Abstract
The lack of simple, non-invasive tests for a sub-clinical decline in insulin production hampers detection of early-stage type 1 pre-diabetes. Pressure pain withdrawal threshold (PPT) is a sensitive index of insulinopenia in diabetic and ‘pre-diabetic’ rats, but its ability to detect human insulin insufficiency is not known; if predictive, PPT testing of those at risk for diabetes would be warranted. To address this question, we used meta-analyses to demonstrate (i) a similar relationship between blood glucose and insulin levels in humans and diabetic rats and (ii) the predictive value of PPT for insulinopenia in a composite group (n=53) of control, streptozotocin (STZ)-diabetic (STZ-HG), and normoglycemic (STZ-NG) rats. The frequency distributions of pooled insulin levels (ng/ml) consisted of three sub-populations, with peak values of < 0.5, 1.5 ± 0.05, and 3.2 ± 0.04. Using the 2.3rd percentile of the sub-population with the highest insulin level (2.81 ng/ml) as a cut-off to define insulinopenia, 40 animals (98% of STZ and 25% of controls) were identified with compromised insulin production. The frequency distribution of pooled PPT values also consisted of three sub-populations (peaks at 75.9 ± 0.6g, 97 ± 0.3g and 122 ± 0.8g), and when 106 g (the 2.3rd percentile of the most pressure-tolerant sub-population) was used as a cut-off, PPT measurements identified 92% of STZ-injected rats and 83% of rats with insulinopenia, as defined by 2.81 ng/ml insulin cut-off. Assuming similar between-species pain mechanisms, these findings support the potential usefulness of PPT measurements for detection of early-stage human type 1 diabetes.
Keywords: pre-diabetes, type 1 diabetes, pain, pressure hyperalgesia, insulin
Introduction
Onset of type 1 diabetes (T1DM) is preceded by several years of sub-clinical progression of the disease, a stage called pre-diabetes, but existing tools for the detection of early stages of T1DM, i.e., assays for insulin and islet cell antibodies, are, unfortunately, too costly to be employed in large-scale screening studies. Our studies using the streptozotocin (STZ; β-cell toxin) rat model of T1DM appear to have direct relevance to the clinical problem of identification of subjects with pre-diabetes, by emphasizing two important issues [1-3]. First in rats, like in humans, hyperglycemia does not occur until insulin production falls to less than 25% of normal. Second, measurements of pain on pressure withdrawal thresholds (PPT) in rats appear to be a substantially more sensitive indicator of insulinopenia than either fasting or random glucose measurements. Because these previous observations were made using relatively limited datasets, the present study used meta-analysis to (i) compare blood-insulin profiles in diabetic patients and diabetic rats and (ii) re-analyze insulin – glucose – PPT relationships after combining data from our previous and more recent, unpublished experiments. The aim of present analysis was to evaluate whether a simple non-invasive test, the pain pressure threshold assay, has prognostic value and reliably identifies subjects whose putative insulin levels need to be tested to verify their status as pre-diabetic.
Materials and Methods
Human data
The PUBMED search was conducted for the abstracts containing combination of “prospective / longitudinal / follow up”, “study / studies”, “type 1 / insulin- dependent diabetes”, “first degree relatives / siblings” and “FPIR / first phase insulin response” words or phrases. Follow-up studies with at least one FPIR measurement made before the diagnosis of clinical diabetes were selected for further analysis. The back-bone study used in our work is that by Vardi and co-authors [4] containing tabulated results of up to 30 per subject first phase insulin response and fasting glucose measurements made in the study of 35 non-diabetic and non-obese first degree relatives of T1DM patients, 18 of which had developed T!DM during average 43 months of follow up. The data collected in this latter group of subjects were ranked and averaged accordingly to time before onset of diabetes or FPIR and presented as circles in the Figures 1A and B, respectively. The data from other sources were added to the Figure 1A as they published (triangles)
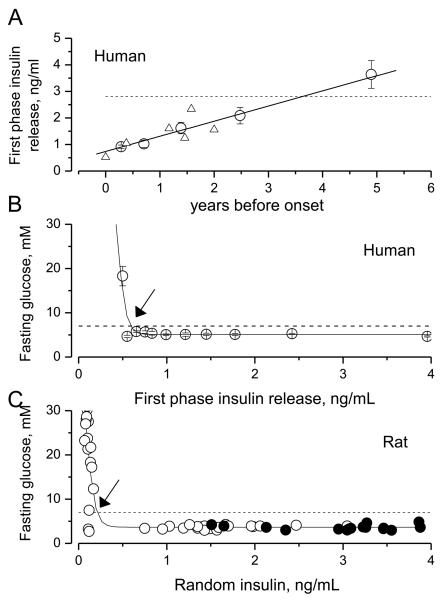
Figure 1.
Decline in first phase insulin release (FPIR) before the onset of human type 1 diabetes (A) and relationships between FPIR (humans; B), random insulin (rat; C) and fasting glucose (FG).
(A) FPIR starts to decline long time before the onset of human type 1 disease (zero of X-axis). Circles represent FPIR data from patients who progressed to overt diabetes during an average of 3.6 years of the follow-up studies of first-degree relatives of subjects with T1DM (ranked and averaged data from Table 2 in [4]). The dashed line is a 10th percentile (2.8 ng/ml) of FPIR of normal non-obese individuals with no family history of diabetes from the study above. Triangles are results from similar studies in humans [5-8]. The solid line is the linear regression model fit to the data shown by circles.
(B) In humans, fasting glucose crosses the threshold for diabetes (7 mM, dashed line) within 6 months prior to onset of diabetes, when FPIR declines to about 0.5 ng/ml (arrow). Circles represent re-arranged and re-calculated data from Table 2 in [4]. Solid line is drawn by eye.
(C) In rats with STZ-induced pancreatic injury (open circles), fasting glucose exceeds the threshold for diabetes (7 mM, dashed line and arrow) in only those animals in which random plasma insulin was decreased below 10% of average insulin level of control rats (~3.5 ng/ml, filled circles). Data are from the current study. Each symbol represents individual animal. Solid curve is drawn by eye.
Animal data presented here are data obtained between 2004 and 2009 ([1;2] and unpublished) and selected under conditions that all PPT, glucose and insulin were measured for given animal within 24 hours of experiment. All experiments were conducted in accord with National Institute of Health Guide for the Care and Use of Laboratory Animals and protocols were approved by UAMS Animal Use Committee.
Male Sprague-Dawley rats (200 – 350 g., Harlan Inc., Indianapolis, IN) were used in all experiments. After one week of acclimation to the animal facilities and behavioral test environment, rats were randomly assigned to control and experimental groups (vehicle and STZ, respectively). STZ was dissolved in a citrate buffer (pH = 4.5) immediately before injection and given intraperitoneally (65 mg/kg) to rats fed ad libitum. On day 3 after injection of STZ random blood glucose was measured and rats that developed hyperglycemia (> 11 mM) or remained normoglycemic were designated as STZ-HG and STZ-NG animals, respectively. During the 2 – 4 week long experiment, PPT values were determined at regular, 2-3 days intervals. Random and/or fasting glucose levels were measured at 3-7 day intervals using tail blood samples obtained by a pin-prick technique and colorimetric Accu-Chek blood glucose monitoring system (Roche Diagnostics Corporation, Indianapolis).
In behavioral tests, dorsal hind-limb paw pressure pain withdrawal thresholds (PPT) were determined using Randal-Selitto analgesia-meter and a standard for our laboratory technique [1;2]. Briefly, 10 determinations of PPT per animal (5 per each hind limb with an interval between sequential measurements greater than 10 minutes) were collected in each test session, filtered using mean +/- SD cut-off, averaged for both limbs and expressed in grams. Threshold force of linearly increasing pressure (16 g/s) was defined as a force that induces the first physical attempt of the animal to escape the stimulus. To avoid tissue injury the cut-off force was set to 250 g of pressure. In terminal experiments, blood was collected by a ventricular puncture technique, and plasma separated by centrifugation (5000g, 5 min) and stored at −20°C until insulin was measured. Insulin was determined using Ultra Sensitive Rat Insulin ELISA Kit (Crystal Chem Inc., Downers Grove, IL) following the manufacturer's protocol.
Best fit analysis of frequency distributions was conducted using a Levenberg-Marguardt algorithm of χ2 minimization (Origin, Microcal, Northampton, MA). During the fitting procedure all independent parameters of the fitted Gaussian functions (area, mean and standard deviation) were allowed to vary.
Results
Figures 1A and 1B present the results of our meta-analysis of data derived from several independent publications of studies by others [4-8]). Analysis of follow-up studies carried out in first-degree relatives of patients with T1DM reveals that insulin production declines without noticeable abnormalities in glucose metabolism for at least a 3 year-long period prior to onset of hyperglycemia (Fig. 1A). Hyperglycemia only becomes manifest after the first phase insulin release (FPIR) falls below about 0.6 ng/mL (Fig. 1B). Notably, the fasting glucose and 2 h glucose levels obtained in oral glucose tolerance tests only cross the thresholds (i.e., 5.6 mM for fasting glucose and 7.8 mM for 2h oral glucose tolerance test; [9]) recommended for diagnosis of pre- diabetes by the American Diabetes Association (ADA) about 6 months before the onset of overt disease [10]. Thus, ‘silent’ and progressive decrements in insulin release are not readily detectable by glucose concentration or clearance assays until disturbances in glucose metabolism become more robust, onset of diabetes is imminent, and treatment protocols require insulin replacement as obligatory step.
When fasting glucose and plasma insulin concentrations were determined in the rats (21 control and 51 STZ rats, open and filled circles, respectively), the pattern of insulin dependence on manifestation of hyperglycemia appears closely resembling that observed in humans (compare Fig. 1B and C). The threshold insulin level is somewhat lower in the rat (Fig. 1C), and this difference could, at least in part, be attributed to the differences of the first response and random insulin values (human and rat experiments, respectively). Regardless of this difference, blood glucose concentration in both humans and rats crosses the level considered to be diagnostic of T1DM (7 mM for fasting glucose [FG], dashed lines in Fig. 1B and C) only after insulin production fells below 25% of normal. Our data suggest that same is also true for the insulin – random glucose level relationships in rats because random hyperglycemia (resting glucose level > 11 mM) was never detected in rats in which plasma insulin exceeded 0.5 ng/ml (13 control and 30 STZ rats; data not shown). Thus, both in humans and rats, measurement of blood glucose concentration is a poor indicator of the state of insulin production.
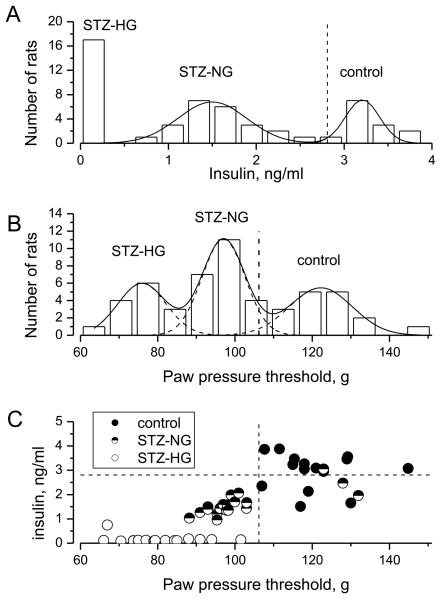
To evaluate the predictive value of PPT measurements for insulinopenia, we next selected and pooled together our data from previous and unpublished experiments in which PPT and plasma insulin were determined within the 24 hour period between tests (53 rats; Fig. 2). First, the frequency distribution of pooled insulin levels was evaluated and found to consist of three sub-populations; the first group (insulin < 0.5 ng/ml) encompassed all 17 STZ-HG rats, whereas the second and third groups (mean insulin 1.5±0.05 ng/ml and 3.2 ± 0.04 ng/ml, respectively) comprised the STZ-NG and control rats (Fig. 2A). The majority, 75% of control rats appeared belonging to third sub-population peaked at 3.2 ng/ml, therefore the 2.3rd percentile of this sub-population (2.81 ng/ml; Fig. 2A, vertical dashed line) was defined as a cut-off separating “normal” and “abnormal” random insulin levels. In accord with this definition, all but one (95%) STZ-NG rats and four (25%) of control rats could be considered as having suppressed insulin production or categorized as pre-diabetic.
Figure 2.
Frequency distributions of random blood plasma insulin (A), PPT (B) and relationship between PPT and insulin values measured in control and STZ-injected rats (total 53 animals).
(A) Frequency distribution of insulin values measured in the pooled population of STZ and control rats and fit with two Gaussian distributions model (solid curves). Data on insulin levels in STZ-HG, diabetic rats (left-most column) were not included during the fit procedure. The vertical dashed line denotes 2.3rd percentile for the right-most distribution, which was used as a cut-off value to define abnormal, reduced insulin production.
(B) Frequency distribution of PPT values measured in pooled population of STZ and control rats and fit with the sum of three Gaussian curves (solid and dashed curves). The vertical dashed line represents the 2.3rd percentile for the right-most distribution, which was used as a cut-off value to separate rats with a decreased tolerance in the paw-pressure test from those with normal tolerance.
(C) PPT and insulin values measured in control, STZ-NG and STZ-HG rats (closed, half-filled and opened circles, respectively; each symbol represents an individual animal). Horizontal and vertical dashed lines indicate respectively insulin and PPT cut-off values, defined as above.
Neither fasting (3.7 mM) nor random (6.6 mM) mean glucose levels differed between the STZ-NG and control groups. Therefore, the frequency distribution of PPT was next analyzed (Fig. 2B). As above, the best-fit procedure identified three Gaussian sub-populations (peaks at 75.9 ± 0.6g, 97 ± 0.3g and 122 ± 0.8g), with a cut-off PPT value of 106 g (2.3rd percentile of sub-population of most pressure-tolerant animals) that separated normal and potentially metabolically-compromised animals (Fig. 2B, vertical dashed line). Using this value, PPT measurements identified 100% of STZ-HG and 83% of animals with insulin levels below 2.81 ng/ml cut-off (Fig. 2C).
Discussion
The major finding of this work is that in rats PPT constitutes very sensitive but indirect measure of the level of circulating insulin. This simple and non-invasive test does not substitute for the need for the hormone level measurements, but with about 80% certainty and zero of false positive events, it allows identification of subjects (rats) in which tests for insulin are highly recommended. Importantly, PPT identified 16 of 20 STZ-NG rats studied, which otherwise, and unlike STZ-HG rats, can not be distinguished from age-matched naïve control rats by appearance, behavior, weight, plasma glucose level, or glucose tolerance [1;2]. These observations raise two critical questions: Are mechanisms linking insulin and sensitivity to deep pressure similar between different species, and do PPT measurements in humans have a similar predictive of insulinopenia value as observed in rats?
Overt hyperglycemia develops after insulin production fells below 25% of normal in both humans and in rats (Fig. 1B and C). In rats, PPT starts to decline at substantially milder degree of insulinopenia (~2 ng/ml vs. ~0.5 ng/ml of random plasma insulin; Fig. 2C). Exaggerated pain on pressure is mostly a manifestation of abnormal activity of non-myelinated and thinly myelinated muscle nociceptor pathways [11], but it is not clear what determines high sensitivity of these nerve pathways to the level of circulating insulin compared to the much lower sensitivity of systemic glucose homeostasis. Insulin might control function of muscle nociceptors directly acting via nervous system cognate insulin receptors (IR) or hybrid, insulin-insulin-like growth factor receptors (IR-IGFR), or indirectly by promoting muscle nutritive blood flow and oxygen supply. Differences in densities of IR and IR-IGFR, having high and low affinities to insulin, respectively, and differences in the strength of intrinsic anti-oxidant defenses may dictate level of vulnerability of specific tissues and mechanisms to insulinopenia. Importantly, poorly-compensated systemic insulin resistance should have essentially the same effect on PPT as insulinopenia (reviewed in [3]). Thus, pain on pressure may be a warning sign of T1DM as well as T2DM, and our measurements of PPT in Zucker rat models of T2diabetes and pre-diabetes support this suggestion [12]. Since basic mechanisms of metabolic control by insulin are similar between vertebrate species, it is likely that close association between the level of insulin signaling and pressure tolerance observed in rats will also be discovered in humans. With this respect however, one of the major concerns is that STZ-NG rat is a model of induced acute insulinopenia and because of the short life span even the rodent models of spontaneous diabetes/pre-diabetes (such as NOD mice) are limited in mimicking slowly progressing human disease.
In people with diabetes and painful neuropathy, deep muscle pain and pain on pressure constitute common symptoms that are experienced by 70-85% of patients [13;14]. Furthermore, prevalence of fibromyalgia in the general population of people with diabetes exceeds that in healthy control adults by a factor of 6 - 10 [15]. As the pain from deep somatic structures is a generally a frequent human clinical complaint, the techniques for assessment of such pain are well developed [12;16]. In particular, pressure algometry is frequently used for measurement of pressure hyperalgesia in people with fibromyalgia [15-17] and reference threshold data and data on intra- and inter-individual variability of PPT measurements in fingers and toes of control human subjects are available [18]. PPT has not, however, been consistently measured in diabetic subjects, and it has never been evaluated in people with pre-diabetes and in insulinopenic or insulin resistant first degree relatives of diabetic patients. Therefore, the actual relationships between a decline in peripheral action of insulin (whether it results from insulinopenia or insulin resistance) and skeleton-muscular pain in humans remain to be established in studies that are not complicated by effects of hyperglycemia, dyslipidemia, and neurodegenerative process. Such studies are also the only way to determine whether PPT measurements may add to the limited arsenal of existing tools allowing detection of human diabetes at its earliest stages of progression.
Acknowledgements
This work was supported by NIH NIDDK (grant DK067284) and by UAMS COM funds. We thank Dr. Gerald A. Dienel (UAMS, Department of Neurology) for helpful insights and discussions regarding this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Romanovsky D, Hastings SL, Stimers JR, Dobretsov M. Relevance of hyperglycemia to early mechanical hyperalgesia in streptozotocin-induced diabetes. J. Peripher. Nerv. Syst. 2004;9:62–69. doi: 10.1111/j.1085-9489.2004.009204.x. [DOI] [PubMed] [Google Scholar]
- 2.Romanovsky D, Cruz NF, Dienel GA, Dobretsov M. Mechanical hyperalgesia correlates with insulin deficiency in normoglycemic streptozotocin-treated rats. Neurobiol. Dis. 2006;24:384–394. doi: 10.1016/j.nbd.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Dobretsov M, Romanovsky D, Smith AG, Stimers JR. Diabetic neuropathy and pain. In: Dobretsov M, Zhang J-M, editors. Mechanisms of pain in peripheral neuropathy Research Signpost. Kerala, India: 2009. pp. 255–294. [Google Scholar]
- 4.Vardi P, Crisa L, Jackson RA. Predictive value of intravenous glucose tolerance test insulin secretion less than or greater than the first percentile in islet cell antibody positive relatives of type 1 (insulin-dependent) diabetic patients. Diabetologia. 1991;34:93–102. doi: 10.1007/BF00500379. [DOI] [PubMed] [Google Scholar]
- 5.Chase HP, Garg SK, Butler-Simon N, Klingensmith G, Norris L, Ruskey CT, O'Brien D. Prediction of the course of pre-type I diabetes. J. Pediatr. 1991;118:838–841. doi: 10.1016/s0022-3476(05)82192-4. [DOI] [PubMed] [Google Scholar]
- 6.Larger E, Rakotoambinina B, Eddouks M, Timsit J, Boitard C, Assan R, Burcelin R, Robert JJ. Normal insulin sensitivity during the late preclinical stage of type 1 diabetes. Diabetes Care. 2004;27:1842–1843. doi: 10.2337/diacare.27.7.1842. [DOI] [PubMed] [Google Scholar]
- 7.Mrena S, Savola K, Kulmala P, Akerblom HK, Knip M. Staging of preclinical type 1 diabetes in siblings of affected children. Childhood Diabetes in Finland Study Group. Pediatrics. 1999;104:925–930. doi: 10.1542/peds.104.4.925. [DOI] [PubMed] [Google Scholar]
- 8.Roder ME, Knip M, Hartling SG, Karjalainen J, Akerblom HK, Binder C. Disproportionately elevated proinsulin levels precede the onset of insulin- dependent diabetes mellitus in siblings with low first phase insulin responses. The Childhood Diabetes in Finland Study Group. J. Clin. Endocrinol. Metab. 1994;79:1570–1575. doi: 10.1210/jcem.79.6.7989457. [DOI] [PubMed] [Google Scholar]
- 9.American Diabetes Association Standards of medical care in diabetes--2010. Diabetes Care. 2010;33(Suppl 1):S11–S61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sosenko JM, Palmer JP, Greenbaum CJ, Mahon J, Cowie C, Krischer JP, Chase HP, White NH, Buckingham B, Herold KC, Cuthbertson D, Skyler JS. Patterns of metabolic progression to type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care. 2006;29:643–649. doi: 10.2337/diacare.29.03.06.dc05-1006. [DOI] [PubMed] [Google Scholar]
- 11.Simone DA, Marchettini P, Caputi G, Ochoa JL. Identification of muscle afferents subserving sensation of deep pain in humans. J. Neurophysiol. 1994;72:883–889. doi: 10.1152/jn.1994.72.2.883. [DOI] [PubMed] [Google Scholar]
- 12.Romanovsky D, Walker JC, Dobretsov M. Pressure pain precedes development of type 2 disease in Zucker rat model of diabetes. Neurosci. Lett. 2008;445:220–223. doi: 10.1016/j.neulet.2008.08.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Otto M, Bak S, Bach FW, Jensen TS, Sindrup SH. Pain phenomena and possible mechanisms in patients with painful polyneuropathy. Pain. 2003;101:187–192. doi: 10.1016/s0304-3959(02)00313-5. [DOI] [PubMed] [Google Scholar]
- 14.Pfeifer MA, Ross DR, Schrage JP, Gelber DA, Schumer MP, Crain GM, Markwell SJ, Jung S. A highly successful and novel model for treatment of chronic painful diabetic peripheral neuropathy. Diabetes Care. 1993;16:1103–1115. doi: 10.2337/diacare.16.8.1103. [DOI] [PubMed] [Google Scholar]
- 15.Tishler M, Smorodin T, Vazina-Amit M, Ramot Y, Koffler M, Fishel B. Fibromyalgia in diabetes mellitus. Rheumatol. Int. 2003;23:171–173. doi: 10.1007/s00296-002-0279-7. [DOI] [PubMed] [Google Scholar]
- 16.Graven-Nielsen T, Arendt-Nielsen L. Induction and assessment of muscle pain, referred pain, and muscular hyperalgesia. Curr. Pain Headache Rep. 2003;7:443–451. doi: 10.1007/s11916-003-0060-y. [DOI] [PubMed] [Google Scholar]
- 17.Jespersen A, Dreyer L, Kendall S, Graven-Nielsen T, Arendt-Nielsen L, Bliddal H, Danneskiold-Samsoe B. Computerized cuff pressure algometry: A new method to assess deep-tissue hypersensitivity in fibromyalgia. Pain. 2007;131:57–62. doi: 10.1016/j.pain.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Brennum J, Kjeldsen M, Jensen K, Jensen TS. Measurements of human pressure-pain thresholds on fingers and toes. Pain. 1989;38:211–217. doi: 10.1016/0304-3959(89)90240-6. [DOI] [PubMed] [Google Scholar]