Abstract
Overindulgence in easily available energy-dense palatable foods is thought to be an important factor in the current obesity epidemic but the underlying neural mechanisms are not well understood. Here we demonstrate that mu-opioid receptor signaling in the nucleus accumbens may be important. Protracted suppression of endogenous mu-opioid receptor signaling focused on the nucleus accumbens shell for several days by means of microinjected β-funaltrexamine (BFNA) diminished both ‘liking’ of sucrose, as indicated by fewer positive hedonic orofacial responses, and the incentive reinforcement value (‘wanting’) of a food reward, as indicated by lower completion speed and increased time being distracted in the incentive runway. BFNA-treatment also decreased responding to sucrose and corn oil in the brief access lick paradigm, a test measuring a combination of mainly taste-guided ‘liking’ and low-effort ‘wanting’, as well as 4-hr intake of sucrose solution. These effects were not due to nonspecific permanent neuronal changes, as they were fully reversible. We conclude that endogenous mu-opioid signaling in the nucleus accumbens is necessary for the full display of palatable food-induced hyperphagia through mechanisms including hedonic, motivational, and reinforcement processes. Development of obesity could be the result of predisposing innate differences in these mechanisms or overstimulation of these mechanisms by external factors.
Keywords: reward, ventral striatum, taste reactivity, hedonic value, working for food, obesity
Introduction
Parallels have recently been drawn between food and drug addiction, and attention directed to the vulnerability of a common underlying neural circuitry to habitual overindulgence that may result in the development of overweight and obesity (Kelley and Berridge, 2002, Volkow and Wise, 2005, Volkow et al., 2008). The psychological construct of reward is complex and efforts have been made to parse it into distinguishable components with specific neural pathways and mechanisms (Berridge, 1996, Schultz et al., 1997, Wise, 2005 #4957, Ikemoto and Panksepp, 1999, Carelli, 2002, Kelley and Berridge, 2002, Salamone et al., 2009). One view distinguishes ‘liking’ (hedonic value or pleasure) from ‘wanting’ (goal-directed action), and learning about rewards (Berridge, 1996, Berridge and Robinson, 2003). The major task of the ‘liking’ system is to evaluate sensory input regarding its ability to evoke immediate pleasure or more extended well-being, while the major task of the ‘wanting’ system is selection of behavioral action that optimally serves current needs. The neural system encoding ‘liking’ is distributed across the neuraxis, including pathways of taste perception in the brainstem and pons, the nucleus accumbens, ventral pallidum, and prefrontal cortex (Grill and Norgren, 1978b, Pecina and Berridge, 2000, Kringelbach, 2004, Berridge and Kringelbach, 2008). The mesolimbic dopamine system with projections from the ventral tegmental area to the nucleus accumbens, frontal cortex, amygdala, and hippocampus has been implicated in both reinforcement and motivational (‘wanting’) processes of reward-seeking behavior (Mogenson et al., 1980, McFarland and Ettenberg, 1998, Cardinal et al., 2002, Everitt and Robbins, 2005, Wise, 2005, Berridge, 2007).
Opioid signaling, particularly through the mu-receptor, has long been known to be involved in the expression of reward behaviors (Morley et al., 1983, Cooper et al., 1985, Mucha and Iversen, 1986, Glass et al., 1999). The pioneering work of the group around the late Anne Kelley has demonstrated the powerful effects of mu-opioid stimulation of the nucleus accumbens on intake of palatable foods such as sucrose and high-fat diet in rats (Zhang et al., 1998, Will et al., 2003). Injection of the selective mu-opioid receptor agonist DAMGO not only elicited voracious ingestion of high-fat diet and sucrose solution (Zhang and Kelley, 1997, Will et al., 2003, Will et al., 2006), but also made rats work harder for a given food reward as assessed by the break point for progressive ratio lever pressing, often used as a measure of ‘wanting’ (Zhang et al., 2003). Furthermore, a small hedonic hot spot within the accumbens shell was identified where DAMGO amplified positive orofacial hedonic reactions (‘liking’) to the taste of sucrose (Pecina and Berridge, 2005). Thus, the nucleus accumbens, particularly its shell, is one area where encoding for both ‘liking’ and ‘wanting’ appears to be represented.
While these mainly agonist-based studies have provided important insights into the neural organization of food reward, they are not ideally suited to elucidate the role of endogenous opioid-signaling because they do not take into account ongoing signaling in the basal state, and blocking strategies seem more promising. The irreversible mu-opioid receptor antagonist β-funaltrexamine (BFNA) has proven to be particularly useful as it suppresses endogenous mu-opioid receptor signaling for several days (Takemori et al., 1981). Injection of BFNA into the cerebral ventricles leads to sustained suppression of food intake and body weight in rats (Cole et al., 1995, Cole et al., 1997) and injection into the nucleus accumbens reduces sucrose preference in rabbits (Ward and Simansky, 2006). Furthermore, we have previously shown that prolonged suppression with repeated injections of BFNA into the nucleus accumbens significantly reduced palatable food intake and slowed development of diet-induced obesity (Lenard et al., 2010).
Together, these studies suggest that mu-opioid signaling in the nucleus accumbens, particularly its shell, is necessary for the expression of reward-driven overconsumption of palatable foods, contributing to the development of dietary obesity, but it remains unclear which behavioral components of food reward behavior are involved. The aim of the present study was to examine specific components of food reward behavior both during and after mu-opioid receptor blockade and to test the hypothesis that chronic blockade of nucleus accumbens mu-opioid receptor signaling reduces palatable food intake by decreasing both hedonic evaluation (‘liking’) and motivation or incentive salience (‘wanting’).
Experimental Procedures
Animals
Male Sprague-Dawley rats weighing ~250g were purchased from Harlan Industries (Indianapolis, IN) and housed individually in wire-mesh cages at a constant temperature of 21–23°C with a 12-h light-dark cycle (light on at 06:00, off at 18:00). Animals were provided with regular chow ad libitum throughout the study unless specified otherwise. All protocols were approved by the Institutional Animal Care and Use Committee at the Pennington Biomedical Research Center in accordance with guidelines established by the National Institute of Health.
Brain cannulation, accumbens injections, and verification of injection sites
Rats were pretreated with atropine (1 mg/kg, i.p.) and anesthetized with ketamine-acepromazine-xylazine cocktail (80/1.6/5.4 mg/kg, s.c.). Cannulas (24 gauge) were aimed bilaterally at the nucleus accumbens shell (AP +1.5, ML ± 1.0, DV −5.3) and secured to the skull using dental cement and three stainless steel screws. Rats were allowed to recover from surgery for ten days, during which they were maintained on chow, but familiarized to the taste of sucrose and corn oil.
For intra-accumbens injections of mu-opioid receptor antagonist or vehicle, rats were briefly anesthetized with isoflurane and a volume of 0.5 µl was slowly injected over 2 min through an injector cannula protruding the guide cannula by 2 mm. The injector was left in place for another 2 min to prevent backflow and was then replaced by an obturator.
At the end of experiments, blue dye (0.5µl of 1% Chicago Blue) was injected through each cannula before euthanasia and perfusion. Brains were extracted and 30 µm-thick frontal sections were examined under the microscope. Injection sites were mapped on the nearest plates from the Paxinos and Watson rat stereotaxic atlas(Paxinos, 1986).
Behavioral testing
The taste reactivity test of Grill and Norgren (Grill and Norgren, 1978a) was used to quantify ‘liking’ (Berridge, 2000). A 200 µl volume of sucrose solution was placed on the transparent floor of a cylindrical test cage, and the rat’s orofacial expressions were videotaped from below. The number of characteristic tongue protrusions was assessed by inspection of slow-motion videos (Berridge, 2000), averaged over three consecutive bouts of ingestion and for 3 ascending concentrations of sucrose (0.01, 0.1, and 1.0 M) administered on separate days in random order. Positive hedonic orofacial responses included midline tongue protrusions, lateral tongue protrusions, and paw licking. Only a few rats, and only during first exposure, demonstrated one or two aversive responses, such as headshakes, gapes, and forelimb flails.
The incentive runway was used to measure ‘wanting’ (Pecina et al., 2003). The runway consisted of start and goal boxes connected with a 158 cm long running alley and a video camera mounted above. Rats were habituated to the runway during two daily 10 min sessions. During two additional sessions, overnight food deprived rats were enticed to eat a small food reward (Fruit Loop cereals, ~2g) in the goal box. Runway behavior was then assessed in the non food-deprived state in daily sessions of two consecutive trials over a period of 20 days. After placing the rats in the start box, the door was opened and the time to reach the goal box and start consuming the reward (completion time) was measured and transformed into completion speed, so as to reflect the level of performance (‘wanting’). In addition, the latency to leave the start box, the times spent walking/running forward, standing still (pauses), and moving backwards (reversals), as well as the net running speed were assessed by replaying the video recordings in slow motion. Video analysis was initially conducted by two independent observers and after establishing good agreement was continued by a single observer, blind to the stimulus condition.
The brief-access lick test (Davis MS-160, DiLog Instruments, Tallahassee, FL) was used to measure taste-guided reward behavior (Spector et al., 1996). Brief access (10 s) to the spout, allowing a limited number of licks for each concentration, minimizes modulation of reward behavior by postingestive learning. Rats were first adapted to the special cage and trained to lick from the spout filled with highly palatable chocolate Ensure under conditions of mild food and water deprivation. On test days, non-deprived animals were presented with increasing concentrations of either sucrose (0, 0.001µ1.5 M) or corn oil emulsions (0, 0.06–32%, in 1% Emplex emulsifier and distilled water). Each concentration was available for 10 s with 5 s intervals in two consecutive ascending series and the number of licks/10 s was averaged for each concentration.
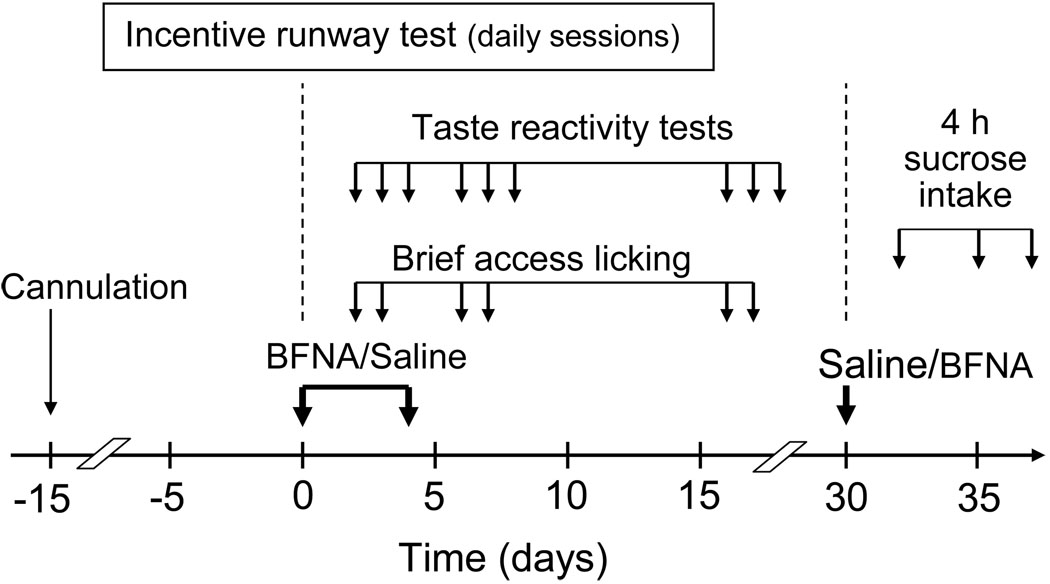
Experimental protocol (Fig. 1)
Fig. 1.
Experimental design. Note that rats initially treated with BFNA on days 0 and 4, received an injection of saline on day 30, while rats initially treated with saline received BFNA on day 30.
After recovery from cannula implantation, rats were matched for body weight and assigned to either bilateral BFNA (10nmol/0.5ul saline/DMSO; Tocris Cookson, Ellisville, MO) or vehicle injection as described above. The dose of BFNA was chosen based on the effectiveness to block DAMGO-induced food intake (Ragnauth et al., 2000) and suppress development of high-fat diet-induced obesity (Lenard et al., 2010). Six days before the first injection, rats begun habituation and pre-training in the incentive runway and daily sessions continued after the first injection. Two days after injection, when incentive runway performance was already suppressed by BFNA, taste reactivity tests and brief access lick tests were administered daily for 3 days. A second injection of BFNA or vehicle was administered 4 days after the first injection and lick tests repeated. Sixteen to eighteen days after the first injection, at a time when incentive runway performance had completely recovered, taste reactivity and brief access lick tests were repeated.
Finally, 30 days after the first injection, treatments were reversed as an additional test for reversibility of effects and non-specific damage to the injection sites. Rats that received saline in the first experimental period now received BFNA (10 nmol/0.5 ml) and rats that received BFNA first, now received saline. Body weight and 4-h 20% sucrose intake were measured before injections and 2, 5, and 7 days after injections.
Statistical analysis
The number of positive hedonic responses in the taste reactivity test and the number of licks in the brief-access test were analyzed by three-way ANOVA with BFNA or saline treatment as between subject factor; tastant concentration and time of testing as within subject factors. Since separate analysis of the two replications of each behavioral test revealed no significant differences, data were collapsed. Completion speed in the incentive runway test was tested by 3-way ANOVA with BFNA or saline treatment as between subject factor; order of run (1st vs. 2nd) and time of testing as within subject factors. All multiple comparisons were made with Bonferroni corrections. Sucrose intake was analyzed with one-way repeated measures ANOVA followed by Bonferroni-adjusted multiple comparisons. Running speed, as well as duration and incidence of latency to get out of the start box, pauses, and reversals were analyzed separately by student’s t-tests. Significant difference was set at p<0.05.
Results
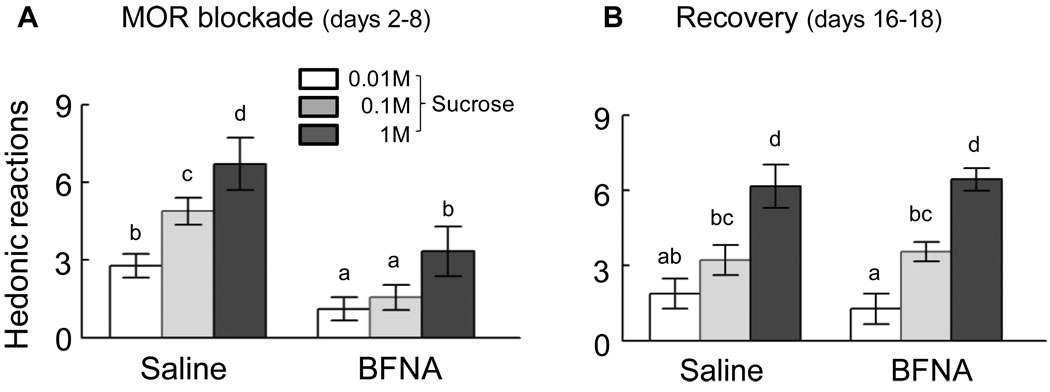
During the first few days after BFNA injections, the number of positive hedonic reactions to the taste of sucrose (‘liking’) was significantly reduced across all 3 concentrations compared with saline treatment (Fig. 2A). While saline-treated rats showed significant concentration-dependent increases in ‘liking’ (all p-values < 0.05), BFNA-treated rats exhibited very few positive hedonic reactions to the two lower concentrations that were not significantly different from each other and a 50% reduction (P < 0.01) of responding to the highest concentration. Sixteen to eighteen days after initial treatment, a significant concentration-response relationship (all p-values < 0.05) was reestablished in the BFNA treated rats, and there were no longer significant differences between BFNA and saline-treated rats for any sucrose concentration (Fig. 2B). The differential effect of treatment during and after mu-opioid receptor blockade was also indicated by a significant treatment x time interaction (F[1,10] = 33.7, p < 0.01). Furthermore, direct pairwaise comparisons of ‘liking’ scores during and after treatment showed that there were no significant changes in saline-treated rats for any sucrose concentration, but the two higher sucrose concentrations were significantly less ‘liked’ (0.1 M, p < 0.01 and 1 M, p < 0.02) during the blockade in BFNA-treated rats.
Fig. 2.
‘Liking’ as measured by the taste reactivity test. Rats were tested 2–8 days (active treatment, A) and 16–18 days (recovery, B) after the first injection of saline (n = 6) or BFNA (n = 6) into the nucleus accumbens shell. The number of positive orofacial hedonic reactions was counted after ingesting a small amount of sucrose solution (<200 µl) and averaged over 3 bouts for each concentration. ‘Liking’ of all three concentrations was significantly (* p < 0.05) reduced during active treatment with BFNA compared to saline, but was not different during the recovery from treatment.
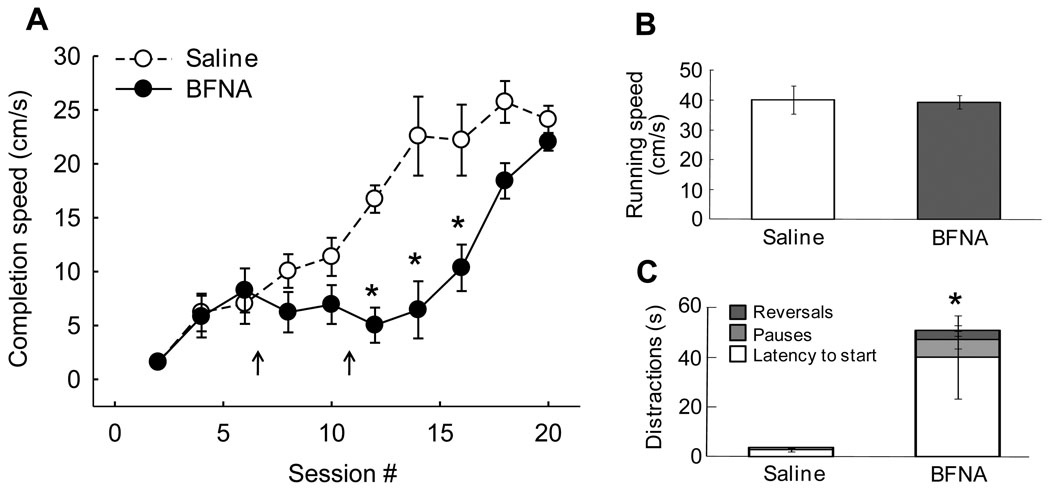
The motivation to obtain a food reward (‘wanting’) was assessed by measuring the evolvement of completion speed in the incentive runway over a period of 20 daily sessions. While saline-treated rats learned the task quite rapidly and reached asymptotic levels after about 15 sessions, BFNA-treated rats learned the task significantly slower (Fig. 3A). Although they initially learned just as fast as the controls, completion speed did not progress during the first 10 days after initiation of the BFNA-treatment and was significantly lower (all p-values < 0.05) from 5–10 days after the first injection. In the last 4 sessions, completion speed was no longer significantly different compared with saline controls.
Fig. 3.
Incentive runway performance as a measure of ‘wanting’. A: Nucleus accumbens BFNA-treatment significantly (* p < 0.05) reduced completion speed up to 8 days after the first BFNA injection. At 14 days after the first injection, completion speed was no longer different between saline and BFNA-treated rats. B: Net running speed was not different, indicating that BFNA-treatment did not affect motor performance per se. C: Distractions including latency to leave the start box, pauses along the runway, and reversals, were significantly (* p < 0.05) longer in duration after BFNA-treatment.
Three-way analysis of variance revealed significant effects of treatment (F[1,10] = 11.3, p < 0.01), session (F[9,90] = 30.8, p < 0.001), and order of trial within each daily session (F[1,10] = 47.4, p < 0.001). A significant day x treatment interaction (F[9,90] = 5.6, p < 0.001) showed that BFNA-treated rats learned slower than saline-treated rats. As expected, there was a highly significant effect of trial order, with completion speed for the second trial significantly faster than for the first trial (data not shown), suggesting that recent memory of being reinforced is an important factor determining runway performance. However, absence of a trial x treatment interaction (F[1,10] = 0.13, n.s.) showed that BFNA-treatment did not differentially influence this effect.
During the period of significantly reduced completion speed, the net running speed was not different between the groups (Fig. 3B), but BFNA-treated animals exhibited significantly more delays and distractions on their way to the goal box (Fig. 3C). Particularly the latency to leave the start box and the time spent pausing was significantly increased.
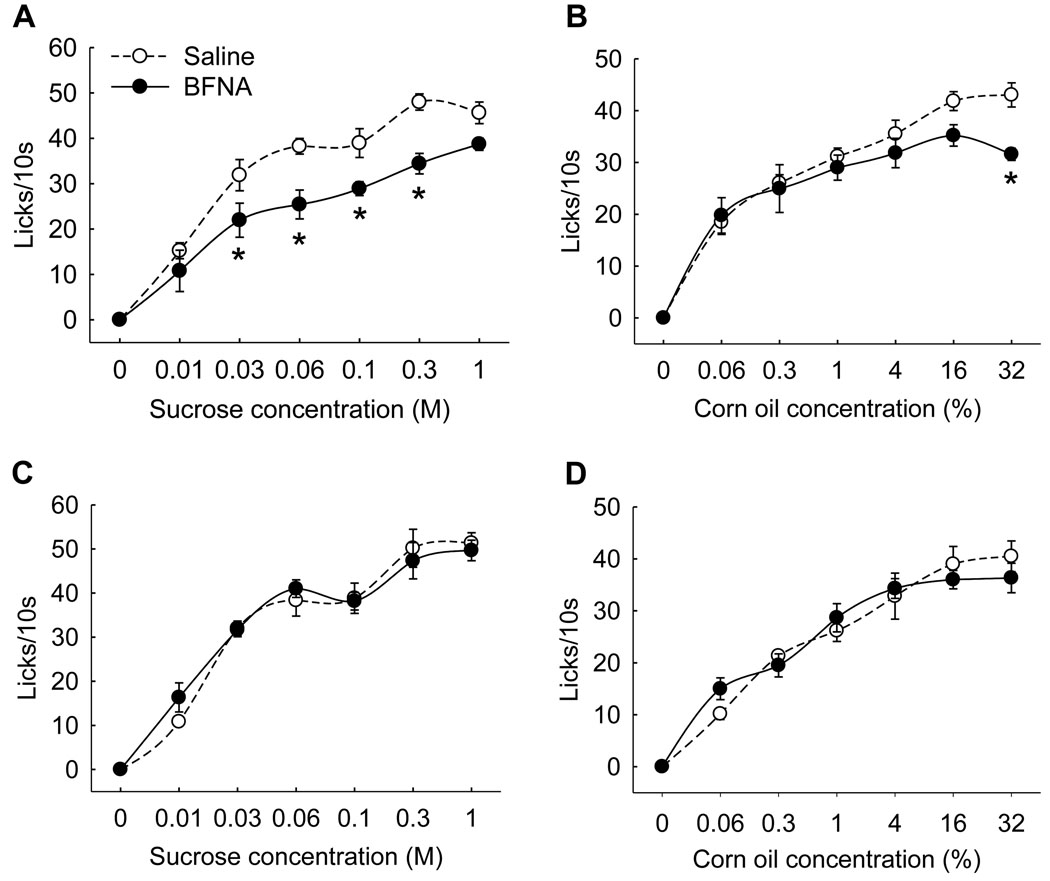
BFNA-treatment also resulted in significant reductions of the number of licks for medium concentrations of sucrose (Fig. 4A) and the highest concentration of corn oil (Fig. 4B). These differences had disappeared when brief access lick testing was repeated 20 days after the first treatment (Fig. 4C,D). Similarly, 4-h intake of 20% sucrose solution during the light period was not different between injection groups at this time (BFNA: 31.4 ± 2.5 ml; saline: 30.9 ± 1.7 ml, n.s.), and 24-h chow intake was also not different (22.8 ± 0.9 vs. 22.1 ± 1.0; n.s.), indicating that the injections did not produce permanent changes in palatable and non-palatable food intake.
Fig. 4.
Brief access lickometer responding as a measure of combined ‘liking’ and taste-guided ‘wanting’, 2–6 days (active treatment, A,B) and 16–18 days (recovery, C,D) after saline (n =6) or BFNA (n =6) treatment. Nucleus accumbens BFNA-treatment significantly (* p < 0.05) reduced responding for middle range of sucrose concentrations (A) and for the highest concentration of corn oil emulsion (B). Responding for both tastants was no longer different after recovery.
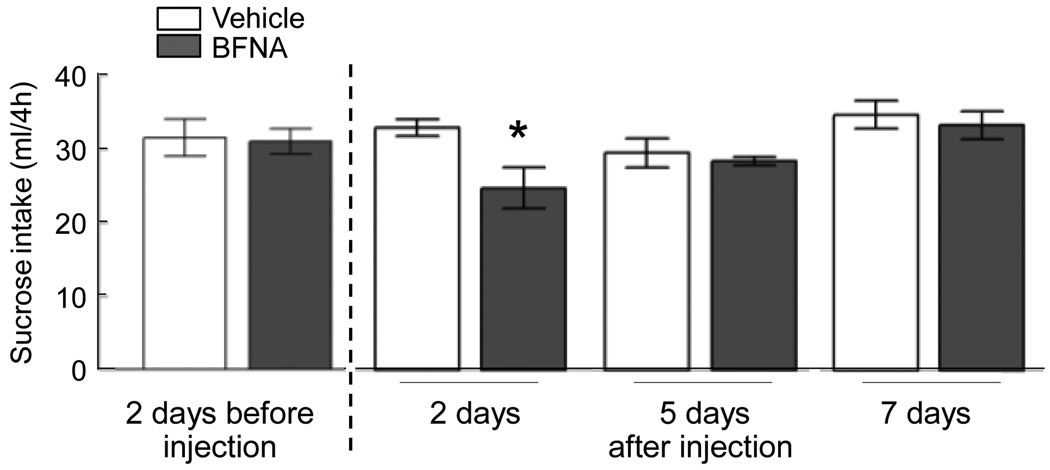
When we then injected BFNA and saline in these ”recovered” animals in reversed fashion (formerly saline-treated rats received BFNA and formerly BFNA-treated rats received saline), sucrose intake was significantly reduced by about 25%, 2 days after BFNA injection compared to saline injection (p < 0.05), an effect that was no longer observed 5 and 7 days after injections (Fig. 5).
Fig. 5.
Sucrose intake before and after nucleus accumbens administration of BFNA (closed bars, n = 6) or vehicle (open bars, n = 6). 20% sucrose solution was made available for 4 h during the light period in ad libitum chow fed rats. * p < 0.05.
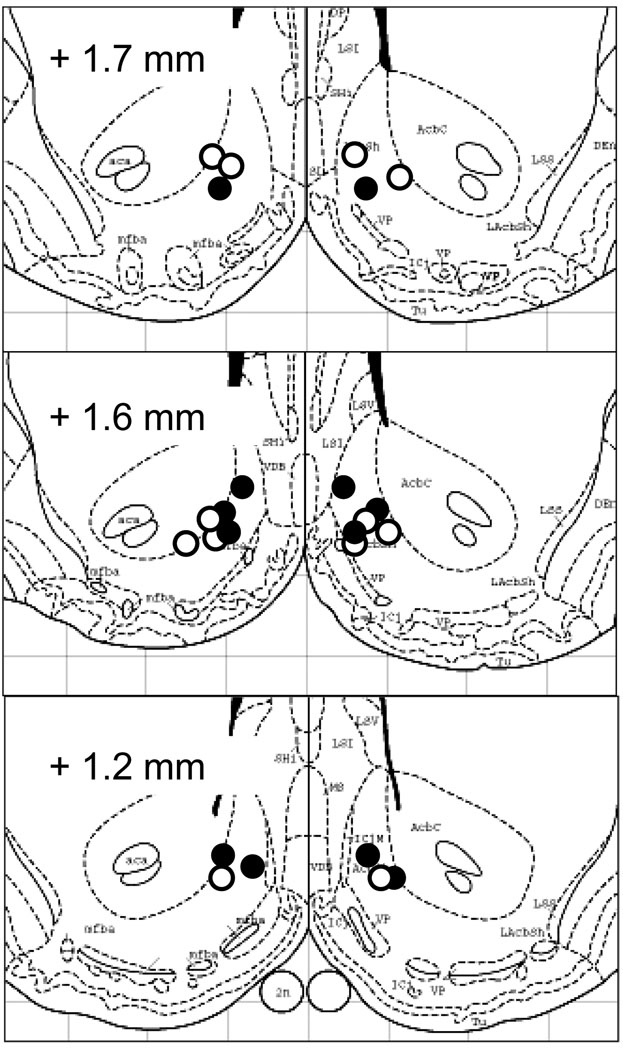
Finally, histological verification of the cannula tips showed that the injection sites were all located within the nucleus accumbens shell or at the border between shell and core (Fig. 6).
Fig. 6.
Histological verification of injection sites. Estimated locations of injector cannula tips for saline – treated (open circles) and BFNA-treated (closed circles) rats, based on recovery of blue dye injected at sacrifice. Sites are superimposed on successive plates 11, 12, and 13 of the Paxinos and Watson stereotaxic atlas (Paxinos and Watson, 1986).
Discussion
The nucleus accumbens has been strongly implicated in processing drug and food reward (Mogenson et al., 1980, Berridge, 1996, Schultz et al., 1997, Ikemoto and Panksepp, 1999, Carelli, 2002, Kelley and Berridge, 2002, Cardinal and Everitt, 2004, Everitt and Robbins, 2005, Wise, 2005, Salamone et al., 2009). Seminal work of the group around the late Anne Kelley has specifically demonstrated that opioid signaling in the nucleus accumbens is selectively driving intake of palatable food, while having little effect on intake of chow in rats (Zhang and Kelley, 1997, Zhang et al., 1998, Will et al., 2003, Zhang et al., 2003, Will et al., 2006). More recently, Berridge and colleagues identified a sub-area of the rat nucleus accumbens shell serving as hotspot for core ‘liking’, measured by the taste reactivity test (Pecina and Berridge, 2005). In addition, mesolimbic dopamine projections from the ventral tegmental area to the nucleus accumbens have been heavily implicated in motivational and reinforcement processes (‘wanting’) of food and other rewards (Schultz et al., 1997, Ikemoto and Panksepp, 1999, Wyvell and Berridge, 2000, Carelli, 2002, Zhang et al., 2003, Wise, 2005). Here we demonstrate transient suppression of both ‘liking’ and ‘wanting’ of palatable food rewards when mu-opioid receptor signaling in the nucleus accumbens shell is semi-chronically antagonized by the alkylating agent BFNA. These findings suggest that endogenous mu-opioid signaling in the nucleus accumbens shell is necessary for the full expression of ‘liking’ (hedonic value) and ‘wanting’ (incentive salience) when making a choice to ingest palatable foods (Pecina and Berridge, 2000). Using a complimentary approach with a long-lasting antagonist, our results fully confirm these earlier observations. Together with our other recent finding that even longer-lasting inhibition of mu-opioid signaling in this nucleus attenuates palatable food intake as well as body weight and body fat gain (Lenard et al., 2010), they suggest that mu-opioid modulation of ‘liking’ and ‘wanting’ in the nucleus accumbens contribute to dietary-induced hyperphagia and obesity.
There is a long history of literature demonstrating involvement of opioid signaling in the control of food intake, with systemically and centrally administered opioid agonists and antagonists, particularly those acting on the mu-opioid receptor, generally increasing and decreasing food intake, respectively (Martin et al., 1963, Holtzman, 1974, Mucha and Iversen, 1986, Kelley et al., 1996, Ragnauth et al., 2000, Statnick et al., 2003, Woolley et al., 2006, South et al., 2007). However, rather surprisingly, the mu-opioid receptor deficient mouse shows only a subtle, and sexually dimorphic, phenotype regarding food intake and body weight/adiposity. Hypophagia is not or only variably observed, but when challenged with palatable high-fat diets, knockout mice do resist development of obesity compared to wildtype controls (Tabarin et al., 2005, Zuberi et al., 2008). These findings suggest that mu-opioid receptor signaling, perhaps in peripheral organs, might also have suppressive effects on food intake and/or stimulatory effects on fat oxidation and energy expenditure. They also demonstrate the need for more localized manipulations of mu-opioid receptor signaling as we attempted here in the nucleus accumbens.
Previous research has shown that a single injection of BFNA into the nucleus accumbens shell in rabbits uncoupled the mu-opioid receptor from its G-protein and selectively decreased daily 4-h sucrose but not chow intake for up to 4 days (Ward et al., 2006), and less specific daily injections of BFNA into the ventricular space reduced food intake and body weight gain in Sprague-Dawley rats on a palatable diet for up to 11 days (Cole et al., 1995) and in obese Zucker rats on a chow diet for 7 days (Cole et al., 1997). In an extensive microinjection mapping study it was further shown that the mu-opioid agonist DAMGO, while robustly increasing eating behavior and food intake throughout the nucleus accumbens, increased positive orofacial hedonic reactions (‘liking’) only in a small portion of the nucleus accumbens, in the rostrodorsal region of the medial shell (Pecina and Berridge, 2005). Here, we show the reverse, that ‘liking’ of sucrose is reduced by mu-opioid antagonism in the shell, strongly suggesting that endogenous mu-opioid receptor signaling in this hedonic hot spot is necessary for rats to fully ‘like’ sucrose in the fed state. The relatively few animals used in our study do not allow us to do fine mapping of the effect, but it is clear that for all our injection sites, the 0.5 µl injection volume affected an area that includes the hot spot defined by Pecina and Berridge. This interpretation gains support by considering the long time course of our experiments, allowing the antagonist to diffuse further (Nicholson, 1985), compared with the acute DAMGO-stimulation studies.
This long term suppression of mu-opioid receptor function made it also possible to assess a potential effect on the other aspect of reward behavior, such as motivational (‘wanting’) and reinforcement processes (Berridge and Robinson, 2003, Ettenberg, 2009). Both currently utilized techniques to assess ‘wanting’ in rodents, food-reinforced incentive runway and lever press performance, require several days of training and/or testing that can only be covered by either chronic infusions of antagonist or the irreversible antagonist used here. Incentive runway completion speed, a measure of ‘wanting’ was significantly attenuated after BFNA-treatment. While it took saline-treated rats 14 sessions to plateau at maximal completion speed, it took BFNA-treated rats almost 20 sessions, and for the 8 sessions after the first injection, there was no increase at all in completion speed. That this was not simply due to potential side effects of the drug-treatment on motor ability is indicated by similar net running speeds for BFNA and saline-treated rats and by significantly increased “distraction” time after BFNA.
The runway paradigm has been used to dissociate primary motivation from secondary, reinforcement-induced effects. While running speed in the first trial is mainly determined by the motivation to obtain the palatable food reinforcer, running speed in the second trial is more determined by the impact of reinforcement on subsequent motivation (Ettenberg, 2009). BFNA-treatment reduced performance equally in the first and second daily trials, suggesting that it equally affected primary motivation and motivation boosted by recently consuming a reinforcer. However, it is possible that the reinforcing power of the fruit loops was reduced because ‘liking’ was simultaneously diminished. It would thus be interesting to test whether a stronger reinforcer such as a very sweet reward would change runway performance.
Performance in the incentive runway test depends on the ability to learn, and we cannot distinguish effects of BFNA-treatment on learning vs. ‘wanting’ in our study. This problem is inherent to all tests of reinforcement learning, including progressive ratio lever pressing. In future experiments, the drug could be administered after rats have learned the task, to eliminate memory formation and consolidation as a confounding factor. However, we would still not be able to distinguish processes related to memory recall from purely motivational processes.
The neurotransmitter most intimately associated with translating motivation into action (‘wanting’), is dopamine acting within the mesolimbic system particularly the nucleus accumbens (Mogenson et al., 1980, Wilson et al., 1995, Berridge and Robinson, 1998). Activation of dopamine receptors by amphetamine in the accumbens shell produced a marked increase in break point of progressive ratio lever pressing for food reward (Zhang et al., 2003), and enhanced incentive salience and cue-triggered ‘wanting’ for sucrose reward in the absence of enhanced ‘liking’ (Wyvell and Berridge, 2000, 2001). Furthermore, hyperdopaminergic mice showed increased motivation in the incentive runway without a change in sucrose ‘liking’ compared with wildtype mice (Pecina et al., 2003). Thus, Berridge and colleagues concluded that accumbens dopamine signaling is specifically involved in ‘wanting’, independent of ‘liking’ (Berridge, 2007). However, other observations with the runway paradigm suggest that rather than interfering with incentive and motivational processes, disruption of dopamine signaling primarily affects the reinforcing consequences of reward consumption and this might only secondarily affect motivation (McFarland and Ettenberg, 1998).
Mu-opioid signaling in the accumbens does not seem to be specific for ‘liking’. Stimulation of mu-opioid signaling by injection of DAMGO in the nucleus accumbens increases the break point of progressive ratio lever pressing for food (Zhang et al., 2003) and robustly increases sucrose or high-fat intake (Zhang and Kelley, 1997, Will et al., 2003, Will et al., 2006, Zheng et al., 2007), without necessarily changing its hedonic value (‘liking’) if not reaching the hotspot (Pecina and Berridge, 2005). Therefore, the present findings of simultaneous suppression of ‘liking’ and ‘wanting’ indicate that the site of action covers parts of the hedonic hot spot and the surrounding, ‘wanting’-related areas of the accumbens shell and core. A limitation of our chronic suppression paradigm is the possibility that decreased ‘wanting’ and/or reinforcement learning is secondary to decreased hedonic evaluation. To avoid this potential confound, additional experiments using more acute suppression of mu-opioid receptor signaling only during the first run of a session, but not during food reward consumption in preceding sessions will be necessary.
The combined effect of decreasing ‘liking’ and ‘wanting’ was also evident in the brief access lick test, with significant suppression of responses for at least some concentrations of sucrose and corn oil. Because this test is mainly taste-guided but also requires the animal to go for and consume the reward, it is best described as combining both components of reward. The relative decreases in lick responses where somewhat smaller than expected from a potential additive or even synergistic effect of combined ‘liking’ and ‘wanting’ on consumption. The discrepancy may be explained by differences in effort or cost/benefit ratio in the two paradigms (Salamone et al., 2009). The incentive runway requires considerably more effort to get to the goal box and consume the reward; the drinking spout is right there in the brief access lick test. In addition, it should be kept in mind that rats were not food deprived during any test, and hunger-induced enhancement of incentive motivation would likely amplify group differences in a test that requires more effort.
Although BFNA irreversibly eliminates functional mu-opioid receptors, all changes in ‘liking’ and ‘wanting’ observed were fully reversible after about 10 days, suggesting that newly formed mu-opioid receptors eventually take over function (Takemori et al., 1981, Ward et al., 1982, Arjune et al., 1990). Alternative explanations include increased recruitment of delta and kappa-opioid receptors (Takemori and Portoghese, 1987, Martin et al., 1995) and/or the formation of mu-delta receptor complexes (Rothman et al., 1988, Heyman et al., 1989).
Conclusion
Mu-opioid signaling has long been recognized as an important driver of palatable food intake , but given the wide distribution of this receptor in the brain and periphery, it has not been clear where the critical action is. From the observation that semi-chronic pharmacological blockade of mu-opioid receptors in the nucleus accumbens shell suppresses both ‘liking’ and ‘wanting’ of rewarding food stimuli, we conclude that endogenous signaling through this receptor at this location is necessary for the full hedonic experience and motivation to consume palatable foods, thus confirming earlier observations with short-acting agonists (Kelley et al., 1996; Pecina and Berridge, 2000). We cannot exclude the possibility that the effect on ‘wanting’ is secondary to decreased ‘liking’. Nevertheless, together with our recent demonstration that even longer suppression of mu-opioid receptors in the nucleus accumbens results in a significant attenuation of cumulative palatable food intake as well as body weight and body fat gain, the findings suggest that mu-opioid signaling in this brain area may be an important contributor to palatable diet-induced obesity.
Acknowledgments
We thank R. Leigh Townsend and Laurel M. Patterson for technical help. Supported by National Institutes of Health grants DK071082 and DK047348.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arjune D, Standifer KM, Pasternak GW, Bodnar RJ. Reduction by central beta-funaltrexamine of food intake in rats under freely-feeding, deprivation and glucoprivic conditions. Brain Res. 1990;535:101–109. doi: 10.1016/0006-8993(90)91828-5. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev. 1996;20:1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neurosci Biobehav Rev. 2000;24:173–198. doi: 10.1016/s0149-7634(99)00072-x. [DOI] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology (Berl) 2008;199:457–480. doi: 10.1007/s00213-008-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Everitt BJ. Neural and psychological mechanisms underlying appetitive learning: links to drug addiction. Curr Opin Neurobiol. 2004;14:156–162. doi: 10.1016/j.conb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Carelli RM. The nucleus accumbens and reward: neurophysiological investigations in behaving animals. Behav Cogn Neurosci Rev. 2002;1:281–296. doi: 10.1177/1534582302238338. [DOI] [PubMed] [Google Scholar]
- Cole JL, Berman N, Bodnar RJ. Evaluation of chronic opioid receptor antagonist effects upon weight and intake measures in lean and obese Zucker rats. Peptides. 1997;18:1201–1207. doi: 10.1016/s0196-9781(97)00074-0. [DOI] [PubMed] [Google Scholar]
- Cole JL, Leventhal L, Pasternak GW, Bowen WD, Bodnar RJ. Reductions in body weight following chronic central opioid receptor subtype antagonists during development of dietary obesity in rats. Brain Res. 1995;678:168–176. doi: 10.1016/0006-8993(95)00181-o. [DOI] [PubMed] [Google Scholar]
- Cooper SJ, Jackson A, Morgan R, Carter R. Evidence for opiate receptor involvement in the consumption of a high palatability diet in nondeprived rats. Neuropeptides. 1985;5:345–348. doi: 10.1016/0143-4179(85)90024-1. [DOI] [PubMed] [Google Scholar]
- Ettenberg A. The runway model of drug self-administration. Pharmacol Biochem Behav. 2009;91:271–277. doi: 10.1016/j.pbb.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Glass MJ, Billington CJ, Levine AS. Opioids and food intake: distributed functional neural pathways? Neuropeptides. 1999;33:360–368. doi: 10.1054/npep.1999.0050. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res. 1978a;143:263–279. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Norgren R. The taste reactivity test. II. Mimetic responses to gustatory stimuli in chronic thalamic and chronic decerebrate rats. Brain Res. 1978b;143:281–297. doi: 10.1016/0006-8993(78)90569-3. [DOI] [PubMed] [Google Scholar]
- Heyman JS, Jiang Q, Rothman RB, Mosberg HI, Porreca F. Modulation of mu-mediated antinociception by delta agonists: characterization with antagonists. Eur J Pharmacol. 1989;169:43–52. doi: 10.1016/0014-2999(89)90815-7. [DOI] [PubMed] [Google Scholar]
- Holtzman SG. Behavioral effects of separate and combined administration of naloxone and damphetamine. J Pharmacol Exp Ther. 1974;189:51–60. [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Brain Res Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Bless EP, Swanson CJ. Investigation of the effects of opiate antagonists infused into the nucleus accumbens on feeding and sucrose drinking in rats. J Pharmacol Exp Ther. 1996;278:1499–1507. [PubMed] [Google Scholar]
- Kringelbach ML. Food for thought: hedonic experience beyond homeostasis in the human brain. Neuroscience. 2004;126:807–819. doi: 10.1016/j.neuroscience.2004.04.035. [DOI] [PubMed] [Google Scholar]
- Lenard NR, Zheng H, Berthoud HR. Chronic suppression of mu-opioid receptor signaling in the nucleus accumbens attenuates development of diet-induced obesity in rats. Int J Obes (Lond) 2010 Jan 12; doi: 10.1038/ijo.2009.297. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TJ, Dworkin SI, Smith JE. Alkylation of mu opioid receptors by beta-funaltrexamine in vivo: comparison of the effects on in situ binding and heroin self-administration in rats. J Pharmacol Exp Ther. 1995;272:1135–1140. [PubMed] [Google Scholar]
- Martin WR, Wikler A, Eades CG, Pescor FT. Tolerance to and Physical Dependence on Morphine in Rats. Psychopharmacologia. 1963;4:247–260. doi: 10.1007/BF00408180. [DOI] [PubMed] [Google Scholar]
- McFarland K, Ettenberg A. Haloperidol does not affect motivational processes in an operant runway model of food-seeking behavior. Behav Neurosci. 1998;112:630–635. doi: 10.1037//0735-7044.112.3.630. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Morley JE, Levine AS, Yim GK, Lowy MT. Opioid modulation of appetite. Neurosci Biobehav Rev. 1983;7:281–305. doi: 10.1016/0149-7634(83)90020-9. [DOI] [PubMed] [Google Scholar]
- Mucha RF, Iversen SD. Increased food intake after opioid microinjections into nucleus accumbens and ventral tegmental area of rat. Brain Res. 1986;397:214–224. doi: 10.1016/0006-8993(86)90622-0. [DOI] [PubMed] [Google Scholar]
- Nicholson C. Diffusion from an injected volume of a substance in brain tissue with arbitrary volume fraction and tortuosity. Brain Res. 1985;333:325–329. doi: 10.1016/0006-8993(85)91586-0. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1986. [Google Scholar]
- Pecina S, Berridge KC. Opioid site in nucleus accumbens shell mediates eating and hedonic 'liking' for food: map based on microinjection Fos plumes. Brain Res. 2000;863:71–86. doi: 10.1016/s0006-8993(00)02102-8. [DOI] [PubMed] [Google Scholar]
- Pecina S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? J Neurosci. 2005;25:11777–11786. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecina S, Cagniard B, Berridge KC, Aldridge JW, Zhuang X. Hyperdopaminergic mutant mice have higher "wanting" but not "liking" for sweet rewards. J Neurosci. 2003;23:9395–9402. doi: 10.1523/JNEUROSCI.23-28-09395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragnauth A, Moroz M, Bodnar RJ. Multiple opioid receptors mediate feeding elicited by mu and delta opioid receptor subtype agonists in the nucleus accumbens shell in rats. Brain Res. 2000;876:76–87. doi: 10.1016/s0006-8993(00)02631-7. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Long JB, Bykov V, Jacobson AE, Rice KC, Holaday JW. beta-FNA binds irreversibly to the opiate receptor complex: in vivo and in vitro evidence. J Pharmacol Exp Ther. 1988;247:405–416. [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar AM, Nunes EJ, Pardo M. Dopamine, behavioral economics, and effort. Front Behav Neurosci. 2009;3:13. doi: 10.3389/neuro.08.013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- South T, Deng C, Huang XF. AM 251 and beta-Funaltrexamine reduce fat intake in a fat-preferring strain of mouse. Behav Brain Res. 2007;181:153–157. doi: 10.1016/j.bbr.2007.03.028. [DOI] [PubMed] [Google Scholar]
- Spector AC, Redman R, Garcea M. The consequences of gustatory nerve transection on taste-guided licking of sucrose and maltose in the rat. Behav Neurosci. 1996;110:1096–1109. [PubMed] [Google Scholar]
- Statnick MA, Tinsley FC, Eastwood BJ, Suter TM, Mitch CH, Heiman ML. Peptides that regulate food intake: antagonism of opioid receptors reduces body fat in obese rats by decreasing food intake and stimulating lipid utilization. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1399–R1408. doi: 10.1152/ajpregu.00632.2002. [DOI] [PubMed] [Google Scholar]
- Tabarin A, Diz-Chaves Y, Carmona Mdel C, Catargi B, Zorrilla EP, Roberts AJ, Coscina DV, Rousset S, Redonnet A, Parker GC, Inoue K, Ricquier D, Penicaud L, Kieffer BL, Koob GF. Resistance to diet-induced obesity in mu-opioid receptor-deficient mice: evidence for a "thrifty gene". Diabetes. 2005;54:3510–3516. doi: 10.2337/diabetes.54.12.3510. [DOI] [PubMed] [Google Scholar]
- Takemori AE, Larson DL, Portoghese PS. The irreversible narcotic antagonistic and reversible agonistic properties of the fumaramate methyl ester derivative of naltrexone. Eur J Pharmacol. 1981;70:445–451. doi: 10.1016/0014-2999(81)90355-1. [DOI] [PubMed] [Google Scholar]
- Takemori AE, Portoghese PS. Evidence for the interaction of morphine with kappa and delta opioid receptors to induce analgesia in beta-funaltrexamine-treated mice. J Pharmacol Exp Ther. 1987;243:91–94. [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci. 2008;363:3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8:555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- Ward HG, Nicklous DM, Aloyo VJ, Simansky KJ. Mu-opioid receptor cellular function in the nucleus accumbens is essential for hedonically driven eating. Eur J Neurosci. 2006;23:1605–1613. doi: 10.1111/j.1460-9568.2006.04674.x. [DOI] [PubMed] [Google Scholar]
- Ward HG, Simansky KJ. Chronic prevention of mu-opioid receptor (MOR) G-protein coupling in the pontine parabrachial nucleus persistently decreases consumption of standard but not palatable food. Psychopharmacology (Berl) 2006;187:435–446. doi: 10.1007/s00213-006-0463-7. [DOI] [PubMed] [Google Scholar]
- Ward SJ, Portoghese PS, Takemori AE. Improved assays for the assessment of kappa- and delta-properties of opioid ligands. Eur J Pharmacol. 1982;85:163–170. doi: 10.1016/0014-2999(82)90461-7. [DOI] [PubMed] [Google Scholar]
- Will MJ, Franzblau EB, Kelley AE. Nucleus accumbens mu-opioids regulate intake of a high-fat diet via activation of a distributed brain network. J Neurosci. 2003;23:2882–2888. doi: 10.1523/JNEUROSCI.23-07-02882.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will MJ, Pratt WE, Kelley AE. Pharmacological characterization of high-fat feeding induced by opioid stimulation of the ventral striatum. Physiol Behav. 2006;89:226–234. doi: 10.1016/j.physbeh.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Wilson C, Nomikos GG, Collu M, Fibiger HC. Dopaminergic correlates of motivated behavior: importance of drive. J Neurosci. 1995;15:5169–5178. doi: 10.1523/JNEUROSCI.15-07-05169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Forebrain substrates of reward and motivation. J Comp Neurol. 2005;493:115–121. doi: 10.1002/cne.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley JD, Lee BS, Fields HL. Nucleus accumbens opioids regulate flavor-based preferences in food consumption. Neuroscience. 2006;143:309–317. doi: 10.1016/j.neuroscience.2006.06.067. [DOI] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward "wanting" without enhanced "liking" or response reinforcement. J Neurosci. 2000;20:8122–8130. doi: 10.1523/JNEUROSCI.20-21-08122.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Incentive sensitization by previous amphetamine exposure: increased cue-triggered "wanting" for sucrose reward. J Neurosci. 2001;21:7831–7840. doi: 10.1523/JNEUROSCI.21-19-07831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Balmadrid C, Kelley AE. Nucleus accumbens opioid, GABaergic, and dopaminergic modulation of palatable food motivation: contrasting effects revealed by a progressive ratio study in the rat. Behav Neurosci. 2003;117:202–211. doi: 10.1037/0735-7044.117.2.202. [DOI] [PubMed] [Google Scholar]
- Zhang M, Gosnell BA, Kelley AE. Intake of high-fat food is selectively enhanced by mu opioid receptor stimulation within the nucleus accumbens. J Pharmacol Exp Ther. 1998;285:908–914. [PubMed] [Google Scholar]
- Zhang M, Kelley AE. Opiate agonists microinjected into the nucleus accumbens enhance sucrose drinking in rats. Psychopharmacology (Berl) 1997;132:350–360. doi: 10.1007/s002130050355. [DOI] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, Berthoud HR. Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci. 2007;27:11075–11082. doi: 10.1523/JNEUROSCI.3542-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuberi AR, Townsend L, Patterson L, Zheng H, Berthoud HR. Increased adiposity on normal diet, but decreased susceptibility to diet-induced obesity in mu-opioid receptor-deficient mice. Eur J Pharmacol. 2008;585:14–23. doi: 10.1016/j.ejphar.2008.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]