Diamination of olefins presents an effective strategy to access vicinal diamine moieties which are contained in various biologically active molecules and are widely used as chiral control elements in asymmetric synthesis.1 Various metal-mediated and metal-catalyzed diamination processes have been reported.1–8 In our earlier studies, we have shown that conjugated dienes can be regioselectively diaminated using Pd(0)9 and Cu(I)10 as catalysts and di-tert-butyldiaziridinone (2)11,12 as nitrogen source. The Pd(0)-catalyzed diamination occurred regioselectively at the internal double bonds of dienes,9 and the Cu(I)-catalyzed diamination occurred primarily at the terminal double bonds with 10 mol% CuCl-P(OPh)3 (Scheme 1).10 The Cu(I)-catalyzed diamination was proposed to proceed via a stepwise radical mechanism involving a Cu(II) species. During our on-going studies, it was a surprise to find that the Cu(I)-catalyzed diamination could occur regioselectively at the internal double bond under certain conditions (e.g. with CuBr as catalyst) (Scheme 1). The reaction is likely to proceed via a concerted mechanism involving a four-membered Cu(III) species. Herein, we wish to report our preliminary studies on this subject.
Scheme 1.
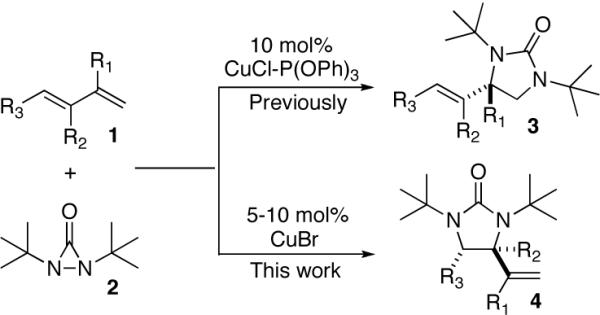
Studies began with the investigation of the effect of reaction conditions on the diamination of (E)-penta-1,3-diene (1a). As shown in Table 1, diene 1a can be selectively diaminated on either double bond. The regioselectivity is highly dependent on the reaction conditions. Terminal diamination product 3a was increased with more phosphorous ligand present (Table 1, entry 2 vs 1). When PCy3 was used, the diamination occurred predominately on the terminal double bond (Table 1, entry 4). On the other hand, internal diamination product 4a was favored in the absence of ligand (Table 1, entries 5 and 6). Essentially only 4a was formed with 5 mol% CuBr as catalyst in CDCl3 at 0 °C (Table 1, entry 6). The control of regioselectivity by simply changing reaction conditions was also observed for additional substrates such as 3-methyl-1-phenylbutadiene (1b) (Table 1, entries 7 and 8).
Table 1.
The effect of reaction conditions on the regioselectivity of the Cu(I)-catalyzed diamination of dienesa
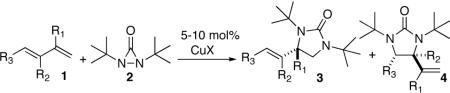
| entry | substrate | catalyst | conv. (%)b | 3:4d |
|---|---|---|---|---|
| 1 |
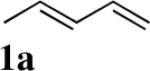
|
CuCl-P(OPh)3 (1:1.2) | 92 | 34:66 |
| 2 | 1a | CuCl-P(OPh)3(1:2) | 92 | 42:58 |
| 3 | 1a | CuCl-PCy3 (1:1.2) | 61 | 78:22 |
| 4 | 1a | CuCl-PCy3 (1:1.5) | 100 (53%)b | 97:3 |
| 5 | 1a | CuCl | 100 | 17:83 |
| 6 | 1a | CuBr | 100 (99%)c | 1:99 |
| 7 |
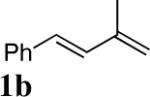
|
CuCl-PCy3 (1:1.5) | 100 (99%)c | >99:1 |
| 8 | 1b | CuBr | 100 (97%)c | 9:91 |
All reactions were carried out with olefin 1a (0.20 mmol), di-tert-butyldiaziridinone (2) (0.40 mmol), and catalyst [0.020 mmol, complex CuCl-P was prepared in situ from CuCl (0.02 mmol) and the corresponding ligand by stirring at rt for 1 h] in C6D6 (0.2 mL) under Ar at rt for 10 h unless otherwise stated. For entry 5, CDCl3 was used. For entry 4, the reaction was carried out with olefin 1a (2.0 mmol) and 2 [0.20 mmol, dissolved in C6D6 (0.1 mL), slow addition over 7 h] at rt for 10 h (total). For entry 6, the reaction was carried out with olefin 1a (0.20 mmol), di-tert-butyldiaziridinone (2) (0.22 mmol), CuBr (0.010 mmol) in CDCl3 (0.4 mL) at 0 °C for 20 h. For entry 7, C6D6 (0.05 mL) was used to dissolve olefin 1b. For entry 8, the reaction was carried out with olefin 1b (0.20 mmol), 2 (0.22 mmol), CuBr (0.010 mmol) in CDCl3 (1.0 mL) at -20 °C for 40 h.
The conversion was based on olefin 1 and determined by analysis of 1H NMR spectrum of the crude reaction mixture except for entry 4 whose conversion was based on 2.
Isolated yield based on olefin 1 for entries 6–8 and based on 2 for entry 4.
The ratio of 3 to 4 was determined by analysis of 1H NMR spectrum of the crude reaction mixture.
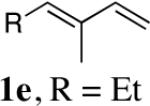
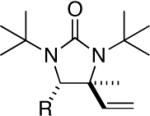
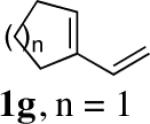
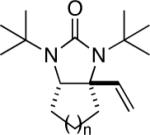
While the Cu(I)-catalyzed terminal diamination of conjugated dienes has been previously shown to be an effective process for various substrates,10 internal diamination did not appear to be a viable process under those conditions. The observation that the internal double bond can be regioselectively diaminated with CuBr prompted us to further explore the substrate scope. As shown in Table 2, various 1-substituted (entries 1 and 2), 1,2-disubstituted (entries 3–9), 1,3-disubstituted (entries 10–12), and 1,2,3-trisubstituted (entries 13 and 14) dienes can be efficiently diaminated at the internal double bonds with high regioselectivity and yield. Cyclic substrates can also be selectively diaminated in high yield (Table 1, entries 5–9). In the case of entry 9, only one diastereoisomer was observed in the 1H NMR spectrum. In most cases, essentially one regioisomer was formed, and the other regioisomer was barely detectable (if there was any) by 1H NMR spectrum of the crude reaction mixture. The reaction is also highly diastereoselective, and essentially only one diastereoisomer was obtained in all cases.
Table 2.
CuBr-catalyzed regioselective diamination of dienesa
| entry | substrate (1) | product (4) | yield (%)b |
|---|---|---|---|
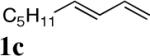
| 1 |
|
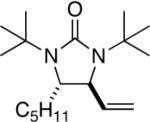
|
97 (96:4)c |
| 2 |
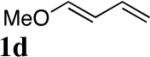
|
|
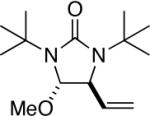
95 |
| 3 |
|
|
81 |
| 4 | 1f,R = Ph | 92 (97:3)c | |
| 5 |
|
|
95 |
| 6 | 1h,n = 2 | 99 | |
| 7 | 1i,n = 3 | 94 | |
| 8 |
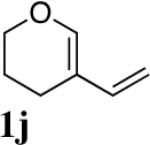
|
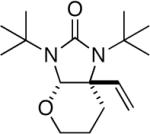
|
85 |
| 9 |
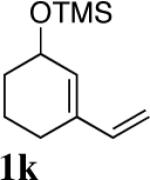
|
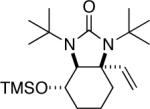
|
95 (97:3)c,d |
| 10 |
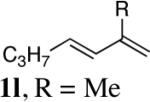
|
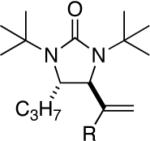
|
98 |
| 11 | 1m,R = Ph | 96 (89:11)c | |
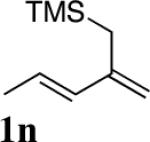
| 12 |
|
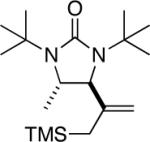
|
94 |
| 13 |
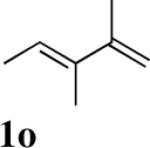
|
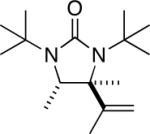
|
90 |
| 14 |
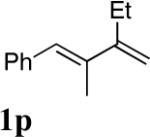
|
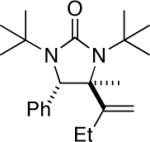
|
95 |
All reactions were carried out with olefin 1 (0.20 mmol), di-tert-butyldiaziridinone (2) (0.22 mmol), CuBr (0.010 mmol) in CDCl3 (0.4 mL) under Ar with vigorous stirring at 0 °C for 20 h unless otherwise stated. For entry 1, 1c (0.19 mmol) was used. For entry 2, olefin 1d (0.25 mmol, E:Z = 15.7:1, E isomer: 0.24 mmol) and 2 (0.2 mmol) were used. For entry 7, the reaction was carried out on 0.40 mmol scale. For entry 9, CuBr (0.020 mmol) was used.
Isolated yield based on 1 except for that of entry 2 which was based on 2.
The ratio of 4 to 3 was determined by analysis of 1H NMR spectrum of the crude reaction mixture.
The stereochemistry was tentatively assigned based upon sterics.
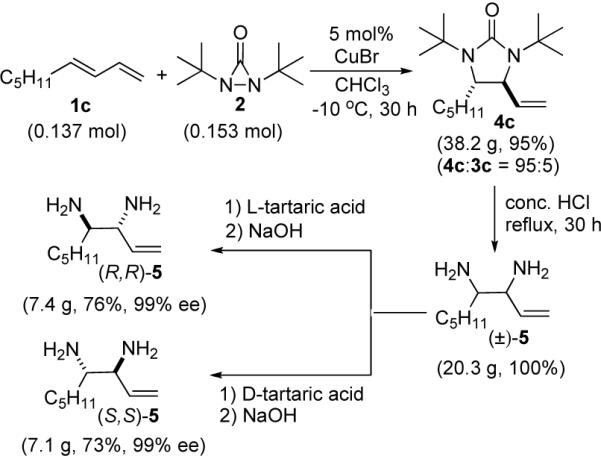
The CuBr-catalyzed diamination can be carried out on relatively large scale (Scheme 2). For example, 38.2 g of diamination product 4c was readily obtained using 5 mol% CuBr as catalyst. The tert-butyl groups and the carbonyl group were cleanly removed by treatment of 4c with concentrated HCl at reflux to give free diamine (±)-5 in quantitative yield. Resolution of racemic diamine 5 with L and D-tartaric acids gave chiral diamines (R,R)-5 (99% ee) and (S,S)-5 (99% ee) in good yield.13
Scheme 2.
Effective substrates require a terminal double bond. Internal dienes such as 1,4-diphenylbutadiene gave insignificant amount of diamination products. Substrates such as 1-phenylbutadiene gave a mixture of both internal and terminal diamination products. When deuterated 1-phenylbutadiene (6) was used, two deuterium isomers 8a and 8b were obtained for the terminal diamination while no deuterium isomerization was observed for the internal diamination (Scheme 3). These results suggest that the internal diamination and terminal diamination likely proceed via two different mechanisms.
Scheme 3.
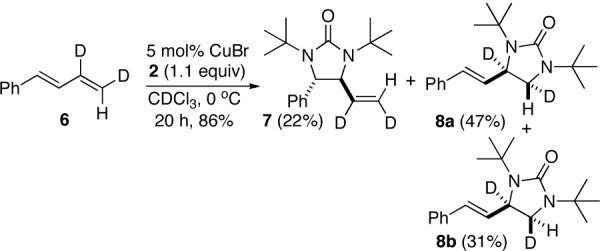
While a precise reaction mechanism awaits further study, a plausible dual mechanism is shown in Scheme 4. The CuX first reductively cleaves the N-N bond of diaziridinone 2 to form four-membered Cu(III) species A and Cu(II) nitrogen radical B, which are likely in equilibrium. As proposed previously,10a radical B adds to the terminal double bond of the diene to form Cu(II) allyl radical species C and/or Cu(III) intermediate D, which subsequently undergo the second C-N bond formation to give the terminal diamination product 3 and regenerate the Cu(I) catalyst. Radical B preferentially adds to the terminal double bond likely due to sterics and the formation of a relatively more stable allyl radical C. The formation of isomers 8a and 8b from deuterated 1-phenylbutadiene (6) (Scheme 3) is consistent with the proposed radical mechanism for the terminal diamination.
Scheme 4.
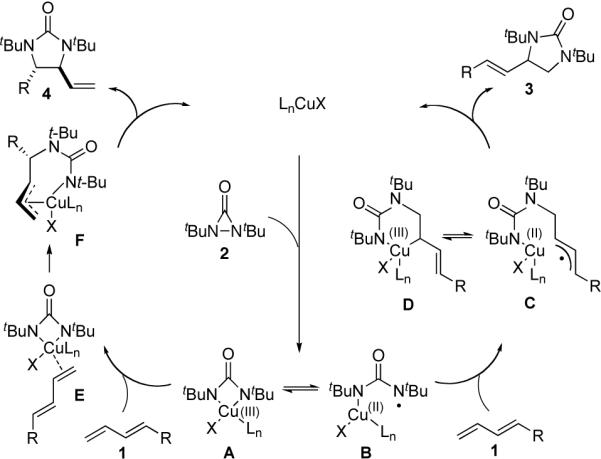
Proposed dual mechanisms for the Cu(I)-catalyzed diamination of conjugated dienes
The fact that no significant amount of diamination product was obtained with internal dienes such as 1,4-diphenylbutadiene suggests that addition of radical B to an internal double bond is unfavorable even in the presence of a radical-stabilizing phenyl group, possibly due to sterics (Scheme 5). A terminal diene should be even less favorable for the addition of radical B to the internal double bond since it does not have the radical-stabilizing phenyl group (radical C2 is less stable than C1) (Scheme 5). Therefore, it is unlikely that the observed internal diamination of terminal dienes proceeds via a radical pathway. The absence of deuterium isomerization in internal diamination product 7 (Scheme 3) also argues against a radical mechanism.
Scheme 5.
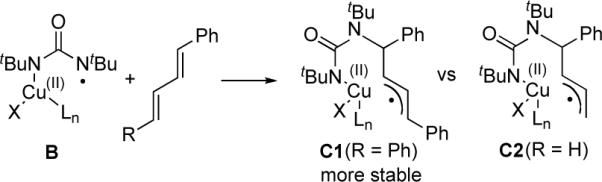
Addition of radical B to the internal double bond
The internal diamination likely proceeds via four-membered Cu(III) species A,14 analogous to the case of Pd-catalyzed diamination.9 Intermediate A coordinates with diene 1 to form complex E, which undergoes a migratory insertion and a subsequent reductive elimination to give internal diamination product 4 and regenerate the Cu(I) catalyst (Scheme 4).
The competition between the two pathways depends on the reaction conditions and types of substrates used. Adding more ligand to the reaction system appears to facilitate the formation of Cu(II) radical B and/or hinder the coordination of diene 1 to Cu(III) species A, thus leading to more terminal diamination product 3. On the other hand, the absence of the ligand may favor Cu(III) species A and subsequent internal diamination to give product 4. The counter anion of the Cu(I) catalyst also has a large influence on the regioselectivity. It appears that CuBr is more favorable toward the internal diamination than CuCl. Substrates with radical stabilizing groups such as 1-phenylbutadiene facilitate the formation of radical C, thus increasing the amount of terminal diamination product 3 even with CuBr. Generally, introduction of electron-donating groups, such as alkyl chains, appears to be more favorable towards formation of the internal diamination product 4.
In summary, studies have shown that the regioselectivity of the Cu(I)-catalyzed diamination of conjugated dienes using di-tert-butyldiaziridinone (2) as nitrogen source can be altered by simply tuning the reaction conditions. Various dienes can be efficiently diaminated at the internal double bonds with high regioselectivity and good yield using 5–10 mol% inexpensive CuBr. The diamination is also amenable to large scale. Chiral diamines can be readily obtained in high optical purity by simple resolution with tartaric acids. Studies also show that the internal diamination and terminal diamination likely proceed via two mechanistic pathways involving Cu(III) and Cu(II) species respectively. The mechanistic duality of the Cu species displayed in this diamination may provide useful insights for Cu-promoted reaction processes.15 Further mechanistic studies and development of an asymmetric diamination process are currently underway.
Supplementary Material
Acknowledgment
We are grateful to the generous financial support from the General Medical Sciences of the National Institutes of Health (GM083944-03).
Footnotes
Supporting Information Available: Experimental procedures, the characterization of diamination products, and the data for determination of enantiomeric excess of diamine 5 along with the 1H NMR and 13C NMR spectra of compounds 3–5, 7, and 8 (54 pages). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).For leading reviews, see: Lucet D, Gall TL, Mioskowski C. Angew. Chem., Int. Ed. 1998;37:2580. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2580::AID-ANIE2580>3.0.CO;2-L.. Mortensen MS, O'Doherty GA. Chemtracts: Org. Chem. 2005;18:555.. Kotti SRSS, Timmons C, Li G. Chem. Biol. Drug Des. 2006;67:101. doi: 10.1111/j.1747-0285.2006.00347.x.. Kizirian J-C. Chem. Rev. 2008;108:140. doi: 10.1021/cr040107v.. Lin G-Q, Xu M-H, Zhong Y-W, Sun X-W. Acc. Chem. Res. 2008;41:831. doi: 10.1021/ar7002623.. de Figueiredo RM. Angew. Chem., Int. Ed. 2009;48:1190. doi: 10.1002/anie.200804362.. Cardona F, Goti A. Nat. Chem. 2009;1:269. doi: 10.1038/nchem.256..
- (2).For examples of metal-mediated diaminations, see: Tl: Aranda VG, Barluenga J, Aznar F. Synthesis. 1974:504.. Os: Chong AO, Oshima K, Sharpless KB. J. Am. Chem. Soc. 1977;99:3420.. Muñiz K. Eur. J. Org. Chem. 2004:2243.. Pd: Bäckvall J-E. Tetrahedron Lett. 1978:163.. Hg: Barluenga J, Alonso-Cires L, Asensio G. Synthesis. 1979:962.. Co: Becker PN, White MA, Bergman RG. J. Am. Chem. Soc. 1980;102:5676.. Mn: Fristad WE, Brandvold TA, Peterson JR, Thompson SR. J. Org. Chem. 1985;50:3647..
- (3).For recent Cu(II)-mediated intramolecular diamination, see: Zabawa TP, Kasi D, Chemler SR. J. Am. Chem. Soc. 2005;127:11250. doi: 10.1021/ja053335v.. Zabawa TP, Chemler SR. Org. Lett. 2007;9:2035. doi: 10.1021/ol0706713..
- (4).For Rh(II), Cu(I), and Fe(III)-catalyzed diamination with TsNCl2 or TsNBr2, see: Li G, Wei H-X, Kim SH, Carducci MD. Angew. Chem., Int. Ed. 2001;40:4277. doi: 10.1002/1521-3773(20011119)40:22<4277::AID-ANIE4277>3.0.CO;2-I.. Wei H-X, Kim SH, Li G. J. Org. Chem. 2002;67:4777. doi: 10.1021/jo0200769.. Han J, Li T, Pan Y, Kattuboina A, Li G. Chem. Biol. Drug. Des. 2008;71:71. doi: 10.1111/j.1747-0285.2007.00604.x..
- (5).For a recent Au(I)-catalyzed intramolecular diamination of allenes via dihydroamination, see: Li H, Widenhoefer RA. Org. Lett. 2009;11:2671. doi: 10.1021/ol900730w..
- (6).For a Pd(II)-catalyzed intermolecular diamination of conjugated dienes, see: Bar GLJ, Lloyd-Jones GC, Booker-Milburn KI. J. Am. Chem. Soc. 2005;127:7308. doi: 10.1021/ja051181d..
- (7).For Pd(II)-catalyzed intramolecular diamination of olefins, see: Streuff J, Hövelmann CH, Nieger M, Muñiz K. J. Am. Chem. Soc. 2005;127:14586. doi: 10.1021/ja055190y.. Muñiz K. J. Am. Chem. Soc. 2007;129:14542. doi: 10.1021/ja075655f.. Muñiz K, Hövelmann CH, Streuff J. J. Am. Chem. Soc. 2008;130:763. doi: 10.1021/ja075041a.. Hövelmann CH, Streuff J, Brelot L, Muñiz K. Chem. Commun. 2008:2334. doi: 10.1039/b719479j.. Muñiz K, Hövelmann CH, Campos-Gómez E, Barluenga J, González JM, Streuff J, Nieger M. Chem. Asian J. 2008;3:776. doi: 10.1002/asia.200700373.. Muñiz K, Streuff J, Chávez P, Hövelmann CH. Chem. Asian J. 2008;3:1248. doi: 10.1002/asia.200800148.. Sibbald PA, Michael FE. Org. Lett. 2009;11:1147. doi: 10.1021/ol9000087.. Sibbald PA, Rosewall CF, Swartz RD, Michael FE. J. Am. Chem. Soc. 2009;131:15945. doi: 10.1021/ja906915w..
- (8).For related Ni(II)-catalyzed intramolecular diamination of olefins, see: Muñiz K, Streuff J, Hövelmann CH, Núñez A. Angew. Chem., Int. Ed. 2007;46:7125. doi: 10.1002/anie.200702160.. Muñiz K, Hövelmann CH, Streuff J, Campos-Gómez E. Pure Appl. Chem. 2008;80:1089..
- (9).Du H, Zhao B, Shi Y. J. Am. Chem. Soc. 2007;129:762. doi: 10.1021/ja0680562.. Du H, Yuan W, Zhao B, Shi Y. J. Am. Chem. Soc. 2007;129:7496. doi: 10.1021/ja072080d.. Du H, Yuan W, Zhao B, Shi Y. J. Am. Chem. Soc. 2007;129:11688. doi: 10.1021/ja074698t.. Du H, Zhao B, Shi Y. J. Am. Chem. Soc. 2008;130:8590. doi: 10.1021/ja8027394.. Zhao B, Du H, Shi Y. J. Am. Chem. Soc. 2010;132:3523. doi: 10.1021/ja909459h..
- (10).Yuan W, Du H, Zhao B, Shi Y. Org. Lett. 2007;9:2589. doi: 10.1021/ol071105a.. Du H, Zhao B, Yuan W, Shi Y. Org. Lett. 2008;10:4231. doi: 10.1021/ol801605w.. Zhao B, Du H, Shi Y. J. Org. Chem. 2009;74:8392. doi: 10.1021/jo901685c..
- (11).For the preparation of di-tert-butyldiaziridinone (2), see: Greene FD, Stowell JC, Bergmark WR. J. Org. Chem. 1969;34:2254.. Du H, Zhao B, Shi Y. Org. Synth. 2009;86:315..
- (12).For a leading review on diaziridinones, see: Heine HW. In: The Chemistry of Heterocyclic Compounds. Hassner A, editor. John Wiley & Sons, Inc.; USA: 1983. p. 547..
- (13).The configurations of chiral diamines were determined by comparing the chiral HPLC chromatogram with the corresponding diamine prepared by Pd(0)-catalyzed asymmetric diamination (ref. 9c).
- (14).For leading references on Cu(III) species and reactions involving Cu(III) intermediates, see: Ribas X, et al. Angew. Chem., Int. Ed. 2002;41:2991. doi: 10.1002/1521-3773(20020816)41:16<2991::AID-ANIE2991>3.0.CO;2-6.. Xifra R, et al. Chem. Eur. J. 2005;11:5146. doi: 10.1002/chem.200500088.. Huffman LM, Stahl SS. J. Am. Chem. Soc. 2008;130:9196. doi: 10.1021/ja802123p.. King AE, Brunold TC, Stahl SS. J. Am. Chem. Soc. 2009;131:5044. doi: 10.1021/ja9006657.. Yao B, Wang D, Huang Z, Wang M. Chem. Commun. 2009:2899. doi: 10.1039/b902946j.. Yang L, Lu Z, Stahl SS. Chem. Commun. 2009:6460. doi: 10.1039/b915487f..
- (15).For a leading review on organocopper reagents, see: Lipshutz BH, Sengupta S. Org. React. 1992;41:135..
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


