Abstract
During many chronic infections the responding CD8 T cells become exhausted as they progressively lose their ability to elaborate key effector functions. Unlike prototypic memory CD8 cells, which rapidly synthesize IFN-γ following activation, severely exhausted T cells fail to produce this effector molecule. Nevertheless, the ontogeny of exhausted CD8 T cells as well as the underlying mechanisms that account for their functional inactivation remains ill-defined. We have utilized cytokine reporter mice, which mark the transcription of IFN-γ mRNA by the expression of Thy1.1, to decipher how activation events during the early stages of a chronic infection dictate the development of exhaustion. We show that virus-specific CD8 T cells clearly respond during the early stages of chronic lymphocytic choriomeningitis virus (LCMV) infection, and that this early T cell response is more pronounced than that initially observed in acutely infected hosts. Thus, exhausted CD8 T cells appear to emerge from populations of potently activated precursors. Unlike acute infections, which result in massive expansion of the responding T cells, there is a rapid attenuation of further expansion during chronic infections. The exhausted T cells that subsequently emerge in chronically infected hosts are incapable of producing the IFN-γ protein. Surprisingly, high levels of the IFN-γ transcript are still present in exhausted cells, demonstrating that ablation of IFN-γ production by exhausted cells is not due to transcriptional silencing. Thus, post-transcription regulatory mechanisms likely disable this effector module.
Introduction
During many chronic viral infections, including hepatitis B virus, hepatitis C virus, human immunodeficiency virus, and lymphocytic choriomeningitis virus (LCMV)4, CD8 T cell responses are initially triggered, but the ability of these cells to elaborate key anti-viral effector functions can erode as they are driven to exhaustion (1–10). The loss of effector activities is thought to occur in a step-wise manner as the anti-viral T cells sequentially lose their polyfunctional capabilities (3, 8–12). This results in a gradation of exhausted states which distinguishes these cells from bona fide memory T cells, that are highly effective at functionally multi-tasking. The establishment of the exhausted state is consequential as it compromises viral control, favoring chronicity and disease development, as well as increasing the likelihood of transmission.
A hallmark of severely exhausted CD8 T cells is their inability to produce IFN-γ. Exhausted CD8 T cells also possess a unique transcriptional profile, express panels of inhibitory receptors including PD-1, and have altered maintenance requirements by comparison with prototypic memory T cells (13–16). Whether exhausted or more functionally competent effector and memory T cells develop is dictated by multiple factors including the sustained presence of viral antigen, availability and levels of cytokines including IL-10, TGF-β, and IL-21, as well as the presence of CD4 T cell help (2, 17–24). Nevertheless, the ontogeny and developmental relationships of exhausted T cells to other effector and memory precursor T cell subsets is less well defined.
One of the most informative systems for analyzing the development of T cell exhaustion is infection of mice with LCMV. A key advantage of the LCMV system is that acute, protracted and chronic infections can be established, depending upon the strain of virus used and immunological status of the host (2, 3, 12, 25). This provides an ideal platform for determining the salient features of anti-viral CD8 T cells which successfully eradicate acute infections, as well delineating the factors that give rise to exhausted responses that are ineffective at clearing prolonged or persistent infections.
Genetically engineered reporter mice are a valuable tool for tracking the development, lineage relationships, and stability of cytokine producing cells. In this study we have harnessed IFN-γ.Thy1.1 Knockin reporter mice (IFN-γ KI) which flag IFN-γ transcript positive cells by expression of the Thy1.1 reporter molecule (26). These mice were generated using homologous recombination to insert an IRES-Thy1.1 expression cassette into the last exon of the IFN-γ gene, following the translational stop codon and prior to the polyadenylation site. In this way the endogenous IFN-γ promotor regulates the expression of a bicistronic mRNA that potentially directs the translation of both IFN-γ and Thy1.1. These mice have been used previously to decipher the ontogeny of memory CD4 T cells, demonstrating that this subset can emerge from IFN-γ+ effector precursors. Herein we use this system to show that, counter-intuitively, the expression of the Thy1.1 reporter molecule is highly upregulated during the early stages of chronic LCMV infections and that the severely exhausted cells which develop retain expression of this reporter. Nevertheless, we demonstrate that the exhausted setting is at least somewhat plastic and that the level of Thy1.1 reporter molecule expression is dictated by the initial precursor frequencies of anti-viral CD8 T cells as well as by the availability of viral antigen.
Materials and Methods
Mice
C57BL/6J (B6), B6.SJL-PtprcaPepcb/BoyJ mice (H2b, CD45.1), and B6.129S2-Cd4tm1Mak/J (Cd4−/−) mice were purchased from Jackson Laboratory (Bar Harbor, ME). P14 TCR transgenic mice (P14) (27) backcrossed onto a B6 background were kindly originally provided by Dr. R. Ahmed (Emory University, GA). The IFN-γ.Thy1.1 Knockin reporter (IFN-γ KI) mice have been previously described (26). P14 TCR transgenic IFN-γ KI (P14.IFN-γ-KI) mice were produced by crossing P14 TCR transgenic mice with homozygous IFN-γ KI mice. P14+ mice that were homozygous for the IFN-γ KI reporter allele (P14.IFN-γ KI) were used in subsequent adoptive transfer studies. All mice were bred and maintained in fully accredited facilities at the University of Alabama at Birmingham.
Infections
For acute infections, mice were infected by i.p. injection with 2×105 p.f.u. LCMV-Armstrong (Arm). Protracted LCMV-clone 13 (cl13) infections were established by i.v. inoculation with 2×106 p.f.u. For chronic infections IFN-γ KI mice were depleted of CD4 T cells by i.p. injection of 300μg of GK1.5 antibody (UAB immunoreagent core facility and BioXCell, West Lebanon, NH) one day before and 3 days after infection with 2×106 p.f.u LCMV-cl13 (17). In certain experiments chronic infections were established in non-reporter Cd4−/− mice by i.v inoculation with 2×106 p.f.u LCMV-cl13 (12). Viral titers were assessed by plaque assays using Vero cell monolayers (28).
Cell preparation
Freshly explanted spleens were disrupted into single cell suspensions, and erythrocytes were removed by lysis using 0.83% (w/v) NH4Cl (10). After washing, cell preparations were finally resuspended in RPMI 1640 medium supplemented with 10% FCS, 50μm 2-mercaptoethanol, 100U/ml penicillin, and 100μg/ml streptomycin. Blood was obtained from mice by retro-orbital bleed and peripheral blood mononuclear cells were isolated by centrifugation over a layer of Histopaque-1083 (Sigma-Aldrich, St. Louis, MO).
MHC class I tetramer staining
Cell suspensions were pretreated with anti-CD16/CD32 mAb (clone 2.4G2). Co-staining was then performed using various combinations of anti-CD43-FITC (clone 1B11), anti-PD-1-FITC (clone RMP1-30), anti-CD127-FITC (clone A7R34), anti-Thy1.1-PE (clone OX-7), anti-Thy1.1-PE-Cy7 (clone HIS51), and anti-KLRG-1-APC (clone 2F1) mAbs (Biolegend, BD Bioscience and eBioscience, San Diego, CA), together with anti-CD8a mAbs and PE or APC-conjugated MHC class I tetramers (produced in house or obtained from the NIAID tetramer core facility, Atlanta, GA). The anti-CD8a clone 53-6.7 (eBioscience) was used in conjunction with H2Db tetramers. For co-stains with H2Kb tetramers, the anti-CD8a clone CT-CD8a antibody (Cedarlane, Burlington, NC) was used. Staining procedures were performed at 4°C in PBS containing 2% (w/v) BSA and 0.2% (w/v) NaN3. After incubation with mAbs, samples were washed and then fixed in PBS containing 2% (w/v) paraformaldehyde. Samples were acquired using an LSR II flow cytometer (BD, San Jose, CA), and data was analyzed using FlowJo software (Tree Star, Ashland, OR).
Intracellular cytokine staining
Intracellular cytokine staining was performed as previously described (12). Briefly, cells were either left untreated or stimulated with LCMV-derived peptide epitopes (1μg/ml) for 5 hours at 37°C in the presence of Brefeldin A (Golgi-Plug; BD Bioscience). Note that stimulation with the GP33 peptide epitope activates both LCMV H2Db-restricted GP33-specific, and H2Kb-restricted GP34-specific CD8 T cells (29). Surface and intracellular staining were then performed using the mAbs anti-CD8a-PE-Cy7 (clone 53-6.7), anti-CD4-PE-Cy7 (clone RM4-5), anti-TNF-α-FITC (clone MP6-XT22), anti-Thy1.1-PE (clone OX-7), anti-IFN-γ-Pacific Blue (clone XMG1.2), and anti-IL-2-APC (clone JES6-5H4). Conjugated mAbs were purchased from BD Bioscience or eBioscience.
Quantitative RT-PCR analysis
Splenic Thy1.1hi and Thy1.1lo CD8 T cells were sorted from IFN-γ KI mice 44–52 days following LCMV-Arm (acute) or cl13 CD4Δ (chronic) infections. In separate experiments LCMV DbGP276-specific CD8 T cells were prepared from non-reporter B6 and Cd4−/− mice 88–93 days following LCMV-Arm (acute) and cl13 (chronic) infections, respectively. These antigen-specific CD8 T cell populations were isolated by cell sorting based upon staining with CD8-PE (53-6.7) and CD44-FITC (IM7) antibodies in conjunction with DbGP276-APC tetramer staining. All cell sorts were performed using a BD FACSAria instrument (BD Biosciences, San Jose, CA) and cells were kept cold throughout the procedure. Total RNA was extracted (RNeasy Plus Micro Kit; Qiagen; Valencia, CA) and cDNA synthesized (iScript cDNA Synthesis Kit; Bio-Rad; Hercules, CA). Real-time PCR was performed using the cDNA with the iQ5 Multicolor real-time PCR Detection System and iQ SYBR Green (Bio-Rad; Hercules, CA). Reactions were run in triplicate, and samples were normalized to the corresponding β2-microglobulin control reaction. The relative difference in IFN-γ mRNA expression between the experimental samples and the level in sorted naive (CD44lo) CD8 T cells prepared from B6 mice was calculated using the 2ΔΔCt method. The following primers were used: IFN-γ, forward 5′-CCTGCGGCCTAGCTCTGAG-3′; reverse 5′-GCCATGAGGAAGAGCTGCA-3′; β2-microblobulin, forward 5′-CCTGCAGAGTTAAGCATGCCAG-3′; reverse 5′-TGCTTGATCACATGTCTCGATCC-3′.
Adoptive transfers
Freshly explanted splenocytes from naïve P14.IFN-γ KI mice were resuspended in PBS containing 1% FCS and stained with anti-CD8-APC (53-6.7), CD44-FITC (IM7) and Thy1.1-PE (OX-7) antibodies. Naive (CD44loThy1.1lo) CD8 T cells were isolated by sorting using a BD FACSAria instrument (BD Biosciences, San Jose, CA). The purified cells were washed in PBS, and different doses of these donor cells were transferred by i.v. injection into CD45.1 mice. Recipient mice were infected with 2×106pfu LCMV-cl13 or the KAA LCMV-cl13 mutant (30) by i.v. injection one day later, and responses were analyzed at day 8 post-infection.
Treatment strategies
Anti-Thy1.1
Cohorts of mice were infected with LCMV-cl13 and either left untreated or administered 250μg anti-Thy1.1 antibodies (31) i.p. (clone 19E1.2, UAB immunoreagent core) at days 0, 2, 4, 6 and 7 following infection. Responses were analyzed at day 8 post-infection.
Anti-IL-10R
Cohorts of mice were infected with LCMV-cl13 and either left untreated or administered 300μg anti-IL-10R antibodies i.p. (clone 1B1.3A, UAB immunoreagent core and BioXCell, West Lebanon NH) at days 0, 1, 3, 5 and 7 following infection. Responses were analyzed at 21 days post-infection.
Anti-PD-L1
Cohorts of mice were infected with LCMV-cl13 and either left untreated or administered 200μg anti-PD-L1 antibodies i.p. (clone 10F.9G2, BioXCell, West Lebanon, NH) at days 18, 21, 24, 27, and 30 following infection. Responses were analyzed at day 32 post-infection.
Statistical analysis
Statistical analysis was performed using an unpaired Mann-Whitney test, and p values were calculated using Prism software (Graph Pad; La Jolla, CA). Statistical significance is defined as * p<0.05, ** p<0.01, or *** p<0.001 unless otherwise indicated.
Results
Accelerated activation of virus-specific CD8 T cells precedes exhaustion
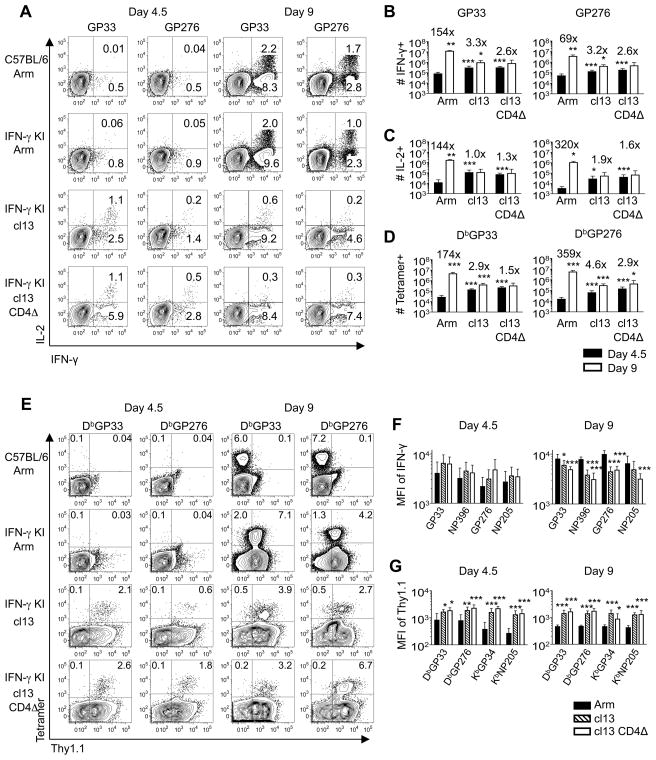
To investigate initial differences in CD8 T cells which may potentially forecast the development of exhaustion, we compared responses at days 4.5 and 9 following acute (LCMV-Arm), protracted (LCMV-cl13), and chronic (LCMV-cl13 in CD4 T cell depleted mice (CD4Δ)) infections (12). In acutely infected mice only minimal virus-specific CD8 T cell responses were detectable by intracellular cytokine analyses and MHC tetramer staining at 4.5 days following infection (Figure 1). By contrast, even at this very early time point, the magnitudes of the responses were consistently higher following infections that are subsequently only slowly (LCMV-cl13) or never contained (LCMV-cl13 with CD4Δ). Thus, the initial emergence of virus-specific CD8 T cells is accelerated during these more persistent viral infections. This pattern reversed by 9 days following infection, as a massive increase in both the numbers of MHC-tetramer binding and cytokine-producing CD8 T cells occurred in acutely infected mice. In mice undergoing protracted and chronic infections this burst was severely attenuated, as during these infections epitope-specific responses experienced minimal to no expansion between days 4.5 and 9 (Figure 1B-D). In addition, by comparison with acutely infected hosts, reductions in the functional quality of the responding CD8 T cells were also observed, as detected by lower mean fluorescence intensities (MFI) of IFN-γ staining at 9 days following infection (Figure 1F).
Figure 1. Virus-specific CD8 T cells during chronic viral infections are hyperactivated before progressing into functional exhaustion.
Cohorts of B6 and IFN-γ KI mice were infected with LCMV-Arm, cl13 or cl13 CD4Δ, and splenic responses were analyzed at days 4.5 and 9 post-infection. A-C, The ability of anti-viral CD8 T cells to produce IFN-γ and IL-2 was assessed by intracellular cytokine staining. A. Plots show gated CD8 T cells following stimulation with GP33 or GP276 peptide epitopes. B-D. Virus-specific CD8 T cells were enumerated by intracellular staining for IFN-γ (B), and IL-2 (C), as well as by direct ex vivo tetramer staining (D). Values above the bars represent the mean fold change in the size of the response between days 4.5 (filled bars) and 9 (open bars) following infection. E, The levels of Thy1.1 reporter expression were determined on MHC tetramer binding CD8 T cells. Plots show gated CD8 T cells and the values indicate the percentages of CD8 T cells in the respective quadrants. F, The MFI of IFN-γ protein production detected by intracellular cytokine staining following stimulation with the indicated viral peptide epitopes. G, MFI of ex vivo Thy1.1 reporter expression on splenic tetramer-binding CD8 T cells at days 4.5 (left panel) and 9 (right panel) following LCMV-Arm (filled bars), cl13 (hatched bars) or cl13 CD4Δ (open bars) infections. For B, C and D, significance denoted above black bars is compared against the Arm response, and above the white bars is compared against the day 4.5 response. For F and G, significance denoted is compared against Arm response; *p<0.05, **p<0.01, ***p<0.001. Error bars are s.d. Representative or composite data are shown from n=6–9 mice from 2–3 independent experiments.
Whereas prototypic effector and memory CD8 T cells are fully capable of producing IFN-γ, one of the hallmarks of severely exhausted T cells is an inability to express this cytokine (8, 9). To track the emergence of exhausted T cells we analyzed expression of the Thy1.1 molecule following acute, protracted and chronic LCMV infections of IFN-γ.Thy1.1 Knockin (IFN-γ KI) reporter mice (26). The anti-viral CD8 T cells detected at day 4.5 in the LCMV-cl13 infected mice had upregulated expression of the Thy1.1/IFN-γ reporter (Figure 1E). Interestingly, by 9 days following infection Thy1.1 reporter expression remained higher on virus-specific CD8 T cells in protracted and chronically infected mice (Figure 1G), despite the attenuation of the response and the lower MFI of IFN-γ protein expression detectable following intracellular staining (Figure 1F). Thus the early stages of T cell exhaustion, which manifest in protracted and chronically infected mice, appear to be preceded by a phase of hyperactivation. Notably, the upregulation of Thy1.1 reporter expression is not bimodal, suggesting that exhausted CD8 T cells may emerge from populations which more uniformly increase IFN-γ transcription even though their capacity to produce IFN-γ protein is reduced (Figure 1A, E, F).
All LCMV-specific CD8 T cells upregulate IFN-γ/Thy1.1 during the initial stages of infection
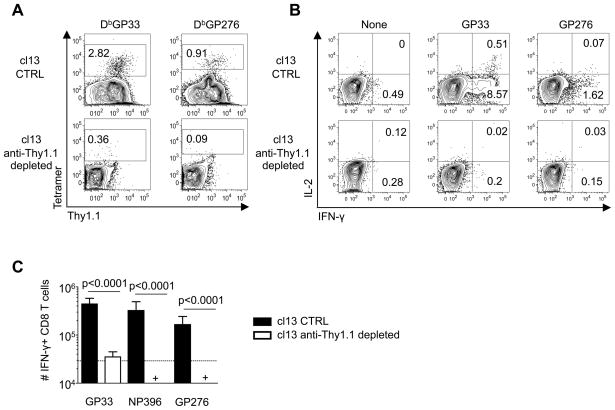
We hypothesized that if all CD8 T cells initially upregulate reporter expression prior to succumbing to exhaustion then the targeted depletion of Thy1.1+ cells should completely ablate the responses. To test this we administered the anti-Thy1.1 depleting antibody, clone 19E1.2 (31), to cohorts of cl13 infected IFN-γ KI reporter mice between days 0–8 following infection. Treatment of reporter mice with this depleting antibody was highly effective at removing MHC-tetramer binding (Figure 2A) as well as IFN-γ and IL-2-producing CD8 T cells (Figure 2B, C). These findings further support the concept that during the initial stages of protracted and chronic infections, all of the virus-specific CD8 T cells vigorously respond by upregulating IFN-γ transcription, even though their subsequent capacity to produce this cytokine may be diminished.
Figure 2. All virus-specific CD8 T cells upregulate Thy1.1/IFN-γ reporter expression following infection.
Cohorts of IFN-γ KI mice were infected with LCMV-cl13 and either left untreated or treated with anti-Thy1.1 depleting antibodies between days 0–7 following infection; responses were analyzed at 8 days following infection. A, The frequencies of splenic virus-specific CD8 T cells were determined by MHC tetramer staining; plots show gated CD8 T cells. B-C, The ability of the anti-viral CD8 T cells to produce IFN-γ and IL-2 was assessed by intracellular cytokine staining. B, Plots show gated CD8 T cells following stimulation with the indicated peptide epitopes. C, Enumeration of IFN-γ producing CD8 T cells specific for three distinct viral epitopes: LCMV-cl13 CTRL (not depleted; filled bars) and anti-Thy.1.1 depleted animals (open bars). Significance denoted is compared against the responses in the control cohort. Error bars are s.d. Representative or composite data are shown from n=8–10 mice from 2 independent experiments. +, below limit of detection.
Exhausted CD8 T cells retain expression of the Thy1.1/IFN-γ reporter
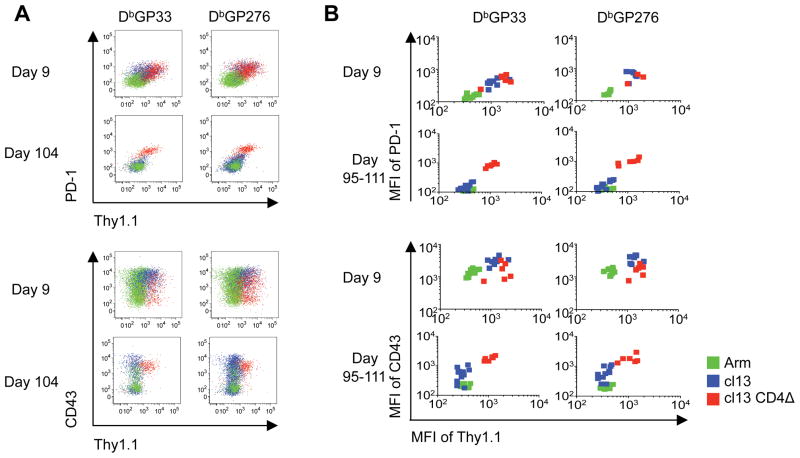
Since exhausted CD8 T cells lose the ability to produce the IFN-γ protein, we further evaluated how the phenotypic and functional changes associated with the development of exhaustion were reflected by expression of the Thy1.1/IFN-γ reporter molecule. As expected, the levels of PD-1 and CD43 (1B11) were most markedly increased on virus-specific CD8 T cells detectable in cl13 (protracted) and cl13 CD4Δ (chronic) infected mice (Figure 3A, B) (11–13). This activated phenotype paralleled the expression of elevated levels of the Thy1.1 reporter, observed 9 days post infection, even though reductions in the capacity to produce IFN-γ were already apparent (Figure 3A, B cf. Figure 1A, F). By 3–4 months post-infection the LCMV-cl13 infection had been brought under control (serum viral loads were below the limit of detection by plaque assay), and PD-1 as well as CD43 expression on virus-specific CD8 T cells were lower, similar to the levels observed on memory CD8 T cells detectable following acute LCMV-Arm infection. Notably, these phenotypic changes coincided with downregulation of Thy1.1 reporter expression. In cl13 CD4Δ infected mice viral loads remained high at these later timepoints (7469 ±3623 pfu/ml in the serum), and the virus-specific CD8 T cells present in these mice retained expression of PD-1 and CD43 (Figure 3). As expected, these cells were exhausted, as their capacity to produce IFN-γ, TNF-α, and IL-2 was extinguished (data not shown). Surprisingly, despite this severe functional impairment the exhausted cells expressed higher levels of the Thy1.1 reporter (Figure 3A, B, red populations). These results indicate that expression of the Thy1.1 reporter is maintained despite development of the exhausted state, but is also dynamic and decreases at later time points, as the availability of viral antigen declines.
Figure 3. Thy1.1/IFN-γ reporter expression is dynamic and retained on exhausted CD8 T cells.
Cohorts of IFN-γ KI mice were infected with LCMV-Arm, cl13 or cl13 CD4Δ, and splenic responses were analyzed at the indicated days post-infection. A-B, Expression of Thy1.1, PD-1 and CD43 by GP33 and GP276 tetramer-positive CD8 T cells following LCMV-Arm (acute/green), cl13 (protracted/blue) or cl13 CD4Δ (chronic/red) infections. A, Overlaid representative staining profiles show gated tetramer+ CD8 T cells. B, MFI of Thy1.1 reporter expression and PD-1 and CD43 levels on the indicated tetramer-positive population following infection; individual mice are shown. Representative or composite data are shown from n=3–11 mice from 2–3 independent experiments.
Exhausted CD8 T cells maintain expression of IFN-γ mRNA
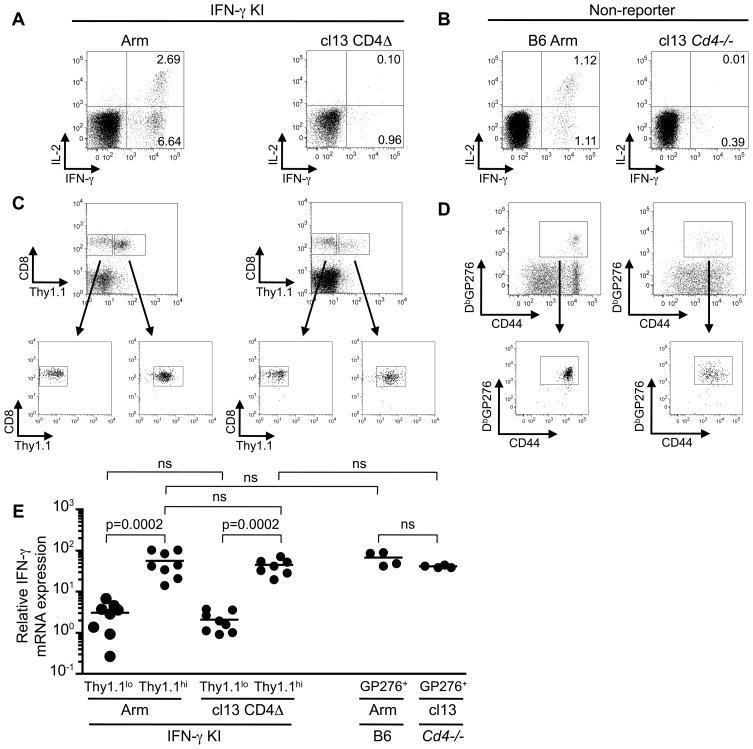
The pronounced upregulation of the Thy1.1 reporter molecule in exhausted CD8 T cells prompted us to perform a comparative analysis of IFN-γ mRNA levels in memory and exhausted CD8 T cells (Figure 4). We verified that memory virus-specific CD8 T cells present in acutely infected IFN-γ KI mice were capable of producing IFN-γ and IL-2 following in vitro stimulation but that this responsiveness was extinguished in chronically CD4Δ cl13 infected IFN-γ KI mice (Figure 4A). In separate experiments we further confirmed that LCMV-specific memory CD8 T cells in non-reporter Arm infected B6 mice were capable of producing IFN-γ whereas virus-specific CD8 T cells in chronically cl13 infected non-reporter Cd4−/− mice were functionally exhausted (Figure 4B).
Figure 4. Exhausted CD8 T cells maintain expression of IFNγ mRNA.
A, IFN-γ KI reporter mice were either infected with LCMV-Arm (acute) or CD4 depleted and infected with LCMV-cl13 (cl 3 CD4Δ; chronic infection) and analyzed 44–59 days post infection. Plots show intracellular cytokine staining profiles for IFN-γ and IL-2 following in vitro restimulation with the LCMV GP33 peptide epitope. Gated CD8 T cells are shown and the values represent the percentages of CD8 T cells present in the respective quadrants. B, In separate experiments memory and exhausted CD8 T cells from non-reporter LCMV-Arm infected B6 mice, and LCMV-cl13 infected Cd4−/− mice, respectively, were analyzed between days 88–93 following infection. Plots show intracellular cytokine staining profiles following stimulation with the LCMV-GP276 peptide epitope. C, CD8 T cells from Arm or cl13 CD4Δ, IFN-γ KI mice were sorted based on Thy1.1 expression as depicted. D, LCMV GP276-specific CD8 T cells from individual Arm infected B6 mice, and pairs of cl13 infected Cd4−/− mice, were isolated by sorting following MHC-tetramer, CD8 and CD44 costaining. Gated CD8 T cells are shown. E, The levels of IFN-γ mRNA expression in the various sorted populations were determined by quantitative real time PCR. The relative difference in expression is compared to that observed in sorted naive CD44lo CD8 T cells using the 2ΔΔct method. Representative or composite data are shown from n=4–12 samples from 2–3 independent experiments. ns, not-significant (p values were >0.1 for all ns samples).
Thy1.1lo and Thy1.1hi splenic CD8 T cells were isolated from acute (Arm) and chronically (cl13 CD4Δ) infected IFN-γ KI reporter mice by FACS (Figure 4C). LCMV DbGP276-specific memory and exhausted CD8 T cells were sorted from non-reporter Arm infected B6 mice and cl13 infected Cd4−/− mice, respectively using MHC tetramer, CD8, and CD44 costaining (Figure 4D). Notably, as previously reported the exhausted CD8 T cells express slightly lower levels of CD44 (3, 12). IFN-γ mRNA levels were then assessed in the Thy1.1lo and Thy1.1hi CD8 T cell populations from acutely (Arm) and chronically (cl13 CD4Δ) infected IFN-γ KI mice and in LCMV GP276-specific memory (B6 Arm) and exhausted (Cd4−/− cl13) CD8 T cells from non-reporter mice using real-time PCR (Figure 4E). Expression was normalized to that observed in sorted naive CD44lo CD8 T cells isolated from control B6 mice. Similar IFN-γ mRNA levels were detected in the Thy1.1hi CD8 T cells, which encompass the virus-specific CD8 T cell population, in both the acute and chronically infected IFN-γ KI reporter mice. Additionally, both memory and exhausted virus-specific CD8 T cells from non-reporter mice also express similar levels IFN-γ mRNA; however, whereas the memory CD8 T cells can rapidly respond to restimuation by producing the IFN-γ protein, this responsiveness is ablated in the exhausted population (Figure 4A, B).
Changes in precursor frequencies skew the initial activation of CD8 T cells during protracted infections
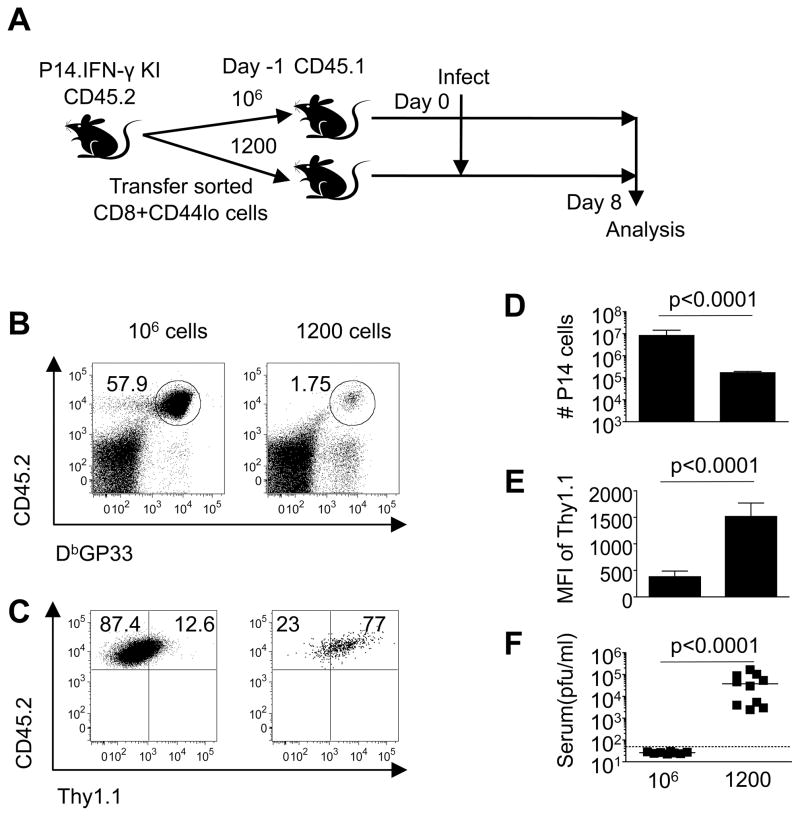
CD8 T cell precursor frequencies have been shown to influence the ensuing development of effector and memory T cells following infections, and also impact viral control (32, 33). To address whether the precursor frequency of virus-specific CD8 T cells impacted the marked upregulation of the Thy1.1 reporter following cl13 infection, we executed a series of adoptive transfer studies using P14 TCR transgenic CD8 T cells, which are specific for the GP33 epitope of LCMV. Different numbers of naive CD44loThy1.1lo LCMV GP33-specific CD8 T cells derived from P14.IFN-γ KI CD45.2 mice were transferred into naive congenic CD45.1 recipients. The recoveries, as well as Thy1.1 reporter expression levels on the P14.IFN-γ KI donor cells, were then evaluated 8 days following cl13 infection (Figure 5). In recipients which received 106 donor P14.IFN-γ KI cells, this population accounted for 98.9% ± 0.22 (SD) of the overall GP33-specific population; however, in recipients which received a more physiological dose of donor cells (1200 P14.IFN-γ KI cells), this population accounted for 40.4% ± 17.7 (SD) of the GP33-specific population. The level of Thy1.1 reporter molecule expression on the donor cells was clearly influenced by the precursor frequency, as the intensity of Thy1.1 expression was lower on donor cells recovered from recipients that received 106 cells, but higher on donor cells recovered from recipients that received 1200 cells (Figure 5B-E). Notably, the overwhelming response resulting from the transfer of 106 cells was associated with significantly lower viral loads than those observed in recipients receiving 1200 cells (Figure 5F). These findings are consistent with the concept that the levels of Thy1.1 reporter expression are dictated by the availability of viral antigen.
Figure 5. Precursor frequency impacts early induction of IFN-γ Thy1.1 reporter expression.
A, Different numbers (106 or 1200) of naive P14.IFN-γ KI cells were transferred into allelically marked CD45.1 recipients which were infected with LCMV-cl13 one day later. Splenic responses were analyzed at day 8 post-infection. B, D, The frequencies and numbers, respectively, of donor P14.IFN-γ KI cells recovered following infection. C, E, The staining profiles and MFI of Thy1.1 expression, respectively, on the donor cells following infection of recipient mice. F, Serum viral loads were determined by plaque assay. Error bars are s.d. Representative or composite data are shown from n=8–10 mice from 2 independent experiments.
Antigenic activation is a principle driver of CD8 T cell hyperactivation
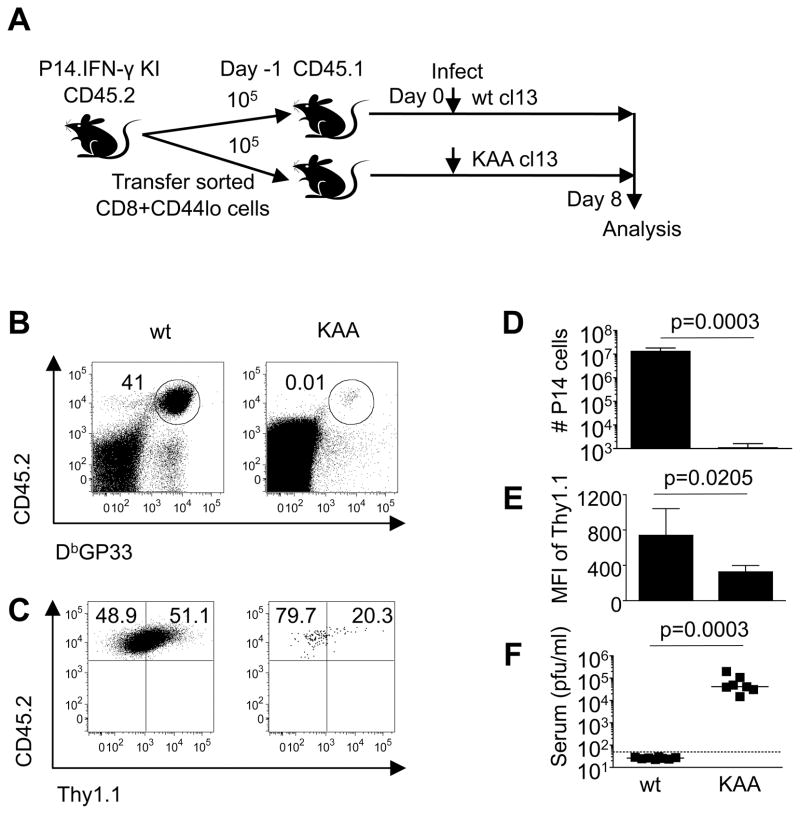
To discriminate between the impact of environmental cues, such as the inflammatory milieu, and the presence of cognate viral antigen in driving CD8 T cell hyperactivation, we took advantage of the availability of epitope escape mutant viruses. Naive donor P14.IFN-γ KI CD45.2 CD8 T cells (105) were adoptively transferred into cohorts of CD45.1 recipient mice which were subsequently infected with either authentic LCMV-cl13 or the KAA cl13 derivative, which contains an escape mutation within the P14 GP33 epitope (30). By 8 days following infection of the recipient mice with authentic cl13, the donor cells had upregulated Thy1.1 reporter expression, markedly expanded, accounting for 94.1% ±3.95 (SD) of the overall GP33 specific response, and the infection was contained (Figure 6). The paramount importance of TCR-driven stimulation in activation of anti-viral CD8 T cells was revealed by analyzing recipient mice infected with the KAA cl13 variant, as the donor cells in these mice failed to expand (Figure 6B-D) and did not strongly upregulate Thy1.1 reporter expression even though viral loads remained high (Figure 6C, E, F). Collectively, these results indicate that the development of the hyperactivated state that precedes exhaustion is primarily driven by the availability of cognate viral antigen rather than by other non-specific infection-associated triggers.
Figure 6. The hyperactivated state is driven by TCR activation.
A, 105 naive P14.IFN-γ KI cells were transferred into allelically marked CD45.1 recipients which were infected with either LCMV-cl13 or the KAA cl13 variant one day later. Splenic responses were analyzed at day 8 post-infection. B, D, The frequencies and numbers, respectively, of donor P14.IFN-γ KI cells recovered following infection. C, E, The staining profiles and MFI of Thy1.1 expression, respectively, on the donor cells following infection of recipient mice. F, Serum viral loads were determined by plaque assay. Error bars are s.d. Representative or composite data are shown from n=7–8 mice from 2 independent experiments.
Strategies to reduce viral loads dampen expression of the Thy1.1 reporter molecule
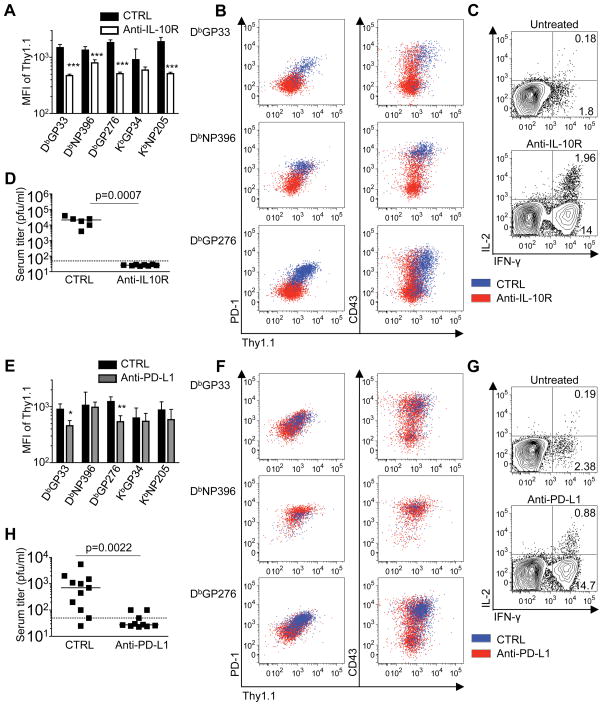
Given the pronounced expression of the Thy1.1 reporter molecule by the exhausted CD8 T cells and the dependence on antigenic signals for Thy1.1 upregulation, we next tested whether Thy1.1 reporter expression was altered by documented approaches to reduce viral loads. To address this we treated mice with anti-IL-10R antibodies during the first week of LCMV-cl13 infection and analyzed the anti-viral CD8 T cell responses at 3 weeks (Figure 7A-D) (18–20). Expression of the Thy1.1 reporter molecule was clearly lower for all epitope-specific responses checked in the treated group (Figure 7A, B), with the levels more closely resembling those observed following acute LCMV-Arm infection (see Figure 1G at day 9 post-infection). This was mirrored by reductions in the levels of PD-1 as well as CD43 (1B11) expression (Figure 7B), indicating a marked divergence between the phenotypic states of the anti-viral CD8 T cells in the anti-IL-10R treated and control groups. Moreover, the decreased Thy1.1 reporter levels and reduced expression of signature markers of T cell exhaustion was associated with enhanced functionality in the treated mice, as assessed by intracellular cytokine staining for IFN-γ and IL-2 (Figure 7C). This treatment strategy also resulted in containment of the infection (Figure 7D). Thus, pronounced upregulation of the Thy1.1 reporter molecule is prevented by this treatment strategy, and lower levels of Thy1.1 expression are associated with improved functional responses.
Figure 7. Treatment strategies promote viral control, dampen exhaustion, and reduce Thy1.1 reporter expression.
A-D, Cohorts of IFN-γ KI mice were infected with LCMV-cl13 and either left untreated or administered anti-IL-10R antibodies between days 0–7 following infection; responses were analyzed at day 21. E-H, LCMV cl13 infected IFN-γ KI mice were either left untreated or treated with anti-PD-L1 antibodies between days 18–30; responses were analyzed at 32 days following infection. A, E. Expression of the Thy1.1 reporter molecule on anti-viral CD8 T cells was determined in the spleens of untreated or treated mice. B, F, The relationship between Thy1.1 reporter and PD-1 or CD43 (1B11) expression; gated tetramer-positive CD8 T cells are shown from a representative untreated mouse (blue) and a treated mouse (red). C, G, The ability of virus-specific CD8 T cells to produce IFN-γ and IL-2 was assessed by intracellular cytokine staining. Plots show gated CD8 T cells following stimulation with the GP33 peptide epitope. D, H, Serum viral loads in the untreated control and treated cohorts were determined by plaque assay. Significance denoted is compared against untreated response; *p<0.05, **p<0.01, ***p<0.001. Error bars are s.d. Representative or composite data are shown from n=6–11 mice (for anti-IL-10R studies) and n=10–11 mice (for anti-PD-L1 studies) from 2 independent experiments for each treatment strategy.
To further evaluate the relationships between viral loads, CD8 T cell function, and Thy1.1 reporter expression, we adopted a therapeutic treatment regime using blocking anti-PD-L1 antibodies (13). This antibody treatment was administered between days 18 and 30 following LCMV cl13 infection of Thy1.1 IFN-γ KI mice and responses were analyzed 2 days later (Figure 7E-H). This therapeutic approach reduced the levels of the Thy1.1 reporter expression on splenic anti-viral CD8 T cells specific for the LCMV-GP33 and GP276 epitopes (Figure 7E); however, levels remained unchanged on the NP396-specific population. These differences in Thy1.1 reporter expression again paralleled the alterations observed in the levels of PD-1 and CD43 expression (Figure 7F), and were also associated with a rejuvenation of cytokine production by the responding CD8 T cells (Figure 7G) and improved viral control (Figure 7H). Collectively, these findings show that the prevention or reversal of T cell exhaustion dampens the activation state of these cells, shifting them to a more resting phenotype. These changes are associated with improved viral control and the levels of Thy1.1/IFN-γ reporter expression are dynamic and decreased on the more functionally competent CD8 T cells. Therefore, the upregulation of the Thy1.1 reporter molecule is likely maintained by continuous antigenic activation in the chronically infected mice, and is indicative of a discordance between IFN-γ mRNA levels and the ability to produce the IFN-γ protein.
Discussion
An unresolved issue in the field of chronic infections and CD8 T cell exhaustion is whether exhausted cells arise from previously activated and functional T cells or represent the outgrowth of cells that were never adequately primed or functionally competent. Our use of IFN-γ KI reporter mice clearly shows that during the initial stages of chronic LCMV infection, all virus-specific CD8 T cells do respond and transcribe IFN-γ, and notably this response is completely abolished by the targeted depletion of reporter expressing cells. Moreover, the early T cell response observed during the course of protracted and chronic infections is more pronounced than that observed in acutely infected hosts. Using a viral escape mutant to which P14 TCR transgenic CD8 T cells are unable to respond, we have demonstrated that this initial activation is antigen-driven; the P14 detector cells are not triggered to respond despite high viral titers in recipients infected with the mutant virus. Thus, the observed hyperactivation may be the first symptom of TCR bombardment, which may ultimately predispose the virus-specific CD8 T cells to functional inactivation. Nevertheless, the fate of these first responders is not set, as the development of the exhausted state can be rescued by approaches that lower viral loads, including by increasing T cell precursor frequencies. This is further supported by studies showing that the transfer of virus-specific CD8 T cells from an LCMV-cl13 (chronic) infected host into an LCMV-Arm (acute) infected animal, at 5 days post-infection, results in the emergence of polyfunctional memory phenotype T cells (34).
During protracted and chronic infections the accelerated and pronounced early activation is followed by an attenuation phase, as the responding cells subsequently fail to markedly expand in number and begin to lose their functional capacity. This restriction of the response is likely to be influenced by both the sustained presence of viral antigen as well as environmental imbalances (35). Rather that receiving a stimulatory “third” signal, increased levels of suppressive cytokines, such as IL-10 and TGF-β, as well as reductions in the availability of CD4 T cell help and lower levels of supportive cytokines, including IL-2 and IL-21, likely limit the response (2, 18–24, 36). Moreover, the rapid differentiation of anti-viral T cells during persistent infections into effector-like cells may arrest their potential transition into memory T cells capable of undergoing antigen-independent homeostatic proliferation (37, 38).
Loss of effector functions by virus-specific CD8 T cells is a defining characteristic of exhaustion (8, 9). The inability to produce IFN-γ represents late stage exhaustion; however, we now document that these cells maintain expression of IFN-γ mRNA, as well as the Thy1.1/IFN-γ reporter molecule. These results are consistent with microarray analyses of the gene expression profiles of exhausted CD8 T cells, which also indicate that these cells retain IFN-γ transcripts (14). Thus, the ablation of IFN-γ production by exhausted cells cannot be due to transcriptional silencing. Conventional memory CD8 T cells differ from exhausted CD8 T cells as memory T cells can rapidly produce abundant levels of the IFN-γ protein following stimulation. Previous studies, and also the results presented in Figure 4, demonstrate that memory CD8 T cells express higher levels of IFN-γ mRNA than naive CD8 T cells (14, 38–40). This is also reflected by the elevated levels of the Thy1.1/IFN-γ reporter molecule observed on the surface of LCMV-specific memory CD8 T cells (Figure 3 and 4). Thus, although IFN-γ mRNA is readily detectable in both memory and exhausted CD8 T cells post-translational control mechanisms likely regulate protein expression.
Discordances between mRNA and protein expression have been documented for several genes in CD8 T cells (41). In certain instances this is due to the rapid degradation of mRNA. IFN-γ mRNA stability is known to be affected by tristetraprolin binding to a putative AU-rich element (ARE) 3 binding site in the 3′ untranslated region (UTR) (42). In addition, activated cells express mRNAs with shortened 3′ UTRs in order to prolong transcript stability (43). Micro RNAs (miRNA) mediate the post-transcriptional control of gene expression in multiple cell types, including cells of the immune system. These miRNA bind to the 3′UTR and reduce gene expression by targeting the mRNA for degradation or by repressing translation (44, 45). The importance of miRNA in regulating T cell development has been highlighted by studies of Dicer-deficient CD4 and CD8 T cells (46, 47). Dicer is a key enzyme required for processing precursor miRNAs into mature miRNAs, which ultimately downregulate gene expression. Deletion of Dicer in the T cell linage dramatically decreased the fraction of CD8 T cells and resulted in marked expression of IFN-γ in CD4 T cells, implicating miRNA in the regulation of IFN-γ expression by CD4 T cells. Since exhausted CD8 T cells are phenotypically and functionally distinct from naive, effector, memory, and also anergic T cells (8, 9, 14), it is possible that a unique set of miRNAs, within exhausted CD8 T cells, participate in controlling this unresponsive state. Nevertheless, the precise roles of miRNAs in repressing IFN-γ levels in exhausted CD8 T cells remains undefined.
Using the IFN-γ KI reporter system we show that exhausted CD8 T cells express the bicistronic IFN-γ reporter mRNA and translate the IRES-dependent Thy1.1 reporter product from this transcript. Nevertheless, the IFN-γ protein is not detectable in the exhausted cells. Consequently, mRNA stability is unlikely to account for the loss of IFN-γ protein production, since the bicistronic transcript is clearly available for translation of Thy1.1. Instead, we speculate that the inability to produce the IFN-γ protein in exhausted T cells may represent a unique blockade of IFN-γ translation. IFN-γ mRNA has been shown to autoregulate its own translation by virtue of its 5′ untranslated region forming a pseudoknot capable of activating the double-stranded RNA dependent protein kinase R (PKR) (48). Once activated, PKR phosphorylates translational initiation factor eIF2α, thereby inhibiting translation. Therefore, these cells may upregulate IFN-γ mRNA in response to TCR and cytokine stimulation in an attempt to combat the infection; however, the ability to initiate cap-dependent translation of this message may be specifically blocked in the exhausted cells. Alternatively, the stability of the IFN-γ protein may be disrupted in exhausted cells resulting in more rapid degradation of this protein once it is produced. As the exhausted state develops during chronic infections the reduction in effector capabilities is not stochastic but instead typically occurs in a step-wise and predictable manner (3, 8–12). Thus, it is plausible that distinct molecular mechanisms may cause effector modules to be successively disabled as exhaustion progresses (49). Differential regulation of these processes during acute and chronic viral infections could therefore account for the divergent T cell functional set-points and polyfunctional profiles apparent at distinct stages of the response.
In this report we show that CD8 T cell responses are rapidly induced during the initial stages of protracted and chronic infections and that this upregulation precedes exhaustion. Exhaustion may, however, be beneficial as it is likely to prevent the development of immunopathology resulting from an over exuberant response to a systemic viral infection. Significantly, exhausted CD8 T cells retain high expression of IFN-γ mRNA despite their inability to produce the IFN-γ protein. Reduction of viral loads results in decreases in Thy1.1-reporter expression on the exhausted cells, implicating antigenic signals in sustaining IFN-γ transcription and IRES-driven Thy1.1 expression. This intriguing uncoupling of the regulation of IFN-γ mRNA and protein expression in exhausted cells suggests that unique mechanisms may regulate IFN-γ translation in exhausted CD8 T cells. Thus, further investigation into the mechanisms of translational control of IFN-γ in exhausted cells may reveal new therapeutic targets to bolster immune responses to chronic infections.
Acknowledgments
We thank all of the members of the Zajac lab for help and advice, and also Robin Hatton and Sunnie Thompson for helpful discussions, and Rafi Ahmed for supplying reagents. We also thank the University of Alabama at Birmingham Molecular Biology, Fermentation, CFAR flow cytometry, and epitope recognition and immunoreagent core facilities for vital services.
Footnotes
This work was supported by grants R01 AI049360, R01 AI067993 and U01 AI082966 from the National Institutes of Health and award TA3025-A-1 from the National Multiple Sclerosis Society.
Abbreviations used in this paper: Arm, LCMV-Armstrong; cl13, LCMV-Clone 13; CD4Δ, CD4 T cell depleted; IRES, internal ribosomal entry site; LCMV, lymphocytic choriomeningitis virus; MFI, mean fluorescence intensity; ns, not significant; UTR, untranslated region.
Publisher's Disclaimer: This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of the The JI, holds the copyright to this manuscript. The manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in the author-produced version of the manuscript or in any version derived from it by the United States National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org
References
- 1.Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 2.Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallimore A, Glithero A, Godkin A, Tissot AC, Pluckthun A, Elliott T, Hengartner H, Zinkernagel R. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J Exp Med. 1998;187:1383–1393. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boni C, Fisicaro P, Valdatta C, Amadei B, Di Vincenzo P, Giuberti T, Laccabue D, Zerbini A, Cavalli A, Missale G, Bertoletti A, Ferrari C. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol. 2007;81:4215–4225. doi: 10.1128/JVI.02844-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lauer GM, Lucas M, Timm J, Ouchi K, Kim AY, Day CL, Schulze Zur Wiesch J, Paranhos-Baccala G, Sheridan I, Casson DR, Reiser M, Gandhi RT, Li B, Allen TM, Chung RT, Klenerman P, Walker BD. Full-breadth analysis of CD8+ T-cell responses in acute hepatitis C virus infection and early therapy. J Virol. 2005;79:12979–12988. doi: 10.1128/JVI.79.20.12979-12988.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Far M, Halwani R, Said E, Trautmann L, Doroudchi M, Janbazian L, Fonseca S, van Grevenynghe J, Yassine-Diab B, Sekaly RP, Haddad EK. T-cell exhaustion in HIV infection. Curr HIV/AIDS Rep. 2008;5:13–19. doi: 10.1007/s11904-008-0003-7. [DOI] [PubMed] [Google Scholar]
- 8.Shin H, Wherry EJ. CD8 T cell dysfunction during chronic viral infection. Curr Opin Immunol. 2007;19:408–415. doi: 10.1016/j.coi.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Yi JS, Cox MA, Zajac AJ. T-cell exhaustion: characteristics, causes and conversion. Immunology. 2010;129:474–481. doi: 10.1111/j.1365-2567.2010.03255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuller MJ, Zajac AJ. Ablation of CD8 and CD4 T cell responses by high viral loads. J Immunol. 2003;170:477–486. doi: 10.4049/jimmunol.170.1.477. [DOI] [PubMed] [Google Scholar]
- 11.Zhou S, Ou R, Huang L, Price GE, Moskophidis D. Differential tissue-specific regulation of antiviral CD8+ T-cell immune responses during chronic viral infection. J Virol. 2004;78:3578–3600. doi: 10.1128/JVI.78.7.3578-3600.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuller MJ, Khanolkar A, Tebo AE, Zajac AJ. Maintenance, loss, and resurgence of T cell responses during acute, protracted, and chronic viral infections. J Immunol. 2004;172:4204–4214. doi: 10.4049/jimmunol.172.7.4204. [DOI] [PubMed] [Google Scholar]
- 13.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 14.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Wherry EJ, Barber DL, Kaech SM, Blattman JN, Ahmed R. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc Natl Acad Sci U S A. 2004;101:16004–16009. doi: 10.1073/pnas.0407192101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuller MJ, Hildeman DA, Sabbaj S, Gaddis DE, Tebo AE, Shang L, Goepfert PA, Zajac AJ. Cutting edge: emergence of CD127high functionally competent memory T cells is compromised by high viral loads and inadequate T cell help. J Immunol. 2005;174:5926–5930. doi: 10.4049/jimmunol.174.10.5926. [DOI] [PubMed] [Google Scholar]
- 17.Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 2006;12:1301–1309. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ejrnaes M, Filippi CM, Martinic MM, Ling EM, Togher LM, Crotty S, von Herrath MG. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J Exp Med. 2006;203:2461–2472. doi: 10.1084/jem.20061462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maris CH, Chappell CP, Jacob J. Interleukin-10 plays an early role in generating virus-specific T cell anergy. BMC Immunol. 2007;8:8. doi: 10.1186/1471-2172-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yi JS, Du M, Zajac AJ. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 2009;324:1572–1576. doi: 10.1126/science.1175194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324:1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frohlich A, Kisielow J, Schmitz I, Freigang S, Shamshiev AT, Weber J, Marsland BJ, Oxenius A, Kopf M. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science. 2009;324:1576–1580. doi: 10.1126/science.1172815. [DOI] [PubMed] [Google Scholar]
- 24.Tinoco R, Alcalde V, Yang Y, Sauer K, Zuniga EI. Cell-intrinsic transforming growth factor-beta signaling mediates virus-specific CD8+ T cell deletion and viral persistence in vivo. Immunity. 2009;31:145–157. doi: 10.1016/j.immuni.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oxenius A, Zinkernagel RM, Hengartner H. Comparison of activation versus induction of unresponsiveness of virus-specific CD4+ and CD8+ T cells upon acute versus persistent viral infection. Immunity. 1998;9:449–457. doi: 10.1016/s1074-7613(00)80628-7. [DOI] [PubMed] [Google Scholar]
- 26.Harrington LE, Janowski KM, Oliver JR, Zajac AJ, Weaver CT. Memory CD4 T cells emerge from effector T-cell progenitors. Nature. 2008;452:356–360. doi: 10.1038/nature06672. [DOI] [PubMed] [Google Scholar]
- 27.Pircher H, Burki K, Lang R, Hengartner H, Zinkernagel RM. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 1989;342:559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- 28.Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984;160:521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hudrisier D, Oldstone MB, Gairin JE. The signal sequence of lymphocytic choriomeningitis virus contains an immunodominant cytotoxic T cell epitope that is restricted by both H-2D(b) and H-2K(b) molecules. Virology. 1997;234:62–73. doi: 10.1006/viro.1997.8627. [DOI] [PubMed] [Google Scholar]
- 30.Blattman JN, Wherry EJ, Ha SJ, van der Most RG, Ahmed R. Impact of epitope escape on PD-1 expression and CD8 T-cell exhaustion during chronic infection. J Virol. 2009;83:4386–4394. doi: 10.1128/JVI.02524-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimonkevitz RP, Bevan MJ. Split tolerance induced by the intrathymic adoptive transfer of thymocyte stem cells. J Exp Med. 1988;168:143–156. doi: 10.1084/jem.168.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Obar JJ, Khanna KM, Lefrancois L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008;28:859–869. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Badovinac VP, Haring JS, Harty JT. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8(+) T cell response to infection. Immunity. 2007;26:827–841. doi: 10.1016/j.immuni.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brooks DG, McGavern DB, Oldstone MB. Reprogramming of antiviral T cells prevents inactivation and restores T cell activity during persistent viral infection. J Clin Invest. 2006;116:1675–1685. doi: 10.1172/JCI26856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Q, Skinner PJ, Ha SJ, Duan L, Mattila TL, Hage A, White C, Barber DL, O’Mara L, Southern PJ, Reilly CS, Carlis JV, Miller CJ, Ahmed R, Haase AT. Visualizing antigen-specific and infected cells in situ predicts outcomes in early viral infection. Science. 2009;323:1726–1729. doi: 10.1126/science.1168676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blattman JN, Grayson JM, Wherry EJ, Kaech SM, Smith KA, Ahmed R. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat Med. 2003;9:540–547. doi: 10.1038/nm866. [DOI] [PubMed] [Google Scholar]
- 37.Volkert M, Marker O, Bro-Jorgensen K. Two populations of T lymphocytes immune to the lymphocytic choriomeningitis virus. J Exp Med. 1974;139:1329–1343. doi: 10.1084/jem.139.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111:837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- 39.Grayson JM, Murali-Krishna K, Altman JD, Ahmed R. Gene expression in antigen-specific CD8+ T cells during viral infection. J Immunol. 2001;166:795–799. doi: 10.4049/jimmunol.166.2.795. [DOI] [PubMed] [Google Scholar]
- 40.Peixoto A, Evaristo C, Munitic I, Monteiro M, Charbit A, Rocha B, Veiga-Fernandes H. CD8 single-cell gene coexpression reveals three different effector types present at distinct phases of the immune response. J Exp Med. 2007;204:1193–1205. doi: 10.1084/jem.20062349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cham CM, Xu H, O’Keefe JP, Rivas FV, Zagouras P, Gajewski TF. Gene array and protein expression profiles suggest post-transcriptional regulation during CD8+ T cell differentiation. J Biol Chem. 2003;278:17044–17052. doi: 10.1074/jbc.M212741200. [DOI] [PubMed] [Google Scholar]
- 42.Ogilvie RL, Sternjohn JR, Rattenbacher B, Vlasova IA, Williams DA, Hau HH, Blackshear PJ, Bohjanen PR. Tristetraprolin mediates interferon-gamma mRNA decay. J Biol Chem. 2009;284:11216–11223. doi: 10.1074/jbc.M901229200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sandberg R, Neilson JR, Sarma A, Sharp PA, Burge CB. Proliferating cells express mRNAs with shortened 3′ untranslated regions and fewer microRNA target sites. Science. 2008;320:1643–1647. doi: 10.1126/science.1155390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 45.O’Connell RM, Rao DS, Chaudhuri AA, Baltimore D. Physiological and pathological roles for microRNAs in the immune system. Nat Rev Immunol. 2101;10:111–122. doi: 10.1038/nri2708. [DOI] [PubMed] [Google Scholar]
- 46.Cobb BS, Nesterova TB, Thompson E, Hertweck A, O’Connor E, Godwin J, Wilson CB, Brockdorff N, Fisher AG, Smale ST, Merkenschlager M. T cell lineage choice and differentiation in the absence of the RNase III enzyme Dicer. J Exp Med. 2005;201:1367–1373. doi: 10.1084/jem.20050572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muljo SA, Ansel KM, Kanellopoulou C, Livingston DM, Rao A, Rajewsky K. Aberrant T cell differentiation in the absence of Dicer. J Exp Med. 2005;202:261–269. doi: 10.1084/jem.20050678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ben-Asouli Y, Banai Y, Pel-Or Y, Shir A, Kaempfer R. Human interferon-gamma mRNA autoregulates its translation through a pseudoknot that activates the interferon-inducible protein kinase PKR. Cell. 2002;108:221–232. doi: 10.1016/s0092-8674(02)00616-5. [DOI] [PubMed] [Google Scholar]
- 49.Agnellini P, Wolint P, Rehr M, Cahenzli J, Karrer U, Oxenius A. Impaired NFAT nuclear translocation results in split exhaustion of virus-specific CD8+ T cell functions during chronic viral infection. Proc Natl Acad Sci USA. 2007;104:4565–4570. doi: 10.1073/pnas.0610335104. [DOI] [PMC free article] [PubMed] [Google Scholar]