P et al demonstrated that 15-HETE bound to PPAR-γ nuclear receptors and induced apoptosis in A549 cells. Moreover, pretreatment of cells with nordihydroguaiaretic acid, a 15-LOX inhibitor, prevented PPAR-γ activation and apoptosis (19). Shappell et al also found that 15-LOX-2-derived 15-HETE may constitute an endogenous ligand for PPAR-γ in the prostate and that loss of this pathway by reduced expression of 15-LOX-2 may contribute to increased proliferation and reduced differentiation in prostate carcinoma (20). We therefore hypothesized that WTE is capable of inducing apoptosis in human NSCLC via modulation of the 15-LOX pathway.
We demonstrate that WTE is effective in inducing apoptosis in both A549 and H520 cells. We also demonstrate that WTE significantly increases 15-HETE release, up-regulates 15-LOX 1 and 2, as well as PPAR-γ mRNA expression, and enhances activation of PPAR-γ by A549 cells. These mechanisms in turn increase caspase 3 activation. Our findings support the future investigation of WTE as a chemopreventive agent for lung cancer.
Material and Methods
Cell Culture
As models to evaluate the impact of WTE on lung cancer, the human lung adenocarcinoma cell lines, A549 and H520 (ATCC, Manassas, VA), were studied in vitro. Cells were maintained as monolayers in an atmosphere of 5% CO2 in air at 37°C in 25-cm2 tissue culture flasks containing 5.0 ml of RPMI-1640 medium supplemented with 10% FBS, 100 units/ml of penicillin, 0.1 mg/ml of streptomycin, and 2 mM of glutamine (JRH Biosciences, Lenexa, KS) for A549 cells, and RPMI 1640 medium with 2 mM L-glutamine adjusted to contain 1.5 g/L sodium bicarbonate, 4.5 g/L glucose, 10 mM HEPES, and 1.0 mM sodium pyruvate, 90%; fetal bovine serum, 10% for H520 cells. 0.1 × 106 A549 or H520 cells were and incubated at 37°C for 2 hours. Different doses of WTE containing 3.5, 5, 7 and 10 μg/ml of Epigallocatechin-3-gallate (EGCG), were added and cells were incubated at 37°C overnight for 16-18 h. The dose range of WTE was chosen based on physiologically relevant doses that have been reported in previous studies (21-23). To ascertain the involvement of PPAR-γ, 15-LOX and caspase 3 in our model systems, PPAR-γ inhibitor (GW9662, Caymen Chemical), exogenous 15-HETE (Cayman Chemical, Ann Arbor, MI), 15-LOX inhibitors (Caffeic Acid, Biomol Research laboratory Inc. Plymouth, PA. and NGDA, Sigma, St. Louis, MO), or caspase 3 inhibitor Z-DEVD-FMK (R &D Systems, Minneapolis, MN), were added one hour prior to addition of WTE. Experiments with dose titrations of each of these inhibitors were first carried out to determine the optimal inhibitory dose without compromising cell viability. Primary normal human bronchial epithelial (NHBE) cells (Cambrex, Walkersville, MD) and human bronchoalveolar lavage (BAL) cells (obtained from chemoprevention trials as previously described (24) were used as control. NHBE cells are maintained according to the manufacturer's instructions. 0.5 × 106 of NHBE cells were plated in wells and incubated at 37°C overnight. 0.5 × 106 of BAL cells were plated in serum free X-vivo medium (Biowhittaker, Walkersville, MD) for 2 h. Similar doses of WTE were then added and cells were incubated at 37°C overnight for 16-18 h.
All conditioned culture supernatants, total RNA or total protein were harvested and stored at -80°C until analysis. Samples of mRNA were collected using the QIAGEN RNeasy Mini Kit (QIAGEN Inc, Valencia, CA). Total protein were obtained by scrapping the cells off the plate in Laemmli buffer and homogenized by repeatedly passing the cells through a 25 gauge syringe.
PPAR- γ shRNA plasmid transfection
Stable transfection of PPAR- γ shRNA plasmid into A549 cells was achieved using the PPAR- γ specific SureSilencing™ shRNA Plasmids kit (SA Bioscience, Frederick, MD). Transfected clones were selected using various concentration of G418 per manufacturer's instruction. Selected clone attained 80% inhibition of PPAR-γ mRNA expression confirmed by qPCR.
Preparation of tea extract and quantification of tea catechin level
WTE and green tea extract (GTE) were purchased from commercially available sources. To ensure same amount of tea extracts with comparable catechin contents from various vendors are used for comparison experiments, a sample of the materials were first analyzed at an independent, commercial Laboratory (Industrial Laboratory, Woodbridge, CO.) for confirmation. Stock solutions of tea extracts standardized to EGCG and total catechin contents were made by dissolving each extract with double distilled water. Aliquots of the stock were stored at −80°C and used only once for each set of experiments.
Quantification of apoptosis in conditioned cells
For quantification of apoptosis, the cell death detection ELISA kits (Roche, Indianapolis, IN) were used according to the manufacturer's instructions to quantify apoptosis in the conditioned cells, as measured by specific determination of mono- and oligonucleosomes in the cytoplasmic fraction of conditioned cell culture lysates.
Real Time (q) PCR
First-strand cDNA was synthesized using 2.5 μg of total RNA (DNase-treated) in a 50 μl reverse transcriptase reaction mixture from the iScript™ cDNA Synthesis Kit (BioRad, Hercules, CA, USA). A region of the β-actin, 15-LOX-1, 15-LOX-2, and PPAR-γ mRNA were amplified using specific primer pairs (IDT, Coralville, IA): β-actin, sense 5′-GTACCACTG-GCATCGTGAT-3′, Anti-sense 5′-ATCTTCATGAGGTAGTCAGTCA-3′; 15-LOX-1, sense 5′-GGGCAAGGAGACAGAACTCAA-3′, Anti-sense 5′- GCACAGAGATCCAGTTGCAGAA-3′; 15-LOX-2, sense 5′-TCGCCTCCCAGTTCCTGAA-3′, Anti-sense 5′-AGTGACGGGGAAGTTCTTTGG -3′; PPAR-γ, sense 5′- CCTATTGACCCAGAAAGCGATT -3′, Anti-sense 5′- CATTACGGAGAGATCCACGGA -3′. The length of the cDNA amplified fragments are: β-actin, 136 bp; 15-LOX-1, 122 bp; 15-LOX-2, 80 bp, PPAR-γ, 135 bp. All real-time PCR reactions were performed in a 25 µl mixture containing 1/25 volume of cDNA preparation (2 µl), and 1× iQ™ SYBR® Green Supermix (BioRad, Hercules, CA, USA). The PCR amplification protocol consists of 1 minute denaturing (95°C), 1 minute annealing (60°C) and 1 minute extension (72°C) for total of 35 cycles. Real-time quantifications were performed using the BIO-RAD iCycler iQ system (BioRad, Hercules, CA, USA). The values were first normalized to β-actin, then to the control values for each experiment. Data are depicted in fold changes normalized to control. The fluorescence threshold value was calculated using the iCycle iQ system software (PCR efficiency ranged from 90-97%). The qPCR reactions for CDKN1A gene were performed using reagents, β-actin and CDKN1A primers from SA Bioscience per the manufacturer's instructions.
Quantification of 15-HETE and 13-S-HODE
Measurement of 15-HETE concentrations in conditioned culture supernatants were performed to assess the effects of WTE on modulating the production of this arachidonic acid metabolite, using enzyme specific immunoassay (EIA) according to the manufacturer's EIA kit protocol (Cayman Chemical, Ann Arbor, MI). Measurements of 13-S-HODE levels in the same conditioned culture supernatants were performed using EIA according to the manufacture's instructions (Assay Design, Ann Arbor, MI).
Immunofluorescene of activated PPAR-γ
Immunofluorescence analyses were performed using A549 cells directly cultured in 8-well chamber slide (Nalge Nunc International, IL, USA). After 8 h of adherence, A549 cells were conditioned with WTE and/or NGDA for 13 h. Cells were then fixed with 3.7% paraformaldehyde for 30 min and subjected to triple labeling of PPAR-γ DNA. A549 cells were incubated with 1:50 mouse monoclonal anti-PPAR-γ antibody (Santa Cruz, CA) for 2 h, 1:500 Cy3-conjugated AffiniPure Goat Anti-Mouse IgG (H+L) (Jackson ImmunoResearch Lab, West Grove, PA) for 30 min and 1:10,000 dilution of 4′,6-diamidino-2-phenylindole (DAPI) stock solution for 5 min. Between each incubation step, the cover glass was rinsed three times with cold PBS. The slides were then mounted in PermaFluor Mountant medium (Thermo Electron Corp., Pittsburgh, PA) for fluorescence microscopic examination. Images were collected using an Olympus U-DO microscope equipped with an Imaging Olympus U-CNAD 3 digital camera.
Quantification of caspase 3 activity
For quantification of caspase 3 activvity, the Caspase 3/CPP32 colormetric Assay Kits (Biovision, Mountainview, CA) were used. The assay is based on spectrophotometric detection of the chromophore p-nitroanilide (pNA) after cleavage from the labeled substrate DEVD-pNA. The pNA light emission was quantified using a microtiter plate reader at 405-nm. Freshly harvested cell extracts from conditioned cultures were assayed per manufacturer's instructions.
Statistical Analysis
Data are expressed as the mean ± SEM in all circumstances where mean values are compared. Data were analyzed by paired Student's t test and/or ANNOVA. Batch analyses were performed for each comparison group to eliminate interassay variability. Differences are considered significant when p < 0.05.
Results
WTE induces apoptosis in NSCLC cells
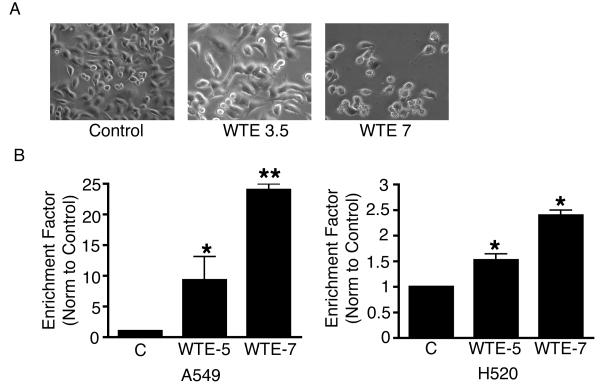
To evaluate the potential of WTE on apoptosis induction, we examined the effects of WTE from various commercially available sources on inducing apoptotic cell death in A549 cells and H520 cells. Treatment with WTE increases apoptosis in both A549 and H520 cells in a dose dependent manner (Fig. 1A & B).
Figure 1.
A) WTE induced morphologic changes in A549 Cells in a dose dependent manner. A549 cells were incubated with varying does of WTE. Representative photo mircographs of conditioned A549 cell culture following 17 hrs of incubation: 1. control; 2. with WTE containing 3.5 μg/ml of EGCG; 3. with WTE containing 7 μg/ml of EGCG. B) Quantification of apoptosis in conditioned A549 and H520 cells by Cell Death Detection ELISA. WTE induced apoptosis in A549 and H520 cells in a dose responsive manner, as measured by specific determination of mono- and oligo-nucleosomes in the cytoplasmic fraction of cell culture lysates. WTE 5 = WTE containing 5 μg/ml of EGCG. WTE 7 = WTE containing 7 μg/ml of EGCG. The mean ± SD absorbance values at 405 nm are reported. Columns, mean (n = 3); bars, SE. *, P < 0.05, **, P <0.01.
To determine whether or not the observed WTE-induced changes are due to nonspecific, direct cytotoxicity, similar experiments were performed on human BAL cells and NHBE cells. Treatment of human BAL and NHBE cells with WTE at the same doses does not result in significant morphologic changes nor increase in apoptosis (data not shown).
Inhibition of PPAR-γ abrogated WTE - induced apoptosis in both A549 and H520 cells
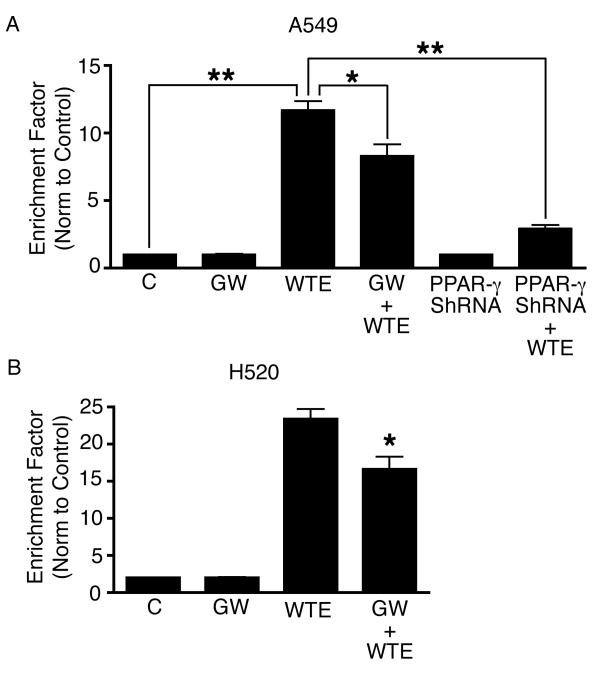
To determine whether WTE-induced apoptosis is mediated via the PPAR-γ pathway, we pretreated A549 and H520 cells with GW9662, a PPAR-γ inhibitor, followed by conditioning with WTE (containing 5 μg/ml of EGCG). Inhibition of PPAR-γ with GW9662 significantly abrogated WTE-induced apoptosis in both A549 and H520 cells (Fig. 2A & B). Inhibition of PPAR-γ mRNA expression with PPAR-γ ShRNA plasmid also abrogated WTE-induced apoptosis in A549 cells (Fig. 2A).
Figure 2.
Inhibition of PPAR-γ with GW9662 significantly abrogated WTE-induced apoptosis in both A549 (Fig. 2A) and H520 cells (Fig. 2B). Inhibition of PPAR-γ mRNA expression with PPAR-γ ShRNA plasmid also abrogated WTE-induced apoptosis in A549 cells (Fig. 2A). Columns, mean (n = 3); bars, SE. *, P < 0.05, **, P <0.01.
WTE, GTE and Exogenous 15-HETE all induce PPAR-γ mRNA expression in A549 cells
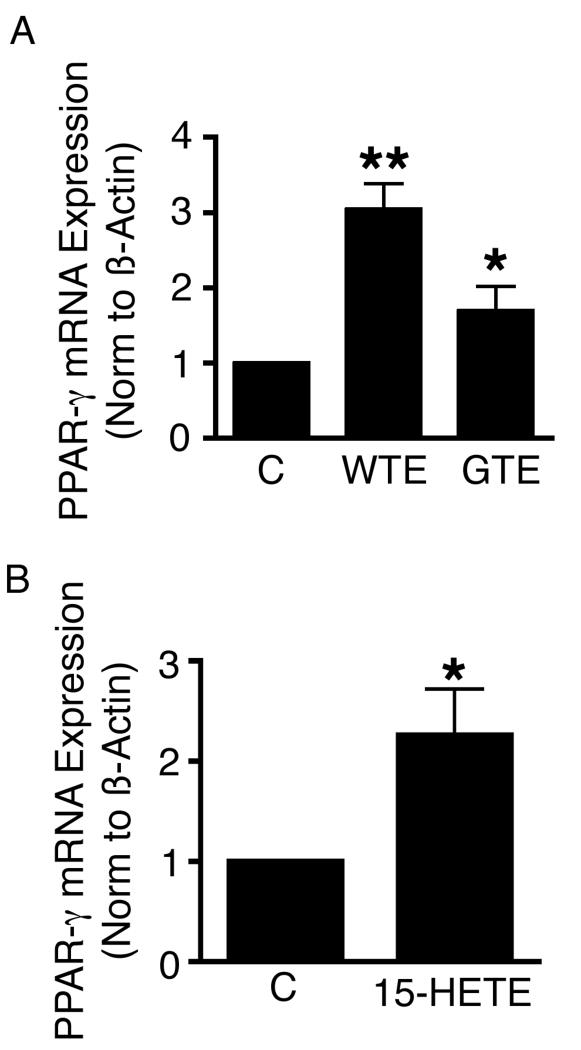
We then looked at the effects of WTE, GTE, and exogenous 15-HETE on PPAR-γ mRNA expression in A549 cells. WTE (containing 7 μg/ml of EGCG), GTE (containing 7 μg/ml of EGCG) and 15-HETE (3 μM) all significantly up-regulated PPAR-γ mRNA expression in A549 cells following 17 h of incubation (Fig. 3A & B). Interestingly, when compared with the same dose of Green tea extract (GTE) with comparable compositions of catechin content (Table I), while WTE and GTE both induced PPAR-γ mRNA expression in A549 cells, WTE was significantly more effective than GTE in the up-regulation of these transcripts.
Figure 3.
WTE (containing 7 μg/ml of EGCG), GTE (containing 7 μg/ml of EGCG) and 15-HETE (3 μM) all significantly up-regulated PPAR-γ mRNA expression in A549 cells following 17 h of incubation. When compared with the same dose of Green tea extract (GTE) with comparable catechin content, WTE was significantly more effective than GTE in the up-regulation of PPAR-γ transcripts. Columns, mean (n = 3); bars, SE. *, P < 0.05, **, P <0.01.
Table I.
| Total catechin | EGCG | |
|---|---|---|
| WTE | 1.73 mg/ml | 0.93 mg/ml |
| GTE | 1.67mg/ml | 0.98 mg/ml |
WTE induces PPAR-γ activation in A549 Cells
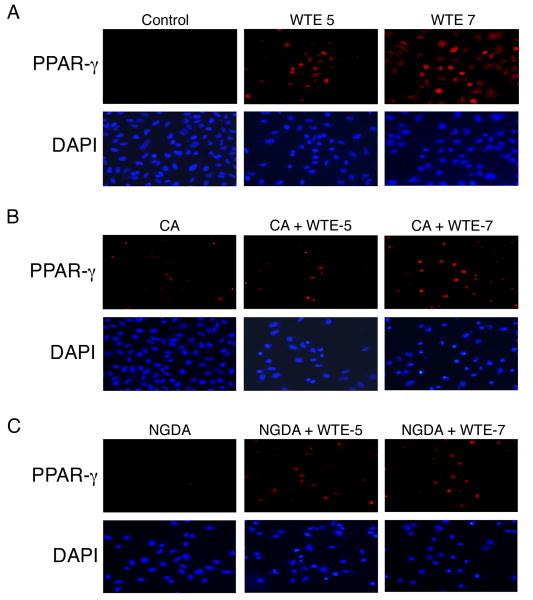
To evaluate the effects of WTE on PPAR-γ activation, Immunofluorescence analysis of PPAR-γ in conditioned cells was performed. Treatment with WTE (containing 5 μg/ml and 7 μg/ml of EGCG) significantly increased nuclear staining of PPAR-γ in a dose dependent manner, and pretreatment with the 15-LOX inhibitors, caffeic acid (CA, 2.2 µM) or NGDA (20 µM) abrogated the WTE-induced nuclear staining of PPAR-γ. Images were taken using a Nikon Eclipe E400 microscope at 20× magnification (Fig. 4).
Figure 4.
To evaluate the effects of WTE on PPAR-γ activation, Immunofluorescence analysis of PPAR-γ in conditioned cells was performed. Nuclear translocation is a necessary step for, and is a marker of, PPAR-γ activation (37, 38). Treatment with WTE significantly increased translocation of PPAR-γ from the cytoplasm to the nucleus in a dose dependent manner and pretreatment with CA or NGDA abrogated the WTE-induced nuclear staining of PPAR-γ. Representative photomicrograph of Images taken at 20 × magnification. (n = 2).
WTE significantly increases 15-HETE production and 15-LOX-1 and -2 expression by A549 cells
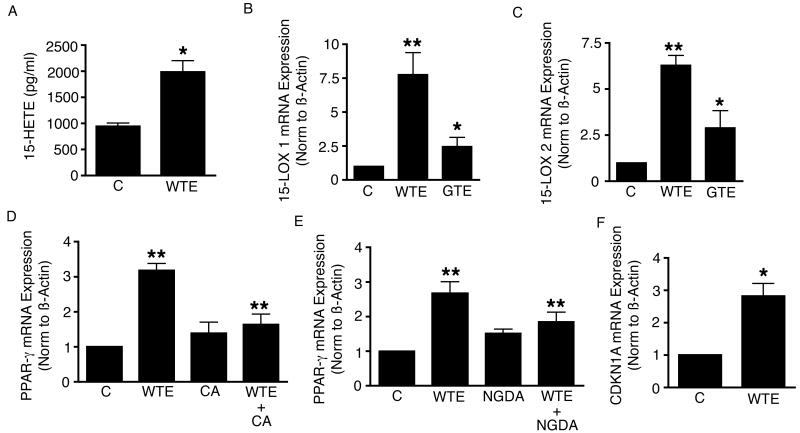
We demonstrate that WTE (containing 5 μg/ml) of EGCG significantly increases 15-HETE production (Fig. 5A), 15-LOX-1 and -2 mRNA expression, corresponding to the increase in apoptotic cell death (Fig. 5B & C). Again, when compared with the same dose of GTE with comparable catechin content, WTE was significantly more effective than GTE in the up-regulation of these transcripts.
Figure 5.
WTE (containing 7 μg/ml of EGCG) significantly increases 15-HETE production (Fig. 5A) and 15-LOX 1 and 2 expression (Fig. 5B & C) by A549 cells. Again, when compared with the same dose of GTE with comparable composition of catechin content, WTE was significantly more effective than GTE in the up-regulation of these transcripts. Preconditioning of A549 cells with CA (Fig. 5D), as well as NGDA (Fig. 5E), abrogate WTE (containing 5 μg/ml of EGCG) - induced up-regulation of PPAR-γ mRNA expression. Columns, mean (n = 3); bars, SE. **, P <0.01. WTE also significantly up-regulated CDKN1A mRNA expression in A549 cells (Fig. 5F). Columns, mean (n = 3); bars, SE. *, P < 0.05.
Inhibition of 15-LOX abrogated the WTE-induced up-regulation of PPAR-γ mRNA expression in A549 cells
When A549 cells were preconditioned with the 15-LOX inhibitors, CA, as well as NGDA, WTE (containing 5 μg/ml of EGCG) loses it's ability to up-regulate PPAR-γ mRNA expression in A549 cells (Fig. 5D & E).
WTE significantly up-regulated CDKN1A mRNA expression in A549 cells
WTE (containing 5 μg/ml of EGCG) also significantly up-regulated the mRNA expression of CDKN1A, a target gene of PPAR-γ in A549 cells (Fig. 5F).
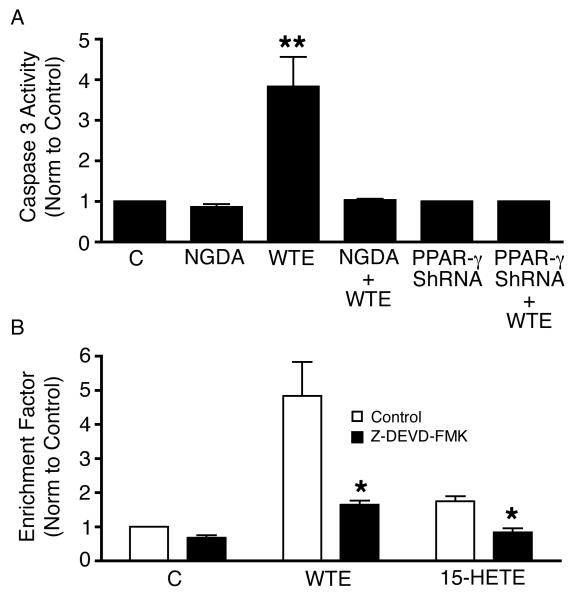
WTE induces Caspase 3 activation in A549 cells
To determine whether the WTE-induced apoptosis in A549 cells involves caspase 3 activation, caspase 3 activities in conditioned cells were measured. Treatment with WTE significantly increased caspase 3 activity; inhibition of 15-LOX with NGDA and PPAR-γ with PPAR-γ ShRNA both abrogated WTE-induced activation of caspase 3 (Fig. 6A). Inhibition of caspase 3 with DEVD-FMK (100 µM) abrogated the WTE-induced apoptosis (Fig. 6B).
Figure 6.
WTE induces Caspase 3 activation in A549 cells. WTE (containing 5 μg/ml of EGCG) induces apoptosis in A549 cells via caspase 3 activation. Inhibition of 15-LOX with NGDA and PPAR-γ with PPAR-γ ShRNA (Fig. 6A) both abrogated WTE-induced activation of caspase 3. Inhibition of caspase 3 with DEVD-FMK abrogated the WTE-induced apoptosis (Fig. 6B). Columns, mean (n = 3); bars, SE. **, P <0.01.
Discussion
In this study, we report the effect of WTE on inducing apoptosis in NSCLC cells. We further demonstrate, for the first time, the roles of novel signaling pathways - PPAR-γ and 15 LOX in mediating tea -induced apoptosis, which involves the activation of caspase 3.
PPARs are nuclear hormone receptors that act as ligand-activated transcription factors. They are involved in lipid transport and metabolism (25). The transcriptional activity of PPARs is regulated by fatty acid binding. PPARs can bind to DNA only as a heterodimer with the retinoid X receptor (RXR). Three PPAR isotypes have been identified: α, β and γ. PPAR-γ stimulates lipolysis of circulating triglycerides and the subsequent uptake of fatty acids into adipose cells. As a nuclear receptor which plays pivotal role in regulating adipocyte differentiation, glucose and lipid homeostasis and intracellular insulin-signaling events, PPAR-γ has been receiving growing interests for it's involvement in carcinogenesis. Reduced PPAR-γ expression within the tumor is associated with poor prognosis in lung cancer patients. Synthetic PPAR-γ agonists such as the thiazolidinedione (TZD) class of anti-diabetic drugs can inhibit growth of NSCLC cells in vitro, and block tumor progression in xenograft models (26). Preclinical studies in NSCLC, colon and prostate cancer cell lines have also implicated the involvement of PPAR-γ in mediating Apoptosis with 15-HETE acting as its ligand (19, 27, 20). Our data indicates that the WTE-induced apoptosis in NSCLC cells is, in part, mediated by increased 15-HETE production, 15-LOX-1, 15-LOX-2 and PPAR-γ expression, resulting in increased PPAR-γ activation.
LOXs are lipid-peroxidizing enzymes that are categorized according to their position in the oxygenation of arachidonic acid, e.g. 15-LOX oxygenates arachidonic acid at C-15 (28). 15-LOX-1 metabolizes not only linoleic acid, the predominant polyunsaturated fatty acid in the human diet, to 13(S)-hydroxyoctadecadienoic acid (13-HODE) but also arachidonic acid to 15-HETE. Numerous studies on LOX expression in human tumors as well as in animal models indicate different roles of the distinct LOX in carcinogenesis (29). Data suggest that the 5-LOX and platelet-type 12-LOX exhibit procarcinogenic activities, whereas 15-LOX-1 and 15-LOX-2 may suppress carcinogenesis. 15-HETE is the most abundant eicosanoid in human bronchi (30). In addition to playing a role in apoptosis, 15-HETE has been shown to be chemotactic and chemokinetic for polymorphonuclear leukocytes and vascular smooth muscle cells. Moreover, 15-HETE also acts as a second messenger in angiotensin II induced aldosterone production. Evidence suggests many other biologic activities, including hyperphosphorylation of several major cytoskeletal proteins and enhancing surface expression of integrin receptors (31). Because 15-LOX-1 is known to preferentially metaboiize linoleic acid to 13-S-HODE (29), we evaluated 13-S-HODE levels in the culture supernatants and found that they were below the detection limit of the EIA (data not shown).
To further substantiate the involvement of PPAR-γ, we measured the effects of WTE on CDKN1A mRNA expression, a known target gene of PPAR-γ (32), and found that WTE significantly up-regulated CDKN1A mRNA expression. CDKN1A gene encodes the P21 (WAF1) protein, which functions as a regulator of cell cycle progression. This protein has been reported to be specifically cleaved by caspase 3-like caspases, which may be instrumental in the execution of apoptosis following caspases activation (33).
Caspase 3 is an intracellular cysteine protease that exists as a proenzyme, becoming activated during the sequence of events associated with apoptosis. Detection of caspase-3 activity in cells is an important mean for evaluating mechanisms of apoptosis induced by a wide variety of apoptotic signals (34). In our study we found that WTE-induced apoptosis in NSCLC cells involves activation of caspase 3 and inhibition of both PPAR-γ and 15-LOX abrogated the effects of WTE on caspase 3 activities, indicating that both PPAR-γ and 15-LOX pathways are involved in caspase 3 activation.
EGCG, the major constituent of tea catechins, which is found in higher concentrations in white tea then green tea, has been shown to inhibit benxo[a]pyerene-induced mutagenesis in the lung of rpsl transgenic mice (35). Santana-Rios et al reported that white tea exhibited greater antimutagenic activity in the Samonella assay than green tea (16). In additional studies, white tea inhibits 2-Amino-1-Methy-6-Phenylimidazo[4,5-b]Pyridine (PhIP)-induced clonic aberrant crypt foci in male F344 rats by altering the expression of carcinogen-metabolizing enzyme (14). White tea also suppressed intestinal polyp formation to a degree that was equivalent to that obtained with sulindac, a potent nonsteroidal anti-inflammatory drug (36). It is important to point out that the prevailing belief of white tea being more effective than green tea is primarily founded on the higher EGCG content in white tea. As such, this advantage over green tea may be lost when the tea extracts are standardized to contain the same amount of EGCG. However, in our study, we found that standardized WTE containing the same amount of total catechin and EGCG levels as GTE from the same vendor, were actually more potent in inducing apoptosis and augmenting 15-LOX-1, 15-LOX-2 and PPAR-γ mRNA expression. What substances in the WTE that may actually be responsible for this phenomenon is unclear. Conceivably, there may be micronutrient or substances that have not yet been characterized and may be a subject of interest for future investigation. Our observations further support the potential advantage of WTE over GTE. This is of particular interests in view of the recent concern over the emerging data showing limited bioavailability of EGCG with green tea studies in vivo (5). Our findings provide important rationales to further investigate the anti-carcinogenic property of WTE and its potential for lung cancer chemoprevention.
Acknowledgments
We wish to thank Miss Deborah Ritter for her excellent technical assistance.
Studies were supported by grants from the National Center for Complementary and Alternative Medicine (JTM, R21AT4503), National Cancer Institute (JTM, U01CA096134), Stop Cancer Miller Family Grant.
References
- 1.Tijburg LB, Mattern T, Folts JD, Weisgerber UM, Katan MB. Tea flavonoids and cardiovascular disease: a review. Crit Rev Food Sci Nutr. 1997;37:771–85. doi: 10.1080/10408399709527802. [DOI] [PubMed] [Google Scholar]
- 2.Dreosti IE. Antioxidant polyphenols in tea, cocoa, and wine. Nutrition. 2000;16:692–4. doi: 10.1016/s0899-9007(00)00304-x. [DOI] [PubMed] [Google Scholar]
- 3.Siddiqui IA, Adhami VM, Saleem M, Mukhtar H. Beneficial effects of tea and its polyphenols against prostate cancer. Mol Nutr Food Res. 2006;50:130–43. doi: 10.1002/mnfr.200500113. [DOI] [PubMed] [Google Scholar]
- 4.Yuan JM, Gao YT, Yang CS, Yu MC. Urinary biomarkers of tea polyphenols and risk of colorectal cancer in the Shanghai Cohort Study. Int J Cancer. 2006 doi: 10.1002/ijc.22460. [DOI] [PubMed] [Google Scholar]
- 5.Yang CS, Sang S, Lambert JD, Hou Z, Ju J, Lu G. Possible mechanisms of the cancer-preventive activities of green tea. Mol Nutr Food Res. 2006;50:170–5. doi: 10.1002/mnfr.200500105. [DOI] [PubMed] [Google Scholar]
- 6.Dashwood RH. Early detection and prevention of colorectal cancer. Oncol Rep. 1999;6:277–81. [PubMed] [Google Scholar]
- 7.Ahmed S, Rahman A, Hasnain A, Lalonde M, Goldberg VM, Haqqi TM. Green tea polyphenol epigallocatechin-3-gallate inhibits the IL-1 beta-induced activity and expression of cyclooxygenase-2 and nitric oxide synthase-2 in human chondrocytes. Free Radic Biol Med. 2002;33:1097–105. doi: 10.1016/s0891-5849(02)01004-3. [DOI] [PubMed] [Google Scholar]
- 8.Metz N, Lobstein A, Schneider Y, et al. Suppression of azoxymethane-induced preneoplastic lesions and inhibition of cyclooxygenase-2 activity in the colonic mucosa of rats drinking a crude green tea extract. Nutr Cancer. 2000;38:60–4. doi: 10.1207/S15327914NC381_9. [DOI] [PubMed] [Google Scholar]
- 9.Luceri C, Caderni G, Sanna A, Dolara P. Red wine and black tea polyphenols modulate the expression of cycloxygenase-2, inducible nitric oxide synthase and glutathione-related enzymes in azoxymethane-induced f344 rat colon tumors. J Nutr. 2002;132:1376–9. doi: 10.1093/jn/132.6.1376. [DOI] [PubMed] [Google Scholar]
- 10.Lu G, Liao J, Yang G, Reuhl KR, Hao X, Yang CS. Inhibition of adenoma progression to adenocarcinoma in a 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis modelin A/J mice by tea polyphenols and caffeine. Cancer Res. 2006;66:11494–501. doi: 10.1158/0008-5472.CAN-06-1497. [DOI] [PubMed] [Google Scholar]
- 11.Record IR, Dreosti IE. Protection by tea against UV-A + B-induced skin cancers in hairless mice. Nutr Cancer. 1998;32:71–5. doi: 10.1080/01635589809514721. [DOI] [PubMed] [Google Scholar]
- 12.Sato D. Inhibition of urinary bladder tumors induced by N-butyl-N-(4-hydroxybutyl)-nitrosamine in rats by green tea. Int J Urol. 1999;6:93–9. doi: 10.1046/j.1442-2042.1999.06239.x. [DOI] [PubMed] [Google Scholar]
- 13.de Boer JG, Yang H, Holcroft J, Skov K. Chemoprotection against N-nitrosomethylbenzylamine-induced mutation in the rat esophagus. Nutr Cancer. 2004;50:168–73. doi: 10.1207/s15327914nc5002_6. [DOI] [PubMed] [Google Scholar]
- 14.Santana-Rios G, Orner GA, Xu M, Izquierdo-Pulido M, Dashwood RH. Inhibition by white tea of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine-induced colonic aberrant crypts in the F344 rat. Nutr Cancer. 2001;41:98–103. doi: 10.1080/01635581.2001.9680618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung FL, Morse MA, Eklind KI, Xu Y. Inhibition of tobacco-specific nitrosamine-induced lung tumorigenesis by compounds derived from cruciferous vegetables and green tea. Ann N Y Acad Sci. 1993;686:186–201. doi: 10.1111/j.1749-6632.1993.tb39174.x. [DOI] [PubMed] [Google Scholar]
- 16.Santana-Rios G, Orner GA, Amantana A, Provost C, Wu SY, Dashwood RH. Potent antimutagenic activity of white tea in comparison with green tea in the Salmonella assay. Mutat Res. 2001;495:61–74. doi: 10.1016/s1383-5718(01)00200-5. [DOI] [PubMed] [Google Scholar]
- 17.Ara G, Teicher BA. Cyclooxygenase and lipoxygenase inhibitors in cancer therapy. Prostaglandins Leukot Essent Fatty Acids. 1996;54:3–16. doi: 10.1016/s0952-3278(96)90075-7. [DOI] [PubMed] [Google Scholar]
- 18.Mao JT, Cui X, Reckamp K, et al. Chemoprevention strategies with cyclooxygenase-2 inhibitors for lung cancer. Clin Lung Cancer. 2005;7:30–9. doi: 10.3816/CLC.2005.n.019. [DOI] [PubMed] [Google Scholar]
- 19.Shankaranarayanan P, Nigam S. IL-4 induces apoptosis in A549 lung adenocarcinoma cells: evidence for the pivotal role of 15-hydroxyeicosatetraenoic acid binding to activated peroxisome proliferator-activated receptor gamma transcription factor. J Immunol. 2003;170:887–94. doi: 10.4049/jimmunol.170.2.887. [DOI] [PubMed] [Google Scholar]
- 20.Shappell SB, Gupta RA, Manning S, et al. 15S-Hydroxyeicosatetraenoic acid activates peroxisome proliferator-activated receptor gamma and inhibits proliferation in PC3 prostate carcinoma cells. Cancer Res. 2001;61:497–503. [PubMed] [Google Scholar]
- 21.Dashwood WM, Orner GA, Dashwood RH. Inhibition of beta-catenin/Tcf activity by white tea, green tea, and epigallocatechin-3-gallate (EGCG): minor contribution of H(2)O(2) at physiologically relevant EGCG concentrations. Biochem Biophys Res Commun. 2002;296:584–8. doi: 10.1016/s0006-291x(02)00914-2. [DOI] [PubMed] [Google Scholar]
- 22.Sartippour M, Heber D, Lu Q, Ma M, Go VL, Nguyen M. Green tea inhibits breast cancer growth and angiogenesis. Nut Cancer. 2001;40:149–56. doi: 10.1207/S15327914NC402_11. [DOI] [PubMed] [Google Scholar]
- 23.Chow HH, Hakim IA, Vining DR, et al. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of Polyphenon E in healthy individuals. Clin Cancer Res. 2005;11:4627–33. doi: 10.1158/1078-0432.CCR-04-2549. [DOI] [PubMed] [Google Scholar]
- 24.Mao JT, Tsu IH, Dubinett SM, et al. Modulation of pulmonary leukotriene B4 production by cyclooxygenase-2 inhibitors and lipopolysaccharide. Clin Cancer Res. 2004;10:6872–8. doi: 10.1158/1078-0432.CCR-04-0945. [DOI] [PubMed] [Google Scholar]
- 25.Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature. 2000;405:421. doi: 10.1038/35013000. [DOI] [PubMed] [Google Scholar]
- 26.Satoh T, Toyoda M, Hoshino H, et al. Activation of peroxisome proliferator-activated receptor-gamma stimulates the growth arrest and DNA-damage inducible 153 gene in non-small cell lung carcinoma. Oncogene. 2002;21:2171–80. doi: 10.1038/sj.onc.1205279. [DOI] [PubMed] [Google Scholar]
- 27.Chen GG, Xu H, Lee JF, et al. 15-hydroxy-eicosatetraenoic acid arrests growth of colorectal cancer cells via a peroxisome proliferator-activated receptor gamma-dependent pathway. Int J Cancer. 2003;107:837–43. doi: 10.1002/ijc.11447. [DOI] [PubMed] [Google Scholar]
- 28.Hsi LC, Wilson L, Nixon J, Eling TE. 15-lipoxygenase-1 metabolites down-regulate peroxisome proliferator-activated receptor gamma via the MAPK signaling pathway. J Biol Chem. 2001;276:34545–52. doi: 10.1074/jbc.M100280200. [DOI] [PubMed] [Google Scholar]
- 29.Shureiqi I, Jiang W, Zuo X, et al. The 15-lipoxygenase-1 product 13-S-hydroxyoctadecadienoic acid down-regulates PPAR-delta to induce apoptosis in colorectal cancer cells. Proc Natl Acad Sci. 2003;100:9968–73. doi: 10.1073/pnas.1631086100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lai CK, Phillips GD, Jenkins JR, Holgate ST. The effect of inhaled 15-(s)-hydroxyeicosatetraenoic acid (15-HETE) on airway calibre and non-specific responsiveness in normal and asthmatic human subjects. Eur Respir J. 1990;3:38–45. [PubMed] [Google Scholar]
- 31.Moreno JJ. New aspects of the role of hydroxyeicosatetraenoic acids in cell growth and cancer development. Biochem Pharmacol. 2009;77:1–10. doi: 10.1016/j.bcp.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 32.Chang TH, Szabo E. Induction of differentiation and apoptosis by ligands of peroxisome roliferator-activated receptor g in non-small cell lung cancer. Cancer Research. 2000;60:1129–38. [PubMed] [Google Scholar]
- 33.Jin YH, Yoo KJ, Lee YH, Lee SK. Caspase 3-mediated cleavage of p21WAF1/CIP1 associated with the cyclin A-cyclin-dependent kinase 2 complex is a prerequisite for apoptosis in SK-HEP-1 cells. J Biol Chem. 2000;275:30256–63. doi: 10.1074/jbc.M001902200. [DOI] [PubMed] [Google Scholar]
- 34.Nicholson DW, Ali A, Thornberry NA, et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 35.Muto S, Yokoi T, Gondo Y, et al. Inhibition of benzo[a]pyrene-induced mutagenesis by (-)-epigallocatechin gallate in the lung of rpsL transgenic mice. Carcinogenesis. 1999;20:421–4. doi: 10.1093/carcin/20.3.421. [DOI] [PubMed] [Google Scholar]
- 36.Orner GA, Dashwood WM, Blum CA, Diaz GD, Li Q, Dashwood RH. Suppression of tumorigenesis in the Apc(min) mouse: down-regulation of beta-catenin signaling by a combination of tea plus sulindac. Carcinogenesis. 2003;24:263–7. doi: 10.1093/carcin/24.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bishop-Bailey D, Hla T. Endothelial cell apoptosis induced by the peroxisome proliferator-activated receptor (PPAR) ligand 15-deoxy-Delta12, 14-prostaglandin J2. J Biol Chem. 1999;274:17042–8. doi: 10.1074/jbc.274.24.17042. [DOI] [PubMed] [Google Scholar]
- 38.Hashimoto K, Ethridge RT, Saito H, Rajaraman S, Evers BM. The PPARgamma ligand, 15d-PGJ2, attenuates the severity of cerulein-induced acute pancreatitis. Pancreas. 2003;27:58–66. doi: 10.1097/00006676-200307000-00009. [DOI] [PubMed] [Google Scholar]