Abstract
Erythropoietin (EPO) promotes functional recovery after traumatic brain injury (TBI). This study was designed to investigate whether EPO treatment promotes contralateral corticospinal tract (CST) plasticity in the spinal cord in rats after TBI. Biotinylated dextran amine (BDA) was injected into the right sensorimotor cortex to anterogradely label the CST. TBI was induced by controlled cortical impact over the left parietal cortex immediately after BDA injections. EPO (5,000 U/kg) or saline was administered intraperitoneally at Days 1, 2, and 3 post injury. Neurological function was assessed using a modified neurological severity score (mNSS) and footfault tests. Animals were sacrificed 35 days after injury and brain sections stained for histological analysis. Compared to the saline treatment, EPO treatment significantly improved sensorimotor functional outcome (lower mNSS and reduced footfaults) from Days 7 to 35 post injury. TBI alone significantly stimulated contralateral CST axon sprouting toward the denervated gray matter of the cervical and lumbar spinal cord; however, EPO treatment further significantly increased the axon sprouting in TBI rats although EPO treatment did not significantly affect axon sprouting in sham animals. The contralesional CST sprouting was highly and positively correlated with sensorimotor recovery after TBI. These data demonstrate that CST fibers originating from the contralesional intact cerebral hemisphere are capable of sprouting into the denervated spinal cord after TBI and EPO treatment, which may at least partially contribute to functional recovery.
Keywords: axonal plasticity, erythropoietin, functional recovery, rats, traumatic brain injury
1. Introduction
Traumatic brain injury (TBI) is the leading cause of mortality and morbidity worldwide, particularly among the young and may result in permanent functional deficits due to both primary and secondary injury mechanisms. The most prevalent and debilitating features in survivors of TBI are cognitive deficits and motor dysfunctions. To date, there is no effective treatment to promote functional recovery except for routine medical intervention and care (Narayan et al., 2002). Erythropoietin (EPO) and the EPO receptor (EPOR), essential for erythropoiesis, are also expressed in the brain (Brines et al., 2000). Previous studies from us and others indicate that EPO-mediated neuroprotection, angiogenesis, and neurogenesis contribute to functional recovery after TBI (Brines et al., 2004; Cherian et al., 2007; Grasso et al., 2007; Lu et al., 2005; Mahmood et al., 2007; Xiong et al., 2008a). Axonal plasticity is also associated with spontaneous motor recovery over time after TBI (Oshima et al., 2009; Ramic et al., 2006; Smith et al., 2007). In principle, a treatment that promotes axonal plasticity could be beneficial to functional recovery after the primary injury (Smith et al., 2007). EPO promotes axonal growth after peripheral nerve injury in the rat (Ransome and Turnley, 2008; Yin et al., 2009); however, it is not known whether EPO treatment promotes axonal plasticity of the corticospinal tract (CST) in the spinal cord after TBI. The CST is a major fiber bundle arising from layer V pyramidal neurons of the frontal motor cortex and connects via the corticospinal or pyramidal tracts to contralateral motor neurons of the spinal cord to control voluntary movements (Stanfield, 1992). Our recent study demonstrates that CST fibers originating from the contralesional intact cortex sprout into the denervated spinal cord after stroke and bone marrow stromal cell treatment, which may contribute to spontaneous and treatment-mediated functional recovery (Liu et al., 2008). Because delayed EPO treatment can improve neurological functional recovery without affecting lesion volume after TBI, we hypothesize that EPO may improve neurological recovery partially through enhancement of neuroplasticity. In the present study, we used a controlled cortical impact TBI rat model to assess the midline axonal crossing in the cervical and lumbar spinal cord and sensorimotor functional recovery in rats after TBI and EPO treatment. We found that EPO treatment of TBI significantly increases axonal crossing in the CST, which is significantly correlated with sensorimotor recovery.
2. Results
2.1. Hematocrit (HCT)
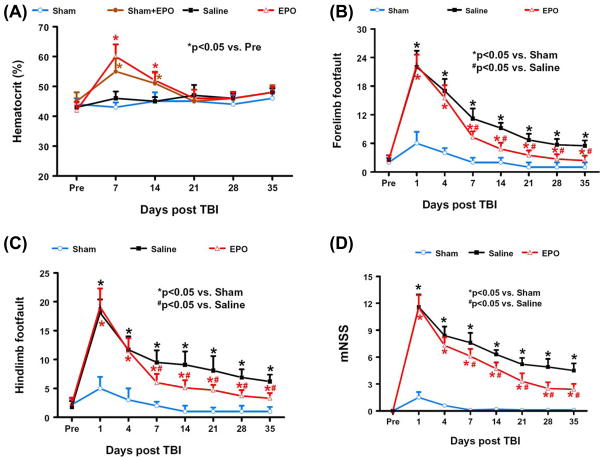
The baseline of HCT was similar for all animals before injury or sham-surgery (Fig. 1A). Compared to preinjury levels, after EPO treatment HCT increased significantly for up to 2 weeks (P < 0.0001 for Days 7 and 14 in the Sham + EPO and EPO group), and returned to normal thereafter. Half of the animals (4 out of 8) in the sham control group receiving EPO treatment was used to investigate potential effects of EPO alone on the axon crossing and functional tests. Our present study found that EPO does not affect axonal crossing and functional outcome in sham animals. Therefore, the data from sham controls were pooled for the following functional and histological analyses.
FIG. 1. Hematocrit and neurological functional tests.
A. Hematocrit before and after EPO treatment. “Pre” represents preinjury level. Four out of 8 sham animals also received EPO treatment to investigate whether EPO alone would affect the axon sprouting. EPO treatment significantly increases hematocrit up to 2 weeks (P < 0.05). Data represent mean ± SD. N = 4 (Sham); 4 (Sham + EPO); 8 (Saline); 8 (EPO). B: Effect of EPO on sensorimotor function (forelimb footfault) after TBI. “Pre” represents pre-injury level. TBI significantly increases the incidence of forelimb footfaults (P < 0.05) compared with sham controls. Delayed EPO treatment significantly reduces forelimb footfaults at Days 7–35 compared with the saline group (P < 0.05). C: Effect of EPO on sensorimotor function (hindlimb footfault) after TBI. “Pre” represents pre-injury level. TBI significantly increases the incidence of hindlimb footfaults (P < 0.05) compared with sham controls. Delayed EPO treatment significantly reduces hindlimb foot faults at Days 7–35 compared with the saline group (P < 0.05). D: The line graph shows the functional improvement detected on the modified neurological severity scores (mNSS). EPO treatment significantly lowers mNSS scores at Days 7–35 compared to saline group (P < 0.05). Data represent mean ± SD. N = 8 (Sham); 8 (Saline); 8 (EPO) in Fig. 1B–D.
2.2. Footfault test
The incidence of forelimb footfaults during baseline (preoperatively) was approximately 5% (Fig. 1B). TBI significantly increased the incidence of right forelimb footfaults contralateral to the TBI on 1 – 35 days postinjury compared with the pre-injury baseline (P < 0.05). Compared to sham controls, TBI significantly increased the incidence of contralateral forelimb footfaults on 1 – 35 days post-injury (P < 0.05). Treatment with EPO significantly reduced the number of contralateral forelimb footfaults on Days 7 – 35 (P < 0.01) after TBI compared to treatment with saline.
Similar results were found for the contralateral hindlimb (Fig. 1C). As compared to preinjury baseline and sham controls, TBI significantly increased the incidence of contralateral hindlimb footfaults at 1 to 35 days post-injury (P < 0.05). Treatment with EPO significantly reduced the number of contralateral hindlimb footfaults on Days 7–35 (P < 0.05) after TBI compared to treatment with saline.
2.3. Modified neurological severity score (mNSS)
Sham animals did not show any significant neurological deficits in this test. TBI significantly increased the mNSS score during the 35-day observation period (P < 0.05). TBI rats showed spontaneous functional recovery over the time (Fig. 1D), which was also observed in the footfault tests (Fig. 1B and 1C). Figure 1D shows that mNSS scores were reduced significantly in the EPO-treated group on Days 7–35 (P < 0.01) after TBI compared to the saline-treated group.
2.4. CST axons crossing over the midline into the denervated side of spinal gray matter
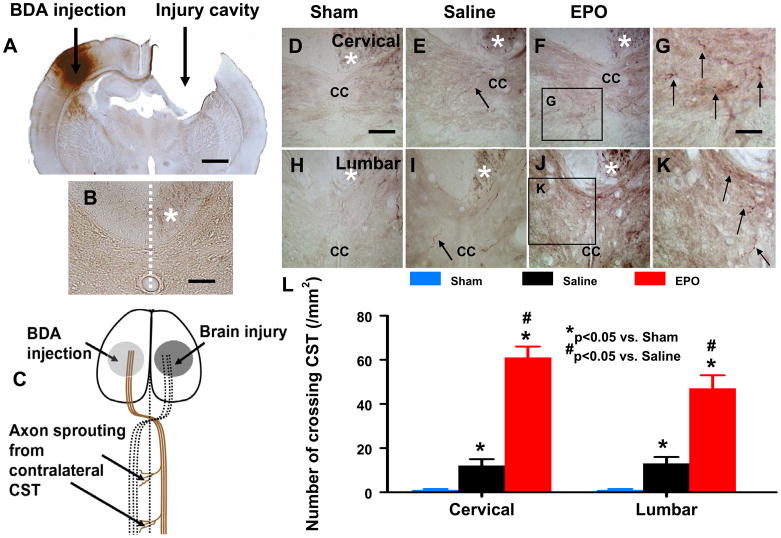
At the cervical and lumbar levels of the spinal cord, the descending CST motor fibers originating from the contralateral cortical pyramidal cells extend into the same side of gray matter to form neural circuits from spinal interneurons to spinal motoneurons for the control of forelimb and hindlimb movement (Liu et al., 2007). We and others have shown that BDA is reliable for anterogradely labeling axons (Liu et al., 2008; Reiner et al., 2000). BDA was injected stereotactically into the pyramidal cell layer in the contralesional hemisphere immediately before injury in this study. Animals were sacrificed at Day 35 post injury to examine the BDA labeling in the cervical and lumbar spinal cords. No obvious BDA-labeled CST fibers crossing over the midline were found in the opposite side of the spinal cord on transverse sections at both cervical (Fig. 2D) and lumbar levels (Fig. 2H) in sham rats, including those treated with EPO. In TBI rats treated with saline, increased axonal sprouting from the intact CST was observed in the denervated side of the gray matter of both cervical (Fig. 2E, P < 0.0001) and lumbar (Fig. 2I, P < 0.0001) levels of the spinal cord. However, the CST sprouting was significantly enhanced at both cervical (12 ± 3 vs 61 ± 5/mm2 for Saline- and EPO-treated groups, respectively; P < 0.0001) and lumbar (13 ± 3 vs 47 ± 6/ mm2 for Saline- and EPO-treated groups, respectively; P < 0.0001) spinal cord in the EPO-treated TBI animals (Fig. 2F–L) compared to the saline-treated TBI animals.
FIG. 2. BDA-labeling of CST originating from the contralesional intact hemisphere.
A. A representative coronal section showing BDA injection and injury cavity. B: A representative transverse section showing the BDA-labeled CST in the ventral-most part of the dorsal funiculus (white asterisk, also applicable to D–F and H–J). C: Simplified schematic illustration of TBI, BDA injection, and CST. Unilateral TBI causes cortical injury which affects the CST (dotted lines). BDA was injected into the contralesional intact cortex (stereotaxic coordinates: 1 and 2 mm posterior to the bregma, 3 and 4 mm lateral to the midline, at a depth of 1.5 to 1.7 mm from the cortical surface) to anterogradely label the CST which sprouts axons and crosses the midline into the denervated spinal cord after TBI and EPO treatment. D–G: Representative pictures from the cervical spinal cord showing midline-crossing BDA-labeled CST axons (arrow in E) sprouting into denervated side of the ventral gray matter in a rat after TBI. EPO treatment significantly increases the axon crossing (arrow in G). H–K: Representative pictures from the lumbar spinal cord showing midline-crossing BDA-labeled CST axons (arrow in I) sprouting into denervated side of the ventral gray matter in a rat after TBI. EPO treatment significantly increases the axon crossing (arrow in K). L: Quantitative data showing that no obvious BDA-labeled axons were observed in the opposite site of the cervical and lumbar spinal cord in sham rats, whereas the number of contralesional CST in the denervated cervical and lumbar gray matter were significantly increased by traumatic injury (P < 0.05 vs. Sham) and EPO treatment (P < 0.05 vs. Saline). CC: central canal. Scale bar = 2 mm in A, 200 μm in B, 100 μm in D (applicable to E, F, H, I, and J) and 50 μm in G (applicable to K). Data represent mean ± SD. N = 8 (Sham); 8 (Saline); 8 (EPO).
2.5. Lesion volume
Rats were sacrificed at 35 days post TBI for histological measurements. EPO treatment (24 h post injury) did not affect the lesion volume after TBI (P > 0.05). For TBI rats treated with saline and EPO, the lesion volume was 16.9 ± 1.4% and 16.3 ± 1.5%, respectively.
2.6. Correlation of axonal crossing with functional recovery
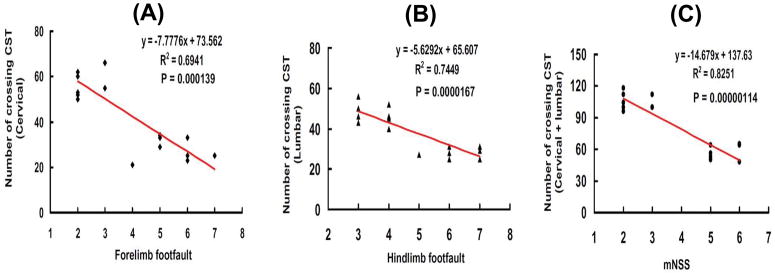
Although delayed EPO therapy did not reduce lesion volume caused by TBI, EPO treatment significantly improved functional recovery assessed by footfault and mNSS tests. EPO may improve neurological recovery through enhancement of neuroplasticity. Our present data show that the number of axons crossing at the cervical and lumbar spinal cord was highly and inversely correlated to the incidence of forelimb (P < 0.001) and hindlimb (P < 0.0001) footfaults examined at Day 35 after TBI (Fig. 3A and 3B). Similarly, the total number of axons crossing at cervical and lumbar spinal cord was highly and inversely correlated to the mNSS score assessed at Day 35 after TBI (Fig. 3C, P < 0.0001).
FIG. 3. Correlation of functional recovery with the number of midline axon crossings.
A. The line graph shows that the number of midline axons crossing at cervical level of the spinal cord is significantly and inversely correlated with the incidence of forelimb footfaults measured at Day 35 in rats after TBI and EPO treatment (P < 0.001). B: The line graph shows that the number of midline axons crossing at the lumbar level of the spinal cord is significantly and inversely correlated with the incidence of hindlimb footfaults measured at Day 35 in rats after TBI and EPO treatment (P < 0.001). C: The line graph shows that the total number of midline axon crossings at both cervical and lumbar levels of the spinal cord is significantly and inversely correlated with the mNSS score examined at Day 35 in rats after TBI and EPO treatment (P < 0.001). Data represent mean ± SD. N = 8 (Sham); 8 (Saline); 8 (EPO).
3. Discussion
Using a unilateral cortical injury model in rodents, we have demonstrated that EPO administration significantly improves functional outcome after TBI (Lu et al., 2005; Mahmood et al., 2007; Xiong et al., 2007). Our previous studies showed that delayed EPO treatment promotes angiogenesis and neurogenesis, which may partially contribute to functional recovery after TBI (Lu et al., 2005; Xiong et al., 2009). To the best of our knowledge, the present study provides the first evidence that EPO treatment promotes CST axons originating from the contralesional intact cortex to cross the midline into the denervated gray matter of cervical and lumbar spinal cord in rats after TBI, suggesting that the EPO-enhanced axonal plasticity may be attributed in part to sensorimotor recovery after TBI.
Unlike axons in the peripheral nervous system, axonal regeneration in the central nervous system (CNS) after brain injury is limited in the adult, often leading to devastating long-term disability (Huebner and Strittmatter, 2009). However, functional recovery occurs with time in animals and patients after brain injury (Lu et al., 2005; Sidaros et al., 2009). This spontaneous recovery after injury may partially be due to the reorganization of the neural network in the remaining compromised brain tissue (Dancause et al., 2005). The plastic reorganization between motor cortex and target motoneurons may include unmasking of existing but functionally inactive pathways, sprouting of fibers from surviving neurons and formation of new synapses, and redundancy of CNS circuitry allowing alternative pathways to take over functions (Navarro, 2009). The noninjured intact hemisphere is capable of sending axon projections to the ipsilateral spinal cord following a unilateral cortical lesion after TBI (Lenzlinger et al., 2005; Smith et al., 2007) and stroke (Chen et al., 2002; Liu et al., 2008). Our stroke model causes extensive brain damage with lesion volume approaching 40% of hemisphere, including both cortical and subcortical structure (striatum), and this injury induces a significant increase in axonal sprouting across the cervical spinal cord (Liu et al., 2008). A recent study shows that a stroke confined to cortex alone is also sufficient to trigger axon sprouting in the spinal cord in mice (Lapash Daniels et al., 2009). In the present study, TBI increases axonal sprouting in both cervical and lumbar spinal cord although it causes relatively smaller lesion volume (16% of hemisphere). Our results are in agreement with recent studies where increased midline axonal sprouting in the cervical spinal cord was observed in rats after lateral fluid percussion brain injury (Lenzlinger et al., 2005) and in mice after lateral controlled cortical impact brain injury (Oshima et al., 2009). However, there was no significant increase in the midline axonal sprouting found in the cervical spinal cord in rats after controlled cortical impact (Smith et al., 2007). This discrepancy may derive mainly from the difference in injury severity (i.e., injuries are more severe in our study) and different time points studied after injury.
Nearly all phase III clinical trials in neuroprotection have failed to show any consistent improvement in outcome for TBI patients (Narayan et al., 2002). The disappointing clinical trials may be due to heterogeneity of the population of TBI patients and variability in treatment approaches. Another important aspect is that TBI evokes many different pathophysiologic cascades leading to secondary injury. Some unsuccessful clinical trials targeted only one aspect of the cascades (Narayan et al., 2002). EPO is a multifunctional agent targeting many TBI-induced cascades including apoptosis, oxidation, and inflammation after brain injury (Brines et al., 2000; Cherian et al., 2007; Grasso et al., 2007; Lu et al., 2005; Mahmood et al., 2007; Wang et al., 2004; Xiong et al., 2008a; Zhang et al., 2005). EPO treatment reduces lesion volume and improves functional recovery when administered within 6 h after experimental TBI (Brines et al., 2004; Brines et al., 2000; Cherian et al., 2007; Xiong et al., 2008a). Within the area of neurotrauma, changes in lesion volume after a controlled cortical impact injury are often used to imply recovery of function. However, despite progressive atrophy, spontaneous clinical improvement occurs in TBI patients (Sidaros et al., 2009). Our previous studies also show that delayed EPO therapy without reduction of lesion is capable of improving functional recovery after TBI and stroke in rats (Lu et al., 2005; Wang et al., 2004). Delayed EPO treatment provides therapeutic benefits possibly through angiogenesis, neurogenesis, and synaptogenesis after brain injury (Cherian et al., 2007; Grasso et al., 2007; Lu et al., 2005; Mahmood et al., 2007; Wang et al., 2004; Xiong et al., 2008a; Zhang et al., 2005). In the present study, we have focused on the descending axons of the CST - the only direct pathway from the sensorimotor cortex to the spinal cord and the major neural pathway for precise control of voluntary movements (Martin, 2005; Stanfield, 1992). Neural reconnection of the denervated side of spinal cord from the brain may play an important role in the neuroanatomical foundation of functional recovery after brain injury. Inosine treatment significantly increases the number of crossing axons of the spinal cord and improves skilled forelimb use in rats after brain insults including stroke (Chen et al., 2002) and controlled cortical impact (Smith et al., 2007). Our recent study demonstrates that bone marrow stromal cell administration promotes the sprouting of CST fibers from the contralesional motor cortex into the denervated spinal cord examined after stroke induced by middle cerebral artery occlusion (Liu et al., 2008), which may partially contribute to functional recovery. Our present study demonstrates that there is a significant correlation between sensorimotor functional recovery and axonal crossing in the cervical and lumbar spinal cord after TBI and EPO treatment. These data strongly suggest that enhanced CST axonal plasticity may at least partially contribute to the therapeutic benefits of EPO treatment for TBI. Our recent study has shown that bone marrow stromal cells enhance inter- and intracortical axonal connections after ischemic stroke in adult rats, suggesting that, after stroke, cortical neurons surviving in the peri-infarct motor cortex also undergo axonal sprouting to restore connections between different cerebral areas (Liu et al., 2010). Our present study does not exclude the possibility of inter- and intracortical axonal connections and other neuronal pathways including ipsilateral ventral CST, corticorubral tract, and other brainstem–spinal systems that contribute to functional recovery after TBI and EPO treatment. It is warranted to investigate these possibilities and effects of EPO dose and therapeutic time on axonal plasticity in the brain and spinal cord after TBI. In addition, as shown in the present study spontaneous functional recovery occurs over time after moderate TBI. Although there is a significant difference in functional recovery between the saline- and EPO-treated groups at 35 days after TBI, the difference between these 2 groups becomes relatively small. Future studies using extended survival times and more severe TBI are warranted.
Previous studies by us (Lu et al., 2007; Mahmood et al., 2007; Xiong et al., 2009) and others (Brines et al., 2000; 2004; Grasso et al., 2007) demonstrate EPO treatment improves functional recovery after TBI. However, the mechanism behind this effect has not been fully investigated. Our previous studies (Xiong et al., 2009) indicate that EPO-induced angiogenesis and neurogenesis may play an important role in functional recovery after TBI. We have also demonstrated that EPO increases the expression of brain-derived neurotrophic factor after TBI (Mahmood et al., 2007), indicating that this growth factor may play a role in functional recovery. EPO stimulated the phosphoinositide 3-kinase (PI3K)/Akt pathway and co-incubation of the PI3K/Akt pathway inhibitor LY294002 abolished EPO effects on Akt phosphorylation and axonal growth in vitro (Ransome and Turnley, 2008). EPO treatment decreases nuclear factor κB and increases phosphorylation of Akt and STAT5 which may participate in regenerating peripheral nervous system axons (Toth et al., 2008). After brain injury, the adult CNS induces the expression of growth-promoting factors such as brain-derived neurotrophic factor, basic fibroblast growth factor, nerve growth factor, neurotrophin-3 and the neurotrophin-4/5 that promote axonal regeneration and lead to partial functional recovery (Cui, 2006; Logan et al., 1994). EPO treatment enhances the expression of these growth factors after neural injury (Mahmood et al., 2007; Zhang et al., 2005). The functional recovery after EPO treatment likely relies on the ability of EPO to activate mechanisms associated with the formation of new motor pathways. Our present study provides the first direct evidence of EPO-induced axonal sprouting from the contralesional cortex to cross the midline into the denervated side of the cervical and lumbar spinal cord after TBI. Thus, EPO treatment may offer a unique multifunctional strategy for neuroprotection and neurorestoration including axonal plasticity (Brines et al., 2004; Cherian et al., 2007; Grasso et al., 2009; Lu et al., 2005; Ransome and Turnley, 2008; Toth et al., 2008; Xiong et al., 2009; Zhang et al., 2009). Additional studies on the role of growth factors in EPO-induced axonal sprouting after TBI by using different specific inhibitors of proposed signaling pathways including LY294002 (PI3 Kinase Inhibitor), are warranted.
In conclusion, delayed EPO treatment significantly improved sensorimotor functional outcome without affecting lesion volume and enhanced the contralateral CST axon midline-sprouting in the spinal cord after TBI. The axon sprouting is highly correlated with sensorimotor recovery after TBI and EPO treatment. These data demonstrate that CST fibers originating from the contralesional intact cerebral hemisphere are capable of sprouting into the denervated spinal cord after TBI and EPO treatment, which may partially contribute to functional recovery.
4. Experimental procedures
4.1. Surgical procedures
All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Henry Ford Hospital. Investigators blinded to the treatment status performed all functional tests and biotinylated dextran amine (BDA)-labeled axon counting.
Young adult male Wistar rats (300–400 g) were anesthetized with an intraperitoneal injection of chloral hydrate (350 mg/kg body weight). The animals’ rectal temperature was controlled at 37 °C by using a feedback-regulated water-heating pad. A CCI device was used to induce the injury. The rats were placed in a stereotactic frame. Two 10-mm-diameter craniotomies were performed adjacent to the central suture, midway between the lambda and bregma. The second craniotomy allowed for the movement of cortical tissue laterally. The dura mater was kept intact over the cortex. Then, 10% solution of BDA (10,000 MW; Molecular Probes, Eugene, OR) in PBS was injected through a finely drawn glass capillary into 4 points in the right cortex (100 nl per injection; stereotaxic coordinates: 1 and 2 mm posterior to the bregma, 3 and 4 mm lateral to the midline, at a depth of 1.5 to 1.7 mm from the cortical surface) to anterogradely label the CST originating from this area. The micropipette remained in place for 4 min after completion of the injection. A controlled cortical impact model of TBI was employed in the present study (Dixon et al., 1991; Mahmood et al., 2004; Xiong et al., 2009). Immediately after the BDA injection, controlled cortical impact was delivered by impacting the left (ipsilateral) cortex with a pneumatic piston containing a 6-mm-diameter tip at the rate of 4 m/s and 2.5 mm of compression. Velocity was measured with a linear velocity displacement transducer.
4.2. Experimental groups and treatment
Young adult male Wistar rats were randomly divided into three groups: Group 1, Sham control (n = 8); Group 2, TBI + Saline (n =8), and Group 3, TBI + EPO (n = 8). TBI was induced by controlled cortical impact over the left parietal cortex. Rats in the sham control group received surgery without injury. EPO at a dose of 5000 U/kg body weight (Epoetin alpha, AMGEN, Thousand Oaks, CA) was administered intraperitoneally at Days 1, 2 and 3 after TBI or sham surgery (Xiong et al., 2008a). To investigate whether EPO alone affects the axon crossing and functional tests in sham controls, half (4 out of 8) of the sham animals received EPO treatment. Animals in the saline-treated group received an equal volume of saline at Days 1, 2 and 3 after surgery. Allrats were sacrificed at 35 days after TBI or surgery.
4.3. Hematocrit
To determine the effects of EPO on hematocrit (HCT), a blood sample (50 μl) was collected via tail vein before injury, at Day 4 and then weekly after TBI. HCT was measured in micro-HCT capillary tubes (Fisher Scientific, Pittsburgh, PA) using standard procedures (Readacrit Centrifuge, Clay Adams, Parsippany, NJ) (Xiong et al., 2007; Xiong et al., 2008b).
4.4. Footfault test
To evaluate sensorimotor function, the footfault test was carried out before TBI and at 1, 4, 7, 14, 21, 28 and 35 days after TBI. The rats were allowed to walk on a grid (Xiong et al., 2007). With each weight-bearing step, a paw might fall or slip between the wires and, if this occurred, it was recorded as a footfault (Baskin et al., 2003). A total of 50 steps were recorded for each right forelimb and hindlimb.
4.5. Modified neurological test
An assessment of neurological functional recovery was performed using the modified neurological severity score (mNSS) test (Chen et al., 2001). The test was carried out on all rats preinjury and on Days 1, 4, 7, 14, 21, 28, and 35 after TBI. The mNSS is a composite of the motor (muscle status and abnormal movement), sensory (visual, tactile and proprioceptive), reflex, and beam-walking tests, and has been employed in previous studies (Lu et al., 2007). In this TBI model, injury in the left hemispheric cortex of rats causes sensory and motor functional deficiency with elevated scores on motor, sensory, and Beam Balance Tests in the early phase after injury (Day 1 after injury). Absent reflexes and abnormal movements can be measured in rats with severe injury. Slow recovery in asymmetry deficiency as reflected by Beam Balance Test results has been reported in unilateral brain injuries (Lu et al., 2007). The mNSS score test is suitable for evaluating long-term neurological function after unilateral brain injury (Mahmood et al., 2008; Xiong et al., 2009).
4.6. Tissue preparation and measurement of lesion volume
At Day 35 after TBI, rats were anesthetized intraperitoneally with overdosed chloral hydrate, and perfused transcardially first with saline solution, followed by 4% paraformaldehyde in 0.1 M phosphate buffered saline (PBS), pH 7.4. Their brains and cervical and lumbar spinal cords were removed and post-fixed in 4% paraformaldehyde for 2 days at room temperature. The brain was cut into seven equally spaced (2-mm) coronal blocks using a rat brain matrix (Activational System, Warren, MI). The 100-μm-thick sections of brain blocks (bregma +1 to −3) were sectioned using a vibratome for BDA staining to verify the tracer injection. The remaining brain blocks were embedded in paraffin. A series of adjacent 6-μm-thick sections were cut from each block and stained with hematoxylin and eosin for lesion volume measurement. For lesion volume measurement, one 6-μm-thick section from each of 7 coronal blocks was traced by a microcomputer imaging device (MCID) (Imaging Research, St. Catharine’s, Ontario, Canada), as previously described (Chen et al., 2005). The indirect lesion area was calculated (i.e., the intact area of the ipsilateral hemisphere is subtracted from the area of the contralateral hemisphere) (Swanson et al., 1990), and the lesion volume presented as a volume percentage of the lesion compared with the contralateral hemisphere to correct for shrinkage of the brain tissue which resulted from the wax-embedding process. The cervical (C1-6) and lumbar (L1-6) spinal cord segments were processed for vibratome traverse sections (100 μm). Sections were incubated with 0.5% H2O2 for 20 min followed with avidin-biotin-peroxidase complex (Vector Laboratories Inc., Burlingame, CA) at 4°C for 48 h, and BDA-labeled axons were visualized with 3,3′-diaminobenzidine-nickel (Vector) for light microscopy examination (Liu et al., 2008). For counting axons crossing the midline of cervical and lumbar spinal cord, the central canal and dorsal median fissure were used as landmarks with the number of labeled axons projecting into the denervated TBI-impaired side of the ventral gray matter. For each animal, the total number of midline-crossing axons was counted on 50 consecutive sections. To avoid possible interanimal variation induced by tracing efficiency and staining, the crossed axon number in each animal was corrected with a quotient of mean BDA-labeled CST number in the dorsal funiculus of spinal cord divided by the individual CST number (Liu et al., 2008).
4.7. Statistical analysis
All data are presented as means with standard deviations (SD). Data on mNSS were first evaluated for normality. The rank data were used for the analysis since data were not normal. Analysis of variance (ANCOVA), PROC MIXED with CONTRAST statement in SAS, was employed to test group differences on mNSS. The analysis began with testing the overall group effect, followed by pair-wise group comparisons if the overall group effect was detected at the 0.05 level; otherwise the pair-wise group comparisons would be considered as exploratory analyses. Data on HCT and sensorimotor function were analyzed by ANOVA for repeated measurements. For lesion volume and axonal counting, a one-way ANOVA followed by post hoc SNK tests were used to compare the differences between the EPO-treated, saline-treated and sham groups. Statistical significance was set at P < 0.05.
Acknowledgments
This work was supported by NIH grants RO1 NS 62002 (YX) and PO1 NS42345 (MA and MC). Special thanks to Ms. Susan MacPhee-Gray for editorial assistance.
Sources of financial support: NINDS grants RO1 NS62002 (Ye Xiong) and PO1 NS42345 (Asim Mahmood, Michael Chopp).
References
- Baskin YK, Dietrich WD, Green EJ. Two effective behavioral tasks for evaluating sensorimotor dysfunction following traumatic brain injury in mice. J Neurosci Methods. 2003;129:87–93. doi: 10.1016/s0165-0270(03)00212-7. [DOI] [PubMed] [Google Scholar]
- Brines M, Grasso G, Fiordaliso F, Sfacteria A, Ghezzi P, Fratelli M, Latini R, Xie QW, Smart J, Su-Rick CJ, Pobre E, Diaz D, Gomez D, Hand C, Coleman T, Cerami A. Erythropoietin mediates tissue protection through an erythropoietin and common beta-subunit heteroreceptor. Proc Natl Acad Sci U S A. 2004;101:14907–14912. doi: 10.1073/pnas.0406491101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brines ML, Ghezzi P, Keenan S, Agnello D, de Lanerolle NC, Cerami C, Itri LM, Cerami A. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci U S A. 2000;97:10526–10531. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, Sanchez-Ramos J, Chopp M. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001;32:2682–2688. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, Katakowski M, Lu M, Chopp M. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005;25:281–290. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Goldberg DE, Kolb B, Lanser M, Benowitz LI. Inosine induces axonal rewiring and improves behavioral outcome after stroke. Proc Natl Acad Sci U S A. 2002;99:9031–9036. doi: 10.1073/pnas.132076299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherian L, Goodman JC, Robertson C. Neuroprotection with erythropoietin administration following controlled cortical impact injury in rats. J Pharmacol Exp Ther. 2007;322:789–794. doi: 10.1124/jpet.107.119628. [DOI] [PubMed] [Google Scholar]
- Cui Q. Actions of neurotrophic factors and their signaling pathways in neuronal survival and axonal regeneration. Mol Neurobiol. 2006;33:155–179. doi: 10.1385/MN:33:2:155. [DOI] [PubMed] [Google Scholar]
- Dancause N, Barbay S, Frost SB, Plautz EJ, Chen D, Zoubina EV, Stowe AM, Nudo RJ. Extensive cortical rewiring after brain injury. J Neurosci. 2005;25:10167–10179. doi: 10.1523/JNEUROSCI.3256-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon CE, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods. 1991;39:253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- Grasso G, Sfacteria A, Meli F, Fodale V, Buemi M, Iacopino DG. Neuroprotection by erythropoietin administration after experimental traumatic brain injury. Brain Res. 2007;1182:99–105. doi: 10.1016/j.brainres.2007.08.078. [DOI] [PubMed] [Google Scholar]
- Grasso G, Graziano F, Sfacteria A, Carletti F, Meli F, Maugeri R, Passalacqua M, Certo F, Fazio M, Buemi M, Iacopino DG. Neuroprotective effect of erythropoietin and darbepoetin alfa after experimental intracerebral hemorrhage. Neurosurgery. 2009;65:763–769. doi: 10.1227/01.NEU.0000347475.73347.5F. [DOI] [PubMed] [Google Scholar]
- Huebner EA, Strittmatter SM. Axon regeneration in the peripheral and central nervous systems. Results Probl Cell Differ. 2009;48:339–351. doi: 10.1007/400_2009_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapash Daniels CM, Ayers KL, Finley AM, Culver JP, Goldberg MP. Axon sprouting in adult mouse spinal cord after motor cortex stroke. Neurosci Lett. 2009;450:191–195. doi: 10.1016/j.neulet.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzlinger PM, Shimizu S, Marklund N, Thompson HJ, Schwab ME, Saatman KE, Hoover RC, Bareyre FM, Motta M, Luginbuhl A, Pape R, Clouse AK, Morganti-Kossmann C, McIntosh TK. Delayed inhibition of Nogo-A does not alter injury-induced axonal sprouting but enhances recovery of cognitive function following experimental traumatic brain injury in rats. Neuroscience. 2005;134:1047–1056. doi: 10.1016/j.neuroscience.2005.04.048. [DOI] [PubMed] [Google Scholar]
- Liu Z, Li Y, Qu R, Shen L, Gao Q, Zhang X, Lu M, Savant-Bhonsale S, Borneman J, Chopp M. Axonal sprouting into the denervated spinal cord and synaptic and postsynaptic protein expression in the spinal cord after transplantation of bone marrow stromal cell in stroke rats. Brain Res. 2007;1149:172–180. doi: 10.1016/j.brainres.2007.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Li Y, Zhang X, Savant-Bhonsale S, Chopp M. Contralesional axonal remodeling of the corticospinal system in adult rats after stroke and bone marrow stromal cell treatment. Stroke. 2008;39:2571–2577. doi: 10.1161/STROKEAHA.107.511659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Li Y, Zhang ZG, Cui X, Cui Y, Lu M, Savant-Bhonsale S, Chopp M. Bone marrow stromal cells enhance inter- and intracortical axonal connections after ischemic stroke in adult rats. J Cereb Blood Flow Metab. 2010 doi: 10.1038/jcbfm.2010.8. cbfm20108 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan A, Oliver JJ, Berry M. Growth factors in CNS repair and regeneration. Prog Growth Factor Res. 1994;5:379–405. doi: 10.1016/0955-2235(94)00008-9. [DOI] [PubMed] [Google Scholar]
- Lu D, Mahmood A, Qu C, Goussev A, Schallert T, Chopp M. Erythropoietin enhances neurogenesis and restores spatial memory in rats after traumatic brain injury. J Neurotrauma. 2005;22:1011–1017. doi: 10.1089/neu.2005.22.1011. [DOI] [PubMed] [Google Scholar]
- Lu D, Mahmood A, Qu C, Hong X, Kaplan D, Chopp M. Collagen scaffolds populated with human marrow stromal cells reduce lesion volume and improve functional outcome after traumatic brain injury. Neurosurgery. 2007;61:596–602. doi: 10.1227/01.NEU.0000290908.38438.B2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood A, Lu D, Chopp M. Marrow stromal cell transplantation after traumatic brain injury promotes cellular proliferation within the brain. Neurosurgery. 2004;55:1185–1193. doi: 10.1227/01.neu.0000141042.14476.3c. [DOI] [PubMed] [Google Scholar]
- Mahmood A, Lu D, Qu C, Goussev A, Zhang ZG, Lu C, Chopp M. Treatment of traumatic brain injury in rats with erythropoietin and carbamylated erythropoietin. J Neurosurg. 2007;107:392–397. doi: 10.3171/JNS-07/08/0392. [DOI] [PubMed] [Google Scholar]
- Mahmood A, Goussev A, Lu D, Qu C, Xiong Y, Kazmi H, Chopp M. Long-lasting benefits after treatment of traumatic brain injury (TBI) in rats with combination therapy of marrow stromal cells (MSCs) and simvastatin. J Neurotrauma. 2008;25:1441–1447. doi: 10.1089/neu.2007.0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JH. The corticospinal system: from development to motor control. Neuroscientist. 2005;11:161–173. doi: 10.1177/1073858404270843. [DOI] [PubMed] [Google Scholar]
- Narayan RK, Michel ME, Ansell B, Baethmann A, Biegon A, Bracken MB, Bullock MR, Choi SC, Clifton GL, Contant CF, Coplin WM, Dietrich WD, Ghajar J, Grady SM, Grossman RG, Hall ED, Heetderks W, Hovda DA, Jallo J, Katz RL, Knoller N, Kochanek PM, Maas AI, Majde J, Marion DW, Marmarou A, Marshall LF, McIntosh TK, Miller E, Mohberg N, Muizelaar JP, Pitts LH, Quinn P, Riesenfeld G, Robertson CS, Strauss KI, Teasdale G, Temkin N, Tuma R, Wade C, Walker MD, Weinrich M, Whyte J, Wilberger J, Young AB, Yurkewicz L. Clinical trials in head injury. J Neurotrauma. 2002;19:503–557. doi: 10.1089/089771502753754037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro X. Chapter 27: Neural plasticity after nerve injury and regeneration. Int Rev Neurobiol. 2009;87:483–505. doi: 10.1016/S0074-7742(09)87027-X. [DOI] [PubMed] [Google Scholar]
- Oshima T, Lee S, Sato A, Oda S, Hirasawa H, Yamashita T. TNF-alpha contributes to axonal sprouting and functional recovery following traumatic brain injury. Brain Res. 2009;1290:102–110. doi: 10.1016/j.brainres.2009.07.022. [DOI] [PubMed] [Google Scholar]
- Ramic M, Emerick AJ, Bollnow MR, O’Brien TE, Tsai SY, Kartje GL. Axonal plasticity is associated with motor recovery following amphetamine treatment combined with rehabilitation after brain injury in the adult rat. Brain Res. 2006;1111:176–186. doi: 10.1016/j.brainres.2006.06.063. [DOI] [PubMed] [Google Scholar]
- Ransome MI, Turnley AM. Erythropoietin promotes axonal growth in a model of neuronal polarization. Mol Cell Neurosci. 2008;38:537–547. doi: 10.1016/j.mcn.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Reiner A, Veenman CL, Medina L, Jiao Y, Del Mar N, Honig MG. Pathway tracing using biotinylated dextran amines. J Neurosci Methods. 2000;103:23–37. doi: 10.1016/s0165-0270(00)00293-4. [DOI] [PubMed] [Google Scholar]
- Sidaros A, Skimminge A, Liptrot MG, Sidaros K, Engberg AW, Herning M, Paulson OB, Jernigan TL, Rostrup E. Long-term global and regional brain volume changes following severe traumatic brain injury: a longitudinal study with clinical correlates. Neuroimage. 2009;44:1–8. doi: 10.1016/j.neuroimage.2008.08.030. [DOI] [PubMed] [Google Scholar]
- Smith JM, Lunga P, Story D, Harris N, Le Belle J, James MF, Pickard JD, Fawcett JW. Inosine promotes recovery of skilled motor function in a model of focal brain injury. Brain. 2007;130:915–925. doi: 10.1093/brain/awl393. [DOI] [PubMed] [Google Scholar]
- Stanfield BB. The development of the corticospinal projection. Prog Neurobiol. 1992;38:169–202. doi: 10.1016/0301-0082(92)90039-h. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- Toth C, Martinez JA, Liu WQ, Diggle J, Guo GF, Ramji N, Mi R, Hoke A, Zochodne DW. Local erythropoietin signaling enhances regeneration in peripheral axons. Neuroscience. 2008;154:767–783. doi: 10.1016/j.neuroscience.2008.03.052. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35:1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Lu D, Qu C, Goussev A, Schallert T, Chopp M. Role of gender in outcome after traumatic brain injury and therapeutic effect of erythropoietin in mice. Brain Res. 2007;1185:301–312. doi: 10.1016/j.brainres.2007.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Lu D, Qu C, Goussev A, Schallert T, Mahmood A, Chopp M. Effects of erythropoietin on reducing brain damage and improving functional outcome after traumatic brain injury in mice. J Neurosurg. 2008a;109:510–521. doi: 10.3171/JNS/2008/109/9/0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Lu D, Qu C, Kazmi H, Goussev A, Zhang ZG, Noguchi CT, Schallert T, Chopp M. Histological and functional outcomes after traumatic brain injury in mice null for the erythropoietin receptor in the central nervous system. Brain Res. 2008b;1230:247–257. doi: 10.1016/j.brainres.2008.06.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Meng Y, Zhang Y, Qu C, Schallert T, Chopp M. Delayed administration of erythropoietin reducing hippocampal cell loss, enhancing angiogenesis and neurogenesis, and improving functional outcome following traumatic brain injury in rats: comparison of treatment with single and triple dose. J Neurosurg. 2009 doi: 10.3171/2009.9.JNS09844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin ZS, Zhang H, Gao W. Erythropoietin Promotes Functional Recovery and Enhances Nerve Regeneration after Peripheral Nerve Injury in Rats. AJNR Am J Neuroradiol. 2009 doi: 10.3174/ajnr.A1820. ajnr.A1820 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Li Y, Cui Y, Chen J, Lu M, Elias SB, Chopp M. Erythropoietin treatment improves neurological functional recovery in EAE mice. Brain Res. 2005;1034:34–39. doi: 10.1016/j.brainres.2004.11.036. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xiong Y, Mahmood A, Meng Y, Qu C, Schallert T, Chopp M. Therapeutic effects of erythropoietin on histological and functional outcomes following traumatic brain injury in rats are independent of hematocrit. Brain Res. 2009;1294:153–164. doi: 10.1016/j.brainres.2009.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]