Abstract
Immune responsiveness declines with age in part due to the development of CD8+ T cell expansions (TCE) that can dominate the peripheral T cell pool. Although some TCE arise due to persistent antigen stimulation from chronic infections, others arise in the apparent absence of chronic infection. We have recently shown that this latter class of TCE can arise over time from the memory CD8+ T cell pool established by an acute viral infection. Unlike TCE driven by chronic infections, these age-related TCE do not display the phenotypic and in vitro functional characteristics of exhausted cells. However, the rate at which these age-related TCE develop from the memory CD8+ T cell pool, and their ability to mount a recall response to secondary pathogen challenge in vivo, are not known. In the current study, we analyzed large cohorts of mice over time for the development of TCE following Sendai virus infection and found a progressive increase in the appearance of TCE such that the majority of mice showed evidence of TCE within the memory T cell pool by two years post-infection. Using a dual adoptive transfer approach to address the recall potential of virus-specific TCE, we also demonstrate that the majority of TCE examined are poorly responsive to a secondary infection. Therefore, we provide evidence that the development of TCE is a common occurrence due to the progressive dysregulation of the virus-specific memory T cell pool with age, but many TCE are profoundly defective in their ability to mediate recall responses.
Keywords: Lung, Viral, CD8+ T cells, Aging
Introduction
Aging is associated with the progressive dysregulation of the immune system and a general decline in immune responsiveness to infection and vaccination (1–6). One feature of this dysregulation is the appearance of CD8+ T cell clonal expansions (TCE) which are non-malignant monoclonal populations of CD8+ T cells that appear with increasing frequency as individuals age (7–13). The sizes of these expansions are variable, but they can sometimes represent up to 90% of the entire peripheral T cell repertoire. TCE in humans are typically observed in individuals that are seropositive for chronic virus infections, such as cytomegalovirus, suggesting that persistent antigenic stimulation drives these expansions (14, 15). However, TCE have also been found in the apparent absence of chronic infections leading to the suggestion that they can be classified into two hypothetical groups that are either dependent or independent of chronic antigen stimulation (16). The sheer size of these expansions has led to speculation that they impact immune responses to both new and recall antigens.
TCE are frequently observed in the mouse, thus providing a means to study the genesis and consequences of these clonal populations. As in humans, there appear to be two classes of TCE in mice that are either dependent or independent of chronic antigen stimulation, and the properties of these murine TCE are essentially identical to those observed in humans. Recently, we analyzed long-term memory in mice that had recovered from an intranasal Sendai or influenza virus infection and conclusively demonstrated that antigen-specific TCE can progressively develop from the memory pool as the mice age (17). These TCE are indistinguishable from normal memory T cells in terms of phenotype and function, suggesting that they develop stochastically from the existing memory T cell pool.
The impact of TCE on host immunity is an important issue that is not well understood. It has previously been shown that TCE found in models of chronic infection are typically non-functional (in terms of cytokine production) and presumably reduce overall immunocompetence (10, 12, 14, 15, 18). For example, recent analysis of a TCE that showed no cross-reactivity to HSV-1 nevertheless impaired the de novo response to HSV-1 infection by restricting the naïve T cell repertoire (11). Conversely, antigen-specific TCE which arise in the absence of persistent antigen appear to retain effector function (17). However, it is not clear whether they retain the capacity to mediate protective immunity to secondary challenge since their high frequencies could either benefit or impair a response to the pathogen for which they are specific. To directly address this issue, we have analyzed the development and function of TCE in mice that have recovered from an acute Sendai virus infection. The data show that memory dysregulation and TCE development occur in the majority of animals over time. Furthermore, we show that memory T cells in many of the mice that exhibit evidence of antigen-specific TCE are profoundly deficient in their capacity to mediate recall responses to secondary virus challenge. Together, these findings demonstrate that T cell memory in aged animals becomes increasingly dysregulated and that this can be associated with a substantially impaired capacity to mount secondary immune responses.
Materials and Methods
Mice, viruses, and infections
C57BL/6, B6.SJL-Ptprca Pep3/BoyJ (CD45.1), and B6.PL-Thy1a/CyJ (CD90.1) mice were purchased from The Jackson Laboratory and re-derived stocks were maintained at the Trudeau Institute. Sendai virus (Enders strain) was grown, stored, and titered as previously described (19). For intranasal infections, 8–12 week-old mice were anesthetized with 2,2,2-tribromoethanol (200mg/kg) and administered 250 50% egg infectious doses (EID50) in a volume of 30μl. All animal studies were approved by the Trudeau Institute Animal Care and Use Committee.
Tissue harvest
For serial bleeds, peripheral blood (approximately 100μl) was obtained by nicking the tail vein and diluted 1:2 in PBS containing 10U/ml heparin. For endpoint assays, cells were isolated from the lung airways by bronchoalveloar lavage (BAL), the lung parenchyma by digestion in collagenase/DNase for 1 hour at 37°C followed by percoll gradient centrifugation, and the MLN and spleen by mechanical disruption. Following red blood cell lysis with ammonium buffered chloride, live cell numbers were determined by counting and trypan blue exclusion.
Flow cytometry and cell sorting
Single cell suspensions were incubated with Fc-block (anti-CD16/32) for 15 minutes on ice followed by staining with SenNP324–332Kb tetramer for 1 hour at room temperature. Tetramer-labeled cells were incubated with antibodies to surface proteins for 30 minutes on ice. Antibodies were purchased from BD Biosciences (CD8, CD49d, CD127, and Ly6c), eBioscience (CD8, CD11a, CD44, CD45.1, CD45.2, CD90.1, CD90.2), BioLegend (CD27 and CD69), and Southern Biotech (KLRG1). All TCR Vβ antibodies were from BD Biosciences. Samples were run on a FACS Canto II flow cytometer (BD Biosciences) and data were analyzed with Flow Jo software (TreeStar). For cell sorting, total splenocytes were stained with antibodies to surface proteins as described above, and bulk memory T cells (CD8+ CD44hi) were isolated. An aliquot of sorted cells from each donor population was stained with SenNP324–332Kb tetramer as described above to determine the number of Sendai-specific CD8+ T cells. Sorting was performed on a FACSVantage cell sorter with DIVA enhancement software (BD Biosciences).
BrdU incorporation and cytokine staining
For measurement of homeostatic turnover, mice were administered drinking water containing 0.8 mg/ml BrdU (Sigma) every 2 days for 14 days. Following sacrifice, single cell suspensions were stained with tetramers and antibodies to surface proteins as described above and BrdU incorporation was detected using the BrdU Flow Kit (BD Biosciences). For measurement of cytokine production, single cell suspensions were incubated with SenNP324–332 or control peptides as previously described (20). Cells were stained for surface markers, fixed and permeabilized (CytoFix/CytoPerm kit, BD Biosciences), and stained for intracellular cytokines with antibodies to IFN-γ and TNF-α.
Dual adoptive transfer
Dual adoptive transfer experiments were performed as previously described (21). Briefly, sorted total memory CD8+ T cells from the donors were combined such that the number of Sendai NP324–332/Kb-specific T cells in each donor population was equal. The combined cells (1×104 Sendai-specific CD8+ T cells from each donor population) were intravenously transferred into naive recipients and then intranasally challenged with 250 EID50 of Sendai virus one day later. On day 11 post-infection, lymphocytes were isolated from various tissues and host and donor Sendai-specific cells were identified by flow cytometry. The relative response in each tissue was calculated from the numbers of Sendai NP324–332/Kb-specific T cells derived from each donor.
Statistics
Statistical analysis was performed with Prism GraphPad software using a Pearson correlation. A p-value of less than 0.05 was considered significant.
Results
Progressive development of TCE in the virus-specific memory T cell pool
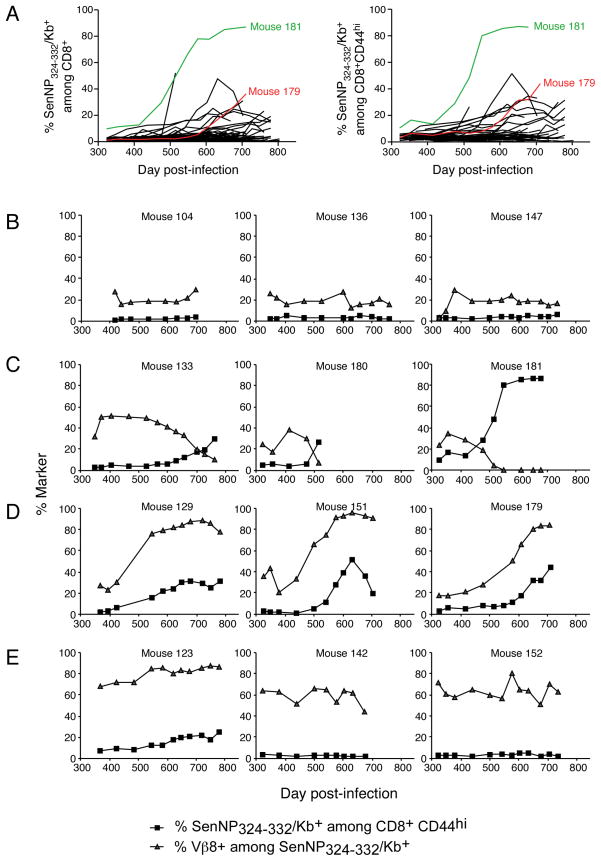
To investigate the kinetics with which antigen-specific TCE develop, we infected a cohort of 100 C57BL/6 mice with Sendai virus and analyzed CD8+ T cell memory to the immunodominant NP324–332/Kb-specific epitope over time. Mice were bled at approximately one month intervals, starting at 11 months post-infection, and the samples analyzed to determine the frequency of NP324–332/Kb-specific T cells. As shown in Figure 1A (left panel), the frequency of NP324–332/Kb-specific T cells among total CD8+ T cells ranged from 0.5–2% in most animals at day 330, although one animal (mouse 181, marked in green) had a frequency of approximately 10%. Over time, the number of animals that expressed elevated frequencies of NP324–332/Kb-specific T cells (>2% of CD8+) increased. The time at which an expansion could be first identified varied for each animal and ranged from day 320 to 700 in this experiment. For example, an expansion in animal 179 was first detected on day 600 (marked in red). However, by day 780, almost all of the mice that were still alive expressed elevated frequencies of NP324–332/Kb-specific T cells. This pattern could not be explained by a change in the ratio of naïve and memory cells in the CD8+ T cell pool since almost identical patterns were observed when the data were calculated with respect to the total memory (CD8+ CD44hi) pool (Figure 1A, right panel). Analysis of individual mice indicated that the increases in NP324–332/Kb-specific T cell frequencies were generally gradual in nature, and that in some cases the frequencies of these cells actually fell after reaching a maximum. In most mice the frequency of NP324–332/Kb-specific T cells rose to between 2 and 40% of the CD8+ CD44hi pool. However in one animal, number 181, the frequency of NP324–332/Kb-specific T cells rose to approximately 90% of the CD8+ CD44hi pool.
Figure 1. The virus-specific memory CD8+ T cell pool becomes increasingly dysregulated over time.
One hundred B6 mice were infected with Sendai virus and rested for 11 months. Serial bleeds were performed on individual animals roughly once a month for the remainder out to 26 months post-infection. (A) The frequency of SenNP324–332Kb+ cells among total CD8+ T cells (left) or total CD8+ CD44hi T cells (right) for each individual mouse is graphed over time. Mice exhibiting TCEs used for further study (#181, green; #179, red) are highlighted. (B-E) Different patterns of dysregulation within the SenNP324–332Kb+ memory T cell pool over time. The data show the frequency of SenNP324–332Kb+ cells among total CD8+ CD44hi T cells (squares) and the frequency of Vβ8+ cells among total SenNP324–332Kb+ cells (triangles) for individual mice.
The increase in NP324–332/Kb-specific T cell frequencies is characteristic of the development of TCE in which single or a limited number of clones of antigen-specific cells expand and dominate the memory pool. Since these expansions are monoclonal, or pauciclonal, in nature, they can be followed by analyzing T cell receptor (TCR) diversity. Given the limited samples available, we followed TCR diversity by analyzing Vβ8 usage (Vβ8.1, Vβ8.2 or Vβ8.3) by NP324–332/Kb-specific T cells. Several distinct patterns of Vβ8 usage were observed and representative examples are illustrated in Figure 1, panels B, C, D, and E. The TCR repertoire of NP324–332/Kb-specific T cells has been previously reported and typically comprises approximately 20% Vβ8+ T cells (22). This is illustrated by three representative animals in Figure 1B where the absolute frequency of NP324–332/Kb-specific T cells and Vβ8 usage by those cells remained relatively constant between days 320 and 780 post-infection. In contrast, Vβ8 usage was varied dramatically in animals that exhibited increases in the frequencies of NP324–332/Kb-specific T cells. In some cases, Vβ8 usage decreased dramatically as the frequency of NP324–332/Kb-specific T cells increased, indicating that the expanded cells expressed some other Vβ element (Figure 1C). In other cases, Vβ8 usage increased dramatically as the frequency of NP324–332/Kb-specific T cells increased, indicating that the expanded cells specifically expressed one of the Vβ8 elements (Figure 1D). Interestingly, some animals exhibited very high levels of Vβ8 usage at the earliest timepoint analyzed, day 320, that did not appear to correlate with increasing frequencies of NP324–332/Kb-specific T cells (Figure 1E). These data suggest that there may be perturbations in the T cell memory pool that do not result in the outgrowth of antigen-specific T cells and further suggest that these perturbations occur earlier than day 320. Taken together, these data indicate that the antigen-independent expansion of virus-specific T cells in these mice are monoclonal or pauciclonal in nature and develop gradually over time in most animals.
Antigen-specific T cell expansions exhibit increased rates of homeostatic proliferation
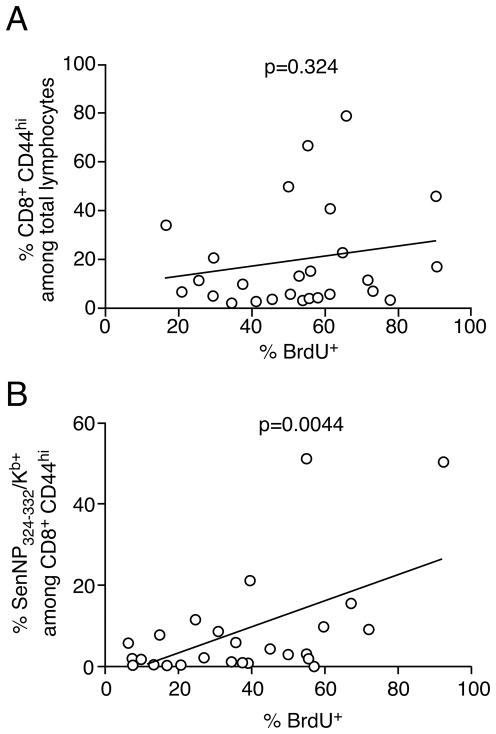
We speculated that the gradual development of antigen-specific T cell expansions correlated with an increased rate of proliferation in specific T cells clones in the memory T cell pool. To investigate this possibility we established a second cohort of Sendai virus infected mice and gave them BrdU in the drinking water on day 600 post-infection. Fourteen days later animals were sacrificed to determine BrdU incorporation in NP324–332/Kb-specific T cells. As shown in Figure 2A, analysis of BrdU incorporation in the total memory CD8+ T cell pool showed no correlation between the relative size of the bulk memory population and homeostatic turnover. In contrast, focusing the analysis on antigen-specific cells showed a very strong correlation between the frequency of NP324–332/Kb-specific T cells in the total memory CD8+ T cell pool and the level of BrdU incorporation. These data suggest that the antigen-independent development of virus-specific T cell expansions is due to an increased rate of homeostatic proliferation in individual clones.
Figure 2. Virus-specific TCE exhibit higher rates of homeostatic turnover.
B6 mice were infected with Sendai virus and rested for 600 days. (A, B) Mice were then administered BrdU in the drinking water for 14 days prior to sacrifice and incorporation was measured by flow cytometry. The data are graphed as the frequency of BrdU+ cells versus (A) the frequency of CD8+ CD44hi cells among total lymphocytes, and (B) the frequency of SenNP324–332Kb+ cells among total CD8+ CD44hi cells. Each symbol represents an individual mouse, and significance was determined using a Pearson correlation.
Antigen-specific TCE vary in their capacity to mediate recall responses
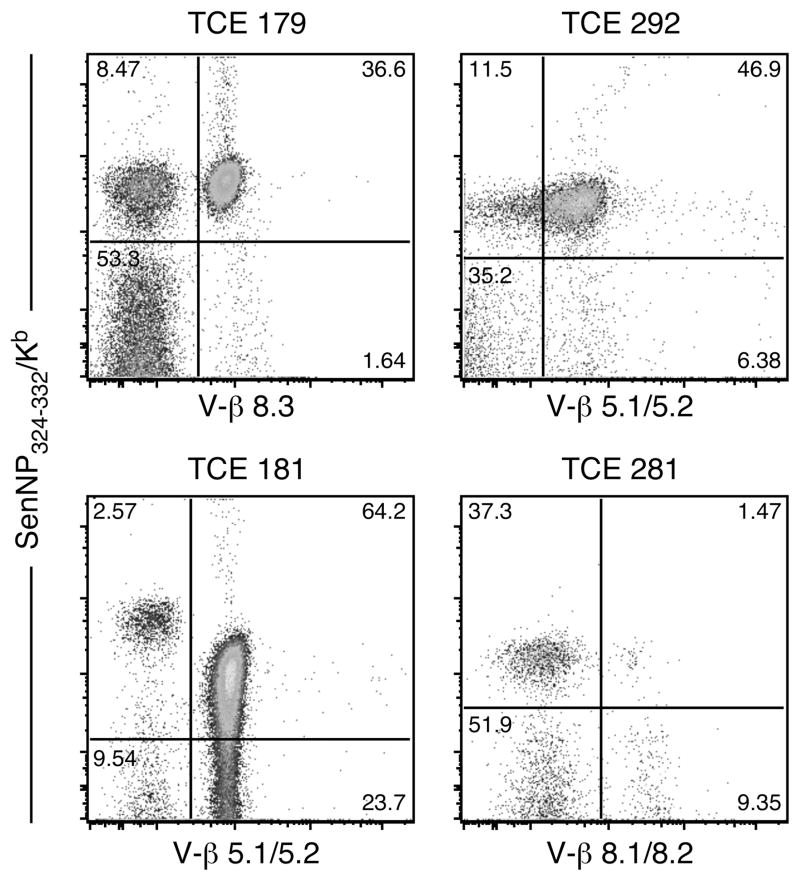
The development of antigen-specific TCE clearly disrupts the normal composition of the antigen-specific memory T cell pool. However, the impact of this dysregulation on the capacity of the memory T cell pool to mediate recall responses is not known. To address this question we focused on four animals (numbers 179, 181, 292, and 281) that exhibited substantial TCE (TCE 292 and 281 were selected based on screening several additional cohorts of Sendai memory mice, see Figure S1). The animals were sacrificed and splenocytes were analyzed using a panel of Vβ-specific antibodies to identify Vβ usage. As shown in Figure 3, animal 179 exhibited a major expansion of NP324–332/Kb-specific T cells that were Vβ8.3+ (comprising 81% of the total NP324–332/Kb-specific T cell pool). Animals 181 and 292 both exhibited expansions that were primarily Vβ5+ (comprising 96% and 80%, respectively, of the total NP324–332/Kb-specific T cell pool). Interestingly, the expanded NP324–332/Kb-specific T cells present in animal 181 expressed reduced TCR levels. Finally, animal 281 exhibited a major expansion of NP324–332/Kb-specific T cells inasmuch as there was a profound loss of Vβ8.1/8.2 usage. However, Vβ usage could not be assigned with the panel of antibodies available.
Figure 3. Characterization of the four TCE chosen to assess recall responses.
B6 mice with TCE identified by serial bleeds were sacrificed and splenocytes were stained to determine Vβ usage among SenNP324–332Kb+ cells. The dominant Vβ element used by SenNP324–332Kb+ cells from each mouse is shown, but the dominant Vβ element could not be identified for mouse #281 using a panel of commercially available Vβ antibodies. The plots shown are gated on total CD8+ cells.
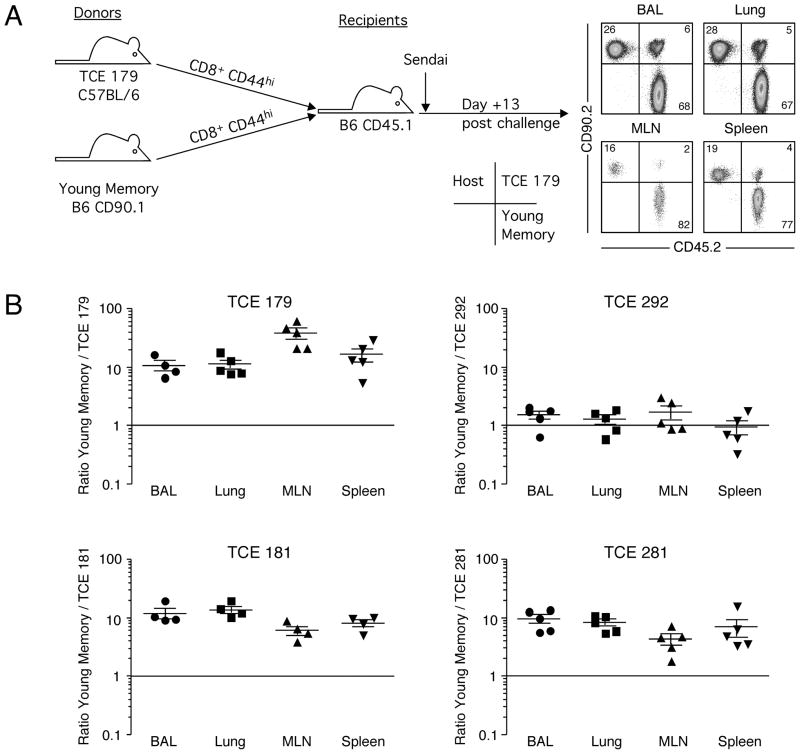
To determine the ability of these virus-specific TCE to respond to a secondary infection, we used a dual adoptive transfer approach in which the responses of two separate populations of memory T cells could be compared in the same animal and under the same infection conditions (21, 23). Spleen cells from animals 179, 181, 292, and 281 were enriched for CD8+ cells on a negative selection column, and then flow cytometrically sorted to isolate CD44+ memory cells. A corresponding population of memory CD8+ T cells was isolated from young B6.CD90.1 mice that had recovered from an intranasal Sendai virus infection (45 days post-infection). The memory T cells from mice exhibiting T cell expansions were then mixed together with young memory T cells such that the ratio of NP324–332/Kb-specific T cells was 1:1 and intravenously injected into B6.CD45.1 recipient mice, as depicted in Figure 4, panel A. One day later, recipient mice were infected with Sendai virus and NP324–332/Kb-specific T cells were analyzed 13 days later in the lung airways (bronchoalveolar lavage, BAL), lung parenchyma (Lung), mediastinal lymph nodes (MLN), and spleen. Cells derived from each donor and from the host were distinguished on the basis of CD90 and CD45 expression and the ratio of the response of the two donors determined (Figure 4A) (24). As shown in Figure 4B, young memory NP324–332/Kb-specific T cells were substantially more efficient at mediating the recall response to Sendai virus infection than memory cells from three of the mice exhibiting TCE (animals 179, 181, and 281). In each of these cases, the young memory T cells mounted responses that were approximately 10-fold greater than those from mice with TCE. Moreover, the reduced responsiveness of these TCE memory cells was exhibited in all tissues tested, suggesting a defect in the capacity of the antigen-specific memory T cells to expand in vivo rather than a defect in migration to the site of infection. There was some minor skewing of the young/TCE ratio among different tissues within the same experiment, suggesting there may be some small differences in trafficking to or survival in infected tissues. In contrast, memory NP324–332/Kb-specific T cells from one animal that exhibited a TCE (#292) mounted a response that was equivalent to that of young memory T cells. These data demonstrate that antigen-specific memory T cells from mice that exhibit dysregulated memory T cell pools vary in their capacity to mount recall responses.
Figure 4. Virus-specific memory T cell populations containing TCEs show mostly poor recall responses in vivo.
(A) Dual adoptive transfer experiments were performed as depicted. Representative staining of the donor and recipient populations from the BAL, lung, MLN, and spleen is shown gated SenNP324–332Kb+ cells. (B) The data from dual adoptive transfer experiments with four different TCEs is graphed as the ratio of young to TCE SenNP324–332Kb+ cells from each tissue on day 13 post-challenge (the cells went into the recipient at a 1:1 ratio). Each symbol represents an individual recipient, and the mean ± s.d. is shown.
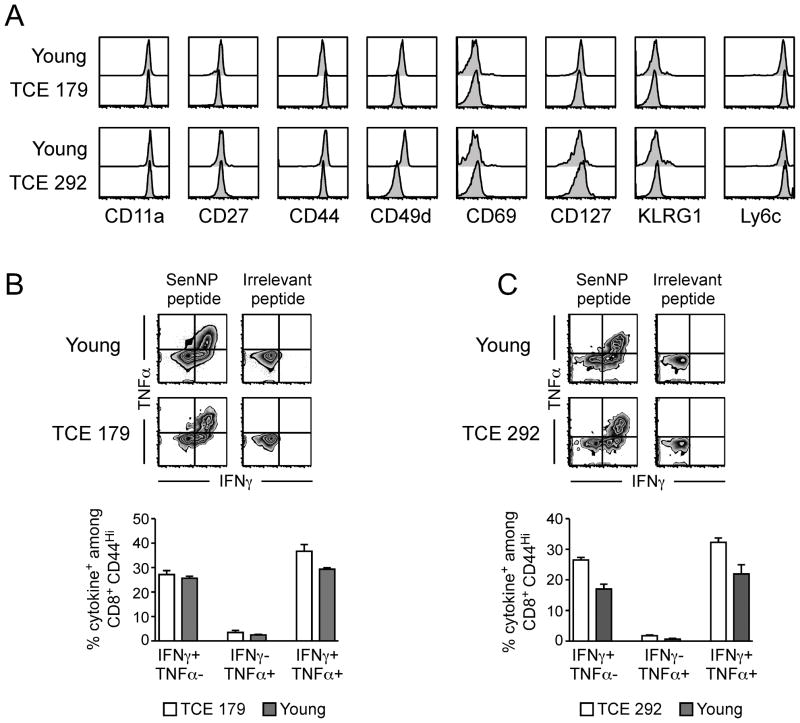
We next analyzed NP324–332/Kb-specific T cells from two of the animals that expressed TCE (#179 and #292) in more detail. Phenotypic analyses (Figure 5A) revealed that the cells were essentially indistinguishable from memory NP324–332/Kb-specific T cells from young mice that did not exhibit expansions. Both of the TCE identified in these mice expressed CD44, an activation marker that is upregulated on effector and memory T cells, and a variety of other activation markers. The only minor difference was that the TCE in these two animals expressed slightly reduced levels of CD49d, suggesting that these TCE were not functionally exhausted (25). To confirm that these TCE retained their functional capacity, we examined cytokine production following stimulation with the NP324–332 peptide. As shown in Figure 5B and 5C, both TCE were able to produce IFNγ and TNFα at levels comparable to those observed in young memory T cells. The production of cytokine was dependent on antigen, since irrelevant Kb-binding peptides did not elicit cytokine production from either TCE or young memory T cells.
Figure 5. T cell expansions that differ in recall potential in vivo show no difference in phenotype or cytokine production in vitro.
(A) Prior to transfer, the phenotype of sorted cells from young and TCE donors was determined by flow cytometry. The histograms shown are gated on SenNP324–332Kb+ cells. (B, C) Sorted splenocytes from young and TCE donors were incubated with specific (SenNP324–332) or irrelevant (FluNP366–374) peptide and cytokine production was assessed by flow cytometry. Representative staining for (B) young and TCE #179 donors, and (C) young and TCE #292 donors, is shown gated on total CD8+ CD44hi cells. The data from replicate wells are graphed as the percentage of cytokine+ cells among total CD8+ CD44hi cells (mean ± s.d.).
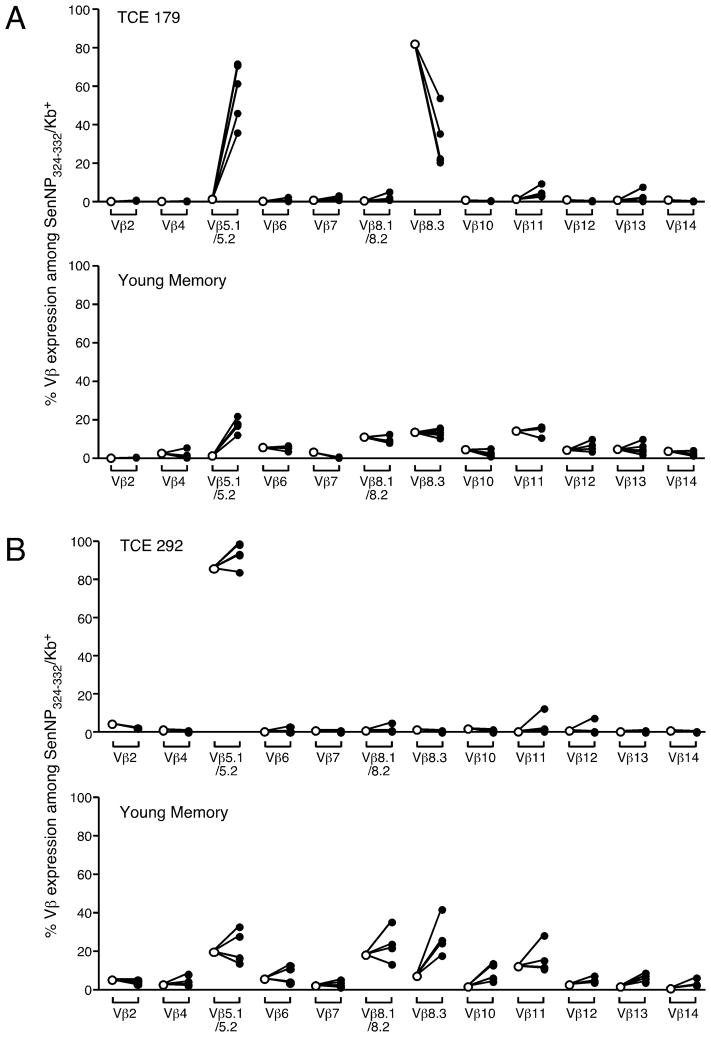
Although recall responses from 3 of 4 animals that exhibited NP324–332/Kb-specific TCE were impaired, they were not completely negative. We speculated that residual, non-expanded, memory in these mice may be mediating this recall response. To investigate this idea, we compared the TCR Vβ usage of the NP324–332/Kb-specific T cells prior to adoptive transfer and following recall to infection in a host animal. We focused on animals #179 and #292, since these memory T cell pools differed in their capacity to mediate recall responses. As shown in Figure 6, and discussed earlier, NP324–332/Kb-specific memory T cells from animal #179 were primarily Vβ8.3+ prior to adoptive transfer into the host animal. This is consistent with the presence of a monoclonal or pauciclonal expansion of part of the virus-specific memory T cell pool in this animal. Thirteen days following a secondary Sendai virus infection, the responding donor population became predominantly Vβ5.1/5.2+ suggesting that a minor subpopulation of T cells in the memory T cell pool had dominated the response. Importantly, it was apparent that the previously dominant Vβ8+ cells, which contained a monoclonal or pauciclonal expansion, were selectively decreased in the recall response. Importantly, the recall of young memory T cells in the same host showed no skewing of the Vβ repertoire, demonstrating that there is no intrinsic bias toward a Vβ5.1/5.2+ response during a secondary infection. In contrast to mouse #179, analysis of mouse #292 revealed a different pattern. In this case, NP324–332/Kb-specific memory T cells were primarily Vβ5.1/5.2+ prior to adoptive transfer into the host animal and this did not change in the recall response to Sendai virus infection. These data suggest that the expanded population of NP324–332/Kb-specific T cells in this animal responded well to secondary infection in vivo and that the presence of one or more expansions did not impact the strength of the response.
Figure 6. TCEs with poor recall responses show a massive skewing of the virus-specific CD8+ T cell repertoire following secondary challenge.
The frequency of different Vβ elements expressed by SenNP324–332Kb+ cells from young and TCE donors before transfer (open symbols) or on day 13 post-challenge (closed symbols). The data are graphed as the percentage of Vβ expression among total SenNP324–332Kb+ cells for the dual transfer with (A) young and TCE #179 donors and (B) young and TCE #292 donors. Each symbol represents an individual recipient mouse.
Discussion
There is increasing evidence that the immune system becomes progressively compromised with age resulting in poor immune responses to newly encountered antigens. In the case of T cell memory to pathogens that elicit acute responses, such as influenza and parainfluenza viruses, we have recently demonstrated in a mouse model there is a progressive dysregulation of antigen-specific memory CD8+ T cells characterized by the outgrowth of antigen-specific T cell clones. This was the first time that pathogen-specific TCE had been linked to the memory T cell pool in the absence of any form of chronic or persistent infection. In the current studies we have extended this finding to show that antigen-specific TCE begin to appear around 12 months post-infection and that they are present in the majority of animals by 24 months post-infection. In addition, we have shown that in many instances, these expansions are not able to participate in recall responses to secondary infection and consequently impair the response of the total memory T cell pool.
An interesting feature of the data is that dysregulation of the memory T cell pool was readily observed in the majority of the surviving animals by day 780 post-infection. This suggests that the outgrowth of specific clones occurs much more frequently than originally anticipated. Furthermore, it is likely that the frequency with which antigen-specific TCE occur may be underestimated in these studies. First, it is possible that the identification of TCE on the basis of elevated frequencies may lead to their underestimation. In the current studies, we identified expansions as populations of NP324–332/Kb-specific memory T cells that were present at frequencies greater than 2% of the total CD8+ T cell pool (2% was selected on the basis that it is three standard deviations above the frequency of recently generated NP324–332/Kb-specific memory T cells in young mice) (17). However, it is apparent from the data that memory T cell populations that fall below this threshold may be dysregulated in terms of their repertoire diversity. For example, at least two of the animals illustrated in Figure 1B exhibit frequencies of NP324–332/Kb-specific memory T cells below the 2% threshold, suggesting that the T cell memory pool is normal. Yet Vβ8+ T cells are overrepresented in this population (60% versus the more normal 20%) indicating substantial dysregulation of the memory pool. Second, the use of Vβ8 expression alone to identify perturbations in the repertoire may miss cases where more than one TCE has developed if two distinct clones happen to both utilize a Vβ8 element. Based on these two possibilities, we believe that many TCE may be overlooked and that dysregulation of the memory T cell pool may be even more dramatic than observed in the current studies. In this regard the large TCE outgrowths simply represent the extremes of a more general pattern of dysregulation in the memory T cell pool. We believe that future studies should be directed at better understanding the true kinetics and extent of memory T cell dysregulation.
An important observation is that memory T cell pools exhibiting substantial dysregulation often mediate a reduced capacity to participate in recall response, despite retaining the capacity to secrete inflammatory cytokines ex vivo (26, 27). It should be noted that the dual-adoptive transfer approach used for these studies effectively integrates all of the factors involved the accumulation of activated effector T cells at the site of infection (such as their capacity to migrate to lymph nodes prior to infection, the overall proliferative and death rates of the responding cells, and the capacity of the cells to migrate to inflammatory sites) (28). Furthermore, the response of the dysregulated memory T cell population is measured in direct comparison to recently generated memory in the same host and under identical conditions of infection and inflammation. Thus, these data suggest that TCE are generally detrimental to a secondary immune response. However, it should be noted that in many cases, there are hugely increased numbers of memory cells and that this increased number may compensate for the overall decrease in proliferative potential in individual animals. A key question in this regard is the extent to which TCE displace normal memory T cells. It should also be noted that not all examples of dysregulated T cell memory correlate with impaired recall responses. One of the four animals that we tested, #292, elicited a response that was clearly equivalent to recently elicited T cell memory. More studies are needed to determine the true frequencies of responsive and non-responsive TCE and the underlying mechanisms associated with non-responsiveness.
The mechanisms that control the development of TCE are currently undefined. In the case of chronic infections, it has been speculated that persistent stimulation by antigen results in the outgrowth of specific clones of T cells (29). However, in the current studies the agent that elicited the memory was cleared by the immune response and did not establish a chronic or persistent infection. Moreover, we have shown that antigen specific TCE can be elicited by both Sendai and influenza virus infections, ruling out any specific biological characteristics of a particular pathogen (17). Based on these observations, we hypothesize that antigen-specific TCE are a natural outcome of the long-term homeostatic proliferation of the memory T cell pool. It is interesting that TCE display enhanced homeostatic proliferation despite showing severely impaired proliferation to antigenic stimulation, but this may be due to signaling defects after TCR triggering, or defects in cytoskeletal rearrangements, that are found in aged T cells (30, 31). Since homeostatic T cell proliferation is asynchronous and individual clones may have slightly different proliferation rates, it might be expected that some clones would tend to overgrow the pool over time. In fact, one could argue that it would be unexpected for the immune system to maintain normal TCR diversity within any given memory CD8+ T cell pool given the relatively rapid rate of cell turnover. The large TCE that develop in some individuals and mice may represent one extreme of a spectrum of gradual degradation of the memory T cell repertoire. In these cases, the system has selected for a clone that exhibits a slightly higher rate of homeostatic proliferation compared to the average. Our data support this view by showing that there is a higher rate of homeostatic proliferation in mice that exhibit large TCE. Furthermore, the phenotype of large antigen-independent TCE is consistent with normal memory cells indicating that they are not substantially different from other memory T cells. A central facet of this hypothesis is that homeostatic cytokines should play a key role in TCE development and consistent with this, TCE that arise from the normal memory T cell pool typically express receptors for cytokines that are involved in CD8+ T cell homeostasis, namely CD122 (IL2Rβ/IL15Rβ) and CD127 (IL7Rα) (16). In addition, TCE isolated from mice can be maintained in the absence of antigen by transfer into β2-microglobulin-deficient mice (10). While the TCE that develop in Sendai-immune mice are clearly specific for viral antigen, it seems likely that expansions of unknown specificity in specific pathogen free mice also develop from the memory T cell pools that are invariably present. Although the idea that TCE represent homeostatic outgrowths of otherwise normal memory T cells is attractive, further investigation is needed to explain why some TCE exhibit an impaired capacity to mount recall responses.
In conclusion, the data we present here demonstrate that T cell memory in aged animals becomes increasingly dysregulated and that this can lead to clonal expansions with a substantially impaired capacity to mount secondary immune responses.
Supplementary Material
Acknowledgments
We thank Drs. Marcy Blackman and Michael Freeman for critical review of the manuscript, the Trudeau Institute Molecular Biology Core for the production of MHC I tetramers, and Brandon Sells and Ron Lacourse for cell sorting.
Abbreviations
- EID50
50% egg infectious dose
- BAL
bronchoalveolar lavage
- MLN
mediastinal lymph node
- TCE
T cell clonal expansion
Footnotes
This work was supported by National Institutes of Health grants AI67967, AI76499, AG21600, and T32 AI49823 (to D.L.W.), AI83610 (to J.E.K.), and funds from the Trudeau Institute.
References
- 1.LeMaoult J, Messaoudi I, Manavalan JS, Potvin H, Nikolich-Zugich D, Dyall R, Szabo P, Weksler ME, Nikolich-Zugich J. Age-related dysregulation in CD8 T cell homeostasis: kinetics of a diversity loss. J Immunol. 2000;165:2367–2373. doi: 10.4049/jimmunol.165.5.2367. [DOI] [PubMed] [Google Scholar]
- 2.Wick G, Jansen-Durr P, Berger P, Blasko I, Grubeck-Loebenstein B. Diseases of aging. Vaccine. 2000;18:1567–1583. doi: 10.1016/s0264-410x(99)00489-2. [DOI] [PubMed] [Google Scholar]
- 3.Miller RA. The aging immune system: primer and prospectus. Science. 1996;273:70–74. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- 4.Haynes L, Cambier J, Fulder R. Aging and immune function. Summary of a workshop held at Trudeau Institute, Saranac Lake, NY. Mech Ageing Dev. 2005;126:822–825. doi: 10.1016/j.mad.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Yager EJ, Ahmed M, Lanzer K, Randall TD, Woodland DL, Blackman MA. Age-associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. J Exp Med. 2008;205:711–723. doi: 10.1084/jem.20071140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Decman V, Laidlaw BJ, Dimenna LJ, Abdulla S, Mozdzanowska K, Erikson J, Ertl HC, Wherry EJ. Cell-intrinsic defects in the proliferative response of antiviral memory CD8 T cells in aged mice upon secondary infection. J Immunol. 2010;184:5151–5159. doi: 10.4049/jimmunol.0902063. [DOI] [PubMed] [Google Scholar]
- 7.Callahan JE, Kappler JW, Marrack P. Unexpected expansions of CD8-bearing cells in old mice. J Immunol. 1993;151:6657–6669. [PubMed] [Google Scholar]
- 8.Hingorani R, I, Choi H, Akolkar P, Gulwani-Akolkar B, Pergolizzi R, Silver J, Gregersen PK. Clonal predominance of T cell receptors within the CD8+ CD45RO+ subset in normal human subjects. J Immunol. 1993;151:5762–5769. [PubMed] [Google Scholar]
- 9.Posnett DN, Sinha R, Kabak S, Russo C. Clonal populations of T cells in normal elderly humans: the T cell equivalent to “benign monoclonal gammapathy”. J Exp Med. 1994;179:609–618. doi: 10.1084/jem.179.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clambey ET, van Dyk LF, Kappler JW, Marrack P. Non-malignant clonal expansions of CD8+ memory T cells in aged individuals. Immunol Rev. 2005;205:170–189. doi: 10.1111/j.0105-2896.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- 11.Messaoudi I, Lemaoult J, Guevara-Patino JA, Metzner BM, Nikolich-Zugich J. Age-related CD8 T cell clonal expansions constrict CD8 T cell repertoire and have the potential to impair immune defense. J Exp Med. 2004;200:1347–1358. doi: 10.1084/jem.20040437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Posnett DN, Yarilin D, Valiando JR, Li F, Liew FY, Weksler ME, Szabo P. Oligoclonal expansions of antigen-specific CD8+ T cells in aged mice. Ann N Y Acad Sci. 2003;987:274–279. doi: 10.1111/j.1749-6632.2003.tb06061.x. [DOI] [PubMed] [Google Scholar]
- 13.Effros RB, Cai Z, Linton PJ. CD8 T cells and aging. Crit Rev Immunol. 2003;23:45–64. doi: 10.1615/critrevimmunol.v23.i12.30. [DOI] [PubMed] [Google Scholar]
- 14.Khan N, Shariff N, Cobbold M, Bruton R, Ainsworth JA, Sinclair AJ, Nayak L, Moss PA. Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J Immunol. 2002;169:1984–1992. doi: 10.4049/jimmunol.169.4.1984. [DOI] [PubMed] [Google Scholar]
- 15.Koch S, Solana R, Dela Rosa O, Pawelec G. Human cytomegalovirus infection and T cell immunosenescence: a mini review. Mech Ageing Dev. 2006;127:538–543. doi: 10.1016/j.mad.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Messaoudi I, Warner J, Nikolich-Zugich J. Age-related CD8+ T cell clonal expansions express elevated levels of CD122 and CD127 and display defects in perceiving homeostatic signals. J Immunol. 2006;177:2784–2792. doi: 10.4049/jimmunol.177.5.2784. [DOI] [PubMed] [Google Scholar]
- 17.Ely KH, Ahmed M, Kohlmeier JE, Roberts AD, Wittmer ST, Blackman MA, Woodland DL. Antigen-specific CD8+ T cell clonal expansions develop from memory T cell pools established by acute respiratory virus infections. J Immunol. 2007;179:3535–3542. doi: 10.4049/jimmunol.179.6.3535. [DOI] [PubMed] [Google Scholar]
- 18.Lang A, Brien JD, Messaoudi I, Nikolich-Zugich J. Age-related dysregulation of CD8+ T cell memory specific for a persistent virus is independent of viral replication. J Immunol. 2008;180:4848–4857. doi: 10.4049/jimmunol.180.7.4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hou S, Doherty PC, Zijlstra M, Jaenisch R, Katz JM. Delayed clearance of Sendai virus in mice lacking class I MHC- restricted CD8+ T cells. J Immunol. 1992;149:1319–1325. [PubMed] [Google Scholar]
- 20.Kohlmeier JE, Cookenham T, Miller SC, Roberts AD, Christensen JP, Thomsen AR, Woodland DL. CXCR3 directs antigen-specific effector CD4+ T cell migration to the lung during parainfluenza virus infection. J Immunol. 2009;183:4378–4384. doi: 10.4049/jimmunol.0902022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts AD, Ely KH, Woodland DL. Differential contributions of central and effector memory T cells to recall responses. J Exp Med. 2005;202:123–133. doi: 10.1084/jem.20050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cole GA, Hogg TL, Woodland DL. The MHC class I-restricted T cell response to Sendai virus infection in C57BL/6 mice: a single immunodominant epitope elicits an extremely diverse repertoire of T cells. Int Immunol. 1994;6:1767–1775. doi: 10.1093/intimm/6.11.1767. [DOI] [PubMed] [Google Scholar]
- 23.Roberts AD, Woodland DL. Cutting edge: effector memory CD8+ T cells play a prominent role in recall responses to secondary viral infection in the lung. J Immunol. 2004;172:6533–6537. doi: 10.4049/jimmunol.172.11.6533. [DOI] [PubMed] [Google Scholar]
- 24.Badovinac VP, Messingham KA, Hamilton SE, Harty JT. Regulation of CD8+ T cells undergoing primary and secondary responses to infection in the same host. J Immunol. 2003;170:4933–4942. doi: 10.4049/jimmunol.170.10.4933. [DOI] [PubMed] [Google Scholar]
- 25.Clambey ET, White J, Kappler JW, Marrack P. Identification of two major types of age-associated CD8 clonal expansions with highly divergent properties. Proc Nat Acad Sci USA. 2008;105:12997–13002. doi: 10.1073/pnas.0805465105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, Fujii H, Kishimoto H, LeRoy E, Surh CD, Sprent J. Aging leads to disturbed homeostasis of memory phenotype CD8+ cells. J Exp Med. 2002;195:283–293. doi: 10.1084/jem.20011267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Zhi W, Wareski P, Weng N-p. IL-15 Activates Telomerase and Minimizes Telomere Loss and May Preserve the Replicative Life Span of Memory CD8+ T Cells In Vitro. J Immunol. 2005;174:4019–4024. doi: 10.4049/jimmunol.174.7.4019. [DOI] [PubMed] [Google Scholar]
- 28.Klonowski KD, Marzo AL, Williams KJ, Lee SJ, Pham QM, Lefrancois L. CD8 T cell recall responses are regulated by the tissue tropism of the memory cell and pathogen. J Immunol. 2006;177:6738–6746. doi: 10.4049/jimmunol.177.10.6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ouyang Q, Wagner WM, Walter S, Muller CA, Wikby A, Aubert G, Klatt T, Stevanovic S, Dodi T, Pawelec G. An age-related increase in the number of CD8+ T cells carrying receptors for an immunodominant Epstein-Barr virus (EBV) epitope is counteracted by a decreased frequency of their antigen-specific responsiveness. Mech Ageing Dev. 2003;124:477–485. doi: 10.1016/s0047-6374(03)00026-5. [DOI] [PubMed] [Google Scholar]
- 30.Garcia GG, Miller RA. Single-cell analyses reveal two defects in peptide-specific activation of naive T cells from aged mice. J Immunol. 2001;166:3151–3157. doi: 10.4049/jimmunol.166.5.3151. [DOI] [PubMed] [Google Scholar]
- 31.Garcia GG, Sadighi Akha AA, Miller RA. Age-related defects in moesin/ezrin cytoskeletal signals in mouse CD4 T cells. J Immunol. 2007;179:6403–6409. doi: 10.4049/jimmunol.179.10.6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.