Abstract
The liver is unique for its ability to regenerate after injury, however, critical injuries or disease cause it to lose this quality. Stem cells have been explored as a possibility to restore the function of seriously damaged livers, based on their self-renewability and multiple differentiation capacity. These experiments examine the ability of muscle derived stem cells (MDSCs) to differentiate into hepatocyte-like cells in vitro and acquire functional liver attributes for repairing damaged livers. In vitro experiments were performed using MDSCs from postnatal mice and mouse hepatocyte cell lines. Our data revealed that MDSCs differentiated into hepatocyte-like cells and expressed liver cell markers, albumin, hepatocyte nuclear factor 4α, and alpha feto-protein, both at the RNA and protein level. Additionally, in vivo studies showed successful engraftment of MDSCs into hepatectomized mouse livers of mice. These results provide evidence suggesting that MDSCs have the capacity to differentiate into liver cell-like cells and may serve as potential candidates to aid in liver regeneration.
Keywords: Liver, Hepatectomy, Muscle Derived Stem Cells, Differentiation
Introduction
The liver plays a central role in metabolic processes such as the synthesis of serum proteins (e.g. albumin), storage of glycogen, maintaining homeostasis, and immunological functions [1]. The most notable characteristic of the liver is its regenerative ability and investigations have shown that the liver can regenerate to its full size even after a 67% hepatectomy [2-3]. Additionally, studies have shown that liver transplantations into comparatively larger or smaller recipients resulted in the transplanted liver adjusting to the appropriate size needed for the recipient. The liver's unique regenerative capacity is often impeded when it is seriously diseased or damaged. Chronic liver injury impairs the liver's functions by causing extracellular matrix proteins such as fibrillar collagens type I and type III to accumulate, while the liver attempts to regenerate itself [4-5]. An increased production of collagen fibers, known as fibrosis, is caused by the activation of hepatic stellate cells (HSC), which proliferate and undergo collagen synthesis [6]. Persistent injury to the liver by alcohol abuse or hepatitis B and C, will transform the liver architecture, into an irregularly nodular rather than a uniformly smooth organ [5, 7]. At advanced stages of liver disorder, the end stage of liver failure is known as cirrhosis, where liver transplants or hepatocyte transplantations are the only options for survival [5, 8]. Since not every patient with liver disease immediately qualifies for transplant surgery, alternative treatments are used to delay the progression of the disease or even reverse it [9-10].
Potential therapies to regenerate damaged liver tissue and improve its function include stem cell therapy, which has stirred immense interest because of their capacity of self-renewal and multipotency [11]. Investigators have found promising results showing human and mouse embryonic stem (ES) cells and adult stem cells derived from bone marrow, skin, adipose tissue and even the liver can differentiate in vitro into hepatocyte-like cells [12-15]. In vivo experiments have proven that murine ES cells can incorporate themselves into hepatectomized livers [16-17]. Unfortunately, the limitation of using ES cells is their potential to develop teratomas after transplantation. However, the incidence for this occurrence was reduced as ES cells from embryoid bodies were allowed to differentiate into hepatocyte-like cells [18]. Adult stem cells have also been used to enhance regeneration of hepatectomized livers derived from bone marrow and adipose tissue, where results did not indicate teratoma formation or immune-rejection from autologous transplantation [19-21].
One group of stem cells isolated from post-natal skeletal muscle tissue has been investigated for its impressive mutlilineage differentiation capacity and self-renewal ability [22]. These cells were isolated from muscle tissue using a modified preplate technique by enzymatic dissociation from a muscle biopsy and divided into 6 populations, based on adhesion characteristics in collagen coated flasks [23-24]. Later pre-plates were regarded as muscle derived stem cells (MDSCs) and identified using flow cytometry for the expression of stem cell antigen 1 (Sca1), CD34, fetal liver kinase 1 (Flk1) and measurable amounts of desmin, but not c-kit nor CD45 [22, 25-26]. Experimental studies have demonstrated the multipotency of MDSCs through differentiation into cell lineages of the three germ layers: mesoderm, ectoderm and endoderm. Multiple investigations have revealed differentiation of MDSCs along the mesoderm lineage into osteocytes, adipocytes, chondrocytes and hematopoietic cells [25, 27-29]. Other studies have demonstrated MDSCs differentiation into ectoderm cell lineages by detecting the expression of both neuronal and glial cell markers. Differentiation along the same germ layer as hepatocytes, the endoderm, has been observed through differentiation into urinary bladder cells for the purpose of treating patients suffering from urinary incontinence [30-33]. The non-invasive isolation procedure of MDSCs has made them advantageous for self-autologous cell transplantation therapies. The purpose of this study was to examine the ability of MDSCs to differentiation into liver-like cells through co-culturing with hepatocyte cell-lines and identifying their expression of specific liver cell markers. Furthermore, in vivo studies were used to determine whether MDSCs could successfully engraft into hepatectomized mouse livers for repair.
Materials and methods
MDSCs isolation and culture
Muscle tissue was isolated from the gastrocnemius muscle of C5710J, wildtype neonatal male mice (3 - 4 weeks old). MDSCs isolation was performed by tissue digestion using a series of collagenase, dispase, and trypsin enzymes. Separation was performed based on their adhesion characteristics using a modified preplate technique [23-24]. Following isolation, a pre-plated population of MDSCs was cultured in proliferation media made of Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% Fetal Bovine Serum (FBS), 10% Horse Serum (HS), 1% Penicillin-Streptomycin (P/S) antibiotics, and 0.5% chicken embryo extract (CEE); and incubated in 5% CO2 at 37°C.
Retroviral LacZ transfection
A LacZ retrovirus was isolated from the Tel-6 cell line with a titer of 8×107 and diluted with proliferation medium [35]. 1 μg/mL of polybrene stock solution (8 mg/mL) was added for a final concentration of 8 μg/mL polybrene. This mixture was added to the MDSCs and incubated at 37°C for 6 hours. This process was repeated three times to ensure maximal yield gene transfer efficiency to produce LacZ-MDSCs.
LacZ staining
LacZ staining was performed by fixing cells with buffered 4% formalin for 5 minutes. Afterward, cells were incubated with X-gal diluted 1:50 in the LacZ staining solution (K4Fe(CN)6 [0.5mmol/L], K3Fe(CN)6 [0.5 mmol/L], MgCl2 [1.0 mmol/L]) for 2 hours at 37°C. LacZ positive cells will express beta-galactosidase (β-Gal) and appear blue under bright field microscopy [35].
Liver cell line culture
Mouse hepatocyte, AML12 (ATCC, Manassas, VA) and tumor hepatocyte lines, Hepa1-6 (ATCC, Manassas, VA) were cultured in DMEM and Ham's F-12 medium at a 1:1 ratio with 10% FBS, 1% P/S, 5μg/mL insulin, 5 μg/mL transferrin, 5 ng/mL sodium selenite and 40 ng/mL dexamethasone.
Cell co-culture
MDSCs were plated in a multi-well dish with either AML12 or Hepa1-6 cells at a ratio of 1:10 (MDSCs:hepatocyte cell line). All cells in the multi-well were incubated with liver cell medium (described above) for periods of 1 and 7 days at 37°C and 5% CO2. MDSCs and hepatocyte lines were also co-cultured using a transwell setup, where MDSCs were plated on 24 mm diameter transwells with 0.4 μm pore size (Corning Inc., NY) placed within a 6 multi-well dish. The hepatocyte cell lines were placed in the bottom of separate wells in the multi-well dish.
Immunocytochemistry
Cells were fixed with 4 % formalin for 5 minutes at room temperature with unspecific binding blocked using HS for 1 hour. Primary antibodies (anti-albumin [1:100, ICN Biomedicals], anti-alpha fetoprotein(αFP) [1:200, Santa Cruz], anti -HNF-4α [1:200, Santa Cruz]) diluted in PBS with 2% HS were incubated overnight at 4°C. Cells were incubated with secondary antibodies (Alexa Fluor 488 anti-rabbit Ab [1:200, Invitrogen Molecular Probes], Alexa Fluor 488 anti-mouse Ab [1:200, Invitrogen Molecular Probes], Cy3 anti-goat Ab [1:200, Sigma]) the following day for 1 hour at room temperature. 4',6diamidine-2-phenylindole (DAPI) was diluted in PBS (100 ng/mL) and administered for 5 minutes. Between each step, cells were washed with PBS. All cells were visualized using a Leica DM IRB fluorescent microscope.
Reverse-Transcription Polymerase Chain Reaction (RT-PCR)
Total RNA was extracted from the cultured MDSCs using the RNeasy plus mini kit (Qiagen), and complimentary DNA was generated using the reverse transcription kit (Retroscript, Ambient Bio Systems) as per the manufacturer's instructions. Polymerase chain reaction (PCR) amplifications were performed using specific sense and antisense primers, shown in Table 1.
Tables.
Primer Used
| αFP | S: 5’ – GTGAAACAGACTTCCTGGTCCT – 3’ |
| A: 5’ – GCCCTACAGACCATGAAACAAG – 3’ | |
| Albumin | S: 5’ – TCAACTGTCAGAGCAGAGAAGC – 3’ |
| A: 5’ – AGACTGCCTTGTGTGGAAGACT – 3’ | |
| CK18 | S: 5’ – TGGTACTCTCCTCAATCTGCTG – 3’ |
| A: 5’ – CTCTGGATTGACTGTGGAAGTG – 3’ | |
| G6P | S: 5’ – CAGGACTGGTTCATCCTT – 3’ |
| A: 5’ – GTTGCTGTAGTAGTCGGT – 3’ | |
| HNF1α | S: 5’ – TTCTAAGCTGAGCCAGCTGCAGACG – 3’ |
| A: 5’ – GCTGAGGTTCTCCGGCTCTTTCAGA – 3’ | |
| TAT | S: 5’ – TCCCGACTGGATAGGTAG – 3’ |
| A: 5’ – ACCTTCAATCCCATCCGA – 3’ | |
| GAPDH | S: 5’ – CCTCTGGAAAGCTGTGGCGT – 3’ |
| A: 5’ – TTGGCAGGTTTCTCCAGGCG – 3’ |
Cell Transplantation
Cell transplantations were performed on 6-8 week old female Nod/LtSz-Prkdcscid mice (The Jackson Laboratory). Initially, mice underwent a 50% hepatectomy, removing left and median lobes, and cells were infused immediately following the surgical procedure through the tail vein. Each mouse received approximately 106 male mouse LacZ-MDSCs. At various time points (3, 7, and 90 days) after cell injections, mice were euthanized, and the liver was collected for histological analysis.
Fluorescence in situ hybridization (FISH)
FISH was performed on sectioned livers as described previously by Matsumoto et al [36]. Briefly, the mouse Y-chromosome-specific probe directly labeled with FITC (ID Laboratories, London, Ontario) and a hybridization mixture were denatured at 75°C for 10 minutes and allowed to reanneal for 60-90 minutes at 37°C following a modification of the manufacturer's protocol. Ten microliters of this mixture was applied to the target area and a plastic cover slip applied carefully. The edges of the cover slip were sealed with rubber cement to prevent evaporation. The slides were stored in a humid chamber at 37°C for 18-24 hours. On day 2, the cover slips were removed and the slides were washed by several steps of 2× sodium chloride-sodium citrate (SSC) for 1 minute and 50% Formamide-2×SSC for 12 minutes and 2×SCC-1% Tween mixture for 5 minutes at 45°C. The nuclei were revealed by counterstaining with DAPI stain (100 ng/ml; Sigma), and all sections were mounted with Vectashield medium (Vector). The hybridized Y-chromosome specific probe was detected using a fluorescent microscope (Nikon E800).
Results
MDSCs differentiation into hepatocyte-like cells by direct cell co-culture
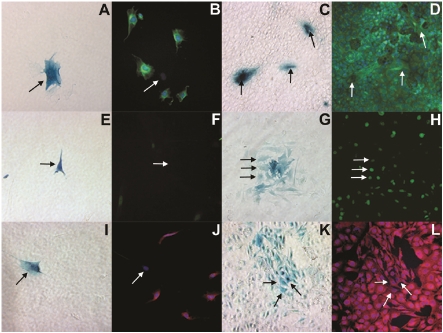
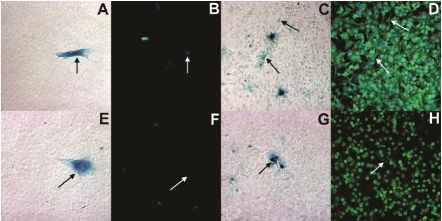
LacZ-MDSCs were plated in a 12 multiwell dish at a 1:10 ratio with a normal mouse hepatocyte cell line (Figure 1), AML12, or a tumurigenic mouse hepatocyte cell line, Hepa1-6 (Figure 2). After 1 and 7 days of co-culture, cells were fixed and fluorescently labeled with liver specific cell markers. Images A, C, E, G, I, and K of figures 1 and 2 were taken using bright field microscopy indicating β-galactosidase (blue) positive MDSCs, while images B, D, F, H, J, and L were taken at the same respective locations using fluorescent microscopy. After 1 day in co-culture with either of the hepatocyte cell lines, MDSCs did not express albumin as illustrated in figure 1B and 2B. By day 7 of co-culture, however, MDSCs begun expressing albumin (green), as shown in Figures 1D and 2D.
Figure 1.
Bright field (A, C, E, G, I, K) and fluorescent microscopy (B, D, F, H, J, L) images demonstrating MDSCs differentiation into hepatocyte-like cells when co-cultured with the AML12 hepatocyte cell line after 1 day (A, B, E, F, I, J) and 7 days (C, D, G, H, K, L). In the bright field images, MDSCs are denoted by β-galactosidase expression (blue) and by black arrows whereas in fluorescent images, they are denoted by white arrows at the same location. In the fluorescent images, the co-cultured cells are stained for albumin (green: B, D), HNF4α (green: F, H) and αFP (red: J, L). The cell nuclei were stained with DAPI (blue).
Figure 2.
Bright field (A, C, E, G) and fluorescent microscopy (B, D, F, H) images demonstrating MDSCs differentiation into hepatocyte-like cells when co-cultured with the Hepa1-6 hepatocyte cell line after 1 day (A, B, E, F) and 7 days (C, D, G, H). MDSCs are denoted by β-galactosidase expression (blue) and black arrows in bright field microscopy images. Fluorescent images, were taken at the exact location, where MDSCs are denoted by white arrows and stained for albumin (green: B, D) and HNF4α (red: F, H). The cell nuclei were stained with DAPI (blue).
Similar results were found for MDSCs labeled for hepatocyte nuclear factor 4 alpha (HNF4α) as shown in Figures 1 and 2, E through H. On day 7 of co-culturing MDSCs with either AML12 or Hepa1-6 cells, MDSCs began expressing HNF4α in the cell nucleus, as shown in Figure 1H and 2H with the white arrows. HNF4α is a nuclear transcription factor that binds to DNA as a homodimer to control the expression of several genes including the hepatocyte nuclear factor 1 (HNF1α), which is another transcription factor that regulates the expression of several hepatic genes. In comparison with day 7, MDSCs co-cultured with either AML12 (Figures 1E & 1F) or Hepa1-6 (Figures 2E & 2F) cells for only 1 day were negative for HNF4α expression, where black arrows show the location for the β-galactosidase positive MDSCs (Figures 1E & 2E) in relation to the mouse liver cells, and the white arrows show the location of the MDSCs (Figure 1F & 2F).
αFP expression with muscle derived stem cells is shown in Figure 1 when co-cultured with AML12 cells. On day 1 of co-culture, β-galactosidase positive MDSCs (Figure 1I, black arrow) do not express αFP (Figure 1J), while the cell nuclei are stained with DAPI (blue). By day 7, MDSCs began to express αFP, similarly to the AML12 cells (Figures 1K & 1L).
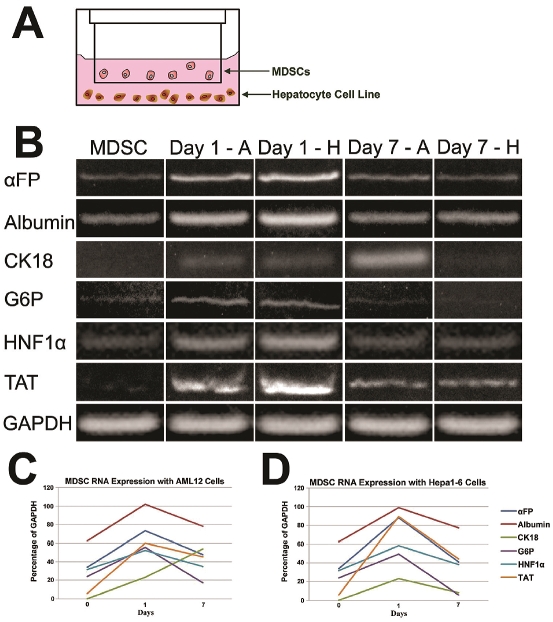
MDSCs differentiation into hepatocyte-like cells using transwell cell co-culture
Using a transwell setup, hepatocyte cell lines, AML12 or Hepa1-6 plated at the bottom of well dishes were separated from MDSCs by transwell inserts and co-cultured for either 1 or 7 days (Figure 3A). The temporal expression of hepatocyte marker genes, such as αFP, albumin, CK18, G6P, TAT and hepatocyte nuclear factor (HNF)-1α were analyzed by RT-PCR (Figure 3B). The expression of these liver marker genes in MDSCs alone was very minimal, except in CK18 and TAT, where their expression was not detected. One day following the co-culture of co-culture, the expression of most genes was MDSCs with both hepatocyte cell lines, the ex-reduced in MDSCs, with the exception of MDSCs pression of all the liver marker genes was no-co-cultured with AML12 cells, where CK18 exticeably upregulated. However, after 7 days of pression increased (Figures 3C & 3D). GAPDH was used as a loading control.
Figure 3.
Representation of the transwell co-culture setup where MDSCs were placed in the transwell and the hepatocyte cell lines (AML12 and Hepa1-6) were placed in the bottom of the multiwell dish. Gene expression profile of mouse MDSCs co-culture with either AML12 (Day 1 - A and Day 7 - A) or Hepa1-6 (Day 1 - H and Day 7 - H) hepatocyte cell lines using a transwell plate after 1 and 7 days (B). The expression of hepatocyte cell markers in MDSCs when co-cultured with AML12 (C) and Hepa1-6 (D) cell lines for different periods of time are shown with normalization to the loading control, GAPDH.
MDSCs differentiation into hepatocyte-like cells in vivo
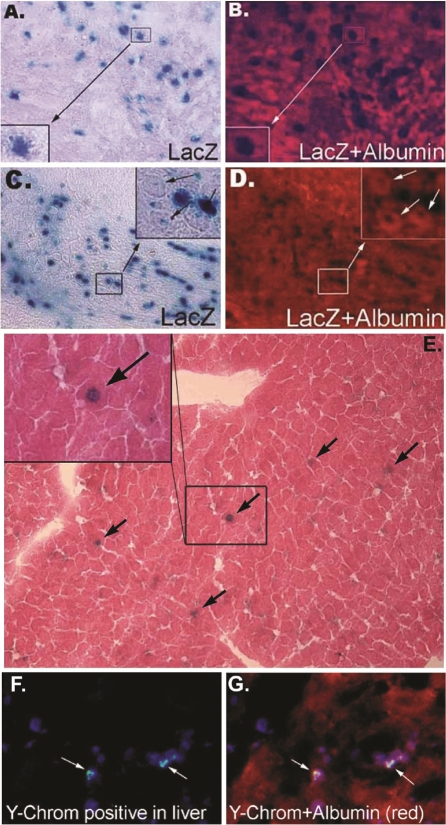
Male LacZ-MDSCs were transplanted (106 cells) into 6-8 week old female mice through the tail vein following a 50% hepatectomy to remove the left and median lobes of the liver. Three and 7 days after cell transplantation, mice were euthanized, and their livers were extracted. Livers were sectioned onto slides and stained for β-galactosidase (blue) at both 3 (Figure 4A) and 7 (Figure 4C) days after cell transplantation. MDSCs also began expressing liver specific marker, albumin (red), 3 (Figure 4B) and 7 (Figure 4D) days after transplantation. These results demonstrate MDSCs survival and engraftment into the remaining liver tissue after the partial hepatectomy.
Figure 4.
MDSCs differentiate into liver-like cells in vivo. Images of liver tissue harvested 3 days after MDSCs transplantation and stained for β-galactosidase (A, blue) or albumin (B, red). Similarly, liver tissue harvested 7 days after MDSC transplantation was also stained for β-galactosidase (C, blue) or albumin (D, red). Long term MDSCs engraftment into hepatectomized livers was observed after 3 months (E). As indicated by the black arrows, β-galactosidase positive (blue) MDSCs are found in the liver tissue subjected to hemotoxylin and eosin staining. A magnified image is shown in the inset of this figure. FISH analysis revealing the donor signal (Ychromosome) within injured liver tissue of female recipients, 7 days after male MDSCs were transplanted (F, G). MDSCs were stained for cell nuclei, blue; Y-chromosome, green; and albumin, red.
After 3 months following liver hepatectomy and MDSCs transplantation, mouse livers were extracted and sectioned to examine long term cell survival. As illustrated in Figure 4E, sectioned mouse livers were subjected to hemotoxylin and eosin staining and stained for β-galactosidase. The positive expression of β-galactosidase (dark blue) indicated the presence of MDSCs within the liver after 3 months from the initial cell transplantation. Magnification of this is shown in the inset of Figure 4E. As described above, the donor MDSCs were obtained from male mice and injected into female mice 24 hours following partial hepatectomy. FISH analysis revealed Y chromosomes within the nuclei of hepatocytes (albumin-positive cells) within the injured liver tissue of female recipients (Figures 4F & 4G), 7 days after male MDSCs transplantation.
Discussion
A number of studies have examined the differentiation of adult and embryonic stem cells isolated from mice and humans into hepatocyte-like cells [12-15]. Generally, the methods used to differentiate stem cells can be divided into several categories, including different serum-free and serum-rich medias, co-culture with hepatocyte-like cells, transfection of a liver-specific gene through a viral vector or a combination of growth factors [37-43]. Both types of stem cells, when transplanted into the animal recipients, were found to improve the function of damaged livers. While adult stem cells are a promising option for cellular transplantations since they do not invoke an immune response, they are, however, challenged by the limited number of stem cells that may be isolated from a patient's tissue [44]. The benefit of MDSCs for cell based therapies is their easy accessibility through non-invasive procedures and multi-differentiation capacity. The MDSCs used in this report have previously been demonstrated to have the multipotency characteristic of stem cells by differentiating into myogenic, hematopoietic, osteogenic, adipogenic, chondrogenic, neural and endothelial cells [30].
In the present study, MDSCs demonstrated their potential to differentiate into hepatocyte-like cells using a co-culture system with two different liver cell types. The results of our immunocytochemistry investigations (Figure 1 & 2) illustrated the MDSCs protein expression is similar to that of AML12 and Hepa1-6 cells. Day 1 of MDSCs co-cultured with AML12 or Hepa1-6 cells showed no expression of liver marker proteins: albumin, αFP, and HNF-4α. At longer times of co-culturing (7 days), MDSCs had comparable expression of liver specific proteins to both hepatocyte cell lines. Because we initially transfected the MDSCs with a LacZ viral vector, we were able to distinguish MDSCs from the hepatocytes. Furthermore, these images show no indication of cell fusion of a β-galactosidase positive MDSCs with LacZ negative cell hepatocytes. Our qualitative results demonstrate that MDSCs differentiate into liver-like cells without fusion, as mentioned in previous publications where hematopoietic stem cells and bone marrow derived cells were shown to differentiate into hepatocyte-like cells of rodents [20, 45]. However, conflicting data has also indicated that transplanted bone marrow cells aid in regenerating the damaged livers of mice through fusion with local hepatocytes [46-47]. While the use of similar cell types repaired liver damage through two different mechanisms (fusion/no fusion), ultimately the model used for liver damage may influence the regenerative process of the transplanted cells. For instance, in the studies where transplanted cells facilitated regeneration by cell fusion and not differentiation, FAH-deficient mice were examined. Thus, the observed fusion most likely resulted from a genetic alteration. In studies where cell differentiation contributed to liver repair, the damaged liver model under investigation was either a partial hepatectomy or chemically induced liver damage by carbon tetrachloride. These results suggest that perhaps adult stem cells have different fundamental signaling patterns to determine which underlying mechanism is impaired and proceed with the proper method to restore function to the liver.
The results of the transwell co-culture further provide evidence that the MDSCs begin to express hepatocyte cell markers when not in contact with two different hepatocyte cell lines (AML12 and Hepa1-6). As early as 1 day following co-culture, an upregulation in the RNA of liver cell markers are found within MDSCs. By day 7 of co-culture, the upregulation of liver cell marker RNA has subsided. Our data show that during the early stages of co-culture, the RNA expression is influenced to increase dramatically, which promoted protein expression several days later. This initial increase in the liver cell markers at the RNA level actively causes the MDSCs to differentiate into a liver cell and to maintain its protein expression of hepatocyte markers. Once the MDSCs are activated for hepatocyte differentiation, an elevated expression of liver cell markers at the RNA level are no longer needed to maintain the protein expression.
These studies have provided evidence that stem cells derived from adult tissue can differentiate and aid in the regeneration of injured livers. Cell migration and proliferation during liver tissue regeneration is often impaired by the presence of fibrosis [8]. In order to properly restore the function of the liver, it would be essential to combine the beneficial attributes of stem cell regeneration with alternative means to reduce fibrotic scar tissue. Matrix metalloproteinases (MMPs) have been identified as a valuable source in remodeling damaged tissue by promoting cell migration, proliferation, and differentiation, as well as degrading fibrotic scar tissue [48-49]. While MMPs are highly expressed by injured livers during repair, MDSCs appear as suitable candidates for cell based therapies of the liver, not only for their capacity to differentiate into hepatocyte-like cells, but because of their expression of MMPs (data not shown) [2, 50]. MDSCs, as demonstrated in our studies, can differentiate into cells along the endoderm lineage and actively promote tissue regeneration in injured liver.
Acknowledgments
The authors thank Haiying Pan, Christopher Vos, Shani Alston and Deeba Mahmood for technical assistance. The authors would also like to thank the Children's Hospital of Pittsburgh of UPMC for financial support from the Research Advisory Committee.
References
- 1.Ebrahimkhani MR, Elsharkawy AM, Mann DA. Wound healing and local neuroendocrine regulation in the injured liver. Expert Rev Mol Med. 2008;10:e11. doi: 10.1017/S146239940800063X. [DOI] [PubMed] [Google Scholar]
- 2.Alwayn IP, Verbesey JE, Kim S, Roy R, Arsenault DA, Greene AK, Novak K, Laforme A, Lee S, Moses MA, Puder M. A critical role for matrix metalloproteinases in liver regeneration. J Surg Res. 2008;145:192–198. doi: 10.1016/j.jss.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 4.Iimuro Y, Nishio T, Morimoto T, Nitta T, Stefanovic B, Choi SK, Brenner DA, Yamaoka Y. Delivery of matrix metalloproteinase-1 attenuates established liver fibrosis in the rat. Gastroenterology. 2003;124:445–458. doi: 10.1053/gast.2003.50063. [DOI] [PubMed] [Google Scholar]
- 5.Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838–851. doi: 10.1016/S0140-6736(08)60383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Svegliati Baroni G, D'Ambrosio L, Ferretti G, Casini A, Di Sario A, Salzano R, Ridolfi F, Saccomanno S, Jezequel AM, Benedetti A. Fibrogenic effect of oxidative stress on rat hepatic stellate cells. Hepatology. 1998;27:720–726. doi: 10.1002/hep.510270313. [DOI] [PubMed] [Google Scholar]
- 7.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wallace K, Burt AD, Wright MC. Liver fibrosis. Biochem J. 2008;411:1–18. doi: 10.1042/BJ20071570. [DOI] [PubMed] [Google Scholar]
- 9.Sekido H, Matsuo K, Takeda K, Ueda M, Morioka D, Kubota T, Tanaka K, Endo I, Togo S, Shimada H. Usefulness of artificial liver support for pretransplant patients with fulminant hepatic failure. Transplant Proc. 2004;36:2355–2356. doi: 10.1016/j.transproceed.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 10.O'Leary JG, Lepe R, Davis GL. Indications for liver transplantation. Gastroenterology. 2008;134:1764–1776. doi: 10.1053/j.gastro.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 11.Oertel M, Shafritz DA. Stem cells, cell transplantation and liver repopulation. Biochim Biophys Acta. 2008;1782:61–74. doi: 10.1016/j.bbadis.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, Dong XJ, Zhang GR, Shao JZ, Xiang LX. In vitro differentiation of mouse bone marrow stromal stem cells into hepatocytes induced by conditioned culture medium of hepatocytes. J Cell Biochem. 2007;102:52–63. doi: 10.1002/jcb.21275. [DOI] [PubMed] [Google Scholar]
- 13.Lysy PA, Smets F, Sibille C, Najimi M, Sokal EM. Human skin fibroblasts: From mesodermal to hepatocyte-like differentiation. Hepatology. 2007;46:1574–1585. doi: 10.1002/hep.21839. [DOI] [PubMed] [Google Scholar]
- 14.Banas A, Teratani T, Yamamoto Y, Tokuhara M, Takeshita F, Quinn G, Okochi H, Ochiya T. Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes. Hepatology. 2007;46:219–228. doi: 10.1002/hep.21704. [DOI] [PubMed] [Google Scholar]
- 15.Najimi M, Khuu DN, Lysy PA, Jazouli N, Abarca J, Sempoux C, Sokal EM. Adult-derived human liver mesenchymal-like cells as a potential progenitor reservoir of hepatocytes? Cell Transplant. 2007;16:717–728. doi: 10.3727/000000007783465154. [DOI] [PubMed] [Google Scholar]
- 16.Caballero M, Lightfoot HM, Jr, Lapaglia M, Pleasant A, Hatada S, Cairns BA, Fair JH. Detection and characterization of hepatic engraftment of embryonic stem derived cells by fluorescent stereomicroscopy. J Surg Res. 2007;141:134–140. doi: 10.1016/j.jss.2006.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi N, Ando M, Kosaka Y, Yong C, Okitsu T, Arata T, Ikeda H, Kobayashi K, Ueda T, Kurabayashi Y, Tanaka N. Partial hepatectomy and subsequent radiation facilitates engraftment of mouse embryonic stem cells in the liver. Transplant Proc. 2004;36:2352–2354. doi: 10.1016/j.transproceed.2004.08.141. [DOI] [PubMed] [Google Scholar]
- 18.Chinzei R, Tanaka Y, Shimizu-Saito K, Hara Y, Kakinuma S, Watanabe M, Teramoto K, Arii S, Takase K, Sato C, Terada N, Teraoka H. Em-bryoid-body cells derived from a mouse embryonic stem cell line show differentiation into functional hepatocytes. Hepatology. 2002;36:22–29. doi: 10.1053/jhep.2002.34136. [DOI] [PubMed] [Google Scholar]
- 19.Aurich I, Mueller LP, Aurich H, Luetzkendorf J, Tisljar K, Dollinger MM, Schormann W, Walldorf J, Hengstler JG, Fleig WE, Christ B. Functional integration of hepatocytes derived from human mesenchymal stem cells into mouse livers. Gut. 2007;56:405–415. doi: 10.1136/gut.2005.090050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oh SH, Witek RP, Bae SH, Zheng D, Jung Y, Piscaglia AC, Petersen BE. Bone marrow-derived hepatic oval cells differentiate into hepatocytes in 2-acetylaminofluorene/partial hepatectomy-induced liver regeneration. Gastroenterology. 2007;132:1077–1087. doi: 10.1053/j.gastro.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Sgodda M, Aurich H, Kleist S, Aurich I, Konig S, Dollinger MM, Fleig WE, Christ B. Hepatocyte differentiation of mesenchymal stem cells from rat peritoneal adipose tissue in vitro and in vivo. Exp Cell Res. 2007;313:2875–2886. doi: 10.1016/j.yexcr.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 22.Deasy BM, Gharaibeh BM, Pollett JB, Jones MM, Lucas MA, Kanda Y, Huard J. Long-term self-renewal of postnatal muscle-derived stem cells. Mol Biol Cell. 2005;16:3323–3333. doi: 10.1091/mbc.E05-02-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Pan HY, Huard J. Isolating stem cells from soft musculoskeletal tissues. Journal of Visualized Experiments (JoVE) 2010;4(41) doi: 10.3791/2011. July. pii 2011, doi: 10.3791/2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gharaibeh B, Lu A, Tebbets J, Zheng B, Feduska J, Crisan M, Peault B, Cummins J, Huard J. Isolation of a slowly adhering cell fraction containing stem cells from murine skeletal muscle by the preplate technique. Nat Protoc. 2008;3:1501–1509. doi: 10.1038/nprot.2008.142. [DOI] [PubMed] [Google Scholar]
- 25.Lee JY, Qu-Petersen Z, Cao B, Kimura S, Jankowski R, Cummins J, Usas A, Gates C, Rob-bins P, Wernig A, Huard J. Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing. J Cell Biol. 2000;150:1085–1100. doi: 10.1083/jcb.150.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jankowski RJ, Haluszczak C, Trucco M, Huard J. Flow cytometric characterization of myogenic cell populations obtained via the preplate technique: potential for rapid isolation of muscle -derived stem cells. Hum Gene Ther. 2001;12:619–628. doi: 10.1089/104303401300057306. [DOI] [PubMed] [Google Scholar]
- 27.Cao B, Zheng B, Jankowski RJ, Kimura S, Ikezawa M, Deasy B, Cummins J, Epperly M, Qu-Petersen Z, Huard J. Muscle stem cells differentiate into haematopoietic lineages but retain myogenic potential. Nat Cell Biol. 2003;5:640–646. doi: 10.1038/ncb1008. [DOI] [PubMed] [Google Scholar]
- 28.Wright V, Peng H, Usas A, Young B, Gearhart B, Cummins J, Huard J. BMP4-expressing muscle-derived stem cells differentiate into osteogenic lineage and improve bone healing in immunocompetent mice. Mol Ther. 2002;6:169–178. doi: 10.1006/mthe.2002.0654. [DOI] [PubMed] [Google Scholar]
- 29.Kuroda R, Usas A, Kubo S, Corsi K, Peng H, Rose T, Cummins J, Fu FH, Huard J. Cartilage repair using bone morphogenetic protein 4 and muscle-derived stem cells. Arthritis Rheum. 2006;54:433–442. doi: 10.1002/art.21632. [DOI] [PubMed] [Google Scholar]
- 30.Deasy BM, Li Y, Huard J. Tissue engineering with muscle-derived stem cells. Curr Opin Biotechnol. 2004;15:419–423. doi: 10.1016/j.copbio.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Romero-Ramos M, Vourc'h P, Young HE, Lucas PA, Wu Y, Chivatakarn O, Zaman R, Dunkelman N, el-Kalay MA, Chesselet MF. Neuronal differentiation of stem cells isolated from adult muscle. J Neurosci Res. 2002;69:894–907. doi: 10.1002/jnr.10374. [DOI] [PubMed] [Google Scholar]
- 32.Lavasani M, Lu A, Peng H, Cummins J, Huard J. Nerve growth factor improves the muscle regeneration capacity of muscle stem cells in dystrophic muscle. Hum Gene Ther. 2006;17:180–192. doi: 10.1089/hum.2006.17.180. [DOI] [PubMed] [Google Scholar]
- 33.Smaldone MC, Chancellor MB. Muscle derived stem cell therapy for stress urinary incontinence. World J Urol. 2008;26:327–332. doi: 10.1007/s00345-008-0269-9. [DOI] [PubMed] [Google Scholar]
- 34.Huard J, Acsadi G, Jani A, Massie B, Karpati G. Gene transfer into skeletal muscles by isogenic myoblasts. Hum Gene Ther. 1994;5:949–958. doi: 10.1089/hum.1994.5.8-949. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Huard J. Differentiation of muscle-derived cells into myofibroblasts in injured skeletal muscle. Am J Pathol. 2002;161:895–907. doi: 10.1016/S0002-9440(10)64250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsumoto T, Cooper GM, Gharaibeh B, Meszaros LB, Li G, Usas A, Fu FH, Huard J. Cartilage repair in a rat model of osteoarthritis through intraarticular transplantation of muscle-derived stem cells expressing bone morphogenetic protein 4 and soluble Flt-1. Arthritis Rheum. 2009;60:1390–1405. doi: 10.1002/art.24443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duan Y, Catana A, Meng Y, Yamamoto N, He S, Gupta S, Gambhir SS, Zern MA. Differentiation and enrichment of hepatocyte-like cells from human embryonic stem cells in vitro and in vivo. Stem Cells. 2007;25:3058–3068. doi: 10.1634/stemcells.2007-0291. [DOI] [PubMed] [Google Scholar]
- 38.Saito K, Yoshikawa M, Ouji Y, Moriya K, Nishiofuku M, Ueda S, Hayashi N, Ishizaka S, Fukui H. Promoted differentiation of cynomolgus monkey ES cells into hepatocyte-like cells by coculture with mouse fetal liver-derived cells. World J Gastroenterol. 2006;12:6818–6827. doi: 10.3748/wjg.v12.i42.6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soto-Gutierrez A, Navarro-Alvarez N, Zhao D, Rivas-Carrillo JD, Lebkowski J, Tanaka N, Fox IJ, Kobayashi N. Differentiation of mouse embryonic stem cells to hepatocyte-like cells by coculture with human liver nonparenchymal cell lines. Nat Protoc. 2007;2:347–356. doi: 10.1038/nprot.2007.18. [DOI] [PubMed] [Google Scholar]
- 40.Teratani T, Yamamoto H, Aoyagi K, Sasaki H, Asari A, Quinn G, Terada M, Ochiya T. Direct hepatic fate specification from mouse embryonic stem cells. Hepatology. 2005;41:836–846. doi: 10.1002/hep.20629. [DOI] [PubMed] [Google Scholar]
- 41.Yamada T, Yoshikawa M, Kanda S, Kato Y, Nakajima Y, Ishizaka S, Tsunoda Y. In vitro differentiation of embryonic stem cells into hepatocyte-like cells identified by cellular uptake of indocyanine green. Stem Cells. 2002;20:146–154. doi: 10.1634/stemcells.20-2-146. [DOI] [PubMed] [Google Scholar]
- 42.Schwartz RE, Reyes M, Koodie L, Jiang Y, Blackstad M, Lund T, Lenvik T, Johnson S, Hu WS, Verfaillie CM. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest. 2002;109:1291–1302. doi: 10.1172/JCI15182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heo J, Factor VM, Uren T, Takahama Y, Lee JS, Major M, Feinstone SM, Thorgeirsson SS. Hepatic precursors derived from murine embryonic stem cells contribute to regeneration of injured liver. Hepatology. 2006;44:1478–1486. doi: 10.1002/hep.21441. [DOI] [PubMed] [Google Scholar]
- 44.Bellayr IH, Li Y. Stem Cells: It's Good To Have Choices. Journal of the American College of Certified Wound Specialists. 2009;1:92–94. doi: 10.1016/j.jcws.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jang YY, Collector MI, Baylin SB, Diehl AM, Sharkis SJ. Hematopoietic stem cells convert into liver cells within days without fusion. Nat Cell Biol. 2004;6:532–539. doi: 10.1038/ncb1132. [DOI] [PubMed] [Google Scholar]
- 46.Vassilopoulos G, Wang PR, Russell DW. Transplanted bone marrow regenerates liver by cell fusion. Nature. 2003;422:901–904. doi: 10.1038/nature01539. [DOI] [PubMed] [Google Scholar]
- 47.Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Olson S, Grompe M. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422:897–901. doi: 10.1038/nature01531. [DOI] [PubMed] [Google Scholar]
- 48.Bellayr IH, Mu X, Li Y. Biochemical insights into the role of matrix metalloproteinases in regeneration: challenges and recent developments. Future Med Chem. 2009;1:1095–1111. doi: 10.4155/fmc.09.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- 50.Roderfeld M, Hemmann S, Roeb E. Mechanisms of fibrinolysis in chronic liver injury (with special emphasis on MMPs and TIMPs) Z Gastroenterol. 2007;45:25–33. doi: 10.1055/s-2006-927388. [DOI] [PubMed] [Google Scholar]