Abstract
Background
Whereas thyroid nodules are less common among children than among adults, the anxiety generated by the finding of a thyroid nodule is high because 20% of nodules found in children contain thyroid cancer. Discovery of a nodule in the context of hyperthyroidism is usually comforting due to the presumption that the nodule represents a benign toxic adenoma.
Summary
An 11-year-old girl presented with heavy menses, fatigue, and a right thyroid mass. Laboratory evaluation revealed elevated triiodothyronine and undetectable thyroid-stimulating hormone. Thyroid ultrasonography revealed a 3.5 cm nonhomogenous nodule, and scintigraphy was consistent with an autonomous hyper-functioning nodule. Fine-needle aspiration biopsy could not rule out malignancy, and patient underwent right hemithyroidectomy and isthmusectomy. Pathology was consistent with papillary thyroid carcinoma.
Conclusions
We report the discovery of papillary thyroid carcinoma in an autonomously hyperfunctioning nodule in an 11-year-old girl. Detection of an autonomously functioning thyroid nodule in children and adolescents does not exclude the possibility of thyroid carcinoma and warrants careful evaluation and appropriate therapy.
Introduction
Thyroid cancer is uncommon among children and adolescents. Nevertheless, with the increased use of imaging techniques to evaluate the thyroid, the number of asymptomatic thyroid nodules being detected has escalated (1). Nodules can be solitary or multiple. They can be cystic, solid, or mixed. Thyroid nodules can occur in the presence of autoimmune thyroid disease, but the major concern, particularly in the pediatric population, is whether the nodule harbors malignancy. Incidental identification of microcarcinomas has increased among adults; most are differentiated papillary thyroid carcinomas (PTC). Yet, among adults, the majority of nodules are unlikely to lead to significant morbidity or mortality (2). Even so, identification of a nodule generates anxiety for both patient and clinician regarding the risk for neoplasia.
Thyroid nodules are less common in children than among adults (3). The angst regarding the possibility of thyroid cancer is greater for children and adolescents because 20% of nodules in children are found to contain thyroid cancer (4). Thus, one major goal of the diagnostic evaluation of thyroid nodules is to differentiate thyroid cancers, especially aggressive lesions, from benign adenomas (5). A number of features raise the suspicion regarding malignancy. These features include a history of radiation exposure, family history of thyroid cancer or multiple endocrine neoplasia syndrome, dysphagia, hoarseness, dyspnea, rapid growth of a nodule, and cervical lymphadenopathy (6).
The discovery of a thyroid nodule is usually reassuring within the context of hyperthyroidism associated with a suppressed thyroid-stimulating hormone (TSH), as the thyroid nodule is presumed to be a toxic adenoma. Almost all are benign and surgical excision is an effective and definitive treatment (7). Indeed, algorithms for management of thyroid nodules in children indicate that in the presence of suppressed TSH levels and a hot nodule, fine-needle aspiration biopsy (FNAB) is not necessary (7). Here, we present an 11-year-old girl with a hot nodule found to contain PTC. Informed consent was obtained for this case report.
Patient
An 11-year-old Caucasian girl presented to a local gynecologist for evaluation of heavy menses and fatigue. On physical examination, she was noted to have a right thyroid mass. She was referred to the Pediatric Endocrinology Division at the Children's Hospital of Pittsburgh of University of Pittsburgh Medical Center for additional evaluation. The referring physician had obtained a thyroid ultrasonography and thyroid scintigraphy. The thyroid ultrasonography showed a large nonhomogenous 3.5 cm nodule in the right lobe, a smaller nodule in upper portion of the right lobe, and several hypoechoic nodules in the left lobe. The thyroid scintigraphy revealed an uptake of 18% (low normal) with predominant autonomous activity in the right lower lobe.
At the time of her evaluation at the Children's Hospital of Pittsburgh, no change in her appetite or weight had been noted. She denied hoarseness, heat intolerance, changes in bowel habits, hair loss, difficulty swallowing, or prior radiation exposure. Her medical history included a normal birth history, tonsillectomy and adenoidectomy, and an episode of pneumonia at 3 years of age. Her review of systems was otherwise unremarkable. Her family history was significant for a right thyroidectomy in her mother for a noncancerous tumor and Graves' disease in a maternal aunt. No history was available regarding her father's family.
Upon initial evaluation, her height was 163.6 cm (>97th percentile), her weight was 77.8 kg (>97th percentile), and body mass index was 29.1 kg/m2 (>97th percentile; Z score 2.23). Her blood pressure was 142/82 and her heart rate 106. She had a visible swelling of the right lobe of her thyroid with a palpable nontender 1.5 × 1 cm nodule. The thyroid moved with swallowing. No lymph nodes were palpated. She had no lid lag or tongue fasciculations. Heart, lung, and abdominal examinations were normal. On neurological examination she had normal deep tendon reflexes and no weakness. Her skin was warm and moist. She had Tanner stage V breast development, and Tanner stage IV pubic hair. No acanthosis nigricans was noted.
Thyroid function tests revealed that her TSH was undetectable, free thyroxine was 1.14 ng/dL (reference range, 0.73–1.84; International System of Units [SI], 14.6 pmol/L), and her triiodothyronine (T3) was 384 ng/dL (reference range, 123–211; SI, 5.90 nmol/L). Her thyroid-stimulating hormone receptor antibody (TRAb) was undetectable (<6%). Her thyroid peroxidase antibody was 6 IU/mL (negative) and her thyroglobulin antibody was 21 IU/mL (negative). Her thyroglobulin concentration was 47 ng/mL (reference range, 3–40; SI, 47 mcg/L).
Correlation of her clinical features, imaging results, and her laboratory data led to the conclusion that she had an autonomous hot nodule. She was referred to pediatric surgery for excision of the autonomously functioning thyroid nodule (AFTN). Due to the remote possibility of a thyroid malignancy, a fine-needle aspiration was performed in the surgeon's office. The features noted in the aspirate did not fit a specific diagnostic pattern. The differential diagnosis included a cellular hyperplastic nodule or neoplasia. Although no definitive features diagnostic of PTC were observed, carcinoma could not be completely excluded based on the results of the aspirate. Thus, based on the clinical finding of a complex solid and cystic 3 cm nodule and the nonspecific cytologic pattern, conservative excision was recommended.
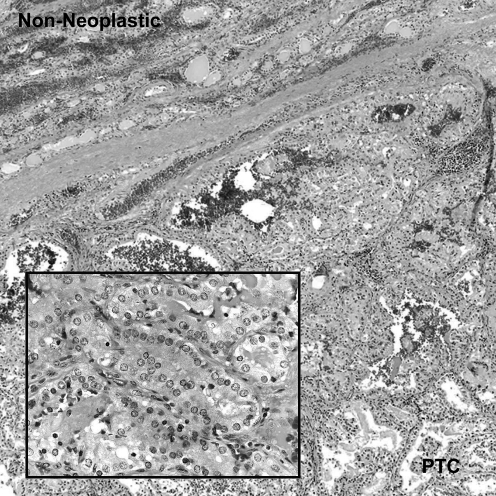
Subsequently, she underwent a right thyroid lobectomy and isthmusectomy. The microscopic sections showed an encapsulated neoplastic nodule containing central papillary structures and a peripheral follicular growth pattern (Fig. 1). The follicular tumor cells showed characteristic nuclear features of PTC including crowding or overlapping, grooves, pale or cleared chromatin, and occasional intranuclear pseudoinclusions. In some areas, the neoplastic cells demonstrated a tall cell pattern. A rare psammoma body was noted. The non-neoplastic thyroid tissue consisted of variable-sized follicles. Scattered lymphoid aggregates, including some with germinal centers, were present in the non-neoplastic parenchyma, and intraepithelial lymphocytes were noted in some adjacent follicles. The final pathologic diagnoses were (i) PTC with follicular and tall cell areas and (ii) focal lymphocytic thyroiditis.
FIG. 1.
At low magnification, the PTC, which has a predominantly follicular pattern, is separated from the non-neoplastic thyroid by a fibrous capsule. The high-magnification inset demonstrates the classic nuclear features of PTC including crowding and overlapping, pale chromatin, nuclear grooves, and a pseudoinclusion (hematoxylin and eosin; original magnifications: 40 × ; inset 400 × ). PTC, papillary thyroid carcinoma.
Upon diagnosis of PTC, additional evaluation was conducted. Chest X-ray showed no evidence of metastatic disease. Approximately 2 weeks after her initial surgery, a completion thyroidectomy was performed. The final pathology from the left thyroid completion thyroidectomy showed multinodular hyperplasia with lymphocytic thyroiditis and no PTC. Subsequently, she underwent 131I radioactive ablation. Her pretreatment scan showed intense iodine-avid tissue in the neck. All iodine-avid tissue appeared to be located within the thyroid bed. There was no evidence of metastatic disease. One year after the thyroidectomy, thyroid scan showed no significant uptake in the thyroid bed or elsewhere in her body, and thyroglobulin concentration was <0.2 ng/mL (reference range, 2–35; SI, <0.2 mcg/L) consistent with remission. She is maintained on thyroid hormone replacement therapy for thyroidectomy and postablative hypothyroidism.
Thyroid mutation panel testing performed on the surgical specimen was negative for mutations in BRAF, NRAS61, HRAS61, and KRAS12/13. Similarly, fluorescent in situ hybridization (FISH) testing was negative for rearranged during transfection proto-oncogene (RET)/PTC rearrangement.
Discussion
This 11-year-old girl presented with a hyperfunctioning thyroid nodule, undetectable TSH concentration, and an elevated T3 concentration. Her clinical presentation was consistent with T3 toxicosis. According to Dinauer et al.'s algorithm for thyroid nodule management in children, medical or surgical therapy is currently recommended for hot nodules (7), as the probability for malignancy in hot nodules in children and adolescents is considered to be very low (8). Alexander echoed that opinion, stating that toxic adenomas are “almost universally benign, and management is primarily directed at hormone control” (2). Consistent with these recommendations, our patient was referred to a pediatric surgeon for definitive surgical management of her AFTN. Surprisingly, FNAB was suspicious for thyroid malignancy. Subsequently, she underwent hemi-thyroidectomy at which time PTC was identified. In the presence of thyroid nodules in the left lobe and the suspicious FNAB from the right lobe, an initial total thyroidectomy could have been justified in our patient. On the other hand, the pathology report on the fine-needle aspirate was suspicious, but not diagnostic for PTC validating the surgeon's decision to perform a lobectomy and potentially avoid permanent hypothyroidism.
Thyroid cancer is considered to be rare among children, particularly in cases of hyperthyroidism due to AFTNs. A low frequency (0.34%) of thyroid cancer in AFTNs was reported for 296 adult Turkish patients, with mean age 54.9 ± 12.4 years, leading the authors to conclude that AFTNs can be treated by 131I radioactive ablation and do not require routine FNAB (9). Nevertheless, several case reports describe thyroid cancer found within hyperfunctioning nodules (10–14). One Japanese study reported that among 17 patients, aged 13–68 years, with AFTNs, thyroid carcinoma was identified in two individuals (1 follicular variant of papillary carcinoma and 1 follicular carcinoma); both children included in this series had benign adenomas (15). There was no correlation of nodule size with thyroid function (15). Ascertained based on a diagnosis of hyperthyroidism, 263/325 Turkish adults were found to have thyroid nodules; thyroid carcinoma was identified in 6.4% of patients with AFTNs, 16% of patients with toxic multinodular goiters, and in 12.6% of patients with Graves' disease (16). Among American children with AFTNs, well-differentiated thyroid carcinoma was identified in 6/53 (11.3%) children (17). Even though finding a thyroid carcinoma located within an AFTN is infrequent, these reports support our recommendation that AFTNs deserve thorough evaluation in all age groups, including children.
Although early reports suggested that concomitant Graves' disease and thyroid cancer were uncommon, the reported frequency of thyroid cancer among patients presenting with Graves' disease varies (18,19). Cantalamessa et al. reported that 106 (33.6%) of 315 patients with Graves' disease had thyroid nodules >8 mm identified by ultrasonography. Since only a single patient was found to have thyroid cancer, these investigators concluded that thyroid cancer rarely coexists with Graves' disease (20). In another series involving 3502 adult patients with hyperthyroidism treated by thyroidectomy, thyroid cancer was identified in one of 265 patients found to have AFTNs (21).
Yet, other reports suggest a higher incidence of thyroid cancer within nodules detected in adult patients with Graves' disease. Thyroid nodules were detected in 86 of 245 consecutive previously untreated Korean adults with hyperthyroidism associated with diffuse goiters; 8 PTC were identified in the 62 patients who had undergone FNAB (22). Thyroid ultrasonography identified nodules in 277 of 32,200 Japanese patients with Graves' disease evaluated over a 10-year period; PTC was diagnosed in 154 (55.6%) of these patients (23). Of 720 Greek patients ascertained by a diagnosis of thyroid cancer, 60 had a contemporaneous diagnosis of hyperthyroidism with the hyperthyroidism being attributed to a solitary AFTN in 17 of these 60 patients. Thyroid carcinoma was identified within the solitary hot nodule in 10/17 (58.8%) patients in this series (24). Thus, frequencies of thyroid carcinoma identified within a solitary AFTN were high among the adult subjects described in these two large series (23,24). Among 25 children and adolescents aged 11–20 years with Graves' disease treated by total thyroidectomy, only 2 (8%) were found to have thyroid carcinomas (25).
Despite the central role of activating TRAb in the pathogenesis of Graves' disease, the significance of these antibodies in the genesis of thyroid cancer is unclear. Cantalamessa et al. found no apparent relationship between TRAb concentrations and nodules in their patient population (20). Activating TSH receptor (TSHR) gene mutations are associated with hyperthyroidism. In rare instances, activating TSHR mutations have been identified in patients with concomitant AFTN and thyroid carcinomas (26,27). A somatic cell heterozygous TSHR mutation predicted to generate a missense mutation, M453T, was identified in a thyroid nodule containing papillary carcinoma that had been excised from an 11-year-old girl with hyperthyroidism, negative thyroid stimulating immunoglobulins (TSI), and hyperfunctioning nodule on scintigraphy (28). This case is reminiscent of our patient. Genetic testing for the common thyroid cancer mutations, including BRAF and RET/PTC rearrangements, in our patient was negative. Unfortunately, molecular genetic analysis for TSHR mutation was not available for our patient.
Thus, the presence of a thyroid nodule, especially in children and adolescents, raises concern regarding thyroid cancer. In our patient, a suppressed TSH and hyperfunctioning nodule on a radionuclide scan suggested an AFTN, which upon further evaluation was found to contain PTC. This case illustrates that thyroid cancer can occur in children with hyperthyroidism due to AFTNs. Hence, we caution others that diagnosis of an AFTN does not exclude the possibility of thyroid carcinoma in children and adolescents. Such children deserve careful evaluation and appropriate targeted treatment (29–31).
Footnotes
This work was presented in part at the 90th Annual Endocrine Society Meeting in June 2008 in San Francisco, California.
Acknowledgments
This work was supported in part by funding from the National Institutes of Health (T32-DK007729) and the David Nicholas Scholarship.
Disclosure Statement
The authors have nothing to disclose. No competing financial interests exist.
References
- 1.Gharib H. Papini E. Paschke R. Thyroid nodules: a review of current guidelines, practices, and prospects. Eur J Endocrinol. 2008;159:493–505. doi: 10.1530/EJE-08-0135. [DOI] [PubMed] [Google Scholar]
- 2.Alexander EK. Approach to the patient with a cytologically indeterminate thyroid nodule. J Clin Endocrinol Metab. 2008;93:4175–4182. doi: 10.1210/jc.2008-1328. [DOI] [PubMed] [Google Scholar]
- 3.Niedziela M. Pathogenesis, diagnosis and management of thyroid nodules in children. Endocr Relat Cancer. 2006;13:427–453. doi: 10.1677/erc.1.00882. [DOI] [PubMed] [Google Scholar]
- 4.Hung W. Solitary thyroid nodules in 93 children and adolescents. a 35-years experience. Horm Res. 1999;52:15–18. doi: 10.1159/000023426. [DOI] [PubMed] [Google Scholar]
- 5.Yeung MJ. Serpell JW. Management of the solitary thyroid nodule. Oncologist. 2008;13:105–112. doi: 10.1634/theoncologist.2007-0212. [DOI] [PubMed] [Google Scholar]
- 6.Dinauer C. Francis GL. Thyroid cancer in children. Endocrinol Metab Clin North Am. 2007;36:779–806. doi: 10.1016/j.ecl.2007.04.002. vii. [DOI] [PubMed] [Google Scholar]
- 7.Dinauer CA. Breuer C. Rivkees SA. Differentiated thyroid cancer in children: diagnosis and management. Curr Opin Oncol. 2008;20:59–65. doi: 10.1097/CCO.0b013e3282f30220. [DOI] [PubMed] [Google Scholar]
- 8.Ruggieri M. Scocchera F. Genderini M. Mascaro A. Luongo B. Paolini A. Hyperthyroidism and concurrent thyroid carcinoma. Eur Rev Med Pharmacol Sci. 1999;3:265–268. [PubMed] [Google Scholar]
- 9.Erdogan MF. Anil C. Ozer D. Kamel N. Erdogan G. Is it useful to routinely biopsy hot nodules in iodine deficient areas? J Endocrinol Invest. 2003;26:128–131. doi: 10.1007/BF03345140. [DOI] [PubMed] [Google Scholar]
- 10.Uludag M. Yetkin G. Citgez B. Isgor A. Basak T. Autonomously functioning thyroid nodule treated with radioactive iodine and later diagnosed as papillary thyroid cancer. Hormones (Athens) 2008;7:175–179. doi: 10.1007/BF03401510. [DOI] [PubMed] [Google Scholar]
- 11.Kim TS. Asato R. Akamizu T. Harada D. Nakashima Y. Higashi T. Yamamoto N. Tamura Y. Tamaki H. Hirano S. Tanaka S. Ito J. A rare case of hyperfunctioning papillary carcinoma of the thyroid gland. Acta Otolaryngol Suppl. 2007;557:55–57. doi: 10.1080/03655230601066785. [DOI] [PubMed] [Google Scholar]
- 12.Nishida AT. Hirano S. Asato R. Tanaka S. Kitani Y. Honda N. Fujiki N. Miyata K. Fukushima H. Ito J. Multifocal hyperfunctioning thyroid carcinoma without metastases. Auris Nasus Larynx. 2008;35:432–436. doi: 10.1016/j.anl.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Bitterman A. Uri O. Levanon A. Baron E. Lefel O. Cohen O. Thyroid carcinoma presenting as a hot nodule. Otolaryngol Head Neck Surg. 2006;134:888–889. doi: 10.1016/j.otohns.2005.03.054. [DOI] [PubMed] [Google Scholar]
- 14.Majima T. Doi K. Komatsu Y. Itoh H. Fukao A. Shigemoto M. Takagi C. Corners J. Mizuta N. Kato R. Nakao K. Papillary thyroid carcinoma without metastases manifesting as an autonomously functioning thyroid nodule. Endocr J. 2005;52:309–316. doi: 10.1507/endocrj.52.309. [DOI] [PubMed] [Google Scholar]
- 15.Mizukami Y. Michigishi T. Nonomura A. Yokoyama K. Noguchi M. Hashimoto T. Nakamura S. Ishizaki T. Autonomously functioning (hot) nodule of the thyroid gland. A clinical and histopathologic study of 17 cases. Am J Clin Pathol. 1994;101:29–35. doi: 10.1093/ajcp/101.1.29. [DOI] [PubMed] [Google Scholar]
- 16.Gul K. Di Ri Koc A. Ki Yak G. Ersoy PE. Ugras NS. Ozdemi D. Ersoy R. Cakir B. Thyroid carcinoma risk in patients with hyperthyroidism and role of preoperative cytology in diagnosis. Minerva Endocrinol. 2009;34:281–288. [PubMed] [Google Scholar]
- 17.Croom RD., 3rd Thomas CG., Jr. Reddick RL. Tawil MT. Autonomously functioning thyroid nodules in childhood and adolescence. Surgery. 1987;102:1101–1108. [PubMed] [Google Scholar]
- 18.Dobyns BM. Sheline GE. Workman JB. Tompkins EA. McConahey WM. Becker DV. Malignant and benign neoplasms of the thyroid in patients treated for hyperthyroidism: a report of the cooperative thyrotoxicosis therapy follow-up study. J Clin Endocrinol Metab. 1974;38:976–998. doi: 10.1210/jcem-38-6-976. [DOI] [PubMed] [Google Scholar]
- 19.Belfiore A. Russo D. Vigneri R. Filetti S. Graves' disease, thyroid nodules and thyroid cancer. Clin Endocrinol (Oxf ) 2001;55:711–718. doi: 10.1046/j.1365-2265.2001.01415.x. [DOI] [PubMed] [Google Scholar]
- 20.Cantalamessa L. Baldini M. Orsatti A. Meroni L. Amodei V. Castagnone D. Thyroid nodules in Graves disease and the risk of thyroid carcinoma. Arch Intern Med. 1999;159:1705–1708. doi: 10.1001/archinte.159.15.1705. [DOI] [PubMed] [Google Scholar]
- 21.Chao TC. Lin JD. Jeng LB. Chen MF. Thyroid cancer with concurrent hyperthyroidism. Arch Surg. 1999;134:130–134. doi: 10.1001/archsurg.134.2.130. [DOI] [PubMed] [Google Scholar]
- 22.Kim WB. Han SM. Kim TY. Nam-Goong IS. Gong G. Lee HK. Hong SJ. Shong YK. Ultrasonographic screening for detection of thyroid cancer in patients with Graves' disease. Clin Endocrinol (Oxf ) 2004;60:719–725. doi: 10.1111/j.1365-2265.2004.02043.x. [DOI] [PubMed] [Google Scholar]
- 23.Yano Y. Shibuya H. Kitagawa W. Nagahama M. Sugino K. Ito K. Ito K. Recent outcome of Graves' disease patients with papillary thyroid cancer. Eur J Endocrinol. 2007;157:325–329. doi: 10.1530/EJE-07-0136. [DOI] [PubMed] [Google Scholar]
- 24.Pazaitou-Panayiotou K. Perros P. Boudina M. Siardos G. Drimonitis A. Patakiouta F. Vainas I. Mortality from thyroid cancer in patients with hyperthyroidism: the Theagenion Cancer Hospital experience. Eur J Endocrinol. 2008;159:799–803. doi: 10.1530/EJE-08-0468. [DOI] [PubMed] [Google Scholar]
- 25.Shapiro SJ. Friedman NB. Perzik SL. Catz B. Incidence of thyroid carcinoma in Graves' disease. Cancer. 1970;26:1261–1270. doi: 10.1002/1097-0142(197012)26:6<1261::aid-cncr2820260613>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 26.Niepomniszcze H. Suarez H. Pitoia F. Pignatta A. Danilowicz K. Manavela M. Elsner B. Bruno OD. Follicular carcinoma presenting as autonomous functioning thyroid nodule and containing an activating mutation of the TSH receptor (T620I) and a mutation of the Ki-RAS (G12C) genes. Thyroid. 2006;16:497–503. doi: 10.1089/thy.2006.16.497. [DOI] [PubMed] [Google Scholar]
- 27.Camacho P. Gordon D. Chiefari E. Yong S. DeJong S. Pitale S. Russo D. Filetti S. A Phe 486 thyrotropin receptor mutation in an autonomously functioning follicular carcinoma that was causing hyperthyroidism. Thyroid. 2000;10:1009–1012. doi: 10.1089/thy.2000.10.1009. [DOI] [PubMed] [Google Scholar]
- 28.Mircescu H. Parma J. Huot C. Deal C. Oligny LL. Vassart G. Van Vliet G. Hyperfunctioning malignant thyroid nodule in an 11-year-old girl: pathologic and molecular studies. J Pediatr. 2000;137:585–587. doi: 10.1067/mpd.2000.108437. [DOI] [PubMed] [Google Scholar]
- 29.Hopwood NJ. Carroll RG. Kenny FM. Foley TP., Jr. Functioning thyroid masses in childhood and adolescence. Clinical, surgical, and pathologic correlations. J Pediatr. 1976;89:710–718. doi: 10.1016/s0022-3476(76)80788-3. [DOI] [PubMed] [Google Scholar]
- 30.De Rosa G. Testa A. Maurizi M. Satta MA. Aimoni C. Artuso A. Silvestri E. Rufini V. Troncone L. Thyroid carcinoma mimicking a toxic adenoma. Eur J Nucl Med. 1990;17:179–184. doi: 10.1007/BF00811447. [DOI] [PubMed] [Google Scholar]
- 31.Nikiforova MN. Nikiforov YE. Molecular genetics of thyroid cancer: implications for diagnosis, treatment and prognosis. Expert Rev Mol Diagn. 2008;8:83–95. doi: 10.1586/14737159.8.1.83. [DOI] [PubMed] [Google Scholar]