Introduction
Abdominal aortic aneurysms (AAAs) are dynamic vascular lesions that represent a major cause of cardiovascular-related morbidity and mortality. Their underlying pathogenesis is complex, but a potentially significant contributor to their formation is protease-driven destruction of collagen and elastin fibers in the aortic media and adventitia, resulting in a weakening of the vessel wall and a progressive expansion of its diameter1–5. This destructive process is likely due in part to a disruption in the balance between proteolytic enzymes and their inhibitors, leading to excessive degradation of the connective tissue that maintains the aortic wall’s structural integrity. One family of proteolytic enzymes highly implicated in the development and progression of AAAs are the matrix metalloproteinases (MMPs), particularly MMP-2 (gelatinase A), MMP-9 (gelatinase B), MMP-12, and MMP-13. Their expression and concentration levels have been found to be elevated in aneurysmal tissue histologically2, 5–11. These endopeptidases are secreted as zymogens and undergo activation in the extracellular space. Their activity is further regulated by numerous inhibitors of their function, both non-specific, such as alpha 1-antitrypsin, and specific, such as tissue inhibitors of matrix metalloproteinases (TIMPs). Studies using knock-out mice9, 12 and doxycycline13, 14, a non-selective MMP inhibitor, have additionally shown that MMP-2 and MMP-9 are key components in the formation of AAAs.
Investigations into the contributions of MMPs to AAA pathophysiology have focused primarily on analysis of enzyme concentrations and expression levels. However, such approaches are less than ideal methods to fully characterize intramural proteolysis, as they are unable to capture the interplay between MMPs, their activators, and their inhibitors that result in the true elevated enzyme activity, rather than expression alone, that is likely ultimately responsible for the over-exuberant elastin- and collagenolysis seen in aneurysmal tissue. Immunohistologic techniques and biochemical assays that measure enzyme concentration or expression provide only a limited perspective on the role of these enzymes in AAA pathophysiology.
Current techniques to study MMP activity in AAAs include in situ zymography8, 15, 16, which provides a topographic assessment of enzymatic activity in histologic specimens. However, this ex vivo approach requires tissue explantation and processing for fluorescence microscopic evaluation, artificial manipulations that have the potential to cause alterations in enzyme activity from the true, in vivo functional levels. Moreover, this technique only allows for the measurement of MMP activity within a very limited, several micron-wide tissue section. An in vivo method to quantify MMP activity over a larger scale would potentially provide a more accurate global evaluation of the proteolytic processes occurring within the aneurysmal wall, and would alleviate concerns regarding processing steps required for ex vivo analysis. Moreover, an in vivo imaging approach could pave the way for improvements in the pre-clinical assessment of new medical therapies for AAAs, by serving as a quantifiable end-point for the evaluation of, for example, novel selective MMP inhibitors as immunomodulatory therapy for the suppression of AAA growth.
In this paper, we present an optical molecular imaging-based method to measure MMP activity in AAAs in vivo. We apply this technology to an animal model of AAAs, in which calcium chloride was topically applied to the surface of the aorta to generate AAAs, the growth of which were then modulated by the administration of doxycycline. We first corroborate the intravital molecular imaging data with in situ zymography results in a mouse model and then demonstrate that this imaging can be performed endovascularly in a rat model given the larger aortic luminal diameter, opening the door for potential pre-clinical applications.
Methods
AAA generation
All animal experiments were approved by the institutional animal care committee. Abdominal aortic aneurysms were generated in mice and rats using a previously published method14, 17. In each case, the rodent was anesthetized with 2% isoflurane in 1L/min O2 and placed in a supine position. The fur was shaved with clippers, and a single midline incision in the abdominal wall was made. The abdominal viscera were retracted and the abdominal aorta was isolated with blunt dissection. A 0.5cm × 0.5cm piece of gauze soaked in 1.0M CaCl2 was then placed directly on the abdominal aorta between the renal arteries and iliac bifurcation for 15 minutes. Following this, the gauze was removed; the abdominal cavity was thoroughly lavaged with warm sterile saline; and the abdomen was closed. A sham surgery was also performed on mice to serve as a control for the surface reflectance imaging experiment described below; in this surgery, the gauze was pre-soaked in saline rather than CaCl2. All animals survived the surgery, and there were no deaths related to the procedure.
Histology and in situ zymography
The AAA generation procedure described above was performed on (n = 10) mice (BALB/c; Charles River Laboratories, Wilmington, MA). The mice were then randomized into either a treatment or a non-treatment group: the former group received 100mg/kg/day doxycycline in their drinking water, while the latter group received normal drinking water. The doxycycline water was changed every 3 days. After one month, the animals were sacrificed, and the infrarenal segment of the abdominal aortas were harvested and stored in OCT media at −80°C. Two serial cryostat sections (10um) were cut from the tissue samples for subsequent in situ zymography analysis; a third serial section (5um) was used for hematoxylin and eosin staining to assess structural morphology.
In situ zymography to detect gelatinolytic activity was performed on the serial tissue sections using a previously published method15, 16. Low-gelling temperature (LGT) agarose (1%; Sigma-Aldrich, St. Louis, MO) was dissolved in PBS at 80°C and then cooled to 37°C. Dye-quenched gelatin (DQ-gelatin) (Invitrogen, Carlsbad, CA) was dissolved to achieve a concentration of 1mg/mL in distilled water, and then diluted 1:10 in the LGT agarose solution. Agarose solution with DQ-gelatin (40uL) was then placed on top of one of the tissue sections, which was then covered with a microscope coverslip; agarose solution without DQ-gelatin (40uL) was placed on the second tissue section so that tissue autofluorescence could be measured. The sections were then placed at 4°C for 30 minutes to allow the agarose to gel; after this, they were kept at room temperature for 4 hours to allow the tissue gelatinases to incubate with the DQ-gelatin substrate. The sections were then imaged using a fluorescence microscope at an excitation wavelength of 480nm and at 20× magnification. Autofluorescence sections were imaged at an exposure time of 500msec; DQ-gelatin-containing sections were imaged at an exposure time of 50msec.
MMP activity was estimated from the in situ zymography fluorescence data in the following manner. The thresholding tool in the ImageJ software package was used to generate a mask representing the area in the fluorescence microscopy image that contained only the aortic wall, and the fluorescence intensity was measured within this area. This procedure was performed for the treatment and non-treatment aorta sections, as well as for the thoracic aorta sections. The MMP activity in the treatment group was calculated by subtracting the mean fluorescence from the thoracic aorta sections from the mean fluorescence of the treatment aorta sections; likewise, the MMP activity in the non-treatment group was calculated by subtracting the mean fluorescence from the thoracic aorta sections from the mean fluorescence of the non-treatment aorta sections. The percent change in MMP activity between the two groups was then calculated to quantify the decrement in enzyme activity secondary to doxycycline therapy.
MMP-activatable optical molecular probe
The MMP-activatable probe MMPSense18–20 (VisEn Medical, Woburn, MA) was used for the in vivo and ex vivo macroscopic scale imaging experiments. The probe’s design consists of a poly-l-lysine backbone studded with multiple methoxypolyethylene glycol chains to reduce immunogenicity and augment blood half-life; attached to this backbone are near-infrared (NIR) fluorochromes linked by a peptide sequence (GPLGVRGKC) that is cleavable by MMPs. Due to a fluorescence resonance energy transfer-like interaction between the fluorochromes when they are bound to the probe’s poly-l-lysine scaffold, the fluorescence of the fluorochromes is suppressed. However, following cleavage of the peptide linkers binding them to the backbone by MMPs, the fluorochromes regain their fluorescence many-fold. MMPSense has been shown to be specific for MMP-2, MMP-9, MMP-12, and MMP-13, and to not be activatable by other enzymes such as plasmin that are found in the walls of AAAs. The probe fluoresces in the NIR, with excitation at 680nm and emission at 700nm. Previous studies20 have demonstrated that the ideal time for MMP activity measurement is 24 hours post-injection, and therefore all imaging was performed with this probe at that time point.
Surface reflectance imaging
Abdominal aortic aneurysm generation surgery was performed on (n = 9) mice (balb/c; Charles River Laboratories, Wilmington, MA), with (n = 2) additional mice undergoing a sham procedure in which saline, rather than CaCl2, was applied to the aortic surface; the animals used in this experiment were distinct from the ones used in the histology experiment described previously. During the initial surgery, the infrarenal aortic diameter was measured to 0.01mm accuracy using digital calipers. The mice that underwent the AAA generation surgery were then randomized into three doxycycline treatment groups: 0mg/kg/day, 50mg/kg/day, and 100mg/kg/day. The animals received the appropriate dose of the medication in their drinking water, which was replaced every three days, for a total of 3 months. After this time period, 2nmol of MMPSense was injected into the animals intravenously. Twenty-four hours following injection, the animals were anesthetized with 2% isoflurane in 100% O2 at 1L/min, and the abdominal aortas were once again surgically exposed. The diameters of the infrarenal aorta were measured using digital calipers to assess for aneurismal growth over the experimental time period. The abdominal aortas were then imaged for NIR fluorescence generated from the optical probe localizing to and undergoing activation within the aortas. Assessment was made using a commercial surface reflectance fluorescence imaging system (Olympus Small Animal Imaging System OV100; Olympus Corp., Tokyo, Japan). Fluorescence intensity from the thoracic aorta was also measured to serve as a baseline control; imaging on the thoracic aorta was performed ex vivo after the in vivo abdominal aorta imaging had been performed. The animals’ diaphragms prevented any CaCl2 from entering the thoracic cavity; the thoracic aorta would therefore not have undergone any inflammatory changes mediated by exposure to CaCl2.
Regions of interest (ROIs) were drawn within the NIR fluorescence images to measure the mean fluorescence intensity of the infrarenal aortas. This mean fluorescence was then normalized for each mouse by subtracting from it the mean fluorescence intensity measured from the animal’s thoracic aorta. The fluorescence intensity was also normalized for variations in acquisition time by dividing the pixel counts by the image exposure time to arrive at an exposure time-independent counts/msec value.
Endovascular imaging
Abdominal aortic aneurysm surgery was performed on (n = 4) female Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA). Two rats were then randomly assigned to a treatment group in which they received doxycycline 100mg/kg/day in their drinking water, which was changed every three days, whereas the other two rats were given normal drinking water. After one month, 15nmol of MMP sensitive imaging probe was administered intravenously. Twenty-four hours following administration, the animals were anesthetized with 2% isoflurane in 100% O2 at 1L/min, and their abdominal aortas were surgically exposed. A 0.4mm diameter fiber-optic imaging catheter was then introduced into the aorta just proximal to the iliac bifurcation in the following manner. Proximal and distal control around the catheter insertion site was first established with vascular clamps. A nick was made in the aorta using microsurgical scissors between the clamps, and a 24-gauge plastic angiocatheter sleeve was introduced through this nick into the aorta, directed rostrally. The imaging catheter was then introduced through the plastic sleeve and advanced proximally until the tip was well within the thoracic aorta. Blood loss through the angiocatheter sleeve was minimal, as the inner diameter of the sleeve was similar to the outer diameter of the imaging catheter. The catheter was retracted from the thoracic aorta into the abdominal cavity and along the length of the aneurysm at a constant rate by a pullback device. Fluorescent signal intensity collected by the catheter was displayed and stored by a custom-designed fluorescence imaging system that allows for real-time and high-resolution catheter-based optical imaging21, 22.
Subsequent to the endovascular imaging studies, the animals were sacrificed, and their abdominal and thoracic aortas were harvested. Surface reflectance imaging was performed on these tissue samples using the OV100 imaging system, as above. ROIs were drawn within the NIR images of the thoracic and infrarenal aortas, and mean fluorescence intensities were measured to assess for differential MMP activity in baseline versus aneurysmal tissue, respectively.
Data interpretation and statistical analysis
ROIs were drawn for the surface reflectance imaging experiment as circles with diameters equal to the width of the aorta at its greatest diameter and centered on the aneurysm. If the circle contained areas of the image beyond the edge of the aorta (i.e. dark background), the diameter was reduced in size until the circle was of maximal size and contained only aorta. For the endovascular imaging experiment, an ROI of a fixed size was drawn within the section of the images corresponding to the aortic wall; the same ROI was propagated throughout all the images for both the treated and untreated animal imaging data sets.
The data presented in this study represents means or percent changes plus or minus the standard error or percent standard error. All fluorescent images within each figure are shown with identical window and level settings; the acquisition times are identical for all the images within an experiment as well unless they are explicitly stated to be different, as in Figure 1.
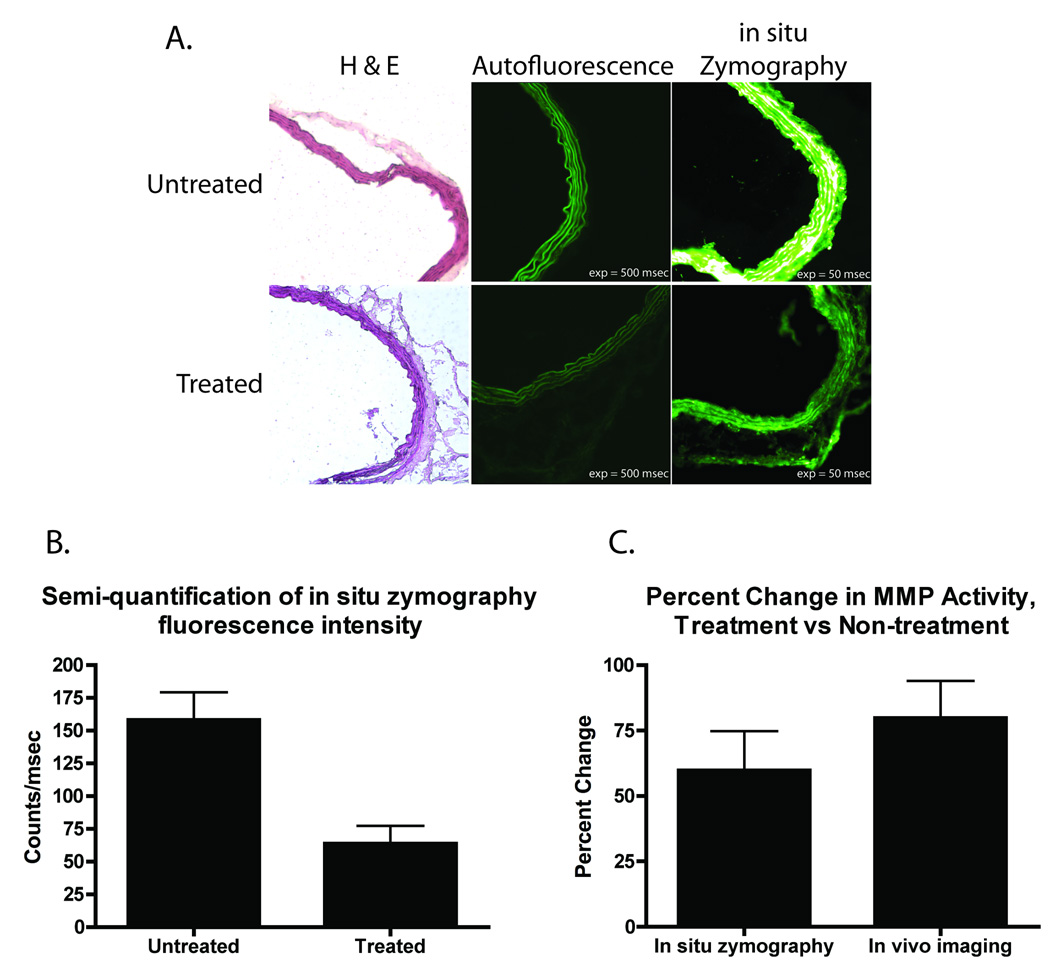
Figure 1.
Histologic assessment of MMP activity. A, histology with hematoxylin and eosin (H & E) staining and in situ zymography of CaCl2-induced AAA treated with doxycycline. The top row of images corresponds to serial sections acquired from the abdominal aorta of one representative animal that received no doxycycline therapy. The bottom row corresponds to serial sections acquired from the abdominal aorta of one representative animal received doxycycline 100mg/kg/day. Of note, autofluorescence in the in situ zymography experiment was minimal. Both autofluorescence images are displayed with identical window/level settings, as are both of the in situ zymography images. B, semi-quantitative evaluation of MMP activity by in situ zymography. In situ zymography reveals a marked increase in MMP activity in the untreated animal versus the treated animal after subtraction of the tissue autofluorescence as measured from the thoracic aorta. C, comparison of percent change in MMP activity as determined by in situ zymography and in vivo optical imaging. The percent decrement in fluorescence between treated and untreated animals was calculated for both in situ zymography and in vivo optical imaging. In both methods, the percent change was similar, with no statistical difference between the two.
Results
Histologic determination of MMP activity
Ex vivo histologic analysis of intramural MMP activity in aneurysmal tissue was performed. As Figure 1A illustrates, there is a significant decrease in gelatinolytic activity as measured by in situ zymography in the aortas of mice with surgically generated AAAs treated with the nonspecific MMP inhibitor doxycycline versus untreated mouse aortas. The images presented in this Figure are representative of the data collected from the (n = 10) mice used in this experiment. These results are in concordance with findings that have been reported previously using conventional gelatin zymography14. Figure 1B offers a semi-quantitative analysis of the relative fluorescence intensities observed by in situ zymography for the treated and untreated animals; the aortas of the animals that received doxycycline demonstrated lower MMP activity when compared to the aortas of the animals that received no doxycycline therapy. Figure 1C compares the percent change in MMP activity between the treated and untreated animals as measured by in situ zymography and in vivo MMP imaging (discussed in more detail in the following subsection). Both techniques revealed an approximate 65% decrease in MMP activity between the treated and untreated groups, with no statistically significant difference between the two techniques’ measurements (in vivo imaging: 59.8% [95% CI: 29.8 – 89.8]; in situ zymography: 79.8% [95% CI: 51.3 – 108.1]).
Surface reflectance imaging of MMP activity
Figure 2 summarizes the results of the surface reflectance imaging experiment, in which (n = 11) mice were treated with three different doses of doxycycline for three months after AAA generation surgery; following that time period, MMP activity within the aneurysmal wall was measured intravitally using the MMP sensitive imaging probe. The representative images in Figure 2A show that MMP activity, as reflected in the fluorescence signal intensity, is markedly decreased in the aorta of the animal that received doxycycline 100mg/kg/day versus in that of the untreated animal. As depicted in Figure 2B, and as has been shown previously 14, doxycycline causes a dose-dependent reduction in the rate of aneurysmal growth, with high doses of the drug resulting in nearly complete suppression of aneurysm formation. Figure 2C compares the imaged MMP activity across the treatment groups and illustrates a similar doxycycline dose-dependent response, with high doses of doxycycline reducing MMP activity to the extent that there is no statistically significant difference in the proteolytic degradative processes within the aortic walls of mice that received a dose of 100mg/kg/day to the mice that underwent sham AAA surgery.
Figure 2.
Effects of doxycycline therapy on AAA growth and MMP activity. A, surface reflectance imaging performed intravitally on abdominal aortas of animals 3 months after AAA generation surgery. The left set of images is of a representative animal that received no doxycycline therapy, while the right set corresponds to an animal that received doxycycline 100mg/kg/day. Standard white-light (WL) analysis demonstrates a focal dilatation of the aortic diameter in the untreated animal that is not present in the treated animal. Moreover, there is significantly increased fluorescence signal, and therefore MMP activity, in the untreated animal versus the treated animal. Fluorescence images were acquired at identical exposure times and are displayed with identical window/level and color look up table settings. B, mice following AAA generation surgery were randomized to receive doxycycline at doses of either 0 (n = 3), 50 (n = 3), or 100mg/kg/day (n = 3) for 3 months. A dose-dependent response to AAA development and progression was found; animals that received the highest dose showed no statistically significant aneurysmal growth compared to the animals that underwent the sham AAA generation procedure (n = 2). C, in vivo surface reflectance imaging of MMP activity 24 hours following administration of MMP sensitive probe likewise exhibited a dose-dependent response to doxycycline therapy, with MMP activity significantly suppressed with increased doses of doxycycline.
Correlation of AAA growth rate with MMP activity
The relationship between AAA growth rate and MMP activity was further evaluated by comparing the former against the latter for each individual mouse in the scatter plot shown in Figure 3. There is a linear relationship between the rate at which the AAA expands and the intensity of fluorescent signal from the MMP selective probe, suggesting that the greater the MMP activity within the wall of an aneurysm, the faster the aneurysm grows. By modulation of the AAA growth rate with doxycycline, a direct correlation is shown over a wide range of growth rates between the rate of AAA growth and the degree of MMP activity.
Figure 3.
Correlation of MMP activity with AAA growth rate. The rate of growth of AAA for each individual mouse across the 4 experimental groups in Figure 2 was plotted against the fluorescence measured from the animal’s abdominal aorta in this scatter plot. A linear relationship (r = 0.78) was found between MMP activity, as measured by the optical reporter fluorescence intensity, and AAA growth rate. Each experimental group’s data points tended to cluster along the best-fit line, with overlap between the sham surgery and 100mg/kg/day doxycycline treatment groups.
Endovascular assessment of MMP activity in AAA
To assess whether the in vivo surface reflectance imaging of MMP activity in AAAs could be applied in a minimally invasive paradigm, AAAs were generated in (n = 4) rats. Following surgery, two rats received doxycycline 100mg/kg/day for a period of one month, and two rats received no drug. Endovascular imaging of the gelatinolytic activity within the AAA was then performed by introducing a 0.4mm fluorescent imaging catheter into the aorta and using a constant pullback device to retract the catheter tip from the thoracic aorta, into the abdominal aorta, and along the length of the aneurysm. The fluorescence intensity profile as the catheter tip passed along the aneurysm is shown in Figure 4. The red squares, which denote data collected from one representative non-treatment rat, demonstrate a focal elevation in MMP activity at the location of the aneurysm. The fluorescence profile of a representative treatment group rat exhibits no such elevation (blue triangles). These data confirm the feasibility of performing in vivo assessment of MMP activity within AAAs through a minimally invasive, endovascular approach.
Figure 4.
Endovascular optical imaging of MMP activity. AAAs were generated in rats, and endovascular imaging with the optical MMP reporter was performed one month later. A constant pull-back device was used to retract the imaging catheter tip along the length of the aorta where the aneurysm was induced. Two representative profiles are shown, with the red squares corresponding to an animal that received no doxycycline treatment, and the blue triangles corresponding to an animal that received doxycycline 100mg/kg/day. The fluorescence intensity plot profile shows a focal elevation in MMP activity in the former animal, whereas the no elevation is seen in the treated animal’s abdominal aorta.
Following the endovascular imaging, the animals’ aortas were harvested so that surface reflectance imaging could be performed on the samples; Figure 5 presents these results. MMP activity measured within the aortas of the treatment animals was markedly decreased compared to the non-treatment animals, and in fact was statistically indistinct from the MMP activity occurring within the thoracic aorta, which served as a negative control.
Figure 5.
Ex vivo analysis of MMP activity in rat AAAs. A, following endovascular imaging, abdominal and thoracic aortas were harvested from the animals so that surface reflectance imaging of the fluorescence activity from the optical reporter could be performed. The left-most set of images show white light (WL) and near infrared (NIR) images from one representative animal’s thoracic aorta; the center set shows an abdominal aorta from an animal that received doxycycline 100mg/kg/day; the right-most set shows an abdominal aorta from an animal that received no doxycycline. Fluorescence intensity is markedly increased in the untreated animal’s abdominal aorta. All fluorescence images were acquired at identical exposure times and are presented at identical window and level settings. B, across all experimental animals, the treated animals’ abdominal aortas displayed MMP activity similar to the negative control, as represented by the animal’s thoracic aorta, while the untreated animals’ abdominal aortas had significantly increased MMP activity.
Discussion
In this study an in vivo method to measure and quantify MMP activity within AAAs on the macroscopic scale is presented that combines optical reporter probes with near infrared imaging devices. The optical imaging probe used in this study increases its fluorescence many-fold following cleavage by activated MMPs and thus functions as a sensitive marker for MMP activity. Fluorescent signal from this and similarly designed probes has been shown previously to co-localize with MMP-2 and MMP-920, as well as MMP-12 and MMP-13. MMP sensitive probes have been successfully applied to several areas of cardiovascular imaging, including the imaging of inflammation within atherosclerosis using optical20 or MR approaches23, 24, as well as the optical imaging of MMP activity in post-myocardial infarction remodeling19. Advantages of imaging optically, as in the current study, include high spatial resolution, real-time imaging capabilities, low relative cost, and lack of ionizing radiation.
In this study, we modeled variable disease severity and progression through the use of doxycycline therapy to diminish MMP-dependent proteolysis. Doxycycline, a non-selective MMP inhibitor, has been shown to reduce aneurysmal growth in a dose dependent manner, with concomitant suppression of MMP activity as measured by gelatin zymography12, 14. We have presented in vivo data here that verifies these previous findings, validated by histologic assessment of MMP activity using in situ zymography. Furthermore, we quantified the intramural MMP activity and assessed its relationship with the rate of AAA growth for each mouse, demonstrating a direct correlation between the two processes. Thus, with a single measurement of MMP activity, one could potentially predict the time course of aneurysmal growth, to a first order, linear approximation. These results add to the evidence for the causal relationship between elevated MMP activity and AAA progression. The association has been hypothesized based on ex vivo analyses of protein concentration or enzymatic activity in microscopic sections; however, the intravital, whole organ data presented in this report has the advantage of no potential additional confounding factors associated with sample preparation and processing.
We expanded upon the clinical relevance of the in vivo optical molecular imaging approach by applying it to the endovascular imaging of MMP activity within AAAs. The ability to minimally invasively assess for proteolytic activity, and thus predict the rate of AAA growth, may augment the management of AAAs by avoiding the need for serial imaging to retrospectively evaluate aneurysm growth velocity. The prospect of medical therapy has been explored through a number of pharmacologic agents25, 26, and the in vivo measurement of MMP activity would represent an ideal end-point to monitor the efficacy of a drug in modulating aneurysmal pathophysiology. Several clinical trials25, 27, 28 have suggested that doxycycline administration for AAA therapy is well-tolerated and reduces MMP-9 plasma levels25, reduces expansion rate28, and reduces aneurysmal neutrophil, cytotoxic T-cell, and MMP-9 protein content27. We believe that our in vivo imaging approach corroborates these prior results and offers a practical, clinically relevant end-point for clinical trials evaluating this drug and other MMP inhibitors for treatment of AAAs.
Additionally, though we demonstrate MMP imaging endovascularly in n = 4 rats, and in a manner that requires arterial catheterization for direct visualization of fluorescence intensity, we believe that the methodology presented herein may have important clinical ramifications, such as in the management of “small” aortic aneurysms. Rather than basing the need for surgical intervention of AAAs on size criteria alone, the inclusion of inflammatory activity data into the clinical decision paradigm may provide for a more informed risk-benefit analysis. For example, “small” aneurysms less than 5.5cm in diameter are generally not intervened upon but are still at risk of rupture. Although two randomized trials have demonstrated no improved survival benefit for elective surgical repair of small aneurysms29, a consideration of the degree of MMP proteolytic function within the aneurysms may help identify those lesions that are at the highest risk of rupturing and therefore should be repaired, as MMPs including MMP-9 are significantly increased at the site of aneurysmal rupture30.
Acknowledgments
Funding:
This research was supported in part by NIH grant RO1-EB001872.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest:
The authors declare no conflicts of interest.
References
- 1.Anidjar S, Dobrin PB, Eichorst M, Graham GP, Chejfec G. Correlation of inflammatory infiltrate with the enlargement of experimental aortic aneurysms. J Vasc Surg. 1992;16:139–147. doi: 10.1067/mva.1992.35585. [DOI] [PubMed] [Google Scholar]
- 2.Freestone T, Turner RJ, Coady A, Higman DJ, Greenhalgh RM, Powell JT. Inflammation and matrix metalloproteinases in the enlarging abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 1995;15:1145–1151. doi: 10.1161/01.atv.15.8.1145. [DOI] [PubMed] [Google Scholar]
- 3.Lopez-Candales A, Holmes DR, Liao S, Scott MJ, Wickline SA, Thompson RW. Decreased vascular smooth muscle cell density in medial degeneration of human abdominal aortic aneurysms. Am J Pathol. 1997;150:993–1007. [PMC free article] [PubMed] [Google Scholar]
- 4.Shah PK. Inflammation, metalloproteinases, and increased proteolysis: an emerging pathophysiological paradigm in aortic aneurysm. Circulation. 1997;96:2115–2117. doi: 10.1161/01.cir.96.7.2115. [DOI] [PubMed] [Google Scholar]
- 5.Shimizu K, Mitchell RN, Libby P. Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2006;26:987–994. doi: 10.1161/01.ATV.0000214999.12921.4f. [DOI] [PubMed] [Google Scholar]
- 6.Ailawadi G, Eliason JL, Upchurch GR., Jr Current concepts in the pathogenesis of abdominal aortic aneurysm. J Vasc Surg. 2003;38:584–588. doi: 10.1016/s0741-5214(03)00324-0. [DOI] [PubMed] [Google Scholar]
- 7.Davies MJ. Aortic aneurysm formation: lessons from human studies and experimental models. Circulation. 1998;98:193–195. doi: 10.1161/01.cir.98.3.193. [DOI] [PubMed] [Google Scholar]
- 8.Knox JB, Sukhova GK, Whittemore AD, Libby P. Evidence for altered balance between matrix metalloproteinases and their inhibitors in human aortic diseases. Circulation. 1997;95:205–212. doi: 10.1161/01.cir.95.1.205. [DOI] [PubMed] [Google Scholar]
- 9.Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest. 2002;110:625–632. doi: 10.1172/JCI15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMillan WD, Tamarina NA, Cipollone M, Johnson DA, Parker MA, Pearce WH. Size matters: the relationship between MMP-9 expression and aortic diameter. Circulation. 1997;96:2228–2232. doi: 10.1161/01.cir.96.7.2228. [DOI] [PubMed] [Google Scholar]
- 11.Thompson RW, Parks WC. Role of matrix metalloproteinases in abdominal aortic aneurysms. Ann N Y Acad Sci. 1996;800:157–174. doi: 10.1111/j.1749-6632.1996.tb33307.x. [DOI] [PubMed] [Google Scholar]
- 12.Pyo R, Lee JK, Shipley JM, Curci JA, Mao D, Ziporin SJ, Ennis TL, Shapiro SD, Senior RM, Thompson RW. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J Clin Invest. 2000;105:1641–1649. doi: 10.1172/JCI8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petrinec D, Liao S, Holmes DR, Reilly JM, Parks WC, Thompson RW. Doxycycline inhibition of aneurysmal degeneration in an elastase-induced rat model of abdominal aortic aneurysm: preservation of aortic elastin associated with suppressed production of 92 kD gelatinase. J Vasc Surg. 1996;23:336–346. doi: 10.1016/s0741-5214(96)70279-3. [DOI] [PubMed] [Google Scholar]
- 14.Prall AK, Longo GM, Mayhan WG, Waltke EA, Fleckten B, Thompson RW, Baxter BT. Doxycycline in patients with abdominal aortic aneurysms and in mice: comparison of serum levels and effect on aneurysm growth in mice. J Vasc Surg. 2002;35:923–929. doi: 10.1067/mva.2002.123757. [DOI] [PubMed] [Google Scholar]
- 15.Frederiks WM, Mook OR. Metabolic mapping of proteinase activity with emphasis on in situ zymography of gelatinases: review and protocols. J Histochem Cytochem. 2004;52:711–722. doi: 10.1369/jhc.4R6251.2004. [DOI] [PubMed] [Google Scholar]
- 16.Mook OR, Van Overbeek C, Ackema EG, Van Maldegem F, Frederiks WM. In situ localization of gelatinolytic activity in the extracellular matrix of metastases of colon cancer in rat liver using quenched fluorogenic DQ-gelatin. J Histochem Cytochem. 2003;51:821–829. doi: 10.1177/002215540305100613. [DOI] [PubMed] [Google Scholar]
- 17.Chiou AC, Chiu B, Pearce WH. Murine aortic aneurysm produced by periarterial application of calcium chloride. J Surg Res. 2001;99:371–376. doi: 10.1006/jsre.2001.6207. [DOI] [PubMed] [Google Scholar]
- 18.Bremer C, Tung CH, Weissleder R. In vivo molecular target assessment of matrix metalloproteinase inhibition. Nat Med. 2001;7:743–748. doi: 10.1038/89126. [DOI] [PubMed] [Google Scholar]
- 19.Chen J, Tung CH, Allport JR, Chen S, Weissleder R, Huang PL. Near-infrared fluorescent imaging of matrix metalloproteinase activity after myocardial infarction. Circulation. 2005;111:1800–1805. doi: 10.1161/01.CIR.0000160936.91849.9F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deguchi JO, Aikawa M, Tung CH, Aikawa E, Kim DE, Ntziachristos V, Weissleder R, Libby P. Inflammation in atherosclerosis: visualizing matrix metalloproteinase action in macrophages in vivo. Circulation. 2006;114:55–62. doi: 10.1161/CIRCULATIONAHA.106.619056. [DOI] [PubMed] [Google Scholar]
- 21.Sheth RA, Upadhyay R, Weissleder R, Mahmood U. Real-time multichannel imaging framework for endoscopy, catheters, and fixed geometry intraoperative systems. Mol Imaging. 2007;6:147–155. [PubMed] [Google Scholar]
- 22.Upadhyay R, Sheth RA, Weissleder R, Mahmood U. Quantitative real-time catheter-based fluorescence molecular imaging in mice. Radiology. 2007;245:523–531. doi: 10.1148/radiol.2452061613. [DOI] [PubMed] [Google Scholar]
- 23.Amirbekian V, Aguinaldo JG, Amirbekian S, Hyafil F, Vucic E, Sirol M, Weinreb DB, Le Greneur S, Lancelot E, Corot C, Fisher EA, Galis ZS, Fayad ZA. Atherosclerosis and matrix metalloproteinases: experimental molecular MR imaging in vivo. Radiology. 2009;251:429–438. doi: 10.1148/radiol.2511080539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lancelot E, Amirbekian V, Brigger I, Raynaud JS, Ballet S, David C, Rousseaux O, Le Greneur S, Port M, Lijnen HR, Bruneval P, Michel JB, Ouimet T, Roques B, Amirbekian S, Hyafil F, Vucic E, Aguinaldo JG, Corot C, Fayad ZA. Evaluation of matrix metalloproteinases in atherosclerosis using a novel noninvasive imaging approach. Arterioscler Thromb Vasc Biol. 2008;28:425–432. doi: 10.1161/ATVBAHA.107.149666. [DOI] [PubMed] [Google Scholar]
- 25.Baxter BT, Pearce WH, Waltke EA, Littooy FN, Hallett JW, Jr, Kent KC, Upchurch GR, Jr, Chaikof EL, Mills JL, Fleckten B, Longo GM, Lee JK, Thompson RW. Prolonged administration of doxycycline in patients with small asymptomatic abdominal aortic aneurysms: report of a prospective (Phase II) multicenter study. J Vasc Surg. 2002;36:1–12. doi: 10.1067/mva.2002.125018. [DOI] [PubMed] [Google Scholar]
- 26.Yoshimura K, Aoki H, Ikeda Y, Fujii K, Akiyama N, Furutani A, Hoshii Y, Tanaka N, Ricci R, Ishihara T, Esato K, Hamano K, Matsuzaki M. Regression of abdominal aortic aneurysm by inhibition of c-Jun N-terminal kinase. Nat Med. 2005;11:1330–1338. doi: 10.1038/nm1335. [DOI] [PubMed] [Google Scholar]
- 27.Lindeman JH, Abdul-Hussien H, van Bockel JH, Wolterbeek R, Kleemann R. Clinical trial of doxycycline for matrix metalloproteinase-9 inhibition in patients with an abdominal aneurysm: doxycycline selectively depletes aortic wall neutrophils and cytotoxic T cells. Circulation. 2009;119:2209–2216. doi: 10.1161/CIRCULATIONAHA.108.806505. [DOI] [PubMed] [Google Scholar]
- 28.Mosorin M, Juvonen J, Biancari F, Satta J, Surcel HM, Leinonen M, Saikku P, Juvonen T. Use of doxycycline to decrease the growth rate of abdominal aortic aneurysms: a randomized, double-blind, placebo-controlled pilot study. J Vasc Surg. 2001;34:606–610. doi: 10.1067/mva.2001.117891. [DOI] [PubMed] [Google Scholar]
- 29.Lederle FA, Wilson SE, Johnson GR, Reinke DB, Littooy FN, Acher CW, Ballard DJ, Messina LM, Gordon IL, Chute EP, Krupski WC, Busuttil SJ, Barone GW, Sparks S, Graham LM, Rapp JH, Makaroun MS, Moneta GL, Cambria RA, Makhoul RG, Eton D, Ansel HJ, Freischlag JA, Bandyk D. Immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346:1437–1444. doi: 10.1056/NEJMoa012573. [DOI] [PubMed] [Google Scholar]
- 30.Wilson WR, Anderton M, Schwalbe EC, Jones JL, Furness PN, Bell PR, Thompson MM. Matrix metalloproteinase-8 and -9 are increased at the site of abdominal aortic aneurysm rupture. Circulation. 2006;113:438–445. doi: 10.1161/CIRCULATIONAHA.105.551572. [DOI] [PubMed] [Google Scholar]