Abstract
Clinical observations suggest that depressed patients were less sensitive to experimental pain than healthy subjects. However, few animal studies are reported concerning the association of depression and pain. The purpose of this study was to investigate the effects of unpredictable chronic mild stress (UCMS) induced depression on the perceived intensity of painful stimulation in rats. We measured the thermal and mechanical paw withdrawal thresholds (PWT) of normal and spinal nerve ligated (SNL) rats using hot plate test and von Frey test, respectively. The results showed that rats exposed to UCMS exhibited significantly higher thermal and mechanical pain thresholds in comparison to the non-depressed controls. In particular, the PWT of the SNL group was restored to nearly normal level after three weeks of UCMS, and even comparable to that of the control group. These results strongly suggest that the depressed subjects have decreased sensitivity to externally applied noxious stimulation, which is consistent with our previous findings.
Research Highlight
▶ Unpredictable chronic mild stress (UCMS) induces depressive behaviors in rats
▶ UCMS elevates contact heat paw withdrawal threshold in normal rats
▶ UCMS elevates mechanical paw withdrawal threshold in normal rats
▶ UCMS elevates mechanical paw withdrawal threshold in SNL rats
Keywords: unpredictable chronic mild stress, depression, hot plate test, neuropathic pain, von Frey filament, allodynia
1. Introduction
Depression and pain are two of the most common health problems and usually occur together in clinical practice. Studies have shown that a considerable proportion of patients with depressive disorders suffer from chronic pain (Dworkin and Gitlin, 1991; Fishbain et al., 1997; Rush et al., 2000) and vice versa, patients with chronic pain have an increased risk of developing depressive disorders (Corruble and Guelfi, 2000; Ohayon and Schatzberg, 2003). In contrast to this close clinical association of pain and depression, experimental pain thresholds in depressive patients are persistently increased (Adler and Gattaz, 1993; Bair et al., 2003; Bar et al., 2005; Davis et al., 1979; Dickens et al., 2003; Gormsen et al., 2004; Graff-Guerrero et al., 2008; Lautenbacher et al., 1994; Lautenbacher et al., 1999). The paradoxical phenomenon may be explained by the fact that the clinical pain was stimulus independent while the experimental pain was evoked by externally applied stimulation. Animal research has provided evidence that depression exerts different effects on stimulus-evoked pain and stimulus-independent pain, with alleviation in the former while aggravation in the latter (Shi et al. 2010).
Numerous human studies have been reported concerning the relationship between depression and experimentally evoked pain (Adler and Gattaz, 1993; Bar et al., 2003; Bar et al., 2005; Davis et al., 1979; Gormsen et al., 2004; Graff-Guerrero et al., 2008; Lautenbacher et al., 1994; Lautenbacher et al., 1999; Schwier et al.). A consistent finding was that depressed patients exhibited reduced sensitivity to painful stimuli such as thermal, electrical, or mechanical stimuli. In contrast to ample evidence from human studies, animal research measuring nociceptive thresholds in depressed condition have rarely been reported. With the tail-flick test, Pinto-Ribeiro et al. (Pinto-Ribeiro et al., 2004) revealed that rats exposed to chronic unpredictable stress were less sensitive to noxious heat stimuli. An inconsistent result reported by Zeng and his colleague showed that the presence of depression-like behavior in rats exacerbated mechanical allodynia under the condition of chronic neuropathic pain (Zeng et al., 2008).
In our previous work, we found that rats exposed to unpredictable chronic mild stress (UCMS) exhibited decreased sensitivity to noxious radiant heat stimulation under both normal and complete Freund’s adjuvant (CFA)-induced chronic pain conditions (Shi et al., 2010). The present study was aimed to further clarify the effects of depression on the stimulus-evoked pain, especially on mechanical allodynia, and confirm previous findings that the evoked pain can be attenuated under the condition of depression. The UCMS depression model was employed in this study to produce depressive-like behavior in rats. The chronic stress-induced changes in the nociceptive thresholds of normal and spinal nerve injured rats were examined using hot plate test and von Frey hair test, respectively.
2. Results
2.1 Effect of UCMS-induced depression upon contact heat-elicited pain
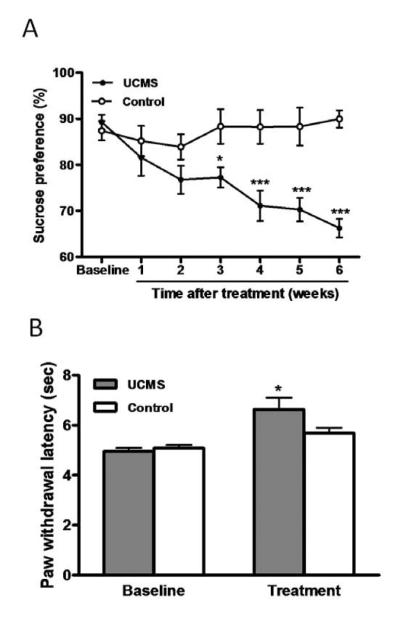
The results of Experiment 1 are shown in Fig. 2. The sucrose consumption of the control group (not exposed to stressors) remained unchanged throughout the observation period (Fig. 2A). In contrast, the group treated with UCMS exhibited a significant reduction in sucrose consumption after three weeks of stress exposure (2-way ANOVA, time effect: F(6,20) = 3.00, P < 0.01; stress effect: F(1,20) = 32.80, P < 0.001; stess*time interaction: F(6,20) = 4.996, P < 0.001), which persisted until the end of the procedure (six weeks). This result indicates that the depression animal model has been successfully established
Fig2.
Effect of UCMS-induced depression upon sucrose consumption and contact heat-elicited pain. (A) Sucrose preference test. Sucrose consumption was measured before and weekly after UCMS onset. Three weeks following stressor application, there was a significant reduction in sucrose solution intake in the UCMS-exposed rats as compared to the control rats (n=11). (B) Hot plate test. The paw withdrawal latency to noxious contact heat stimuli was examined before (baseline) and 6 weeks after UCMS procedure (treatment). A significant increase was observed in the withdrawal latency after UCMS exposure (n=11). Data are presented as mean ± SEM. * P < 0.05; *** P < 0.001, compared to the control group.
Hot plate tests found no differences between control and experimental groups in the baseline withdrawal latency (t-test, 4.96 ± 014 vs. 5.08 ± 0.13 s, P = 0.52; see Fig. 2B). However, UCMS treatment produced significantly increase in the paw withdrawal latency when compared to the control group (t-test, 6.20 ± 0.23 s vs. 5.59 ± 0.19 s, P < 0.05), suggesting that depressed subjects were less sensitive to the contact thermal stimuli.
2.2 Effect of UCMS-induced depression upon mechanical sensitivity of rats
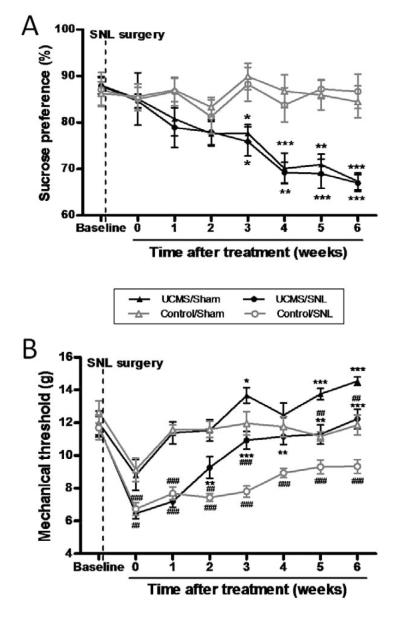
The results of Experiment 2 showed the effects of UCMS treatment on the mechanical sensitivity of rats (Fig. 3 & Table 2, 3). Fig. 3A presents the intake of sucrose solution over the course of 6 weeks. As expected, the sucrose consumption in UCMS groups were significantly decreased as compared to that in control groups (3-way ANOVA, time effect: F(7, 385) = 6.930, P < 0.001; stress effect: F(1, 55) = 56.73, P < 0.001; time*stress effect: F(7, 385) = 6.520, P < 0.001, Fig. 3A). No surgery effect was observed on the depressive like behaviors (surgery effect: F(1, 55) =0.278, P =0.600; surgery*stress interaction: F(1, 55) = 0.04, P= 0.84; surgery*time interaction: F(7, 385) =0.108, P =0.998).
Fig 3.
Effect of UCMS-induced depression upon sucrose consumption and mechanical sensitivity of rats. (A) Sucrose consumption throughout the experimental period. Chronic stressors were applied one week after the surgery. Sucrose preference test were performed before and weekly after the surgery. A significant reduction can be seen in sucrose consumption in the UCMS-exposed rats as compared to the control rats (n=13-16). (B) Mechanical threshold throughout the experimental period. ANOVA analysis revealed significant decrease in the mechanical thresholds of SNL rats in comparison to those of sham-operated rats. More importantly, chronic stress exposure significantly decreased the mechanical sensitivity to von Frey stimulation in both sham- and nerve ligated rats. In particular, the paw withdrawal threshold of the UCMS/SNL group was restored to nearly control levels after three weeks of UCMS (n=13-16). Data are presented as mean ± SEM. * P < 0.05, ** P < 0.01, *** P < 0.001, compared with their respective control group. ## P < 0.01, ### P < 0.001, compared with their respective sham group. Baseline tests were performed initially. Then rats received SNL or sham surgery, as the dashed line indicates. The chronic stress protocol began one week after the surgery. Point 0 at the x axis indicates the start of the UCMS procedure.
Table 2.
Analysis of variance for von Frey threshold measurement
| Effect | Degree of Freedom |
Mean Square | F-value | P-Value |
|---|---|---|---|---|
| Stress | 1 | 229.256 | 19.105 | <0.0001 |
| Surgery | 1 | 721.487 | 60.125 | <0.0001 |
| Time | 7 | 96.886 | 29.672 | <0.0001 |
| Stress*Surgery | 1 | 5.899 | 0.492 | 0.4870 |
| Stress*Time | 7 | 18.176 | 5.567 | <0.0001 |
| Surgery*Time | 7 | 9.636 | 2.951 | 0.0053 |
| Stress*Time*Surgery | 7 | 1.471 | 0.451 | 0.8695 |
Table 3.
Post hoc comparison of von Frey thresholds between subgroups and across time points
| Baseline | Op. | UCMS procedure |
||||||
|---|---|---|---|---|---|---|---|---|
| 1w | 1w | 2w | 3w | 4w | 5w | 6w | ||
| UCMS/SNL vs Con/SNL | ns | ns | ns | 0.017 | <0.001 | 0.003 | 0.006 | <0.001 |
| UCMS/Sham vs Con/Sham | ns | ns | ns | ns | 0.003 | 0.068 | <0.001 | <0.001 |
| UCMS/SNL vs UCMS/Sham | ns | 0.003 | <0.001 | 0.002 | <0.001 | 0.02 | <0.001 | 0.003 |
| Con/SNL vs Con/Sham | ns | 0.007 | <0.001 | <0.001 | <0.001 | <0.001 | 0.01 | <0.001 |
| UCMS/SNL vs baseline | - | <0.001 | <0.001 | 0.004 | ns | ns | ns | ns |
| UCMS/Sham vs baseline | - | <0.001 | ns | ns | 0.02 | ns | 0.02 | 0.003 |
| Con/SNL vs baseline | - | <0.001 | <0.001 | <0.001 | 0.001 | 0.03 | 0.06 | 0.05 |
| Con/Sham vs baseline | - | <0.001 | ns | ns | ns | ns | ns | ns |
The values in the table represent P values. Con=control; Op.=operation.
The analysis on von Frey pain test, however, revealed a strong effect of SNL surgery (3-way ANOVA, surgery effect: F(1, 55) < 60.125, P < 0.0001; see Fig. 3B & Table 2). A significant drop in the mechanical thresholds of SNL rats was found in comparison to those of sham-operated rats, indicating the development of chronic neuropathic pain. Within-subject contrasts showed that the nerve-ligated rats without stress exposure (control/SNL) exhibited remarkably decreased thresholds (allodynia) to von Frey stimulation one week after surgery when compared to the baseline level (post hoc test, P < 0.001; see Table 3), and maintained the low-level thresholds throughout the observation period. In contrast, the von Frey thresholds in the sham-operated rats (control/sham) were transiently lowered and returned to normal level two weeks after surgery. This suggests that spinal nerve ligation caused persistent injury in rats while sham operation does not essentially affect the sensory transmission.
It is important to note that chronic stress exposure significantly suppressed the mechanical sensitivity in both sham- and nerve ligated rats. ANOVA analysis revealed significantly higher von Frey thresholds in UCMS-treated rats than in normal treated rats (stress effect: F(1.55) = 19.105, P < 0.0001, Fig. 3B & Table 2). In particular, the paw withdrawal threshold of the UCMS/SNL group was restored to nearly normal level after three weeks of UCMS, and even comparable to that of the control/sham group. These results strongly suggest that similar as to acute mechanical pain, the depressive subjects also become insensitive to neuropathic mechanical allodynia.
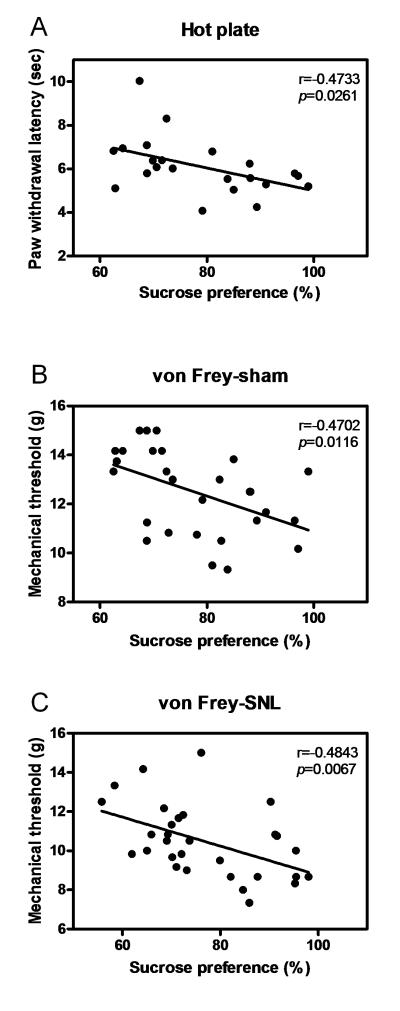
Additionally, correlations were calculated to investigate whether the changes in pain-related behaviors during stress protocol were associated with the state of depression (Fig. 4). Consistently, significant negative correlations were found between sucrose preference and pain thresholds under acute contact-heat pain (r = −0.4733, P < 0.05; see Fig. 4A), mechanical pain (r = −0.4702, P < 0.05; see Fig. 4B) and chronic mechanical allodynia conditions (r = −0.4843, P < 0.01; see Fig. 4C). These results further confirmed that the decreased sensitivity of the subjects to environmental noxious events may be related with the depressive emotional state.
Fig 4.
The correlation between nociceptive behaviors and sucrose preference. A consistent negative correlation was observed between sucrose consumption and nociceptive thresholds under acute contact-heat pain (A), acute mechanical pain (B), and chronic neuropathic pain (C) conditions.
3. Discussion
The present study was aimed to further investigate pain perception in rats with depressive-like behaviors in comparison to non-depressed controls. The results showed that the chronic stress exposed rats exhibited decreased pain sensitivity to contact thermal stimulation in hot plate test, and to mechanical stimulation in von Frey filament test under both normal (sham surgery) and chronic neuropathic pain conditions. These findings corroborate previous reports that depressed subjects have stronger tolerance to externally applied painful stimulation.
Although patients with depressive disorders often report pain, their sensitivity to experimental pain is controversial. Most authors indicated that depressive patients were less sensitive to experimental pain. Evidence exists that patients suffering depressive disorder showed significantly elevated thresholds to cutaneous thermal, electrical, and mechanical stimuli than healthy subjects (Bar et al., 2005; Bar et al., 2006; Graff-Guerrero et al., 2008; Lautenbacher et al., 1994; Lautenbacher et al., 1999). In a recent study, Schiwier et al explored cold-pain thresholds in patients with depression and found a decreased sensitivity for cold pain as compared to controls (Schwier et al., 2010). Similar results were also obtained in animal studies. In a tail-flick test, rats submitted to a chronic unpredictable stress paradigm displayed hypoalgesic responses to noxious thermal stimuli (Pinto-Ribeiro et al., 2004; Wang et al., 2010). Using UCMS and olfactory bulbectomy depression models, we have found that rats with depressive-like behaviors showed significantly decreased sensitivity to noxious radiant heat stimuli under both normal and complete Freund’s adjuvant (CFA) induced inflammatory pain conditions (Shi et al.; 2010; Wang et al., 2010). Thus, the behavioral changes (depressive behavior and nociceptive sensitivity) were not specifically induced by the UCMS procedure. In the present study, we employed different kinds of stimulations (contact thermal and mechanical stimuli) and pain model (chronic neuropathic pain). The results also confirmed previous findings by showing that the chronic stress induced depression suppressed the nocifensive responses of rats with acute or chronic pain. More surprisingly, the mechanical allodynia caused by nerve injury was totally reversed by chronic mild stress exposure, as indicated by the elevated von Frey threshold after three weeks of UCMS. Given the fact that nerve ligation may cause more severe injury thus more intense pain than CFA injection (tactile allodynia vs. hyperalgesia), the recovery of mechanical sensitivity of SNL rats after stress exposure strongly corroborated that depression can decrease the somatic sensitivity to externally applied painful stimulation.
Several putative mechanisms underlining this phenomenon might be proposed. It is suggested that loss of “motivation” is one of the core symptoms in depressed patients. The impairment of central dopaminegic system is supposed to be the mechanism of this amotivation (Willner, 1995), since administration of dopamine reuptake inhibitors and dopamine receptor agonists can exert antidepressant effects in placebo-controlled studies (Kundermann et al., 2009; Willner, 1995; Zarate et al., 2004). Moreover, escaping from noxious stimulation is believed a motivation-driven behavior. Thus, the lowered sensitivity and responsiveness to experimental stimulation in depressed rats observed in this study may be a result of lack of motivation.
Dopaminergic dysfunction is also known to generate motor deficit, including akinesia. Therefore, the lack of responsiveness to noxious stimuli observed in the current study might also be due to motor deficit. However, we have previously confirmed that our UCMS treated rats showed elevated open field activity (Tavares, 2003), which is in consistent with other reports (Gronli et al., 2005; Mineur et al., 2006). Thus, it is not likely that the slowness in response to nociceptive stimulation is due to a motor deficit of dopaminergic dysfunction.
A third possible mechanism of what the observed lack of responsiveness to pain is cognitive impairment, which is a characteristic of depressive disorder and is included in the diagnostic criteria for depression. Studies have reported a wide-range of deficits including impairment in early information processing, attention, memory, and executive function (Ottowitz et al., 2002; Tavares, 2003) in depressed patients. As a result, the decreased response to noxious stimulation under depressed condition may in part result from the dysfunction of cognition and attention of rats.
Serotoninergic neurotransmission has been regarded to be involved in the nociceptive processing as well as in the pathophysiology of depression (Delgado, 2004). For example, it has been accepted that serotonin-1A receptor (5-HT1A) plays a predominant role in the pain conducting pathway. Some researchers have reported that 5-HT1A antagonists increased the antinociceptive potency of the antidepressant (Ardid et al., 2001; Berrocoso and Mico, 2009; Hernandez et al., 2004). On the other hand, abnormalities of serotoninergic system have been proposed as part of a neurochemical imbalance in depression. Decreased activity of brain serotoninergic neurotransmission has been described in both human subjects with depression and different animal models (Csernansky and Sheline, 1993; Lesch and Heils, 2000). However, inconsistent changes of serotonin receptor have been reported in depressed state. Lower 5-HT1A receptor densities were found in depressed rats (Nishi et al., 2009; Sato et al., 2008) as well as depressed patients (Drevets et al., 1999; Drevets et al., 2007; Parsey et al., 2002; Sargent et al., 2000). In contrast, the density of 5-HT2A receptors has been found to be increased in subjects with depression (Mendelson, 2000). From the above, 5-HT1A-mediated pathway may participate in the interaction between depression and experimentally evoked pain. However, this possible explanation needs to be tested in the future studies.
The hypothalamo-pituitary-adrenal (HPA) axis may be another mechanism involved in the pain processing modulated by depression. In patients suffering from depression, the over-activity of HPA system is one of the most consistent neurobiological findings (Holsboer, 2000; Schuld et al., 2003). In animal studies, the stress-induced analgesia was significantly attenuated by systemic injection of an inhibitor of HPA axis function or intravenous administration of hydrocortisone (Filaretov et al., 1995; Mousa et al., 1983). Thus, depressive state might also induce analgesia partially via this HPA route.
Inconsistent data were also reported concerning the response of depressed subject to experimental neuropathic pain. Zeng and colleagues reported that the mechanical allodynia after chronic constriction nerve injury (CCI) was exacerbated in Wistar-Kyoto (WKY) rats, which is born with a depressive state, as compared with normal Wistar rats (Zeng et al., 2008). The most possible explanation for the inconsistency between Zeng’s and our results is the difference in depression models (i.e., the congenital depressive WKY strain vs. the stress-induced UCMS model). The reasons are as follows. First, while WKY rats might be more close to the patients with inherited depression, the UCMS model is thought to better reflect the status of most clinical depression. The WKY strain is proposed as an animal model of depression due to that they are hyper-reactive to stress, show dysregulation of the hypothalamic-pituitary-adrenal (HPA), and exhibit depressive-like behavior in a wide range of accepted behavioral paradigms. However, clinical depressive disorders are usually caused by various environmental stresses. Second, as compared to WKY rats, the UCMS treated rats are more sensitive to clinical antidepressant drugs. Several studies have found that the WKY strain is resistant to antidepressants, e.g. fluoxetine, the selective serotonin (5-HT) reuptake inhibitor (SSRI), when compared to other strains (Will et al., 2003). This indicates that the WKY depression model may not be mediated by a 5-HT mechanism. Numerous studies have supported a role of 5-HT in depression and favor the hypothesis that a deficiency in brain serotonergic activity contribute to the symptoms of clinical depressive disorders. Besides, the serotonergic projections are involved in the modulation of nociceptive transmission. The painful symptoms in depressed patients are believed to be caused by the dysfunction of 5-HT pathways (Kundermann et al., 2009). Therefore, it would not be surprising considering the different behavioral response to experimental pain between WKY and UCMS models. This also indicated that the WKY strain may not be a feasible model for investigating the relationship between depression and pain in general.
In conclusion, the present study showed that rats exposed to chronic unpredictable stress exhibited both a decreased sensitivity to acute contact heat and mechanical nociceptive stimuli, as well as an alleviated mechanical allodynia in chronic SNL model. This confirms our previous findings and makes it more robust that depression can exert inhibitory effect on the stimulus-evoked pain in general, including neuropathic allodynia.
4. Experimental Procedures
4.1. Animals
Eighty-one male Wistar rats (purchased from the Academy of Military Medical Science, Beijing, China) weighing 220-250 g at the beginning of experiment were used. All rats were housed individually with food and water freely available, and were kept in separated rooms in order to independently manipulate the environments. The rooms were maintained at 22 ± 2°C with a standard 12 h light-dark cycle (lights on at 07:00 am). Testing was performed during the light cycle. Animals were allowed to habituate to the environment for 1 week before experiments, and softly handled 3-5 minutes per day by the experimenter. The Principles of laboratory animal care (NIH publication No. 86-23, revised 1985) were followed in all our experiments. The experimental procedures were approved by the Internal Review Board of the Institute of Psychology, Chinese Academy of Sciences.
4.2. UCMS procedure and behavioral tests
Stressors were unpredictable in their nature, duration, and frequency. The procedure lasted for several weekly cycles and consisted of two to five different stressors per day. The stress procedure was illustrated in Table 1. Stressors included: 22- and 40-h periods of water deprivation, 20- and 22-h periods of food deprivation, one 1-h period of empty water bottle (exposed to empty water bottle immediately after one 40-h period of water deprivation), one 3-h period of restricted access to food (two small pieces of pellet in each cage) following one 20-h period of food deprivation, 8- and 16-h periods of cage tilt (45°), 7- and 8-h periods of strobe light, two 16-h periods of soiled bedding, one 16-h group housing (8 rats in a cage), two 16-h periods of overnight illumination, 2- and 5-h periods of intermittent white noise (75 dB), two 16-h periods of novel odor, two 30 min periods of exposure to a hot room (40°C) and two 30-min periods of exposure to a cold room (10°C). The stressors were presented in a pseudo-random order.
Table 1.
UCMS protocol
| Monday | Tuesday | Wednesday | Thursday | Friday | Saturday | Sunday | |
|---|---|---|---|---|---|---|---|
| Water deprivation |
1700  0900 0900 |
1600  1400 1400 |
|||||
| Empty water bottle |
0900 →1000 | ||||||
| Food deprivation | 0900  1700 1700 |
1600  1400 1400 |
|||||
| Restricted access to food |
1700→2000 | ||||||
| Cage tilt (45°) | 0900 →1700 | 1700  0900 0900 |
|||||
| Strobe light | 0900→1600 | 0900 →1700 | |||||
| Soiled cage | 0900 | 1700  0900 0900 |
1700 → | ||||
| Paired housing | 1700  0900 0900 |
||||||
| Continuous illumination |
1700  0900 0900 |
1700  0900 0900 |
|||||
| Cold room (10°C) |
30 min | 30 min | |||||
| Hot room (40°C) | 1600 →1630 | 0900 →0930 | |||||
| Crowding | 1700  0900 0900 |
||||||
| Odour | 1700  0900 0900 |
1700  0900 0900 |
|||||
| Sucrose preference test |
1400 →1500 | ||||||
Values in the table are time points in a 24-h period.
At the start of the experiment, animals were trained to consume a 1% sucrose solution for 48 h. The sucrose preference test was performed as a baseline value. Then a 6-week UCMS procedure was conducted. Sucrose consumption was monitored weekly throughout the experiment. Sucrose preference test took place at 14:00 h on Sunday in their home cage, following 22-h food and water deprivation. Each animal was presented simultaneously with 2 bottles, one containing sucrose solution (1%) and the other containing water. The percent preference for sucrose consumption was calculated according to the following formula: % sucrose preference = (sucrose solution consumption/ (sucrose solution consumption + water consumption)) × 100.
4.3. Pain tests
4.3.1. Hot plate test
The hot plate test (RB-200, Cheng Du Technology & Market Corp., LTD, China) was carried out according to the method described by Eddy and Leimbach (Duman et al., 2006; Eddy and Leimbach, 1953). Rats were placed into a glass cylinder (height 33 cm × diameter 20 cm) standing on a metal plate heated to 55±0.5°C. The latency to the first hindpaw withdrawal/licking was measured in seconds as an index of nociceptive threshold. To minimize tissue damage, a cut-off time of 30 s was adopted.
4.3.2. von Frey test
The mechanical allodynia was evaluated by withdrawal response using von Frey filaments (Semmes-Weinstein Monofilaments, North Coast Medical, Inc. Morgan Hill, USA). The animal model of neuropathic pain was established by L5 spinal nerve ligation (SNL) according to the procedure of Kim and Chung (Kim and Chung, 1992). Rats were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and placed in the prone position. The skin was incised in the midline over the lumbar spine, and the left transverse process of the L6 vertebra was removed. The left L5 spinal nerve was isolated and tightly ligated with 3-0 silk thread. A complete hemostasis was confirmed and the wound was sutured. In the sham-operated animals, the same surgical procedure was performed except for the ligation of the L5 spinal nerve.
The mechanical withdrawal thresholds of the paw were determined with the method described by Kaufmann et al. (Kaufmann et al., 2008). Animals were placed under plastic domes on a metal mesh floor, and von Frey filaments were applied from underneath the mesh floor to the plantar surface of the operated paw. The initial bending force of the filaments was (in grams): 0.6, 1.4, 2, 4, 6, 8, 10, 15. The filament of 15 g was chosen as the maximal probe. Stimulation began with the 0.6 g filament, using a perpendicular force to the skin that was just sufficient to bend the filament. If the animal failed to respond with a brief paw withdrawal to at least 3 out of 5 stimuli, the next stiffer filament was tested, and so forth using an ascending staircase protocol until the 3 of 5 response criterion was reached. This procedure was repeated twice, spaced at no less than 5 min apart. The response threshold for the trial was set as the average of the minimal force required to obtain a criterion response on the two repeats. If the animal failed to respond to the stiffest filament in the set, threshold for the trial was arbitrarily recorded as 15 g.
4.4. Experimental protocol
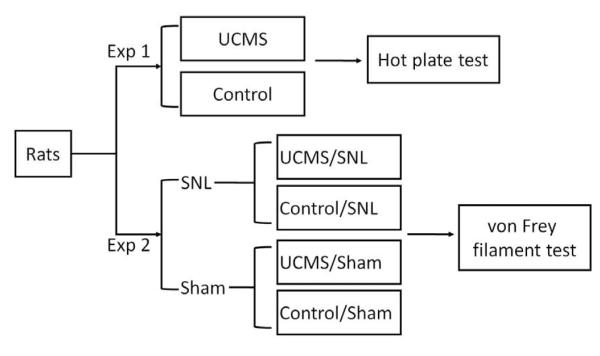
Two experiments were performed. A general scheme of the experimental protocol was shown in Fig 1. In Experiment 1, the effect of UCMS-induced depression on the contact heat-elicited pain was examined by the hot plate test. Rats were randomly assigned to 2 groups, UCMS group (n=11) and control group (n=11), which received UCMS exposure and no-stress treatment respectively. Both groups were subjected to the hot plate test before (baseline) and after the 6-week UCMS procedure.
Fig 1.
Experimental protocol. Two experiments were performed. In Experiment 1, the effect of UCMS-induced depression on the contact heat-elicited pain was examined. In Experiment 2, the influence of depression on the mechanical sensitivity of rats with or without neuropathic pain was investigated.
In Experiment 2, we explored the influence of depression on the mechanical sensitivity of rats with or without neuropathic pain. Animals were randomly divided into 2 groups, receiving L5 spinal nerve ligation (SNL) and sham surgery respectively. Then each group was subdivided into 2 groups (UCMS vs. control) according to the different treatment. The UCMS/SNL group (n=15) and UCMS/Sham group (n=13) formed the experimental portion of the design; the Control/SNL group (n=16) and Control/Sham group (n=15) served as controls. The chronic stress protocol began one week after SNL or sham surgery. Mechanical paw withdrawal thresholds to von Frey filaments were measured before, and once a week after surgery until the end of the entire experiment.
4.5. Statistical analysis
GraphPad prism 5.0 and Statistica 5.1 were used for statistical analyses and graph generation. Data affected by two or three factors were analyzed with multi-factor analysis of variance (ANOVA). Duncan’s test was used for post hoc test. Student’s t-test was employed when two groups were compared. Relationships between nociceptive behaviors and sucrose preference were examined with Pearson correlation coefficients. The data was presented as means ± SEM. The statistical significance was set at P < 0.05.
Acknowledgments
This work was funded by a NNSF grant (30700223) to JYW, NNSF grants (30770688 and 30970959), a Beijing NNSF grant (5082008), the 100 Talented Plan of the Chinese Academy of Sciences, the National Hi-Tech Research and Development Program of China (Grant No.2008AA022604), and a grant from NIH Fogarty International Center (R03TW008038) to FL.
Abbreviations
- UCMS
unpredictable chronic mild stress
- PWT
paw withdrawal threshold
- SNL
spinal nerve ligation
- CFA
complete Freund’s adjuvant
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Adler G, Gattaz WF. Pain perception threshold in major depression. Biol Psychiatry. 1993;34:687–9. doi: 10.1016/0006-3223(93)90041-b. [DOI] [PubMed] [Google Scholar]
- Ardid D, Alloui A, Brousse G, Jourdan D, Picard P, Dubray C, Eschalier A. Potentiation of the antinociceptive effect of clomipramine by a 5-ht(1A) antagonist in neuropathic pain in rats. Br J Pharmacol. 2001;132:1118–26. doi: 10.1038/sj.bjp.0703897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163:2433–45. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- Bar KJ, Greiner W, Letsch A, Kobele R, Sauer H. Influence of gender and hemispheric lateralization on heat pain perception in major depression. J Psychiatr Res. 2003;37:345–53. doi: 10.1016/s0022-3956(03)00051-7. [DOI] [PubMed] [Google Scholar]
- Bar KJ, Brehm S, Boettger MK, Boettger S, Wagner G, Sauer H. Pain perception in major depression depends on pain modality. Pain. 2005;117:97–103. doi: 10.1016/j.pain.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Bar KJ, Brehm S, Boettger MK, Wagner G, Boettger S, Sauer H. Decreased sensitivity to experimental pain in adjustment disorder. Eur J Pain. 2006;10:467–71. doi: 10.1016/j.ejpain.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Berrocoso E, Mico JA. Role of serotonin 5-HT1A receptors in the antidepressant-like effect and the antinociceptive effect of venlafaxine in mice. Int J Neuropsychopharmacol. 2009;12:61–71. doi: 10.1017/S1461145708008766. [DOI] [PubMed] [Google Scholar]
- Corruble E, Guelfi JD. Pain complaints in depressed inpatients. Psychopathology. 2000;33:307–9. doi: 10.1159/000029163. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Sheline YI. Abnormalities of serotonin metabolism and nonpsychotic psychiatric disorders. Ann Clin Psychiatry. 1993;5:275–81. doi: 10.3109/10401239309148828. [DOI] [PubMed] [Google Scholar]
- Davis GC, Buchsbaum MS, Bunney WE., Jr. Analgesia to painful stimuli in affective illness. Am J Psychiatry. 1979;136:1148–51. doi: 10.1176/ajp.136.9.1148. [DOI] [PubMed] [Google Scholar]
- Delgado PL. Common pathways of depression and pain. J Clin Psychiatry. 2004;65(Suppl 12):16–9. [PubMed] [Google Scholar]
- Dickens C, McGowan L, Dale S. Impact of depression on experimental pain perception: a systematic review of the literature with meta-analysis. Psychosom Med. 2003;65:369–75. doi: 10.1097/01.psy.0000041622.69462.06. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Frank E, Price JC, Kupfer DJ, Holt D, Greer PJ, Huang Y, Gautier C, Mathis C. PET imaging of serotonin 1A receptor binding in depression. Biol Psychiatry. 1999;46:1375–87. doi: 10.1016/s0006-3223(99)00189-4. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Thase ME, Moses-Kolko EL, Price J, Frank E, Kupfer DJ, Mathis C. Serotonin-1A receptor imaging in recurrent depression: replication and literature review. Nucl Med Biol. 2007;34:865–77. doi: 10.1016/j.nucmedbio.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman EN, Kesim M, Kadioglu M, Ulku C, Kalyoncu NI, Yaris E. Effect of gender on antinociceptive effect of paroxetine in hot plate test in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:292–6. doi: 10.1016/j.pnpbp.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, Gitlin MJ. Clinical aspects of depression in chronic pain patients. Clin J Pain. 1991;7:79–94. doi: 10.1097/00002508-199106000-00004. [DOI] [PubMed] [Google Scholar]
- Eddy NB, Leimbach D. Synthetic analgesics. II. Dithienylbutenyl- and dithienylbutylamines. J Pharmacol Exp Ther. 1953;107:385–93. [PubMed] [Google Scholar]
- Filaretov AA, Bogdanov AI, Iarushkina NI. [Stress-induced analgesia. The role of the hormones of the hypophyseal-adrenocortical system] Fiziol Zh Im I M Sechenova. 1995;81:40–6. [PubMed] [Google Scholar]
- Fishbain DA, Cutler R, Rosomoff HL, Rosomoff RS. Chronic pain-associated depression: antecedent or consequence of chronic pain? A review. Clin J Pain. 1997;13:116–37. doi: 10.1097/00002508-199706000-00006. [DOI] [PubMed] [Google Scholar]
- Gormsen L, Ribe AR, Raun P, Rosenberg R, Videbech P, Vestergaard P, Bach FW, Jensen TS. Pain thresholds during and after treatment of severe depression with electroconvulsive therapy. Eur J Pain. 2004;8:487–93. doi: 10.1016/j.ejpain.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Graff-Guerrero A, Pellicer F, Mendoza-Espinosa Y, Martinez-Medina P, Romero-Romo J, de la Fuente-Sandoval C. Cerebral blood flow changes associated with experimental pain stimulation in patients with major depression. J Affect Disord. 2008;107:161–8. doi: 10.1016/j.jad.2007.08.021. [DOI] [PubMed] [Google Scholar]
- Gronli J, Murison R, Fiske E, Bjorvatn B, Sorensen E, Portas CM, Ursin R. Effects of chronic mild stress on sexual behavior, locomotor activity and consumption of sucrose and saccharine solutions. Physiol Behav. 2005;84:571–7. doi: 10.1016/j.physbeh.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Hernandez A, Constandil L, Laurido C, Pelissier T, Marchand F, Ardid D, Alloui A, Eschalier A, Soto-Moyano R. Venlafaxine-induced depression of wind-up activity in mononeuropathic rats is potentiated by inhibition of brain 5-HT1A receptor expression in vivo. Int J Neurosci. 2004;114:229–42. doi: 10.1080/00207450490269453. [DOI] [PubMed] [Google Scholar]
- Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- Kaufmann D, Yagen B, Minert A, Tal M, Devor M, Bialer M. Evaluation of the enantioselective antiallodynic and pharmacokinetic profile of propylisopropylacetamide, a chiral isomer of valproic acid amide. Neuropharmacology. 2008;54:699–707. doi: 10.1016/j.neuropharm.2007.11.020. [DOI] [PubMed] [Google Scholar]
- Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–63. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- Kundermann B, Hemmeter-Spernal J, Strate P, Gebhardt S, Huber MT, Krieg JC, Lautenbacher S. Pain sensitivity in major depression and its relationship to central serotoninergic function as reflected by the neuroendocrine response to clomipramine. J Psychiatr Res. 2009;43:1253–61. doi: 10.1016/j.jpsychires.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Lautenbacher S, Roscher S, Strian D, Fassbender K, Krumrey K, Krieg JC. Pain perception in depression: relationships to symptomatology and naloxone-sensitive mechanisms. Psychosom Med. 1994;56:345–52. doi: 10.1097/00006842-199407000-00010. [DOI] [PubMed] [Google Scholar]
- Lautenbacher S, Spernal J, Schreiber W, Krieg JC. Relationship between clinical pain complaints and pain sensitivity in patients with depression and panic disorder. Psychosom Med. 1999;61:822–7. doi: 10.1097/00006842-199911000-00015. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Heils A. Serotonergic gene transcriptional control regions: targets for antidepressant drug development? Int J Neuropsychopharmacol. 2000;3:67–79. doi: 10.1017/S1461145700001747. [DOI] [PubMed] [Google Scholar]
- Mendelson SD. The current status of the platelet 5-HT(2A) receptor in depression. J Affect Disord. 2000;57:13–24. doi: 10.1016/s0165-0327(99)00177-9. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Belzung C, Crusio WE. Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav Brain Res. 2006;175:43–50. doi: 10.1016/j.bbr.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Mousa S, Miller CH, Jr., Couri D. Dexamethasone and stress-induced analgesia. Psychopharmacology (Berl) 1983;79:199–202. doi: 10.1007/BF00427812. [DOI] [PubMed] [Google Scholar]
- Nishi K, Kanemaru K, Diksic M. A genetic rat model of depression, Flinders sensitive line, has a lower density of 5-HT(1A) receptors, but a higher density of 5-HT(1B) receptors, compared to control rats. Neurochem Int. 2009;54:299–307. doi: 10.1016/j.neuint.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon MM, Schatzberg AF. Using chronic pain to predict depressive morbidity in the general population. Arch Gen Psychiatry. 2003;60:39–47. doi: 10.1001/archpsyc.60.1.39. [DOI] [PubMed] [Google Scholar]
- Ottowitz WE, Dougherty DD, Savage CR. The neural network basis for abnormalities of attention and executive function in major depressive disorder: implications for application of the medical disease model to psychiatric disorders. Harv Rev Psychiatry. 2002;10:86–99. doi: 10.1080/10673220216210. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Oquendo MA, Simpson NR, Ogden RT, Van Heertum R, Arango V, Mann JJ. Effects of sex, age, and aggressive traits in man on brain serotonin 5-HT1A receptor binding potential measured by PET using [C-11]WAY-100635. Brain Res. 2002;954:173–82. doi: 10.1016/s0006-8993(02)03243-2. [DOI] [PubMed] [Google Scholar]
- Pinto-Ribeiro F, Almeida A, Pego JM, Cerqueira J, Sousa N. Chronic unpredictable stress inhibits nociception in male rats. Neurosci Lett. 2004;359:73–6. doi: 10.1016/j.neulet.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Polatin P, Gatchel RJ. Depression and chronic low back pain: establishing priorities in treatment. Spine (Phila Pa 1976) 2000;25:2566–71. doi: 10.1097/00007632-200010150-00004. [DOI] [PubMed] [Google Scholar]
- Sargent PA, Kjaer KH, Bench CJ, Rabiner EA, Messa C, Meyer J, Gunn RN, Grasby PM, Cowen PJ. Brain serotonin1A receptor binding measured by positron emission tomography with [11C]WAY-100635: effects of depression and antidepressant treatment. Arch Gen Psychiatry. 2000;57:174–80. doi: 10.1001/archpsyc.57.2.174. [DOI] [PubMed] [Google Scholar]
- Sato H, Skelin I, Debonnel G, Diksic M. Chronic buspirone treatment normalizes open field behavior in olfactory bulbectomized rats: assessment with a quantitative autoradiographic evaluation of the 5-HT1A binding sites. Brain Res Bull. 2008;75:545–55. doi: 10.1016/j.brainresbull.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Schuld A, Schmid DA, Haack M, Holsboer F, Friess E, Pollmacher T. Hypothalamo-pituitary-adrenal function in patients with depressive disorders is correlated with baseline cytokine levels, but not with cytokine responses to hydrocortisone. J Psychiatr Res. 2003;37:463–70. doi: 10.1016/s0022-3956(03)00054-2. [DOI] [PubMed] [Google Scholar]
- Schwier C, Kliem A, Boettger MK, Bar KJ. Increased cold-pain thresholds in major depression. J Pain. 2010;11:287–90. doi: 10.1016/j.jpain.2009.07.012. [DOI] [PubMed] [Google Scholar]
- Shi M, Wang JY, Luo F. Depression shows divergent effects on evoked and spontaneous pain behaviors in rats. J Pain. 2010;11:219–29. doi: 10.1016/j.jpain.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares J, Drevets WC, Sahakian BJ. Cognition in mania and depression. Psychol Med. 2003;33:959–967. doi: 10.1017/s0033291703008432. [DOI] [PubMed] [Google Scholar]
- Wang W, Qi WJ, Xu Y, Wang JY, Luo F. The differential effects of depression on evoked and spontaneous pain behaviors in olfactory bulbectomized rats. Neurosci Lett. 2010;472:143–7. doi: 10.1016/j.neulet.2010.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will CC, Aird F, Redei EE. Selectively bred Wistar-Kyoto rats: an animal model of depression and hyper-responsiveness to antidepressants. Mol Psychiatry. 2003;8:925–32. doi: 10.1038/sj.mp.4001345. [DOI] [PubMed] [Google Scholar]
- Willner P. Dopaminergic mechanisms in depression and mania. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: the Fourth Generation of Progress. Raven Press; New York: 1995. pp. 921–931. [Google Scholar]
- Zarate CA, Jr., Payne JL, Singh J, Quiroz JA, Luckenbaugh DA, Denicoff KD, Charney DS, Manji HK. Pramipexole for bipolar II depression: a placebo-controlled proof of concept study. Biol Psychiatry. 2004;56:54–60. doi: 10.1016/j.biopsych.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Zeng Q, Wang S, Lim G, Yang L, Mao J, Sung B, Chang Y, Lim JA, Guo G. Exacerbated mechanical allodynia in rats with depression-like behavior. Brain Res. 2008;1200:27–38. doi: 10.1016/j.brainres.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]