Abstract
A film paradigm was developed to examine baseline and emotion modulated startle across a broad age range from preschool to adulthood. The paradigm was tested in children (3-, 5-, 7- and 9-year-olds) and adults (total N =122). The paradigm elicited a similar startle potentiation pattern across age groups; however, baseline startle changed with age: 3- and 5-year olds showed lower response probability and magnitude of baseline startle than adults. Females exhibited larger baseline startle response probability and overall magnitude than did males; however, no sex by emotion modulated startle interaction was noted. Anxiety measures were obtained for all children. Individual differences in anxiety were associated with baseline startle magnitude among older but not younger children. No association of anxiety with startle potentiation was noted. Overall the film paradigm was applicable across a wide developmental span, revealing potential developmental and gender differences in baseline startle magnitude and response probability.
Keywords: Emotion Modulated and Baseline Startle, Typically Developing Children, Gender Differences, Anxiety, New Experimental Paradigm
The study of defensive motivations, particularly of fear behaviors, is an important area of research with ramifications for several domains of inquiry including research on temperament and psychopathology. The eye blink startle response can be used as a measure of defensive motivation and as a noninvasive physiological index of fear (Bradley, Cuthbert, & Lang, 1999). Studies of defensive motivations with young children have been impeded by problems with the elicitation of fear states. Experimental startle paradigms use fear-provoking stimuli such as threats of electric shock (Grillon, Ameli, Goddard, Woods, & Davis, 1994), periods of complete darkness (Grillon et al. 2005), and intensely threatening/disgusting still pictures (Bernat, Patrick, Benning, & Tellegen, 2006). Such stimuli are not appropriate in child studies. Therefore, the first objective of this study was to develop an appropriate and effective paradigm to sample the startle response (baseline and emotion modulated) from early childhood to adulthood.
The lack of experimental paradigms that are appropriate and effective across development confounds the interpretation of research using the startle response as a physiological measure of fear and anxiety. Differences between children and adults in components of the startle response are reported often across studies. This could be due to actual developmental change or to experimental parameters such as use of less aversive stimuli in studies of young children. Using the same paradigm with young children and adults permitted us to address our second objective: to examine whether components of the startle response (baseline and emotion modulated) would show differences across development. Finally, the association of the startle response with fear and anxiety is inconsistent (at times paradoxical) in young populations partly due to difficult elicitation of startle in small children and the dearth of appropriate paradigms. Thus the third objective was to test the association of startle (baseline and/or potentiation) with anxiety in typically developing children and to explore the putative role of development in this association.
The Startle Response: Neurobiology of its Components and Association with Fear Behaviors
There are two advantages to using the startle response as a noninvasive measure of defensive motivations. First, its neural circuitry is well described (Davis, Gendelman, Tischler, & Gendelman, 1982; Tischler & Davis, 1983), and second, its components co-vary with fear and anxiety behaviors (Davis, 2006; Pissiota et al., 2003). When evoked by a loud sound, the baseline startle response involves an acoustic pathway that synapses in the cochlear nucleus and next in the nucleus reticularis pontis caudalis (Wu, Suzuki, & Siegel, 1988). Damaging this pathway eliminates baseline startle completely (Davis et al., 1982). At the level of the nucleus reticularis pontis caudalis, input from the amygdala is received. This modulates the nuanced processing of stimuli both pleasant and frightening. Startle potentiation due to exposure to aversive stimuli (i.e. heightened reflex amplitude) and its suppression via exposure to pleasant stimuli requires this pathway. Damaging the amygdala pathway to the nucleus reticularis pontis caudalis eliminates emotion modulation of the startle response without disrupting baseline startle (Davis, Falls, Campeau, & Kim, 1993).
Both baseline and emotion modulated startle are linked to typical and pathological fear in adult samples. Elevated baseline startle magnitude discriminates assorted anxiety disorders (See review by Grillon, 2002); and heightened startle potentiation, elicited via emotion modulated startle paradigms, is associated with elevated neuroticism, trait anxiety, harm avoidance, and fearfulness (Cook, Hawk, Davis, & Stevenson, 1991; Corr, Wilson, Fotiadou, Kumari, & et al., 1995; Temple & Cook, 2007; Wilson, Kumari, Gray, & Corr, 2000). In child studies associations between baseline, emotion modulated startle and anxiety have also been reported. Children and adolescents were exposed to two trial conditions: a pre-pulse (70 dB, 30 ms duration) 120 ms or 4000 ms prior to a startle probe (106 dB, 40 ms duration white noise) over a 65 dB background of noise with an inter-trial interval (ITI) mean of 25000 ms. Baseline startle magnitude typified those with a parental history of anxiety disorders (Grillon, Dierker, & Merikangas, 1997). Furthermore, both baseline and emotion modulated startle (102 dB, 40 ms duration white noise, ITI 17000 - 42000 ms) discriminated adolescents and young adults at high and low risk for anxiety disorders (Grillon, Dierker, & Merikangas, 1998). In summary, baseline startle reflects basic reactivity to unexpected/frightening events and emotion modulated startle reflects more nuanced emotional processing; both these components are peripheral signs of central neural pathways that orchestrate normal and pathological fear behaviors.
Developing a Novel Startle Paradigm Appropriate from Early Childhood through Adulthood
Baseline startle has been examined in early childhood (Balaban, Snidman, & Kagan, 1997), but with few exceptions (See Balaban, 1995), studies have seldom examined emotion modulated startle in groups of very young children. Emotion-modulated startle paradigms have been developed and used successfully with 10- to 17-year old children and adolescents (Grillon et al., 1997), and 12- to 22- year old adolescents and young adults (Grillon et al., 1998), but to our knowledge there is no emotion-modulated startle paradigm that has been used successfully from early childhood to adulthood.
To elicit emotion modulated startle in older children researchers have used stimulus sets, developed with adults (ex. Lang & Bradley, 1999). But for ethical reasons, the stimuli that are most effective with adults are not used in child studies and as a result the available paradigms are not equivalent in valence and arousal to those used with adults (Grillon et al., 1999). This may explain why many child studies fail to elicit startle potentiation to aversive stimuli (Cook, Hawk, Hawk, & Hummer, 1995; Essex et al., 2003; McManis, Bradley, Berg, Cuthbert, & Lang, 2001; Waters & Ornitz, 2005). In additions to difficulty with eliciting startle potentiation in children, there is dearth of startle studies during early childhood because young children are difficult to test. The small muscles and faces of young children hamper placing sensors while minimizing contribution from other muscles. Attrition rates (due to noncompliance and/or high motor activity) are higher for young children (31%-50%) than for adults (5%-10%) (Balaban & Berg, 2007). Thus employing still pictures is ineffectual with young children as they do not elicit the rapt attention required to minimize their motor activity for long periods. The paradigm presented in this study used movie clips to modulate emotion as it was expected that these would capture and hold the attention of young children.
Developmental Factors may Play a Role in Components of the Startle Response
There are inconsistent empirical results with regard to the occurrence of emotion modulated startle in children. McManis et al. (1995) found no startle potentiation to aversive stimuli in 7- to 10- year-old children in one study, and potentiation only for girls in another study (McManis et al., 2001). Both studies employed valenced slides (95 dB, 50 ms white noise, 2800-5500 ms after picture onset, ITI 12000 - 30000 ms). Several other studies have also failed to elicit startle potentiation in school-age children (Cook et al., 1995; Waters, Neumann, Henry, Craske, & Ornitz, 2008b; Waters & Ornitz, 2005). In one of these studies, Waters, Neumann et al. (2008b) tested 4- to 8- year-old high anxious and non-anxious children using still pictures of emotional faces (102 dB, 50 ms white noise, 3000-5000 ms after face onset, 22000 ms mean ITI); neither group exhibited emotion modulation of the startle reflex. By contrast, the same researchers (Waters & Ornitz, 2005), elicited an emotion-modulated response from 6- to 12-year-old anxious and non-anxious children using valenced slides (105 dB, 50 ms white noise). However, they noted emotion-modulation only at a very short interval after picture onset (60 ms) but not at longer intervals (240, 3500, and 5000 ms). Overall it appears that the presence of emotion modulated startle in children is inconsistent but it is unknown whether studies' experimental parameters or developmental factors explain conflicting results.
Given the limitations of prior studies, the second objective of this study is to examine possible developmental differences in components of startle (baseline and emotion modulated). Obviously, this second objective is intertwined with the first goal of validating a paradigm appropriate and effective from early childhood through adulthood so that existing developmental differences, if any, can be detected with the same paradigm.
The Association between Anxiety and Startle in Child Samples is Inconsistent
There is extensive literature in adult samples linking increased baseline startle and/or startle potentiation to individual differences in fear behaviors (Grillon, 2002), but studies examining the association between startle and fear behaviors in children are scarce, and those available yield inconsistent and sometimes paradoxical results. For example, although Schmidt and Fox (1998) noted greater startle potentiation among 9-month-olds who were, versus who were not, at risk for behavioral inhibition (a temperamental disposition reflecting social anxiety), other researchers studying older children failed to observe this association (McManis, Bradley, Cuthbert, Schupp, & Lang, 1996), or have noted the opposite association (van Brakel, Muris, & Derks, 2006). And while some researchers have noted larger overall startle magnitudes among high anxious relative to non-anxious children (Waters et al., 2008b), others have noted that high fearful children exhibit smaller and not the expected larger startle magnitudes to aversive relative to neutral stimuli (Cook et al., 1995). While some of these inconsistent and paradoxical findings may be due to differences in paradigms, the use of children of different ages in these studies may also be a factor. For example, in one study of 7- to 12-year-olds, the association between baseline startle and anxiety increased across this age period (Waters et al., 2008a). The third and final goal of the present study was to employ the same startle paradigm with children of different ages to examine whether associations with measures of anxiety differ as a function of child age. If so, then some of the inconsistency in the literature may be due to the use of participants who are at different developmental levels in to one age group (children).
Methods
Participants
The study involved a total of 122 participants, see Table 1 for demographics. Child and adult participants were from middle- to upper-income socioeconomic groups with the adult participants and the parents of the child participants having at least some college education. The participants were primarily non-Hispanic, Caucasians. All of the participants and their parents reported having no history of psychiatric illness, brain trauma or prominently anxiety disorder. Thirteen child subjects (not part of the 122 employed in this study) were excluded from the analyses. Five 3-year olds, two 5-year olds, two 7-year olds and one 9-year old refused the experimental procedures. Two 9-year olds and a 5 year-old were excluded due to experimenter error. Excluded participants were significantly younger than those retained, t(36.34)= 3.48, p <.001, but did not differ by gender. Refusal by child participants generally occurred during sensor placement, prior to being shown the film clips.
Table 1.
Age and Sex Demographics
| Age Group | Male | Female | Total | Mean Age (SD) |
|---|---|---|---|---|
| 3-year-olds | 13 | 12 | 25 | 3.64 (.50) |
| 5-year-olds | 11 | 12 | 23 | 5.58 (.27) |
| 7-year-olds | 12 | 13 | 25 | 7.48 (.22) |
| 9-year-olds | 13 | 11 | 24 | 9.54 (.19) |
| Adults | 10 | 15 | 25 | 22.16 (6.20) |
| Total | 65 | 70 | 122 |
Startle Experimental Paradigm
Participants viewed six (2 minutes) film clips previously rated by a panel of adult judges to be to be neutral, positive, or aversive in emotional valence. Film, film clips deemed to be pure exemplars of the target valences were selected from a variety of movies marketed for school-age children. Selected film clips were then rated by graduate students and experts in child development on arousal, valence, and appropriateness for 3 year old children. The film clips retained for use were the ones rated highest in arousal and target valence among those deemed appropriate for children as young as three years. Film clip fragments were extracted from the following movies: “Honey I Shrunk the Kids,” “The Goonies,” “Homeward Bound,” and two nature films titled, “Eye Witness Trees” and “Real Sanctuary.”
Participants viewed one of three possible orders of film clip presentation. The order of film clips was systematically varied such that films of the same valence were never presented sequentially: instead film valences alternated within the three possible orders. Film orders versions were counterbalanced across age and gender. Preceding the valenced films, a film clip with a pleasant nature scene lasting 1 minute was used for habituation and three startle probes were delivered. After each film, including the habituation film, a blue screen was presented for 10 seconds and startle probes were delivered 2, 4 or 5 seconds after film offset. During each valenced film clip, 4 startle probes were strategically administered at times that, according to the experimenters' judgment, coincided with moments exemplifying the peak emotional valence of the clip in order to assess effects during states of peak emotion and arousal. For example, during aversive film clips, a probe was placed when bats suddenly surround a group of scared children, as opposed to when the children argue about removing the stone; or at the precise time that a child flashes a frightened face in close-up as a giant scorpion's sting strikes. Whenever possible the probes were delivered when very frightened or very happy faces filled the screen, given the well replicated power of such stimuli to elicit amygdala activity (Thomas et al., 2001). A total of 36 probes were given throughout the experiment: 3 during habituation, 4 probes during each of the 6 valenced film clips (e.g. 8 per valence) and 9 during the blue screen/baseline condition. Probes delivered during the blue screen allowed for the assessment of baseline startle response, while the probes delivered during the negative, neutral, and positive films allowed for the assessment of the emotion modulated startle response.
Behavioral Measures
Children of age 7 and older and all parents completed age appropriate versions of the Spence Children's Anxiety Scale (SCAS, Muris, Merckelbach, Ollendick, King, & Bogie, 2002; Nauta et al., 2004). All parents completed the Behavior Assessment System for Children, 2nd Edition (BASC-2, Reynolds & Kamphaus, 1998). Parents completed these questionnaires while their children were being tested. Children completed the SCAS after the psychophysiological testing ended.
Procedures
Adult participants were recruited through undergraduate psychology courses to receive extra credits for taking part in research studies. Parents of child participants were contacted using a phone registry of families interested in participating in research in child development. After describing the experiment, participants were scheduled for appointments at the research center. When they arrived, participants were guided through the informed consent and/or assent process and taken to a child-friendly room where electrodes were applied. Researchers adjusted and placed headphones on the participant's head making sure that the ear was completely encircled and inside the cavity created by the cushion. Researchers turned off the lights and positioned the participant 22 inches from a 21-in computer monitor. Researchers remained in the room and sat behind and away from the participant's view. All parents waited outside of the testing room. Both the film (VHS tapes) and the psychophysiology software (ERP32, New Boundary Technologies, Minneapolis, MN) were simultaneously started. ERP32 presented the startle probes via the headphones and acquired the electromyogram (EMG) data. To ensure that the probes were presented accurately during the film clips, researchers noted the timing of the first probe during habituation and restarted the experiment if the timing was off. Before and after completion of data collection, impedances were recorded and electrodes were removed. After data collection, participants were then instructed in the use of rating scales used to assess valence and arousal. Valence and arousal ratings were collected after watching all clips were seen and sensors were removed, to ensure comfort and compliance from the youngest participants given the possible discomfort associated with having sensors attached to sensitive areas of the face. All participants rated the films using the Self-Assessment Manikin (SAM) system (Bradley & Lang, 1994). The SAM is an inexpensive non-verbal pictorial self report assessment technique that measures via self report the pleasure, arousal, and dominance (not collected) associated with a person's affective reaction to stimuli. Degrees of arousal and pleasure are depicted in a cartoon and participants point to the figure that best represents their emotional state while watching the films. Participants also rated whether they had seen the movies from which the clips had been extracted (and how much did they remember) in a scale from 0 (not seen the movie/no memory) to 7 (remember all). Instructions to administer the SAM and to rate memory were comparable for all participants but developmentally appropriate scripts were used for adults and children to administer the SAM and to rate memory. For adults and children, fragments of the films were replayed to identify the film and aid in recalling how they felt when they watched the clip. At the end of the session, participants were debriefed. Small toys and gift cards for the value of 10 dollars were given as compensation to the children.
Startle Response Data Acquisition and Coding
EMG activity was recorded in response to auditory probes to measure startle response. EMG was recorded from two Ag/AgCl mini electrodes placed over right-sided orbicularis oculi (sampled at 1000 Hz, bandpass filtered 30-300 KHz, and amplified 20K), while a third electrode placed in the forehead served as ground. Impedances were below 10,000 ohms. There were no significant differences between impedances measured before and after data collection. Auditory probes were presented binaurally via headphones. Probes were 40 ms white noise (M= 98.6 dB, SD = 1.6 dB) with nearly instantaneous rise, presented with an average inter-trial interval across all conditions of 18197 ms, SD = 4891 ms. There were no differences between the recorded intensity of probes delivered for children and adults, (t =−1.18, p=0.24). There were no differences in inter-trial interval between the film valence conditions but the average inter-trial interval for the blue screen/baseline condition was shorter than during the valenced films F(3,28)=6.6, p<0.01. This was expected given that the blue screen/baseline condition lasted 10 seconds while the film clips lasted 2 minutes. The soundtrack of the films (not delivered through the headphones but as part of the film clips) was kept at approximately 65db. The headphones used were commercially available and had large cushioned ear-shells selected because of their attractive features for very young children. They could be adjusted to the head size of both adults and children and the speakers encircled and covered the auditory canal for participants of all ages. Data were rectified and integrated with a 20 ms time constant, during 20-175 ms of EMG activity post-probe onset. Coding parameters to determine the presence of a clear startle were as follows. A clear startle response was defined as a sharp increase in EMG signal amplitude within a window of 20-175 ms after probe onset. This exemplar response would have a quiet baseline period during 0-20 ms, and typically resolved to near zero level of EMG by 200 ms. The morphology of the raw and rectified startle response EMG is depicted elsewhere (Blumenthal et al., 2005). Data were scored via visual inspection by trained coders to determine the presence (coded 1) or absence (coded 0) of a clear startle response. Coders were blind to gender and age group and were trained to define clear startle responses and “artifactual trials” by pattern recognition to greater than 90% agreement. Coders were ranked ordered by greatest agreement, and the higher ranking coder's data were retained for final analyses. Mean Kappa reliability among coders was 0.8 computed on 30% of cases. Trials containing evidence of gross motor movement (i.e. EMG amplitude was very elevated, sustained and it obscured any potential startle response) during the post-probe window or containing response latencies of less than 20 ms were determined to be “artifactual trials” and eliminated. Finally, it must be underscored that the number of artifactual trials eliminated from all startle EMG measures did not differ by age, gender or condition. All groups, on average, contributed equal frequencies of data points for the analyses of emotion modulated and baseline startle.
Startle EMG Measures
For both baseline and emotion modulated startle responses, the response size in microvolts (μV) was adjusted by subtracting mean EMG activity during the 100 ms. prior to the auditory probe onset. Two measures were obtained for baseline and emotion modulated startle.
Startle response probability was computed as total number of non-artifactual startle responses divided by the total number of possible trials in each condition. For valenced trials the possible range was 0 to 8 trials, while for baseline (blue screen condition) the possible range was 0 to 9 trials. For all conditions there was at least 1 startle response thus no response probabilities were calculated with a 0 as denominator.
Startle magnitude was computed as the averaged (baseline EMG activity corrected) response of all non-artifactual trials for each film valence and, separately, for the blue screen condition (baseline). As customary, this measure included scores for subjects with no discernible startle response. Thus, magnitude values include trials showing near-zero amplitudes and were non-normally distributed, with skew values ranging 1.4 – 2.2 and kurtosis values ranging 1.9 – 5.0. Therefore magnitude values were log-transformed.
Anxiety Measures
Parent-reported SCAS and the BASC-2 anxiety scale scores were significant correlated and thus were standardized and averaged to yield one parent report measure of child anxiety (ANXparent). If only the parent report measure was available (i.e. for the 3- and 5-year-olds) the anxiety measure was employed in the analyses and based only the parent report data. Child reported SCAS scores were available for the 7- and 9-year old children. These were highly correlated with the parent SCAS reports, r=.87 p<.001 therefore it was thought reasonable to combine them with the ANXparent variable. To create an overall composite anxiety score (ANXcomposite) that included child report measures, the child SCAS score was standardized and averaged with ANXparent. Anxiety measures were no available for adult participants.
Analysis Plan
Effects of sex and age group were analyzed using 2(sex) by 5 (age group) by 3 (film valence) repeated measures analyses of variance (RM-ANOVA). Main effects of age group were then followed-up with LSD post-hoc tests and planned contrasts to test for linear trends. Associations between anxiety scores and startle measures were analyzed using Pearson correlation coefficients for the younger and older child participants separately to examine possible age changes in the strength of these associations.
Results
Valence, Arousal and Memory Ratings
To aid in interpretation of the startle results, possible age group or sex differences in how participants rated the valence and arousal of the films were examined. For valence ratings, there was a significant age group by valence interaction, F(8,234) = 4.15, p<.01, η2p= 0.12, as well as significant valence effect was also noted, F(1.5,178.8) = 163.64, p <.01, η2p= 0.58. Planned contrasts showed that, as expected, negative films were rated more negatively than neutral films, which were rated more negatively than positive films (p's<.05) within all age groups. However, examining age groups within each valence of films, the interaction was limited to negative film clips F(4,122) = 5.13, p<.01, with 9-year olds rating the negative films as less aversive than did the other groups (significant differences between 9-year olds and both 3-year olds and adults). Finally, a sex by valence interaction for valence ratings was obtained, F(1.5,178.8) = 5.13, p < .05, η2p= 0.04 with females rating positive films more positively and negative ones more negatively than males, F(1,117) = 7.36, p <.05.
For arousal ratings, a significant age group by valence interaction was obtained, F(8,224) = 5.33, p<.01, η2p= 0.16 which reflected age group differences for both neutral, F(4,118) = 5.54, p<.01, and positive film arousal ratings, F(4,118) = 5.41, p<.01. Three- and 5-year olds rated neutral and positive films as more arousing than did 9-year-olds. Additionally, 3-year-old children rated neutral films as more arousing than adults. Finally, the expected main effect of film valence F(1.7, 190.2) = 38.26, p <.01, η2p= 0.26 was examined through planned contrasts, and showed that all age groups rated neutral films as less arousing than either negative or positive film clips (p's<.05).
For memory ratings, a significant age group effect F(4, 120)=20.9, p<.01, η2p= 0.41 was obtained. Post- hoc analysis with Bonferroni correction indicated that older children and adults reported that they had seen the films (1.“Homeward Bound”, 2.“Honey I Shrunk the Kids”, 3.“The Goonies”, 4.“Eye Witness Trees”, 5.“Real Sanctuary”) and remembered them better than did 3- and 5-year-old children. Additionally, adults reported seeing and remembering the films better than all children (Overall memory: Mage 3 = 0.1, sd=0.4, Mage 5 = 0.44, sd=0.99, Mage 7 = 1.13, sd= 1.3, Mage 9 = 1.31, sd= 0.9, Madults =2.4, sd= 0.84). A significant effect of film F(4, 472)=35.8, p<.01, η2p= 0.23, indicated that films 1, 2 and 3 had been seen and were better remembered than films 4 and 5. No other main or interaction effects were significant.
Baseline Startle
Before conducting baseline startle analyses, it was verified that baseline startle measures (response probability and magnitude) were independent of the valence of the clips shown prior to them, as no significant effect of valence of prior clip was found for baseline startle measures.
Baseline response probability
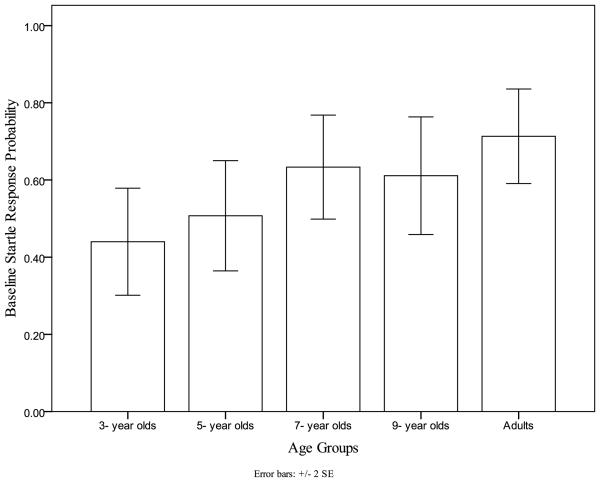
A significant effect of age group on baseline startle response probability, was found, F(4, 112)=2.5, p<.05, η2p= 0.08. Post-hoc multiple comparisons showed that both 3- and 5-year-olds had lower baseline startle response probability than adults, and 3- year olds had lower baseline startle response probability than 7- year olds, (Error! Reference source not found.). Planned contrasts with the following weights per age group (3 yr: −0.2, 5 yr:−0.2, 7yr: 0.05, 9yr: 0.15, adults: 0.2) yielded a significant linear trend characterized by increased baseline response probability with increasing age (t = 2.91, p<.01). A main effect of sex on baseline startle response probability was also noted, F(1,112)=5.4, p<.05, η2p= 0.05, with females exhibiting higher response probability of baseline startle responses than males. No significant age group by sex interaction was noted. The response probability differences were not due to more boys (8 of 59) than girls (6 of 57) failing to produce any startles.
Baseline magnitude
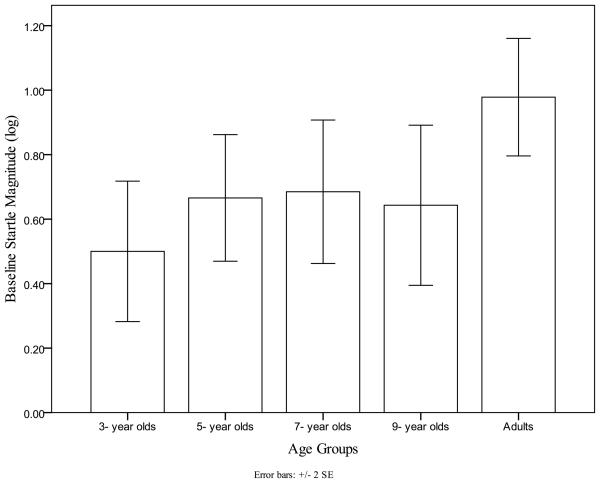
There was a significant age group effect, F(4,117)=2.7, p<.05, η2p= 0.09 on baseline startle magnitude. Post hoc multiple comparisons showed that baseline startle magnitudes for adults were larger than for 3-, 5-, and 9-year-old children, (Error! Reference source not found.). Planned contrasts using the same weights previously employed yielded a significant linear trend characterized by increased baseline response magnitude with increasing age (t = 2.27, p<0.05). There were no other significant main effects or interactions for baseline startle magnitude.
Emotion Modulated Startle (EMS)
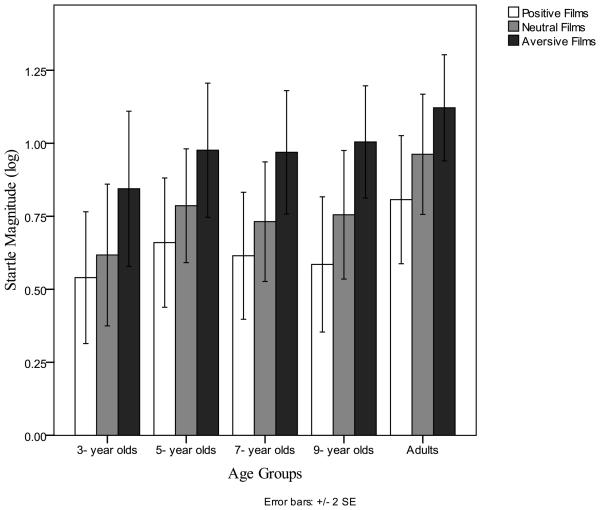
There were no main effects of age group for any of the EMS startle measures: response probability, F(4,112)=1.57, ns and magnitude, F(4,111)=.79, ns. Critically, there were no significant valence by age group interactions for any of the EMS measures: response probability, F(8,224)=1.26, ns and magnitude, F(8,222)=.45, ns. Thus, all age groups showed heightened magnitude and more probable startle responses to negative films and startle inhibition to positive films, (Error! Reference source not found.).
There were main effects of valence in startle measures: response probability, F(1.9,207.3)= 43.85, p <.001, η2p= 0.38 and magnitude, F(1.9, 199.69)=80.16, p<.01, η2p= 0.42. Specifically, aversive films provoked higher probabilities of responses and larger startles than neutral films: response probability, F(1,112) = 44.41, p <.001 and magnitude, F(1,111)=82.08. Neutral films elicited higher probabilities of responses and larger startles than positive films, response probability, F(1,112) = 65.25, p <.001 and magnitude, F(1,111)=23.95, p<.01. Log transformed magnitude means (SD) in microvolts for film valence across all groups were: Aversive = 0.99 (Sd=0.53); Neutral = 0.77 (Sd=0.53); Positive = 0.64 (Sd=0.55).
Main effects of sex were noted for response probability, F(1,112) = 5.03, p < .05, η2p=0.04 and magnitude, F(1,111)=5.7, p<.001, η2p= 0.03 with females showing higher overall startle magnitudes and higher overall response probabilities than males during the emotion valenced portion of the paradigm. These main effects on overall response probability and magnitude were not qualified by interactions with age for the emotion modulated startle response.
There were valence rating differences between genders and ages and arousal ratings differences between ages. Therefore, all the previous analyses were repeated with valence and arousal ratings as covariates and similar significant effects of film valence and gender in startle response probability and magnitude were found. Finally, although the memory rating differences (better memory of films for adults than for children and for more known films) may conceivably have resulted in less physiological reactivity for adults due to predictability and habituation to the films this response pattern was not observed. Furthermore, analyses were repeated co-varying overall memory ratings and again the main reported results were obtained.
Associations of Startle Components with Anxiety
Prior to examining associations between anxiety and startle, the composite anxiety scores (ANXcomposite and ANXparent) were examined for sex, age group and interaction effects. No differences were found in anxiety scores between ages or gender (ANXcomposite: Mage 3 = −0.3, sd=0.6, Mage 5 = 0.19, sd=0.7, Mage 7 = 0.2, sd=0.8, Mage 9 = − 0.02, sd=1). In addition, no associations were noted between this composite anxiety score and startle potentiation (aversive – neutral) measures: response probability, r=−.16, df=94 and magnitude, r=−.04, df=94, ns. However, significant positive correlations were noted between ANXcomposite and baseline startle: magnitude, r=.33, df=96, p<.001, but not response probability, r=.12, df=94, ns. To extend these results, the correlation of ANXcomposite with startle magnitude during the neutral film clips was examined: magnitude, r=.29, df=96, p<.01. Similar results were found for correlations between ANXparent and baseline startle magnitude. These findings were not altered when partial correlations were computed controlling for age group and sex.
To test if baseline startle was indexing the anxiogenic nature of the laboratory testing; low vs. high anxious children groups (median split of ANXcomposite) were compared on baseline startle magnitude during the first or the second half of the experimental paradigm. There were no differences between low (M= 7.4) and high anxious children (M= 9.2) during the first half of the experiment, t(95) = 0.31 ns. However, low anxious (M=5.3) baseline startle magnitude was significantly lower than high anxious children (M=8.4) during the second half of the experiment, t(95) = −2,08, p<.05. This was confirmed within anxiety groups given that baseline startle magnitude significantly diminished between the first part and the second part of the experiment for low F(1,47)=12.5 p<.01, but not for high anxious children F(1,48)=.697, ns; confirming the role of the laboratory testing as a mildly anxiogenic for high anxious children.
To examine whether the associations of startle measures with anxiety were moderated by age, the children were split into Younger (3- and 5-year olds) and Older (7- and 9-year olds) groups similar to procedures employed by Waters et al (2008a). Both the parent report anxiety measure (ANXparent) and the anxiety composite including child report symptoms (ANXcomposite) were employed for these analyses. Correlations were computed for baseline startle magnitude and anxiety. Baseline response probability was not employed due to initial lack of relationship with anxiety. The correlations between ANXparent and baseline startle magnitude were as follows: Younger children, r = 0.076, p<0.61, Older children r = 0.46 p<0.01. These correlations are significantly different, Fisher's test, z = −1.97, p<0.05. Showing that the associations between anxiety and baseline startle magnitude is significantly larger among older than among younger children. Similar results were obtained with the ANXcomposite measure Younger children r = 0.08, p<0.6, Older children r = 0.47 p<0.01.Fisher's test z = −2.07, p<0.05.
Discussion
A Novel Startle Paradigm Appropriate from Early Childhood through Adulthood
The film paradigm elicited emotion modulated startle in 3-year-olds to 9-year-olds and adults. Experimenter's initial judgments of the films' valences were confirmed: aversive films were rated by participants as more negative than neutral ones and neutral as more negative than positive films. A robust effect of film valence was obtained: aversive films elicited larger and more likely startles than neutral and positive films, and positive films suppressed the probability and magnitude of startle. Valence, arousal and memory rating differences between age groups and gender did not influence the expected emotion modulation of startle. These results show that an engaging film format can be employed to modulate the startle response in very young children. Additionally, the films reliably activated startle potentiation in adults, while being appropriate for the younger participants. Interestingly, adult ratings showed that these films were rated as low to only moderately arousing and comparably rated still pictures do not elicit startle potentiation in adults (Cuthbert, Bradley, & Lang, 1996). This suggests that film clips may be a means of extending the range of arousal intensities that are effective in studies of adults. Overall this paradigm appears to thoroughly and safely permit the exploration baseline and emotion-modulated startle responses across a wide age spectrum.
The Role of Development on Components of the Startle Response
Young children, particularly 3- and 5-year olds, showed a lower response probabilities and magnitude of baseline startles than did adults, while these components of the startle response did not differ between adults and 7- year old children. The reasons for these age differences in baseline startle magnitude and response probability are not clear. It might be that the younger children experienced less intense startle probes than adults. However, we have no indication that this was the case. There were no differences between groups in the recorded intensity of startle probes, and the headphones fitted the ear and head anatomy of all age groups. Thus, it seems unlikely that the differences are due to unreliable sensory input for the youngest children. Another possibility is the impact of different facial musculature in the startle amplitude and frequency, given that muscle size and endurance are known to increase with advancing sexual maturation (Derman, Yalcin, Kanbur, & Kinik, 2002). If this were the explanation, then we would also expect older males to exhibit larger and more frequent startles than older females in our paradigm as the direction of muscle growth favors males as development proceeds. Because we found that females exhibited larger and more frequent startles than males, this does not support a size-of-musculature explanation of the developmental findings, although this explanation cannot be ruled out.
Finally, age differences in baseline startle may be of developmental importance. Age differences in baseline startle measures may represent developmental changes in primary neural pathways of the startle response and in basic reactivity of defensive behaviors. For example, lower response probability of response and magnitude of baseline startle response has been found in infants compared with adults, supporting the hypothesis that underlying biological systems may be immature in very young children (Blumenthal, Avendano, & Berg, 1987). Additional evidence shows that components of the startle response, such as startle inhibition by a pre-pulse, are immature in early childhood (Balaban, Anthony, & Graham, 1989; Ornitz, Guthrie, Kaplan, Lane, & Norman, 1986; Ornitz, Guthrie, Sadeghpour, & Sugiyama, 1991). Overall these findings are interpreted as ongoing neural development of the primary brain stem pathways supporting baseline startle, and this study's results appear to fit with this explanation. Changes in baseline startle response between early childhood and adulthood may occur during the transitional period of adolescence. Increases in startle overall magnitude have previously been reported with more advanced pubertal stage (Quevedo, Benning, Gunnar, & Dahl, 2009). This increase may reflect maturation of brainstem pathways regulating the startle response such that differences in baseline startle magnitude between children and adults may emerge with puberty. Finally, the lower probability of startle responses in preschool-aged children has implications for developmental research. It may be necessary to deliver more probes and/or recruit larger samples in order to use startle as a measure with young children.
Regardless of differences in probability and magnitude of baseline startles, emotion modulation was similar for all age groups: the startle response varied with the emotional valence of the film clips. Most notably for research on fear and anxiety, the size of the change in startle from neutral to aversive film clips (startle potentiation) was comparable across groups. Thus it seems safe to assume that nuanced affective processing of threat contexts is established early and conserved throughout development. This conclusion is consistent with evidence that fear potentiation to threatening faces shows as early as 5 months of age (Balaban, 1995).
Sex Differences in the Startle Response
The results also showed that females exhibited higher baseline startle response probability than did males. Larger magnitude and higher response probability of startle was also noted for female versus males during the film portion of the paradigm. None of these gender differences in startle measures were explained away by co-varying rating differences. However, there was no evidence that females of any age exhibited greater startle potentiation to negative films than males.
Although no gender differences in startle response components had been anticipated; these findings are both similar to and different from results in the available literature. McManis et al. (2001) found enhanced startle potentiation for girls compared to boys. Gender differences in reactivity to aversive contents employing startle have been described in adults (Bradley, Codispoti, Sabatinelli, & Lang, 2001; Lang, Greenwald, Bradley, & Hamm, 1993). Additionally, women show a more active central processing to threatening and aversive material than males (Wrase et al., 2003). It is not clear why this study's findings differ with regards to startle potentiation. A possible explanation is that the aversiveness and arousal value of the employed film clips were less extreme than those of stimuli generally used in adult studies of gender differences in startle potentiation might reflect the less extreme nature of the threat stimuli.
In the context of prior research higher baseline startle response probability and larger startle magnitude for females may reflect sex differences in the processing of threatening cues. Animal research suggests that gonadal hormones play a strong role in sex differences in emotional reactivity and fear learning (Toufexis, Myers, & Davis, 2006). This is consistent with research that shows, that although during childhood more boys than girls are depressed, a higher prevalence of anxiety and depression in girls emerges after mid-puberty (Ge, Conger, & Elder, 2001; Zahn-Waxler, Shirtcliff, & Marceau, 2008).
Associations of Startle Components with Anxiety
Individual differences in anxiety were related to startle baseline magnitude but not to startle potentiation, and this association was less robust for younger than older children at least by parent report. Similar evidence of associations between anxiety and baseline but not fear potentiation have been reported in adults (Grillon, 2002). There are several explanations for these results. First, it appears that for more anxious individuals and perhaps especially for more anxious children, the context of laboratory testing is in itself mildly anxiogenic. Low anxious and high anxious children showed similar baseline startle magnitude during the first half of the experimental paradigm, yet while low anxious children's showed less startle reactivity as they adapted to the laboratory, high anxious children showed no significant change in reactivity throughout the experiment. This mean that “baseline” is not a true baseline for all individuals, particularly for those who are more dispositionally anxious. With regards to the lack of relationship between startle potentiation and anxiety, the aversive films in this study may have been of sufficient intensity to provoke similar heightened startle across children of differing dispositional anxiety. This might be particularly true in samples where anxiety is within the normal range, because suboptimal stimuli (such as baseline startle) may be more effective than supra-optimal stimuli in revealing individual differences in physiological reactivity.
The larger association between baseline startle and parent reported anxiety in older versus younger children must be interpreted with care. Either the anxiety or the startle measures, or both, may simply become more reliable indices of stable individual traits with age, thus increasing the likelihood of observing significant associations. Conversely, the emergence of a more coherent emotion-physiological association may reflect development of the emotional appraisal system. Notably, we are not the first to note an increase in startle-anxiety correlations with increasing age, as this was previously reported for children at risk for anxiety disorders (Waters, Craske et al. 2008). Explication of changes in these links requires longitudinal data.
Finally, as previously discussed overall and baseline startle differentiate at risk and clinically anxious children from healthy controls but startle potentiation has failed to differentiate such groups (Grillon et al., 1997; Waters et al., 2008b). Given a scarcity of startle studies comparing anxious and healthy school-age children, in both baseline and emotion modulated startle, it is clear that more research is needed to elucidate this question. Overall these data provide support for the value of assessing eye-blink startle in studies of emotional development.
Limitations and Future Directions
The generalizability of the present results may be limited by the socio-demographics of the sample employed, consisting mostly of Caucasian middle class families. Although the results speak to the robustness of the experimental paradigm across the age span, the characteristics of startle components elicited by this paradigm might be different in a more diverse sample. Future studies are needed to determine if these findings can be extended to children in lower economic strata and to those of other race/ethnicities. For example, children growing in communities and/or families where there is less protection from threatening stimulus might show different physiological response patterns to the films employed in this paradigm. Additionally, it will be important to employ this paradigm in both children exhibiting the normal range of anxiety and children exhibiting clinical levels of anxiety symptoms. This paradigm may also be of value in longitudinal research. Given the noted relation between baseline startle and normative anxiety symptoms it is possible that typically developing children who currently exhibit larger baseline startle frequency and magnitude might be at greater risks for developing clinically significant anxiety symptoms.
Figure 1.
Younger Children show Lower Baseline Startle Response Probability than Adults.
Figure 2.
Children show Lower Baseline Startle Magnitude than Adults.
Figure 3.
Similar Emotion Modulated Startle in Children and Adults.
References
- Balaban MT. Affective influences on startle in five-month-old infants: Reactions to facial expressions of emotion. Child Development. 1995;66:28–36. doi: 10.1111/j.1467-8624.1995.tb00853.x. [DOI] [PubMed] [Google Scholar]
- Balaban MT, Anthony BJ, Graham FK. Prestimulation effects on blink and cardiac reflexes of 15-month human infants. Developmental Psychobiology. 1989;22(2):115–127. doi: 10.1002/dev.420220203. [DOI] [PubMed] [Google Scholar]
- Balaban MT, Berg WK. Measuring the Electromyographic Startle Response: Developmental Issues and Findings. In: McMaster LAS, Brock SJS, editors. Developmental Psychophysiology Theory, Systems, and Methods. Cambridge University Press; Cambridge, New York: 2007. pp. 257–285. [Google Scholar]
- Balaban MT, Snidman N, Kagan J. Attention and orienting: Sensory and motivational processes. Lawrence Erlbaum Associates Publishers; Mahwah, NJ: 1997. Attention, emotion, and reactivity in infancy and early childhood; pp. 369–391. [Google Scholar]
- Bernat E, Patrick CJ, Benning SD, Tellegen A. Effects of picture content and intensity on affective physiological response. Psychophysiology. 2006;43(1):93–103. doi: 10.1111/j.1469-8986.2006.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal TD, Avendano A, Berg WK. The startle response and auditory temporal summation in neonates. Journal of Experimental Child Psychology. 1987;44(1):64–79. doi: 10.1016/0022-0965(87)90022-1. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, van Boxtel A. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42(1):1–15. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Sabatinelli D, Lang PJ. Emotion and motivation II: Sex differences in picture processing. Emotion. 2001;1(3):300–319. [PubMed] [Google Scholar]
- Bradley MM, Cuthbert BN, Lang PJ. Affect and the startle reflex. In: Dawson ME, Schell AM, Bohmelt AH, editors. Startle modification: Implications for neuroscience, cognitive science, and clinical science. Cambridge University Press; New York, NY: 1999. pp. 157–183. [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: the Self-Assessment Manikin and the Semantic Differential. Journal of Behavior Therapy and Experimental Psychiatry. 1994;25(1):49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Cook EW, Hawk LW, Davis TL, Stevenson VE. Affective individual differences and startle reflex modulation. Journal of Abnormal Psychology. 1991;100(1):5–13. doi: 10.1037//0021-843x.100.1.5. [DOI] [PubMed] [Google Scholar]
- Cook EW, Hawk LW, Hawk TM, Hummer K. Affective modulation of startle in children. Psychophysiology. 1995;32:S25. Abstract. [Google Scholar]
- Corr PJ, Wilson GD, Fotiadou M, Kumari V, et al. Personality and affective modulation of the startle reflex. Personality and Individual Differences. 1995;19(4):543–553. [Google Scholar]
- Cuthbert BN, Bradley MM, Lang PJ. Probing picture perception: Activation and emotion. Psychophysiology. 1996;33(2):103–111. doi: 10.1111/j.1469-8986.1996.tb02114.x. [DOI] [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. The American Psychologist. 2006;61(8):741–756. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- Davis M, Falls WA, Campeau S, Kim M. Fear-potentiated startle: a neural and pharmacological analysis. Behavioural Brain Research. 1993;58(1-2):175–198. doi: 10.1016/0166-4328(93)90102-v. [DOI] [PubMed] [Google Scholar]
- Davis M, Gendelman DS, Tischler MD, Gendelman PM. A primary acoustic startle circuit: lesion and stimulation studies. Journal of Neuroscience. 1982;2(6):791–805. doi: 10.1523/JNEUROSCI.02-06-00791.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derman O, Yalcin SS, Kanbur N, Kinik E. The influence of the sexual stages of adolescent boys on the circumference of the arm, muscle area and skinfold measurements. International Journal of Adolescent Medicine and Health. 2002;14(1):19–26. doi: 10.1515/IJAMH.2002.14.1.19. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Goldsmith H, Smider NA, Dolski I, Sutton SK, Davidson RJ. Comparison of video- and EMG-based evaluations of the magnitude of children's emotion-modulated startle response. Behavior Research Methods, Instruments & Computers. 2003;35(4):590–598. doi: 10.3758/bf03195538. [DOI] [PubMed] [Google Scholar]
- Ge X, Conger RD, Elder GH., Jr. Pubertal transition, stressful life events, and the emergence of gender differences in adolescent depressive symptoms. Developmental Psychology. 2001;37(3):404–417. doi: 10.1037//0012-1649.37.3.404. [DOI] [PubMed] [Google Scholar]
- Grillon C. Startle reactivity and anxiety disorders: Aversive conditioning, context, and neurobiology. Biological Psychiatry. 2002;52(10):958–975. doi: 10.1016/s0006-3223(02)01665-7. [DOI] [PubMed] [Google Scholar]
- Grillon C, Ameli R, Goddard A, Woods SW, Davis M. Baseline and fear-potentiated startle in panic disorder patients. Biological Psychiatry. 1994;35(7):431–439. doi: 10.1016/0006-3223(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Grillon C, Dierker L, Merikangas KR. Startle modulation in children at risk for anxiety disorders and/or alcoholism. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36(7):925–932. doi: 10.1097/00004583-199707000-00014. [DOI] [PubMed] [Google Scholar]
- Grillon C, Dierker L, Merikangas KR. Fear-potentiated startle in adolescent offspring of parents with anxiety disorders. Biological Psychiatry. 1998;44(10):990–997. doi: 10.1016/s0006-3223(98)00188-7. [DOI] [PubMed] [Google Scholar]
- Grillon C, Merikangas KR, Dierker L, Snidman N, Arriaga RI, Kagan J, et al. Startle potentiation by threat of aversive stimuli and darkness in adolescents: A multi-site study. International Journal of Psychophysiology. 1999;32(1):63–73. doi: 10.1016/s0167-8760(99)00002-1. [DOI] [PubMed] [Google Scholar]
- Grillon C, Warner V, Hille J, Merikangas KR, Bruder GE, Tenke CE, et al. Families at High and Low Risk for Depression: A Three-Generation Startle Study. Biological Psychiatry. 2005;57(9):953–960. doi: 10.1016/j.biopsych.2005.01.045. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, editors. International affective picture system (IAPS): Instruction manual and affective ratings. University of Florida, The Center for Research in Psychophysiology; Gainesville, FL: 1999. (Tech. Rep. No. A-4). [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: Affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30(3):261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- McManis MH, Bradley MM, Berg WK, Cuthbert BN, Lang PJ. Emotional reactions in children: verbal, physiological, and behavioral responses to affective pictures. Psychophysiology. 2001;38(2):222–231. [PubMed] [Google Scholar]
- McManis MH, Bradley MM, Cuthbert BN, Lang PJ. Kids have feelings too: children's physiological responses to affective pictures. Psychophysiology. 1995;33:S53. Abstract. [PubMed] [Google Scholar]
- McManis MH, Bradley MM, Cuthbert BN, Schupp H, Lang PJ. Kid's cortical ERP's: emotion and attention in picture processing. Psychophysiology. 1996;34:S61. Abstract. [Google Scholar]
- Muris P, Merckelbach H, Ollendick T, King N, Bogie N. Three traditional and three new childhood anxiety questionnaires: Their reliability and validity in a normal adolescent sample. Behaviour Research and Therapy. 2002;40(7):753–772. doi: 10.1016/s0005-7967(01)00056-0. [DOI] [PubMed] [Google Scholar]
- Nauta MH, Scholing A, Rapee RM, Abbott M, Spence SH, Waters A. A parent-report measure of children's anxiety: psychometric properties and comparison with child-report in a clinic and normal sample. Behaviour Research & Therapy. 2004;42:813–839. doi: 10.1016/S0005-7967(03)00200-6. [DOI] [PubMed] [Google Scholar]
- Ornitz EM, Guthrie D, Kaplan AR, Lane SJ, Norman RJ. Maturation of startle modulation. Psychophysiology. 1986;23(6):624–634. doi: 10.1111/j.1469-8986.1986.tb00681.x. [DOI] [PubMed] [Google Scholar]
- Ornitz EM, Guthrie D, Sadeghpour M, Sugiyama T. Maturation of prestimulation-induced startle modulation in girls. Psychophysiology. 1991;28(1):11–20. doi: 10.1111/j.1469-8986.1991.tb03381.x. [DOI] [PubMed] [Google Scholar]
- Pissiota A, Frans O, Michelgard A, Appel L, Langstrom B, Flaten MA, et al. Amygdala and anterior cingulate cortex activation during affective startle modulation: A PET study of fear. European Journal of Neuroscience. 2003;18(5):1325–1331. doi: 10.1046/j.1460-9568.2003.02855.x. [DOI] [PubMed] [Google Scholar]
- Quevedo KM, Benning SD, Gunnar MR, Dahl RE. The onset of puberty: Effects on the psychophysiology of defensive and appetitive motivation. Development & Psychopathology. 2009;21:27–45. doi: 10.1017/S0954579409000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CR, Kamphaus RW. Behavior Assessment System for Children; Behavioral assessment of children. American Guidance Service; Circle Pines: MN: 1998. [Google Scholar]
- Schmidt LA, Fox NA. Fear-potentiated startle responses in temperamentally different human infants. Developmental Psychobiology. 1998;32(2):113–120. doi: 10.1002/(sici)1098-2302(199803)32:2<113::aid-dev4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Temple RO, Cook EW., III. Anxiety and defensiveness: Individual differences in affective startle modulation. Motivation and Emotion. 2007;31(2):115–123. [Google Scholar]
- Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH, et al. Amygdala response to fearful faces in anxious and depressed children. Arch Gen Psychiatry. 2001;58(11):1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- Tischler MD, Davis M. A visual pathway that mediates fear-conditioned enhancement of acoustic startle. Brain Research. 1983;276(1):55–71. doi: 10.1016/0006-8993(83)90548-6. [DOI] [PubMed] [Google Scholar]
- Toufexis DJ, Myers KM, Davis M. The effect of gonadal hormones and gender on anxiety and emotional learning. Hormones and Behavior. 2006;50(4):539–549. doi: 10.1016/j.yhbeh.2006.06.020. [DOI] [PubMed] [Google Scholar]
- van Brakel AM, Muris P, Derks W. Eye blink startle responses in behaviorally inhibited and uninhibited children. International Journal of Behavioral Development. 2006;30(5):460–465. [Google Scholar]
- Waters AM, Craske MG, Bergman RL, Naliboff BD, Negoro H, Ornitz EM. Developmental changes in startle reactivity in school-age children at risk for and with actual anxiety disorder. International Journal of Psychophysiology. 2008a;70(3):158–164. doi: 10.1016/j.ijpsycho.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Waters AM, Neumann DL, Henry J, Craske MG, Ornitz EM. Baseline and affective startle modulation by angry and neutral faces in 4--8-year-old anxious and non-anxious children. Biological Psychology. 2008b;78(1):10–19. doi: 10.1016/j.biopsycho.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Waters AM, Ornitz EM. When the orbicularis oculi response to a startling stimulus is zero, the vertical EOG may reveal that a blink has occurred. Clinical Neurophysiology. 2005;116(9):2110–2120. doi: 10.1016/j.clinph.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Wilson GD, Kumari V, Gray JA, Corr PJ. The role of neuroticism in startle reactions to fearful and disgusting stimuli. Personality and Individual Differences. 2000;29(6):1077–1082. [Google Scholar]
- Wrase J, Klein S, Gruesser SM, Hermann D, Flor H, Mann K, et al. Gender differences in the processing of standardized emotional visual stimuli in humans: a functional magnetic resonance imaging study. Neuroscience Letters. 2003;348(1):41–45. doi: 10.1016/s0304-3940(03)00565-2. [DOI] [PubMed] [Google Scholar]
- Wu MF, Suzuki SS, Siegel JM. Anatomical distribution and response patterns of reticular neurons active in relation to acoustic startle. Brain Research. 1988;457(2):399–406. doi: 10.1016/0006-8993(88)90716-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn-Waxler C, Shirtcliff EA, Marceau K. Disorders of childhood and adolescence: gender and psychopathology. Annual Review of Clinical Psychology. 2008;4:275–303. doi: 10.1146/annurev.clinpsy.3.022806.091358. [DOI] [PubMed] [Google Scholar]