Abstract
Medullary thymic epithelial cells (mTECs) play a critical role in thymic negative selection of autoreactive thymocytes, especially for thymocytes specific for peripheral tissue-restricted self-antigens (TRA). Deficiency in LTβR is associated with peripheral tissue inflammation but whether it is caused by defective negative selection has been unclear; the significance of the LTβR pathway for negative selection is evident in some models but not others. In this opinion, we revisit the data and clarify the role of LTβR in mTECs development and function and thymic TRA expression. These processes are discussed as potential mechanisms for LTβR-mediated control of negative selection.
Medullary thymic epithelial cells (mTECs), Aire and thymic negative selection
Negative selection of autoreactive thymocytes is a central mechanism for establishing self-tolerance. During this process self-antigens are presented mainly by mTECs and/or thymic dendritic cells (DCs) to developing thymocytes to induce apoptosis of thymocytes with a high affinity TCR against self-antigens 1–6. Although it is easy to understand how autoreactive T cells against ubiquitous self-antigens are purged, it had been a mystery how the same mechanism might forestall autoimmunity against peripheral tissue-restricted self-antigens (TRA). The explanation began to emerge by the demonstration that a myriad of genes classified as peripheral tissue-restrictive are also expressed in thymic epithelial cells, especially in medullary thymic epithelial cells (mTECs) 7,8.
The importance of mTECs and mTECs TRA expression in the establishment of central tolerance is demonstrated mainly by the following two aspects. Firstly, abnormal mTECs development and organization is often associated with autoimmunity. Examples include Relb−/− mice 9,10; aly/aly mice 11; Ikka−/− embryonic-thymi-grafted nude mice 12, Traf6−/− mice 13, Nfkb2−/− mice 14,15, Ltbr−/− mice 16 and Nfkb2−/−Bcl3−/− mice 17. All these mice have disorganized or reduced cellularity of mTECs to different degrees; they also possess autoantibody and/or peripheral organ lymphocyte infiltration, the prototypical phenotype of autoimmunity. Additional evidence underlying the importance of organized mTECs in preventing autoimmunity is that in several autoimmune models, the disruption of thymic medulla (e.g. reduced mTECs, aberrant mTECs location in cortex) is often associated with or proceeds the development of autoimmunity 18,19.
Secondly, genetically-altered mice with reduced TRA thymic expression develop autoimmunity. A typical case is the autoimmune regulator (Aire) deficient mouse. The AIRE gene was first identified and cloned from patients with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy syndrome (APECED) 20,21. A subsequent study in mice revealed that Aire is a master regulator of ectopic expression of a large number of peripherally expressed genes in the thymus, and that Aire deficiency in mice leads to autoimmunity against peripheral organs 22. This was initially attributed solely to reduced ectopic expression of thymic TRA 22,23. However, it was later found that Aire might possess additional roles other than regulation of TRA expression, such as regulation of antigen processing and presentation, mTECs differentiation and thymocyte migration 24–28. Thus, the relative contribution of each Aire-related mechanism in mediating negative selection needs to be fully unraveled. Even so, a critical role for TRA expression in mTECs has been recently demonstrated; investigators found that lack of a single protein, interphotoreceptor retinoid-binding protein (IRBP) in the thymus, even in the presence of Aire, is sufficient to trigger spontaneous eye-specific autoimmunity as found in Aire deficient mice 29.
Given the critical roles of mTECs and thymic TRA expression in negative selection, their regulation has been an actively investigated. In this area of research, the lymphotoxin β receptor (LTβR) has received much attention given its important, yet complicated, role in thymic negative selection. This article attempts to revisit the data and clarify the controversial role of LTβR in mTECs development and function and thymic TRA expression.
Can the LTβR pathway control negative selection of TRA-reactive T cells?
LTβR belongs to the TNFR superfamily and is extensively expressed on stromal cells as well as DCs and macrophages, but not on T or B cells. Two ligands of LTβR have been identified so far: lymphotoxin (LT) and LIGHT. LT is expressed mainly on B, T and NK cells, while LIGHT is expressed on immature DCs, activated T cells and NK cells. The LTβR pathway plays a critical role in secondary lymphoid organ development and function30,31. LTβR deficiency is associated with increased numbers of lymphocytes in peripheral organs, which when first described was presumed to be due to the lack of lymph nodes (LNs) in these mice 32. However, further careful studies by two groups challenged this view with data showing lymphocyte infiltration in peripheral organs was independent of defective LNs and instead dependent on thymic defects 16,32,33. This opened a new line of investigation into the control of T cell negative selection. So far, four antigen-specific TCR transgenic and neo-self Ag transgenic systems have been employed to directly address the role of LTβR in thymic negative selection: (1) OT-I/RIP-mOVA; (2) OT-II/RIP-mOVA; (3) TAG-I/TRAMP; (4) TGB/TRAMP (Box 1). Intriguingly, the results obtained from these different studies are somewhat divergent. In one study using the OT-II/RIP-mOVA system LTβR had little influence on thymic negative selection 34. However, in other studies using three different CD8+ transgenic TCR systems (1, 3, and 4 above), a significant role of LTβR on thymic negative selection was revealed 14,35. These different results, as well as a controversial role for LTβR and control of TRA and Aire expression, have lead to some confusion in the field regarding the role of LTβR in negative selection of TRA-specific T cells. This may be due to the different models used in the respective studies, such as CD4 versus CD8 T cells (DCs are believed to be the prime antigen presenting cells (APCs) to CD4 T cells whereas mTECs are for CD8 T cells 36) as well as analysis of different TRAs (with different promoters and mechanisms of regulation) for induction of thymic negative selection. More antigens and models are needed to have comprehensive view on this.
Box 1. TCR and neo-self TRA transgenic systems used in the study of negative selection.
Both OT-I and TAG-I are transgenic CD8+ TCRs, recognizing ovabumin AA257-264 and SV40-Tag, respectively, in the context of H2Kb; OT-II is a CD4+ transgenic TCR recognizing ovabumin AA323-339 in the context of H2Ab; TAG-I and TGB are both CD8+ transgenic TCRs recognizing different SV40-T antigen epitopes in the context of H2Db and H2Kk, respectively. RIP-mOVA transgenic mice bear membrane bound ovabumin under rat insulin 1 promoter. TRAMP mice bear SV40-T antigen under probasin promoter. Both insulin 1 and probasin are considered TRA. Thus, mOVA and SV40-Tag driven by these promoters are considered to be expressed in a way mimicking the TRA expression.
Can the LTβR pathway control mTECs development and organization?
mTECs development is generally considered a step-wise process, where several key subsets of mTECs are defined depending on their maturation status (represented by MHC-II or CD80 expression level) and Aire expression (CD80lowAire−, CD80highAire−, CD80highAire+) 37. Different subsets of mTECs are presumed to have different TRA expression patterns and antigen presentation functions as well as a different rates of turnover 8,38–40.
The role of LTβR pathway in thymus had been largely overlooked and lagged behind its well-defined role in peripheral lymphoid organogenesis and development 30,41. This was partially due to the grossly normal size and architecture of thymi from LT and LTβR deficient mice. However, in one study where the thymic medulla was examined in more detail, significant reductions of mTECs subsets expressing UEA-1 and nonpolymorphic MHC-II antigen I-O were observed in Ltbr−/− mice16. When defined as CD45−G8.8+ CDR1−B7.1+, the total number of mTECs was also dramatically reduced in Ltbr−/− thymi. LTβ deficiency was found to have a non-identical phenotype in this regard. In fact, when studying LTβR ligands, it was found that in LTβ and LIGHT double-deficient mice, in which both known ligands of LTβR are ablated, the thymic phenotype found in Ltbr−/− mice was only partially reproduced. Thus it was hypothesized that additional unknown ligand(s) of LTβR exist. A role for the LTβR pathway on mTECs development was also observed by two other groups 34,42, and the milder effect of LTα, compared with LTβR, on mTECs development was also noted by the former study. Furthermore, the development of Aire+MHC-II high mTECs population was also found to be dependent on LTβR 34,42. It is now generally agreed upon that LTβR is required for proper mTECs development. It remains unclear, however, exactly how, and at which differentiation stage, LTβR regulates mTECs development.
It must be noted that other TNFR superfamily members, CD40 and RANK, are also important for mTECs development and central tolerance 43–45. This is not surprising, as both CD40 and RANK can deliver signals through the non-canonical NF-κB pathway. However, it is surprising that so many TNFR family members are involved in mTECs development. This coordinated regulation pattern of mTECs by different molecules is probably based on different ligand-receptor spatial and temporal expression patterns 46. This also highlights that the finely-tuned regulation of mTECs is critical for establishing central tolerance.
It is important to note that, in addition to the regulation of mTECs development, the LTβR pathway is also involved in mTECs organization. In immunofluoresence microscopy experiments not all mTECs markers reveal identical defects in thymic medulla organization; obvious disorganization is detected using UEA-1 staining but is less clear with MTS-10 staining16,32. Lectin UEA-1-expressing mTECs were found in clumps, and the connective mTECs network was disrupted in mice with deficiency of LTβR 16, opposed to the broad and even distribution in WT thymi. A similar finding was also noted in Nfkb2−/−, plt/plt and Ccr7−/− mice 47,48. Thymocyte migration in the thymus is a highly organized process and the developing thymocytes need to patrol the thymic medulla for antigen to undergo negative selection 49,50. Whether disrupted mTECs/medulla organization itself also influences negative selection of autoreactive thymocytes remains largely unclear and awaits further investigation.
Is expression of Aire and TRA controlled by the LTβR pathway directly or indirectly?
Given the similar autoimmune phenotypes between Ltbr−/− and Aire−/− mice, it was proposed that LTβR might regulate thymic central tolerance in an Aire-dependent manner or via regulation of TRA gene expression. This led to the initial finding of dramatically reduced Aire, Insulin 1 and Collagen II gene expression in total thymi of Ltbr−/− or Lta−/− mice compared to WT thymi by quantitative real-time PCR32,51. However, in a separate study, normal Aire expression was found in mTECs isolated from Ltbr−/− thymi by semiquantitative RT-PCR16. Supporting the latter, normal Aire and TRA expression in Lta−/− thymi was found by semiquantitative PCR and Lta−/− thymi showed largely normal Aire+ mTECs frequency by tissue immunofluoresence staining 11,52. Thus these studies led to the suggestion that the LT-LTβR pathway regulates Aire and TRA expression in thymus through indirect mechanisms.
More recent studies have attempted to clarify this controversial issue by analyzing Aire and TRA gene expression in more detail on a per cell basis 34,53. In these studies, purified mTECs from Ltbr−/− and/or Lta−/− mice showed no reduction of Aire or TRA gene expression, compared with WT mTECs by both gene array and quantitative real-time PCR. Thus, these studies concluded that LTβR signaling is not directly required for TRA expression in mTECs. Instead, based on gene profiling, it was proposed that the role of LTβR on Aire expression might not be direct but indirect through the regulation of mTECs development 34,53. The data suggest that the reduction of Aire or TRA in whole thymic tissues by earlier studies is likely associated with the reduced number of total or subsets of mTECs rather than reduced Aire expression in individual cells.
To study this further, MHC-IIhi (mature) versus MHC-IIlo (immature) mTECs were separated, by cell sorting, from Lta−/− and Ltb−/− mice and Aire and TRA expression were determined. While Aire expression was not reduced in either subset of mTECs from Lta−/− or Ltb−/− mice compared with WT, some Aire-dependent (including insulin 2) and -independent TRAs were reduced in both the Lta−/− and Ltb−/− mTECs subsets 42. This supports a direct role for LT signaling in regulating expression of some TRA in mTECs. It is worthy to note that this study also found that LT deficiency has a much more pronounced effect on TRA expression in the MHC-IIlo mTECs subset than in the MHC-IIhi mTECs subset, which raises the possibility that LT signaling may be more important for TRA expression in some mTECs subsets than others 42.
The studies described above mostly focused on the essential role of LT-LTβR pathway in Aire and TRA expression. Whether LTβR signaling pathway is sufficient to upregulate Aire and TRA expression is a different question. Efforts to address this issue have been somewhat limited due to lack of proper reagents and low expression of antigens in mTECs. However, an early study showed that treatment of mice with the 3C8 clone of agonistic anti-LTβR upregulated in vivo thymic Aire, Insulin 1 and Collagen II transcript expression after several hours 32,51. Further more, in vitro experiments showed that 3C8 treatment of the mTECs cell line 427.1 can also upregulate the expression of these genes, suggesting a direct impact of LTβR signaling on Aire and TRA expression in mTECs 32. However, upregulation of Aire by agonisitic LTβR antibody was not found in a recent study using2-deoxyglucose (DG) treated fetal thymic organ culture (FTOC) while Crp (an Aire-independent TRA) was significantly upregulated 45. Thus, different conclusions have been drawn based on these two studies. It should be kept in mind that different models, reagents, and stimulation time were used in these studies and the role of LT-LTβR might be different in different scenarios.
It is worth noting several issues when interpreting the data described above. Firstly, the induction or upregulation of Aire and TRA expression by direct LTβR signaling raises the interesting question of whether TRA-specific TCR-pMHC interactions during thymocyte development feed back on mTECs to upregulate TRA via stabilized LT-LTβR signaling on an individual cell basis. Given the rare interaction events between TRA-specific TCR and TRA presented by mTECs, this crosstalk between individual thymocytes and mTECs, mediated by an LT-LTβR interaction, might help to increase the efficiency of negative selection. Secondly, it is possible that LTβR might play a more essential role in certain subsets of mTECs than in others 42. This effect could be compromised when the whole mTECs population is analyzed instead of mTECs subsets 34,53. Thirdly, LTβR seems essential for expression of only a subset of TRAs. Several other TNF family members, similar to LTβR, are essential for mTECs development 43–45. Do they also control TRA expression directly? If yes, how do they cooperate with LTβR? These are interesting questions to which answers should be determined in future. Last, but not least, one can argue that agonistic antibodies that regulate TRA expression might provide means for clinical intervention to enhance negative selection thus providing better central tolerance. However, the clinical relevance of “sufficiency” for TRA expression and the amount of TRA upregulation have not been tested.
As discussed in the previous section, it is clear that LTβR plays an essential role in mTECs development/organization 14–16,34,53 and by doing so the LTβR pathway can control thymic TRA expression indirectly. Thus, although the direct role of LT-LTβR signaling on Aire expression could be limited at steady state, by regulating mTECs development and organization LT-LTβR signaling would indirectly induce Aire to control negative selection.
Could LTβR pathway regulate thymocyte migration?
We have unexpectedly identified another role for LTβR in central tolerance that is regulation of mTECs chemokine expression and thymocyte migration 14. This study originated from an unexpected finding in the OT-I/RIP-mOVA system used to address the role of LTβR in thymic negative selection. Although thymic mOVA expression remains normal in RIP-mOVAtg/Ltbr−/− mice, we still found defective thymic negative selection of OT-I cells when LTβR was deficient. This finding, together with previous data showing that LTβR controls chemokine expression in peripheral tissues, and the important role of chemokines in central tolerance, led us to examine whether LTβR controls chemokine expression in the thymus, thereby altering migration of developing thymocytes. Indeed, we found impaired secondary lymphoid organ chemokine (SLC) and EBI1-ligand chemokine (ELC) expression in mTECs from Ltbr−/− mice, which resulted in defective thymocyte migration to the medulla. To further evaluate the role of the SLC and ELC defect itself on thymic negative selection, we used plt/plt mice, in which SLC and ELC are both deficient, and found that SLC and ELC deficiency alone is sufficient to lead to a thymic negative selection defect. These findings have also been confirmed by others 48, 42.
Implications of LTβR-regulated negative selection
Although the underlying mechanisms are not fully understood, the role of LTβR on negative selection of TRA-reactive T cells is clear, at least for certain TRA-reactive T cells. Given the significant influence of LTβR on negative selection of TRA-reactive CD8+ T cells and the fact that many tumors express organ-restricted self-antigens, a recent study creatively applied this knowledge to the prevention of tumor development 35. In this study, ablation of LT signaling either LTα deficiency or by administering an LTβR-human Ig fusion protein dramatically rescued most high affinity tumor/self-specific TCR clones, which was associated with inhibited/reduced spontaneous tumor development in the TRAMP prostate cancer model. This study not only reveals a significant role for the LTβR pathway in negative selection, but together with other studies as discussed above, also suggests that the degree of LTβR involvement in thymic negative selection might depend on the type of TRA and/or the type of promoter regulating the TRA, as well as the mTECs subsets involved. In fact, LTβR signaling ablation showed a more dramatic rescue in TAG-I-TRAMP system than in OT-I-RIP-mOVA system (20 vs. 3 fold). There are at least three models to explain this data: 1) RIP driven mOVA and probasin promoter driven SV40-Tag are expressed in different subsets of mTECs; 2) the transcription or translation of the two genes are differentially regulated by LTβR; 3) the affinity of the antigenic epitopes of the two proteins to TCR is different. Those models remain to be tested in future.
It is noteworthy that blocking the LTβR pathway could have multiple effects in addition to rescue of high-affinity TRA-reactive T cells. Blockade of the LTβR pathway has been shown to reduce inflammation in several models 54–56, and inflammation has been considered a factor promoting cancer development 57. Additionally, the LTβR pathway was found to promote tumor growth by inducing angiogenesis 58. A recent study also demonstrated that the LTβR signaling pathway is upregulated in chronic HBV or HCV infection-induced hepatitis and hepatocellular carcinoma59. Thus it cannot be excluded that additional mechanisms contributed to tumor prevention.
Concluding remarks
The studies on the role of LTβR in thymic negative selection have raised interesting new questions about how T cells are negatively selected and how LTβR signaling is required for the control negative selection of some TRA-reactive T cells, but not others. Past studies help to clarify the complicated roles of LTβR in various aspects of thymic negative selection (Figure 1). As discussed above, while evaluating the role of LTβR in negative selection, it is worthwhile considering the experimental model and methods used to modulate LTβR signaling (Table 1). Thus, it is not surprising that the role of LTβR in thymic negative selection of TRA-specific T cells is revealed in some studies but not in others. The different results obtained under different scenarios not only underscore the complicated regulation of thymic negative selection but also help to point out future directions to discover novel factors in this important thymic process. Some key questions that should be addressed in future are outlined in Box 1. The increased understanding of the mTECs differentiation program, the role of LTβR, and more broadly, all TNFR superfamily receptors will help us to have more comprehensive view on thymic negative selection to various TRA. Furthermore, we can expect to see more preclinical studies employing techniques to regulate negative selection for the combat of cancer and autoimmune disease.
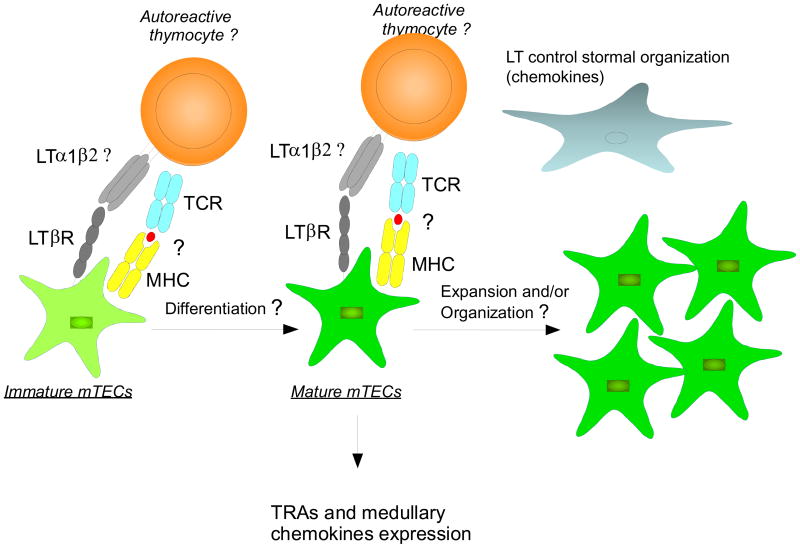
Figure 1. Direct and indirect roles of LTβR in mTECs development and function.
LTβR signaling regulates mTECs development and function in various ways. LTβR controls mTECs differentiation, however, it remains unknown at which stage and how LTβR controls this process. LTβR is not essential for Aire thymic expression but is indeed essential for expression of some Aire-dependent and Aire-independent TRA on a per cell basis. In addition, LTβR might have more impact on TRA expression in certain subsets of mTECs than others. The underlying molecular mechanism remains to be determined. LTβR signaling also controls mTECs organization and chemokine production, which may indirectly regulate thymocyte migration or TRA expression and presentation to developing thymocytes. It is unclear which cells deliver which ligand(s) to LTβR for control of mTEC differentiation, TRA and chemokine expression; LT seems to play only a partial role. It is also intriguing whether TCR-pMHC interaction between thymocytes and mTECs is required for LTβR to exert its roles.
Table 1.
Summary of current data on the role of LTβR in thymic negative selection
| Data for a role | Data against a role | |||||
|---|---|---|---|---|---|---|
| Experimental model | Observation | Ref | Experimental model | Observation | Ref | |
| Number of mTECs | Ltbr−/− thymi tissue staining, mTECs isolation and FACS | ↓ | 14,16,34 | Lta−/− mice, tissue staining | = | 32 |
| Ltb−/− mice, UEA1 tissue staining | ↓ | 16 | LTa−/− mice, mTECs isolation and FACS | = | 34 | |
| Aire expression | Whole thymi from Ltbr−/−, Lta−/− mice, RT-PCR | ↓ | 14,32 | Whole thymi from Lta−/− mice, semi-quantitative PCR | = | 11 |
| Whole thymi, in vivo agonistic LTβR antibody | ↑ | 32,47 | Isolated mTECs from Lrbr−/−, Lta−/− mice, RT-PCR | = | 34,53 | |
| 2-DG FTOC, in vitro agonistic LTβR antibody | ↑ | 32,45 | ||||
| Aire-dependent TRAs | Whole thymi from Ltbr−/− mice, RT-PCR | ↓ | 14,32 | Isolated mTECs from Lta −/− and Ltbr−/− mice by CD45-CDR1-G8.8+MHC-IIhi, RT-PCR | = | 34 |
| Whole thymi, in vivo agonistic LTβR antibody | ↑ | 32,47 | Isolated mTECs from Ltbr−/− mice by CD45-CDR1-G8.8+, RT-PCR | = | 53 | |
| Isolated mTECs from Lta−/−, Ltb−/− mice by CD45-UEA1+MHC-IIhi or CD45-UEA1+MHC-IIlo, RT-PCR | ↓ | 42 | ||||
| Aire-independent TRAs | Whole thymi from Ltbr−/− mice, RT-PCR | ↓ | 33 | |||
| Isolated mTECs from Lta−/−, Ltb−/− mice by CD45-UEA1+MHC-IIhi or CD45-UEA1+MHC-IIlo, RT-PCR | ↓ | 42 | ||||
| Whole thymi, in vivo agonistic LTβR antibody | ↑ | 33 | ||||
| 2-DG FTOC, in vitro agonistic LTβR antibody | ↑ | 32,45 | ||||
| mTECs chemokines | Isolated mTECs from Ltbr−/− mice by CD45-G8.8+CD80+, RT-PCR, SLC and ELC | ↓ | 47 | |||
| Isolated mTECs from Lta−/−, Ltb−/− mice by CD45-UEA1+MHC-IIhi or CD45-UEA1+MHC-IIlo, RT-PCR, SLC and ELC | ↓ | 42 | ||||
| Negative selection | OT-I/RIP-mOVA, Ltbr−/− | ↓ | 14 | OT-II/RIP-mOVA, Ltbr−/− | = / ↓ | 34 |
| TAG-I/TRAMP, Lta−/− | ↓ | 35 | ||||
| TGB/TRAMP, LTβ R-hIg blockade | ↓ | 35 | ||||
Box 2. Outstanding questions.
At what stage of differentiation does LTβR regulate mTECs? What are the cellular and molecular mechanisms for regulation of mTECs development by LTβR signaling?
How does LTβR cooperate with other TNFR superfamily receptors, e.g. CD40 and RANK, to regulate mTECs development and function?
How does LTβR regulate TRA (both Aire-dependent and -independent) expression?
Is the LTβR-mediated regulation of mTECs dependent on TCR-pMHC interaction?
How does LTβR regulate mTECs and thymic medulla organization and is this a factor in control of central tolerance?
Acknowledgments
This work was supported by grants from National Institutes of Health (to Y-X.F.) and Fund from Chinese Academy of Sciences (M.Z, and Y-X.F).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goodnow CC. Multistep Pathogenesis of Autoimmune Disease. Cell. 2007;130 (1):25–35. doi: 10.1016/j.cell.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 2.Mathis D, Benoist C. Aire. Annual Review of Immunology. 2009;27 (1):287–312. doi: 10.1146/annurev.immunol.25.022106.141532. [DOI] [PubMed] [Google Scholar]
- 3.Mathis D, Benoist C. Back to Central Tolerance. 2004;20(5):509–516. doi: 10.1016/s1074-7613(04)00111-6. [DOI] [PubMed] [Google Scholar]
- 4.Gallegos AM, Bevan MJ. Central tolerance: good but imperfect. Immunol Rev. 2006;209:290–296. doi: 10.1111/j.0105-2896.2006.00348.x. [DOI] [PubMed] [Google Scholar]
- 5.Klein L, et al. Antigen presentation in the thymus for positive selection and central tolerance induction. Nat Rev Immunol. 2009;9 (12):833–844. doi: 10.1038/nri2669. [DOI] [PubMed] [Google Scholar]
- 6.Sakaguchi S, et al. Regulatory T Cells and Immune Tolerance. 2008;133(5):775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Derbinski J, et al. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol. 2001;2 (11):1032–1039. doi: 10.1038/ni723. [DOI] [PubMed] [Google Scholar]
- 8.Gotter J, et al. Medullary epithelial cells of the human thymus express a highly diverse selection of tissue-specific genes colocalized in chromosomal clusters. J Exp Med. 2004;199 (2):155–166. doi: 10.1084/jem.20031677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weih F, et al. Multiorgan inflammation and hematopoietic abnormalities in mice with a targeted disruption of RelB, a member of the NF-[kappa]B/Rel family. Cell. 1995;80 (2):331–340. doi: 10.1016/0092-8674(95)90416-6. [DOI] [PubMed] [Google Scholar]
- 10.Burkly L, et al. Expression of relB is required for the development of thymic medulla and dendritic cells. Nature. 1995;373 (6514):531–536. doi: 10.1038/373531a0. [DOI] [PubMed] [Google Scholar]
- 11.Kajiura F, et al. NF-{kappa}B-Inducing Kinase Establishes Self-Tolerance in a Thymic Stroma-Dependent Manner. J Immunol. 2004;172 (4):2067–2075. doi: 10.4049/jimmunol.172.4.2067. [DOI] [PubMed] [Google Scholar]
- 12.Kinoshita D, et al. Essential role of IkappaB kinase alpha in thymic organogenesis required for the establishment of self-tolerance. Journal of Immunology. 2006;176 (7):3995–4002. doi: 10.4049/jimmunol.176.7.3995. [DOI] [PubMed] [Google Scholar]
- 13.Akiyama T, et al. Dependence of self-tolerance on TRAF6-directed development of thymic stroma. Science. 2005;308 (5719):248–251. doi: 10.1126/science.1105677. [DOI] [PubMed] [Google Scholar]
- 14.Zhu M, et al. Lymphotoxin Receptor Is Required for the Migration and Selection of Autoreactive T Cells in Thymic Medulla. J Immunol. 2007;179 (12):8069–8075. doi: 10.4049/jimmunol.179.12.8069. [DOI] [PubMed] [Google Scholar]
- 15.Zhang B, et al. NF-kappaB2 Is Required for the Control of Autoimmunity by Regulating the Development of Medullary Thymic Epithelial Cells. Journal of Biological Chemistry. 2006;281 (50):38617–38624. doi: 10.1074/jbc.M606705200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boehm T, et al. Thymic Medullary Epithelial Cell Differentiation, Thymocyte Emigration, and the Control of Autoimmunity Require Lympho-Epithelial Cross Talk via LT{beta}R. J Exp Med. 2003;198(5):757–769. doi: 10.1084/jem.20030794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, et al. A Role for the I[kappa]B Family Member Bcl-3 in the Control of Central Immunologic Tolerance. Immunity. 2007;27 (3):438–452. doi: 10.1016/j.immuni.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atlan-Gepner C, et al. Disorganization of thymic medulla precedes evolution towards diabetes in female NOD mice. Autoimmunity. 1999;31 (4):249–260. doi: 10.3109/08916939908994070. [DOI] [PubMed] [Google Scholar]
- 19.Takeoka Y, et al. Thymic Microenvironmental Abnormalities in MRL/MP-lpr/lpr, BXSB/MpJYaaand C3H HeJ-gld/gldMice. Journal of Autoimmunity. 1995;8 (2):145–161. doi: 10.1006/jaut.1995.0012. [DOI] [PubMed] [Google Scholar]
- 20.Aaltonen J, et al. An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat Genet. 1997;17 (4):399–403. doi: 10.1038/ng1297-399. [DOI] [PubMed] [Google Scholar]
- 21.Nagamine K, et al. Positional cloning of the APECED gene. Nat Genet. 1997;17 (4):393–398. doi: 10.1038/ng1297-393. [DOI] [PubMed] [Google Scholar]
- 22.Anderson MS, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298 (5597):1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 23.Liston A, et al. Aire regulates negative selection of organ-specific T cells. Nat Immunol. 2003;4 (4):350–354. doi: 10.1038/ni906. [DOI] [PubMed] [Google Scholar]
- 24.Anderson MS, et al. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005;23 (2):227–239. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Kuroda N, et al. Development of Autoimmunity against Transcriptionally Unrepressed Target Antigen in the Thymus of Aire-Deficient Mice. J Immunol. 2005;174 (4):1862–1870. doi: 10.4049/jimmunol.174.4.1862. [DOI] [PubMed] [Google Scholar]
- 26.Niki S, et al. Alteration of intra-pancreatic target-organ specificity by abrogation of Aire in NOD mice. The Journal of Clinical Investigation. 2006;116 (5):1292–1301. doi: 10.1172/JCI26971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gray D, et al. Proliferative arrest and rapid turnover of thymic epithelial cells expressing Aire. J Exp Med. 2007;204(11):2521–2528. doi: 10.1084/jem.20070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yano M, et al. Aire controls the differentiation program of thymic epithelial cells in the medulla for the establishment of self-tolerance. J Exp Med. 2008;205(12):2827–2838. doi: 10.1084/jem.20080046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeVoss J, et al. Spontaneous autoimmunity prevented by thymic expression of a single self-antigen. J Exp Med. 2006;203(12):2727–2735. doi: 10.1084/jem.20061864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fu YX, Chaplin DD. Development and maturation of secondary lymphoid tissues. Annu Rev Immunol. 1999;17:399–433. doi: 10.1146/annurev.immunol.17.1.399. [DOI] [PubMed] [Google Scholar]
- 31.Randall TD, et al. Development of Secondary Lymphoid Organs. Annual Review of Immunology. 2008;26 (1):627–650. doi: 10.1146/annurev.immunol.26.021607.090257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chin RK, et al. Lymphotoxin pathway directs thymic Aire expression. Nat Immunol. 2003;4 (11):1121–1127. doi: 10.1038/ni982. [DOI] [PubMed] [Google Scholar]
- 33.Chin RK, et al. Lymphotoxin Pathway-Directed, Autoimmune Regulator-Independent Central Tolerance to Arthritogenic Collagen. J Immunol. 2006;177 (1):290–297. doi: 10.4049/jimmunol.177.1.290. [DOI] [PubMed] [Google Scholar]
- 34.Venanzi ES, et al. Lymphotoxin Pathway and Aire Influences on Thymic Medullary Epithelial Cells Are Unconnected. J Immunol. 2007;179 (9):5693–5700. doi: 10.4049/jimmunol.179.9.5693. [DOI] [PubMed] [Google Scholar]
- 35.Zhou P, et al. Targeting lymphotoxin-mediated negative selection to prevent prostate cancer in mice with genetic predisposition. Proceedings of the National Academy of Sciences. 2009;106 (40):17134–17139. doi: 10.1073/pnas.0905707106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gallegos AM, Bevan MJ. Central Tolerance to Tissue-specific Antigens Mediated by Direct and Indirect Antigen Presentation. J Exp Med. 2004;200(8):1039–1049. doi: 10.1084/jem.20041457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tykocinski LO, et al. The Thymus Medulla Slowly Yields Its Secrets. Annals of the New York Academy of Sciences. 2008;1143 :105–122. doi: 10.1196/annals.1443.018. The Year in Immulogy 2008. [DOI] [PubMed] [Google Scholar]
- 38.Derbinski J, et al. Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. J Exp Med. 2005;202(1):33–45. doi: 10.1084/jem.20050471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gabler J, et al. Promiscuous gene expression and the developmental dynamics of medullary thymic epithelial cells. European Journal of Immunology. 2007;37 (12):3363–3372. doi: 10.1002/eji.200737131. [DOI] [PubMed] [Google Scholar]
- 40.Kyewski B, Klein L. A central role for central tolerance. Annu Rev Immunol. 2006;24:571–606. doi: 10.1146/annurev.immunol.23.021704.115601. [DOI] [PubMed] [Google Scholar]
- 41.Weih F, Caamano J. Regulation of secondary lymphoid organ development by the nuclear factor-κB signal transduction pathway. Immunological Reviews. 2003;195 (1):91–105. doi: 10.1034/j.1600-065x.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- 42.Seach N, et al. The Lymphotoxin Pathway Regulates Aire-Independent Expression of Ectopic Genes and Chemokines in Thymic Stromal Cells. J Immunol. 2008;180 (8):5384–5392. doi: 10.4049/jimmunol.180.8.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Irla M, et al. Autoantigen-Specific Interactions with CD4+ Thymocytes Control Mature Medullary Thymic Epithelial Cell Cellularity. Immunity. 2008;29 (3):451–463. doi: 10.1016/j.immuni.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 44.Hikosaka Y, et al. The Cytokine RANKL Produced by Positively Selected Thymocytes Fosters Medullary Thymic Epithelial Cells that Express Autoimmune Regulator. Immunity. 2008;29 (3):438–450. doi: 10.1016/j.immuni.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 45.Akiyama T, et al. The Tumor Necrosis Factor Family Receptors RANK and CD40 Cooperatively Establish the Thymic Medullary Microenvironment and Self-Tolerance. Immunity. 2008;29 (3):423–437. doi: 10.1016/j.immuni.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 46.Zhu M, Fu YX. Coordinating Development of Medullary Thymic Epithelial Cells. Immunity. 2008;29 (3):386–388. doi: 10.1016/j.immuni.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 47.Zhu M, et al. NF-kappaB2 is required for the establishment of central tolerance through an Aire-dependent pathway. The Journal of Clinical Investigation. 2006;116 (11):2964–2971. doi: 10.1172/JCI28326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nitta T, et al. CCR7-mediated migration of developing thymocytes to the medulla is essential for negative selection to tissue-restricted antigens. Proceedings of the National Academy of Sciences. 2009;106 (40):17129–17133. doi: 10.1073/pnas.0906956106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takahama Y. Journey through the thymus: stromal guides for T-cell development and selection. Nat Rev Immunol. 2006;6 (2):127–135. doi: 10.1038/nri1781. [DOI] [PubMed] [Google Scholar]
- 50.Petrie HT, Zuniga-Pflucker JC. Zoned Out: Functional Mapping of Stromal Signaling Microenvironments in the Thymus. Annual Review of Immunology. 2007;25 (1):649–679. doi: 10.1146/annurev.immunol.23.021704.115715. [DOI] [PubMed] [Google Scholar]
- 51.Chin RK, et al. Lymphotoxin pathway-directed, autoimmune regulator-independent central tolerance to arthritogenic collagen. Journal of Immunology. 2006;177 (1):290–297. doi: 10.4049/jimmunol.177.1.290. [DOI] [PubMed] [Google Scholar]
- 52.Rossi SW, et al. RANK signals from CD4+3- inducer cells regulate development of Aire-expressing epithelial cells in the thymic medulla. The Journal of Experimental Medicine. 2007;204 (6):1267–1272. doi: 10.1084/jem.20062497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martins VC, et al. Lt{beta}r Signaling Does Not Regulate Aire-Dependent Transcripts in Medullary Thymic Epithelial Cells. J Immunol. 2008;181 (1):400–407. doi: 10.4049/jimmunol.181.1.400. [DOI] [PubMed] [Google Scholar]
- 54.Mackay F, et al. Both the lymphotoxin and tumor necrosis factor pathways are involved in experimental murine models of colitis. Gastroenterology. 1998;115 (6):1464–1475. doi: 10.1016/s0016-5085(98)70025-3. [DOI] [PubMed] [Google Scholar]
- 55.Fava RA, et al. A Role for the Lymphotoxin/LIGHT Axis in the Pathogenesis of Murine Collagen-Induced Arthritis. J Immunol. 2003;171 (1):115–126. doi: 10.4049/jimmunol.171.1.115. [DOI] [PubMed] [Google Scholar]
- 56.Ruddell RG, et al. Lymphotoxin-beta receptor signaling regulates hepatic stellate cell function and wound healing in a murine model of chronic liver injury. Hepatology. 2009;49 (1):227–239. doi: 10.1002/hep.22597. [DOI] [PubMed] [Google Scholar]
- 57.Karin M. Nuclear factor-[kappa]B in cancer development and progression. Nature. 2006;441 (7092):431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 58.Hehlgans T, et al. Lymphotoxin-{beta} Receptor Immune Interaction Promotes Tumor Growth by Inducing Angiogenesis. Cancer Res. 2002;62 (14):4034–4040. [PubMed] [Google Scholar]
- 59.Haybaeck J, et al. A Lymphotoxin-Driven Pathway to Hepatocellular Carcinoma. Cancer Cell. 2009;16 (4):295–308. doi: 10.1016/j.ccr.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]