Reactive oxygen species (ROS) have historically been viewed as toxic metabolic byproducts and causal agents in a myriad of human pathologies. More recent work, however, indicates that ROS are critical intermediates of cellular signaling pathways. Although it is clear that dedicated cellular ROS producers such as NADPH oxidases participate in signaling, evidence suggests that mitochondrial production of ROS is also a tightly controlled process, and plays a role in the maintenance of cellular oxidative homeostasis and propagation of cellular signaling pathways. Production of ROS at mitochondria thus integrates cellular energy state, metabolite concentrations, and other upstream signaling events and has important implications in cellular stress signaling, maintenance of stem cell populations, cellular survival, and oncogenic transformation.
Production of ROS by the Mitochondrial Electron Transport Chain
Mitochondria are a principal source of cellular reactive oxygen species (ROS). Whereas mitochondrial ROS production has commonly been thought of solely as the result of inefficiencies in the electron transport chain, a role for mitochondrial ROS in the propagation of cellular signaling pathways has emerged, leaving the questions of if and how ROS production at mitochondria is specifically regulated by these pathways in order to dictate biological outcomes. This review discusses pathways which impinge on and depend on mitochondrial ROS production and their important implications for biology both at the cellular and organismal level.
ROS are produced by mitochondria during oxidative metabolism through the one-electron reduction of molecular oxygen (O2), forming superoxide anion (O2•−). Superoxide is the proximal ROS produced by mitochondria and is converted to hydrogen peroxide (H2O2) through the action of superoxide dismutases (SODs) both within the mitochondria and in the cytosol. Complexes I, II, and III of the electron transport chain contain sites wherein electrons can prematurely reduce oxygen, resulting in the formation of superoxide 1, 2. Although complexes I and II produce ROS only into the matrix, complex III can produce ROS on both sides of the mitochondrial inner membrane 1, 3. This is of interest in the field of signaling, as ROS produced into the intermembrane space theoretically have an easier route to the cytosol to act as signaling molecules than do ROS produced into the matrix 4. There are other non-respiratory chain enzymes that produce superoxide in mitochondria, including glycerol-3-phosphate dehydrogenase; however the contribution of these enzymes to total mitochondrial ROS production remains unclear 2, 5.
For a given cell, the rate of ROS production from the electron transport chain varies with the amount of electron carriers present in a state capable of reacting with O2. This is affected by the concentration of a given electron carrier in a cell as well as the rate of electron supply and release from each carrier 2. These factors can change depending on the biological state of a given cell, respiratory rate, mitochondrial inner membrane potential, and posttranslational modifications or damage to the respiratory chain. Several groups have measured the rate of mitochondrial superoxide production in vitro, leading to the estimation that between 0.15% and 2% of cellular oxygen consumption results in superoxide 2, 5. Unfortunately, little is known about the regulation of mitochondrial function in vivo; thus it is unknown how much superoxide is produced by mitochondria in vivo 2.
There are many excellent reviews that discuss in detail the production of ROS by each mitochondrial complex, as well as the biochemical mechanisms of oxidative signaling modifications 1, 2, 5–7. The purpose of this review is to discuss specific cellular signaling pathways that impact or require mitochondrial ROS production. We attempt to explain the current evidence for ROS involvement in these pathways, although in many cases, direct biochemical evidence has yet to be provided. Future work must focus on providing a greater understanding of how upstream signals impinge on mitochondrial ROS production, as well as identifying the proximal targets of ROS in each pathway.
Hypoxia-induced production of mitochondrial ROS is required for the cellular response to hypoxia
Exposure of cells to low oxygen (hypoxia) leads to the activation of signaling pathways that promote adaptive transcriptional programs, reduce cellular oxygen usage, and decrease cellular energy consumption. Paradoxically, hypoxia leads to an increase in mitochondrial production of ROS. Current evidence suggests that this H2O2 emission from mitochondria during hypoxia is a central upstream regulator of many of the cellular responses to hypoxia 8–14.
The cellular response to hypoxia requires the induction of the hypoxia inducible transcription factors (HIFs) that consist of a stable β-subunit and one of three labile α-subunits (HIF-1α, HIF-2α, and HIF-3α) 15. During hypoxia, the normally degraded HIF-α subunits become stabilized, allowing for transcriptional transactivation and expression of genes regulating erythropoiesis, glycolysis, angiogenesis, cell cycle, and survival 15. The normoxic turnover of HIF-α subunits requires proline-directed hydroxylation of HIF-α subunits that targets them for recognition by the von Hippel-Lindau tumor suppressor protein and subsequent proteasomal degradation 16. This hydroxylation reaction is carried out by a family of 2-oxoglutarate-dependent dioxygenases termed prolyl hydroxylases 1, 2, and 3 (PHD1-3). During hypoxia, PHD activity is inhibited, allowing for stabilization of HIF-α subunits.
The requirement of mitochondrial ROS for hypoxia-mediated HIF stabilization was first suggested by studies in ρ0 Hep3B cells. These cells do not contain mitochondrial DNA and thus demonstrate no electron transport or ROS production; moreover, they are incapable of HIF-mediated transcription during hypoxia due to a lack of HIF-α subunit stabilization 8. Further experiments using mitochondrial inhibitors, antioxidants, and genetic targeting of mitochondrial proteins suggested that hypoxia induces ROS production from mitochondrial complex III, creating a cytosolic signal that stabilizes HIF 11, 17–19. Later work demonstrated that the ability of complex III to pump protons or to participate in oxidative phosphorylation is not required for ROS generation at this complex. Thus, cells that express a mutant cytochrome b subunit are unable to consume oxygen for oxidative phosphorylation, but are able to produce complex III-derived ROS and stabilize HIF during hypoxia 20. HIF stabilization afforded by mitochondrial ROS is a result of PHD inhibition; however, the mechanism of this inhibition is not yet known 20.
In addition to the transcriptional regulation mediated by HIFs, hypoxia also requires that cells expend less energy to minimize oxygen usage. A major consumer of cellular ATP is the Na/K-ATPase, which can account for 20–80% of oxygen expenditure in mammalian cells 21. Exposure of cells to hypoxia reversibly suppresses Na/K-ATPase activity via a mitochondrial ROS-mediated process that promotes the rapid endocytosis of this protein complex 12. The cellular function of the Na/K-ATPase is to transport sodium and potassium ions across the plasma membrane to maintain cellular ionic gradients. Thus, hypoxic downregulation of Na/K-ATPase activity has important implications for airway fluid absorption during hypoxia 22.
Hypoxia-induced Na/K-ATPase endocytosis requires the activity of AMP-activated protein kinase (AMPK) 23. AMPK is ubiquitously expressed and facilitates ATP production while suppressing ATP usage in energy-stressed cells. During hypoxia, AMPK phosphorylates and activates protein kinase C zeta (PKCζ) 23, which then phosphorylates Na/K-ATPase α-subunits on serine-18, leading to endocytosis of the complex 12. Mitochondrial ROS production triggers AMPK activation during hypoxia, providing a link between ROS and Na/K-ATPase inhibition 13, 23. In addition to regulating the Na/K-ATPase, AMPK phosphorylates and activates the tuberous sclerosis complex during hypoxia. This leads to inhibition of mTOR activity and further conservation of cellular energy by inhibiting the energetically-costly process of protein translation 24.
Another important organismal adaptation to hypoxia is constriction of pulmonary arteries which allows for the diversion of blood from poorly oxygenated regions of the lung. Hypoxia triggers contraction of pulmonary artery smooth muscle cells through an increase in cytosolic calcium from both intracellular and extracellular stores 25. This increase in calcium and subsequent pulmonary artery vasoconstriction are both dependent on hypoxic mitochondrial ROS production 9, 14. Thus, the cellular response to hypoxia requires the mitochondrial generation of ROS to propagate signaling events that regulate transcription, calcium stores, and energy stores at the cellular level. At the organismal level, mitochondrial ROS regulate airway fluid absorption and oxygen exchange in the pulmonary vasculature.
Hypoxic generation of ROS at mitochondrial complex III occurs through a process termed the Q-cycle. Complex III accepts electrons donated to coenzyme Q by mitochondrial complexes I and II and transfers them to cytochrome c. Whereas coenzyme Q accepts two electrons from either complex I or II, cytochrome c is only capable of reduction with one electron. Thus complex III must sequentially remove each electron from reduced coenzyme Q (ubiquinol, QH2). Removal of the first electron of ubiquiol results in the formation of the radical ubisemiquinone (QH•). Normally, the unpaired electron of ubisemiquinone is transferred to the cytochrome b center of complex III; however, this electron can also react with molecular oxygen to form superoxide 2, 26. Thus, genetic targeting of the complex III subunit Rieske iron-sulfur protein, the protein required for electron transfer from ubiquiol, abolishes ROS formation during hypoxia, preventing the activation of downstream signaling effectors 17, 19, 20.
Although much work has gone into demonstrating the role of mitochondrial ROS in hypoxic signaling, it remains unclear whether ROS induction during hypoxia is an intrinsic property of mitochondria, or if other cellular factors are required. Indeed, it is counterintuitive that a decrease in the concentration of a reactant would lead to an increase in product formation; thus upstream pathways might be required. If hypoxic ROS emission is intrinsic to the electron transport chain, possible explanations include increased half-life of ubisemiquinone during hypoxia or increased access of ubisemiquinone to oxygen in the inner membrane during hypoxia. It is also possible that complex III produces relatively more superoxide on the outer side of the mitochondrial inner membrane during hypoxia than during normoxia 27. This could result from a conformational change of complex III within the inner membrane during hypoxia leading to more ROS emitted into the intermembrane space without affecting total ROS levels produced by complex III. The mechanism of hypoxic induction of mitochondrial ROS production will be the subject of intense study in the years to come.
The PI3-Kinase pathway induces mitochondrial ROS emission
Activation of phosphoinositide-3-kinase (PI3K) by ligation of growth factors to their cellular receptors promotes recruitment of Akt to the plasma membrane and its subsequent activation 28. Akt positively regulates the mammalian target of rapamycin (mTOR), which is a key regulator of mitochondrial oxygen consumption and oxidative capacity 29. Akt activation is associated with increased oxygen consumption as well as an increase in total cellular ATP derived from both glycolytic and oxidative sources 30, 31. Conversely, rapamycin-mediated inhibition of mTOR activity results in reduced oxygen consumption and oxidative capacity 29.
Akt activation is also associated with the accumulation of mitochondrial ROS 31. This ROS induction might stem from an increase in mitochondrial metabolism, but other factors might also play a role 32, 33. Regardless of the effect of Akt activation on the creation of mitochondrial ROS, Akt activity is a key inhibitor of mitochondrial ROS scavenging. Akt phosphorylates forkhead box O (FOXO) transcription factors on three conserved residues, resulting in cytosolic sequestration through interaction with 14-3-3 adaptor proteins 34. FOXOs comprise a family of transcription factors that promote the expression of genes associated with cell cycle arrest, stress resistance, apoptosis, and tumor suppression. Of note, FOXOs robustly regulate the expression of mitochondrial manganese superoxide dismutase (SOD2) and catalase 35, 36. Thus, activation of the PI3K-Akt pathway not only results in increased production of ROS from mitochondria through metabolic pathways, but also the inability to scavenge mitochondrial ROS through inhibition of FOXOs.
FOXOs regulate mitochondrial ROS, proliferation, survival, and differentiation
FOXO-mediated transcription is activated upon cellular stress, including oxidative stress, via phosphorylation events mediated by JNK (c-Jun N-terminal kinase), MST1 (mammalian Sterile20-like 1), or AMPK 34.
The physiological significance of FOXO-dependent regulation of antioxidants has been demonstrated in the ability of FOXOs to block the cardiac hypertrophic response. As mammalian cardiomyocytes do not proliferate, increased workloads are met with cell growth. Prolonged hypertrophy, however, leads to congestive heart failure and death due to arrhythmias 37. The hypertrophic response is associated with increased production of ROS from mitochondrial and non-mitochondrial sources, which leads to aberrant signaling via downstream pro-growth pathways 38, 39. Induction of FOXO3a activity during the hypertrophic response thus increases mitochondrial ROS scavenging, attenuating pro-growth signaling and protecting cardiomyocytes from prolonged hypertrophic growth 40.
Recently, FOXO-mediated antioxidant transcription has been implicated in the maintenance of stem cell populations. Deletion of Foxo1, 3, and 4 in hematopoietic stem cells (HSCs) results in cell cycle entry and terminal differentiation 41. Remarkably, administration of antioxidants to FOXO-deficient mice rescued this defect in the HSC compartment, suggesting the necessity of ROS regulation in stem cell maintenance. This observation corresponds with the HSC defects observed in mice deficient for the DNA damage activated kinase Atm (ataxia telangiectasia mutated) 42. Atm deficiency leads to increased cellular ROS levels in HSCs, resulting in activation of p38 mitogen-activated protein kinase (MAPK) and mitotic entry; treatment of mice with antioxidants or p38 inhibitors rescued this stem cell hyperproliferation 42.
The regulation of ROS in stem cell populations is not restricted to the hematopoietic compartment. Deletion of the polycomb gene Bmi1 leads to mitochondrial dysfunction and increased ROS production in thymocytes 43. The thymocyte maturation defect characteristic of Bmi1−/− mice is largely rescued by treatment with antioxidants. Cellular ROS levels also increase upon the differentiation of both neural and epithelial stem cells 44–46. Thus, a paradigm appears to be set in which ROS levels increase as a population of stem cells differentiates. Low levels of ROS are required for quiescence and stem cell maintenance, whereas ROS induction leads to proliferation and differentiation programs. Indeed, work in Drosophila melanogaster has corroborated the essential role of ROS regulation in stem cell populations. ROS levels increase as Drosophila multipotent hematopoietic progenitors differentiate 47; overproduction of mitochondrial ROS leads to precocious differentiation into mature blood cells whereas increased scavenging ability inhibits differentiation.
ROS regulate the activity of phosphatases
In the cytosol, oxidation of cysteines remains the best-studied oxidative signaling modification 6. Oxidation of the cysteine sulfhydryl group can alter protein–protein interactions, the DNA binding activity of transcription factors, and the catalytic activity of enzymes. Additionally, oxidation of two intra- or intermolecular cysteines forms disulfide bridges allowing for conformational changes or oligomerization of proteins 6, 7. Mitochondrial ROS play a large role in maintaining the oxidative homeostasis of the proteome as inhibition of mitochondrial ROS production drastically decreases the total number of cellular protein disulfide bonds 48.
The best-described class of ROS targets is phosphatases. These enzymes oppose the activity of protein kinases and possess a reactive cysteine in their catalytic domain that is required for enzymatic activity. The low pKa of this cysteine allows it to act as a nucleophile for catalysis of the dephosphorylation reaction, but also makes it a target for oxidation by ROS 6, 7. Protein tyrosine phosphatase 1B was the first phosphatase demonstrated to be inhibited by ROS under physiological conditions 49, 50. Since then, ROS have been shown to inhibit other classes of phosphatases, including phosphatase and tensin homolog (PTEN, lipid phosphatase) and MAPK phosphatases 51–53.
Mitochondrial ROS regulate NF-κB and TNFα-mediated cell death
Tumor necrosis factor alpha (TNFα) is a pleiotropic cytokine that acts on its receptors (TNFRs) to promote either cell survival or cell death 54. The outcome of TNFα exposure on cell fate depends on the activities of two separate TNFR complexes, one of which mediates survival through activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) 55. The c-Jun N-terminal kinase (JNK) is an important mediator of pro-apoptotic signaling downstream of TNFα. Upon binding of TNFα to its receptors, JNK activity is rapidly, but transiently, stimulated 56. However, in the absence of NF-κB, this JNK activation is greatly extended, allowing for increased cell death 57, 58.
Although NF-κB regulates the expression of several anti-apoptotic genes, the key regulator of NF-κB-mediated inhibition of the JNK pathway is the mitochondrial antioxidant protein SOD2 53. Upon treatment with TNFα, mitochondrial production of ROS is increased 59. Cells without intact NF-κB lack SOD2 induction and accumulate ROS at much higher levels than their wild-type counterparts when treated with TNFα, corresponding with increased JNK activation 53, 60. SOD2 is thus essential for cellular survival following TNFα treatment 61–63, and enforced expression of SOD2 in the absence of NF-κB can counteract TNFα-induced cell death 53. Furthermore, treatment of cells with antioxidants inhibits TNFα-induced killing of NF-κB-deficient cells 60, 64.
The mechanism by which SOD2 confers survival to cells treated with TNFα lies in the ability of ROS to inactivate JNK phosphatases 53. Treatment of cells with TNFα thus leads to mitochondrial ROS production, oxidation of JNK phosphatase catalytic cysteines, and activation of JNK signaling and cell death. SOD2 provides a protective effect by lowering cellular ROS levels and decreasing JNK activation after TNFα treatment 53. Thus, NF-κB promotes survival through regulation of mitochondrial antioxidant protein expression during the response to TNFα.
Mitochondrial ROS contribute to cellular transformation
Cellular transformation is a multistep process requiring acquisition of activated oncogenes and loss of tumor-suppressor genes, leading to usurpation of cellular signaling pathways controlling proliferation, survival, and metabolism 65, 66. Transformed cells acquire capabilities such as anchorage-independent growth, limitless proliferation, and evasion of apoptotic signals. Another common trait of transformed cells is oxidative stress 67, 68. Activation of oncogenes, aberrant metabolism, mitochondrial dysfunction, and loss of the tumor suppressor p53 can all promote increased ROS accumulation in cancer cells 69.
Traditionally, the high ROS levels observed in tumors were viewed as triggers for high mutation rates and genomic instability which promote tumor progression. Although DNA damage undoubtedly contributes to cellular transformation, the role of ROS as signaling intermediates is also crucial. In a mouse model of myc-driven tumorigenesis, tumor growth is inhibited by treatment with antioxidants 70. However, the effect of antioxidant treatment was a result of the inhibition of HIF accumulation: expression of a stable HIF mutant alone could provide resistance to antioxidant treatment. Similarly, antioxidants inhibited tumor growth in a mouse model of Kaposi’s sarcoma. In this case, treatment was associated with reduced Akt and HIF activities in tumors71.
Recent work demonstrates that mitochondrial ROS directly contribute to cellular transformation induced by either Ras or Myc overexpression. The NAD-dependent deacetylase sirtuin 3 (SIRT3) acts as a tumor suppressor through its regulation of FOXO activity and ROS maintenance 72. SIRT3 deacetylates FOXO3a, promoting its nuclear localization and transcriptional activation 40, 72. Moreover, Sirt3−/− murine fibroblasts display increased levels of mitochondrial ROS accumulation (compared to wild-type cells) when either Myc or Ras oncogenes are expressed. This ROS accumulation is associated with decreased flux through mitochondrial complexes I and III (possibly allowing for increased escape of electrons to O2), and reduced cellular levels of SOD2 72. Sirt3−/− cells become transformed after expression of either Myc or Ras, whereas wild-type cells require expression of both oncogenes. Expression of SOD2 inhibited this oncogene-mediated transformation of Sirt3−/− cells, suggesting that mitochondrial ROS play an important role in the Sirt3−/− phenotype. These finding are consistent with observations in Sod2−/− fibroblasts, which like their Sirt3−/− counterparts, are capable of transformation with single oncogenes 72. Interestingly, SIRT3 is present in mitochondria, where it regulates the acetylation and activity of a variety of metabolic enzymes, suggesting additional ways that it might affect mitochondrial ROS production 73.
The available data suggests that elevated ROS levels are required for tumor growth and can promote transformation, even acting as the “second hit” in the Knudson two-hit model of carcinogenesis. One can then envision a model in which oncogene expression leads to increased levels of mitochondrial ROS production. In normal cells, high ROS levels activate tumor suppressors, leading to senescence or apoptosis. However, loss of tumor suppressor function allows cells to sustain high levels of ROS, which further activates proliferative, angiogenic, and survival pathways, which allow increased ROS accumulation and the continuation of a ‘vicious cycle’. The equilibrium of ROS concentration will thus be reached at the point where maximal signaling is permitted without causing irreversible damage to cellular components. Indeed, this seems to be the case as compounds that raise ROS levels can effectively and selectively kill a variety of tumor cell lines 31, 74, 75.
ROS-mediated signaling is not only involved in tumor initiation and maintenance; it also promotes metastasis. Cells harboring the G13997A mutation in the gene encoding the ND6 subunit of mitochondrial complex I are characterized by respiratory deficiency and high ROS production. This mutation is also associated with high HIF activity and increased metastatic potential 76, 77. Transfer of mutant mitochondria to a poorly metastatic tumor cell line was sufficient to increase the metastatic potential of the recipient cell line. Notably, this metastasis was inhibited by treatment with antioxidants 76.
Concluding remarks: Mitochondrial ROS regulate the fitness of organisms- A heretical model
The common notion of ROS solely as damaging agents likely stems from two factors. First, the discovery of the first antioxidant enzymes (superoxide dismutase) came decades before the discovery that non-phagocytic cells also possess enzymes (NADPH oxidases) whose sole biological function is to produce ROS 78, 79. Second, high cellular ROS levels are associated with cancer, diabetes, inflammatory diseases, ischemia-related diseases, and neurodegeneration, and are thus commonly thought to contribute to human pathologies and aging 80–83. This line of thinking has led to multiple clinical trials using antioxidants to scavenge ROS, yet these trials have consistently failed, and in some cases, actually increased mortality 84–87.
In addition to these clinical trials, genetic experiments in mouse models have cast doubt on the idea that less is better when it comes to ROS. Sod2−/− mice die before birth, and their heterozygous littermates exhibit mitochondrial damage and oxidative modifications to both nuclear and mitochondrial DNA. However, these heterozygous mice have a normal lifespan 88, 89. Furthermore, Sod2 transgenic mice do not have an increased lifespan compared to wild-type, and depending on the level of transgene expression, these mice display growth retardation and decreased fertility 90, 91. Various combinations of Sod2, cytosolic Sod1, or catalase transgenes also fail to extend the lifespan of mice 92.
To make matters more complicated, recent publications draw a correlation between high oxidative stress and lifespan extension. Mice heterozygous for the mitochondrial glutathione peroxidase 4 or the ubiquinone biosynthesis protein CLK1 display high levels of mitochondrial oxidative stress, yet live longer than their wild-type counterparts 93, 94. These results correlate with those from Caenorhabditis elegans, in which inactivation of clk-1 or sod-2 is associated with increased lifespan 95, 96. Furthermore, in C. elegans, lifespan extension mediated by glucose restriction is associated with increased mitochondrial metabolism and ROS production. Treatment of worms with antioxidants abolished this life extension 97.
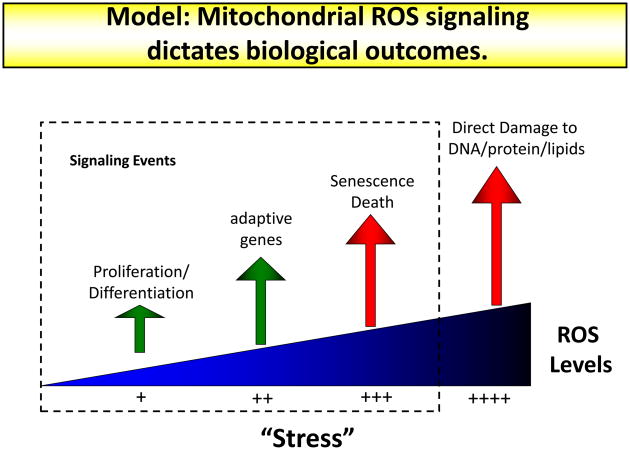
Thus, there is no unified model of mitochondrial ROS as a detrimental or beneficial agent in biology. The term hormesis is used to describe nonlethal stress doses that induce stress responses and an adaptive, beneficial effect on organismal fitness. This theory can be applied to mitochondrial ROS 97, and indeed, part of the fitness of Clk1+/− mice is attributed to the ability of mitochondrial ROS to modulate HIF function and inflammation 98. Based on the entirety of published observations, we propose a new view of mitochondrial ROS in which low levels of ROS are required for cellular processes such as proliferation and differentiation. Cellular stress can cause increased ROS levels, which can promote adaptation and possibly organismal fitness. Even higher levels of ROS will trigger senescence or cell death. Levels of ROS that cause irreversible damage to cellular proteins, DNA, or lipids might only be seen under super-physiological conditions such as UV irradiation or direct treatment of cells in culture with oxidants. Thus, future work might center on the modulation (as opposed to focusing on reduction) of mitochondrial ROS production to achieve a beneficial therapeutic outcome.
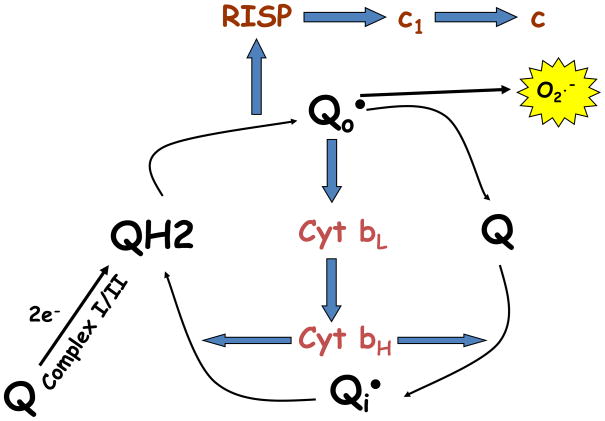
Box 1. Mitochondrial complex III produces superoxide through the Q-cycle.
Mitochondrial complexes I and II transfer two electrons to coenzyme Q (ubiquinone, Q), forming reduced coenzyme Q (ubiquinol, QH2). These electrons are transferred from ubiquinol to cytochrome c at mitochondrial complex III. As cytochrome c is only capable of reduction by a single electron, complex III function occurs through a bifurcated process termed the Q-cycle. Complex III binds coenzyme Q at two sites, the intermembrane space-proximal Qo site, and the matrix-proximal Qi site. Complex III receives ubiquinol at the Qo site. The Rieske iron-sulfur protein (RISP) then removes one electron from ubiquinol and transfers it to cytochrome c1, from which it is then transferred to cytochrome c, and finally to complex IV (cytochrome oxidase). This one electron oxidation of ubiquinone results in the transient formation of ubisemiquinone (QH•).
The remaining electron of ubisemiquinone is then transferred by RISP to cytochrome b where it passes through two heme groups (bL and bH), before it is subsequently used to reduce another molecule of ubiquinone at the Qi site, forming ubisemiquinone. After a second round of this cycle, coenzyme Q at the Qi site is fully reduced and can then be oxidized at the Qo site. By oxidizing coenzyme Q on the intermembrane space side of the inner membrane, and reducing it on the matrix side, complex III produces a net flux of protons from the matrix to the intermembrane space.
Ubisemiquinone formed at the Qo site of complex III is capable of donating its free electron directly to oxygen, forming superoxide. Genetic targeting of RISP expression prevents formation of ubisemiquinone at the Qo site, rendering cells both respiratory incompetent and incapable of superoxide formation at complex III. Mutation of cytochrome b severely decreases electron flow through complex III, rendering cells respiratory incompetent. However, the presence of RISP allows for ubisemiquinone formation and superoxide is formed in cells lacking cytochrome bactivity.
Figure I.
Mitochondrial complex III produces superoxide through the Q-cycle.
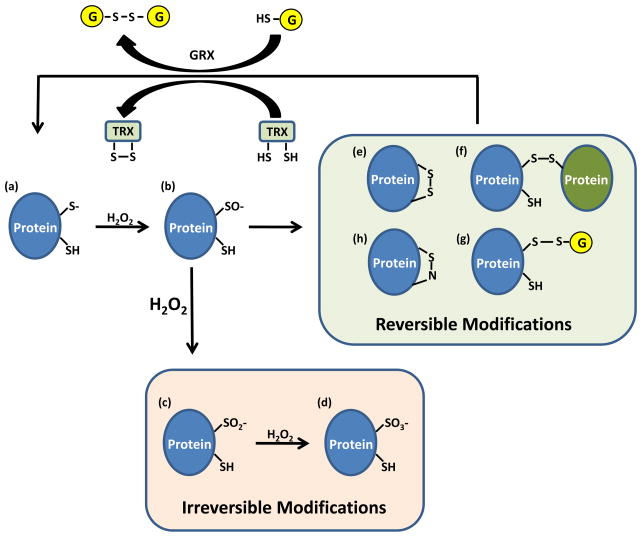
Box 2. Regulation of phosphatases by hydrogen peroxide-mediated oxidation of cysteine residues.
The catalytic cysteine of phosphatases has a low pKa, allowing the cysteine thiol group (SH) to exist as a thiolate anion (Figure Ia, S−). Thiolate anions are better nucleophiles than thiols, allowing these catalytic cysteines to participate in dephosphorylation reactions, but also making them more susceptible to oxidation than thiols. Hydrogen peroxide (H2O2) readily oxidizes thiolate, yielding sulfenic acid (Figure Ib, SO−), inhibiting enzyme activity. Under high concentrations of H2O2, SO− can undergo further oxidation to generate sulfinic (Figure Ic, SO2−) and sulfonic (Figure Id, SO3−) acids. Sulfinic and sulfonic acids represent irreversible oxidative modifications, preventing reactivation of the phosphatase. A common mechanism to prevent irreversible oxidation of catalytic cysteines is to encorporate the SO− intermediate into a disulfide (S-S) bond or into a sulfenic-amide (S-N) bond. Disulfides are formed by reaction of SO− with either an inter- or intra- molecular cysteine (Figure Ie, f), or with glutathione (Figure Ig). Sulfenic-amide bonds are formed by nucleophilic attack of the backbone nitrogen atom of the adjacent residue on SO− (Figure Ih). The actions of glutathione reductase (GRX) or thioredoxin (TRX) restores the oxidized enzymes back to their reduced state.
Figure I.
Cysteine oxidation regulates phosphatase activity.
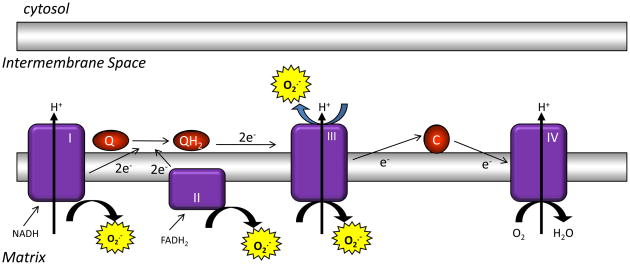
Figure 1. The mitochondrial electron transport chain produces ROS.
Mitochondrial complexes I and II use electrons donated from NADH and FADH2 to reduce coenzyme Q. Coenzyme Q shuttles these electrons to complex III, where they are transferred to cytochrome c. Complex IV uses electrons from cytochrome c to reduce molecular oxygen to water. The action of complexes I, III, and IV produce a proton electrochemical potential gradient, the free energy of which is used to phosphorylate ADP at ATP synthase. Complexes I, II, and III produce superoxide through the incomplete reduction of oxygen to superoxide. Whereas complexes I and II produce superoxide only into the mitochondrial matrix, complex III produces superoxide into both the matrix and the intermembrane space.
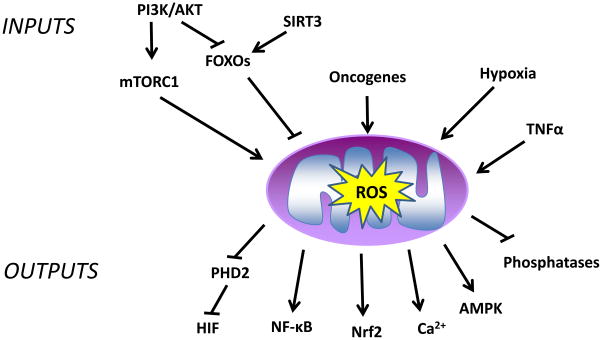
Figure 2. Signaling inputs and outputs of mitochondrial ROS signaling.
There are multiple inputs that regulate the generation of mitochondrial ROS (e.g., hypoxia, PI3K, TNFα, and oncogenes). These ROS activate multiple outputs including phosphatases, transcription factors, and kinases.
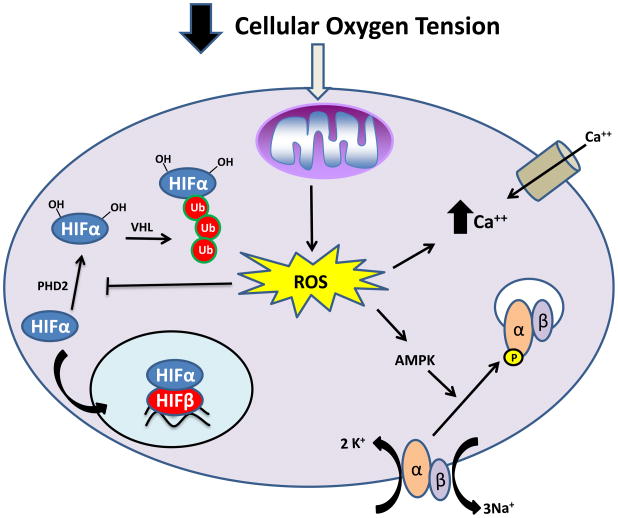
Figure 3. Mitochondrial ROS regulate the cellular response to hypoxia.
Hypoxia leads to an induction in the production of mitochondrial ROS. These ROS inhibit the activity of PHD2, leading to stabilization of HIFα subunits (blue) and transcriptional activation. Mitochondrial ROS generated during hypoxia regulate increases in cellular calcium uptake and contraction of pulmonary arteries. Mitochondrial ROS also lead to activation of AMPK, allowing increased cellular energy conservation. AMPK phosphorylates the α-subunit (peach) of the Na/K ATPase leading to endocytosis.
Figure 4. Mitochondrial ROS levels are crucial for biological outcomes.
Low levels of mitochondrial ROS production are required for cellular processes such as proliferation and differentiation. An induction in ROS production will lead to adaptive programs including the transcriptional upregulation of antioxidant genes. Even higher levels of ROS will signal the initiation of senescence and apoptosis. Non-signaling, irreversible damage to cellular components is only observed under the highest levels of cellular ROS.
Acknowledgments
This work was supported by a NIH Grant R01CA123067-03 and RO1GM60472-10 to N.S.C. R.B.H. was supported by a post-doctoral training grant T32CA070085-13. We thank members of the Chandel and Ridge labs for critical proofreading. Due to the brevity of this review, we apologize to those whose important work we could not discuss.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muller FL, et al. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J Biol Chem. 2004;279:49064–49073. doi: 10.1074/jbc.M407715200. [DOI] [PubMed] [Google Scholar]
- 4.Han D, et al. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J Biol Chem. 2003;278:5557–5563. doi: 10.1074/jbc.M210269200. [DOI] [PubMed] [Google Scholar]
- 5.Brand MD. The sites and topology of mitochondrial superoxide production. Exp Gerontol. doi: 10.1016/j.exger.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janssen-Heininger YM, et al. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic Biol Med. 2008;45:1–17. doi: 10.1016/j.freeradbiomed.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandes N, et al. Thiol-based redox switches in eukaryotic proteins. Antioxid Redox Signal. 2009;11:997–1014. doi: 10.1089/ars.2008.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandel NS, et al. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci U S A. 1998;95:11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waypa GB, et al. Model for hypoxic pulmonary vasoconstriction involving mitochondrial oxygen sensing. Circ Res. 2001;88:1259–1266. doi: 10.1161/hh1201.091960. [DOI] [PubMed] [Google Scholar]
- 10.Bell EL, et al. Mitochondrial reactive oxygen species trigger hypoxia-inducible factor-dependent extension of the replicative life span during hypoxia. Mol Cell Biol. 2007;27:5737–5745. doi: 10.1128/MCB.02265-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chandel NS, et al. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000;275:25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 12.Dada LA, et al. Hypoxia-induced endocytosis of Na,K-ATPase in alveolar epithelial cells is mediated by mitochondrial reactive oxygen species and PKC-zeta. J Clin Invest. 2003;111:1057–1064. doi: 10.1172/JCI16826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emerling BM, et al. Hypoxic activation of AMPK is dependent on mitochondrial ROS but independent of an increase in AMP/ATP ratio. Free Radic Biol Med. 2009;46:1386–1391. doi: 10.1016/j.freeradbiomed.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waypa GB, et al. Increases in mitochondrial reactive oxygen species trigger hypoxia-induced calcium responses in pulmonary artery smooth muscle cells. Circ Res. 2006;99:970–978. doi: 10.1161/01.RES.0000247068.75808.3f. [DOI] [PubMed] [Google Scholar]
- 15.Weidemann A, Johnson RS. Biology of HIF-1alpha. Cell Death Differ. 2008;15:621–627. doi: 10.1038/cdd.2008.12. [DOI] [PubMed] [Google Scholar]
- 16.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Brunelle JK, et al. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab. 2005;1:409–414. doi: 10.1016/j.cmet.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Mansfield KD, et al. Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-alpha activation. Cell Metab. 2005;1:393–399. doi: 10.1016/j.cmet.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guzy RD, et al. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005;1:401–408. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Bell EL, et al. The Qo site of the mitochondrial complex III is required for the transduction of hypoxic signaling via reactive oxygen species production. J Cell Biol. 2007;177:1029–1036. doi: 10.1083/jcb.200609074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michiels C. Physiological and pathological responses to hypoxia. Am J Pathol. 2004;164:1875–1882. doi: 10.1016/S0002-9440(10)63747-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou G, et al. Regulation of alveolar epithelial function by hypoxia. Eur Respir J. 2008;31:1107–1113. doi: 10.1183/09031936.00155507. [DOI] [PubMed] [Google Scholar]
- 23.Gusarova GA, et al. Alpha1-AMP-activated protein kinase regulates hypoxia-induced Na,K-ATPase endocytosis via direct phosphorylation of protein kinase C zeta. Mol Cell Biol. 2009;29:3455–3464. doi: 10.1128/MCB.00054-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu L, et al. Hypoxia-induced energy stress regulates mRNA translation and cell growth. Mol Cell. 2006;21:521–531. doi: 10.1016/j.molcel.2006.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ward JP, et al. Calcium, mitochondria and oxygen sensing in the pulmonary circulation. Cell Calcium. 2004;36:209–220. doi: 10.1016/j.ceca.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 26.Turrens JF, et al. Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch Biochem Biophys. 1985;237:408–414. doi: 10.1016/0003-9861(85)90293-0. [DOI] [PubMed] [Google Scholar]
- 27.Guzy RD, Schumacker PT. Oxygen sensing by mitochondria at complex III: the paradox of increased reactive oxygen species during hypoxia. Exp Physiol. 2006;91:807–819. doi: 10.1113/expphysiol.2006.033506. [DOI] [PubMed] [Google Scholar]
- 28.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 29.Schieke SM, et al. The mammalian target of rapamycin (mTOR) pathway regulates mitochondrial oxygen consumption and oxidative capacity. J Biol Chem. 2006;281:27643–27652. doi: 10.1074/jbc.M603536200. [DOI] [PubMed] [Google Scholar]
- 30.Gottlob K, et al. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev. 2001;15:1406–1418. doi: 10.1101/gad.889901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nogueira V, et al. Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer Cell. 2008;14:458–470. doi: 10.1016/j.ccr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robey RB, Hay N. Is Akt the “Warburg kinase”?-Akt-energy metabolism interactions and oncogenesis. Semin Cancer Biol. 2009;19:25–31. doi: 10.1016/j.semcancer.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nemoto S, et al. Role for mitochondrial oxidants as regulators of cellular metabolism. Mol Cell Biol. 2000;20:7311–7318. doi: 10.1128/mcb.20.19.7311-7318.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hedrick SM. The cunning little vixen: Foxo and the cycle of life and death. Nat Immunol. 2009;10:1057–1063. doi: 10.1038/ni.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kops GJ, et al. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419:316–321. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
- 36.Nemoto S, Finkel T. Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science. 2002;295:2450–2452. doi: 10.1126/science.1069004. [DOI] [PubMed] [Google Scholar]
- 37.Frey N, et al. Hypertrophy of the heart: a new therapeutic target? Circulation. 2004;109:1580–1589. doi: 10.1161/01.CIR.0000120390.68287.BB. [DOI] [PubMed] [Google Scholar]
- 38.Tsutsui H, et al. Mitochondrial oxidative stress, DNA damage, and heart failure. Antioxid Redox Signal. 2006;8:1737–1744. doi: 10.1089/ars.2006.8.1737. [DOI] [PubMed] [Google Scholar]
- 39.Seddon M, et al. Oxidative stress and redox signalling in cardiac hypertrophy and heart failure. Heart. 2007;93:903–907. doi: 10.1136/hrt.2005.068270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sundaresan NR, et al. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest. 2009;119:2758–2771. doi: 10.1172/JCI39162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tothova Z, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 42.Ito K, et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- 43.Liu J, et al. Bmi1 regulates mitochondrial function and the DNA damage response pathway. Nature. 2009;459:387–392. doi: 10.1038/nature08040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith J, et al. Redox state is a central modulator of the balance between self-renewal and differentiation in a dividing glial precursor cell. Proc Natl Acad Sci U S A. 2000;97:10032–10037. doi: 10.1073/pnas.170209797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsatmali M, et al. Newborn neurons acquire high levels of reactive oxygen species and increased mitochondrial proteins upon differentiation from progenitors. Brain Res. 2005;1040:137–150. doi: 10.1016/j.brainres.2005.01.087. [DOI] [PubMed] [Google Scholar]
- 46.Diehn M, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–783. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Owusu-Ansah E, Banerjee U. Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature. 2009;461:537–541. doi: 10.1038/nature08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang Y, et al. Regulation of the protein disulfide proteome by mitochondria in mammalian cells. Proc Natl Acad Sci U S A. 2007;104:10813–10817. doi: 10.1073/pnas.0702027104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee SR, et al. Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. J Biol Chem. 1998;273:15366–15372. doi: 10.1074/jbc.273.25.15366. [DOI] [PubMed] [Google Scholar]
- 50.Meng TC, et al. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol Cell. 2002;9:387–399. doi: 10.1016/s1097-2765(02)00445-8. [DOI] [PubMed] [Google Scholar]
- 51.Kwon J, et al. Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc Natl Acad Sci U S A. 2004;101:16419–16424. doi: 10.1073/pnas.0407396101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Levinthal DJ, Defranco DB. Reversible oxidation of ERK-directed protein phosphatases drives oxidative toxicity in neurons. J Biol Chem. 2005;280:5875–5883. doi: 10.1074/jbc.M410771200. [DOI] [PubMed] [Google Scholar]
- 53.Kamata H, et al. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 54.Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 55.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 56.Liu ZG, et al. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-kappaB activation prevents cell death. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 57.Tang G, et al. Inhibition of JNK activation through NF-kappaB target genes. Nature. 2001;414:313–317. doi: 10.1038/35104568. [DOI] [PubMed] [Google Scholar]
- 58.De Smaele E, et al. Induction of gadd45beta by NF-kappaB downregulates pro-apoptotic JNK signalling. Nature. 2001;414:308–313. doi: 10.1038/35104560. [DOI] [PubMed] [Google Scholar]
- 59.Chandel NS, et al. Reactive oxygen species are downstream products of TRAF-mediated signal transduction. J Biol Chem. 2001;276:42728–42736. doi: 10.1074/jbc.M103074200. [DOI] [PubMed] [Google Scholar]
- 60.Sakon S, et al. NF-kappaB inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. EMBO J. 2003;22:3898–3909. doi: 10.1093/emboj/cdg379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wong GH, Goeddel DV. Induction of manganous superoxide dismutase by tumor necrosis factor: possible protective mechanism. Science. 1988;242:941–944. doi: 10.1126/science.3263703. [DOI] [PubMed] [Google Scholar]
- 62.Wong GH, et al. Manganous superoxide dismutase is essential for cellular resistance to cytotoxicity of tumor necrosis factor. Cell. 1989;58:923–931. doi: 10.1016/0092-8674(89)90944-6. [DOI] [PubMed] [Google Scholar]
- 63.Jones PL, et al. Tumor necrosis factor alpha and interleukin-1beta regulate the murine manganese superoxide dismutase gene through a complex intronic enhancer involving C/EBP-beta and NF-kappaB. Mol Cell Biol. 1997;17:6970–6981. doi: 10.1128/mcb.17.12.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pham CG, et al. Ferritin heavy chain upregulation by NF-kappaB inhibits TNFalpha-induced apoptosis by suppressing reactive oxygen species. Cell. 2004;119:529–542. doi: 10.1016/j.cell.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 65.Hahn WC, Weinberg RA. Rules for making human tumor cells. N Engl J Med. 2002;347:1593–1603. doi: 10.1056/NEJMra021902. [DOI] [PubMed] [Google Scholar]
- 66.Jones RG, Thompson CB. Tumor suppressors and cell metabolism: a recipe for cancer growth. Genes Dev. 2009;23:537–548. doi: 10.1101/gad.1756509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- 68.Toyokuni S, et al. Persistent oxidative stress in cancer. FEBS Lett. 1995;358:1–3. doi: 10.1016/0014-5793(94)01368-b. [DOI] [PubMed] [Google Scholar]
- 69.Trachootham D, et al. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 70.Gao P, et al. HIF-dependent antitumorigenic effect of antioxidants in vivo. Cancer Cell. 2007;12:230–238. doi: 10.1016/j.ccr.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ma Q, et al. Antitumorigenesis of antioxidants in a transgenic Rac1 model of Kaposi’s sarcoma. Proc Natl Acad Sci U S A. 2009;106:8683–8688. doi: 10.1073/pnas.0812688106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim HS, et al. SIRT3 Is a Mitochondria-Localized Tumor Suppressor Required for Maintenance of Mitochondrial Integrity and Metabolism during Stress. Cancer Cell. 17:41–52. doi: 10.1016/j.ccr.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hirschey MD, et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 464:121–125. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yu R, et al. Chemopreventive isothiocyanates induce apoptosis and caspase-3-like protease activity. Cancer Res. 1998;58:402–408. [PubMed] [Google Scholar]
- 75.Trachootham D, et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10:241–252. doi: 10.1016/j.ccr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 76.Ishikawa K, et al. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science. 2008;320:661–664. doi: 10.1126/science.1156906. [DOI] [PubMed] [Google Scholar]
- 77.Koshikawa N, et al. Reactive oxygen species-generating mitochondrial DNA mutation up-regulates hypoxia-inducible factor-1alpha gene transcription via phosphatidylinositol 3-kinase-Akt/protein kinase C/histone deacetylase pathway. J Biol Chem. 2009;284:33185–33194. doi: 10.1074/jbc.M109.054221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 79.Suh YA, et al. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- 80.Finkel T, et al. The common biology of cancer and ageing. Nature. 2007;448:767–774. doi: 10.1038/nature05985. [DOI] [PubMed] [Google Scholar]
- 81.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 82.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 83.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 84.Storch A, et al. Randomized, double-blind, placebo-controlled trial on symptomatic effects of coenzyme Q(10) in Parkinson disease. Arch Neurol. 2007;64:938–944. doi: 10.1001/archneur.64.7.nct60005. [DOI] [PubMed] [Google Scholar]
- 85.Kang JH, et al. Vitamin E, vitamin C, beta carotene, and cognitive function among women with or at risk of cardiovascular disease: The Women’s Antioxidant and Cardiovascular Study. Circulation. 2009;119:2772–2780. doi: 10.1161/CIRCULATIONAHA.108.816900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lin J, et al. Vitamins C and E and beta carotene supplementation and cancer risk: a randomized controlled trial. J Natl Cancer Inst. 2009;101:14–23. doi: 10.1093/jnci/djn438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bjelakovic G, et al. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 88.Li Y, et al. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 89.Lebovitz RM, et al. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc Natl Acad Sci U S A. 1996;93:9782–9787. doi: 10.1073/pnas.93.18.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Van Remmen H, et al. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol Genomics. 2003;16:29–37. doi: 10.1152/physiolgenomics.00122.2003. [DOI] [PubMed] [Google Scholar]
- 91.Raineri I, et al. Strain-dependent high-level expression of a transgene for manganese superoxide dismutase is associated with growth retardation and decreased fertility. Free Radic Biol Med. 2001;31:1018–1030. doi: 10.1016/s0891-5849(01)00686-4. [DOI] [PubMed] [Google Scholar]
- 92.Perez VI, et al. The overexpression of major antioxidant enzymes does not extend the lifespan of mice. Aging Cell. 2009;8:73–75. doi: 10.1111/j.1474-9726.2008.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ran Q, et al. Reduction in glutathione peroxidase 4 increases life span through increased sensitivity to apoptosis. J Gerontol A Biol Sci Med Sci. 2007;62:932–942. doi: 10.1093/gerona/62.9.932. [DOI] [PubMed] [Google Scholar]
- 94.Liu X, et al. Evolutionary conservation of the clk-1-dependent mechanism of longevity: loss of mclk1 increases cellular fitness and lifespan in mice. Genes Dev. 2005;19:2424–2434. doi: 10.1101/gad.1352905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wong A, et al. Mutations in the clk-1 gene of Caenorhabditis elegans affect developmental and behavioral timing. Genetics. 1995;139:1247–1259. doi: 10.1093/genetics/139.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Van Raamsdonk JM, Hekimi S. Deletion of the mitochondrial superoxide dismutase sod-2 extends lifespan in Caenorhabditis elegans. PLoS Genet. 2009;5:e1000361. doi: 10.1371/journal.pgen.1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schulz TJ, et al. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007;6:280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 98.Wang D, et al. Elevated mitochondrial reactive oxygen species generation affects the immune response via hypoxia-inducible factor-1alpha in long-lived Mclk1+/− mouse mutants. J Immunol. 184:582–590. doi: 10.4049/jimmunol.0902352. [DOI] [PubMed] [Google Scholar]