Abstract
EphB4 is a transmembrane receptor tyrosine kinase that plays an important role in neural plasticity and angiogenesis. EphB4 is overexpressed in ovarian cancer and is predictive of poor clinical outcome. However, the biological significance of EphB4 in ovarian cancer is not known and is the focus of the current study. Here, we examined the biological effects of two different methods of EphB4 targeting (a novel monoclonal antibody, EphB4-131 or siRNA) using several ovarian cancer models. EphB4 gene silencing significantly increased tumor cell apoptosis, and decreased migration (p<0.001) and invasion (p<0.001). Compared to controls, EphB4 siRNA-DOPC alone significantly reduced tumor growth in the A2780-cp20 (48%, p<0.05) and IGROV-af1 (61%, p<0.05) models. Combination therapy with EphB4 siRNA-DOPC and docetaxel resulted in the greatest reduction in tumor weight in both A2780-cp20 and IGROV-af1 models (89-95% reduction versus controls; p<0.05 for both groups). The EphB4-131 antibody, which reduced EphB4 protein level, decreased tumor growth by 80-83% (p<0.01 for both models) in the A2780-cp20 and IGROV-af1 models. Combination of EphB4-131 and docetaxel resulted in the greatest tumor reduction in both A2780-cp20 and IGROV-af1 models (94-98% reduction versus controls; p<0.05 for both groups). Compared to controls, EphB4 targeting resulted in reduced tumor angiogenesis (p<0.001), proliferation (p<0.001), and increased tumor cell apoptosis (p<0.001), which likely occurs through modulation of PI3K signaling. Collectively, these data identify EphB4 as a valuable therapeutic target in ovarian cancer and offer two new strategies for further development.
Keywords: EphB4, angiogenesis, receptor tyrosine kinase, EphrinB2, ovarian carcinoma
Introduction
Ovarian cancer is the fifth leading cause of death among women in the United States and remains the most common cause of death from a gynecologic malignancy (1). There is no effective screening tool, and in the majority of cases, the diagnosis is not made until disease is advanced. Despite the use of primary cytoreductive surgery and combination chemotherapy regimens, most patients eventually develop recurrent cancer and die of their disease. Thus, there is a critical need for novel therapeutic strategies to improve the outcome of this deadly disease.
EphB4 is a protein tyrosine kinase located on chromosome 7q22, a member of the largest family of receptor tyrosine kinases with at least 16 Eph receptors and 9 ephrin ligands (2). Ephrin-B2 is the sole known ligand for EphB4, and binding of EphB4 to ephrin-B2 requires cell-to-cell contact, which initiates bidirectional signaling in both EphB4 and ephrin-B2 expressing cells (3). EphB4 also plays an important role in angiogenesis as well as a variety of processes during embryonic development including cell aggregation and migration, segmentation, neural development and vascular remodeling (4, 5). EphB4 is normally expressed on venous endothelial cells while its cognate ligand, ephrin-B2, is expressed on arterial endothelial cells. Ligand - receptor binding is required to produce vascular hierarchy and vessel maturation (3). Studies have shown that EphB4 and ephrin-B2 expression persists in adult vasculature and plays an important role in adult angiogenesis in wound healing and in the female reproductive system (6).
Increased EphB4 expression has been demonstrated in a number of human cancers including breast (7), prostate (8), bladder (2), and lung carcinoma (9). EphB4 has been shown to provide a survival advantage for tumor cells by interfering with apoptotic pathways, and by promoting tumor cell migration and invasion (2, 10). We have previously shown that EphB4 overexpression is predictive of poor clinical outcome in ovarian cancer patients (11). In addition, we demonstrated decreased tumor cell viability, migration, and invasion associated with inhibition of EphB4 expression. The purpose of this study was to further elucidate the biological function of EphB4 in ovarian cancer growth and downstream pathway modulation, and to develop therapeutically relevant approaches for EphB4 targeting.
Materials and Methods
Cell lines and cultures
A2780-par, A2780-cp20, HeyA8, SKOV3ip1, and IGROV-af1 cell lines were cultured in RPMI-1640 media supplemented with 10-15% fetal bovine serum (FBS) and 0.1% gentamicin sulfate (Gemini Bioproducts, Calabasas, CA). The derivation and sources of the cell lines have been reported previously (12, 13). The IGROV-af1 variant was derived from ascites arising in a nude mouse given an intraperitoneal injection of IGROV cells. All experiments were performed at 60-80% confluence and cell lines were routinely tested for mycoplasma.
SiRNA transfections in vitro
EphB4 targeted siRNA was purchased from Qiagen (Valencia, CA) and used to silence EphB4 expression in ovarian cancer cell lines (target sequence: 5’-CCCAGCCAATAGCCACTCTAA-3’). Control non-targeting siRNA (target sequence: 5’-AATTCTCCGAACGTGTCACGT-3’; confirmed to have no sequence homology with any known human mRNA by BLAST analysis) was used as control siRNA for all in vitro and in vivo experiments. For in vitro transfections, 2 × 106 cells/well were plated in 6-well plates. After cells were attached, medium was replaced and cells were incubated with 5 μg siRNA (EphB4 or control) with 30 μl of RNAiFect transfection reagent (Qiagen, Valencia, CA). The following day, medium was replaced and cells were incubated in 3 μg siRNA (EphB4 or control) with 18 μl of RNAiFect transfection reagent. Medium was again replaced 6 hours after transfection.
Liposomal siRNA preparation for in vivo delivery
For in vivo experiments, siRNA constructs were incorporated into neutral nanoliposomes (DOPC; 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine), lyophilized, and stored at -20°C as previously described (14). Prior to in vivo delivery, siRNA-DOPC preparations were rehydrated with PBS to appropriate concentration.
Anti-EphB4 therapy in orthotopic murine ovarian cancer models
Female athymic nude mice were purchased from the National Cancer Institute-Frederick Cancer Research and Development Center (Frederick, MD) and housed in specific pathogen-free conditions. The animals were cared for in accordance with the guidelines set forth by the American Association for Accreditation for Laboratory Animal Care and the U.S. Public Health Service Policy on Human Care and Use of Laboratory Animals, and all studies were approved and supervised by the MDACC Institutional Animal Care and Use Committee.
Development and characterization of the orthotopic mouse model of advanced ovarian cancer used in these experiments has been previously described by our laboratory (14, 15). Therapy trials were designed to test the effects of EphB4 siRNA alone and in combination with docetaxel. EphB4 siRNA-DOPC dosing schedule was determined through in vivo downregulation studies (data not shown). Tumor cells were trypsinized, resuspended in PBS, and injected into the peritoneal cavity (1×106 A2780-cp20 cells or 2x106 IGROV-af1 cells per mouse). One week after tumor cell injection, mice were randomized into 4 groups (n=10 mice/group) and treated with intraperitoneal (i.p.) injections of the following agents: control siRNA-DOPC (5.0 μg/mouse i.p., twice weekly), EphB4 siRNA-DOPC (5.0 μg/mouse i.p., twice weekly), docetaxel only (docetaxel 35 μg/mouse i.p., weekly), or EphB4 siRNA-DOPC plus docetaxel (EphB4 siRNA-DOPC 5 μg/mouse i.p., twice weekly; docetaxel 35 μg/mouse i.p., weekly). To demonstrate a persistent decrease in EphB4 expression in xenografts at completion of the in vivo experiments, Ephb4 mRNA levels were evaluated for the different treatment groups using quantitative RT-PCR for the A2780-cp20 cell line.
Additional therapy trials were designed to test the effects of EphB4-131, a murine IgG1 monoclonal antibody the recognizes the FN-1 domain of EphB4 and induces degradation of the receptor (developed by Vasgene Therapeutics Inc.)(16), alone and in combination with docetaxel. EphB4-131 dosing schedule was determined through in vivo downregulation studies (data not shown). One week after tumor cell injection (A2780-cp20 or IGROV-af1), mice were randomized into 4 groups (n=10 mice/group) and treated with i.p. injections of the following agents: control antibody (10 mg/kg/mouse i.p., twice weekly), EphB4-131 (10 mg/kg/mouse i.p., twice weekly), docetaxel alone (docetaxel 35 μg/mouse i.p., weekly), or EphB4-131 plus docetaxel (EphB4-131 10 mg/kg/mouse i.p., twice weekly; docetaxel 35 μg/mouse i.p., weekly).
During the therapy experiments, mice were monitored daily for adverse effects and sacrificed when moribund. Mouse and tumor weight, tumor distribution, number of tumor nodules, and amount of ascites were recorded at necropsy. While longitudinal growth curves are frequently used with subcutaneously injected tumors, it is difficult to perform serial measurements with intraperitoneal tumors. Therefore, final tumor weights and nodule counts were performed at the completion of the experiments. Mice that did not develop tumor after i.p. injection were excluded from analysis. Tissue specimens were fixed in formalin for paraffin embedding or snap frozen in optimal cutting medium (OCT; Miles, Inc., Elkhart, IN) for frozen slide preparation.
Western blot analysis
Whole cell lysates were prepared from HeyA8, SKOV3ip1, A2780-par, A2780-cp20 and IGROV-af1 ovarian cancer cell lines. To assess the effects of EphB4 siRNA on EphB4 silencing, whole cell lysates were collected from A2780-cp20 and IGROV-af1 cell lines 24-72 hours following EphB4 siRNA transfection. To assess the effects of EphB4-131 on EphB4 downregulation, whole cell lysates were collected from A2780-cp20 and IGROV-af1 cell lines 48 hours following treatment with control antibody or EphB4-131 antibody at varying doses (1 μg/ml, 5 μg/ml, 10 μg/ml, and 20 μg/ml). Cell lysates were also collected from cells treated with 10 μg/ml control antibody or EphB4-131 at varying time points (12, 24, 36, 48, and 72 hours). To investigate the effects of EphB4 stimulation on phospho-AKT levels, A2780-cp20 cells were first starved in serum free medium. Eight hours after the starvation began, 20 nM rapamycin (mTOR inhibitor, Calbiochem) or 3 μM LY294002 (PI3K inhibitor; Calbiochem) were added for 16 hours. Cells were then stimulated with 2.5 ug/ml EphrinB2-Fc (Vasgene Therapeutics) for 30 min before lysis. To determine the effects of EphB4 silencing on phosphoinositide-3 kinase (PI3K) signaling, A2780-cp20 cells were transfected with EphB4 siRNA (GFP siRNA as control) and then treated 48 hours later with 20 nM rapamycin or 3 uM LY294002 for additional 16 hours. Cells were then lysed. To prepare whole cell lysate, cells were washed with PBS and then lysed with radioimmunoprecipitation assay (RIPA) lysis buffer supplemented with 1 × protease inhibitor cocktail (Roche, Mannheim, Germany) and 1 mM sodium orthovanadate for 20 minutes on ice. Lysates were collected and then centrifuged at 13,000 rpm for 20 minutes at 4°C. The protein concentration of the samples was determined by a bicinchoninic acid Protein Assay Reagent kit. Typically, 50 μg of protein from whole-cell lysate was fractionated by 10% SDS-PAGE, transferred to nitrocellulose membrane. The membrane was then blocked with 5% nonfat milk for 1 hour at room temperature, and probed with primary antibody against EphB4 (MAb 265, Vasgene Therapeutics) or AKT protein phosphorylated at serine 473 (Cell Signaling) at 4°C overnight. Blots were then incubated with appropriate horseradish peroxidase-conjugated secondary antibodies (The Jackson Laboratory, Bar Harbor, ME), and developed with an enhanced chemiluminescence detection kit (ECL; Amersham Pharmacia Biotech, Piscataway, NJ). To ensure equal protein loading, a monoclonal beta-actin antibody (Chemicon International, Temecula, CA) was used.
Reverse transcription – polymerase chain reaction (RT – PCR)
Total RNA was isolated from cells using the RNAeasy minikit (Qiagen, Valencia, CA) according to the manufacturer's instructions. cDNA was synthesized from 5 μg of total RNA using the Superscript First-Strand Kit (Invitrogen, Carlsbad, CA) as per manufacturer's instructions. The cDNA was subjected to PCR amplification of EphB4 with β-Actin as a housekeeping gene (Primers: human EphB4 sense: 5’-GCTGGACTACGAGGTCAAAT-3’, human EphB4 antisense: 5’-TCATAAGTGAAGGGGTCGAT-3’, murine EphB4 sense: 5’-CAGCAGTGTTCGTTTCCTGA-3’, murine EphB4 antisense: 5’-TCTTTGGCAAATTCCCTCAC-3’). The PCR cycling condition was: 94 °C for 2 minutes, followed by 25 cycles of 94 °C for 30 seconds, 55 °C for 45 seconds, and 72 °C for 2 minutes. Amplified PCR products were analyzed by electrophoresis on 1% agarose gel with Tris-borate-EDTA buffer and visualized under UV light after staining with ethidium bromide. For real-time RT-PCR, we obtained quantitative values (each sample was normalized on the basis of its 18S content), as previously described (17).
Immunohistochemistry (IHC)
Tumor tissue was fixed with formalin and embedded in paraffin. Tissue sections (5-μm thick) were deparaffinized and hydrated. For antigen retrieval, sections were brought to a boil in 10 mM sodium citrate buffer (pH 6.0) and then maintained at a sub-boiling temperature for 10 minutes. Endogenous peroxidase activity was blocked by incubation in 3% H2O2, followed by blocking of nonspecific sites with blocking buffer (5% normal goat serum in TBST (Tris-buffered saline, 0.1% Tween-20)) at room temperature for 1 hour. Sections were then incubated with primary antibody diluted in SignalStain antibody diluent (Cell Signaling) overnight at 4°C. After washing in TBST, sections were incubated with biotinylated secondary antibodies for 1 hour at room temperature. ABC reagent kit (Vector laboratories, Burlingame, CA) was used to develop signal following manufacturer's procedure. Nuclei were counterstained with hematoxylin. Antibodies against AKT, phosphorylated AKT (Ser473), S6, and phosphorylated S6 (Ser235/236) were from Cell Signaling (Danvers, MA). Antibodies against CD31 and PCNA were from PharMingen (San Diego, CA) and DAKO (Carpinteria, CA), respectively. For quantification of PCNA expression, the number of PCNA-positive tumor cells was counted in 10 random fields at X200 magnification. To quantify microvessel density (MVD), microvessel-like structures consisting of endothelial cells that were stained with the anti-CD31 antibody were counted in similar fields.
TUNEL assay
After deparaffinizing, samples were treated with proteinase K (1:500 dilution). One set of slides was treated with DNase (1:50 dilution) as a positive control. Samples were incubated with terminal (1:400) and biotin-16-dUTP (1:200) in terminal deoxynucleotidyl transferase buffer at 37°C for 1 hour and then incubated with 2% bovine serum albumin/normal horse serum in water. Samples were then incubated with peroxidase streptavidin 1:400 in house detection diluent at 37°C for 40 minutes. For visualization, 3,3'-diaminobenzidine and counterstaining with Gill's hematoxylin was utilized. For quantification of TUNEL signal, the number TUNEL-positive tumor cells were counted in 10 random fields at X200 magnification.
Staining of EphB4 or EphB2 expessing cells with EphB4-131
Full length EphB4 or EphB2 were transiently expressed in 293T cells and stained with EphB4 or EphB2 antibodies. EphB4-131 specific staining was examined in EphB4 and EphB2 expressing cells, while EphB2 specific MAb 110 was used as a positive control. Nuclei were counter-stained with DAPI (blue).
Endocytosis of EphB4-131
A2780-cp20 ovarian cancer cells were incubated with 10 μg/ml of biotinylated EphB4-131 for 1 hour at 4 °C or 37 °C. Cells were then fixed with 4% paraformaldehyde and EphB4-131 was localized using streptavidin-FITC. Nuclei were counter-stained with DAPI (blue). Confocal images were taken at 100x with a Zeiss confocal microscope.
Migration assay
To determine the effects of EphB4 downregulation on tumor cell migration, 1 × 105 ovarian cancer cells (A2780-cp20), pretreated with EphB4 siRNA for 48 hrs or EphB4-131 , were resuspended in serum-free media (SFM) and plated onto a 0.1% gelatin coated membrane matrix using the membrane invasion culture system (MICS) (18). Bottom wells were filled with SFM, and chambers were incubated for 6 hours at 37°C. At completion, cells in bottom chambers were removed with 0.1% EDTA, loaded onto a 3.0 micron polycarbonate filter (Osmonics, Livermore, CA) using an S&S Minifold I Dot-Blot System (Schleicher & Schuell, Keene, NH), fixed, stained, and counted by light microscopy (18). Cells from ten random fields (final magnification= 400x) were counted by two investigators (W.A.S. and A.K.S). Experiments were performed in duplicate and repeated once.
Invasion Assay
The effect of EphB4 downregulation on tumor cell invasion was performed using a membrane invasion culture system as previously described (18, 19). In brief, A2780-cp20 cells were pretreated with EphB4 siRNA for 48 hours or EphB4-131. The following day, 1 × 105 viable cells (resuspended in SFM) were placed into the top wells onto a defined basement membrane matrix consisting of human laminin (Sigma), type IV collagen (Sigma), and gelatin (ICN Biomedical, Aurora, CO), used as the intervening barrier to invasion. SFM or 5% FBS containing media was placed into bottom wells as chemoattractant and chambers were then incubated for 24 hours at 37°C. Analysis of invaded cells was performed as previously described for migration assay (18). The invasion assays were performed in duplicate and repeated once.
Cell viability assay (MTT assay)
In order to determine the in vitro effects of EphB4 downregulation on ovarian cancer cell viability, 2,000 cells per well were plated in a 96-well plate after EphB4 siRNA transfection, with experimental conditions set in triplicate. Cells were then incubated for 72 hours at 37°C. To assess cell viability, 50 μL of 0.15% MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, Sigma) was added to each well, and incubated for 2 hours at 37°C. The medium was then removed from each well and replaced with 100 μl DMSO. Absorbance at 470 nm was analyzed with Ceres UV 900C (Bio-Tek Instrument, Winooski, VT) within 30 minutes.
Apoptosis assay
Apoptosis was studied using the Annexin V-PE apoptosis detection kit I (BD Biosciences, San Jose, CA). 24, 48, and 72 hours following EphB4 siRNA transfection, A2780-cp20 cells were washed twice with cold PBS and then resuspended in 1X Binding Buffer at a concentration of 1 × 106 cells/ml. 1 × 105 cells were then incubated with 5 μl Annexin V-PE and 5 μl of 7-AAD. Cells were gently vortexed and then incubated for 15 min at RT (25°C) in the dark. After adding 400ul 1x binding buffer, samples were analyzed by flow cytometry.
Clonogenic assays
A2780-cp20 cells (1 × 106) were plated in 10 cm plates. After cells were attached, medium was replaced and cells were incubated with 10 μg siRNA (EphB4 or control) with 60 μl of RNAiFect transfection reagent (Qiagen, Valencia, CA). The following day, medium was replaced and cells were incubated in 8 μg siRNA (EphB4 or control) with 48 μl of RNAiFect transfection reagent. Medium was again replaced 6 hours after transfection. Twenty-four hours after transfection, cells were trypsinized and re-plated in 6-well plates (5 × 103 cells per well). After approximately one week, when control dishes had formed sufficiently large clones, colonies were fixed and stained. After washing with PBS, 1ml of a mixture of 4% formaldehyde and 1% crystal violet was added to each well for 5 minutes. Cells were then rinsed with PBS and allowed to dry at room temperature. Colonies of at least 50 cells were then counted in 10 random fields (final magnification = 40x).
Cell proliferation assay
A2780-cp20 cells (3 × 103) were plated in 96-well plates and then treated with EphB4 siRNA for 24, 48, and 72 hours. To measure the effect of EphB4 siRNA on DNA synthesis, cells were pulse-labeled with [3H]thymidine (MP Biomedicals) for 2 hours followed by lysis in 100 μL of 0.1 mol/L KOH. Cells were harvested onto fiberglass filters and incorporated tritium was quantified by β-counter.
Statistics
All results were expressed as mean ± SD unless indicated otherwise. Continuous variables were compared using Student's t test or ANOVA. For non-normally distributed data sets, the Mann-Whitney rank sum test was used. Survival curves were plotted by Kaplan-Meier method, and differences were determined by log-rank test. A p < 0.05 was considered statistically significant.
Results
EphB4 downregulation with EphB4-131 monoclonal antibody
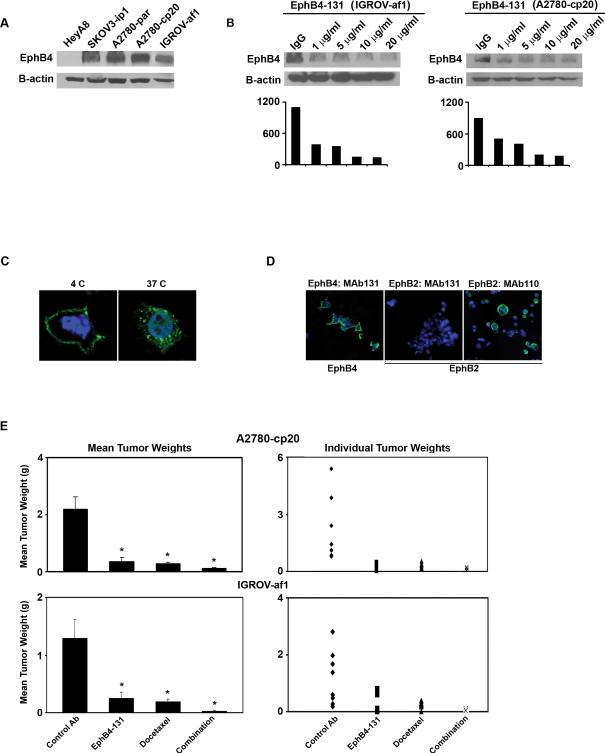
We first characterized EphB4 expression in ovarian cancer cell lines. Western blot analysis demonstrated EphB4 expression in SKOV3ip1, A2780-par, A2780-cp20 and IGROV-af1 cell lines, with highest expression noted in the A2780-cp20 and IGROV-af1 cells (Fig. 1A). Compared to control antibody, treatment with EphB4-131 antibody significantly reduced EphB4 protein levels in A2780-cp20 and IGROV-af1 cells at 48 hours (Fig.1B). EphB4-131 (10 μg/ml) led to peak downregulation of EphB4 in both cell lines and further increases in antibody concentration (20 μg/ml) did not result in more downregulation (Fig. 1B). To see the time response with EphB4-131 treatment, A2780-cp20 and IGROV-af1 cells were then treated with 10 μg/ml EphB4-131 antibody at varying time points. The greatest EphB4 downregulation was noted at 72 hours (Supplementary Fig. 1A). Antibody triggered endocytosis of cell surface receptor is a well-known mechanism for the degradation of receptor, thus we sought to determine if EphB4-131 induces EphB4 endocytosis. A2780-cp20 ovarian cancer cells were incubated with biotinylated EphB4-131, which was localized using streptavidin-FITC. Confocal images demonstrate EphB4-131 bound to cell surface at 4 °C, and was internalized at 37 °C (Fig. 1C).
Figure 1. Targeting EphB4 with EphB4-131 monoclonal antibody in ovarian cancer cell lines.
A) Western blot analysis of EphB4 expression in ovarian cancer cell lines. B) A2780-cp20 and IGROV-af1 cells were treated by indicated amount of EphB4-131 antibody for 48 hours. Cells were then lysed and subjected to Western blot analysis to assess EphB4 expression. Corresponding densitometry graphs are included. C) Biotinylated EphB4-131 was localized in A2780-cp20 cells using streptavidin-FITC. Confocal images were taken at 100x. EphB4 bound MAb131 (green) was internalized at 37 °C only. D) EphB4-131 specific staining was seen in cells expressing EphB4 (left panel) but not in cells expressing EphB2 only (middle and right panel). EphB2 specific antibody MAb 110 was used as a positive control. Nuclei were counterstained with DAPI (blue). E) EphB4-131 alone and in combination with docetaxel significantly decreased tumor growth in A2780-cp20 and IGROV-af1 tumor models (*p<0.05 compared to control antibody group). An unrelated control antibody was used as negative control.
Specificity of EphB4-131 binding
Full length EphB4 or EphB2 were transiently expressed in 293T cells and stained with appropriate antibodies. EphB4-131 specifically stained EphB4 expressing cells but not EphB2 expressing cells (Fig. 1D). EphB4-131 also doesn't recognize other EphB receptors (data not shown).
EphB4 downregulation with siRNA
Prior to performing in vivo experiments, efficacy of EphB4 siRNA was also tested in vitro with A2780-cp20 and IGROV-af1 cancer cell lines. Compared to control siRNA, treatment with EphB4 siRNA resulted in significantly reduced EphB4 mRNA (Supplementary Fig. 1B) and protein (Fig. 2A) levels in the A2780-cp20 cells 72 hours after transfection. Similar results were noted in the IGROV-af1 cells, with maximum (>80%) downregulation of EphB4 protein expression at 72 hours (data not shown).
Figure 2. EphB4 silencing with EphB4 siRNA.
A) EphB4 siRNA effectively reduced EphB4 protein expression in the A2780-cp20 cell line. B) EphB4 siRNA-DOPC alone and in combination with docetaxel significantly reduced tumor growth in A2780-cp20 and IGROV-af1 tumor models (*p<0.05 compared to control siRNA group).
EphB4-131 antibody reduced ovarian cancer growth in orthotopic murine model
To assess potential efficacy of targeting EphB4, we first carried out experiments with the EphB4-131 antibody in the A2780-cp20 and IGROV-af1 models of ovarian carcinoma. Seven days following tumor cell injection, treatment was started according to the following groups (n=10 mice per group): 1) control antibody, 2) EphB4-131 antibody, 3) docetaxel alone, or 4) EphB4-131 antibody plus docetaxel. After 5 weeks of therapy, mice were sacrificed and necropsies were performed. Compared to controls, EphB4-131 alone decreased tumor growth by 83% in A2780-cp20 (p<0.01; Fig. 1E) and 80% in IGROV-af1 (p<0.001; Fig. 1E) cell lines. Combination therapy with EphB4-131 and docetaxel resulted in the greatest tumor reduction in both A2780-cp20 and IGROV-af1 models (94-98% reduction versus controls; p<0.05 for both groups). Compared to docetaxel alone, combination treatment with EphB4-131 and docetaxel significantly decreased tumor growth in both models (87% vs. 94%, p<0.05, and 85% vs. 98%, p<0.001, respectively). There was not a significant difference between treatment with single agent EphB4-131 or single agent docetaxel in either cell line. In both experiments, there was no significant difference in feeding behavior or average mouse weights among the various treatment groups (data not shown).
EphB4 siRNA reduced ovarian cancer growth in orthotopic murine model
To examine for consistency of biological effects with EphB4 targeting, we also tested the effects of EphB4 gene silencing using siRNA incorporated in DOPC nanoliposomes (14, 20). EphB4 siRNA-DOPC was used in the A2780-cp20 and IGROV-af1 models. The sequence being utilized here is specific for the human sequence (i.e., tumor cells) and does not affect mouse EphB4 (Supplementary Fig. 1C). Seven days following tumor cell injection, treatment was started according to the following groups: 1) control siRNA-DOPC, 2) EphB4 siRNA-DOPC, 3) docetaxel alone, or 4) EphB4 siRNA-DOPC plus docetaxel. EphB4 siRNA-DOPC was compared alone and in combination with docetaxel (n=10 mice per group). After 4 to 5 weeks of therapy, mice were sacrificed and necropsies were performed. Compared to controls, EphB4 siRNA-DOPC alone decreased tumor growth by 48% in A2780-cp20 (p<0.05; Fig. 2B) and 61% in IGROV-af1 (p<0.05; Fig. 2B) cell lines. Combination therapy with EphB4 siRNA-DOPC and docetaxel resulted in the greatest tumor reduction in both A2780-cp20 and IGROV-af1 models (89-95% reduction versus controls; p<0.05 for both groups). Compared to docetaxel alone, combination treatment with EphB4 siRNA-DOPC and docetaxel significantly decreased tumor growth in both models (56% vs. 89% and 77% vs. 95%, respectively; p<0.05 for both cell lines). There was not a significant difference between treatment with single agent EphB4 siRNA-DOPC or docetaxel in either cell line. Quantitative RT-PCR confirmed decreased EphB4 expression at the completion of in vivo experiments (Supplementary Fig. 1D). In both experiments, there was no significant difference in feeding behavior or average mouse weights among the various treatment groups (data not shown).
Biological effects of EphB4 gene silencing
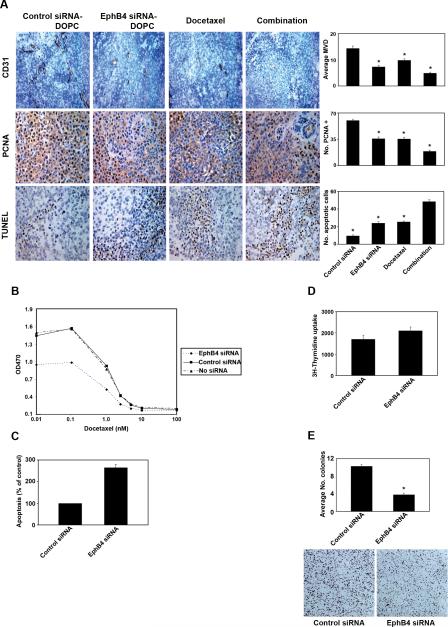
Based on the role of EphB4 in angiogenesis (4, 5), we assessed microvessel density (MVD) in A2780-cp20 tumors by CD31 staining (Fig. 3A). Compared to controls, EphB4 siRNA-DOPC treatment significantly decreased MVD by 49% (p<0.001). The greatest decrease in MVD was seen in the group with combination of EphB4 siRNA-DOPC and docetaxel (66% decrease; p<0.001).
Figure 3. Biological effects of EphB4 silencing.
A) Effects of EphB4 siRNA-DOPC with or without docetaxel on MVD (CD31 staining), proliferation (PCNA staining), and apoptosis (TUNEL assay). Bar graphs correspond to the figures on the left. All photographs were taken at original magnification X200 (error bars represent SEM; *p<0.01 compared to the control siRNA-DOPC group). B) Effect of EphB4 siRNA on A2780-cp20 cell viability in vitro at 72 hrs. C) Effect of EphB4 siRNA on A2780-cp20 apoptosis analyzed by Annexin V-PE staining. D) Effect of EphB4 siRNA in vitro on A2780-cp20 tumor cell proliferation ([3H] thymidine uptake). E) Effect of EphB4 siRNA on A2780-cp20 colony formation (*p<0.05 compared to the control siRNA group). Representative pictures are shown below the quantitation bar graphs.
To determine whether EphB4 affects tumor cell viability, we examined both in vitro and in vivo effects of EphB4 silencing. A2780-cp20 cells were treated in vitro with EphB4 siRNA or control siRNA, in combination with docetaxel, and the number of viable cells was assessed by the MTT assay. EphB4 siRNA treatment led to a 37% reduction in A2780-cp20 cell number compared to untreated cells at 72 hrs (Fig. 3B). To determine the cause for reduced cell numbers following EphB4 knockdown as observed by the MTT assay, we examined the in vitro effects of EphB4 silencing on tumor cell apoptosis using Annexin V-PE labeling. Compared to control, EphB4 siRNA treatment increased apoptosis (9% vs 25%) at 72hrs, a 2.5-fold increase (Fig. 3C).
We next examined the effects of EphB4 siRNA-DOPC, docetaxel, and combination therapy on apoptosis in vivo by immunohistochemical analysis of TUNEL assay in tumor tissues (Fig. 3A). Compared to treatment with control siRNA-DOPC, EphB4 siRNA-DOPC alone led to a 153% increase in tumor cell apoptosis (p<0.001). A similar increase in apoptosis (169%; p<0.001) was observed in the group treated with docetaxel alone. The greatest increase in tumor cell apoptosis was observed in the group with combination of EphB4 siRNA-DOPC and docetaxel (415%; p<0.001).
To determine if EphB4 siRNA has direct anti-proliferative effects on tumor cells, A2780-cp20 cells were treated with either control or EphB4 siRNA for 48 hours, and cell proliferation was determined by pulse-labeling of cells with [3H]thymidine. EphB4 downregulation did not affect in vitro tumor cell proliferation (Fig. 3D). However, EphB4 silencing did decrease tumor cell proliferation in vivo, as evidenced by PCNA staining (Fig. 3A). Both EphB4 siRNA-DOPC and docetaxel alone significantly reduced tumor cell proliferation compared to controls (40% and 41%; p<0.001; Fig. 3A). The greatest effect was observed with combination treatment (69%; p<0.001). Given the absence of direct in vitro effects, these results suggest an indirect effect, possibly due to reduced angiogenesis.
EphB4 has been shown to regulate the long-term clonogenic potential of colorectal tumor cells (21). To determine if EphB4 silencing affects the capacity of cells to produce colonies in ovarian cancer, A2780-cp20 cells were treated with either control or EphB4 siRNA and then plated in 6-well plates for 1 week. Compared to control, EphB4 siRNA treatment decreased colony formation by 63% (p < 0.001; Fig. 3E).
EphB4 regulates cell migration and invasion
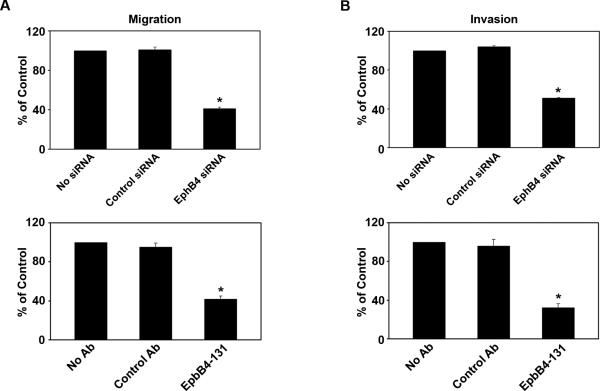
Tumor growth and metastasis is regulated, in part, by the ability of tumor cells to degrade surrounding matrix and migrate. EphB4 has been shown to promote the migration of various cells, including endothelial cells (22). Therefore, we next determined whether EphB4 siRNA could decrease tumor cell migration and invasion using a membrane invasion culture system (MICS) (18, 19). Compared to control, tumor cell migration was reduced by 60% following treatment with EphB4 siRNA and 58% following treatment with EphB4-131 (p<0.001; Fig. 4A). Similarly, compared to controls, tumor cell invasion was decreased by 51% with EphB4 siRNA and 67% with EphB4-131 (p<0.001; Fig. 4B).
Figure 4. EphB4 silencing decreased tumor cell migration and invasion.
Effect of EphB4 siRNA and EphB4-131 on A2780-cp20 tumor cell migration (A) and invasion (B) was assessed. No treatment and treatment with non-silencing siRNA or control antibody were used as negative controls. (*p<0.001 compared to control siRNA group).
EphB4 stimulates PI3K activation
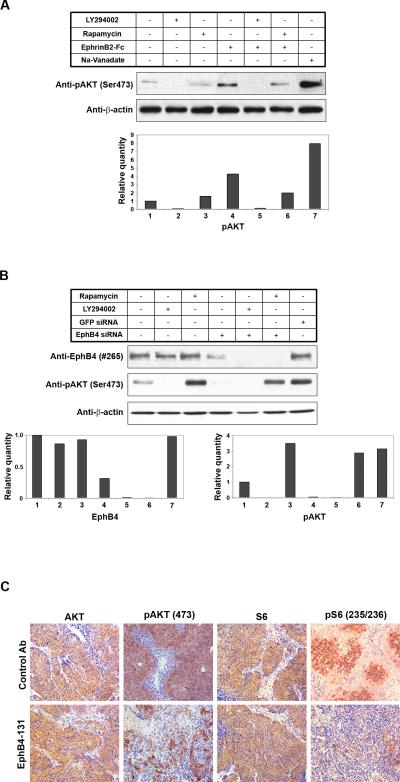
Phosphoinositide-3 kinase (PI3K) pathway plays a pivotal role in ovarian cancer growth and progression. Therefore, we examined the effects of EphB4 activation on PI3K signaling in ovarian cancer cells. Before stimulation, A2780-CP20 cells had been starved for 24 hours to reduce background pAKT (AKT phosphorylated at Ser473, an important marker of PI3K pathway activation) signal. In some settings, cells were also treated by LY294002 (PI3K inhibitor) or rapamycin (mTOR inhibitor) for 16 hours. Cells were then stimulated with 2.5 μg/ml EphrinB2-Fc for 30 min. This stimulation resulted in increased pAKT level (Fig. 5A). Treatment with LY294002 (PI3K/AKT inhibitor) effectively blocked this increase in pAKT level, while rapamycin had no effect. This lack of effect by rapamycin is likely due to the interrupted feedback mechanisms in serum starved cells (23).
Figure 5. EphB4 signals through PI3K/AKT in ovarian cancer cells.
(A) A2780-cp20 cells were starved overnight and then stimulated with 2.5 ug/ml ephrin-B2-Fc for 30 min. In some settings, cells were also treated with LY294002 (PI3K inhibitor) or rapamycin (mTOR inhibitor) for 16 hours. (B) Cells were transfected with EphB4 siRNA or GRP siRNA as control. 48 hours post-transfection, cells were treated with LY294002 or rapamycin for another 16 hours. In both (A) and (B), whole cell lysates were subjected to Western blot analysis with EphB4, pAKT, and beta-actin antibodies. Na3VO4, an inhibitor of phosphatase, was used as a positive control to show phosphorylation of AKT. Quantitation was done by Image J (NIH) and the bar graphs were shown below the picture. The number labels in the bar graph correspond to the number labels in the picture. (C) IHC staining of tumor tissues treated with control or EphB4-131 antibody. Pictures of AKT, pAKT, S6, and phospho-S6 (Ser235/236) were taken at original magnification X200.
Basal AKT activation was observed in A2780-cp20 cells cultured in FBS containing complete growth medium, indicating constitutive activation (Fig. 5B). This basal AKT (PI3K) activation was significantly decreased by LY294002, as well as EphB4 silencing by siRNA (Fig. 5B). Thus, AKT activation in these cells is for the most part mediated by EphB4. In addition, LY294002 also reduced EphB4 level (Fig. 5B). We also examined the effect of EphB4-131 antibody on PI3K signaling in vivo. IHC staining was performed to check the level of total AKT and pAKT in ovarian xenograft tumor tissues treated by control antibody or EphB4-131. The level of pAKT was significantly reduced in EphB4-131 antibody treated tumors, while no obvious difference in total AKT was observed between control group and EphB4-131 antibody treated tumors (Fig. 5C). Consistently, phosphorylation (Ser235/236) of ribosomal protein S6, another important downstream marker of PI3K signaling, was also significantly reduced by EphB4-131 antibody treatment, while total S6 protein level was not affected (Fig. 5C).
Discussion
In this study, we found that EphB4 siRNA-DOPC and EphB4-131 therapy alone and in combination with chemotherapy substantially reduced tumor growth in ovarian cancer models. These effects appear to be due to a multitude of biological effects on ovarian cancer cells including decreased tumor cell proliferation and invasion, induction of apoptosis, and decreased tumor angiogenesis.
EphB4 plays an important role in a number of diverse cellular functions including cell migration, axon guidance, angiogenesis, and vascular remodeling. Aberrant EphB4 expression has been reported in multiple malignancies, including breast (7), colon (21), prostate (8), bladder (2), and lung carcinoma (9). In a previous study, we demonstrated EphB4 overexpression in a substantial proportion of ovarian cancers (11). While we were able to silence EphB4 expression using antisense oligonucleotides previously, we wanted to develop more clinically relevant therapeutic approaches against EphB4. Therefore, in the current study, we utilized two parallel approaches that have potential for clinical development.
EphB4 overexpression has been shown to provide a survival advantage for cancer cells and promote tumor cell invasion, metastasis and angiogenesis by ligand-dependent and independent mechanisms. To evaluate the effects of EphB4 targeting in ovarian cancer, we utilized a novel monoclonal antibody as well as a novel method of systemic siRNA delivery to target EphB4 in orthotopic ovarian cancer models. We showed that targeting EphB4 using EphB4 siRNA-DOPC or EphB4-131 significantly decreased tumor growth. Combination therapy with EphB4 siRNA-DOPC or EphB4-131 and taxane-based chemotherapy had the greatest effect, with no observed side effects. Our results are consistent with other tumor xenograft studies that have shown a marked reduction in tumor growth associated with EphB4 silencing (8, 10).
Angiogenesis is a complex, and highly regulated process that is critical for tumor growth and metastasis. EphB4 expression on tumor cells has been shown to promote tumor vascularization through binding with its ligand, ephrin-B2, on endothelial cells (22). Targeting EphB4 on tumor cells is thought to decrease interactions with ephrin-B2-positive endothelial cells, resulting in decreased tumor angiogenesis (22). Our experiments demonstrated a significant decrease in vessel density following EphB4 gene silencing. These results are supported by previous studies showing that blocking EphB4 signaling interferes with tumor angiogenesis and vessel organization (24). In a murine breast tumor xenograft model, EphB4 knockdown significantly decreased tumor growth, with a 44% reduction in tumor angiogenesis (7). Therefore, targeting EphB4 may have a dual benefit in ovarian cancer by directly targeting tumor cells and indirectly targeting the tumor vasculature.
In addition to decreased angiogenesis, EphB4 targeting led to a significant decrease in tumor cell proliferation and viability in our in vivo experiments. These findings are also supported by studies that have shown EphB4 to function as a survival factor. For example, Xia and colleagues showed a decrease in tumor cell proliferation and increase in apoptosis following EphB4 knockdown in a murine bladder cancer model (2). Downregulation of EphB4 expression in breast cancer cell lines led to decreased cell viability and activation of caspase-8 mediated apoptosis (7). Consistent with our in vivo results, EphB4 silencing led to a significant reduction in A2780-cp20 ovarian cancer cell viability compared to untreated cells, accompanied by induction of apoptosis.
Given the pro-survival role of EphB4 in ovarian cancer, we suspected that EphB4 may promote other features of malignant disease, such as tumor cell migration and invasion. Munarini and colleagues have shown that transgenic mice with mammary tumors resulting from targeted overexpression of neuT have localized tumors, while EphB4/neuT double transgenic mice frequently develop lung metastasis (25). Yang and colleagues demonstrated the role of EphB4 in melanoma cell invasion and migration through its influence on RhoA-mediated actin cytoskeleton reorganization. EphB4 silencing decreased RhoA activity and cell migration (26). Our in vitro studies confirm that EphB4 silencing leads to a significant decrease in ovarian cancer cell migration and invasion. While EphB4 silencing also contributed to loss of tumor cell viability, the effects on invasion and migration are likely to be independent since they were observed at shorter time points prior to the observed effects on apoptosis.
There is little known information regarding the downstream signaling of EphB4 in ovarian cancer cells, although activation of the PI3K pathway, including AKT phosphorylation, has been observed in endothelial cells (27). Here, we demonstrate increased levels of Akt phosphorylation following activation of EphB4 in ovarian cancer cells. In addition, ovarian cancer cells demonstrated basal levels of AKT phosphorylation, and EphB4 silencing with siRNA effectively blocked this phosphorylation. Moreover, ovarian cancer xenograft tumors treated with the EphB4-131 antibody had decreased levels of phosphorylated AKT and downstream S6 protein. These suggest PI3K signaling in ovarian cancer is modulated by targeting EphB4 in vivo. Thus, the role of EphB4 in ovarian cancer cell migration, proliferation, and survival is likely through activation of the PI3K pathway.
In summary, targeting EphB4 with siRNA-DOPC or EphB4-131 was highly effective in reducing tumor growth in orthotopic ovarian cancer models. Thus, EphB4 may represent a novel target for biological therapy in ovarian cancer.
Supplementary Material
Suppl Figure 1. Downregulation of EphB4 with EphB4-131 in the A2780-cp20 ovarian cancer cell line was most significant at 72 hours (A). EphB4 siRNA effectively silenced EphB4 mRNA expression in the A2780-cp20 ovarian cancer cell line (B). EphB4 siRNA-DOPC used in mouse orthotopic ovarian cancer models is specific for the human sequence and does not affect mouse EphB4 (C). EphB4 mRNA levels show a persistent decrease in EphB4 expression at completion of the A2780-cp20 in vivo model (D).
Acknowledgements
This work was supported in part by the U.T.M.D. Anderson Ovarian Cancer Spore (P50 CA083639), NIH grants (CA110793 and CA109298), the Ovarian Cancer Research Fund Program Project Development Grant, the Zarrow Foundation, the Marcus Foundation, and the Betty Ann Asche Murray Distinguished Professorship. This work was also supported by the Entertainment Industry Foundation, and the Blanton-Davis Ovarian Cancer Research Program. WMM, YGL, AMN, and WAS are supported by the National Cancer Institute - DHHS - NIH T32 Training Grant (T32 CA101642). PSG is supported by NIH grant (R01CA79218), and Women's Cancer Research Fund.
References
- 1.Cancer Facts and Figures. American Cancer Society; 2007. [Google Scholar]
- 2.Xia G, Kumar SR, Stein JP, et al. EphB4 receptor tyrosine kinase is expressed in bladder cancer and provides signals for cell survival. Oncogene. 2006;25:769–80. doi: 10.1038/sj.onc.1209108. [DOI] [PubMed] [Google Scholar]
- 3.Holland SJ, Gale NW, Mbamalu G, Yancopoulos GD, Henkemeyer M, Pawson T. Bidirectional signalling through the EPH-family receptor Nuk and its transmembrane ligands. Nature. 1996;383:722–5. doi: 10.1038/383722a0. [DOI] [PubMed] [Google Scholar]
- 4.Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nature reviews. 2005;6:462–75. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- 5.Gerety SS, Wang HU, Chen ZF, Anderson DJ. Symmetrical mutant phenotypes of the receptor EphB4 and its specific transmembrane ligand ephrin-B2 in cardiovascular development. Molecular cell. 1999;4:403–14. doi: 10.1016/s1097-2765(00)80342-1. [DOI] [PubMed] [Google Scholar]
- 6.Gale NW, Baluk P, Pan L, et al. Ephrin-B2 selectively marks arterial vessels and neovascularization sites in the adult, with expression in both endothelial and smooth-muscle cells. Developmental biology. 2001;230:151–60. doi: 10.1006/dbio.2000.0112. [DOI] [PubMed] [Google Scholar]
- 7.Kumar SR, Singh J, Xia G, et al. Receptor tyrosine kinase EphB4 is a survival factor in breast cancer. The American journal of pathology. 2006;169:279–93. doi: 10.2353/ajpath.2006.050889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xia G, Kumar SR, Masood R, et al. EphB4 expression and biological significance in prostate cancer. Cancer research. 2005;65:4623–32. doi: 10.1158/0008-5472.CAN-04-2667. [DOI] [PubMed] [Google Scholar]
- 9.Tang XX, Brodeur GM, Campling BG, Ikegaki N. Coexpression of transcripts encoding EPHB receptor protein tyrosine kinases and their ephrin-B ligands in human small cell lung carcinoma. Clin Cancer Res. 1999;5:455–60. [PubMed] [Google Scholar]
- 10.Xia G, Kumar SR, Masood R, et al. Up-regulation of EphB4 in mesothelioma and its biological significance. Clin Cancer Res. 2005;11:4305–15. doi: 10.1158/1078-0432.CCR-04-2109. [DOI] [PubMed] [Google Scholar]
- 11.Kumar SR, Masood R, Spannuth WA, et al. The receptor tyrosine kinase EphB4 is overexpressed in ovarian cancer, provides survival signals and predicts poor outcome. British journal of cancer. 2007;96:1083–91. doi: 10.1038/sj.bjc.6603642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sood AK, Seftor EA, Fletcher MS, et al. Molecular determinants of ovarian cancer plasticity. The American journal of pathology. 2001;158:1279–88. doi: 10.1016/S0002-9440(10)64079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoneda J, Kuniyasu H, Crispens MA, Price JE, Bucana CD, Fidler IJ. Expression of angiogenesis-related genes and progression of human ovarian carcinomas in nude mice. Journal of the National Cancer Institute. 1998;90:447–54. doi: 10.1093/jnci/90.6.447. [DOI] [PubMed] [Google Scholar]
- 14.Landen CN, Jr., Chavez-Reyes A, Bucana C, et al. Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer research. 2005;65:6910–8. doi: 10.1158/0008-5472.CAN-05-0530. [DOI] [PubMed] [Google Scholar]
- 15.Halder J, Kamat AA, Landen CN, Jr., et al. Focal adhesion kinase targeting using in vivo short interfering RNA delivery in neutral liposomes for ovarian carcinoma therapy. Clin Cancer Res. 2006;12:4916–24. doi: 10.1158/1078-0432.CCR-06-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krasnoperov V, Kumar SR, Ley E, et al. Novel EphB4 monoclonal antibodies modulate angiogenesis and inhibit tumor growth. The American journal of pathology. 176:2029–38. doi: 10.2353/ajpath.2010.090755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thaker PH, Han LY, Kamat AA, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nature medicine. 2006;12:939–44. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 18.Sood AK, Coffin JE, Schneider GB, et al. Biological significance of focal adhesion kinase in ovarian cancer: role in migration and invasion. The American journal of pathology. 2004;165:1087–95. doi: 10.1016/S0002-9440(10)63370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sood AK, Bhatty R, Kamat AA, et al. Stress hormone-mediated invasion of ovarian cancer cells. Clin Cancer Res. 2006;12:369–75. doi: 10.1158/1078-0432.CCR-05-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merritt WM, Lin YG, Spannuth WA, et al. Effect of interleukin-8 gene silencing with liposome-encapsulated small interfering RNA on ovarian cancer cell growth. Journal of the National Cancer Institute. 2008;100:359–72. doi: 10.1093/jnci/djn024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davalos V, Dopeso H, Castano J, et al. EPHB4 and survival of colorectal cancer patients. Cancer research. 2006;66:8943–8. doi: 10.1158/0008-5472.CAN-05-4640. [DOI] [PubMed] [Google Scholar]
- 22.Noren NK, Lu M, Freeman AL, Koolpe M, Pasquale EB. Interplay between EphB4 on tumor cells and vascular ephrin-B2 regulates tumor growth. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:5583–8. doi: 10.1073/pnas.0401381101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Sun SY. Enhancing mTOR-targeted cancer therapy. Expert opinion on therapeutic targets. 2009;13:1193–203. doi: 10.1517/14728220903225008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martiny-Baron G, Korff T, Schaffner F, et al. Inhibition of tumor growth and angiogenesis by soluble EphB4. Neoplasia (New York, NY. 2004;6:248–57. doi: 10.1593/neo.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munarini N, Jager R, Abderhalden S, et al. Altered mammary epithelial development, pattern formation and involution in transgenic mice expressing the EphB4 receptor tyrosine kinase. Journal of cell science. 2002;115:25–37. doi: 10.1242/jcs.115.1.25. [DOI] [PubMed] [Google Scholar]
- 26.Yang NY, Pasquale EB, Owen LB, Ethell IM. The EphB4 receptor-tyrosine kinase promotes the migration of melanoma cells through Rho-mediated actin cytoskeleton reorganization. The Journal of biological chemistry. 2006;281:32574–86. doi: 10.1074/jbc.M604338200. [DOI] [PubMed] [Google Scholar]
- 27.Steinle JJ, Meininger CJ, Forough R, Wu G, Wu MH, Granger HJ. Eph B4 receptor signaling mediates endothelial cell migration and proliferation via the phosphatidylinositol 3-kinase pathway. The Journal of biological chemistry. 2002;277:43830–5. doi: 10.1074/jbc.M207221200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl Figure 1. Downregulation of EphB4 with EphB4-131 in the A2780-cp20 ovarian cancer cell line was most significant at 72 hours (A). EphB4 siRNA effectively silenced EphB4 mRNA expression in the A2780-cp20 ovarian cancer cell line (B). EphB4 siRNA-DOPC used in mouse orthotopic ovarian cancer models is specific for the human sequence and does not affect mouse EphB4 (C). EphB4 mRNA levels show a persistent decrease in EphB4 expression at completion of the A2780-cp20 in vivo model (D).