Abstract
Resveratrol has been shown to exhibit cancer preventive activities in preclinical studies. We conducted a clinical study to determine the effect of pharmacological doses of resveratrol on drug and carcinogen metabolizing enzymes. Forty-two healthy volunteers underwent baseline assessment of cytochrome P450 (CYP) and Phase II detoxification enzymes. CYP 1A2, 2D6, 2C9, and 3A4 enzyme activities were measured by the metabolism of caffeine, dextromethorphan, losartan, and buspirone, respectively. Blood lymphocyte glutathione S-transferase (GST) activity and GST-π level and serum total and direct bilirubin, a surrogate for UDP-glucuronosyl transferase (UGT) 1A1 activity, were measured to assess Phase II enzymes. After the baseline evaluation, study participants took 1 gm of resveratrol once daily for 4 wks. Enzyme assessment was repeated upon intervention completion. Resveratrol intervention was found to inhibit the phenotypic indices of CYP3A4, 2D6, and 2C9, and to induce the phenotypic index of 1A2. Overall, GST and UGT1A1 activities were minimally affected by the intervention, although an induction of GST-π level and UGT1A1 activity was observed in individuals with low baseline enzyme level/activity. We conclude that resveratrol can modulate enzyme systems involved in carcinogen activation and detoxification, which may be one mechanism by which resveratrol inhibits carcinogenesis. However, pharmacological doses of resveratrol could potentially lead to increased adverse drug reactions or altered drug efficacy due to inhibition or induction of certain CYPs. Further clinical development of resveratrol for cancer prevention should consider evaluation of lower doses of resveratrol to minimize adverse metabolic drug interactions.
Keywords: resveratrol, Cytochrome P450 enzymes, cancer prevention, phase II enzymes
INTRODUCTION
Resveratrol, or 3, 4’, 5-trihydroxystilbene, is produced by a restricted number of plant species. It belongs to a class of defense molecules called phytoalexins that are produced in response to stress such as infection or UV irradiation. Resveratrol was identified in the dried roots of Polygonum cuspidatum (1), called Ko-jo-kon in Japanese, which is used in traditional Asian medicine for treatment of fungal infection, inflammation, hypertension, dermatitis, and hyperlipidemia. Resveratrol is also found in several edible natural products such as grapes, peanuts, and berries. Since its presence was first reported in red wine, scientists have speculated that the cardioprotective effects of red wine (so called “French Paradox”) may be attributed to resveratrol (2).
Resveratrol has been shown to inhibit carcinogenesis by affecting various molecular events in the initiation, promotion and progression stages. Some of these studies are summarized in recent reviews (3–7). The anti-initiation activity of resveratrol has been linked to the suppression of the metabolic activation and/or induction of detoxification of carcinogens via modulation of enzymes involved in either Phase I reactions (i.e., cytochrome P450 enzymes (CYP)) or Phase II conjugation reactions. A number of in vitro studies have shown that resveratrol inhibits CYP 1A1 and 1A2 enzyme activities (8, 9). Administration of resveratrol in mice resulted in suppressed expression of CYP1A1 and inhibited benzo[a]pyrene induced DNA adduct formation (10). Resveratrol has also been shown to inhibit the activity of CYP 1B1 (11, 12), 3A4 (13, 14), and 2E1 (13) in in vitro systems. Resveratrol has been shown to induce Phase II detoxification enzymes including UDP glucuronosyltransferase (UGT), glutathione S-transferase (GST), and quinone reductase activity in in vitro and in vivo systems (15–18). It is postulated that resveratrol activates the Phase II enzyme gene expression via modulation of the mitogen-activated protein kinase pathway.
Modulation of enzyme systems involved in carcinogen activation and detoxification could be one of the biochemical mechanisms responsible for the cancer preventive effect of resveratrol. However, such changes may also affect drug efficacy and toxicity because these enzymes are also responsible for drug metabolism. Unlike pharmaceutical drugs, information on metabolic drug interactions is often lacking when bioactive food components (BAFC) are developed for clinical indications. Such information is critically important in early phase clinical development of BAFC for cancer prevention, especially when studied at pharmacological doses. Here, we report a clinical study conducted in healthy volunteers to determine the effect of 1 gram QD dosing of resveratrol on the activity of drug and carcinogen metabolizing enzymes. This dose of resveratrol is much higher than that from dietary exposure as a 5-oz glass of red wine contains 0.29 – 1.89 mg of resveratrol and a cup of red grapes contain 0.24–1.25 mg of resveratrol. Nevertheless, this dose was chosen because it is currently being investigated clinically for cancer prevention.
MATERIALS AND METHODS
Study Drugs
Resveratrol drug product was supplied by Pharmascience Inc. through the Chemoprevention Agent Development Research Group, Division of Cancer Prevention, National Cancer Institute. Resveratrol caplets were manufactured by Pharmascience Inc. using synthetic resveratrol. Resveratrol purity was assessed by HPLC with UV detection. Each study caplet contains 500 mg resveratrol plus inert pharmaceutical excipients. The study caplets were stored at room temperature and protected from environmental extremes.
Study Participants
Male and female participants were recruited from the Tucson metropolitan area. Participants were eligible if they were ≥ 18 years of age who were nonsmokers or had stopped smoking for more than 1 year. Participants had normal liver and renal function. Participants were excluded if they were pregnant or breast feeding, had invasive cancers within the past 5 years, had uncontrolled severe metabolic disorders or other serious acute or chronic diseases, consumed more than 3 drinks of alcohol per week on average, had known hypersensitivity to resveratrol or CYP metabolic probe drugs (caffeine, dextromethorphan, losartan or buspirone), were taking medications/supplements that are known CYP enzyme inducers or inhibitors, or had participated in other clinical research studies within the past 3 months. The study was approved by the University of Arizona Human Subjects Protection Program. Written informed consent was obtained from all participants.
Study Design
During the initial visit, study participants completed a medical history form and underwent a brief physical exam. A fasting blood sample was collected for complete blood count and blood chemistry. Eligible subjects underwent a minimum of 2 weeks of washout in which they were required to limit resveratrol containing foods and products, refrain from herbal/botanical supplements, and minimize the consumption of cruciferous vegetables. After the washout period, study subjects underwent baseline evaluation of CYP and Phase II enzyme activities. Subjects were required to abstain from caffeine-containing products and food items that have been reported to affect drug metabolizing enzymes (such as grapefruit juice, cruciferous vegetables, and food cooked over charcoal), and over-the-counter medications beginning 72 hours before and until 8 hours after the CYP probe drug administration. Study subjects were instructed to fast overnight for 8 hours before and until 4 hours after the administration of the probes. For CYP enzyme activity determination, low doses of four CYP metabolic probe drugs were co-administered orally: caffeine (100 mg), dextromethorphan (30 mg), losartan (25 mg), and buspirone (10 mg) to assess the activity of CYP1A2, 2D6, 2C9, and 3A4, respectively. A standardized lunch was provided to the subjects at 4 hours after probe drug administration. Blood samples were collected before and at 0.5, 1, 2, 4, 6, and 8 hours after dosing. Lymphocytes were isolated from the pre-dose sample for assessment of baseline GST activity and GST-π level. Serum was isolated from the pre-dose sample for assessment of total and direct bilirubin level. Plasma was isolated from each collection for measurement of CYP probe drug and metabolite levels. Total voided urine was collected for 8 hours after probe drug administration for measurement of CYP probe drug and metabolite levels.
Following the completion of baseline enzyme activity determination, study participants took the first resveratrol dose (1 gm) on an empty stomach after an overnight fast to reduce variability in GI absorption. Blood samples were collected before and 1 hour after resveratrol administration for measurement of plasma resveratrol and resveratrol metabolite concentrations. Subsequently, participants underwent 4 weeks of daily resveratrol intervention at a dose of 1 gm once a day. Participants were instructed to take the daily dose with food.
Study participants returned to the clinic the day after completing the resveratrol intervention for post-intervention assessment of CYP and Phase II enzyme activities. A fasting blood sample was collected for lymphocyte GST activity/level and serum bilirubin levels. Study participants underwent post-intervention CYP assessment as described for baseline activity assessment. In addition, a fasting blood sample was collected for blood count and blood chemistry. Following the post-intervention assessment, study participants were followed for 2 weeks for any adverse reactions.
Analytical Methods for Metabolic Probe Drugs
For each assay, baseline and post-treatment samples of the same individual were paired in the analysis (i.e., analyzed in the same batch). Caffeine and paraxanthine in 4-hr post-dose plasma samples were determined using reversed-phase HPLC with ultraviolet detection (19). Dextromethorphan and dextrorphan in urine were analyzed using reversed-phase HPLC with fluorescence detection (20, 21). Losartan and its metabolite, E3174 (5-carboxylic acid of losartan (22)), in urine were analyzed using reversed-phase HPLC with fluorescence detection (22). Plasma buspirone levels were determined using a HPLC-tandem mass spectrometry method (23).
Measurements of GST Activities and GST-π Levels
Cell lysates were prepared by sonicating the lymphocyte pellets twice at 15-s intervals. The lysed cells were centrifuged at 10,000 × g at 4°C for 30 min. The supernatant was collected and stored at −80°C prior to the analysis. Total GST activity in lymphocyte lysates was determined using a GST assay kit (Cayman Chemical, Ann Arbor, MI) by measuring the conjugation of 1-chloro-2,4-dinitrobenzene (CDNB) with reduced glutathione. This GST mediated conjugation reaction is accompanied by an increase in absorbance at 340 nm. GST activity was expressed as nmol/min/mg protein, with protein concentrations assayed by the Bio-Rad protein assay kit (Bio-Rad Lab, Hercules, CA). GST-π concentrations in lymphocyte lysates were measured using an enzyme-linked immunoassay kit (Human pi GST EIA assay, Argutus Medical, Dublin, Ireland). The assay procedure is based on sequential addition of diluted samples or standards, anti-GST-π IgG conjugated with horseradish peroxidase, and substrate to microassay wells coated with anti-GST-π IgG. The assay range is 3.12 to 100 ng/ml. Standards of known concentrations were included in every run, and the enzyme levels were calculated from a standard curve. GST-π concentrations were normalized to protein concentrations and expressed as pg/mg protein.
Measurements of Bilirubin Levels
Total and direct serum bilirubin levels were determined in a certified commercial laboratory (Sonora Quest, Phoenix, AZ).
Resveratrol Assay
Plasma resveratrol and metabolite concentrations were determined using a published HPLC method with UV detection (24). Plasma concentrations of resveratrol metabolites were estimated based on the calibration curve established with resveratrol standard because authentic standards of resveratrol metabolites were not commercially available. The identity of resveratrol and its metabolites was confirmed by HPLC in tandem with mass spectrometry by monitoring the parent/product ion transitions of resveratrol and metabolites (24).
Data Analysis
CYP1A2 phenotypic index was assessed by the caffeine to paraxanthine concentration ratio in plasma samples collected 4 hours after probe cocktail dosing (19). CYP3A4 phenotypic index was determined by the area under the plasma buspirone concentration-time profile (AUC) (25) obtained after probe drug administration with the AUC estimated using the WINNONLIN program (version 5.0). CYP2D6 phenotypic index was assessed by the urinary recovery of dextromethorphan to dextrorphan molar ratio in urine collected up to 8 hours after probe drug administration (26). CYP2C9 phenotypic index was determined by the urinary recovery of losartan to E3174 ratio in urine collected up to 8 hours after probe drug administration (19). The distribution of these phenotypic indices was normalized by logarithmic transformation prior to tests of significance. The log-transformed indices determined after resveratrol intervention administration were compared to those determined at baseline using the paired t-test. GST activity, GST-π, total bilirubin, and direct bilirubin were analyzed similar to those described for the CYP indices.
RESULTS
Forty-two eligible participants initiated the study intervention. There were 11 men and 31 women. Average age was 40 years (range of 19 – 64 years) and average body mass index was 25.4 kg/m2 (range of 20.0 – 42.3 kg/m3). Safety data were analyzed on all 42 participants. CYP and Phase II enzyme activity were assessed in 40 participants because one participant withdrew participation after taking the first resveratrol dose and one participant was unavailable for the post-dosing enzyme activity assessment.
Table 1 summarizes the CYP phenotypic indices determined before and after 4 weeks of resveratrol administration. For CYP1A2 activity, assessed by the metabolic ratio of caffeine and paraxanthine, data from four participants were not included in the analysis because of the presence of high caffeine and paraxanthine levels in the predosing samples. Similar to those reported previously (19), there were large between-subject variations in CYP1A2 activity. The geometric mean baseline caffeine/paraxanthine ratio was 3.95 with values ranging from 1.31 to 21.4. After 4 weeks of daily resveratrol administration, the caffeine/paraxanthine ratio decreased significantly (p = 0.0005), suggesting an induction of CYP1A2 activity. The geometric mean ratio of post- over pre-intervention CYP1A2 index was 0.84 (90% CI, 0.78–0.91), representing a geometric mean change of 16%.
Table 1.
CYP phenotypic indices before and after 4 weeks of daily resveratrol administration (1 gm QD).
| CYP Isozyme |
Index Values1 | Post- to Pre-Intervention Ratio | P value2 | |
|---|---|---|---|---|
| Pre | Post | |||
| 1A2 | 3.95 ± 0.383 (N=36) |
3.33 ± 0.29 (N=36) |
0.84 (0.78, 0.91)4 | 0.0005 |
| 3A4 | 73.2 ± 12.8 (N=39) |
97.1 ± 16.7 (N=39) |
1.33 (1.11, 1.59) | 0.01 |
| 2D6 | 0.07 ± 0.02 (N=38) |
0.12 ± 0.04 (N=35) |
1.70 (1.23, 2.19) | 0.01 |
| 2C9 | 1.02 ± 0.11 (N=39) |
2.78 ± 0.36 (N=39) |
2.71 (2.22, 3.31) | < 0.0001 |
caffeine/paraxanthine ratio in 4-h postdose plasma as CYP1A2 index; buspirone AUC [(ng/ml)·min] as CYP3A4 index; dextromethorphan/dextrorphan molar ratio in 0–8 hr postdose urine as CYP2D6 index; losartan/E3174 ratio in 0–8 h postdose urine as CYP2C9 index
paired t-test on log-transformed indices
geometric mean ± standard error; standard error was derived from a delta method.
geometric mean ratio (90% confidence interval)
For CYP3A4 activity, assessed by the AUC of buspirone, data from one participant was not obtained because the plasma buspirone concentrations of this individual were highly variable, not allowing for the AUC calculation. Large between-subject variations were also observed for CYP3A4 enzyme activity. The geometric mean baseline buspirone AUC was 73.2 [(ng/ml)·min] with values ranging from 7.9 to 1,322 [(ng/ml)·min]. Four weeks of resveratrol administration resulted in a statistically significant increase in buspirone AUC (p = 0.01), suggesting inhibition of CYP3A4 activity. The geometric mean ratio of post- over pre-intervention buspirone AUC was 1.33 (90% CI, 1.11 – 1.59), representing a geometric mean change of 33%.
For CYP2D6 activity, assessed by the metabolic ratio of dextromethorphan and dextrorphan, data for one participant was not available due to a missed collection. In addition, dextromethorphan levels were not detectable in one participant in the baseline sample and in four participants in the post-intervention sample. These data were not included in the analysis. The baseline geometric mean dextromethorphan/dextrorphan molar ratio was found to be 0.07 with values ranging from 0.002 to 7.96. Resveratrol intervention resulted in significantly increased post-intervention dextromethorphan/dextrorphan molar ratio (p = 0.01), suggesting inhibition of CYP2D6 activity. The geometric mean ratio of post- over pre-intervention CYP2D6 index was 1.70 (90% CI, 1.23 – 2.19), representing a geometric mean change of 70%.
For CYP2C9 activity, assessed by the metabolic ratio of losartan and E3174, data was unavailable for one participant due to a missed collection. The baseline geometric mean losartan/E3174 ratio was 1.02 with values ranging from 0.37 to 5.06. Four weeks of resveratrol intervention resulted in a significant increase in CYP2C9 phenotypic index (p < 0.0001). The geometric mean ratio of post- over pre-intervention CYP2C9 index was 2.71 (90% CI, 2.22–3.31), representing a geometric mean change of 171%.
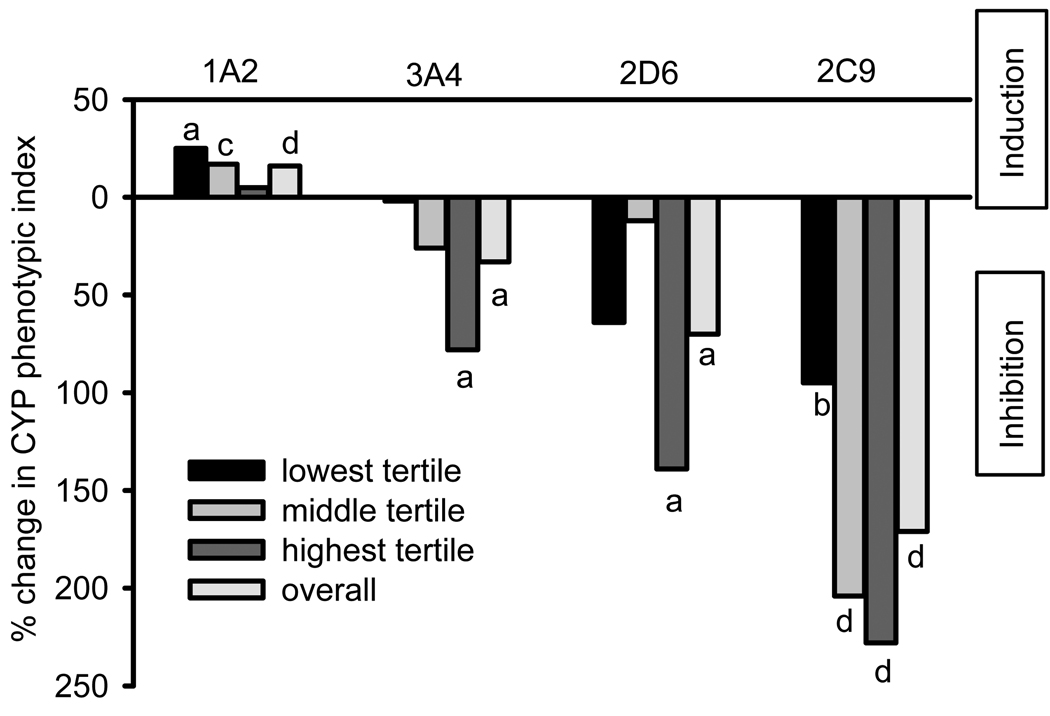
We examined the change in CYP indices stratified by baseline level because univariate regression analysis showed that the change in CYP indices was dependent on baseline values. Figure 1 illustrates the geometric mean percent change in CYP indices by tertile based on the baseline level. CYP1A2 is induced in individuals with enzyme activity in the lowest and middle tertiles. Inhibition of CYP3A4 and 2D6 indices was statistically significant in individuals with enzyme activity in the highest tertile, whereas inhibition of CYP2C9 index was significant in all tertiles.
Figure 1.
Percent change in CYP phenotypic indices by baseline activity tertile. a: p <0.05, b: p < 0.01, c: p < 0.005, d: p < 0.001.
Table 2 summarizes the GST activity and GST-π level in peripheral blood lymphocytes before and after 4 weeks of daily resveratrol administration. Resveratrol intervention had minimal effects on overall GST activity (p = 0.77) and GST-π level (p = 0.10). Table 3 summarizes the total and direct bilirubin determined before and after 4 weeks of daily resveratrol administration. Because bilirubin is primarily cleared from the body by the liver through conjugation reactions mediated by UGT1A1, individuals with high bilirubin levels tend to have low UGT1A1 activity and vice versa. Resveratrol intervention did not affect the overall serum bilirubin level (p = 0.90 for total bilirubin; p = 0.27 for direct bilirubin).
Table 2.
Blood lymphocyte GST enzyme activity and GST-π levels before and after 4 weeks of daily resveratrol administration (1 gm QD).
| GST | Pre | Post | Post- to Pre- Intervention Ratio |
P value3 |
|---|---|---|---|---|
| Total activity (nmol/min/mg protein) |
31.5 ± 2.51 (N = 38) |
32.1 ± 2.5 (N = 38) |
1.02 (0.92, 1.12)2 | 0.77 |
| GST-π level (ng/mg protein) |
4468 ± 310 (N = 36) |
5029 ± 218 (N = 36) |
1.13 (1.00, 1.27) | 0.10 |
geometric mean ± standard error; standard error was derived from a delta method.
geometric mean ratio (90% confidence interval)
paired t-test on log-transformed measurements
Table 3.
Serum total and direct bilirubin levels before and after 4 weeks of daily resveratrol administration (1 gm QD).
| Serum bilirubin | Pre | Post | Post- to Pre- Intervention Ratio |
P value3 |
|---|---|---|---|---|
| Total bilirubin (mg/dL) |
0.50 ± 0.041 (N = 40) |
0.50 ± 0.04 (N = 40) |
0.99 (0.91, 1.08)2 | 0.90 |
| Direct bilirubin (mg/dL) |
0.13 ± 0.01 (N = 40) |
0.12 ± 0.01 (N = 40) |
0.93 (0.83, 1.04) | 0.27 |
geometric mean ± standard error; standard error was derived from a delta method.
geometric mean ratio (90% confidence interval)
paired t-test on log-transformed measurements
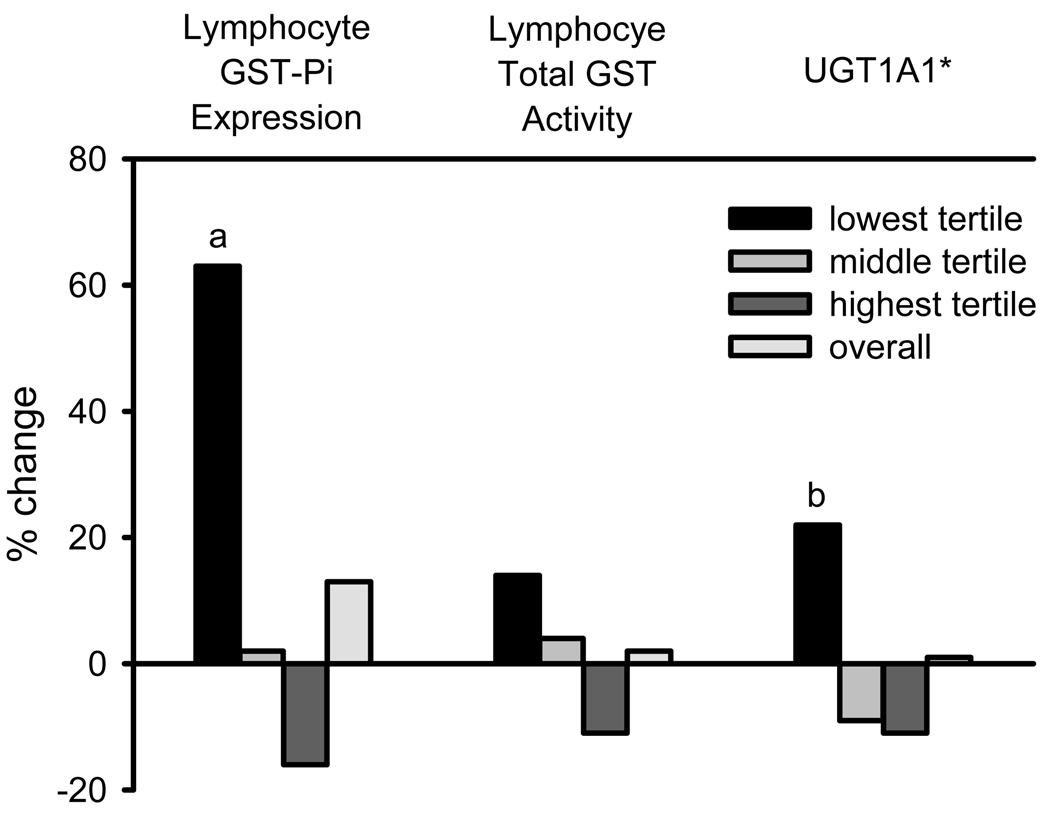
Similarly, we also examined the change in Phase II enzyme measurements stratified by baseline level because univariate regression analysis showed that the change in Phase II enzymes was dependent on baseline value. Figure 2 illustrates the change in Phase II enzyme measurements by tertile based on the baseline level. Individuals with low baseline GST-π showed a statistically significant increase with resveratrol intervention. Induction of UGT1A1 activity, assessed by serum total bilirubin level, was significant in those with low baseline activity.
Figure 2.
Percent change in GST pi expression, total GST activity, and UGT1A1 activity, by baseline expression/activity level. UGT1A1 activity was assessed by serum total bilirubin levels. a: p < 0.005, b: p < 0.01.
Table 4 summarizes the plasma resveratrol and metabolite concentrations 1 hr after a single oral dose of 1 gm of resveratrol. Previous studies have shown that plasma resveratrol concentrations reach maximum concentration approximately 1 hr after a single oral dose (27). The mean 1 hr post dosing plasma resveratrol concentration was 72.7 ng/ml with concentration values ranging from 8.3 to 404.4 ng/ml. The 1 hr post dose plasma concentrations of resveratrol metabolites were much higher than those observed for the parent compound. The major resveratrol metabolites were identified by liquid chromatography-tandem mass spectrometry as described previously (24, 27), with one metabolite conjugated with a glucuronide and a sulfate moiety (glucuronide-sulfate), two metabolites with a glucuronide moiety (monoglucuronide 1 and monoglucuronide 2), one metabolite with two sulfate moieties (disulfate), and one metabolite with a sulfate moiety at the 3-hydroxyl position (3-sulfate). The mean plasma concentrations of glucuronide-sulfate, monoglucuronide 1, monoglucuronide 2, disulfate, and 3-sulfate metabolite were 339.6, 619.5, 767.9, 359.3, 2,376.6 ng/ml, respectively, significantly higher than that of the parent compound.
Table 4.
Plasma resveratrol and metabolite concentrations 1 hr post a single oral dose of 1 gm of resveratrol.
| Plasma concentration (ng/ml) 1 hr post single dose |
|
|---|---|
| Resveratrol | 72.7 ± 11.71 (8.3 – 404.4) |
| Glucuronide- sulfate |
339.6 ± 42.62 (50 – 1,113.2) |
| Mono- glucuronide 1 |
619.5 ± 62.42 (160.4 – 2,237.2) |
| Mono- glucuronide 2 |
767.9 ± 97.72 (69.3 – 3,352.3) |
| Disulfate | 359.3 ± 64.52 (14.2 – 2,197.9) |
| 3-sulfate | 2,376.6 ± 320.22 (479.1 – 10,993.1) |
mean ± standard error (concentration range)
resveratrol metabolite concentrations were estimated based on the resveratrol standard
Table 5 lists the reported adverse events deemed possibly or probably related to study agent because of temporal proximity. Four weeks of daily administration of pharmacological doses of resveratrol was well tolerated in healthy participants. All reported adverse events were CTC Grade 1 or 2, and many were very mild and transient. One participant withdrew from study participation after the first dose of resveratrol due to diarrhea. One post-menopausal woman (BMI 36.9 kg/m2) experienced new onset, persistent peri-menopausal symptoms (hot flashes, insomnia) which required a 50% dose reduction. Four weeks of resveratrol dosing did not result in any clinically significant changes in blood chemistry and hematology measurements (data not shown).
Table 5.
Summary of adverse events possibly or probably related to resveratrol.
| Adverse event | Number (%) of subjects reporting the AE (total N = 42) |
|---|---|
| Diarrhea | 4 (9.5%) |
| Heartburn | 3 (7.1%) |
| Increased appetite | 2 (4.8%) |
| Mood alteration | 2 (4.8%) |
| Menstrual changes | 2 (4.8%) |
| Vivid dreams | 1 (2.4%) |
| Hot flashes | 1 (2.4%) |
| Insomnia | 1 (2.4%) |
| Decreased appetite | 1 (2.4%) |
| Flatulence | 1 (2.4%) |
| Nausea | 1 (2.4%) |
| Abdominal pain | 1 (2.4%) |
| Urine odor | 1 (2.4%) |
DISCUSSION
This clinical study showed that intervention with 1 gm of resveratrol once daily for 4 weeks modulated the phenotypic indices of multiple human CYP isozymes. In this study, we used probe drugs (or the associated metabolic ratios) to assess the activity of selected CYP isozymes. The selected probe drugs and the associated metabolic ratios have been previously validated for assessing the activity of each of the studied CYP isozymes (19, 25, 26). However, it is worth noting that additional P450s may also play a role for the metabolism of the probe drugs (or the associated metabolic ratios).
We found that four weeks of daily resveratrol administration resulted in a 16% decrease in the caffeine/paraxanthine metabolic ratio, suggesting an induction of CYP1A2 activity. Many environmental carcinogens, such as polycyclic aromatic hydrocarbons, are generally thought to be activated by the CYP1A and 1B subfamilies to form genotoxic epoxide metabolites, which can bind to DNA, forming adducts. Therefore, inhibition of these enzymes is thought to be an important mechanism in the prevention of carcinogenesis. However, utilizing Cyp1a1(−/−) knockout mice, studies have shown that the inducible CYP1A1 is far more important in detoxification than in metabolic activation of benzo[a]pyrene (28, 29). Therefore, the impact of CYP1A induction on carcinogen activation or detoxification may depend on the particular study context.
We showed that four weeks of resveratrol intervention resulted in 33%, 70%, and 171% increases in buspirone plasma AUC, metabolic ratio of dextromethorphan to dextrorphan, and metabolic ratio of losartan to E3174, respectively. These changes suggested the inhibition of the activity of 3A4, 2D6, and 2C9, respectively. Among the CYP enzymes, CYP3A4 metabolizes the vast majority of drugs, including immunosuppressive drugs for transplant patients, HIV protease inhibitors, cholesterol lowering statin drugs, and chemotherapeutics. Inhibition of CYP3A4 would result in elevation of the systemic blood levels of drugs metabolized by this isozyme which could lead to increased drug toxicity. Among the CYP enzymes, CYP2D6 shows the largest phenotypical variability. CYP2D6 is responsible for converting tamoxifen to the potent anti-estrogen, endoxifen. Studies have shown that individuals with decreased CYP2D6 metabolism due to genetic variations or enzyme inhibition have reduced plasma endoxifen concentration and increased risk of breast cancer relapse (30, 31). It is possible that resveratrol could decrease the formation of endoxifen, thus affecting the chemopreventive or anticancer activity of tamoxifen. CYP2C9 is the second most abundant CYP in the liver and small intestine. It is involved in the metabolic clearance of a wide variety of therapeutic drugs, including many NSAIDs, COX-2 inhibitors, oral anticoagulants and oral hypoglycemics. This raises the question of whether resveratrol would decrease the clearance of these drugs, possibly increasing the toxicity of these compounds.
Phase II enzymes mediate conjugation reactions which generally lead to detoxification of electrophilic active carcinogens. Resveratrol has been shown to induce UDP glucuronosyltransferase (UGT), glutathione S-transferase (GST), and quinone reductase activity in in vitro and in vivo systems (15–18). It has been suggested that resveratrol activates the Phase II enzyme gene expression via modulation of the mitogen-activated protein kinase pathway. We measured the clinical effects of resveratrol on GST expression in blood lymphocytes and serum bilirubin, a surrogate for hepatic UGT1A1 (32, 33) and found that overall GST and UGT1A1 activity were minimally affected by the resveratrol intervention. However, individuals with low baseline GST-π levels and UGT1A1 activity showed a significant increase in enzyme activity following resveratrol intervention. This appears to be consistent with previous observations that induction of Phase II enzymes is more pronounced in individuals with low baseline enzyme activity (32, 34). Nevertheless, precautions are needed in interpreting these data because the observed changes could be due to regression to the mean.
We conclude that four weeks of daily resveratrol dosing at 1 gm QD was well tolerated in healthy individuals. Resveratrol intervention modulated the enzyme systems involved in carcinogen activation and detoxification, which may be one mechanism by which resveratrol inhibits carcinogenesis. However, pharmacological doses of resveratrol could lead to increased adverse drug reactions or altered drug efficacy due to suppression of drug metabolism mediated by CYP3A4, 2D6, and 2C9. Given that resveratrol is being studied in a variety of chemoprevention settings and is available for use as a dietary supplement, further studies are needed to determine resveratrol-drug interactions to assess the effects of resveratrol on drug efficacy and safety. In addition, further clinical development of resveratrol for cancer prevention should consider evaluation of lower doses of resveratrol to minimize adverse metabolic drug interactions.
Acknowledgements
The authors would like to acknowledge Bonita Weible, Heidi Fritz, and Roberta Kline, NP, for their excellent assistance in participant recruitment and clinical specimen collection and processing. The authors also would like to acknowledge Steve Rodney for his efforts in data management.
This work was supported by a contract (N01-CN-35158) from the National Cancer Institute
Glossary
The abbreviations used are
- CYP
cytochrome P450
- GST
glutathione S-transferase
- UGT
UDP-glucuronosyl transferase
- AUC
area under the plasma concentration-time curve.
REFERENCES
- 1.Vastano BC, Chen Y, Zhu N, Ho CT, Zhou Z, Rosen RT. Isolation and identification of stilbenes in two varieties of Polygonum cuspidatum. J Agric Food Chem. 2000;48:253–256. doi: 10.1021/jf9909196. [DOI] [PubMed] [Google Scholar]
- 2.Kopp P. Resveratrol, a phytoestrogen found in red wine. A possible explanation for the conundrum of the 'French paradox'? Eur J Endocrinol. 1998;138:619–620. doi: 10.1530/eje.0.1380619. [DOI] [PubMed] [Google Scholar]
- 3.Delmas D, Lancon A, Colin D, Jannin B, Latruffe N. Resveratrol as a chemopreventive agent: a promising molecule for fighting cancer. Curr Drug Targets. 2006;7:423–442. doi: 10.2174/138945006776359331. [DOI] [PubMed] [Google Scholar]
- 4.Signorelli P, Ghidoni R. Resveratrol as an anticancer nutrient: molecular basis, open questions and promises. J Nutr Biochem. 2005;16:449–466. doi: 10.1016/j.jnutbio.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 5.Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Research. 2004;24:2783–2840. [PubMed] [Google Scholar]
- 6.Athar M, Back JH, Tang X, et al. esveratrol: a review of preclinical studies for human cancer prevention. Toxicol Appl Pharmacol. 2007;224:274–283. doi: 10.1016/j.taap.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bishayee A. Cancer prevention and treatment with resveratrol: from rodent studies to clinical trials. Cancer Prev Res (Phila Pa) 2009;2:409–418. doi: 10.1158/1940-6207.CAPR-08-0160. [DOI] [PubMed] [Google Scholar]
- 8.Ciolino HP, Daschner PJ, Yeh GC. Resveratrol inhibits transcription of CYP1A1 in vitro by preventing activation of the aryl hydrocarbon receptor. Cancer Res. 1998;58:5707–5712. [PubMed] [Google Scholar]
- 9.Yueh MF, Kawahara M, Raucy J. Cell-based high-throughput bioassays to assess induction and inhibition of CYP1A enzymes. Toxicol In Vitro. 2005;19:275–287. doi: 10.1016/j.tiv.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Revel A, Raanani H, Younglai E, et al. Resveratrol, a natural aryl hydrocarbon receptor antagonist, protects lung from DNA damage and apoptosis caused by benzo[a]pyrene. J Appl Toxicol. 2003;23:255–261. doi: 10.1002/jat.916. [DOI] [PubMed] [Google Scholar]
- 11.Mollerup S, Ovrebo S, Haugen A. Lung carcinogenesis: resveratrol modulates the expression of genes involved in the metabolism of PAH in human bronchial epithelial cells. Int J Cancer. 2001;92:18–25. [PubMed] [Google Scholar]
- 12.Chen ZH, Hurh YJ, Na HK, et al. Resveratrol inhibits TCDD-induced expression of CYP1A1 and CYP1B1 and catechol estrogen-mediated oxidative DNA damage in cultured human mammary epithelial cells. Carcinogenesis. 2004;25:2005–2013. doi: 10.1093/carcin/bgh183. [DOI] [PubMed] [Google Scholar]
- 13.Piver B, Berthou F, Dreano Y, Lucas D. Inhibition of CYP3A, CYP1A and CYP2E1 activities by resveratrol and other non volatile red wine components. Toxicol Lett. 2001;125:83–91. doi: 10.1016/s0378-4274(01)00418-0. [DOI] [PubMed] [Google Scholar]
- 14.Chang TK, Yeung RK. Effect of trans-resveratrol on 7-benzyloxy-4-trifluoromethylcoumarin O-dealkylation catalyzed by human recombinant CYP3A4 and CYP3A5. Can J Physiol Pharmacol. 2001;79:220–226. [PubMed] [Google Scholar]
- 15.Sainz RM, Mayo JC, Tan DX, Lopez-Burillo S, Natarajan M, Reiter RJ. Antioxidant activity of melatonin in Chinese hamster ovarian cells: changes in cellular proliferation and differentiation. Biochem Biophys Res Commun. 2003;302:625–634. doi: 10.1016/s0006-291x(03)00230-4. [DOI] [PubMed] [Google Scholar]
- 16.Szaefer H, Cichocki M, Brauze D, Baer-Dubowska W. Alteration in phase I and II enzyme activities and polycyclic aromatic hydrocarbons-DNA adduct formation by plant phenolics in mouse epidermis. Nutr Cancer. 2004;48:70–77. doi: 10.1207/s15327914nc4801_10. [DOI] [PubMed] [Google Scholar]
- 17.Gerhauser C, Klimo K, Heiss E, et al. Mechanism-based in vitro screening of potential cancer chemopreventive agents. Mutat Res. 2003;523–524:163–172. doi: 10.1016/s0027-5107(02)00332-9. [DOI] [PubMed] [Google Scholar]
- 18.Yen GC, Duh PD, Lin CW. Effects of resveratrol and 4-hexylresorcinol on hydrogen peroxide-induced oxidative DNA damage in human lymphocytes. Free Radic Res. 2003;37:509–514. doi: 10.1080/1071576031000083099. [DOI] [PubMed] [Google Scholar]
- 19.Christensen M, Andersson K, Dalen P, et al. The Karolinska cocktail for phenotyping of five human cytochrome P450 enzymes. Clin Pharmacol Ther. 2003;73:517–528. doi: 10.1016/S0009-9236(03)00050-X. [DOI] [PubMed] [Google Scholar]
- 20.Park YH, Kullberg MP, Hinsvark ON. Quantitative determination of dextromethorphan and three metabolites in urine by reverse-phase high-performance liquid chromatography. J Pharm Sci. 1984;73:24–29. doi: 10.1002/jps.2600730107. [DOI] [PubMed] [Google Scholar]
- 21.Lam YWF, Rodriguez SY. High-performance liquid chromatography determination of dextromethorphan and dextrorphan for oxidation phenotyping by fluorescence and ultraviolet detection. Ther Drug Monit. 1993;15:300–304. doi: 10.1097/00007691-199308000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Ritter MA, Furtek CI, Lo MW. An improved method for the simultaneous determination of losartan and its major metabolite, EXP3174, in human plasma and urine by high-performance liquid chromatography with fluorescence detection. J Pharm Biomed Anal. 1997;15:1021–1029. doi: 10.1016/s0731-7085(96)01948-6. [DOI] [PubMed] [Google Scholar]
- 23.Chew W, Xu M-J, Cordova C, Chow H-H. Quantification of a cytochrome P450 3A4 substrate, buspirone, in human plasma by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;844:235–239. doi: 10.1016/j.jchromb.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Boocock D, Patel KR, Faust GES, et al. Quantitation of trans-resveratrol and detection of its metabolites in human plasma and urine by high performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;848:182–187. doi: 10.1016/j.jchromb.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lilja J, Kivisto K, Backman J, Lamberg T, Neuvonen P. Grapefruit juice substantially increases plasma concentrations of buspirone. Clin Pharmacol Ther. 1998;64:655–660. doi: 10.1016/S0009-9236(98)90056-X. [DOI] [PubMed] [Google Scholar]
- 26.Wang Z, Gorski JC, Hamman MA, Huang SM, Lesko LJ, Hall SD. The effect of St. John's wort (Hypericum perforatum) on human cytochrome P450 activity. Clin Pharmacol Ther. 2001;70:317–326. [PubMed] [Google Scholar]
- 27.Boocock DJ, Faust GE, Patel KR, et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prev. 2007;16:1246–1252. doi: 10.1158/1055-9965.EPI-07-0022. [DOI] [PubMed] [Google Scholar]
- 28.Uno S, Dalton TP, Shertzer HG, et al. Benzo[a]pyrene-induced toxicity: paradoxical protection in Cyp1a1(−/−) knockout mice having increased hepatic BaP-DNA adduct levels. Biochem Biophys Res Commun. 2001;289:1049–1056. doi: 10.1006/bbrc.2001.6110. [DOI] [PubMed] [Google Scholar]
- 29.Uno S, Dalton TP, Derkenne S, et al. Oral exposure to benzo[a]pyrene in the mouse: detoxication by inducible cytochrome P450 is more important than metabolic activation. Mol Pharmacol. 2004;65:1225–1237. doi: 10.1124/mol.65.5.1225. [DOI] [PubMed] [Google Scholar]
- 30.Jin Y, Desta Z, Stearns V, et al. CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst. 2005;97:30–39. doi: 10.1093/jnci/dji005. [DOI] [PubMed] [Google Scholar]
- 31.Goetz MP, Knox SK, Suman VJ, et al. The impact of cytochrome P450 2D6 metabolism in women receiving adjuvant tamoxifen. Breast Cancer Res Treat. 2007;101:113–121. doi: 10.1007/s10549-006-9428-0. [DOI] [PubMed] [Google Scholar]
- 32.Navarro SL, Peterson S, Chen C, et al. Cruciferous vegetable feeding alters UGT1A1 activity: diet-and genotype-dependent changes in serum bilirubin in a controlled feeding trial. Cancer Prev Res (Phila Pa) 2009;2:345–352. doi: 10.1158/1940-6207.CAPR-08-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bosma PJ, Chowdhury JR, Bakker C, et al. The genetic basis of the reduced expression of bilirubin UDP-glucuronosyltransferase 1 in Gilbert's syndrome. N Engl J Med. 1995;333:1171–1175. doi: 10.1056/NEJM199511023331802. [DOI] [PubMed] [Google Scholar]
- 34.Chow HH, Hakim IA, Vining DR, et al. Modulation of human glutathione s-transferases by polyphenon e intervention. Cancer Epidemiol Biomarkers Prev. 2007;16:1662–1666. doi: 10.1158/1055-9965.EPI-06-0830. [DOI] [PubMed] [Google Scholar]