Abstract
Background
Multifocal visual evoked potentials (mfVEP) measure local response amplitude and latency in the field of vision
Objective
To compare the sensitivity of mfVEP, Humphrey visual field (HVF) and optical coherence tomography (OCT) in detecting visual abnormality in multiple sclerosis (MS) patients.
Methods
MfVEP, HVF, and OCT (retinal nerve fiber layer [RNFL]) were performed in 47 MS-ON eyes (last optic neuritis (ON) attack ≥ 6 months prior) and 65 MS-no-ON eyes without ON history. Criteria to define an eye as abnormal were: mfVEP 1) amplitude/latency: either amplitude or latency probability plots meeting cluster criteria with 95% specificity 2) amplitude or latency alone (specificity: 97% and 98%, respectively); HVF and OCT, mean deviation and RNFL thickness meeting p < 0.05, respectively.
Results
MfVEP (amplitude/latency) identified more abnormality in MS-ON eyes (89%) than HVF (72%), OCT (62%), mfVEP amplitude (66%) or latency (67%) alone. 18% of MS-no-ON eyes were abnormal for both mfVEP (amplitude/latency) and HVF compared to 8% with OCT. Agreement between tests ranged from 60% to 79%. MfVEP (amplitude/latency) categorized an additional 15% of MS-ON eyes as abnormal compared to HVF and OCT combined.
Conclusions
MfVEP, which detects both demyelination (increased latency) and neural degeneration (reduced amplitude) revealed more abnormality than HVF or OCT in MS patients.
Keywords: multiple sclerosis, optic neuritis, multifocal visual evoked potentials, optical coherence tomography, subclinical, standard automated perimetry
Introduction
Multiple sclerosis (MS) is a chronic demyelinating and neurodegenerative disease of the central nervous system. About 85% of patients experience a relapsing-remitting disease course initially. Current immunomodulatory treatments reduce the number of relapses and may delay conversion to clinically definite MS if treatment is initiated when symptoms are first suggestive of MS (i.e., in patients diagnosed with clinically isolated syndrome) [1–4]. However, such treatments may have limited effect in preventing neurodegeneration and the resultant disabilities [5]. Extensive research on the immunology, neurobiology and genetics of MS have led to a better understanding of the disease mechanisms and have set the stage for developing new therapeutic and protective strategies [6,7]. Sensitive tests are needed for early detection of the disease as well as for evaluating the efficacy of current and new treatments.
Pathology affecting the anterior visual pathway, in particular optic neuritis (ON), is prevalent in MS patients, and is often the initial manifestation of the disease [8–10]. The retinal nerve fiber layer (RNFL) which consists of unmyelinated axons of retinal ganglion cells that become myelinated past the lamina cribrosa and form the optic nerve, can be visualized using retinal imaging techniques. The RNFL will show retrograde degeneration following damage to the optic nerve or the optic tract in the brain. The eye therefore provides a window for assessing quantitatively, axonal damage associated with ON.
Both structural and functional tests can be used to assess damage to the axons of the optic nerve. For structural evaluation, optical coherence tomography (OCT) is a relatively recent optical imaging technique that measures cross-sectional RNFL thickness with high resolution (8–10 microns for Stratus OCT 3000 used in this study) and good reproducibility [11,12]. OCT is easy to perform, time-efficient, and is less costly than magnetic resonance imaging (MRI), a standard evaluative approach in MS patients. OCT has shown promise as a potential surrogate measure of axonal loss and neuro-protection in MS [13–20].
Functional testing can be subjective or objective. Standard automated perimetry (SAP), such as the Humphrey visual field (HVF) test, provides a subjective measure of visual function that is considered to be a clinical “gold standard” for documenting loss of sensitivity. The visual loss documented by the HVF test in various optic nerve diseases is correlated, to a greater or lesser extent depending upon the study and patient population being assessed, with results from imaging approaches such as OCT [17,21–23].
The multifocal visual evoked potential (mfVEP) is a relatively new objective approach for assessing early visual pathway integrity. This noninvasive electrodiagnostic technique records many (typically 60) local visual evoked responses simultaneously from over 40 degree field of vision. In addition to providing response amplitudes, the mfVEP also provides information about nerve conduction velocity (latency) which is useful for assessing the extent of demyelination. The mfVEP has been shown to have good repeatability, even slightly better than that of the HVF in some cases [24–27], and it detects local defects which would not be possible to find using the traditional VEP which tests global function over a large central region of the visual field [28,29]. For example, one study reported that the mfVEP detected 20% more local abnormalities in the visual field than the HVF in patients with ON [30]. Another advantage of the mfVEP is its potential to detect subclinical demyelination, indicated by prolonged latencies in local areas. Prolonged latencies could indicate increased risk of clinically definite MS in a patient with clinically isolated syndrome who has presented only with ON [31,32]. Klistorner et al (2009) recently compared results from OCT and mfVEP in patients with unilateral ON and found that the mfVEP detected more abnormality than the OCT RNFL thickness in both affected eyes and fellow eyes [33].
The main purpose of this study was to compare the sensitivity of the mfVEP in detecting abnormalities in the visual pathway of MS patients with that provided by standard automated perimetry, using HVF testing, and/or imaging of the nerve fiber layer, using OCT. Test results were compared for eyes of 69 MS patients with and without a history of ON. Qualitative, quantitative, and topographic comparisons among the three tests were performed to determine how well the tests agreed, and whether the mfVEP detected pathological changes in visual pathways in MS patients that were missed by the other tests. We used both amplitude (amp) and latency (lat) information for assessment of the mfVEP’s overall performance in detecting any nerve (axonal and/or myelin) pathology. In addition, we analyzed the mfVEP using amplitude or latency alone for revealing axonal damage or demyelination, respectively. Some of the findings in this study appeared previously in abstract form (Laron, Invest. Ophthalmol. Vis. Sci. 2007: E-Abstract 3761; Cheng, Invest. Ophthalmol. Vis. Sci. 2007: E-Abstract 3765).
Methods
Subjects
Sixty nine patients with clinical definite MS (67 with relapsing-remitting MS and 2 with secondary progressive MS) participated in the study [34,35]. Data from thirty four of the patients were included in a previous study that looked only at the relation between results of SAP and the RNFL thickness [21]. The mfVEP data from all patients were reported as a part of a study confined to looking at the sensitivity of the multifocal VEP in detecting abnormality in the visual pathway [36]. Patients ranged in age from 21 to 57 years (mean 39 ± 9.6 SD, M:F = 1:3.7). The time from MS diagnosis ranged from just diagnosed to 21 years (mean ± SD: 6.1 ± 5.6). Patients underwent a thorough eye examination by experienced ophthalmologists at the MS Eye CARE clinic, University of Houston. Patients with any ocular conditions (such as glaucoma, cataract or macular diseases) or systemic conditions (such as diabetes) other than ON/MS that could affect the visual system were excluded. A history of ON was determined based on clinical signs and symptoms [37,38]. There were two study groups. The first group, referred to as the MS-ON group, included 47 eyes that had a history of ON. In each case, the last attack of ON was at least six months prior to data collection. The waiting period of six months ensured adequate time for complete retrograde axonal degeneration to occur in the RFNL after an ON event [13,39]. The second group, referred to as the MS-no-ON group, included 65 eyes with no history of ON. Fifteen eyes with acute ON, 7 eyes with unreliable HVF results (over 33% fixation losses/false positives/false negatives) 2 eyes with unclear history regarding ON, and two eyes with severe loss of vision from ON (unable to perform visual fields), were excluded from the study. In the MS-ON group, the average number of ON attacks was 1.3 ± 0.6 SD. Thirteen eyes had experienced more than one episode of ON and four, more than two. The average time elapsed from the last ON attack was 5.9 ± 6.7 SD years.
For all eyes, the best-corrected distance visual acuity (VA) was measured using high contrast Snellen acuity chart. In 26 MS-ON eyes and 33 MS-no-ON eyes, we also measured monocular contrast sensitivity (CS) using a Pelli-Robson chart at 1 meter. The Pelli-Robson chart contains 16 triplets of Sloan letters each subtending 2.8 degrees. The three letters within each triplet have the same contrast, and each successive triplet declines in contrast from 0 to 2.25 log units in 0.15 log unit steps. The test was terminated when two out of the triplet letters were named incorrectly, and each letter read correctly was counted as 0.05 log unit [40].
Informed consent was obtained from all patients. Procedures adhered to the tenets of Declaration of Helsinki, and the protocol was approved by the University of Houston Committee for the Protection of Human Subjects.
Multifocal visual evoked potential
Recording
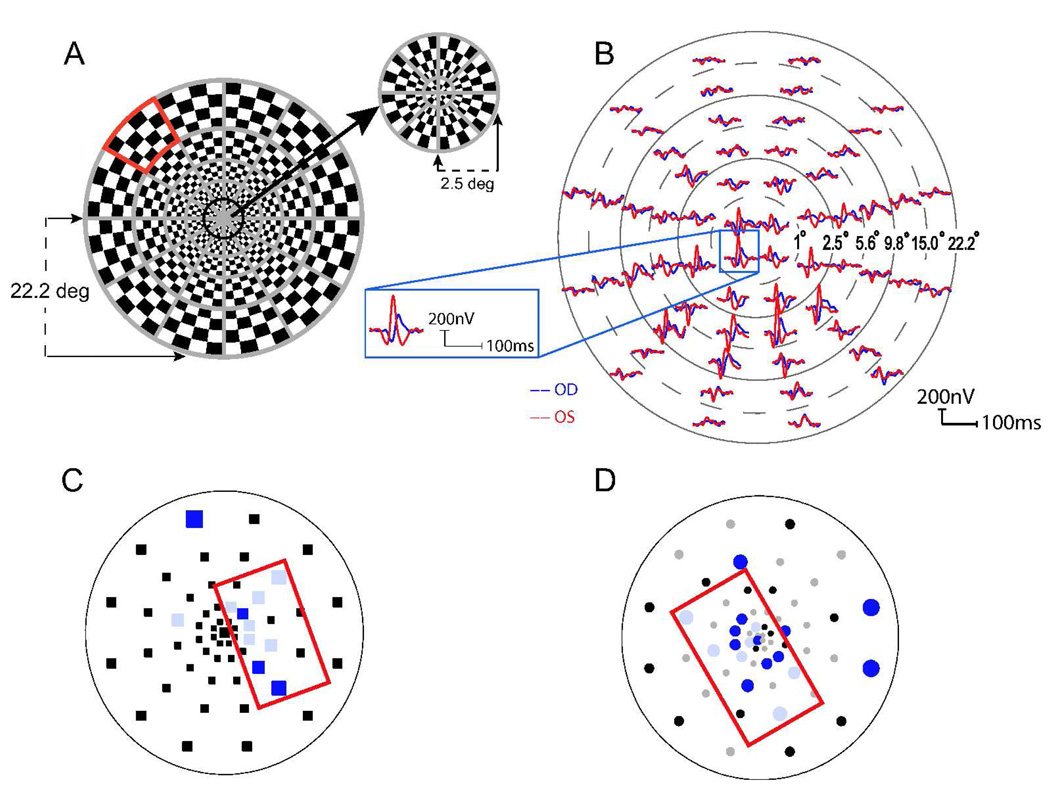
The mfVEP was recorded monocularly for each eye of every patient with VERIS 5.1 (Electro-Diagnostic Imaging Inc. San Mateo, CA). The stimulus was a 60 sector, 44 degree diameter cortically scaled dartboard pattern (Figure 1A) with a mean luminance of 66 cd/m2 and Michelson contrast of 95%. Each sector had 16 checks (8 black and 8 white). The black and white checks in each sector were reversed in contrast using a pseudorandom sequence of reversal (m-sequence) at a frame rate of 75 Hz [41]. Subjects were instructed to fixate at the center of the dartboard pattern (marked with an “X”) with their best refractive correction in place, and the eye position was monitored continuously by the examiner via the camera display provided in the VERIS package. Three channels were recorded simultaneously. Active electrodes, all referenced to inion, were placed 4 cm above inion, and 1 cm above and 4 cm lateral to the inion on either side of the midline. Three additional channels were derived offline [42,43]. Two 7-minute recordings from each eye were obtained, and the averaged responses (the first slice of the second-order kernel) were used.
Figure 1.
(A) The mfVEP 60 sector dartboard stimulus with a peripheral sector marked in red. (B) The mfVEP waveforms from an MS patient with a clinical history of optic neuritis (ON) in the right eye. Blue and red traces represent responses from the right and left eye respectively. The position of the waveforms for the 60 sectors has been adjusted for better visibility. The inset shows waveforms from one of the sectors scaled in size. The monocular probability plots for the same patient are shown for response amplitudes (C) and latencies (D). Sectors marked in black indicate no significant difference from the normative database. Colored sectors denote significantly reduced amplitudes (C) and delayed responses (D). Saturated color: p < 0.01; desaturated color: p < 0.05. Red rectangles in C and D outline clusters of adjacent abnormal sectors. Sections A and B were reproduced with permission from Laron et al (2009) [36]
Response analysis
All data analyses were based on ‘best channel’ responses using customized software written in MATLAB (The MathWorks Inc. Natick, MA) [42,44,45]. For each sector, the following parameters were calculated. The monocular amplitude (MAMP) represented by the signal to noise ratio (SNR) was calculated as the root mean square (RMS) of the sector’s waveform in the signal window (45–150 ms) divided by the mean RMS from the noise windows (325–430 ms) of all 60 sectors [42]. The log SNR has been reported in this study because previous publications, as well as our own normative database, showed that responses with log transformation follow a normal distribution [36,42]. The interocular amplitude (IAMP) was ratio of the RMS of the signal window in the right eye to that in the left eye [42]. The monocular latency (MLAT) was calculated using cross correlation (i.e., ‘xcorr’ function from MATLAB 7.1) between the waveform and a template derived from the Portland 100 normal controls (Devers Eye Institute, Portland, OR) [44], and is reported as the shift in milliseconds (ms) needed to achieve the best cross correlation. The interocular latency (ILAT) was also calculated using the ‘xcorr’ function and represents the shift in milliseconds to achieve the best cross correlation between a subject’s responses from his/her two eyes [45]. An example of the array of mfVEP waveforms from monocular stimulation of the right eye (blue) and left eye (red) is shown in Figure 1B for an MS patient with history of ON in the right eye.
mfVEP probability plots and cluster criteria
For each subject, the customized software calculated probability plots that were analogous to the total deviation plot of the Humphrey visual field. The four types of probability plot, MAMP, IAMP, MLAT and ILAT, were calculated by comparing the amplitude or latency of the mfVEP from a particular sector of the stimulus array to the mean and standard deviation of the Portland normative data for the corresponding location, and assigning a probability value [42,44,45]. As illustrated in Figure 1C (MAMP) and D (MLAT), the probability map is comprised of 60 points representing the 60 mfVEP sectors, each marked as ‘normal’ (p>0.05) or ‘abnormal’ (p<0.05 or p<0.01; 1.96 or 2.58 standard deviations below mean values respectively). Black points indicate responses within normal limits. Colored points denote abnormal responses (desaturated colors for p<0.05, and saturated colors for p<0.01). The gray symbols indicate sectors with invalid measurements due to low signal to noise ratios [42,45]. To assess the overall performance of the mfVEP, we used the mfVEP (amp/lat) criterion, which defined an eye as abnormal if one of the four probability plots met the following criteria: at least five adjacent points with p<0.05 for MAMP and IAMP plots, and at least four adjacent points with p<0.01 or cluster size larger than 7 for MLAT and ILAT plots. These cluster criteria were found to provide a specificity of 95% (or false positive rate of 5%) in a previous study based on 100 eyes from control subjects recorded in our lab (see details in ref [36]). In addition, the mfVEP (amp) criterion (which defined an eye as abnormal when either MAMP or IAMP plot met the cluster criterion described above) had a specificity of 97% and was used for comparison of the mfVEP and OCT in detecting axonal damage; the mfVEP (lat) criterion (which defined an eye as abnormal when either MLAT or ILAT plot met the cluster criterion described above) had a specificity of 98%, and was used for comparison of mfVEP and HVF to assess the correspondence between demyelination and sensitivity loss.
Standard automated perimetry
Visual fields were tested with the Humphrey field analyzer 750 (Carl Zeiss Meditec, Inc.) using the SITA (Swedish interactive threshold algorithm) 24-2 (18 patients) or 30-2 (51 patients) protocols. For the 30-2 protocol, only test locations that overlapped with the 24-2 map were used for analysis. The stimulus was a Goldman size III (0.43 deg) with a background luminance of 31.5 Apostilb in all cases. The visual field was defined as being abnormal if the mean deviation of the total deviation plot had p<0.05 (false positive rate of 5%).
Optical coherence tomography
Stratus OCT 3000 (Carl Zeiss Meditec, Inc., Dublin, CA) was used to acquire RNFL thickness measurements. The average of three 3.4 mm diameter circular scans centered on the optic disc was calculated (fast RNFL thickness protocol). OCT measurements were taken with a dilated pupil. The OCT result was defined as being abnormal if the overall RNFL thickness was significantly different (i.e. thinner) than norms provided with the instrument p<0.05 (false positive rate of 5%). An additional quadrant criterion was examined to assess abnormality revealed by OCT. Specifically, the ‘OCT ≥1 quadrant’ criterion defines an eye as abnormal when any OCT quadrant is classified by the instrument as abnormal (either p < 0.05 or p < 0.01). A drawback of using the quadrant criterion to classify a whole eye as abnormal is the unknown false positive rate (we used machine norms only). Statistically, the false positive rate should fall between 1% to 4% for ‘OCT ≥ 1 (p < 0.01)’ criterion, and 5% to 20% for ‘OCT ≥ 1 (p < 0.05)’ criterion. Therefore, to ensure a specificity of 95% for all tests, we selected overall RNFL thickness (p < 0.05) as the OCT criterion to compare different test sensitivity and assess test agreement.
Topographic mapping of mfVEP, HVF, and OCT
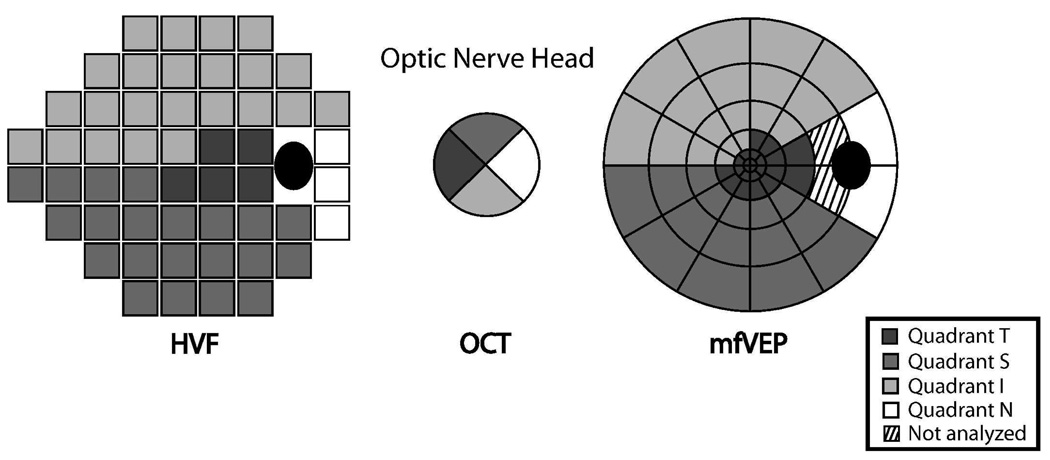
The topographic relationship between the HVF and OCT was based on a previous study by Garway-Heath et al. [46] that mapped each test location of the HVF to the entry location of the axons of local retinal ganglion cells, in degrees, to the optic nerve head. The HVF and OCT were divided into four quadrants corresponding to the four quadrant output of the OCT [21]. The mfVEP output array was also divided into four quadrants, in this study using the interpolation of the HVF 24-2 test locations to the mfVEP sectors described by Hood et al. [47] Figure 2 illustrates the corresponding quadrants for the three tests. In this study, topographic analysis has been confined to the three sectors, superior, inferior, and temporal for which there was substantial overlap for the three tests. The nasal sector was excluded from topographic comparisons among the three tests due to limited representation by the HVF and mfVEP tests. The two mfVEP sectors adjacent to the optic nerve head on the temporal side also were excluded from analysis because responses were unreliable in that region.
Figure 2.
A topographic map relating nominal quadrants from OCT measurements of RNFL thickness and the corresponding quadrants from the HVF and mfVEP tests. For example, quadrant S denotes the superior RNFL quadrant and the corresponding quadrant of the 24-2 Humphrey visual field and multifocal VEP. For patients tested with the HVF 30-2 paradigm, only the areas corresponding to the 24-2 map were analyzed.
Criteria used to define abnormal quadrants for each technique
OCT
A quadrant was defined as being abnormal if the RFNL thickness was significantly thinner than the norms, p<0.05.
HVF
A quadrant was defined as being abnormal using one of the following cluster criteria: at least two adjacent test points in a sector depressed by p<0.01, or at least three adjacent points depressed by p<0.05 with one of the points depressed by p<0.01. HVF inferior and superior sector clusters could not include more than one peripheral rim test location. These criteria were used previously by other investigators for fields of glaucoma patients, and were shown to reduce the false positive rate [48,49]. Good agreement between the HVF and the OCT was also shown topographically in MS patients using the above criteria in a previous study in our lab in which 34 of patients in the current study were described [21].
MfVEP
A quadrant was defined as being abnormal if either MAMP or IAMP met the following cluster criteria: at least two adjacent test points in a sector depressed by p<0.01, or at least three adjacent points depressed by p<0.05. The cluster criteria described above provides a specificity of 95% based on our own normal controls (100 eyes). Only mfVEP amplitude was used for analysis in this section because the purpose of topographic analysis was to reveal the structure-function relationship for retinal ganglion cell axons, and the latency was presumed to be a measure of demyelination rather than axonal loss.
Statistical analysis
To compare results from the MS-ON and MS-no-ON groups the Student’s t-test statistic was used. The chi-square test was used for comparisons between samples with categorical outcomes, i.e. normal or abnormal. An agreement coefficient AC1 was used to adjust for chance agreement in 2×2 agreement tables among the OCT, HVF, and mfVEP [50]. Compared to a commonly used Kappa statistic, AC1 is less dependent on the prevalence of a trait [50]. Calculation of AC1 statistic was described in detail in Cheng et al. [21] for our findings, and details of the test can be found in [ref. 50]. Linear regression analysis was performed between several parameters to assess relationships among test results.
Results
Snellen visual acuity (VA), contrast sensitivity and its relationship with other tests
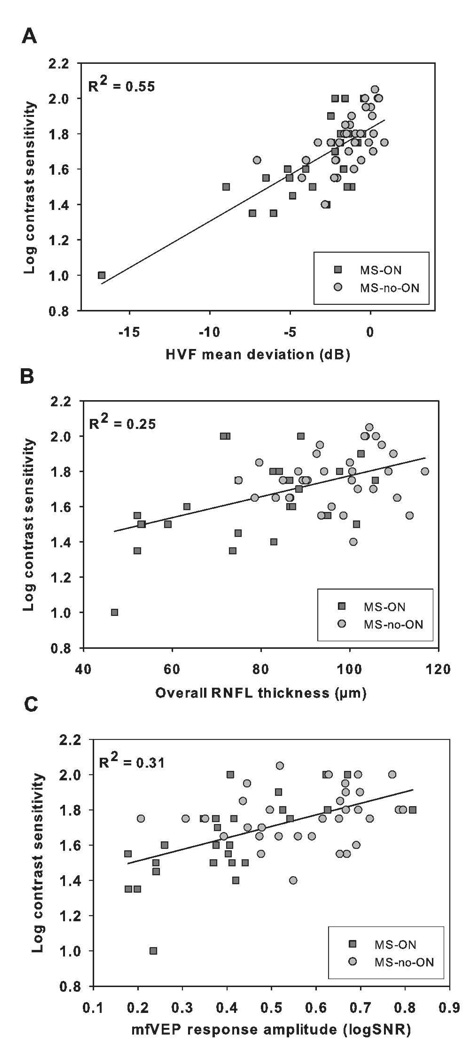
The median VA was 20/20 in both MS-ON and MS-no-ON groups. All eyes (n = 47) in the MS-ON group had visual acuities of 20/30 or better except for five eyes with VA between 20/40 – 20/60, while in the MS-no-ON group all eyes (n = 65) had VA of 20/25 or better except two eyes with VA of 20/40. Contrast sensitivity (CS) was measured with Pelli-Robson chart for 26 MS-ON and 33 MS-no-ON eyes. Mean log CS (± SE) for MS-ON eyes was 1.63 ± 0.05, about one line lower than 1.77 +/− 0.03 for MS-no-ON eyes (Student’s t test, p < 0.05). Linear regression analysis (Fig. 3) showed significant (p < 0.0001) correlation between log CS and HVF mean deviation (MD) (R2 = 0.55), OCT overall RNFL thickness (R2 = 0.24), and mfVEP response amp (R2 = 0.31). Similar results were obtained if only one eye from a patient was used for regression analysis (R2 = 0.60, 0.31, 0.32 for MD, overall RNFL thickness, and mfVEP amp, respectively; p < 0.0001 for all). The relationship between log CS and latency was weak or not significant: R2 = 0.10 (p = 0.014) when both MS-ON (n = 26) and MS-no-ON (n = 33) eyes were included; R2 = 0.04 (p = 0.31) when only MS-ON eyes (n = 26) were used for linear regression.
Figure 3.
Scatter plots and linear regressions for Pelli-Robson contrast sensitivity (log unit) and (A) HVF mean deviation (MD), (B) overall OCT RNFL thickness, and (C) mfVEP response amplitude (log SNR); all reaching p < 0.0001. Each data point represents an MS-ON eye (square) or an MS-no-ON eye (circle).
Global measurements of OCT, HVF, and mfVEP for the MS-ON and MS-no-ON groups
The averaged retinal nerve fiber layer (RNFL) thickness measured by OCT, MD measured by HVF, and response amplitude (calculated as log SNR) and latency measured by mfVEP are shown for the MS-ON (n = 47) and MS-no-ON (n = 65) groups in Table 1. For all parameters there were statistically significant differences between the MS-ON group and the MS-no-ON group with p < 0.0001 for RNFL thickness, p = 0.0005 for MD, p < 0.0001 for mfVEP response amplitude, and p = 0.0002 for mfVEP latency (Student’s t-test).
Table 1.
Global measurements for the two groups of patients (Mean ± SE): MS-ON and MS-no-ON.
| OCT RNFL thickness (µm) |
HVF mean deviation (dB) |
mfVEP log SNR |
mfVEP latency (ms) |
|
|---|---|---|---|---|
| MS-ON (n = 47 eyes) |
79.1 ± 2.5 | -5.6 ± 1.0 | 0.4 ± 0.03 | 11.7 ± 1.6 |
| MS-no-ON (n = 65 eyes) |
96.3 ± 1.4 | -2.1 ± 0.4 | 0.5 ± 0.02 | 4.4 ± 1.1 |
| Normal control (n = 100 eyes)* |
age matched machine norms |
age matched machine norms |
0.6 ± 0.01 | 0.3 ± 0.4 |
Based on our own lab controls, see details in Laron et al 2009[36].
Abnormality detected by the OCT, HVF and mfVEP in the MS-ON and MS-no-ON groups
To document the performance of the OCT, HVF, and mfVEP in detecting abnormality in the visual pathway for the MS-ON and MS-no-ON groups, the percent of eyes defined as abnormal by each of the three tests, is reported in Table 2A. Eyes with abnormal OCT varied from 51% to 79% depending on the criterion used. To compare the relative sensitivity for each test, we chose criteria that had a specificity of 95% (see Methods), and for OCT, the overall thickness (p < 0.05) criterion was used. In the MS-ON group, using the mfVEP (amp/lat) criterion, which defined an eye as abnormal when either the amplitude or latency was abnormal, the mfVEP detected substantially more abnormality (89% of eyes) than either HVF (72%) (p = 0.036, Chi square) or OCT (62%) (p = 0.002, Chi square). The abnormality detected by mfVEP amplitude (66%) or latency (67%) alone was less than that revealed by HVF (p = 0.002, Chi square), but similar to that of OCT (p = 0.07, Chi square).
Table 2.
(A) Percent of eyes in each group defined as being abnormal by each test, using the criteria described in the Methods. (B) Percent of eyes in the MS-ON group (n = 47 eyes) defined as being abnormal by combined findings from pairing of tests: HVF and OCT, HVF and mfVEP, OCT and mfVEP (left column), and when adding results from all three tests (right column). Here an eye’s OCT result was defined as being abnormal when its overall RNFL thickness fell below the instrument norms (p < 0.05).
| A. | |||||||
|---|---|---|---|---|---|---|---|
| Group | OCT (overall thickness p < 0.05) |
OCT≥1 quadrant (p < 0.01) |
OCT≥1 Quadrant (p < 0.05) |
HVF | mfVEP (amp) |
mfVEP (lat) |
mfVEP (amp/lat) |
| MS-ON (n = 47) |
62% | 51% | 79% | 72% | 66% | 67% | 89% |
| MS-no-ON (n = 65) |
8% | 9% | 31% | 38% | 15% | 25% | 29% |
| MS-no-ON in both eyes (n = 31) |
3% | 10% | 29% | 45% | 16% | 23% | 26% |
| B. | ||
|---|---|---|
| Two tests combined | Three tests combined HVF + OCT + mfVEP (amp/lat) |
|
| HVF + OCT | 83% | |
| HVF + mfVEP (amp/lat) | 91% | 98% |
| OCT + mfVEP (amp/lat) | 96% | |
In the MS-no-ON group, more eyes were found to be abnormal based on HVF (38%), or mfVEP (amp/lat: 29%, amp: 15%, lat: 25%), than OCT (8%). In 18% of the eyes in the MS-no-ON group, abnormality was found by both HVF and mfVEP (amp/lat) tests. This suggests that 20–40 % of MS patients’ eyes with no clinical history of ON are likely to have had a subclinical event somewhere along the visual pathway. To test whether clinical history of ON in the fellow eye had an effect on the status of visual responses driven by the MS-no-ON eye, we separately analyzed the data from the MS-no-ON subgroup with no clinical history of ON in either eye (n = 31, Table 2A). No apparent differences were found compared to the entire MS-no-ON group.
Agreement between the mfVEP, HVF, and OCT in the MS-ON group
Agreement between the mfVEP and the HVF
The mfVEP (amp/lat) results defined 89% (42/47) of eyes as abnormal compared to 72% (34/47) found by the HVF (Table 3). The agreement between the two tests was 79% (AC1 = 0.69). For 19% of MS-ON eyes, the mfVEP (amp/lat) was abnormal while the HVF was normal, compared to only 2% the other way. When the mfVEP (amp) was used, the agreement between the two tests was 77% (AC1 = 0.59) with slightly more (6%) abnormality identified by HVF than mfVEP (amp). When HVF was compared to mfVEP (lat), the agreement was not as strong, 64% (AC1 = 0.35).
Table 3.
Agreement between mfVEP and HVF results for MS-ON eyes (n = 47)
| mfVEP (amp/lat) |
mfVEP (amp) |
mfVEP (lat)* |
||||
|---|---|---|---|---|---|---|
| Normal | Abnormal | Normal | Abnormal | Normal | Abnormal | |
| HVF Normal | 4 (9%) | 9 (19%) | 9 (19%) | 4(9%) | 6 (15%) | 7 (18%) |
| HVF Abnormal | 1 (2%) | 33 (70%) | 7(15%) | 27(57%) | 7 (18%) | 19 (49%) |
Agreement: 79% (AC1 = 0.69) for HVF vs. mfVEP (amp/lat), 77% (AC1 = 0.59) for HVF vs. mfVEP (amp), 64% (AC1 = 0.35) for HVF vs. mfVEP (lat).
n = 39 for mfVEP (lat) due to response amplitudes being too low to measure latency in eight MS-ON eyes.
Agreement between the OCT and the HVF or mfVEP
RNFL thickness was found to be abnormal in 62% (29/47) of MS-ON eyes using the overall thickness (p < 0.05) as a criterion (Table 4). OCT and HVF agreement was 68% (AC1 = 0.43) in classifying eyes as normal or abnormal. The agreement between the OCT results and the mfVEP was 60% (AC1 = 0.36) for mfVEP (amp/lat) and 66% (AC1 = 0.37) for mfVEP (amp).
Table 4.
Agreement between OCT results and each of the two functional tests, HVF and mfVEP for MS-ON eyes (n = 47)
| HVF |
mfVEP (amp/lat) |
mfVEP (amp) |
||||
|---|---|---|---|---|---|---|
| Normal | Abnormal | Normal | Abnormal | Normal | Abnormal | |
| OCT Normal | 8(17%) | 10(21%) | 2 (4%) | 16 (34%) | 9 (19%) | 9 (19%) |
| OCT Abnormal | 5(11%) | 24(51%) | 3 (6.5%) | 26 (55.5%) | 7 (15%) | 22 (47%) |
Agreement: 68% (AC1 = 0.43) for OCT vs. HVF, 60% (AC1 = 0.36) for OCT vs. mfVEP (amp/lat); 66% (AC1 = 0.37) for OCT vs. mfVEP (amp)
Agreement between structural (OCT) and functional (HVF and mfVEP) tests
Table 5 illustrates the relationship between the structural (OCT) and functional (HVF and mfVEP) tests in a subgroup of MS-ON eyes (n = 37) in which the HVF and mfVEP tests agreed (i.e. for each eye both the mfVEP and HVF were found to be either normal or abnormal). The agreement between the OCT results and the combined (and agreed) functional test results was around 70% (AC1 = 0.53). Using mfVEP amplitude alone, functional tests detected slightly more abnormality (6%) than the OCT. When mfVEP amplitude and latency were both considered, the two functional tests detected 16% more abnormality than OCT.
Table 5.
Agreement between OCT results and the two functional tests for the MS-ON subgroup in which mfVEP and HVF agreed (i.e., for each eye both were found to be either normal or abnormal)
| mfVEP (amp/lat) + HVF (n = 37) |
mfVEP (amp) + HVF (n = 36) |
|||
|---|---|---|---|---|
| Normal | Abnormal | Normal | Abnormal | |
| OCT Normal | 1 (3%) | 9 (24%) | 5 (14%) | 6 (17%) |
| OCT Abnormal | 3 (8%) | 24 (65%) | 4 (11%) | 21 (58%) |
Agreement: 68% (AC1 = 0.53) for OCT vs. mfVEP (amp/lat) + HVF; 72% (AC1 = 0.53) for OCT vs. mfVEP (amp) + HVF.
Added contribution of the mfVEP in detecting abnormality
For the MS-ON group, the percent of eyes found to have visual pathway abnormality by either HVF or OCT (i.e. either both or only one of the two test results was abnormal) was 83%. When the mfVEP (amp/lat) was added as a third test, 98% of MS-ON eyes were found to be abnormal (Table 2B). The mfVEP (amp/lat) detected abnormality for an additional 15% of MS-ON eyes compared to findings based on HVF and OCT together.
Topographical relationships between the OCT, mfVEP, and HVF for the MS-ON group
A total of 141 quadrants were analyzed (47 eyes, 3 quadrants per eye, i.e. superior, inferior and temporal). As noted in the methods section, the nasal sector was not well represented in HVF and mfVEP and was therefore excluded from topographic comparison. The mfVEP amplitude alone was used here to examine the structure-function relationship with respect to retinal ganglion cell axonal loss. As shown in Table 6, agreement for the quadrants between the two functional tests was 64% (AC1 = 0.28), which was weaker than the whole field agreement shown in Table 3 (77%, AC1 = 0.59). Agreement for the quadrants for each pairing of the three tests was similar, in the range of 60% to 64% (AC1 0.20 to 0.28).
Table 6.
Topographic agreement between tests for the MS-ON group. The HVF and mfVEP maps were divided into quadrants corresponding to the four nominal quadrants of the OCT results as illustrated in Figure 2. The total number of quadrants analyzed was 141, corresponding to three of the four quadrants (T, S, and I; quadrant N was not analyzed, as explained in the text) for each of the 47 eyes.
| Agreement | AC1 statistic | |
|---|---|---|
| mfVEP(amp) vs. HVF | 64% | 0.28 |
| OCT vs. mfVEP(amp) | 61% | 0.24 |
| OCT vs. HVF | 60% | 0.20 |
Table 7 illustrates the percent of quadrants identified as being abnormal by each test. Interestingly, the total number of abnormal quadrants was similar for all three tests. While the temporal sector, T, was the most affected for the mfVEP test, it was the least affected for the HVF test.
Table 7.
Percent of quadrants identified as being abnormal by each of the three tests: mfVEP (amp), HVF, and OCT
| T | S | I | Total | |
|---|---|---|---|---|
| (n = 47) | (n = 47) | (n = 47) | (all 3 sectors, n = 141) | |
| mfVEP (amp) | 66% | 43% | 47% | 52% |
| HVF | 34% | 66% | 55% | 55% |
| OCT | 60% | 57% | 43% | 53% |
Quantitative comparisons between the OCT, mfVEP, and HVF in the MS-ON group
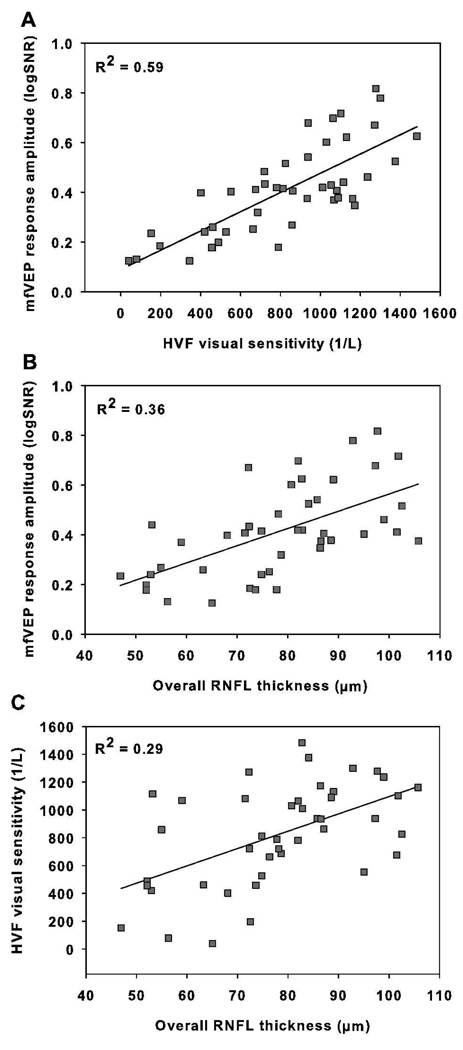
In addition to qualitative analysis, the relationships among the OCT RNFL thickness, mfVEP response amplitude (log SNR), and HVF unlogged visual sensitivity measurements were studied quantitatively using linear regression analysis. For this comparison, visual sensitivity in dB at each test point was divided by 10 and unlogged. This value in 1/Lambert (1/L) was then averaged across the field (dB = 10*log (1/L))[51]. The HVF unlogged sensitivity (1/L) showed moderate correlation with the mfVEP log SNR (R2 = 0.59, Figure 4A). The correlation between the OCT RNFL thickness and the functional measurements was weaker: R2 = 0.36 for the mfVEP log SNR (Figure 4B), and R2 = 0.29 for the HVF unlogged sensitivity (1/L) (Figure 4C). To eliminate any potential contamination from intrasubject correlation, we also performed linear regression analysis using only one eye from each patient (n = 33, the right eye was used for patients with bilateral ON). The coefficient of determination (R2) in this case was R2 = 0.60 for the mfVEP and HVF, but lower for the structure function relationships: R2= 0.38 for the mfVEP and OCT, and R2= 0.38 for the HVF and OCT.
Figure 4.
Scatter plots and linear regressions for mfVEP mean log SNR vs. HVF linear sensitivity (p<0.0001) (A), OCT RNFL thickness vs. mfVEP log SNR (p<0.0001) (B) and OCT RNFL thickness vs. HVF linear sensitivity (p = 0.0002) (C). Each data point represents an MS-ON eye. Parameters denote the mean log SNR across the 60 sectors of the mfVEP stimulus, the mean of the linear sensitivity across all test locations of HVF 24-2, and the overall RNFL thickness.
Discussion
This study evaluated the relationship between a structural measure, the RNFL thickness measured by OCT, and visual function measured subjectively by HVF and objectively by mfVEP in MS patients. For the group of eyes with a history of ON, the MS-ON group, the mfVEP (amp/lat) was more sensitive in detecting abnormality (89% of eyes) than the HVF (72%) and OCT (62%). The mfVEP (amp/lat) found an additional 15% of the MS-ON eyes to be abnormal when compared to the combined results of HVF and OCT. For the eyes without a history of ON, the MS-no-ON group, both the mfVEP (amp/lat) and HVF were more sensitive and detected abnormality in about 20–40% of eyes compared to only 8% detected by OCT. Generally, the agreement between the two functional tests (HVF and mfVEP) was stronger than the agreement between the structural and individual functional tests. For the subgroup of MS-ON eyes, in which the mfVEP (amp/lat) and the HVF agreed, the OCT did not detect abnormality, using our criteria, in 24% of eyes classified as abnormal by both functional tests. Our results indicated that the mfVEP performed better than the OCT or HVF mainly due to its ability to detect demyelination (latency abnormality). The performance of the mfVEP based on response amplitude alone was similar to OCT and worse than HVF in MS-ON eyes. Although it was not the main purpose of this study, we also measured contrast sensitivity in about half of the study population, and found significant correlations between contrast sensitivity and OCT, HVF and mfVEP measurements.
Relating structure (OCT) and function (HVF and mfVEP) in MS
In agreement with the previous reports [33,52,53], we found that functional tests (mfVEP and HVF) detected more abnormality in MS patients than the structural test. Such a result is expected because (1) as noted above, the mfVEP or VEP, by virtue of the latency measurements detects demyelination while the OCT does not; (2) the OCT measurement is limited to the anterior visual pathway assessed at the retinal level whereas the functional tests measure integrity of both anterior and posterior visual pathways. According to the Optic Neuritis Treatment Trial, optic neuritis is retrobulbar in approximately two thirds of patients [54]. The OCT will not detect or underestimate the defects when retrograde axonal degeneration is partial or not significant, and is not expected to detect lesions beyond the lateral geniculate nucleus because these are unlikely to lead to retrograde axonal degeneration in the adult retina. The OCT may also not detect subclinical events which mainly involve demyelination and are associated with subtle or no axonal injury (Table 2, MS-no-ON group). In accordance, our quantitative analysis of the structure-function measurements revealed relatively weak correlation as well (Fig. 4B and C).
A well established model to study the relationship between the retinal ganglion cell’s structure and function is glaucoma, a disease affecting optic nerve only. In general, concordance between structural and functional tests is observed in glaucoma, although the exact quantitative relationship between retinal ganglion cell or RNFL thickness and visual sensitivity is under debate [22,55,56].
Studies of structural and functional relationships are limited by many factors, and one of them is the variability of each test. HVF is a subjective test which may be more variable for ON eyes [42,57]. OCT also has its limitations [56]; it measures the entire retinal nerve fiber layer thickness which includes blood vessels [58,59] and glial tissue. There is evidence suggesting that a linear increase in the glial tissue component with age compensates to some extent for the age-related loss in the neuronal component of the RNFL measure. Should glial tissue also replace dead ganglion cell axons resulting from ON, erroneously higher RNFL thickness measurements could occur [60,61].
Another technique commonly used to measure RNFL thickness is scanning laser polarimetry (GDx). The GDx calculates the RNFL thickness from the phase retardation induced by RNFL birefringence, a property that depends on the integrity of axons’ internal structures such as microtubules and neurofilaments [62,63]. In theory, the GDx can detect structural destruction of axons before thinning [62] and should not be as affected by blood vessels and glial tissues as the OCT and therefore may yield better structural-functional agreement. A recent development in the OCT technology is the spectral domain (SD) OCT which is shown to have higher resolution, and better reproducibility than the time domain (TD) OCT (used in this study) due to automated optic nerve centering and faster scan time. The high resolution of the SD-OCT allows segmental analysis of different retinal layers in the macular region, for example, those involving the retinal ganglion cells, whereas the TD-OCT only measures the entire retinal thickness. More studies are needed to investigate whether the SD-OCT improves the diagnostic performance as well as the accuracy of structure-function correlations in glaucoma or ON or other diseases of the optic nerve compared to the TD-OCT [64,65].
Criteria for defining an eye as abnormal
In this study, the criteria chosen for each test to define an eye, and the visual responses that it drives, as abnormal had a specificity of 95% or better. For the HVF, we used mean deviation with p < 0.05 as our criterion because no significant media opacities were present in any of our patients. Previous studies using standard automated perimetry have shown that defining an eye as abnormal based on cluster criteria (i.e., the presence of adjacent abnormal points on the total deviation plot) improves test specificity and is sensitive in detecting local abnormality [48,49]. To examine the effect of choosing one criterion over the other, we also evaluated the performance of the HVF using cluster criteria on the total deviation plots as described by Cheng et al. [21]. For the MS-ON group, the HVF detected abnormality in 74% of eyes when using cluster criteria compared to 72% when using mean deviation with p < 5% as a criterion. The agreement between HVF and the other two tests was similar regardless of the criterion used.
As shown in Results, the percent of MS-ON eyes defined as abnormal by OCT varied with criterion. More abnormality was detected using the ‘≥1 quadrant (p < 0.05)’ criterion. Unfortunately, we did not have normal controls to determine the specificity of the ‘OCT≥1 quadrant (p < 0.05)’ criterion, which should fall between 80% and 95% of the machine norms statistically and previous studies have reported a specificity of 76% [65] or 95% [67]. Future studies should use the same normal controls for both mfVEP and OCT in order to do a fairer comparison of the sensitivity and specificity of the two tests in detecting RNFL axonal loss.
Topographic comparisons among the three tests
In this study we also evaluated the relationship between the OCT, mfVEP and HVF topographically. For the temporal quadrant, both the OCT and mfVEP were abnormal for about two thirds of the ON eyes while the HVF was abnormal in only one third (Table 7, left column). This discrepancy is likely due to the fact that only five test locations were measured in the HVF 24-2 corresponding to the OCT’s temporal sector, even though the region has a high cell density. In comparison, the mfVEP has 23 test locations (sectors) and the OCT measures 64 pixels in this region, yielding much higher sampling than for the HVF. It is our clinical impression that a HVF 10-2 test detects more abnormality in the central field thus its use should be considered in MS/ON patients. The relatively weak topographical agreement (~60%) observed among the three tests compared to those of whole eye is partially due to the use of cluster criteria to define an abnormal quadrant in both the mfVEP and HVF. A previous study from our lab found that the spatial distribution of visual field defects in ON is relatively diffuse, meaning that defects tend to cross the boundaries defined by the OCT quadrants [21]. Specifically, in 22 out of the 23 ON eyes that showed abnormal clusters in the HVF 24-2 test, the clusters crossed the boundaries of at least 2 quadrants of the field [21]. In the present study, mfVEP clusters crossed the boundaries of at least 2 sectors in 110 out of 113 probability plots that had abnormal clusters. When using cluster criteria to define an abnormal quadrant, relatively small defects located on the boundary between two sectors, may not meet the cluster criteria at each sector and be missed resulting in decreased agreement among the tests. The topographic agreement/correlation is also limited by lack of accurate structure-function maps and the inherent variability of such maps among individuals [46,56,68,69].
Advantages of the mfVEP
At present, both the HVF and OCT tests (and tradition VEP) are used during ophthalmic evaluation of MS/ON patients, whereas the mfVEP is relatively time consuming and not readily available in most clinics. Compared to the HVF, the mfVEP has the advantage that it is an objective test of local visual function that does not require patients to make a decision. Some MS patients, especially those with advanced disease, may suffer from cognitive impairment [70,71], and have slowed reaction time, which adversely affects their performance on the HVF test.
Most importantly, the mfVEP also provides local information about delayed responses (latencies), which is not reflected by either HVF or OCT. The latency information is of high value in MS patients, since a hallmark of the disease is nerve demyelination which disrupts and slows signal conduction. While most cases of ON can be diagnosed clinically, the detection of subclinical demyelination substantially relies on latency measurements. For example, Table 2 of the present study shows, based on mfVEP latency measurements alone, that abnormality was detected in 23% of eyes of patients without a history of ON in either eye. When both the mfVEP amplitude and latency were used for analysis, abnormality was only detected in an additional 3% of the eyes. It is also important to point out that not all (only 67%) of our MS-ON eyes showed latency abnormalities. As discussed in detail elsewhere [36], several factors may have contributed to the relatively low incidence of latency abnormalities in our sample. First, there is short term and long term latency recovery as a result of remyelination [72–75]. Our MS-ON eyes had long recovery time (5.9±6.7 years on average) from the last ON episode. Second, 62% of our MS-ON eyes were from patients where ON had occurred in both eyes, which may decrease the sensitivity in detecting mild latency abnormalities with interocular comparisons. Third, some abnormal latency values would be masked by decreased SNR of the response, making the measurement difficult [76].
Limitations of the current study
There are several limitations to this study. As noted above, we did not use the same subjects as normal controls for all tests; this could impact the fairness of comparisons of test sensitivity. For example, the OCT’s performance may be improved by using a quadrant criterion as shown in glaucoma [66]. The current study only measured the RNFL thickness in the peripapillary region, and did not measure macular volume, which has also been found to reveal RGC neuronal loss in ON/MS [77,78]. More importantly, this was a cross sectional study and did not address the important issue of test-retest variability. A functional test like mfVEP or HVF is more likely to be affected by physiological factors such as fatigue and drug effects in MS patients, than a structural test like OCT [57]. Future studies should evaluate test repeatability in MS patients, and compare test utility longitudinally.
Conclusions
The mfVEP, HVF and OCT provide complementary information in detecting visual pathway abnormalities in MS. The three tests together identified abnormality in 98% of the MS-ON eyes. The functional tests provide both objective (mfVEP) and subjective (HVF) information on axonal pathology; the structural test (OCT) is a valuable tool for documenting axonal loss. The mfVEP latency measure is particularly useful for detection of demyelination in visual pathways which can be subclinical is some cases. Results of both structural and functional tests should be included in longitudinal studies in order to understand the processes involved in the neuronal damage in MS that occurs over time, the repair mechanisms and whether/how therapeutic treatments affect these processes. Further improvements in the mfVEP technique such as the use of sparse stimulation [79,80] may improve mfVEP SNR and shorten the recording time, and make it more applicable with respect to both amplitude and latency measurements in the clinic setting [81,82].
Acknowledgements
This study was supported by NIH grants P30 EY07751, T35 007088, a pilot grant from the National Multiple Sclerosis Society, a University of Houston GEAR grant, and the Minnie Flaura Turner memorial fund for impaired vision research. We wish to thank Dr. Don Hood for his generosity with the software for analysis of mfVEP data, and Dr. Ying Sheng Hu for helpful discussions on statistical analyses.
References
- 1.Comi G, Filippi M, Barkhof F, Durelli L, Edan G, Fernandez O, et al. Effect of early interferon treatment on conversion to definite multiple sclerosis: a randomised study. Lancet. 2001;357:1576–1582. doi: 10.1016/s0140-6736(00)04725-5. (doi:S0140673600047255 [pii]) [DOI] [PubMed] [Google Scholar]
- 2.Kappos L, Freedman MS, Polman CH, Edan G, Hartung HP, Miller DH, et al. Effect of early versus delayed interferon beta-1b treatment on disability after a first clinical event suggestive of multiple sclerosis: a 3-year follow-up analysis of the BENEFIT study. Lancet. 2007;370:389–397. doi: 10.1016/S0140-6736(07)61194-5. (doi:S0140-6736(07)61194-5 [pii] 10.1016/S0140-6736(07)61194-5) [DOI] [PubMed] [Google Scholar]
- 3.Jacobs LD, Beck RW, Simon JH, Kinkel RP, Brownscheidle CM, Murray TJ, et al. Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. CHAMPS Study Group. N Engl J Med. 2000;343:898–904. doi: 10.1056/NEJM200009283431301. [DOI] [PubMed] [Google Scholar]
- 4.Kappos L, Polman CH, Freedman MS, Edan G, Hartung HP, Miller DH, et al. Treatment with interferon beta-1b delays conversion to clinically definite and McDonald MS in patients with clinically isolated syndromes. Neurology. 2006;67:1242–1249. doi: 10.1212/01.wnl.0000237641.33768.8d. (doi:01.wnl.0000237641.33768.8d [pii] 10.1212/01.wnl.0000237641.33768.8d) [DOI] [PubMed] [Google Scholar]
- 5.Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. (doi:S0140-6736(08)61620-7 [pii] 10.1016/S0140-6736(08)61620-7) [DOI] [PubMed] [Google Scholar]
- 6.DeAngelis T, Lublin F. Neurotherapeutics in multiple sclerosis: novel agents and emerging treatment strategies. Mt Sinai J Med. 2008;75:157–167. doi: 10.1002/msj.20030. (doi:10.1002/msj.20030) [DOI] [PubMed] [Google Scholar]
- 7.Imitola J, Chitnis T, Khoury SJ. Insights into the molecular pathogenesis of progression in multiple sclerosis: potential implications for future therapies. Arch Neurol. 2006;63:25–33. doi: 10.1001/archneur.63.1.25. (doi:63/1/25 [pii] 10.1001/archneur.63.1.25) [DOI] [PubMed] [Google Scholar]
- 8.Beck RW, Trobe JD, Moke PS, Gal RL, Xing D, Bhatti MT, et al. High- and low-risk profiles for the development of multiple sclerosis within 10 years after optic neuritis: experience of the optic neuritis treatment trial. Arch Ophthalmol. 2003;121:944–949. doi: 10.1001/archopht.121.7.944. (doi:10.1001/archopht.121.7.944 121/7/944 [pii]) [DOI] [PubMed] [Google Scholar]
- 9.McDonald WI, Barnes D. The ocular manifestations of multiple sclerosis. 1. Abnormalities of the afferent visual system. J Neurol Neurosurg Psychiatry. 1992;55:747–752. doi: 10.1136/jnnp.55.9.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorensen TL, Frederiksen JL, Bronnum-Hansen H, Petersen HC. Optic neuritis as onset manifestation of multiple sclerosis: a nationwide, long-term survey. Neurology. 1999;53:473–478. doi: 10.1212/wnl.53.3.473. [DOI] [PubMed] [Google Scholar]
- 11.Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, et al. Optical coherence tomography. Science. 1991;254:1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Budenz DL, Chang RT, Huang X, Knighton RW, Tielsch JM. Reproducibility of retinal nerve fiber thickness measurements using the stratus OCT in normal and glaucomatous eyes. Invest Ophthalmol Vis Sci. 2005;46:2440–2443. doi: 10.1167/iovs.04-1174. (doi:46/7/2440 [pii] 10.1167/iovs.04-1174) [DOI] [PubMed] [Google Scholar]
- 13.Costello F, Coupland S, Hodge W, Lorello GR, Koroluk J, Pan YI, et al. Quantifying axonal loss after optic neuritis with optical coherence tomography. Ann Neurol. 2006;59:963–969. doi: 10.1002/ana.20851. (doi:10.1002/ana.20851) [DOI] [PubMed] [Google Scholar]
- 14.Frohman EM, Fujimoto JG, Frohman TC, Calabresi PA, Cutter G, Balcer LJ. Optical coherence tomography: a window into the mechanisms of multiple sclerosis. Nat Clin Pract Neurol. 2008;4:664–675. doi: 10.1038/ncpneuro0950. (doi:ncpneuro0950 [pii] 10.1038/ncpneuro0950) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon-Lipkin E, Chodkowski B, Reich DS, Smith SA, Pulicken M, Balcer LJ, et al. Retinal nerve fiber layer is associated with brain atrophy in multiple sclerosis. Neurology. 2007;69:1603–1609. doi: 10.1212/01.wnl.0000295995.46586.ae. (doi:69/16/1603 [pii] 10.1212/01.wnl.0000295995.46586.ae) [DOI] [PubMed] [Google Scholar]
- 16.Kallenbach K, Frederiksen J. Optical coherence tomography in optic neuritis and multiple sclerosis: a review. Eur J Neurol. 2007;14:841–849. doi: 10.1111/j.1468-1331.2007.01736.x. (doi:ENE1736 [pii] 10.1111/j.1468-1331.2007.01736.x) [DOI] [PubMed] [Google Scholar]
- 17.Noval S, Contreras I, Rebolleda G, Munoz-Negrete FJ. Optical coherence tomography versus automated perimetry for follow-up of optic neuritis. Acta Ophthalmol Scand. 2006;84:790–794. doi: 10.1111/j.1600-0420.2006.00724.x. (doi:AOS724 [pii] 10.1111/j.1600-0420.2006.00724.x) [DOI] [PubMed] [Google Scholar]
- 18.Sergott RC, Frohman E, Glanzman R, Al-Sabbagh A. The role of optical coherence tomography in multiple sclerosis: expert panel consensus. J Neurol Sci. 2007;263:3–14. doi: 10.1016/j.jns.2007.05.024. (doi:S0022-510X(07)00383-8 [pii] 10.1016/j.jns.2007.05.024) [DOI] [PubMed] [Google Scholar]
- 19.Zaveri MS, Conger A, Salter A, Frohman TC, Galetta SL, Markowitz CE, et al. Retinal imaging by laser polarimetry and optical coherence tomography evidence of axonal degeneration in multiple sclerosis. Arch Neurol. 2008;65:924–928. doi: 10.1001/archneur.65.7.924. (doi:65/7/924 [pii] 10.1001/archneur.65.7.924) [DOI] [PubMed] [Google Scholar]
- 20.Parisi V, Manni G, Spadaro M, Colacino G, Restuccia R, Marchi S, et al. Correlation between morphological and functional retinal impairment in multiple sclerosis patients. Invest Ophthalmol Vis Sci. 1999;40:2520–2527. [PubMed] [Google Scholar]
- 21.Cheng H, Laron M, Schiffman JS, Tang RA, Frishman LJ. The relationship between visual field and retinal nerve fiber layer measurements in patients with multiple sclerosis. Invest Ophthalmol Vis Sci. 2007;48:5798–5805. doi: 10.1167/iovs.07-0738. (doi:48/12/5798 [pii] 10.1167/iovs.07-0738) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harwerth RS, Vilupuru AS, Rangaswamy NV, Smith EL., 3rd The relationship between nerve fiber layer and perimetry measurements. Invest Ophthalmol Vis Sci. 2007;48:763–773. doi: 10.1167/iovs.06-0688. (doi:48/2/763 [pii] 10.1167/iovs.06-0688) [DOI] [PubMed] [Google Scholar]
- 23.Kanamori A, Naka M, Nagai-Kusuhara A, Yamada Y, Nakamura M, Negi A. Regional relationship between retinal nerve fiber layer thickness and corresponding visual field sensitivity in glaucomatous eyes. Arch Ophthalmol. 2008;126:1500–1506. doi: 10.1001/archopht.126.11.1500. (doi:126/11/1500 [pii] 10.1001/archopht.126.11.1500) [DOI] [PubMed] [Google Scholar]
- 24.Bjerre A, Grigg JR, Parry NR, Henson DB. Test-retest variability of multifocal visual evoked potential and SITA standard perimetry in glaucoma. Invest Ophthalmol Vis Sci. 2004;45:4035–4040. doi: 10.1167/iovs.04-0099. (doi:45/11/4035 [pii] 10.1167/iovs.04-0099) [DOI] [PubMed] [Google Scholar]
- 25.Chen CS, Hood DC, Zhang X, Karam EZ, Liebmann JM, Ritch R, et al. Repeat reliability of the multifocal visual evoked potential in normal and glaucomatous eyes. J Glaucoma. 2003;12:399–408. doi: 10.1097/00061198-200310000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Fortune B, Demirel S, Zhang X, Hood DC, Johnson CA. Repeatability of normal multifocal VEP: implications for detecting progression. J Glaucoma. 2006;15:131–141. doi: 10.1097/00061198-200604000-00010. (doi:00061198-200604000-00010 [pii]) [DOI] [PubMed] [Google Scholar]
- 27.Goldberg I, Graham SL, Klistorner AI. Multifocal objective perimetry in the detection of glaucomatous field loss. Am J Ophthalmol. 2002;133:29–39. doi: 10.1016/s0002-9394(01)01294-6. (doi:S0002939401012946 [pii]) [DOI] [PubMed] [Google Scholar]
- 28.Grover LK, Hood DC, Ghadiali Q, Grippo TM, Wenick AS, Greenstein VC, et al. A comparison of multifocal and conventional visual evoked potential techniques in patients with optic neuritis/multiple sclerosis. Doc Ophthalmol. 2008;117:121–128. doi: 10.1007/s10633-007-9112-7. (doi:10.1007/s10633-007-9112-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klistorner A, Fraser C, Garrick R, Graham S, Arvind H. Correlation between full-field and multifocal VEPs in optic neuritis. Doc Ophthalmol. 2008;116:19–27. doi: 10.1007/s10633-007-9072-y. (doi:10.1007/s10633-007-9072-y) [DOI] [PubMed] [Google Scholar]
- 30.Pakrou N, Casson R, Kaines A, Selva D. Multifocal objective perimetry compared with Humphrey full-threshold perimetry in patients with optic neuritis. Clin Experiment Ophthalmol. 2006;34:562–567. doi: 10.1111/j.1442-9071.2006.01277.x. (doi:CEO1277 [pii] 10.1111/j.1442-9071.2006.01277.x) [DOI] [PubMed] [Google Scholar]
- 31.Fraser C, Klistorner A, Graham S, Garrick R, Billson F, Grigg J. Multifocal visual evoked potential latency analysis: predicting progression to multiple sclerosis. Arch Neurol. 2006;63:847–850. doi: 10.1001/archneur.63.6.847. (doi:63/6/847 [pii] 10.1001/archneur.63.6.847) [DOI] [PubMed] [Google Scholar]
- 32.Klistorner A, Arvind H, Nguyen T, Garrick R, Paine M, Graham S, et al. Fellow eye changes in optic neuritis correlate with the risk of multiple sclerosis. Mult Scler. 2009;15:928–932. doi: 10.1177/1352458509105228. (doi:1352458509105228 [pii] 10.1177/1352458509105228) [DOI] [PubMed] [Google Scholar]
- 33.Klistorner A, Arvind H, Nguyen T, Garrick R, Paine M, Graham S, et al. Axonal loss and myelin in early ON loss in postacute optic neuritis. Ann Neurol. 2008;64:325–331. doi: 10.1002/ana.21474. (doi:10.1002/ana.21474) [DOI] [PubMed] [Google Scholar]
- 34.Freedman MS, Thompson EJ, Deisenhammer F, Giovannoni G, Grimsley G, Keir G, et al. Recommended standard of cerebrospinal fluid analysis in the diagnosis of multiple sclerosis: a consensus statement. Arch Neurol. 2005;62:865–870. doi: 10.1001/archneur.62.6.865. (doi:62/6/865 [pii] 10.1001/archneur.62.6.865) [DOI] [PubMed] [Google Scholar]
- 35.McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121–127. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 36.Laron M, Cheng H, Zhang B, Schiffman JS, Tang RA, Frishman LJ. Assessing visual pathway function in multiple sclerosis patients with multifocal visual evoked potential. Mult Scler. 2009 doi: 10.1177/1352458509350470. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.The clinical profile of optic neuritis. Experience of the Optic Neuritis Treatment Trial. Optic Neuritis Study Group. Arch Ophthalmol. 1991;109:1673–1678. doi: 10.1001/archopht.1991.01080120057025. [DOI] [PubMed] [Google Scholar]
- 38.Hickman SJ, Dalton CM, Miller DH, Plant GT. Management of acute optic neuritis. Lancet. 2002;360:1953–1962. doi: 10.1016/s0140-6736(02)11919-2. (doi:S0140673602119192 [pii]) [DOI] [PubMed] [Google Scholar]
- 39.Costello F, Hodge W, Pan YI, Eggenberger E, Coupland S, Kardon RH. Tracking retinal nerve fiber layer loss after optic neuritis: a prospective study using optical coherence tomography. Mult Scler. 2008;14:893–905. doi: 10.1177/1352458508091367. (doi:1352458508091367 [pii] 10.1177/1352458508091367) [DOI] [PubMed] [Google Scholar]
- 40.Arditi A. Improving the design of the letter contrast sensitivity test. Invest Ophthalmol Vis Sci. 2005;46:2225–2229. doi: 10.1167/iovs.04-1198. (doi:46/6/2225 [pii] 10.1167/iovs.04-1198) [DOI] [PubMed] [Google Scholar]
- 41.Sutter EE. Imaging visual function with the multifocal m-sequence technique. Vision Res. 2001;41:1241–1255. doi: 10.1016/s0042-6989(01)00078-5. (doi:S0042-6989(01)00078-5 [pii]) [DOI] [PubMed] [Google Scholar]
- 42.Hood DC, Greenstein VC. Multifocal VEP and ganglion cell damage: applications and limitations for the study of glaucoma. Prog Retin Eye Res. 2003;22:201–251. doi: 10.1016/s1350-9462(02)00061-7. (doi:S1350946202000617 [pii]) [DOI] [PubMed] [Google Scholar]
- 43.Hood DC, Zhang X, Hong JE, Chen CS. Quantifying the benefits of additional channels of multifocal VEP recording. Doc Ophthalmol. 2002;104:303–320. doi: 10.1023/a:1015235617673. [DOI] [PubMed] [Google Scholar]
- 44.Hood DC, Ohri N, Yang EB, Rodarte C, Zhang X, Fortune B, et al. Determining abnormal latencies of multifocal visual evoked potentials: a monocular analysis. Doc Ophthalmol. 2004;109:189–199. doi: 10.1007/s10633-004-5512-0. [DOI] [PubMed] [Google Scholar]
- 45.Hood DC, Zhang X, Rodarte C, Yang EB, Ohri N, Fortune B, et al. Determining abnormal interocular latencies of multifocal visual evoked potentials. Doc Ophthalmol. 2004;109:177–187. doi: 10.1007/s10633-004-5511-1. [DOI] [PubMed] [Google Scholar]
- 46.Garway-Heath DF, Poinoosawmy D, Fitzke FW, Hitchings RA. Mapping the visual field to the optic disc in normal tension glaucoma eyes. Ophthalmology. 2000;107:1809–1815. doi: 10.1016/s0161-6420(00)00284-0. (doi:S0161-6420(00)00284-0 [pii]) [DOI] [PubMed] [Google Scholar]
- 47.Hood DC, Greenstein VC, Odel JG, Zhang X, Ritch R, Liebmann JM, et al. Visual field defects and multifocal visual evoked potentials: evidence of a linear relationship. Arch Ophthalmol. 2002;120:1672–1681. doi: 10.1001/archopht.120.12.1672. (doi:ecs20019 [pii]) [DOI] [PubMed] [Google Scholar]
- 48.Chauhan BC, Drance SM, Douglas GR, Johnson CA. Visual field damage in normal-tension and high-tension glaucoma. Am J Ophthalmol. 1989;108:636–642. doi: 10.1016/0002-9394(89)90854-4. [DOI] [PubMed] [Google Scholar]
- 49.Johnson CA, Sample PA, Cioffi GA, Liebmann JR, Weinreb RN. Structure and function evaluation (SAFE): I. criteria for glaucomatous visual field loss using standard automated perimetry (SAP) and short wavelength automated perimetry (SWAP) Am J Ophthalmol. 2002;134:177–185. doi: 10.1016/s0002-9394(02)01577-5. (doi:S0002939402015775 [pii]) [DOI] [PubMed] [Google Scholar]
- 50.Gwet K. Statistical Methods For Inter-Rater Reliability Assessment No2 wwwstataxiscom. 2002. May, Inter-rater reliability: dependency on trait prevalence and marginal homogeneity. p^pp. [Google Scholar]
- 51.Garway-Heath DF, Caprioli J, Fitzke FW, Hitchings RA. Scaling the hill of vision: the physiological relationship between light sensitivity and ganglion cell numbers. Invest Ophthalmol Vis Sci. 2000;41:1774–1782. [PubMed] [Google Scholar]
- 52.Klistorner A, Arvind H, Nguyen T, Garrick R, Paine M, Graham S, et al. Multifocal VEP and OCT in optic neuritis: a topographical study of the structure-function relationship. Doc Ophthalmol. 2009;118:129–137. doi: 10.1007/s10633-008-9147-4. (doi:10.1007/s10633-008-9147-4) [DOI] [PubMed] [Google Scholar]
- 53.Naismith RT, Tutlam NT, Xu J, Shepherd JB, Klawiter EC, Song SK, et al. Optical coherence tomography is less sensitive than visual evoked potentials in optic neuritis. Neurology. 2009;73:46–52. doi: 10.1212/WNL.0b013e3181aaea32. (doi:73/1/46 [pii] 10.1212/WNL.0b013e3181aaea32) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beck RW. The Optic Neuritis Treatment Trial. Arch Ophthalmol. 1988;106:1051–1053. doi: 10.1001/archopht.1988.01060140207023. [DOI] [PubMed] [Google Scholar]
- 55.Harwerth RS, Carter-Dawson L, Smith EL, 3rd, Barnes G, Holt WF, Crawford ML. Neural losses correlated with visual losses in clinical perimetry. Invest Ophthalmol Vis Sci. 2004;45:3152–3160. doi: 10.1167/iovs.04-0227. (doi:10.1167/iovs.04-0227 45/9/3152 [pii]) [DOI] [PubMed] [Google Scholar]
- 56.Hood DC, Kardon RH. A framework for comparing structural and functional measures of glaucomatous damage. Prog Retin Eye Res. 2007;26:688–710. doi: 10.1016/j.preteyeres.2007.08.001. (doi:S1350-9462(07)00055-9 [pii] 10.1016/j.preteyeres.2007.08.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wall M, Johnson CA, Kutzko KE, Nguyen R, Brito C, Keltner JL. Long- and short-term variability of automated perimetry results in patients with optic neuritis and healthy subjects. Arch Ophthalmol. 1998;116:53–61. doi: 10.1001/archopht.116.1.53. [DOI] [PubMed] [Google Scholar]
- 58.Hood DC, Fortune B, Arthur SN, Xing D, Salant JA, Ritch R, et al. Blood vessel contributions to retinal nerve fiber layer thickness profiles measured with optical coherence tomography. J Glaucoma. 2008;17:519–528. doi: 10.1097/IJG.0b013e3181629a02. (doi:10.1097/IJG.0b013e3181629a02 00061198-200810000-00003 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hood DC, Salant JA, Arthur SN, Ritch R, Liebmann JM. The Location of the Inferior and Superior Temporal Blood Vessels and Interindividual Variability of the Retinal Nerve Fiber Layer Thickness. J Glaucoma. 2009 doi: 10.1097/IJG.0b013e3181af31ec. (doi:10.1097/IJG.0b013e3181af31ec) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harwerth RS, Wheat JL. Modeling the effects of aging on retinal ganglion cell density and nerve fiber layer thickness. Graefes Arch Clin Exp Ophthalmol. 2008;246:305–314. doi: 10.1007/s00417-007-0691-5. (doi:10.1007/s00417-007-0691-5) [DOI] [PubMed] [Google Scholar]
- 61.Harwerth RS, Wheat JL, Rangaswamy NV. Age-related losses of retinal ganglion cells and axons. Invest Ophthalmol Vis Sci. 2008;49:4437–4443. doi: 10.1167/iovs.08-1753. (doi:iovs.08-1753 [pii] 10.1167/iovs.08-1753) [DOI] [PubMed] [Google Scholar]
- 62.Fortune B, Wang L, Cull G, Cioffi GA. Intravitreal colchicine causes decreased RNFL birefringence without altering RNFL thickness. Invest Ophthalmol Vis Sci. 2008;49:255–261. doi: 10.1167/iovs.07-0872. (doi:49/1/255 [pii] 10.1167/iovs.07-0872) [DOI] [PubMed] [Google Scholar]
- 63.Huang XR, Knighton RW. Microtubules contribute to the birefringence of the retinal nerve fiber layer. Invest Ophthalmol Vis Sci. 2005;46:4588–4593. doi: 10.1167/iovs.05-0532. (doi:46/12/4588 [pii] 10.1167/iovs.05-0532) [DOI] [PubMed] [Google Scholar]
- 64.Leung CK, Cheung CY, Weinreb RN, Qiu Q, Liu S, Li H, et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: a variability and diagnostic performance study. Ophthalmology. 2009;116:1257–1263. doi: 10.1016/j.ophtha.2009.04.013. 1263 e1251-1252. (doi:S0161-6420(09)00365-0 [pii] 10.1016/j.ophtha.2009.04.013) [DOI] [PubMed] [Google Scholar]
- 65.Chang RT, Knight OR, Feuer WJ, Budenz DL. Sensitivity and Specificity of Time-Domain versus Spectral-Domain Optical Coherence Tomography in Diagnosing Early to Moderate Glaucoma. Ophthalmology. 2009 doi: 10.1016/j.ophtha.2009.06.012. (doi:S0161-6420(09)00639-3 [pii] 10.1016/j.ophtha.2009.06.012) [DOI] [PubMed] [Google Scholar]
- 66.Lu AT, Wang M, Varma R, Schuman JS, Greenfield DS, Smith SD, et al. Combining nerve fiber layer parameters to optimize glaucoma diagnosis with optical coherence tomography. Ophthalmology. 2008;115:1352–1357. doi: 10.1016/j.ophtha.2008.01.011. 1357 e1351-1352. (doi:S0161-6420(08)00027-4 [pii] 10.1016/j.ophtha.2008.01.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Budenz DL, Michael A, Chang RT, McSoley J, Katz J. Sensitivity and specificity of the StratusOCT for perimetric glaucoma. Ophthalmology. 2005;112:3–9. doi: 10.1016/j.ophtha.2004.06.039. (doi:S0161-6420(04)01131-5 [pii] 10.1016/j.ophtha.2004.06.039) [DOI] [PubMed] [Google Scholar]
- 68.Gardiner SK, Johnson CA, Cioffi GA. Evaluation of the structure-function relationship in glaucoma. Invest Ophthalmol Vis Sci. 2005;46:3712–3717. doi: 10.1167/iovs.05-0266. (doi:46/10/3712 [pii] 10.1167/iovs.05-0266) [DOI] [PubMed] [Google Scholar]
- 69.Strouthidis NG, Vinciotti V, Tucker AJ, Gardiner SK, Crabb DP, Garway-Heath DF. Structure and function in glaucoma: The relationship between a functional visual field map and an anatomic retinal map. Invest Ophthalmol Vis Sci. 2006;47:5356–5362. doi: 10.1167/iovs.05-1660. (doi:47/12/5356 [pii] 10.1167/iovs.05-1660) [DOI] [PubMed] [Google Scholar]
- 70.Genova HM, Sumowski JF, Chiaravalloti N, Voelbel GT, Deluca J. Cognition in multiple sclerosis: a review of neuropsychological and fMRI research. Front Biosci. 2009;14:1730–1744. doi: 10.2741/3336. (doi:3336 [pii]) [DOI] [PubMed] [Google Scholar]
- 71.Wallin MT, Wilken JA, Kane R. Cognitive dysfunction in multiple sclerosis: Assessment, imaging, and risk factors. J Rehabil Res Dev. 2006;43:63–72. doi: 10.1682/jrrd.2004.09.0120. [DOI] [PubMed] [Google Scholar]
- 72.Jones SJ, Brusa A. Neurophysiological evidence for long-term repair of MS lesions: implications for axon protection. J Neurol Sci. 2003;206:193–198. doi: 10.1016/s0022-510x(02)00428-8. (doi:S0022510X02004288 [pii]) [DOI] [PubMed] [Google Scholar]
- 73.Klistorner A, Graham S, Fraser C, Garrick R, Nguyen T, Paine M, et al. Electrophysiological evidence for heterogeneity of lesions in optic neuritis. Invest Ophthalmol Vis Sci. 2007;48:4549–4556. doi: 10.1167/iovs.07-0381. (doi:48/10/4549 [pii] 10.1167/iovs.07-0381) [DOI] [PubMed] [Google Scholar]
- 74.Prineas JW, Barnard RO, Kwon EE, Sharer LR, Cho ES. Multiple sclerosis: remyelination of nascent lesions. Ann Neurol. 1993;33:137–151. doi: 10.1002/ana.410330203. (doi:10.1002/ana.410330203) [DOI] [PubMed] [Google Scholar]
- 75.Yang EB, Hood DC, Rodarte C, Zhang X, Odel JG, Behrens MM. Improvement in conduction velocity after optic neuritis measured with the multifocal VEP. Invest Ophthalmol Vis Sci. 2007;48:692–698. doi: 10.1167/iovs.06-0475. (doi:48/2/692 [pii] 10.1167/iovs.06-0475) [DOI] [PubMed] [Google Scholar]
- 76.Hood DC, Odel JG, Zhang X. Tracking the recovery of local optic nerve function after optic neuritis: a multifocal VEP study. Invest Ophthalmol Vis Sci. 2000;41:4032–4038. [PubMed] [Google Scholar]
- 77.Trip SA, Schlottmann PG, Jones SJ, Altmann DR, Garway-Heath DF, Thompson AJ, et al. Retinal nerve fiber layer axonal loss and visual dysfunction in optic neuritis. Ann Neurol. 2005;58:383–391. doi: 10.1002/ana.20575. (doi:10.1002/ana.20575) [DOI] [PubMed] [Google Scholar]
- 78.Henderson AP, Trip SA, Schlottmann PG, Altmann DR, Garway-Heath DF, Plant GT, et al. An investigation of the retinal nerve fibre layer in progressive multiple sclerosis using optical coherence tomography. Brain. 2008;131:277–287. doi: 10.1093/brain/awm285. (doi:awm285 [pii] 10.1093/brain/awm285) [DOI] [PubMed] [Google Scholar]
- 79.Maddess T, James AC, Bowman EA. Contrast response of temporally sparse dichoptic multifocal visual evoked potentials. Vis Neurosci. 2005;22:153–162. doi: 10.1017/S0952523805222046. (doi:S0952523805222046 [pii] 10.1017/S0952523805222046) [DOI] [PubMed] [Google Scholar]
- 80.Ruseckaite R, Maddess T, Danta G, Lueck CJ, James AC. Sparse multifocal stimuli for the detection of multiple sclerosis. Ann Neurol. 2005;57:904–913. doi: 10.1002/ana.20504. (doi:10.1002/ana.20504) [DOI] [PubMed] [Google Scholar]
- 81.Fortune B, Demirel S, Bui BV. Multifocal visual evoked potential responses to pattern-reversal, pattern-onset, pattern-offset, and sparse pulse stimuli. Vis Neurosci. 2009;26:227–235. doi: 10.1017/S0952523808080954. (doi:S0952523808080954 [pii] 10.1017/S0952523808080954) [DOI] [PubMed] [Google Scholar]
- 82.Klistorner AI, Graham SL. Effect of eccentricity on pattern-pulse multifocal VEP. Doc Ophthalmol. 2005;110:209–218. doi: 10.1007/s10633-005-7309-1. (doi:10.1007/s10633-005-7309-1) [DOI] [PubMed] [Google Scholar]