Sir, Pseudoxanthoma elasticum (PXE) is a multi-system disorder characterized by ectopic mineralization of extracellular matrix of connective tissues (For review, see ref 1). The disease primarily affects the skin, the eyes, and the cardiovascular system, with considerable mortality and occasional morbidity. The characteristic skin manifestations, i.e., yellowish papules, which tend to coalesce into larger inelastic plaques on the predilection sites, are primarily of cosmetic concern, but they imply the risk for development of serious ocular and vascular complications.
PXE is caused by mutations in the ABCC6 gene which encodes transmembrane transporter expressed primarily in the liver and kidneys2. The precise function of this transporter under physiologic conditions and its ligand molecules in vivo are currently unknown. It has been suggested, however, largely based on animal studies utilizing an Abcc6 “knock-out” mouse as a model system, that PXE is a metabolic disorder which in the absence of functional ABCC6 transporter activity results in deficiency of anti-mineralization factors in the circulation3. This situation then allows progressive mineralization of the peripheral connective tissues to ensue.
Well over 300 mutant ABCC6 alleles have been identified in families with PXE, and mutation analysis has established that PXE is an autosomal recessive disorder4,5. The utility of mutation detection in PXE is emphasized by the fact that the onset of the disease is delayed, and definitive diagnosis in patients with PXE is often not established until late teens or twenties, or even later in life. In families with history of an affected individual with known ABCC6 mutations, genotyping can be used to determine if other members of the family are at risk for developing PXE by presymptomatic DNA testing. In this brief report, we illustrate this utility of mutation analysis in a family with history of PXE.
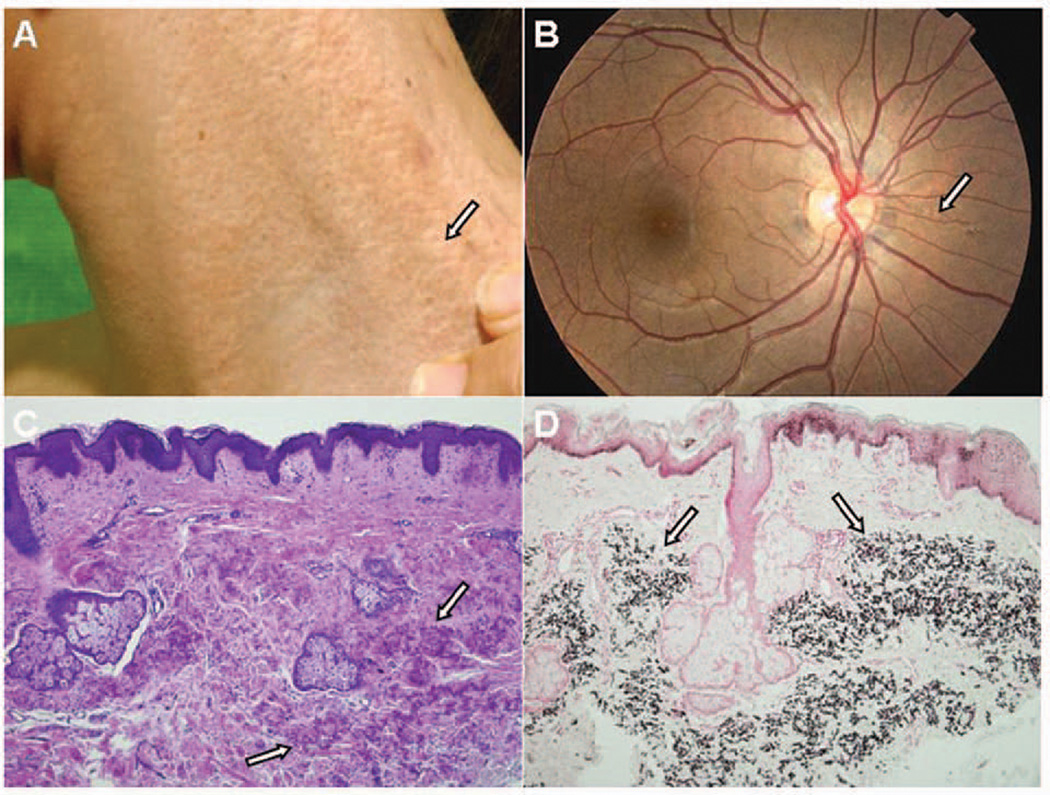
The proband, a 19-year old female, had been diagnosed for PXE on the basis of characteristic cutaneous finding and the presence of angioid streaks at the age of 14 years (Fig. 1A, B). Skin biopsy revealed accumulation of mineralized elastotic material in the mid-dermis by histopathologic examination using Hematoxylin & Eosin and von Kossa stains (Fig. 1C, D). The proband’s parents did not have any evidence of PXE, consistent with autosomal recessive inheritance of this disease (Fig. 2A). The proband had an older sister who refused to participate in this study, and a younger, clinically unaffected brother of 13 years of age, who was concerned of the potential of developing PXE later in his life.
Figure 1.
Diagnosis of PXE in the proband of the family. Note the characteristic cutaneous lesions on the side of the neck (A) and angioid streaks in the eye (B) (arrow). Histopathology reveals accumulation of mineralized elastotic material in mid-dermis when examined by Hematoxylin and Eosin (C) or von Kossa (D) stains (arrows).
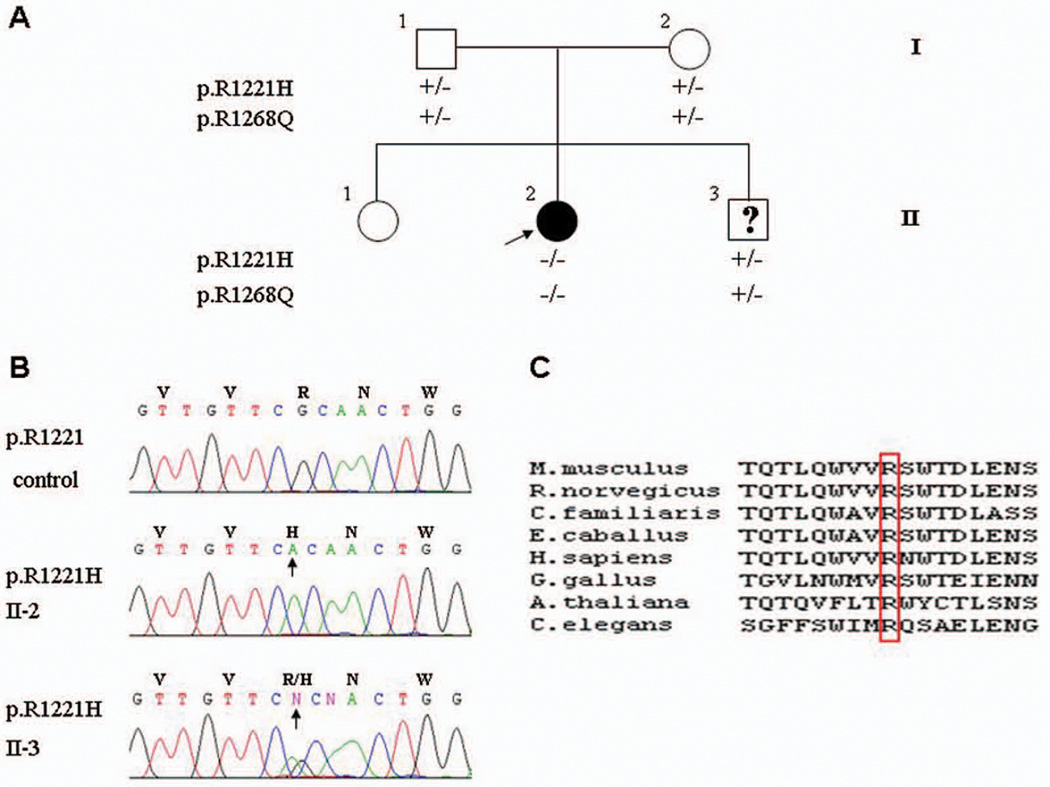
Figure 2.
Mutation analysis of the ABCC6 gene in the family. (A) The proband (arrow; II-2) has a clinically unaffected older sister and a younger brother, and the parents (I-1, I-2) show no clinical signs of PXE. (B) Sequencing of the proband’s ABCC6 gene (II-2) revealed that the arginine in position 1221 in control DNA is replaced by homozygous substitution by histidine (p.R1221H) reflecting G-to-A nucleotide substitution (arrow) and confirming the diagnosis of PXE. The 13-year old younger brother (II-3) is a heterozygous carrier of the p.R1221H substitution and is consequently predicted not to develop PXE, similar to his parents. The mutation p.R1221H co-segregates in the family with an allelic polymorphism, p.R1268Q (A). (C) Note the evolutionary conservation of arginine 1221 in the ABCC6 gene in different species. The alignment was performed with ClustalW2 Program (www.ebi.ac.uk/Tools/clustalW2).
After appropriate counseling and having obtained an informed consent, DNA was isolated from peripheral blood of the proband, her parents, and the younger brother. The analysis of the ABCC6 gene was performed by PCR amplification of all exons and flanking intronic sequences, followed by automated nucleotide sequencing, as described previously2. Two homozygous sequence variants were identified in the ABCC6 gene of the proband: p.R1221H and p.R1268Q. Both parents were heterozygous carriers of these mutations in their DNA (Fig. 2B). Previously, p.R1221H has been identified as pathogenic mutation in a patient with PXE6. Furthermore, the arginine in the position 1221 is evolutionarily highly conserved (Fig. 2C). In contrast, the p.R1268Q sequence variant has been suggested to be a relatively common polymorphism with an allelic frequency of ~30% in healthy Caucasian and Japanese populations7,8. Collectively, it appears that p.R1221H is the pathogenic mutation resulting in PXE, when present in both alleles, as in the case of the proband of this study.
Analysis of the ABCC6 gene in the proband’s younger brother revealed that he was also heterozygous carrier of both sequence variants, analogous to the clinically unaffected parents (Fig. 2A). On the basis of these analyses, we concluded, therefore, that the younger brother was not at risk for developing PXE later in his life.
In summary, in the pedigree examined in this study, the proband had unequivocal diagnosis of PXE on the basis of clinical findings and histopathology, and this was confirmed by demonstration of homozygous mutations in the ABCC6 gene. The proband’s younger brother, 13-years of age, was found to be heterozygous carrier of the same mutation in one allele only, similar to the unaffected parents. These findings suggest that the younger brother is not at risk for development of PXE, thus alleviating concerns of his future health status with respect to PXE. In this context it should be noted that heterozygous carriers of ABCC6 mutations have been suggested to be at an increased risk of premature coronary artery disease9,10. Thus, the younger brother may benefit from careful monitoring of the cardiovascular disease early on and initiation of prevention therapy as necessary. The latter considerations regarding the risks of a heterozygous individual, such as the proband’s younger brother and the parents, should be included in the pretest counseling process.
What’s already known about this topic?
Mutations in the ABCC6 gene have been identified in patients with PXE, an autosomal recessive disorder
The onset of PXE is late, often during the 2nd or 3rd decade of life
What does this study add?
The family illustrates the potential of presymptomatic diagnosis for this, currently intractable disorder
In the present family, heterozygous carrier status suggests that a family member of the proband is not at risk for PXE
Acknowledgements
We thank the family for participation. This study was supported by the United States DHHS, NIH/NIAMS grant R01 AR28450.
Footnotes
The authors state no conflict of interest.
Contributor Information
Q. Li, Department of Dermatology and Cutaneous Biology, Jefferson Medical College, and Jefferson Institute of Molecular Medicine, Thomas Jefferson University, Philadelphia, PA 19107, USA
L. Török, Department of Dermatology, County Hospital Kecskemét, Hungary
L. Kocsis, Department of Dermatology, County Hospital Kecskemét, Hungary
J. Uitto, Department of Dermatology and Cutaneous Biology, Jefferson Medical College, and Jefferson Institute of Molecular Medicine, Thomas Jefferson University, Philadelphia, PA 19107, USA
References
- 1.Li Q, Jiang Q, Pfendner E, et al. Pseudoxanthoma elasticum: clinical phenotypes, molecular genetics and putative pathomechanisms. Exp Derm. 2009;18:1–11. doi: 10.1111/j.1600-0625.2008.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pfendner E, Vanakker O, Terry SF, et al. Mutation detection in the ABCC6 gene and genotype/phenotype analysis in a large international case series affected by pseudoxanthoma elasticum. J Med Genet. 2007;44:621–628. doi: 10.1136/jmg.2007.051094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang Q, Endoh M, Dibra F, Wang F, et al. Pseudoxanthoma elasticum is a metabolic disease. J Invest Dermatol. 2009;129:348–353. doi: 10.1038/jid.2008.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergen AA. Pseudoxanthoma elasticum: the end of the autosomal dominant segregation myth. J Invest Dermatol. 2006;126:704–705. doi: 10.1038/sj.jid.5700129. [DOI] [PubMed] [Google Scholar]
- 5.Ringpfeil F, McGuigan K, Fuchsel L, et al. Pseudoxanthoma elasticum is a recessive disease characterized by compound heterozygosity. J Invest Dermatol. 2006;126:782–786. doi: 10.1038/sj.jid.5700115. [DOI] [PubMed] [Google Scholar]
- 6.Kiec-Wilk B, Surdacki A, Dembinska-Kiec A, et al. Acute myocardial infarction and a new ABCC6 mutation in a 16-year-old boy with pseudoxanthoma elasticum. Int J Cardiol. 2007;116:261–262. doi: 10.1016/j.ijcard.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 7.Germain DP, Perdu J, Remones V, Jeunemaitre X. Homozygosity for the R1268Q mutation in MRP6, the pseudoxanthoma elasticum gene, is not disease-causing. Biochem Biophys Res Commun. 2000;274:297–301. doi: 10.1006/bbrc.2000.3101. [DOI] [PubMed] [Google Scholar]
- 8.Mizutani Y, Nakayama T, Asai S, et al. ABCC6 mutation in patients with angioid streaks. I J B S. 2006;2:9–14. [PMC free article] [PubMed] [Google Scholar]
- 9.Trip MD, Smulders YM, Wegman JJ, et al. Frequent mutation in the ABCC6 gene (R1141X) is associated with a strong increase in the prevalence of coronary artery disease. Circulation. 2002;106:773–775. doi: 10.1161/01.cir.0000028420.27813.c0. [DOI] [PubMed] [Google Scholar]
- 10.Köblös G, Andrikovics H, Prohászka Z, et al. The R1141X loss-of-function mutation of the ABCC6 gene is a strong genetic risk factor for coronary artery disease. Genet Test Mol Biomarkers. 2009;14:75–78. doi: 10.1089/gtmb.2009.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]