Abstract
Mammals navigate a complex environment using a variety of strategies, which can operate in parallel and even compete with one another. We have recently described a variant water maze task in which two of these strategies, hippocampus-dependent spatial learning and striatum-dependent cued learning, can be dissociated. Male rodents perform better at some spatial learning tasks, while female rodents more readily learn certain striatum-dependent behavioral strategies. We therefore predicted that sex would differentially influence spatial and cued learning in the water maze. We trained adult male and female C57Bl/6 mice for seven days in the two-cue variant of the water maze, with probe trials on days 5 and 7. In two independent experiments, males and females performed similarly, with both groups showing good spatial learning after 5 and 7 days of training, and both groups showing trend-level cued learning after 5 days and robust learning after 7. Therefore, contrary to our hypothesis, sex does not significantly affect cued or spatial learning in this task.
Keywords: spatial learning, cued learning, water maze, sex differences, mice
INTRODUCTION
Navigating a complex environment requires flexible use of learning strategies that can accommodate both reliable regularities and unpredictable deviations from the expected (1). The multiple memory systems hypothesis proposes that different brain circuits, which can be dissociated by experimental manipulations, employ different logics of learning and are invoked under different circumstances (2). We have recently described a water maze learning task in mice that permits assessment of cued or spatial learning in two otherwise identical task variants(3). We found spatial learning to depend on the hippocampus, while cued learning depended on the striatum, consistent with earlier literature (4–6). We found that the two systems compete with one another during learning (7), such that disruptions of striatal function can actually enhance spatial learning, while hippocampal disruptions enhance cued learning (3).
Male and female rodents have been shown to learn differentially under some conditions. Male rats show consistently superior performance in hippocampus-dependent spatial reference and working memory tasks, in both the Morris water maze and the radial maze (8). Male mice have also been reported to show better spatial memory in a radial maze (9, 10), though findings in the water maze have been more equivocal (8, 11, 12). Interestingly, ‘sex-reversed’ female mice (which carry the male Y chromosome) perform better in the Morris water maze than normal females, suggesting an important role for male genotype (as opposed to hormonal complement) on hippocampus-dependent spatial learning (13). Several explanations for this sexual dimorphism have been proposed, including evolved, adaptive differences in brain wiring between the two sexes (e.g. 14), differential glucocorticoid responses to new tasks (15), differential responsivity to appetitive motivation (16), and differential response to non-spatial pretraining (17).
In contrast, limited data suggest that female mice more rapidly acquire certain striatum-dependent learning tasks. In an instrumental habit task, which is sensitive to striatal disruptions in both rats and mice (18, 19), genotypically female mice show more rapid acquisition of habitual responding than males or ‘sex-reversed’ females (20). In navigation tasks, it has been suggested that females rely more on landmark cues rather than on spatial cognition (8, 21). Earlier studies in rats (5, 22), our results in a cued water maze task in mice (3), and neuroimaging studies in humans (e.g. 23) suggest that such cue-based navigation depends on the striatum.
We therefore speculated that our variant water maze task, which tests both spatial and cued learning and can detect competition between them (3), would reveal predictable sexual dimorphisms in behavior. Specifically, we predicted that male mice would perform better than females in the spatial task, while female mice would show superior performance in the cued task. We tested this hypothesis in adult C57Bl/6 mice.
MATERIALS AND METHODS
Animals
All experiments were conducted under the supervision of the Yale University Institutional Animal Care and Use Committee (Animal Welfare Assurance Number A3230-1). Food (standard laboratory chow) and water were available ad libitum. All experiments used adult male and female C57Bl/6J mice, aged 2.5 to 4 months; male and female mice were of the same age in each experiment.
Behavioral testing. Water maze task
Animals were trained on either the cued or spatial variant of the water maze, using a slight variation on the protocol described in detail by Lee et al (3). The apparatus consisted of a large circular pool (172 cm diameter) filled with opaque water, which is maintained at 25°C. A 12 cm circular Atlantis platform (Med Associates, Vermont), 1.5 cm below the surface of the water, permits escape from the water. The platform is marked with a visible cue, consisting of a plastic cylinder (2.5 cm diameter × 11 cm high), painted either a uniform gray or with high-contrast 1-cm horizontal or vertical stripes, as detailed below. During two-cue training (see below), as second cue was held on a stand elsewhere in the pool, such that it was not possible to discern from above the water which cue was associated with the platform and thus the possibility of escape.
All training days consisted of four trials with a 20-minute inter-trial interval. The first five days consisted of shaping to the task. On day 1, the gray cue was used and the animal was placed on the platform for 30 seconds for each trial. On days 2–5 the gray cue was again used; animals were placed in the water at the edge of the pool and allowed to search for the escape platform for up to 120 seconds. Any animal unable to find the platform in this time period was guided to it by the experimenter. After 15 seconds on the platform, animals were returned to the home cage. Following shaping, animals were returned to the vivarium and left undisturbed for 1–3 days.
Shaping was followed by 7 days of cued or spatial 2-cue training. Vertically and horizontally striped cues were used for this phase; in each trial, one of these cues marked the location of the platform, while the other was placed elsewhere in the pool and did not permit escape. Each animal was assigned to either cued or spatial learning. In the cued task, the platform was consistently associated with one of the two cues (vertical or horizontal stripes, counterbalanced across animals), but varied in location among the four quadrants of the pool. In the spatial task, the platform was consistently in the same place (counterbalanced across groups), but the cue marking it varied pseudo-randomly. In both tasks, the other cue (termed the lure cue) was placed on the stand, not permitting escape, in an adjacent quadrant. On each training trial, the animal was placed in the pool against the edge, opposite both cues, and permitted to search for 120 seconds for the escape platform. After finding the escape platform, the animal was permitted to rest there for 15 seconds before being removed to its home cage.
Probe trials were performed on the fourth trial of days 5 and 7 of 2-cue training. On a probe trial, the Atlantis platform was lowered so that it did not permit escape. Search was monitored for 60 seconds using an overhead digital camera and automated tracking software (Any-Maze: Stoelting), after which the platform was raised and the animal permitted to escape to it. Any animal failing to escape after 60 seconds (or a total of 120 seconds of search) was guided to the platform. Systematic bias towards one cue in the probe trial was interpreted as evidence of learning, in either the cued or the spatial task. Probe trial performance was quantified as either quadrant occupancy or occupancy in a circular zone, 25 cm in diameter, centered on the goal or lure cue. For probe trials, the time of each animal’s first touch of the goal cue was used as a latency measure in the latency analysis (figure 1).
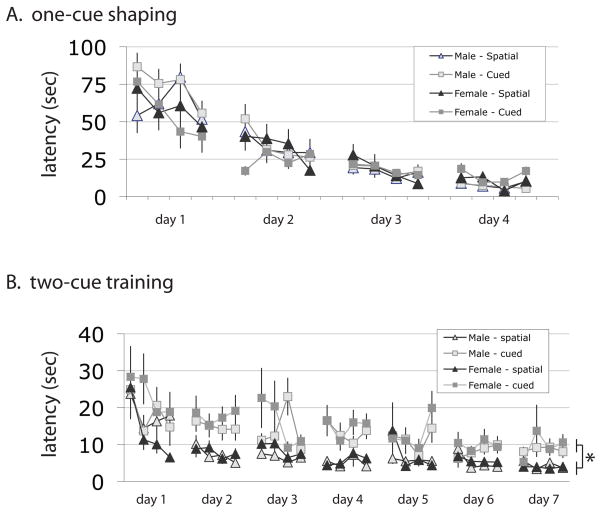
Figure 1. Escape latencies during shaping and training.
A. Male and female mice showed similar escape latencies during 1-cue training during the shaping phase. ‘Cued’ and ‘Spatial’ mice received identical training during this phase but are separated out for illustrative purposes. All values are mean ± SEM. B. In the 2-cue task, animals learning the spatial task showed faster escape than those learning the cued task, but there was no difference between male and female mice. See text for statistical analysis.
Analysis
Analysis of latency and probe trial data was by RM-ANOVA; effects within individual tasks were probed using lower-order ANOVAs. Analysis was performed using SPSS.
RESULTS
61 adult C57Bl/6J mice were trained in the cued and spatial water maze tasks (3) in two independent balanced experiments (n = 16 male/spatial, 16 male/cued, 15 female/spatial, 14 female/cued). Similar results were found in the initial experiment and the replication, and data were pooled for analysis, with experiment number as an independent variable in all primary analyses.
Latency to find the escape platform improved, as expected, across training trials. There was no difference between sexes in escape latencies in the one-cue shaping task (figure 1A). In the two-cue task, latencies improved with training for both the cued and spatial task (figure 1B. RM-ANOVA: day, F[6,318] = 22.6, p < 0.0001; trial, F[3, 159] = 3.53, p < 0.02; day x trial interaction, F[18,954] = 2.17, p < 0.005). Latencies were shorter in the spatial task than in the cued task (F[1, 53] = 34.7; p < 0.0001), consistent with what we have observed previously (3). There was no effect of sex on latency in either task, and no interactions (all p > 0.05). Similarly, there was no effect of experiment number and no interactions (all p > 0.05).
Probe trials were substituted for normal training trials on the fourth trial of days 5 and 7. Analysis of all probe trial data (RM-ANOVA of quadrant occupancy data with sex, task, and experiment as between-subjects factors and with probe trial day and quadrant as within-subject factors) showed a clear bias towards the goal quadrant (main effect of quadrant: F[1,53] = 68.36, p < 0.0001) that differed between spatial and cued tasks (quadrant X task interaction: F[1, 53] = 33.8, p < 0.0001) but was not influenced by sex (quadrant X sex: F[1,53] = 0.036, p > 0.1; interactions of sex with task and trial were likewise non-significant).
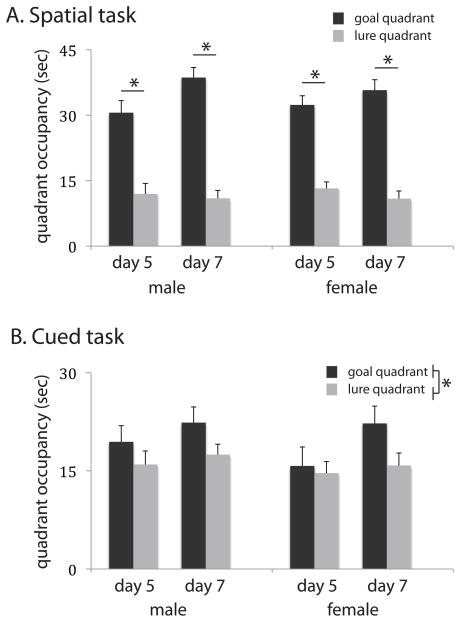
In the spatial task (figure 2A), significant bias towards the goal quadrant was apparent across both probe trials (RM-ANOVA restricted to the spatial task: main effect of quadrant, F[1, 27] = 84.1, p < 0.0001) that increased from day 5 to day 7 (quadrant X trial interaction: F[1, 27] = 9.013, p = 0.006). However, there was no effect of sex on quadrant bias (quadrant X sex: F[1, 27] = 0.03, p > 0.5), or any interactions involving sex. Similarly, significant goal-quadrant bias but no effects of sex were found in ANOVA analysis restricted to day 5 or to day 7.
Figure 2. Probe trial performance.
A. In the spatial task, both males and females showed robust learning on days 5 and 7, illustrated here by a significantly greater search time in the target quadrant relative to the lure quadrant. There was no significant effect of sex in this or any other measure of probe trial performance. B. Learning was less robust in the cued task, but both groups showed preference for the goal quadrant after seven days of training. There was again no effect of sex on learning or probe trial performance. See text for details and statistical analysis. * p < 0.05; all values are mean ± SEM.
Less dramatic learning was apparent in the cued task (figure 2B), consistent with our previous findings (3). Bias towards the goal quadrant was at trend level across the experiment (RM-ANOVA restricted to the cued task: main effect of quadrant: F[1, 26] = 3.732, p = 0.064), emerging as significant on day 7 (day 5, main effect of quadrant: F[1, 26] = 0.82, p > 0.3; day 7: F[1, 26] = 4.85, p < 0.04). The quadrant X sex interaction was non-significant when analyzed either across the whole experiment (F[1,26] = 0.01, p > 0.5) or on independently on days 5 and 7.
We repeated these analyses using other measures of probe trial performance, including occupancy in a circular target zone around the goal and lure cues and mean distance from the goal and lure cues during search (3). The same effects were seen in these other analyses; no significant effect of sex or interaction was found using these alternative measures.
DISCUSSION
We have described a variant water maze task that permits assessment of cued and spatial learning in mice; we have previously shown (3) that the spatial task depends on the hippocampus, while the cued task depends on the dorsal striatum, consistent with a large body of previous work (2, 4, 5, 18). Strikingly, we found that these two memory systems can destructively interfere with one another, or compete, during learning (3, 24). We hypothesized that this task would reveal sexual dimorphism in learning strategy, such that male mice would exhibit superior performance in the spatial task while female mice would be superior in the cued task. However, in two independent experiments with a large number of animals, we did not find any differences between adult male and female mice in either task. Therefore, at least as assayed by this task, adult male and female C57Bl/6 mice do not markedly differ in their ability to use hippocampus-dependent and striatum-dependent strategies during learning.
Superior hippocampus-dependent spatial learning in males has been demonstrated in rats and mice in a variety of tasks (8). For example, in a water escape-motivated radial arm maze, male C57Bl/6 mice have been reported to exhibit better spatial reference and working memory than females (9). However, results in both radial-maze and more standard water maze tasks have varied, with many studies showing no clear difference between sexes, especially in mice (8). Indeed one study actually showed female superiority in spatial learning in aged mice (12), and another recent study found object location memory, which also depends on hippocampal function, to be superior in females (14). Therefore, while the absence of a clear male advantage in spatial learning in our modified water maze task is contrary to our initial hypothesis, it is consistent with a significant body of literature.
It remains possible that details of our training protocol served to mitigate a difference between male and female animals that might be revealed by a different protocol. Although nonspatial shaping did not interfere with our ability to show distinct effects of targeted brain lesions on spatial and cued learning in our original study (3), similar pretraining has been shown to mitigate sex differences in spatial learning in rats (15, 17). This effect was found to correlate with a greater corticosterone response to training in the water in females than in males; pretraining reduced both the increased corticosterone level and the relative deficit in spatial learning (15). Other studies have similarly found that sex differences in spatial and non-spatial learning were eliminated over the course of extensive training (8). Our paradigm includes substantial pretraining in a one-cue water escape task, which may have eliminated a sex difference that would otherwise have been seen in two-cue spatial training. However, it might have been expected that such an effect would manifest as a sex difference in latencies during the early phase of one-cue pretraining; such an effect is not apparent in our data (figure 1A).
It is possible that male and female mice are achieving similar performance in the spatial task by applying different strategies. Consistent with this possibility, a recent study showed C57Bl/6 male mice to have superior spatial memory (as assayed by the classic Morris water maze task), while female mice exhibited superior object recognition (25); this reproduced a similar earlier study in rats (14). Earlier work in rats has similarly suggested that females are more reliant on individual ‘landmark’ cues for efficient spatial navigation (8, 21). Our spatial task is actually a hybrid, in which optimal performance requires using spatial information to pick which of two prominent cues (i.e. landmarks) to swim to; it may be that a strategy of being guided by individual landmarks is better able to compensate for a subtle deficit in spatial learning in such a task than in the classic Morris water maze.
Finally, it may be that a deficit in spatial learning in female mice, relative to males, was masked by variability introduced by the females’ hormonal cycling. However, several observations argue against this possibility. First, training in the spatial task occurs over seven days (nearly two typical estrus cycles), so any such source of variability would be expected to average out over training. Effects of estrus cycling have been observed in spatial water maze tasks in mice only using protocols in which all training occurs during a single day (e.g. 26). Second, this explanation would predict a larger variance in probe trial performance in females than in males; but variance in our experiment was actually smaller in females than in males on day 5 – an effect that approached statistical significance (Levene’s test for equality of variances, day 5 goal quad occupancy, p = 0.05). There was no difference between sexes in variance on day 7. These data argue against the commonly held view that estrus cycling adds variance and thus complicates analysis of behavioral tasks in females. Finally, while an effect of estrus stage on Morris water maze performance has been suggested in rats even when training extends across several days, the effect has been subtle and limited to early in training in some studies (when learning is likely to be non-spatial; e.g. 27), and absent in others (e.g. 28).
We tested striatum-dependent cued learning and hippocampus-dependent spatial learning in parallel. This strategy has proven valuable for dissociating the anatomical substrates of different forms of learning and for examining their interactions during training (3, 5, 22). Female rats and mice have shown better acquisition of striatum-dependent tasks in some contexts (20). In addition, as mentioned above, female rats are thought to depend more on discrete cues during navigation than males (8, 21). We therefore predicted that females would be superior to males in our striatum-dependent cued task; we further anticipated that, since spatial and cued strategies compete with one another during learning in this task (3), the predicted sexual dimorphisms in cued and spatial learning should amplify one another. However, no such female superiority in the cued task was seen.
It is likely that different forms of striatum-dependent-learning will be differently affected by sexual dimorphism both in mnemonic capacity and in other parameters that affect performance, such as attention and motivation. In this water maze task, however, no significant sexual dimorphism in either cued or spatial learning is apparent.
Acknowledgments
This work was supported by NIH grants T32MH014175 (LBR) and K08MH081190 (CP) and by a NARSAD Young Investigator Award (CP)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Daw ND, Niv Y, Dayan P. Nat Neurosci. 2005;8:1704–1711. doi: 10.1038/nn1560. [DOI] [PubMed] [Google Scholar]
- 2.White NM, McDonald RJ. Neurobiol Learn Mem. 2002;77:125–184. doi: 10.1006/nlme.2001.4008. [DOI] [PubMed] [Google Scholar]
- 3.Lee AS, Duman RS, Pittenger C. Proc Natl Acad Sci U S A. 2008;105:17163–17168. doi: 10.1073/pnas.0807749105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Keefe J, Nadel L. The hippocampus as a cognitive map. Clarendon Press; Oxford University Press; Oxford New York: 1978. [Google Scholar]
- 5.Packard MG, McGaugh JL. Behav Neurosci. 1992;106:439–446. doi: 10.1037//0735-7044.106.3.439. [DOI] [PubMed] [Google Scholar]
- 6.Morris RG, Garrud P, Rawlins JN, O’Keefe J. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 7.Poldrack RA, Clark J, Pare-Blagoev EJ, Shohamy D, Creso Moyano J, Myers C, Gluck MA. Nature. 2001;414:546–550. doi: 10.1038/35107080. [DOI] [PubMed] [Google Scholar]
- 8.Jonasson Z. Neurosci Biobehav Rev. 2005;28:811–825. doi: 10.1016/j.neubiorev.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Gresack JE, Frick KM. Brain Res. 2003;982:98–107. doi: 10.1016/s0006-8993(03)03000-2. [DOI] [PubMed] [Google Scholar]
- 10.LaBuda CJ, Mellgren RL, Hale RL. Physiol Behav. 2002;76:213–217. doi: 10.1016/s0031-9384(02)00713-8. [DOI] [PubMed] [Google Scholar]
- 11.Galea LA, Kavaliers M, Ossenkopp KP, Innes D, Hargreaves EL. Brain Res. 1994;635:18–26. doi: 10.1016/0006-8993(94)91419-2. [DOI] [PubMed] [Google Scholar]
- 12.Frick KM, Burlingame LA, Arters JA, Berger-Sweeney J. Neuroscience. 2000;95:293–307. doi: 10.1016/s0306-4522(99)00418-2. [DOI] [PubMed] [Google Scholar]
- 13.Stavnezer AJ, McDowell CS, Hyde LA, Bimonte HA, Balogh SA, Hoplight BJ, Denenberg VH. Behav Brain Res. 2000;112:135–143. doi: 10.1016/s0166-4328(00)00174-1. [DOI] [PubMed] [Google Scholar]
- 14.Saucier DM, Shultz SR, Keller AJ, Cook CM, Binsted G. Anim Cogn. 2008;11:129–137. doi: 10.1007/s10071-007-0096-1. [DOI] [PubMed] [Google Scholar]
- 15.Beiko J, Lander R, Hampson E, Boon F, Cain DP. Behav Brain Res. 2004;151:239–253. doi: 10.1016/j.bbr.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 16.Mishima N, Higashitani F, Teraoka K, Yoshioka R. Physiol Behav. 1986;37:263–268. doi: 10.1016/0031-9384(86)90230-1. [DOI] [PubMed] [Google Scholar]
- 17.Perrot-Sinal TS, Kostenuik MA, Ossenkopp KP, Kavaliers M. Behav Neurosci. 1996;110:1309–1320. doi: 10.1037//0735-7044.110.6.1309. [DOI] [PubMed] [Google Scholar]
- 18.Yin HH, Knowlton BJ. Nat Rev Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- 19.Quinn JJ, Pittenger C, Lee AS, Zaffin M, Duman RS, Taylor JR. (in preparation) [Google Scholar]
- 20.Quinn JJ, Hitchcott PK, Umeda EA, Arnold AP, Taylor JR. Nat Neurosci. 2007;10:1398–1400. doi: 10.1038/nn1994. [DOI] [PubMed] [Google Scholar]
- 21.Roof RL, Stein DG. Physiol Behav. 1999;68:81–86. doi: 10.1016/s0031-9384(99)00162-6. [DOI] [PubMed] [Google Scholar]
- 22.Packard MG, Hirsh R, White NM. J Neurosci. 1989;9:1465–1472. doi: 10.1523/JNEUROSCI.09-05-01465.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartley T, Maguire EA, Spiers HJ, Burgess N. Neuron. 2003;37:877–888. doi: 10.1016/s0896-6273(03)00095-3. [DOI] [PubMed] [Google Scholar]
- 24.Poldrack RA, Packard MG. Neuropsychologia. 2003;41:245–251. doi: 10.1016/s0028-3932(02)00157-4. [DOI] [PubMed] [Google Scholar]
- 25.Bettis TJ, Jacobs LF. Behav Processes. 2009;82:249–255. doi: 10.1016/j.beproc.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Frick KM, Berger-Sweeney J. Behav Neurosci. 2001;115:229–237. doi: 10.1037/0735-7044.115.1.229. [DOI] [PubMed] [Google Scholar]
- 27.Frye CA. Physiol Behav. 1995;57:5–14. doi: 10.1016/0031-9384(94)00197-d. [DOI] [PubMed] [Google Scholar]
- 28.Berry B, McMahan R, Gallagher M. Behav Neurosci. 1997;111:267–274. doi: 10.1037//0735-7044.111.2.267. [DOI] [PubMed] [Google Scholar]