Abstract
We modified our established and clinically relevant ARDS model of smoke inhalation injury and septic shock by administration of combined antibiotics (AB) such as piperacillin and ciprofloxacin, to more closely mimic the clinical intensive care setting. Twenty-three sheep were subjected to the injury, and allocated to 4 groups for a 96hrs study period: sham (n=5 non-injured); control (n=6: injured); AB6h (n=6: injured, antibiotics started 6hrs post-injury); AB12h (n=6: injured, antibiotics started 12hrs post-injury). All sham animals survived 96hrs. Control, AB6h, AB12h groups reached criteria of septic shock within 12hrs post-injury. All controls died within 36hrs. Eighty three percent of AB6h and fifty percent of AB12h survived 96hrs. Median survival times were significantly improved in the treated groups compared with the control group: 24hrs in control vs. 80.5hrs in AB6h, and 65hrs in AB12h animals. Combined Ciprofloxacin and Piperacillin therapy was effective, reduced nitric oxide production and mortality, and will allow future long-term studies in this model.
Introduction
More than 30% of thermally-injured patients admitted to burn centers in the United States have concomitant smoke inhalation injury[1]. Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) following smoke inhalation are frequently associated with pneumonia and sepsis and are a major cause of morbidity and mortality in thermally-injured patients[2]. Appropriate and timely antimicrobial treatment is crucial to improve patients outcome and to reduce mortality rate[3]. The development of pneumonia and sepsis is a common complication in patients who require ventilator support after smoke inhalation injury. Pseudomonas aeruginosa is an opportunistic pathogen that represents one of the most prevalent causes of nosocomial infection in the world[4; 5]. Our group developed a clinically relevant ovine model of septic shock that involves the insufflation of the cotton smoke followed by the instillation of live Ps. aeruginosa into the lung. This model achieves ALI associated with septic shock[6]. In the present study, we investigated the administration of piperacillin and ciprofloxacin as a treatment of Ps. aeruginosa pneumonia and septic shock. This combination is a recommended initial antimicrobial therapy for severe pneumonia due to Ps. aeruginosa[7]. Both antibiotics are broad-spectrum and well-tolerated[8; 9]. This model follows the recommendations of E. A. Deitch, who suggests that animal models for sepsis studies receive adequate fluid resuscitation and effective antibiotic therapy in order to mirror current clinical practice[10]. We hypothesized that this modification of our model could be used as the basis for investigating new strategies for treating septic patients with acute lung injury. A long-term model will allow us to test different treatment modalities—not only the short term effects at the shock stage, but also any long term effects that may present after recovery. In addition, it will allow mortality studies for drug development.
Materials and methods
Animal Care
The Institutional Animal Care and Use Committee at the University of Texas Medical Branch (UTMB) approved this study. The guidelines of the National Institutes of Health (NIH) for the care and use of experimental animals were carefully followed. Animals were individually housed in metabolic cages and, although the injury was accomplished under deep anesthesia, sheep were studied in the awake state.
Surgical preparation and experimental protocol
Twenty-three female Merino sheep (34±1 kg) were included in this study. For the operative procedures, sheep were anesthetized, and under aseptic conditions, the animals were chronically instrumented for hemodynamic monitoring with a right femoral artery catheter, a 7-French Swan-Ganz™ thermodilution catheter, and a left atrial catheter, as previously described[6; 11]. Following the operative procedure, catheters were flushed with heparin, and the animals were allowed to recover for seven days. During this time they had free access to food and water. One day before the experiment was started; catheters were connected to pressure transducers (Model PX3X3, Baxter Edwards Critical Care Division, Irvine, CA) with continuous flushing devices. Electronically calculated mean pressures were recorded on a monitor with graphic and digital displays, Cardiac output (CO), core body temperature, arterial blood gases, and carboxyhemoglobin (COHb) was measured as reported elsewhere[12]. Cardiac index (CI), and systemic vascular resistance index (SVRI) were calculated using standard equations.
For microbial identification, Pseudomonas aeruginosa (Strain 12/4/4) that was isolated and cultured from a male burn patient at Brooke Army Medical Center in San Antonio, Texas, and evaluated for susceptibility to piperacillin and ciprofloxacin, was used as recently described[13; 14]. The microbiology report showed high susceptibility of the strain 12/4/4 to both piperacillin and ciprofloxacin. Following a baseline measurement, sheep were randomly allocated to one of the four groups: sham (n=5: uninjured, untreated), control (n=6: injured, untreated), AB6h (n=6: injured, treated with combined antibiotics beginning from 6h post-injury), and AB12h (n=6: injured, treated with combined antibiotics beginning from 12h post-injury). A tracheotomy was performed under ketamine anesthesia (10 mg/kg), and a Foley urinary retention catheter was placed in all animals. Anesthesia was then maintained using 1.5–2.5% halothane (Vedco Inc., St. Joseph, MO) in O2. The control and treatment animals were subjected to smoke inhalation injury (4•12 breaths of cotton smoke, <40°C), according to an established protocol[15]. The sham group received 4•12 breaths of room air. Arterial COHb plasma concentrations were determined after each set of smoke or air inhalation and served as an index of lung injury.
After smoke inhalation, an experimental bacterial solution was instilled into the lungs of control and treatment animals using a bronchoscope (Model PF-P40, Olympus America Inc. Melville, NY). Live Pseudomonas aeruginosa 3~5•1011 CFU were suspended in 30ml of saline and instilled into the right lower and middle lobes (10ml each) as well as the left lower lobe (10ml). The sham group received only the vehicle (normal saline) administered in the same fashion. Anesthesia was then discontinued and the sheep were allowed to awaken[11]. Combined antibiotics (ciprofloxacin [0.4g, every 12hrs] plus piperacillin [3g, every 6hrs]) were intravenously administered beginning 6 and 12 hrs post injury (Fig. 1A). All animals were mechanically ventilated (Servo-Ventilator 900C, Siemens, Elema, Sweden) with a FiO2 1.0, a tidal volume of 15 ml/kg and a respiration rate of 20/min. For the duration of the 96h study period, ventilator settings were periodically adjusted to maintain arterial pCO2 ~10% below baseline values, because this approach allows invasive ventilation in the awake state. The ventilatory settings were adapted to the physiology of the sheep. Since the lungs of sheep have a higher compliance than those of human’s, a tidal volume of 15ml/kg body weight was used to prevent atelectasis. Such volume result in peak and plateau pressures of approximately 20 mmHg and is similar to an 8–10 ml/kg tidal volume in humans. Positive end-expiratory pressure (PEEP) remained at a fixed level of 6 cmH2O to avoid ventilation-related differences in the study groups. These ventilator settings were chosen in accordance with those originally described for this model by Murakami et al[6]. All animals were fluid resuscitated with lactated Ringer’s solution, started with an infusion rate of 2 mL•kg−1•h−1. The rate of fluid administration was adjusted to maintain hematocrit ± 10% of baseline. During the study period, all animals had free access to food, but not to water to precisely control the fluid balance[14]. Arterial blood samples were frequently taken. The animals were sacrificed if they matched any one of the termination criteria mentioned below; all others were sacrificed at 96hrs post-injury by intravenous injection of 60 mL saturated potassium chloride under ketamine anesthesia (10 mg/kg)[6]. The termination criteria were arterial oxygen partial pressure (PaO2) <50mmHg with fraction of inspired oxygen (FiO2) =1.0, PaCO2>100mmHg, or systolic arterial blood pressure <50mmHg. The cardiopulmonary variables were continuously monitored during the study.
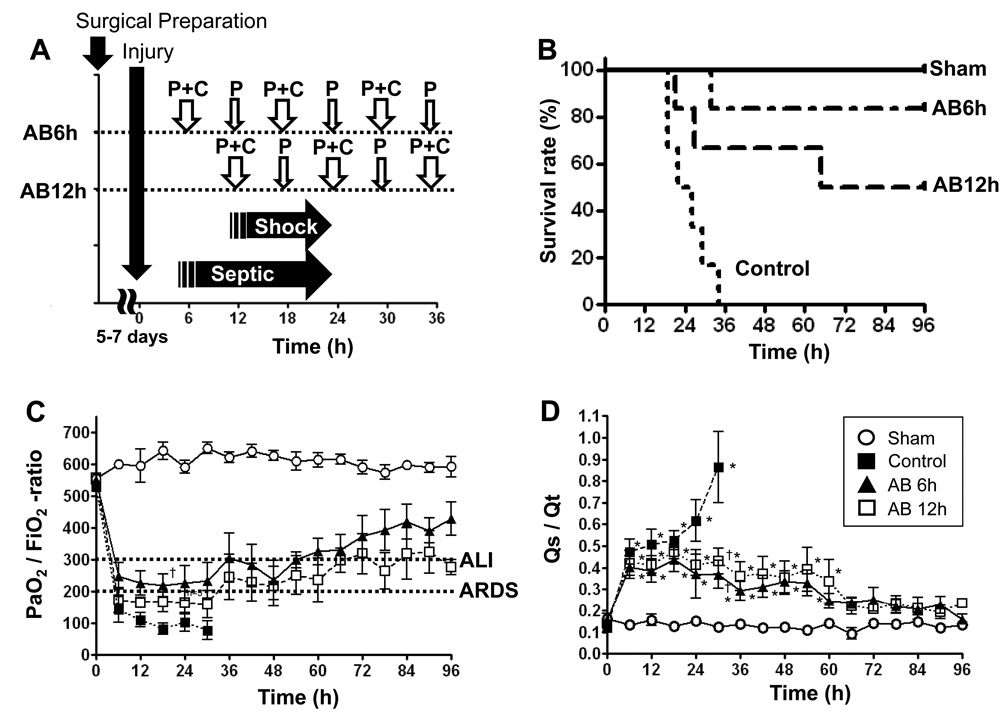
Fig. 1.
A Time point of protocol. After 5 to 7 days of recovery from surgical preparation, injury was induced. The injured group became septic before 6hrs post injury and septic shock before 12hrs post injury. AB6h and AB12h groups started antibiotics at 6hrs and 12hrs, respectively. P+C = Piperacillin + Ciprofloxacin, P = Piperacillin
B Survival in Sham, Control, and Treated animals. Kaplan-Meier curve of sham (n=5), control (n=6), AB6h (n=6), and AB12h (n=6) animals is shown. Animal survival for 96hrs (4days) were considered survivors and sacrificed. There were statistically significant differences for survival between control and AB6h (p=0.0015), and control and AB12h groups (p=0.026) at 96hrs.
C/D Changes in PaO2/FiO2-ratio and pulmonary shunt fraction (Qs/Qt). Data are expressed as mean ± SEM. Significance was assumed when p was less than 0.05; *vs. sham; †vs. control.
The concentration of NOx (total amount of nitric oxide metabolites) in the plasma was measured intermittently. Plasma samples were subjected to NOx reduction using vanadium (III) as a reducing agent in a commercial instrument (model 745, Antek Instruments, Houston, TX). The resulting nitric oxide (NO) was measured with a chemiluminescent NO analyzer (model 7020, Antek) and was recorded by dedicated software as the NO content (in µM) [11].
Statistical analysis
For statistical analysis, GraphPad Prism 4 software (GraphPad Software Inc., San Diego CA) was used. Paired and unpaired t-tests were used to detect differences between and within groups. Furthermore, linear trends within the results were investigated using regression analysis. A logrank test was used to detect differences for survival between control and treated groups. P < 0.05 was considered statistically significant. Data are presented as means ± standard errors of the mean.
Results
Injury and survival
All sham sheep survived over 96hrs. All control sheep died or reached the termination criteria before 36hrs post injury (Fig. 1B). Survival rate of the AB6h and AB12h groups were 83% and 66% at 48hrs and 83% and 50% at 96hrs post-injury, respectively (Fig.1B). Median survival time was significantly improved in treated groups compared to the control group (24hrs in control group vs. 80.5hrs in AB6h group and 65hrs in AB12h group). There were statistically significant differences for survival between control and AB6h (p=0.0015), and control and AB12h groups (p=0.026) at 96hrs. All injured sheep reached the sepsis criteria[16] as described by Bone et al. before 6hrs post injury.
Bacterial culture
Only one of 6 in AB6h and two of 6 in AB12h animals developed bacteremia. All control animals developed bacteremia.
Changes in pulmonary gas exchange
PaO2/FiO2 ratio significantly decreased in control and treated groups compared to baseline values. However, treatment prevented any further decrease in PaO2/FiO2 ratio and showed signs of improvement (Fig. 1C). Results from the surviving sheep in the AB6h and AB12h groups showed a statistically significant increase in the PaO2/FiO2 ratio from 256 ± 28 at 12 hrs to 429 ± 53 at 96 hrs (p<0.0001) and 169 ± 29 at 12 hrs to 278 ± 24 at 96 hrs (p=0.0009) respectively. Pulmonary shunt fraction (Qs/Qt) significantly increased in both control and treated groups compared to baseline value, but slightly attenuated in treated groups after 24hrs post-injury. There were significant differences between control and treated groups at 30hrs post-injury (Fig. 1D). Results from the surviving sheep in the AB6h and AB12h groups showed a statistically significant decrease in the pulmonary shunt fraction from 0.35 ± 0.05 at 12 hrs to 0.16 ± 0.02 at 96 hrs (p=0.0013) and 0.44 ± 0.06 at 12 hrs to 0.24 ± 0.02 at 96 hrs (p<0.0001) respectively.
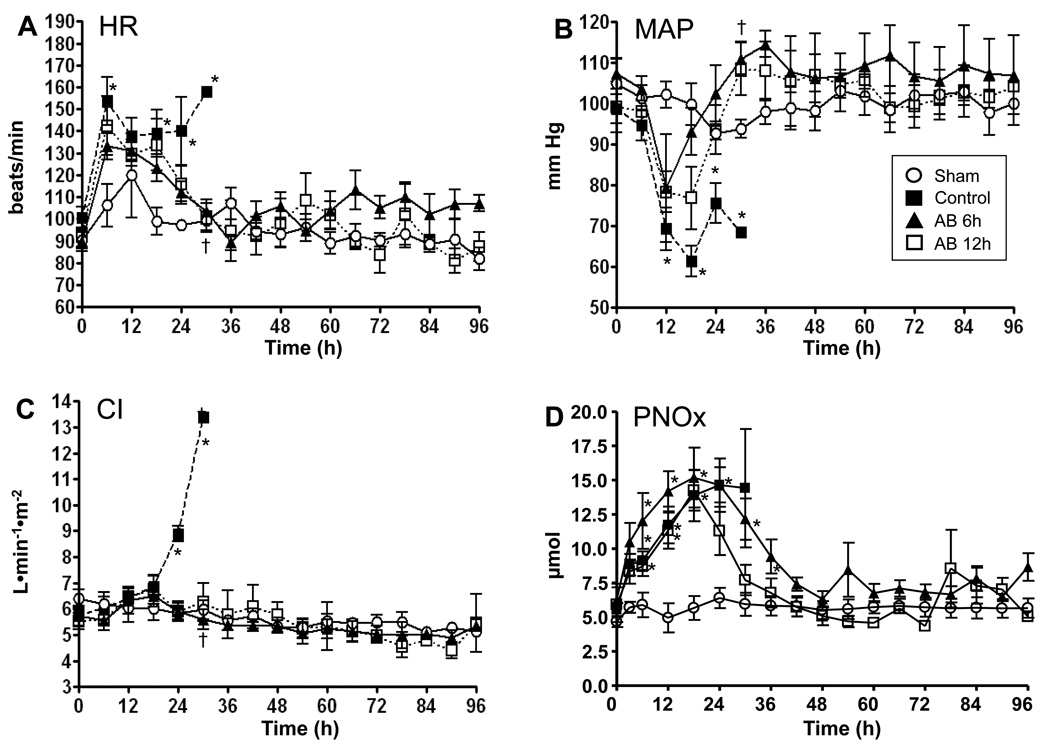
Hemodynamic variables were stable in sham animals. Heart rate significantly increased and reached criteria of sepsis in all injured groups at six hrs. However, this increase in heart rate was reversed by treatment after 12hrs post treatment (18hrs for AB6h and 24hrs for AB12h) (Fig. 2A). MAP significantly decreased in all injured groups before 12hrs. The treated groups recovered from shock 12hrs post treatment (18hrs for AB6h and 24hrs for AB12h) (Fig. 2B). CI increased significantly in the control group, reflecting the typical hyperdynamic response to septic shock after 24hrs (5.7 ± 0.2 at the baseline to 8.9 ± 0.3 at 24hrs) (Fig. 2C). The treated groups did not show a significant increase. CVP, MPAP, PAOP slightly increased in all groups after tracheotomy and mechanical ventilation. There were no significant differences between groups throughout the study (Table).
Fig. 2.
Changes in HR, MAP, CI, and PNOx. HR, heart rate; MAP, mean arterial pressure; CI, cardiac index; PNOx, plasma nitrate-to-nitrite level. Data are expressed as mean ± SEM. Significance was assumed when p was less than 0.05; *vs. sham; †vs. control.
Table.
| Parameter | Groups | BL | 6h | 12h | 24h | 36h | 48h | 72h | 96h |
|---|---|---|---|---|---|---|---|---|---|
| Temp (°C) |
Sham | 38.9±0.1 | 39.1±0.1 | 39.3±0.2 | 39.3±0.1 | 39.5±0.4 | 39.6±0.4 | 39.6±0.4 | 39.3±0.2 |
| n= | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |
| Control | 39.3±0.1 | 40.5±0.4* | 40.0±0.5 | 39.1±0.6 | n/a | n/a | n/a | n/a | |
| n= | 6 | 6 | 6 | 3 | 0 | 0 | 0 | 0 | |
| AB6h | 39.5±0.1 | 40.7±0.2* | 40.5±0.2 | 40.0±0.4 | 40.0±0.1 | 39.8±0.3 | 39.8±0.2 | 40.0±0.3 | |
| n= | 6 | 6 | 6 | 6 | 5 | 5 | 5 | 5 | |
| AB12h | 39.2±0.1 | 40.6±0.3* | 40.4±0.2 | 39.7±0.3 | 40.0±0.3 | 40.1±0.2 | 39.7±0.2 | 39.5±0.1 | |
| n= | 6 | 6 | 6 | 5 | 4 | 4 | 3 | 3 | |
| WBC (×103 cells /mm3) |
Sham | 6.0±0.8 | 6.5±0.8 | 8.0±1.0 | 6.5±0.5 | 7.1±0.0 | 7.8±0.9 | 6.9±1.2 | 4.2±0.3 |
| n= | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |
| Control | 5.2 ± 0.4 | 1.2 ± 0.1* | 1.3 ± 0.4 | 1.2 ± 0.3 | n/a | n/a | n/a | n/a | |
| n= | 6 | 6 | 6 | 3 | 0 | 0 | 0 | 0 | |
| AB6h | 5.0±0.6 | 1.7±0.3* | 2.0±0.4 | 2.1±0.5 | 3.6±1.2 | 4.2±1.3 | 4.0±1.0 | 6.5±0.8 | |
| n= | 6 | 6 | 6 | 6 | 5 | 5 | 5 | 5 | |
| AB12h | 5.4±1.0 | 1.5±0.2* | 1.6±0.2 | 1.7±0.1 | 3.1±0.2 | 3.5±0.3 | 4.9±0.5 | 4.5±0.6 | |
| n= | 6 | 6 | 6 | 5 | 4 | 4 | 3 | 3 | |
| Plt (×104 /mm3) |
Sham | 302±55 | 273±56 | 285±55 | 288±61 | 351±64 | 373±52 | 335±70 | 346±70 |
| n= | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |
| Control | 245±18 | 119±48 | 155±47 | 241±21 | n/a | n/a | n/a | n/a | |
| n= | 6 | 6 | 6 | 4 | 0 | 0 | 0 | 0 | |
| AB6h | 341±43 | 108±34 | 201±46 | 295±56 | 193±38 | 205±40 | 202±50 | 230±44 | |
| n= | 6 | 6 | 6 | 6 | 5 | 5 | 5 | 5 | |
| AB12h | 225±13 | 80±25 | 147±24 | 143±32 | 157±38 | 180±20 | 218±54 | 302±44 | |
| n= | 6 | 6 | 6 | 5 | 4 | 4 | 3 | 3 | |
| CVP (mm Hg) |
Sham | 7.2±1.0 | 10.6±0.6 | 11.4±1.2 | 11.0±1.8 | 10.0±1.7 | 9.8±1.2 | 9.4±1.6 | 10.0±0.7 |
| n= | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |
| Control | 6.5±1.1 | 7.5±0.6 | 11.3±1.0 | 16.3±0.9 | n/a | n/a | n/a | n/a | |
| n= | 6 | 6 | 6 | 3 | 0 | 0 | 0 | 0 | |
| AB6h | 7.7±0.8 | 10.2±1.0 | 11.5±1.7 | 11.8±1.9 | 10.2±1.4 | 10.0±1.6 | 8.2±1.6 | 8.0±1.3 | |
| n= | 6 | 6 | 6 | 6 | 5 | 5 | 5 | 5 | |
| AB12h | 7.3±1.3 | 8.2±1.2 | 10.2±1.0 | 13.2±1.4 | 12.3±1.2 | 10.8±1.5 | 8.3±0.7 | 8.3±1.7 | |
| n= | 6 | 6 | 6 | 5 | 4 | 4 | 3 | 3 | |
| MPAP (mm Hg) |
Sham | 21.4±1.8 | 30.0±2.7 | 29.4±2.5 | 24.6±2.1 | 26.2±1.5 | 23.2±1.7 | 21.0±1.4 | 19.6±2.3 |
| n= | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |
| Control | 20.5±1.0 | 24.2±1.6 | 24.7±1.5 | 30.0±0.7 | n/a | n/a | n/a | n/a | |
| n= | 6 | 6 | 6 | 3 | 0 | 0 | 0 | 0 | |
| AB6h | 19.0±0.7 | 24.5±1.6 | 25.5±2.5 | 29.7±3.1 | 25.0±3.3 | 25.2±3.1 | 21.4±0.7 | 24.6±2.2 | |
| n= | 6 | 6 | 6 | 6 | 5 | 5 | 5 | 5 | |
| AB12h | 19.5±0.5 | 22.7±3.5 | 23.2±2.1 | 30.4±2.7 | 25.8±2.3 | 25.5±2.3 | 25.0±2.5 | 24.3±1.5 | |
| n= | 6 | 6 | 6 | 5 | 4 | 4 | 3 | 3 | |
| PAOP (mm Hg) |
Sham | 11.8±0.7 | 16.0±1.0 | 16.4±1.8 | 13.0±1.2 | 15.2±1.5 | 13.2±0.4 | 14.2±1.0 | 13.0±0.5 |
| n= | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |
| Control | 11.7±0.8 | 13.5±1.2 | 14.3±1.1 | 17.7±0.7 | n/a | n/a | n/a | n/a | |
| n= | 6 | 6 | 6 | 3 | 0 | 0 | 0 | 0 | |
| AB6h | 9.3±0.8 | 14.0±1.6 | 14.5±1.4 | 18.2±2.2 | 14.4±1.4 | 14.6±0.9 | 13.0±0.8 | 12.8±1.8 | |
| n= | 6 | 6 | 6 | 6 | 5 | 5 | 5 | 5 | |
| AB12h | 11.0±0.7 | 13.7±1.7 | 13.5±1.3 | 15.6±1.3 | 15.5±1.6 | 13.0±0.7 | 12.3±1.5 | 11.3±0.9 | |
| n= | 6 | 6 | 6 | 5 | 4 | 4 | 3 | 3 | |
| Peak airway pressure |
Sham | 19.1±1.7 | 18.7±2.2 | 20.0±2.4 | 18.6±1.6 | 17.9±2.2 | 18.7±2.3 | 16.9±1.0 | 16.9±1.6 |
| n= | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |
| Control | 19.3±1.6 | 23.2±0.9 | 26.2±2.4 | 33.4±2.8 | n/a | n/a | n/a | n/a | |
| n= | 6 | 6 | 6 | 3 | 0 | 0 | 0 | 0 | |
| AB6h | 20.1±1.2 | 19.7±0.7 | 23.4±1.1 | 30.8±4.4 | 26.8±2.6 | 26.7±4.1 | 25.5±5.8 | 24.2±5.9 | |
| n= | 6 | 6 | 6 | 6 | 5 | 5 | 5 | 5 | |
| AB12h | 20.5±1.4 | 21.7±1.1 | 23.4±1.8 | 29.9±3.9 | 24.7±4.2 | 27.6±5.7 | 24.9±2.3 | 24.0±1.4 | |
| n= | 6 | 6 | 6 | 6 | 4 | 4 | 3 | 3 | |
| Pause airway pressure |
Sham | 17.1±1.8 | 16.4±1.7 | 16.3±1.3 | 16.2±1.4 | 15.6±1.5 | 16.4±2.2 | 14.8±0.7 | 14.2±0.2 |
| n= | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |
| Control | 18.3±1.5 | 22.0±1.0 | 24.0±2.1 | 27.8±2.7 | n/a | n/a | n/a | n/a | |
| n= | 6 | 6 | 6 | 3 | 0 | 0 | 0 | 0 | |
| AB6h | 18.5±1.4 | 18.0±0.5 | 20.2±0.8 | 25.1±3.8 | 20.3±3.0 | 22.1±2.2 | 20.3±3.2 | 19.8±3.3 | |
| n= | 6 | 6 | 6 | 6 | 5 | 5 | 5 | 5 | |
| AB12h | 19.7±1.6 | 19.2±1.0 | 20.4±1.5 | 25.9±2.7 | 23.3±4.1 | 25.7±5.4 | 23.7±2.5 | 23.5±2.4 | |
| n= | 6 | 6 | 6 | 6 | 4 | 4 | 3 | 3 | |
| Fluid balance |
Sham | 0 | −317±148 | −517±229 | −347±188 | −216±191 | −490±162 | −726±513 | −599±552 |
| n= | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |
| Control | 0 | −264±87 | 512±352 | 3584±1209* | n/a | n/a | n/a | n/a | |
| n= | 6 | 6 | 6 | 3 | 0 | 0 | 0 | 0 | |
| AB6h | 0 | −430±39 | −243±324 | 520±936 | −840±526 | −1184±362 | −1425±438 | −372±885 | |
| n= | 6 | 4 | 4 | 4 | 3 | 3 | 3 | 3 | |
| AB12h | 0 | −497±79 | 161±364 | 1049±1028 | 1240±2233 | 555±1990 | −1522±1036 | −1833±888 | |
| n= | 6 | 6 | 6 | 5 | 4 | 4 | 3 | 3 | |
Temp, temperature; WBC, white blood cell count; Plt, platelet count; CVP, central venous pressure; MPAP, mean pulmonary arterial pressure; PAOP, pulmonary artery occlusion pressure; Fluid balance, Acumulated fluid balance;
p < 0.05, *vs. Sham.
Accumulated fluid balance significantly increased in control groups after 18hrs. AB6h and AB12h groups also showed trend of increase at 18hrs, but the increase was not significant. There were no significant differences between groups throughout the study (Table).
Plasma nitrate/nitrite (NOx) levels increased significantly in both control and treated group. High NOx in the treated groups attenuated after 24hrs and showing no significant difference from baseline after 36hrs in AB6h group and 30hrs in AB12h group (Fig. 2D)
Temperature
All injured groups showed a significant increase in core body temperature in comparison with baseline values. (Table)
White Blood Cells dropped significantly and reached sepsis criteria in all injured groups at 6hrs (Table), but slightly recovered in the treated groups. The WBC levels of the treated groups reached the normal range (4–12 × 103 cells/mm3) at 48hrs in the AB6h group (1.7 ± 0.3 at 6hrs to 4.2 ± 1.3 at 48hrs) and at 60hrs in the AB12h group (1.4 ± 0.1 at 6hrs to 4.8 ± 0.7 at 60hrs; Table)
Discussion
Over the last three decades, many sepsis models were developed in order to mimic human sepsis[17; 18]. Most models were of limited use since they do not mimic the typical fall in systemic vascular resistance. Our group investigated a septic model in sheep by intravenous bacteria infusion[19]. Although animals developed septic shock in that model, the mean arterial pressure and blood gas changes were not as severe as anticipated. In addition, when the bacterial infusion was stopped, the animals rapidly recovered their cardiopulmonary status. E.A. Deitch[10] clearly focused on the problems inherent with sepsis models created through intravenous bacteria infusion, which are as follows: a) they do not correlate with clinical disease; b) they typically produce a hypodynamic circulatory response; c) survival time is generally short and therefore there is limited time for progression of disease; d) the serum cytokine response is transient and much greater in magnitude than that observed in septic patients; and e) antisepsis agents shown to be effective in these types of animal models have not been effective when tested in clinical trials[20]. In order to mimic the clinical scenario as closely as possible, our group developed an ovine ARDS model of smoke inhalation injury and pneumonia that solved most of the problems[6]. However, some of our experimental therapeutics were not successful because the animals would succumb to the ravages of the infection. The addition of antibiotics to our model makes it possible to evaluate survival components of anticipated therapies. A mortality of 50% in the AB12h group is comparable to what is seen in human septic shock[21]. Thus, it is possible to determine if agents that affect inflammation or cardiopulmonary function are effective in further decreasing mortality. In addition, it is possible that a therapeutic agent that appeared to improve some aspects of cardiopulmonary function, i.e. myocardial contractility, may even have an adverse effect on mortality. Similarly an anti-inflammatory agent may have positive effects on cardiopulmonary function but stimulate bacterial growth. Clinical studies support appropriate initial antibiotic treatment that covers all likely organisms[22; 23]. Ps. aeruginosa is, however, intrinsically resistant to many antimicrobial drugs. The most active agents are carbapenems, piperacillin, cefepime, ceftazidime, ciprofloxacin, amikacin, and tobramycin; nonetheless, resistance to available anti-pseudomonal agents is increasing[24]. Traditionally, a combination therapy of two of the above mentioned antibiotics is used for treatment of Ps. aeruginosa pneumonia. This regimen increases the chance of effective initial therapy before receiving susceptibility results. Attributable mortality was significantly higher in patients with inappropriate initial antibiotic therapy[25]. Inadequate empirical therapy has been associated with mortality exceeding 30%, and delays in the initiation of appropriate therapy contribute to increased length of hospital stay and persistence of infection. Initial therapy needs to be a broad-spectrum, well-tolerated treatment with strong therapeutic effects[5].
Patients suffering ALI/ARDS from smoke inhalation injury, frequently have concomitant burn injuries to the skin. In those patients, massive fluid requirements and kidney failure are often observed; pneumonia/sepsis itself causes vascular leakage and kidney failure. As a result, management of the individual patient’s pharmacokinetics is very difficult. Because of its relatively narrow margin of safety, the administration of aminoglycosides often results in side effects, including nephrotoxicity[26]. In addition, the fluoroquinolones have better penetration characteristics, and concentrations in bronchial secretions are between 0.8 and 2.0 times those in serum. By comparison, aminoglycosides have ratios of 0.2 to 0.6[4]. Previously, we have shown in our ovine model of acute lung injury associated with septic shock, that gentamicin improves hemodynamics when administered 6hrs post injury, but had no influence on the pulmonary injury[13].
The production of free radicals plays a major role in the pathogenesis of smoke inhalation injury and sepsis, leading to membrane damage caused by reactive oxygen radicals after stimulation of polymorphonuclear neutrophils[27]. In addition, NO is known to be an inflammatory mediator, especially when it binds to superoxide to form reactive nitrogen species such as peroxynitrite. NO produced in the airway circulation plays a major role in the pathogenesis of increased airway blood flow, which may contribute to the spread of injury from injured airways to the lung parenchyma. NO also contributes to the loss of hypoxic pulmonary vasoconstriction and plays an important role in lung oxidative tissue injury resulting in ARDS[28].
In our previous study, gentamicin did not inhibit the increase in plasma NOx levels, suggesting that it does not affect the formation of NO and the consecutive development of vascular leakage and lung edema. This was considered one reason why gentamicin did not improve the PaO2/FiO2-ratio when administered 6hrs after ALI associated with bacterial challenge in this model of sepsis. We anticipated that most of the lung tissue damage had manifested by 6hrs after the insult, as indexed by a PaO2/FiO2 -ratio <200 in both injured groups. Therefore, the reduction of bacteria and toxins at this time point would have no effect on the course of ARDS because tissue damage caused by reactive oxygen radicals had already been established. In the present study, we can clearly show significant improvements in pulmonary gas exchange and global hemodynamics, because of the 96hrs duration of the experiments, allowing a detailed observation of the pathophysiology of the insult and efficiency of treatments.
A follow up study in the same model using ceftazidime one hour post-injury showed significant improvements in oxygenation and reduction in lung-3- nitrotyrosine production[14]. The NO production in this study had a similar course like in the gentamicin study and could also not be reduced by ceftazidime, indicating that the effects of ceftazidime result from reduction of superoxide release from neutrophils. However, these results may also depend on early bacterial clearance and less lung injury, what made it difficult to differentiate the extent of injury between both studies. In addition we anticipated that the 6hrs post injury time point better reflects the clinical scenario, since it takes approximately 6hrs to evaluate patients, diagnose sepsis and start adequate treatments. Therefore, in the present study, combined ciprofloxacin and piperacillin therapy was started earliest 6hrs post injury. From a clinical standpoint, this might still be considered an early treatment. However, given the symptoms of septic shock, the early start of empiric antibiotics is reasonable and of clinical relevance, since it might reduce mortality[29].
The treatment prevented any further decrease in PaO2/FiO2 ratio and did reverse the deterioration of pulmonary function significantly over time. Most impressively, the NOx levels started to drop 24hrs post-injury in the treatment groups, what is a major finding of this study, and the advantage of a long-term model, showing the acute phase and recovery compared to the 24hrs gentamicin[13] and ceftazidime[14] studies. Our assumption from these previous studies, that the antibiotic treatment had not shown any effect on NOx production was due to the short observation period. In this new long-term model we can show evidence that the improvements of global hemodynamics and gas exchange are related to the release of NO.
In conclusion, the present study shows evidence that the administration of piperacillin combined with ciprofloxacin 6 and 12hrs after smoke inhalation injury and bacterial challenge improved survival, as well as cardiac and pulmonary function in this model.
This modification of our sepsis model with antibiotic therapy mimics more closely the current clinical practice compared to short term models of sepsis. In addition, this modification of our sepsis model is a useful new and clinically relevant approach for future studies of pneumonia and septic shock resulting from smoke inhalation injury, especially for evaluation of the late and recovery stage.
Acknowledgements
The authors thank the staff of the Investigational Intensive Care Unit at the University of Texas Medical Branch for their valuable assistance in conducting these studies. This study was granted by National Institutes of Health (PO12GM066312, R01GM060688) and Shriners Hospitals for Children (8450, 8954, and 8820).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare that they have no competing interests.
References
- 1.Pruitt BA, Jr, Goodwin CW, Mason AD., Jr . Epidemiological, demographic and outcome characteristics of burn injury. London, Edinburgh, New York, Philadelphia, St. Louis, Sydney, Toronto: W. B. Saunders; 2002. [Google Scholar]
- 2.Shirani KZ, Pruitt BA, Jr, Mason AD., Jr The influence of inhalation injury and pneumonia on burn mortality. Ann Surg. 1987;205(1):82–87. doi: 10.1097/00000658-198701000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fiel S. Guidelines and critical pathways for severe hospital-acquired pneumonia. Chest. 2001;119(2 Suppl):412S–418S. doi: 10.1378/chest.119.2_suppl.412s. [DOI] [PubMed] [Google Scholar]
- 4.Chastre J, Trouillet JL. Problem pathogens (Pseudomonas aeruginosa and Acinetobacter) Semin Respir Infect. 2000;15(4):287–298. doi: 10.1053/srin.2000.20944. [DOI] [PubMed] [Google Scholar]
- 5.Ibrahim EH, Ward S, Sherman G, Schaiff R, Fraser VJ, Kollef MH. Experience with a clinical guideline for the treatment of ventilator-associated pneumonia. Crit Care Med. 2001;29(6):1109–1115. doi: 10.1097/00003246-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Murakami K, Bjertnaes LJ, Schmalstieg FC, McGuire R, Cox RA, Hawkins HK, Herndon DN, Traber LD, Traber DL. A novel animal model of sepsis after acute lung injury in sheep. Crit Care Med. 2002;30(9):2083–2090. doi: 10.1097/00003246-200209000-00022. [DOI] [PubMed] [Google Scholar]
- 7.Ohl CA, Pollack M. Infections due to Pseudomonas species and related organisms. In: Braunwald E, Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson JL, editors. Harrison's PRINCIPLES OF INTERNAL MEDICINE. New York: McGraw-Hill; 2001. pp. 963–970. [Google Scholar]
- 8.Holmes B, Richards DM, Brogden RN, Heel RC. Piperacillin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs. 1984;28(5):375–425. doi: 10.2165/00003495-198428050-00002. [DOI] [PubMed] [Google Scholar]
- 9.Davis R, Markham A, Balfour JA. Ciprofloxacin. An updated review of its pharmacology, therapeutic efficacy and tolerability. Drugs. 1996;51(6):1019–1074. doi: 10.2165/00003495-199651060-00010. [DOI] [PubMed] [Google Scholar]
- 10.Deitch EA. Rodent models of intra-abdominal infection. Shock. 2005;24 Suppl 1:19–23. doi: 10.1097/01.shk.0000191386.18818.0a. [DOI] [PubMed] [Google Scholar]
- 11.Maybauer MO, Maybauer DM, Fraser JF, Traber LD, Westphal M, Enkhbaatar P, Cox RA, Huda R, Hawkins HK, Morita N, Murakami K, Mizutani A, Herndon DN, Traber DL. Recombinant human activated protein C improves pulmonary function in ovine acute lung injury resulting from smoke inhalation and sepsis. Crit Care Med. 2006;34(9):2432–2438. doi: 10.1097/01.CCM.0000230384.61350.FA. [DOI] [PubMed] [Google Scholar]
- 12.Westphal M, Morita N, Enkhbaatar P, Murakami K, Traber L, Traber DL. Acute effects of combined burn and smoke inhalation injury on carboxyhemoglobin formation, tissue oxygenation, and cardiac performance. Biochem Biophys Res Commun. 2004;317(3):945–949. doi: 10.1016/j.bbrc.2004.03.135. [DOI] [PubMed] [Google Scholar]
- 13.Maybauer MO, Maybauer DM, Traber LD, Westphal M, Enkhbaatar P, Morita N, Jodoin JM, Heggers JP, Herndon DN, Traber DL. Gentamicin improves hemodynamics in ovine septic shock after smoke inhalation injury. Shock. 2005;24(3):226–231. doi: 10.1097/01.shk.0000174021.95063.f4. [DOI] [PubMed] [Google Scholar]
- 14.Maybauer MO, Maybauer DM, Fraser JF, Traber LD, Westphal M, Cox RA, Huda R, Nakano YY, Enkhbaatar P, Hawkins HK, Herndon DN, Traber DL. Ceftazidime improves hemodynamics and oxygenation in ovine smoke inhalation injury and septic shock. Intensive Care Med. 2007;33(7):1219–1227. doi: 10.1007/s00134-007-0658-3. [DOI] [PubMed] [Google Scholar]
- 15.Westphal M, Morita N, Enkhbaatar P, Murakami K, Traber L, Traber DL. Carboxyhemoglobin formation following smoke inhalation injury in sheep is interrelated with pulmonary shunt fraction. Biochem Biophys Res Commun. 2003;311(3):754–758. doi: 10.1016/j.bbrc.2003.10.063. [DOI] [PubMed] [Google Scholar]
- 16.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine `see comments. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 17.Wichterman KA, Baue AE, Chaudry IH. Sepsis and septic shock--a review of laboratory models and a proposal. J Surg Res. 1980;29(2):189–201. doi: 10.1016/0022-4804(80)90037-2. [DOI] [PubMed] [Google Scholar]
- 18.Fink MP, Heard SO. Laboratory models of sepsis and septic shock. J Surg Res. 1990;49(2):186–196. doi: 10.1016/0022-4804(90)90260-9. [DOI] [PubMed] [Google Scholar]
- 19.Lingnau W, McGuire R, Dehring DJ, Traber LD, Linares HA, Nelson SH, Kilbourn RG, Traber DL. Changes in regional hemodynamics after nitric oxide inhibition during ovine bacteremia. Am J Physiol. 1996;270(1 Pt 2):R207–R216. doi: 10.1152/ajpregu.1996.270.1.R207. [DOI] [PubMed] [Google Scholar]
- 20.Redl H, Schlag G, Bahrami S. Animal models of sepsis and shock: a review and lessons learned. Edwin A Deitch. Shock 9(1):1–11, 1998. Shock. 1998;10(6):442–445. [PubMed] [Google Scholar]
- 21.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest. 2000;118(1):146–155. doi: 10.1378/chest.118.1.146. [DOI] [PubMed] [Google Scholar]
- 23.Dupont H, Mentec H, Sollet JP, Bleichner G. Impact of appropriateness of initial antibiotic therapy on the outcome of ventilator-associated pneumonia. Intensive Care Med. 2001;27(2):355–362. doi: 10.1007/s001340000640. [DOI] [PubMed] [Google Scholar]
- 24.Trouillet JL, Chastre J, Vuagnat A, Joly-Guillou ML, Combaux D, Dombret MC, Gibert C. Ventilator-associated pneumonia caused by potentially drug-resistant bacteria. Am J Respir Crit Care Med. 1998;157(2):531–539. doi: 10.1164/ajrccm.157.2.9705064. [DOI] [PubMed] [Google Scholar]
- 25.Alvarez-Lerma F. Modification of empiric antibiotic treatment in patients with pneumonia acquired in the intensive care unit. ICU-Acquired Pneumonia Study Group. Intensive Care Med. 1996;22(5):387–394. doi: 10.1007/BF01712153. [DOI] [PubMed] [Google Scholar]
- 26.Lashev L, Lasarova S. Pharmacokinetics and side-effects of gentamicin in healthy and pseudomonas aeruginosa infected sheep. J Vet Pharmacol Ther. 2001;24(3):237–240. doi: 10.1046/j.1365-2885.2001.00332.x. [DOI] [PubMed] [Google Scholar]
- 27.Traber DL, Maybauer MO, Maybauer DM, Westphal M, Traber LD. Inhalational and acute lung injury. Shock. 2005;24 Suppl 1:82–87. doi: 10.1097/01.shk.0000191338.39154.73. [DOI] [PubMed] [Google Scholar]
- 28.Traber DL, Herndon DN, Enkhbaatar P, Maybauer MO, Maybauer DM. The pathophysiology of inhalation injury. In: Herndon DN, editor. Total Burn Care. Philadelphia: Saunders; 2007. pp. 248–261. [Google Scholar]
- 29.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36(1):296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]