Abstract
We investigated the functional relationship between the SNARE protein syntaxin 1A (syn 1A) and the dopamine transporter (DAT) by treating rat striatal tissue with Botulinum Neurotoxin C (BoNT/C) and co-transfecting syn 1A with DAT in non-neuronal cells, followed by analysis of DAT activity, phosphorylation, and regulation. Treatment of striatal slices with BoNT/C resulted in elevated dopamine (DA) transport Vmax and reduced DAT phosphorylation, while heterologous co-expression of syn 1A led to reduction in DAT surface expression and transport Vmax. Syn 1A was present in DAT immunoprecipitation complexes, supporting a direct or indirect interaction between the proteins. Phorbol ester regulation of DA transport activity was retained in BoNT/C-treated synaptosomes and syn 1A transfected cells, demonstrating that PKC and syn 1A effects occur through independent processes. These findings reveal a novel mechanism for regulation of DAT activity and phosphorylation, and suggest the potential for syn 1A to impact DA neurotransmission through effects on reuptake.
Keywords: botulinum neurotoxin C, synaptosomes, phorbol ester, transport regulation, synprint domain
The dopamine transporter (DAT) is an integral plasma membrane phosphoprotein that transports extracellular dopamine (DA) from the synapse into presynaptic neurons, controlling the spatial and temporal availability of DA for binding to its effectors (Giros, et al., 1996). DA transport is inhibited by drugs such as cocaine and amphetamine (AMPH) that block reuptake or induce transport efflux, leading to increased synaptic DA levels associated with psychomotor stimulation and addiction (Riddle, et al., 1995, Sulzer, et al., 2005). DAT is subject to complex functional regulation by multiple signaling pathways, substrates, and transport blockers, allowing for conditional modulation of DA clearance (Mortensen and Amara, 2003, Zahniser and Doolen, 2001). It is thought that dysregulation of DAT activity, by leading to inappropriate DA clearance, could play a role in the etiology of dopaminergic disorders such as schizophrenia, depression, and Parkinson’s disease (Miller, et al., 1999).
DAT forward and reverse transport, plasma membrane expression, and phosphorylation state are acutely regulated by many protein kinases and phosphatases (Daniels and Amara, 1999, Fog, et al., 2006, Foster, et al., 2003, Gorentla, et al., 2009, Granas, et al., 2003, Johnson, et al., 2005, Moron, et al., 2003, Vaughan, et al., 1997, Zahniser and Doolen, 2001). Activation of protein kinase C (PKC) leads to reduced DA transport activity via increased endocytosis and reduced recycling of DAT (Loder and Melikian, 2003; Melikian 2004), and by a trafficking-independent mechanism that is cholesterol sensitive and may be related to DAT presence in membrane rafts (Foster, et al., 2008). Substrates and blockers also impact DAT surface expression and phosphorylation via PKC-dependent pathways during feedback regulation of transmitter clearance (Cervinski, et al., 2005, Chi and Reith, 2003, Gorentla and Vaughan, 2005, Sandoval, et al., 2001, Saunders, et al., 2000).
DAT is found within extensive protein complexes that modify its activity and plasma membrane expression (Torres, 2006). One regulatory partner is the neuronal SNARE protein syntaxin 1A (syn 1A) and its homolog UNC64, which have been demonstrated in murine synaptosomes to mediate AMPH-induced DA efflux (Binda, et al., 2008), and in C. elegans to regulate DAT1 ion channel activity (Carvelli, et al., 2008). These studies and others (Lee, et al. 2004), have implicated the transporter cytoplasmic N-terminal domain in syn 1A effects in vivo and/or identified direct syn 1A-N-terminal binding in vitro. The DAT N-terminus contains all known transporter phosphorylation sites (Cervinski, et al., 2005, Foster, et al., 2002, Gorentla, et al., 2009, Granas, et al., 2003), undergoes PKC-dependent ubiquitylation involved with endocytosis (Miranda, et al., 2005), contains residues involved with intracellular gating (Kniazeff, et al., 2008) and plasma membrane expression (Miranda, et al., 2007), and is contiguous with transmembrane domain (TM) 1 which contains crucial elements of the substrate permeation pathway (Beuming, et al., 2008), suggesting the potential for syn 1A to impact these transporter properties.
In this study we investigated the involvement of syn 1A with various transporter functions by treating rat striatal tissue with the syn 1A protease Botulinum Neurotoxin C (BoNT/C) and by co-expressing syn 1A with DAT in non-neuronal cells. Our findings indicate that syn 1A regulates DAT transport activity and phosphorylation in striatal preparations, and transport capacity and surface levels in heterologous systems, but does not affect PMA-dependent regulation of transport activity. The modulation of these DAT functions by syn 1A demonstrates the presence of novel transporter regulatory mechanisms that may function in coordination of DA release and reuptake and be a factor in dopaminergic dysregulation in disease.
EXPERIMENTAL PROCEDURES
Materials
Carrier free 32PO4 was from MP Biochemicals; [3H]DA (41 Ci/mmol) and high range Rainbow molecular mass standards were from Amersham; Botulinum Neurotoxin C was purchased from Metabiologicals Inc., Madison, WI; PMA was from Calbiochem; DA, (–)-cocaine, antibodies for syn 1A, tyrosine hydroxylase, and polyhistidine, and all other reagents were from Sigma-Aldrich. Rats were purchased from Charles River Laboratories and were housed and treated in accordance with regulations established by the University of North Dakota Institutional Animal Care and Use Committee and the National Institute of Health Guide for the Care and Use of Laboratory Animals.
BoNT/C treatment of rat striatal tissue
Male Sprague Dawley rats (175–300 g) were decapitated and the striata were rapidly removed and weighed. The tissue was sliced into 350 μm slices using a McElvain Tissue Chopper, and slices were incubated in oxygenated Krebs-bicarbonate buffer (KBB) (25 mM NaHCO3, 125 mM NaCl, 5 mM KCl, 1.5 mM CaCl2, 5 mM MgSO4, and 10 mM glucose, pH 7.3) containing 10–100 ng/ml BoNT/C at 37 °C for 20–60 min. To minimize variability caused by DAT density differences throughout the striatum, entire striata from each individual hemispheres were used for each treatment condition.
Transport Analysis
For uptake assays, synaptosomes prepared from control and BoNT/C treated striatal slices (Cervinski, et al., 2005, Foster, et al., 2002) were dispensed into tubes containing modified-Krebs phosphate buffer (126 mM NaCl, 4.8 mM KCl, 16 mM potassium phosphate, 1.4 mM MgSO4, 10 mM glucose, 1.1 mM ascorbic acid, and 1.3 mM CaCl2; pH 7.4). For regulation studies PMA or vehicle (0.1% dimethylsulfoxide) were added to synaptosomes for 15 min at 30 °C prior to assay. Transport was initiated by adding [3H]DA to a final concentration of 1 nM except for saturation analyses where [3H]DA was increased to 10 nM and unlabeled DA varied from 10 nM to 10 μM. Uptake was carried out in triplicate for 5 min at 30 °C using 100 μM (−)-cocaine to define non-specific uptake. Transport was stopped by the addition of 5 ml ice-cold sucrose phosphate (SP) buffer (10 mM Na2HPO4, 0.32 M sucrose, pH 7.4) and synaptosomes were harvested using a Brandel tissue harvester and Whatman GF/B filters pre-soaked for 1h in a 0.05% polyethyleneimine. Bound radioactivity was quantified by liquid scintillation counting at 60% efficiency. Proteins were assayed with the BCA protein assay kit (Pierce) using BSA as the standard, and values varied by <10%. Uptake values were corrected for protein, and for comparison across multiple experiments, transport activity from treated tissue was expressed as a fraction of control activity normalized to 100%. Kinetic parameter data were analyzed by nonlinear regression using Prism3 software and values analyzed statistically by student’s t-test. Phosphorylation results were analyzed statistically using Student's t-test with unequal sample size. All other experiments results were analyzed by Analysis of Variance (ANOVA) followed by Tukey post-test. For all analyses significance was set at p<0.05.
Phosphorylation of rat striatal DAT
Rat striatal slices were preincubated in oxygenated KBB for 30 min at 30 °C, followed by exchange with fresh buffer containing 1 mCi/ml 32PO4 and continued incubation at 30 °C for 90–120 min as described (Cervinski, et al., 2005, Foster, et al., 2002). Vehicle or 10–100 ng/ml BoNT/C were added for the final 20–60 min, and tissue slices were transferred to microcentrifuge tubes and pelleted by centrifugation at 500 g for 2 min at 4 °C. The supernatants were removed and replaced with 1 ml ice-cold KBB, tissue was disrupted by 6 passages through a 26 gauge needle, and membranes pelleted by centrifugation at 500 g for 2 min at 4°C. Membranes were solubilized with 0.5% SDS sample buffer(60 mM Tris, pH 6.8, 0.5% SDS, 10% glycerol, 100 mM dithiothreitol) at 50 mg/ml original wet weight and centrifuged at 20,000 g for 20 min to remove insoluble material.
Cloning, transfection and cell culture
A pCMV SPORT 6 plasmid containing the human syn 1A cDNA sequence (Syn 1A pCMV SPORT 6) was obtained from American Type Culture Collection (Manassas, VA) through the IMAGE (Integrated Molecular Analysis of Genomes and their Expression) Consortium program. The syn 1A cDNA sequence was excised from the vector by restriction digestion, ligated into the pcDNA 3.1/Hygro (+) vector, and sequenced for accuracy (Northwoods DNA, Solway, MN). For syn 1A transfection experiments LLCPK1 cells stably expressing 6xHis rDAT (Vaughan, et al., 2005) were grown to 80–90% confluency. Cells were transiently transfected with 1 μg vector or syn 1A cDNA in 2 μl Lipofectamine 2000 and analyzed after 24h.
Transport analysis in DAT expressing cells
6xHis-rDAT-LLCPK1 cells transfected with vector or syn 1A cDNA were washed twice with 1 ml of Krebs-Ringer HEPES (KRH) buffer (25 mM HEPES, 125 mM NaCl, 4.8 mM KCl, 1.2 mM KH2PO4, 1.3 mM CaCl2, 1.2 mM MgSO4, 5.6 mM glucose, pH 7.4), and where indicated were pretreated at 37 °C for 15 min with vehicle (0.1% dimethylsulfoxide) or 1 μM PMA prior to initiation of transport. Uptake was initiated by adding 10 nM [3H]DA plus 3 μM total DA in KRH buffer using 100 μM (−)cocaine to determine non-specific uptake. Uptake assays were carried out in triplicate at 37 °C for 8 min and terminated by rapidly washing the wells three times with 1 ml ice cold KRH. The cells were solubilized in 500 μl of 1% Triton X-100. Lysates were measured for incorporated radioactivity by a liquid scintillation counting at 60% efficiency and aliquots were analyzed for protein, which varied by <10%. Transport activity was normalized for protein, and for comparison across experiments, values from treatment groups were expressed relative to controls normalized to 100%. Results were analyzed by ANOVA with significance set at p<0.05.
Phosphorylation of rDAT in LLCPK1 cells
6xHis-rDAT-LLCPK1 cells were incubated in phosphate-free medium for 30 min followed by the addition of 32PO4 to a final concentration of 0.5 mCi/ml. Cells were labeled for 2–4 h at 37 °C, followed by application of PMA or vehicle for 30 min. At the end of the treatment cells were washed once with 500 μl of ice cold SP, and lysed on ice for 15 min with 500 μl RIPA buffer. Lysates were centrifuged at 20,000 g at 4 °C for 20 min to remove cell debris and the resulting supernatant centrifuged at 100,000 g at 4 °C for 60 min to remove insoluble material.
Immunoprecipitation
Equal amounts of protein from solubilized striatal membranes or cell lysates were immunoprecipitated with DAT antibody (Ab) 16 generated against rDAT N-terminal amino acids 42–59 (Vaughan, 1995) or with commercial anti-His antibodies. Precipitated samples were electrophoresed on 4–20% SDS polyacrylamide gels with high range Rainbow molecular mass standards, and gels were transferred to PVDF membranes for immunoblotting studies or were dried and subjected to autoradiography for 7–14 days for phosphorylation analysis. DAT phosphorylation levels were quantified by densitometry with Molecular Analyst software (BioRad). Phosphorylation intensities of treated samples were expressed as percent of the basal phosphorylation level normalized to 100%, and averaged intensities were analyzed by ANOVA.
Immunoblotting
Equal amounts of protein from cell lysates or solubilized rat striatal membranes were electrophoresed on 4–20% SDS-polyacrylamide gels and proteins transferred to PVDF membranes. DAT and syn 1A expression were detected with DAT monoclonal antibody 16 (mAb 16) (Gaffaney and Vaughan, 2004) and syn 1A monoclonal antibody HPC 1. Immunoreactive bands were visualized using Immun-Star AP substrate (BioRad) and quantified using a Lumi-Imager and LumiAnalyst 3.0 software. Band intensities of treated samples were expressed as percent of the control level normalized to 100%, and averaged intensities were analyzed by ANOVA.
Cell surface biotinylation
6xHis-rDAT-LLCPK1 cells with vector or syn 1A transfection were incubated with the membrane impermeable biotinylating reagent sulfo-NHS-LC-biotin, and biotinylated DATs purified by chromatography on NeutrAvidin beads, separated by SDS-PAGE and quantified by immunoblotting (Foster, et al., 2008). Parallel sets of cells were treated with PMA to induce DAT endocytosis as a control for DAT surface changes. Protein phosphatase 1 was immunoblotted as a cytoplasmic control for membrane integrity.
RESULTS
BoNT/C treatment of rat striatal tissue affects DA transport capacity and DAT phosphorylation
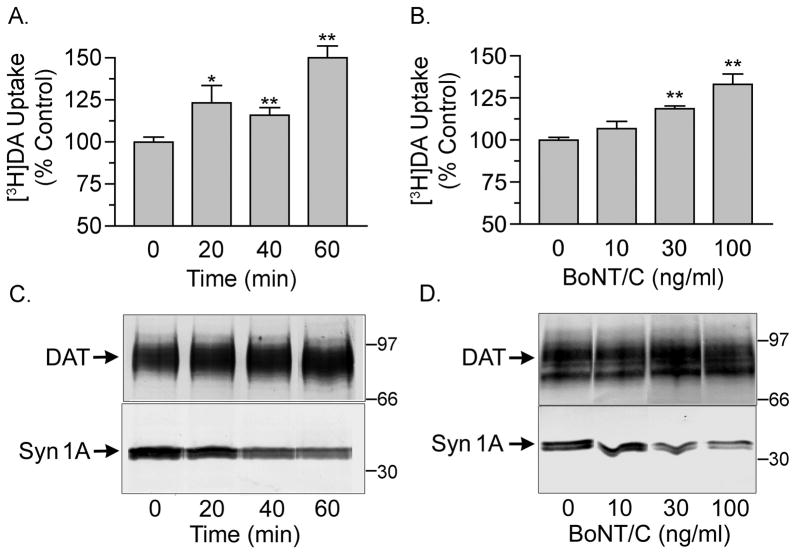
To examine the role of syn 1A on DAT function in neurons, we treated rat striatal tissue with BoNT/C and monitored effects on DA transport and DAT phosphorylation. For uptake experiments, striatal slices were treated with vehicle or BoNT/C followed by preparation of synaptosomes and analysis of [3H]DA transport activity or DAT and syn 1A levels (Fig. 1). Synaptosomes prepared from tissue treated with 100 ng/ml BoNT/C for 20, 40, or 60 min displayed [3H]DA uptake activity that was increased to 123 ± 10%, 116 ± 4%, and 153 ± 14% of control, respectively (p<0.05 or p<0.01) (Fig. 1A). In BoNT/C dose experiments, a slight trend toward increased [3H]DA uptake was seen with 10 ng/ml BoNT/C (107 ± 4% of control), and statistically significant increases in transport were produced by 30 ng/ml and 100 ng/ml BoNT/C (119 ± 2% and 133 ± 6% of control, respectively, p<0.01) (Fig. 1B). Representative syn 1A and DAT immunoblots from these experiments are shown in Figs. 1C and 1D. Syn1A immunoreactivity showed dose- and time-dependent reductions consistent with BoNT/C-mediated proteolysis, with final reductions to 14 ± 11% of control (p<0.001), while DAT levels were not consistently changed by the treatments (average values with BoNT/C, 107 ± 17% of control p>0.05), demonstrating that BoNT/C effects on DAT function were not associated with alterations in total DAT protein.
Fig. 1.
BoNT/C treatment of striatal tissue increases DA transport activity. Rat striatal slices were treated with 100 ng/ml BoNT/C for the indicated times (A, C) or for 1h with the indicated doses (B, D) followed by [3H]DA uptake assay or western blotting for DAT or syn 1A. A. and B., [3H]DA uptake activity (means ± S.E. of 3 independent experiments performed in triplicate). *, p<0.05; **, p<0.01 relative to control by ANOVA. C. and D., representative DAT and syn 1A immunoblots from treated tissue. Molecular mass markers are shown in kDa.
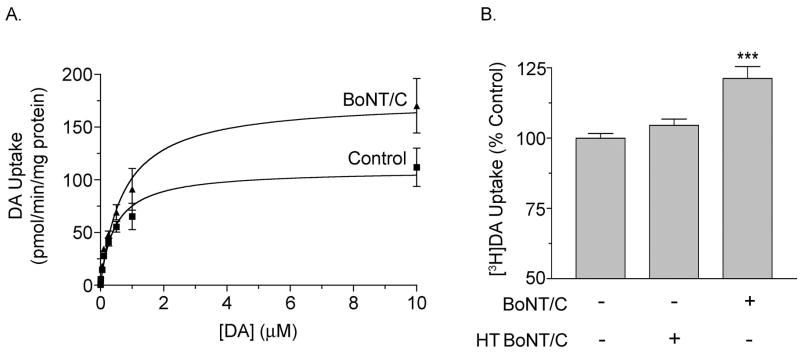
To evaluate BoNT/C effects on DAT kinetic parameters, striatal slices were treated with or without 100 ng/ml BoNT/C for 60 min and synaptosomes prepared from the tissue were assayed for [3H]DA uptake in the presence of increasing DA concentrations (Fig. 2A). Km values obtained from control and BoNT/C treated tissue were not statistically different (514 ± 100 nM and 740 ± 170 nM, respectively; p>0.05), while transport Vmax was significantly increased in the treated samples (control, 116 ± 9 pmol DA/min/mg protein; BoNT/C, 177 ± 17 pmol DA/min/mg protein; p<0.05). To verify that the BoNT/C-induced effects were specific to the actions of the toxin we heat-inactivated BoNT/C at 100 °C for 1h (Arnon, et al., 2001). In two independent experiments [3H]DA transport activity in rat striatal tissue was not affected by heat-treated BoNT/C (105 ± 2% of control; p>0.05), while tissue treated in parallel with control BoNT/C displayed increased transport activity (121 ± 4% of control, p<0.001) (Fig. 2B).
Fig. 2.
Kinetic analysis of BoNT/C effects. A. Rat striatal slices were treated with vehicle or 100 ng/ml BoNT/C for 1h followed by preparation of synaptosomes and [3H]DA transport saturation analysis (points shown are means ± S.E. of results obtained from 2–5 independent experiments performed in triplicate). B. [3H]DA transport activity in synaptosomes prepared from rat striatal tissue treated for 1h with 100 ng/ml control or heat-treated (HT) BoNT/C (means ± S.E. of 2 independent experiments performed in triplicate). ***, p<0.001 relative to control by Student’ t-test.
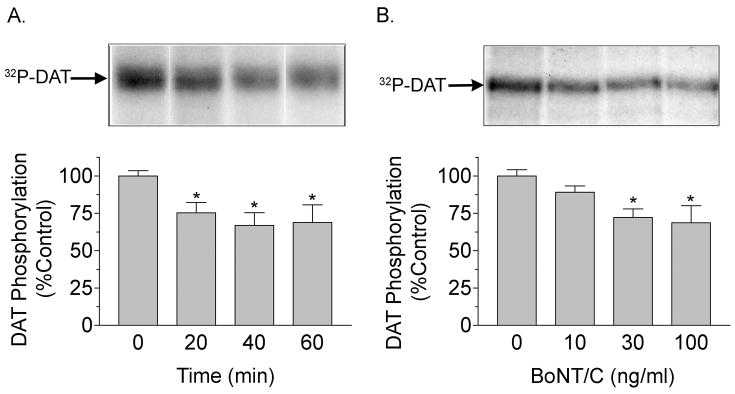
Treatment of rat striatal tissue with BoNT/C also produced time- and dose-dependent changes in DAT phosphorylation (Fig. 3). For these experiments rat striatal slices were labeled with 32PO4 for 2h and given BoNT/C treatment during the final 20–60 min of labeling. Representative autoradiographs of 32P-labeled DAT and results averaged from multiple experiments are shown in Fig. 3. The phosphorylation levels of DAT extracted from rat striatal slices treated with 100 ng/ml BoNT/C for 20, 40, or 60 min were decreased to 75 ± 7%, 67 ± 9%, and 69 ± 12% of the control level, respectively (p<0.05) (Fig. 3A), and in dose experiments, DAT phosphorylation showed a trend toward reduced levels at 10 ng/ml BoNT/C (89 ± 4% of control) and displayed statistically significant decreases to 72 ± 6% and 69 ± 11% of control (p<0.05) with treatments of 30 ng/ml and 100 ng/ml BoNT/C (Fig. 3B).
Fig. 3.
BoNT/C treatment decreases DAT phosphorylation. Rat striatal slices were labeled with 32PO4 and treated for the indicated times with 100 ng/ml BoNT/C (A) or for 1h with the indicated BoNT/C doses (B) followed by immunoprecipitation and SDS-PAGE/autoradiography of DAT. Upper panels show representative autoradiographs of 32P-labeled DAT; histograms show quantification of DAT phosphorylation (means ± S.E. of 3–5 experiments performed in duplicate). *, p<0.05; **, p<0.01 relative to control by student’s t-test.
BoNT/C does not affect PMA-induced transport regulation
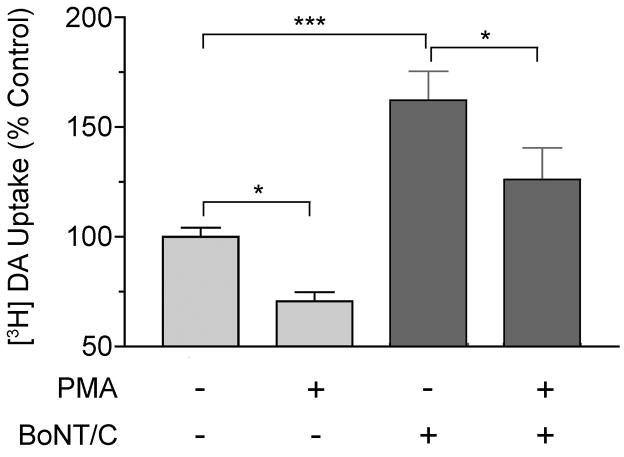
Because DAT transport activity and phosphorylation are also regulated by PKC, we explored the relationship between syn 1A and PKC regulation of DAT. For these experiments rat striatal slices were treated with or without 100 ng/ml BoNT/C for 1h and synaptosomes prepared from the tissue were treated with vehicle or phorbol 12-myristate, 13-acetate (PMA) for 15 min followed by assay for [3H]DA transport (Fig. 4). Synaptosomes from control tissue treated with PMA displayed transport activity that was reduced to 71 ± 4% of control values (p<0.05), as previously found (Vaughan, et al., 1997), while synaptosomes prepared from slices treated with BoNT/C displayed transport that was increased to 162 ± 13% of control (p<0.001), similar to results presented in Figs. 1 and 2. In synaptosomes prepared from BoNT/C-treated tissue and given PMA, DA transport activity was reduced to 79 ± 14% of that of the BoNT/C only synaptosomes (p<0.05), demonstrating that the BoNT/C-increased transport activity was still subject to PMA-induced down-regulation.
Fig. 4.
BoNT/C treatment of striatal tissue does not affect PKC-induced DA transport down–regulation. Rat striatal slices were treated with 100 ng/ml BoNT/C for 1h and synaptosomes prepared from the tissue were treated with vehicle or 1 μM PMA for 15 min followed by analysis of [3H]DA transport. Results shown are means ± S.E. of 4 independent experiments performed in triplicate. *, p<0.05, ***, p<0.001 by ANOVA.
Syn 1A affects DAT transport activity and surface levels in LLCPK1 cells
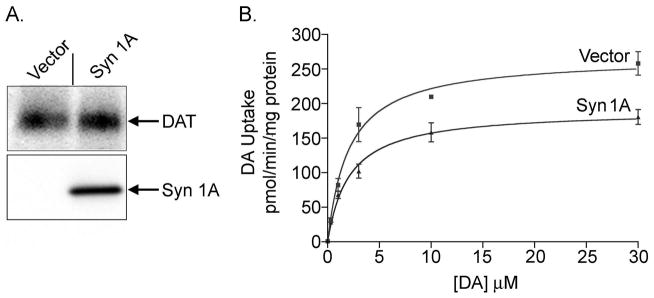
To determine if syn 1A effects on DAT transport activity and phosphorylation could be induced heterologously, we transiently transfected syn 1A into 6xHis-rDAT-LLCPK1 cells, and assessed DAT uptake activity, surface levels, PMA-dependent regulation, and phosphorylation. In four independent experiments we found that exogenous expression of syn 1A caused [3H]DA uptake activity to be reduced to 66 ± 7% of control levels (p<0.01). Immunoblotting of lysates from these experiments confirmed that syn 1A was expressed in transfected but not nontransfected cells (Fig. 5A) and showed that DAT levels were not significantly changed by syn 1A expression (91 ± 3% of control, p>0.05). Saturation analysis showed that decreased activity was the result of reduced transport Vmax (control, 298 ± 15 pmol/min/mg protein; syn 1A, 183 ± 6 pmol/min/mg protein; p<0.05) with no change in Km (control, 2.84 ± 0.45 μM; syn 1A, 2.53 ± 1.06 μM, p>0.05) (Fig. 5B, results representative of three independent experiments).
Fig. 5.
Syn 1A co-expression decreases DA transport activity. 6xHis-rDAT-LLCPK1 cells were transiently transfected with vector or syn 1A cDNA and assayed after 24h. A. DAT and syn 1A immunoblots of vector or syn 1A transfected cell lysates. B. Transport saturation analysis of vector or syn 1A transfected cells (means ± S.E of triplicate determinations). Results shown are representative of three independent experiments. Results were analyzed by Student’s t-test.
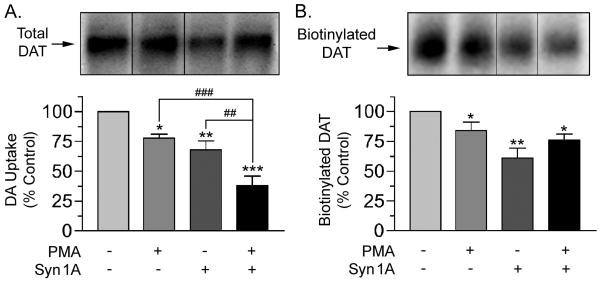
To assess the nature of the transport reduction by syn 1A we analyzed DAT activity and surface levels in combination with PMA treatment to investigate potential regulatory interactions (Fig. 6). Total DAT protein assessed by immunoblotting was not significantly different from control levels for any syn 1A transfection or PMA treatment combination (representative blot Fig. 6A, top; quantification of results from 5 experiments, p>0.05 for all combinations by ANOVA, not shown). DA transport activity in cells given these treatments (Fig. 6A), showed activity reduced to 76 ± 3% of control by PMA, to 63 ± 9% of control by syn 1A transfection, and to 41 ± 8% of control by PMA plus syn 1A transfection (p<0.05–0.001 relative to control). Transport activity in the cells given the syn 1A plus PMA combination was significantly less than that of the PMA only and syn 1A only levels (p<0.01, p<0.001), consistent with activation of independent mechanisms. Plasma membrane levels of DAT in cells given these treatments were analyzed by surface biotinylation (Fig. 6B, representative blot, top). In syn 1A transfected cells, DAT surface levels were reduced to 61 ± 8% of control (p<0.01), suggesting this as the cause of syn 1A-induced transport reduction. In cells given PMA treatment alone or in combination with syn 1A transfection, DAT surface levels were reduced to 84 ± 7% and 76 ± 10% of control, respectively (both p<0.05) (Fig. 6A), consistent with increased endocytosis or reduced membrane insertion driven by PKC (Loder and Melikian, 2003, Melikian, 2004). Although in some individual experiments (Fig. 6B, top), loss of surface DAT with PMA and syn 1A appeared to be additive with treatments, average surface biotinylation results did not show differences between treatment groups, and we did not pursue this observation in further detail. We also assessed phosphorylation of DAT in syn 1A transfected 6xHis-rDAT-LLCPK1 cells but found no consistent alterations relative to controls (not shown), indicating that in these cells, heterologous syn 1A expression does not affect transporter phosphorylation.
Fig. 6.
Regulation of DAT transport activity and surface levels in syn 1A co-expressing cells. 6xHis-rDAT-LLCPK1 cells transiently transfected with vector or syn 1A cDNA were treated with vehicle or 1 μM PMA for 15 min and analyzed for [3H]DA transport activity or subjected to cell surface biotinylation. A. [3H]DA transport activity (means ± S.E. of 5 independent experiments performed in triplicate). *, p<0.05; **, p<0.01, ***, p<0.001 relative to control or indicated treatment by ANOVA. Upper panel, representative blot of total DAT levels. Quantification of blot intensities p>0.05 relative to control by ANOVA. B. Representative immunoblot and quantification of biotinylated DAT (means ± S.E. of 4 independent experiments performed in duplicate). *, p<0.05, **, p<0.01, relative to control by ANOVA.
Syn1A co-immunoprecipitates with DAT
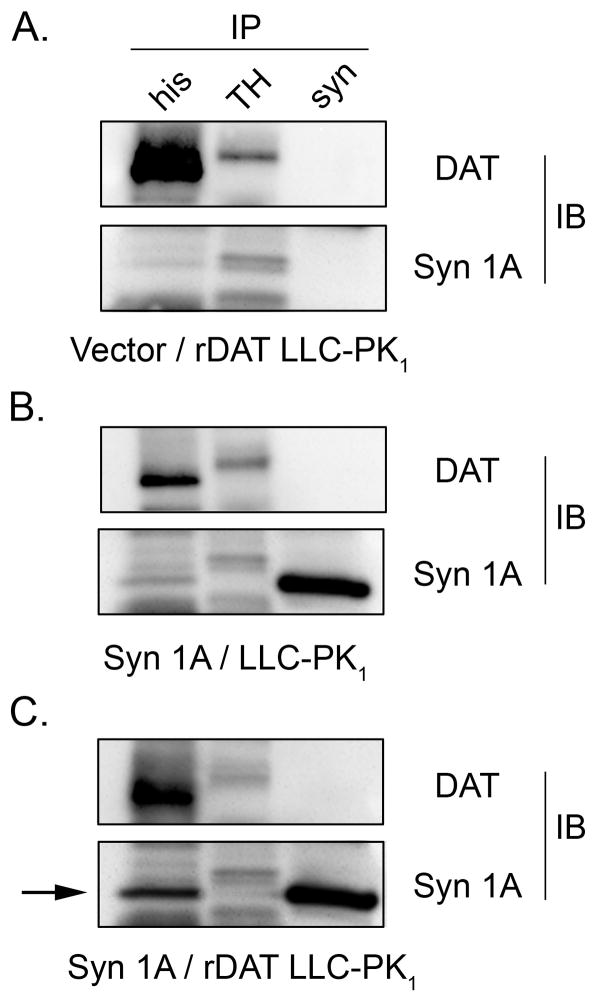
To assess the potential for the syn 1A effects we identified to be mediated by DAT-syn 1A interactions we performed co-immunoprecipitation experiments (Fig. 7). For these studies we used 6xHis-rDAT–LLCPK1 cells (panels A and C) or DAT-null LLCPK1 cells (panel B) transiently transfected with vector (panel A) or syn 1A (panels B and C). Western blotting showed that total levels of DAT and syn1A did not vary with any of the transfection combinations (not shown). Lysates from each cell type were incubated with anti-His to precipitate DAT or anti-syn 1A to precipitate syn 1A, followed by immunoblotting with DAT monoclonal Ab 16 (upper rows) or anti-syn 1A (lower rows). Parallel precipitation of lysates with anti-tyrosine hydroxylase (TH) followed by western blotting was performed as a negative control for signal specificity. Immunoblots showed that anti-His precipitated DAT from 6xHis-rDAT-LLCPK1 cells (A and C, top left lanes) but not from LLCPK1 cells (B), and that anti-syn precipitated syn 1A from syn 1A transfected cells (B and C, bottom right lanes) but not vector-transfected cells (A). Neither DAT nor syn 1A were precipitated from any lysates with anti-TH (A–C, middle lanes), and faint bands present in DAT and syn 1A blots of anti-His and anti TH precipitates are not syn 1A or DAT, as they are present in lysates that lack those proteins.
Fig. 7.
Co-immunoprecipitation of syn 1A with DAT. LLCPK1 or 6xHis-rDAT-LLCPK1 cells were transiently transfected with vector or syn 1A cDNA and lysates were immunoprecipitated for DAT (anti-His), syn 1A (syn) or tyrosine hydroxylase (TH) as indicated (same order for all panels) followed by immunoblotting for DAT (upper panels) and syn1A (lower panels). Arrow indicates presence of syn 1A in DAT immune complex. Blots are representative of three independent experiments.
The specificity of syn 1A presence in DAT immune complexes is demonstrated by syn 1A immunoreactivity in anti-His precipitates prepared from 6xHis-rDAT-LLCPK1 cells transfected with syn 1A (panel C, bottom left, arrow) but not from vector transfected 6xHis-rDAT-LLCPK1 cells or syn 1A transfected LLCPK1 cells (A and B, bottom left lanes). This result has been replicated in two additional experiments. In numerous attempts we were unable to demonstrate the specific presence of syn 1A in DAT immune complexes when extracting expressed or striatal DAT with Ab16 generated against N-terminal residues 42–61 (not shown). Our ability to detect syn 1A in DAT immune complexes when isolating DAT with antibodies against an exogenous epitope but not with those for the native N-terminus suggests that Ab16 may displace syn1A from DAT immune complexes. Similarly, we were unable to detect DAT in syn 1A immune complexes (Fig. 7), possibly because of syn 1A antibody interference with DAT interactions.
DISCUSSION
In this study we found that treatment of rat striatal tissue with BoNT/C led to increased synaptosomal DA transport activity and decreased DAT phosphorylation, while heterologous co-expression of syn 1A with DAT caused reductions in transport capacity and transporter surface expression. In addition we found specific co-precipitation of syn 1A with DAT, demonstrating the presence of a direct or indirect interaction between the proteins. These findings suggest that in neurons, syn 1A interactions with DAT result in suppression of DA uptake and maintenance of transporter phosphorylation, and that some aspect of DAT trafficking may also be controlled by syn 1A. However, the well-characterized regulation of DAT by PKC is not related to syn 1A, as PMA-induced down-regulation of transport activity was maintained in both BoNT/C-treated striatal tissue and syn 1 co-transfected cells. The identification of syn 1A regulation of these DAT functions indicates the potential for syn 1A to modulate mammalian DA neurotransmission through effects on transmitter reuptake and phosphorylation.
In previous studies syn 1A was demonstrated to regulate DAT reverse transport and ion channel activity (Binda, et al., 2008, Carvelli, et al., 2008). The potential for the mechanism of these effects to involve the N-terminus is supported by yeast two-hybrid and fusion protein studies showing in vitro syn 1A interactions with the transporter N-terminal domain (Binda, et al., 2008, Lee, et al., 2004) and N-terminal fluorescent protein tag disruption of C. elegans DAT1-UNC64 interactions (Carvelli, et al., 2008). Our observations that syn 1A co-precipitates with DAT when isolating the transporter with exogenous epitope antibodies but not with endogenous N-terminal antibodies is consistent with these findings and supports the importance of the DAT N-terminal domain in mediating syn 1A effects on the full-length mammalian proteins. Our findings that BoNT/C treatment of striatal tissue leads to increased synaptosomal DA uptake, in conjunction with increased DAT1 ion channel fluxes induced by disruption of UNC64 interactions (Carvelli, et al., 2008), are consistent with a mechanism in which forward DA transport and accompanying ion movements are suppressed by syn 1A binding to the transporter and alleviated by conditions that reduce these interactions. Syn 1A binding to DAT or DAT complexes could decrease transport activity by inducing N-terminal conformational changes that affect orientation of intracellular gate residues (Kniazeff, et al., 2008) or propagate to TM1 active sites (Beuming, et al., 2008) to directly modulate transport kinetic steps. The findings that DAT-syn 1A interactions and their modulation by AMPH occur at the plasma membrane of murine synaptosomes (Binda, et al., 2008) support the potential for direct syn 1A effects on transport kinetics. However, the reduction of DAT surface levels by exogenous syn 1A also suggests the potential for trafficking-dependent syn 1A mechanisms, and DAT N-terminal residues that could be affected by syn 1A interactions have been linked to transporter surface expression (Miranda, et al., 2007). Although syn 1A and PMA caused a clear reduction of DAT surface levels in heterologous cells, and showed additive effects on transport activity, we did not find additivity of DAT surface reductions. It is possible that the significant loss of surface DAT induced by syn 1A (~40%) represents a floor effect that cannot be further down-regulated, that the biotinylation assay is insufficiently sensitive to quantify additivity of small changes in surface levels, or that trafficking independent mechanisms (Foster et al., 2008) contribute to additivity of transport regulation of by these treatments.
The reduction of DAT 32P-labeling with BoNT/C treatment suggests that syn 1A also impacts the N-terminal domain in a way that promotes or stabilizes the transporter phosphorylation level. DAT is phosphorylated under basal, PKC, and AMPH conditions at multiple residues at the distal end of the DAT N-terminal tail (Foster, et al., 2002, Granas, et al., 2003; Cervinski, et al., 2005) and also at a membrane proximal proline-directed site (Gorentla, et al., 2009). It is not known if syn 1A affects the phosphorylation of all or a subset of these sites. Syn 1A could stabilize DAT phosphorylation via induction of N-terminal conformational changes that affect accessibility of phosphorylation sites to kinases or phosphatases, or by regulating binding of scaffolding proteins such as receptor for activated C-kinase, which also binds to the DAT N-terminus (Lee, et al., 2004), that could impact transporter phosphorylation. Alternatively, although the subcellular compartments in which DAT is phosphorylated and dephosphorylated are currently not known, it is possible that syn 1A could impact DAT availability to kinase and phosphatases through effects on trafficking. We have previously shown that DAT is present in cholesterol- and ganglioside-rich membrane rafts, and that regulation of transporter phosphorylation and endocytosis differs between raft and non-raft membranes (Foster et al., 2008). Because BoNT/C is regulated by gangliosides (Rummel et al., 2009), it is possible that its effects on DAT phosphorylation and trafficking may be related to transporter microdomain partitioning.
Surprisingly, the robust and consistent reduction in striatal DAT phosphorylation we obtained with BoNT/C treatment was not mirrored by phosphorylation increases with heterologous syn 1A transfection. We do not know if lack of heterologous syn 1A effects indicates the presence of an impediment such as a pre-existing DAT complex that blocks effects on phosphorylation, or if syn 1A modulation of phosphorylation cannot be detected in long-term transfection studies compared to the rapid effects produced by BoNT/C. It is also possible that BoNT/C effects on striatal DAT phosphorylation are due to cleavage of other syntaxin isoforms (syn 1B, syn 2 or syn 3), potentially resulting in the differences observed in native and heterologous systems. Thus further work will be necessary to clarify the relationship between the rapid transport and phosphorylation responses induced by BoNT/C in striatal tissue and longer-term effects associated with syn 1A transfection.
Syn 1A is also known to modulate activity and subcellular localization of gamma aminobutyric acid, norepinephrine and serotonin transporters (GAT1, NET, and SERT) via binding to transporter N-terminal domains (Beckman, et al., 1998, Deken, et al., 2000, Dipace, et al., 2007, Haase, et al., 2001, Quick, 2002a, Quick, 2002b, Quick, 2003). For GAT1 and NET, syn 1A promotes increased transporter surface expression but overall reduced transport activity, suggesting a mechanism in which syn 1A recruits transporters to the cell surface but maintains them in a suppressed state until the interaction is released (Deken, et al., 2000, Dipace, et al., 2007, Sung, et al., 2003, Sung and Blakely, 2007). Our findings for DAT are consistent with syn 1A functioning to dampen transmitter clearance, but show the opposite direction of surface effects. In addition, for GAT1 and NET, interaction with syn 1A is necessary for PMA-induced transporter down regulation (Beckman et al., 1998; Sung, et al., 2003, Sung and Blakely, 2007), whereas our results clearly demonstrate that DAT down-regulation by PMA in cells or synaptosomes was not affected by syn 1A transfection or BoNT/C treatment. Thus DAT shows both similarities and differences to related transporters with respect to syn 1A regulatory properties.
The finding that DAT-syn1A interactions are increased by AMPH (Binda, et al., 2008) is further consistent with syn 1A promotion of transporter phosphorylation. Because DAT phosphorylation has been associated with enhanced DA efflux (Khoshbouei, et al., 2004), it is possible that the sequence of AMPH → syn 1A binding → DAT phosphorylation represents part of the mechanism underlying AMPH stimulation of reverse DA transport. Other as yet unknown functions of DAT phosphorylation would also be candidates for regulation by syn 1A.
Fusion protein experiments indicate that residues 1–33 of DAT are sufficient to support syn 1A binding (Binda, et al., 2008), thus the syn 1A interaction site is close to or overlaps the major DAT phosphorylation domain. The proximity of these functional domains suggests a similarity to synaptic protein interaction (synprint) sites found in N-type voltage-gated Ca++ channels (Yokoyama, et al., 2005, Yokoyama, et al., 1997) and Sept5 (Taniguchi, et al., 2007). The synprint domains in these proteins contain multiple phosphorylation sites for various kinases that regulate syn 1A binding and associated protein modulation. Potentially similar results have been found for SERT, NET, and GAT1, which show regulation of syn 1A binding by PKC, ERK, and/or calcium-calmodulin dependent protein kinase conditions (Beckman, et al., 1998; Samuvel, et al., 2005; Sung et al., 2003), although it is not known if these effects are mediated by transporter phosphorylation. Thus, a growing body of evidence links phosphorylation processes to syn 1A regulation of neurotransmitter transporters by mechanisms similar to those found in Ca++ channels and other synprint domain proteins.
Syn 1A and other SNARE proteins coordinate molecular events in neurotransmission through their roles as core components of the synaptic vesicle fusion apparatus (Sudhof, 1995), and by interactions with other synaptic proteins such as ion channels (Bezprozvanny, et al., 1995). Increasing evidence from transporter systems has indicated involvement of syn 1A in coordinating neurotransmitter release and reuptake, and our new studies now connect syn 1A to regulation of DA reuptake and DAT phosphorylation, providing previously unknown roles for syn 1A in transporter function and DA neurotransmission. These findings expand the list of molecular events impacted by syn 1A and identify DAT functional and regulatory properties that may be altered in DA disorders via defects in syn 1A function.
Acknowledgments
This work was supported by DA13147, ND EPSCoR Award, and UND Faculty Seed Award (R.A.V.), F31 DA17520 (M.A.C.), and P20 RR016741 to the University of North Dakota from the INBRE program of the National Center for Research Resources, and ND EPSCoR through NSF EPS-0447679. We thank Steven Adkins for excellent technical assistance.
Abbreviations
- DAT
dopamine transporter
- DA
dopamine
- Syn 1A
Syntaxin 1A
- SNARE
soluble N-ethylmaleimide-sensitive factor attachment receptor
- BoNT/C
botulinum neurotoxin C
- AMPH
amphetamine
- PKC
protein kinase C
- PMA
phorbol 12-myristate, 13-acetate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnon SS, Schechter R, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Hauer J, Layton M, Lillibridge S, Osterholm MT, O'Toole T, Parker G, Perl TM, Russel PK, Swerdlow DL, Tonat K. Botulinum toxin as a biological weapon: medical and public health management. JAMA. 2001;285:1059–1070. doi: 10.1001/jama.285.8.1059. [DOI] [PubMed] [Google Scholar]
- Beckman ML, Bernstein EM, Quick MW. Protein kinase C regulates the interaction between a GABA transporter and syntaxin 1A. J Neurosci. 1998;18:6103–6112. doi: 10.1523/JNEUROSCI.18-16-06103.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuming T, Kniazeff J, Bergmann ML, Shi L, Gracia L, Raniszewska K, Newman AH, Javitch JA, Weinstein H, Gether U, Loland CJ. The binding sites for cocaine and dopamine in the dopamine transporter overlap. Nat Neurosci. 2008;11:780–789. doi: 10.1038/nn.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I, Scheller RH, Tsien RW. Functional impact of syntaxin on gating of N-type and Q-type calcium channels. Nature. 1995:623–626. doi: 10.1038/378623a0. [DOI] [PubMed] [Google Scholar]
- Binda F, Dipace C, Robertson SD, Lute BJ, Fog JU, Zhang M, Sen N, Colbran RJ, Gnegy ME, Gether U, Javitch JA, Erreger K, Galli A. Syntaxin 1A interaction with the dopamine transporter promotes amphetamine-induced dopamine efflux. Mol Pharmacol. 2008;74:1101–1108. doi: 10.1124/mol.108.048447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvelli L, Blakely RD, DeFelice LJ. Dopamine transporter/syntaxin 1A interactions regulate transporter channel activity and dopaminergic synaptic transmission. Proc Natl Acad Sci U S A. 2008;105:14192–14197. doi: 10.1073/pnas.0802214105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervinski MA, Foster JD, Vaughan RA. Psychoactive substrates stimulate dopamine transporter phosphorylation and down-regulation by cocaine-sensitive and protein kinase C-dependent mechanisms. J Biol Chem. 2005;280:40442–40449. doi: 10.1074/jbc.M501969200. [DOI] [PubMed] [Google Scholar]
- Chi L, Reith ME. Substrate-induced trafficking of the dopamine transporter in heterologously expressing cells and in rat striatal synaptosomal preparations. J Pharmacol Exp Ther. 2003;307:729–736. doi: 10.1124/jpet.103.055095. [DOI] [PubMed] [Google Scholar]
- Daniels GM, Amara SG. Regulated trafficking of the human dopamine transporter. Clathrin- mediated internalization and lysosomal degradation in response to phorbol esters. J Biol Chem. 1999;274:35794–35801. doi: 10.1074/jbc.274.50.35794. [DOI] [PubMed] [Google Scholar]
- Deken SL, Beckman ML, Boos L, Quick MW. Transport rates of GABA transporters: regulation by the N-terminal domain and syntaxin 1A. Nat Neurosci. 2000;3:998–1003. doi: 10.1038/79939. [DOI] [PubMed] [Google Scholar]
- Dipace C, Sung U, Binda F, Blakely RD. Amphetamine induces a calcium/calmodulin-dependent protein kinase II-dependent reduction in norepinephrine transporter surface expression linked to changes in syntaxin 1A/transporter complexes. Mol Pharmacol. 2007;71:230–239. doi: 10.1124/mol.106.026690. [DOI] [PubMed] [Google Scholar]
- Fog JU, Khoshbouei H, Holy M, Owens WA, Vaegter CB, Sen N, Nikandrova Y, Bowton E, McMahon DG, Colbran RJ, Daws LC, Sitte HH, Javitch JA, Galli A, Gether U. Calmodulin kinase II interacts with the dopamine transporter C terminus to regulate amphetamine-induced reverse transport. Neuron. 2006;51:417–429. doi: 10.1016/j.neuron.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Foster JD, Adkins SD, Lever JR, Vaughan RA. Phorbol ester induced trafficking-independent regulation and enhanced phosphorylation of the dopamine transporter associated with membrane rafts and cholesterol. J Neurochem. 2008;105:1683–1699. doi: 10.1111/j.1471-4159.2008.05262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JD, Pananusorn B, Cervinski MA, Holden HE, Vaughan RA. Dopamine transporters are dephosphorylated in striatal homogenates and in vitro by protein phosphatase 1. Brain Res Mol Brain Res. 2003;110:100–108. doi: 10.1016/s0169-328x(02)00645-9. [DOI] [PubMed] [Google Scholar]
- Foster JD, Pananusorn B, Vaughan RA. Dopamine transporters are phosphorylated on N-terminal serines in rat striatum. J Biol Chem. 2002;277:25178–25186. doi: 10.1074/jbc.M200294200. [DOI] [PubMed] [Google Scholar]
- Gaffaney JD, Vaughan RA. Uptake inhibitors but not substrates induce protease resistance in extracellular loop two of the dopamine transporter. Mol Pharmacol. 2004;65:692–701. doi: 10.1124/mol.65.3.692. [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Gorentla BK, Moritz AE, Foster JD, Vaughan RA. Proline-directed phosphorylation of the dopamine transporter N-terminal domain. Biochemistry. 2009;48:1067–1076. doi: 10.1021/bi801696n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorentla BK, Vaughan RA. Differential effects of dopamine and psychoactive drugs on dopamine transporter phosphorylation and regulation. Neuropharmacology. 2005;49:759–768. doi: 10.1016/j.neuropharm.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Granas C, Ferrer J, Loland CJ, Javitch JA, Gether U. N-terminal truncation of the dopamine transporter abolishes phorbol ester- and substance P receptor-stimulated phosphorylation without impairing transporter internalization. J Biol Chem. 2003;278:4990–5000. doi: 10.1074/jbc.M205058200. [DOI] [PubMed] [Google Scholar]
- Haase J, Killian AM, Magnani F, Williams C. Regulation of the serotonin transporter by interacting proteins. Biochem Soc Trans. 2001;29:722–728. doi: 10.1042/0300-5127:0290722. [DOI] [PubMed] [Google Scholar]
- Johnson LA, Guptaroy B, Lund D, Shamban S, Gnegy ME. Regulation of amphetamine-stimulated dopamine efflux by protein kinase C beta. J Biol Chem. 2005;280:10914–10919. doi: 10.1074/jbc.M413887200. [DOI] [PubMed] [Google Scholar]
- Khoshbouei H, Sen N, Guptaroy B, Johnson L, Lund D, Gnegy ME, Galli A, Javitch JA. N-terminal phosphorylation of the dopamine transporter is required for amphetamine-induced efflux. PLoS Biol. 2004;2:E78. doi: 10.1371/journal.pbio.0020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniazeff J, Shi L, Loland CJ, Javitch JA, Weinstein H, Gether U. An intracellular interaction network regulates conformational transitions in the dopamine transporter. J Biol Chem. 2008;283:17691–17701. doi: 10.1074/jbc.M800475200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Kim MY, Kim DH, Lee YS. Syntaxin 1A and receptor for activated C kinase interact with the N-terminal region of human dopamine transporter. Neurochem Res. 2004;29:1405–1409. doi: 10.1023/b:nere.0000026404.08779.43. [DOI] [PubMed] [Google Scholar]
- Loder MK, Melikian HE. The dopamine transporter constitutively internalizes and recycles in a protein kinase C-regulated manner in stably transfected PC12 cell lines. J Biol Chem. 2003;278:22168–22174. doi: 10.1074/jbc.M301845200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melikian HE. Neurotransmitter transporter trafficking: endocytosis, recycling, and regulation. Pharmacol Ther. 2004;104:17–27. doi: 10.1016/j.pharmthera.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Miller GW, Gainetdinov RR, Levey AI, Caron MG. Dopamine transporters and neuronal injury. Trends Pharmacol Sci. 1999;20:424–429. doi: 10.1016/s0165-6147(99)01379-6. [DOI] [PubMed] [Google Scholar]
- Miranda M, Dionne KR, Sorkina T, Sorkin A. Three ubiquitin conjugation sites in the amino terminus of the dopamine transporter mediate protein kinase C-dependent endocytosis of the transporter. Mol Biol Cell. 2007;18:313–323. doi: 10.1091/mbc.E06-08-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda M, Wu CC, Sorkina T, Korstjens DR, Sorkin A. Enhanced ubiquitylation and accelerated degradation of the dopamine transporter mediated by protein kinase C. J Biol Chem. 2005;280:35617–35624. doi: 10.1074/jbc.M506618200. [DOI] [PubMed] [Google Scholar]
- Moron JA, Zakharova I, Ferrer JV, Merrill GA, Hope B, Lafer EM, Lin ZC, Wang JB, Javitch JA, Galli A, Shippenberg TS. Mitogen-activated protein kinase regulates dopamine transporter surface expression and dopamine transport capacity. J Neurosci. 2003;23:8480–8488. doi: 10.1523/JNEUROSCI.23-24-08480.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen OV, Amara SG. Dynamic regulation of the dopamine transporter. Eur J Pharmacol. 2003;479:159–170. doi: 10.1016/j.ejphar.2003.08.066. [DOI] [PubMed] [Google Scholar]
- Quick M. Role of syntaxin 1A on serotonin transporter expression in developing thalamocortical neurons. Int J Dev Neurosci. 2002a;20:219–224. doi: 10.1016/s0736-5748(02)00021-7. [DOI] [PubMed] [Google Scholar]
- Quick M. Substrates regulate gamma-aminobutyric acid transporters in a syntaxin 1A-dependent manner. Proc Natl Acad Sci U S A. 2002b;99:5686–5691. doi: 10.1073/pnas.082712899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick M. Regulating the conducting states of a mammalian serotonin transporter. Neuron. 2003;40:537–549. doi: 10.1016/s0896-6273(03)00605-6. [DOI] [PubMed] [Google Scholar]
- Riddle EL, Fleckenstein AE, Hason GR. Role of monoamine transporters in mediating psychostimulant effects. Aaps J. 1995;7:E847–851. doi: 10.1208/aapsj070481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rummel A, Häfner K, Mahrhold S, Darashchonak N, Holt M, Jahn R, Beermann S, Karnath T, Bigalke H, Binz T. Botulinum neurotoxins C, E and F bind gangliosides via a conserved binding site prior to stimulation-dependent uptake with botulinum neurotoxin F utilising the three isoforms of SV2 as second receptor. J Neurochem. 2009;110(6):1942–1954. doi: 10.1111/j.1471-4159.2009.06298.x. [DOI] [PubMed] [Google Scholar]
- Samuvel DJ, Jayanthi LD, Bhat NR, Ramamoorthy S. A role for p38 mitogen-activated protein kinase in the regulation of the serotonin transporter: evidence for distinct cellular mechanisms involved in transporter surface expression. J Neurosci. 2005;25:29–41. doi: 10.1523/JNEUROSCI.3754-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval V, Riddle EL, Ugarte YV, Hanson GR, Fleckenstein AE. Methamphetamine-induced rapid and reversible changes in dopamine transporter function: an in vitro model. J Neurosci. 2001;21:1413–1419. doi: 10.1523/JNEUROSCI.21-04-01413.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders C, Ferrer JV, Shi L, Chen J, Merrill G, Lamb ME, Leeb-Lundberg LM, Carvelli L, Javitch JA, Galli A. Amphetamine-induced loss of human dopamine transporter activity: an internalization-dependent and cocaine-sensitive mechanism. Proc Natl Acad Sci U S A. 2000;97:6850–6855. doi: 10.1073/pnas.110035297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC. The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature. 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: A review. Prog Neurobiol. 2005 doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Sung U, Apparsundaram S, Galli A, Kahlig KM, Savchenko V, Schroeter S, Quick MW, Blakely RD. A regulated interaction of syntaxin 1A with the antidepressant-sensitive norepinephrine transporter establishes catecholamine clearance capacity. J Neurosci. 2003;23:1697–1709. doi: 10.1523/JNEUROSCI.23-05-01697.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung U, Blakely RD. Calcium-dependent interactions of the human norepinephrine transporter with syntaxin 1A. Mol Cell Neurosci. 2007;34:251–260. doi: 10.1016/j.mcn.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi M, Taoka M, Itakura M, Asada A, Saito T, Kinoshita M, Takahashi M, Isobe T, Hisanaga S. Phosphorylation of adult type Sept5 (CDCrel-1) by cyclin-dependent kinase 5 inhibits interaction with syntaxin-1. J Biol Chem. 2007;282:7869–7876. doi: 10.1074/jbc.M609457200. [DOI] [PubMed] [Google Scholar]
- Torres GE. The dopamine transporter proteome. J Neurochem. 2006;97(Suppl 1):3–10. doi: 10.1111/j.1471-4159.2006.03719.x. [DOI] [PubMed] [Google Scholar]
- Vaughan RA. Photoaffinity-labeled ligand binding domains on dopamine transporters identified by peptide mapping. Mol Pharmacol. 1995;47:956–964. [PubMed] [Google Scholar]
- Vaughan RA, Huff RA, Uhl GR, Kuhar MJ. Protein kinase C-mediated phosphorylation and functional regulation of dopamine transporters in striatal synaptosomes. J Biol Chem. 1997;272:15541–15546. doi: 10.1074/jbc.272.24.15541. [DOI] [PubMed] [Google Scholar]
- Vaughan RA, Parnas ML, Gaffaney JD, Lowe MJ, Wirtz S, Pham A, Reed B, Dutta SM, Murray KK, Justice JB. Affinity labeling the dopamine transporter ligand binding site. J Neurosci Methods. 2005;143:33–40. doi: 10.1016/j.jneumeth.2004.09.022. [DOI] [PubMed] [Google Scholar]
- Yokoyama CT, Meyers SJ, Fu J, Mockus SM, Scheuer T, Catterall WA. Mechanism of SNARE protein binding and regulation of Cav2 channels by phosphorylation of the synaptic protein interaction site. Mol Cell Neurosci. 2005;28:1–17. doi: 10.1016/j.mcn.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Yokoyama CT, Sheng ZH, Catterall WA. Phosphorylation of the synaptic protein interaction site on N-type calcium channels inhibits interactions with SNARE proteins. J Neurosci. 1997;17:6929–6938. doi: 10.1523/JNEUROSCI.17-18-06929.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahniser NR, Doolen S. Chronic and acute regulation of Na+/Cl−-dependent neurotransmitter transporters: drugs, substrates, presynaptic receptors, and signaling systems. Pharmacol Ther. 2001;92:21–55. doi: 10.1016/s0163-7258(01)00158-9. [DOI] [PubMed] [Google Scholar]