Abstract
Epigallocatechin-3-gallate (EGCG) is the main polyphenol component of green tea. This compound exhibits antioxidant, immunomodulatory, photoprotective, anti-angiogenic, and anti-inflammatory properties. We conducted a small randomized, double blind, split face trial using a cream containing 2.5% w/w of EGCG. Four healthy volunteers with significant erythema and telangiectasia on the face applied EGCG cream to one side of the face, and vehicle control cream to the other, twice daily for six weeks. After six weeks, biopsies were taken from EGCG and vehicle treated sites. Immunohistochemistry was used to measure VEGF and HIF-1α. HIF-1α expression was decreased in EGCG treated sites, such that 28.4% of the epidermis showed positive staining in vehicle treated vs. 13.8% in EGCG treated sites (p<0.001). A similar decrease in VEGF expression was found (6.7% in EGCG vs. 11.0%in in vehicle-treated skin (p<0.005). EGCG topical treatments influence HIF-1α induction and VEGF expression and may serve as a potential agent in the prevention of telangiectasias.
Keywords: VEGF, Green tea, EGCG, skin, Rosacea, HIf-1α, angiogenesis
Introduction
Green tea remains popular as an additive to skin care products, because its main polyphenol component, epigallocatechin-3-gallate (EGCG) has been found to have antioxidant, immunomodulatory, photoprotective, anti-angiogenic, and anti-inflammatory properties [1-4]. Treatment with green tea extracts may benefit patients with a predisposition to skin conditions that present with erythema and telangiectasia. Actinic telangiectasia, for instance, is a common skin condition characterized by prominent vascularity that includes both dilated actinically damaged superficial vessels and associated secondary background erythema. Rosacea is another common condition that presents with chronic facial erythema and telangiectasias. There are currently no topical agents that significantly prevent or reverse these conditions.
The goal of this study was to examine the effects of EGCG applied in humans in vivo on these angiogenic factors, using subjects with facial erythema and telangectasia. Immunohistochemistry was used to measure VEGF and HIF -1α. Our results show that EGCG topical treatments inhibit HIF-1α induction and VEGF expression in the skin.
Materials and methods
In order to evaluate the clinical and molecular response of subjects with erythema and facial telangiestasia to a topical cream containing EGCG, we designed a randomized, placebo-controlled, split face, double blind study. All procedures were approved by the Institutional Review Board of University Hospitals Case Medical Center. After obtaining informed consent, volunteers were evaluated by skin examination and colorimetric assessment (Minolta CR-300, Osaka, Japan). Excluded were subjects with active skin lesions on the face such as acne or pustular rosacea. Only those with a background erythema/telangiectasia were included. During the initial visit, a baseline biopsy was performed. Two topical formulations: 1) 2.5% w/w EGCG in a silicone in water emulsion; and 2) a silicone in water emulsion vehicle control, coded as A and B were given to subjects. In order to blind both subjects and investigators, both creams were similar in smell and color. The subjects underwent a live demonstration and received written directions to ensure proper cream application and avoid cross contamination. Subjects were instructed to apply each cream to separate halves of the face, twice daily for 6 weeks. Both creams were weighed at baseline and on subsequent visits to track patient compliance.
Punch biopsies from EGCG and vehicle control treated sites were obtained after 6 weeks of application. Samples were H &E stained to examine potential differences in vascularity. Separate samples were paraffin-embedded, serially cut into 5 urn sections, and mounted on ThermonShandon slides (Waltham, MA). Sections were deparaffinized in xylene and dehydrated in graded alcohols. After washing in phosphate-buffered saline (PBS), sections were placed in epitope-retrieval buffer (DakoCytomation, Carpenteria, CA) at 95–97°C for 20 minutes and then were subsequently cooled at room temperature for an additional 20 minutes. Sections were then blocked with 10% goat serum in PBS, followed by a block for endogenous peroxidases with peroxidase block solution (DakoCytomation, Carpenteria, CA). Sections were incubated overnight at 4°C with antibodies against hypoxia inducible factor-1-alpha (HIF-1α) (1.0 μg/mL, generously provided by Dr. Faton Agani, Case Western Reserve University) and vascular endothelial growth factor (VEGF) (0.8 μg/mL, Santa Cruz Biotechnology, Santa Cruz, CA). Unbound antibodies were removed the following day by washing the slides three times with PBS. Areas positive for VEGF and HIF-1α induction stained brown after development with diaminobenzidine (DAB), also from DakoCytomation. Slides were counterstained with filtered Mayer's hematoxylin (Sigma-Aldrich, St. Louis, MO), rinsed with distilled water, allowed to dry, and then mounted for viewing purposes. Five 20× brightfield images were taken from each section using an AxioCam HR digital camera and AxioPhot microscope (Carl Zeiss, Germany). The percentage in area of positively stained epidermis for both markers was quantified using Image-Pro image analysis software (Media Cybernetics Inc., San Diego, CA) on 15 measures/ sample. Data were compared using the Student's t-test, with significance determined as p ≤ 0.05.
Results
Four subjects aged 40 to 59; M: F, 3:1, with facial erythema and telangiectasia, on no other active therapies, completed the 6-week treatment period and underwent punch biopsies. H&E staining revealed no significant difference in vascularity between EGCG treated tissue and vehicle control treated samples. Representative immunohistochemical images of skin sections analyzed for HIF-1α and VEGF are shown in figures 1 and 2 respectively. We found a significant difference in HIF-1α expression (28.4% in vehicle control vs. 13.8% in EGCG treated sites (p<0.001) (Figure 1), between vehicle-treated and EGCG sites. A significant difference was also found in VEGF expression (11.0% vs. 6.7% in vehicle treated skin relative to EGCG-treated skin (p<0.005) (Figure 2). These results are summarized in Figure 3. Chromometer assessment of skin redness in vivo showed no significant change. The average baseline erythema was 21.22. After 6 weeks of treatment the chromometer values for erythema in EGCG-treated and vehicle-treated sites were 20.16 (p=0.609) and 20.09, (p=0.743) respectively, indicating no clinically observable difference.
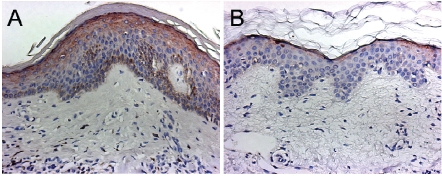
Figure 1.
Representative immunohistochemistry images of HIF-1 α staining in vehicle treated (A) versus EGCG treated skin (B). Tissue sections were stained with the HIF-1 α antibody according to the protocol described in the methods section. Epidermal areas that are positive for HIF-1α are identified by the chromogen DAB (dark brown color). These sections demonstrate that EGCG results in decreased expression of HIF-1 α compared to vehicle.
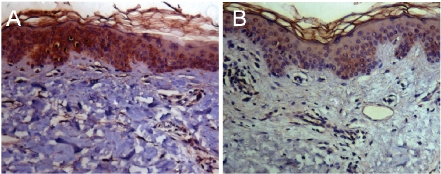
Figure 2.
Representative immunohistochemistry images of VEGF staining in vehicle (A) treated versus EGCG (B) treated skin. Tissue sections were stained with VEGF rabbit polyclonal antibody according to the protocol described in the methods section. Epidermal areas positive for VEGF are identified by the chromogen DAB (dark brown color). These sections demonstrate that EGCG results in decreased expression of VEGF compared to vehicle.
Figure 3.
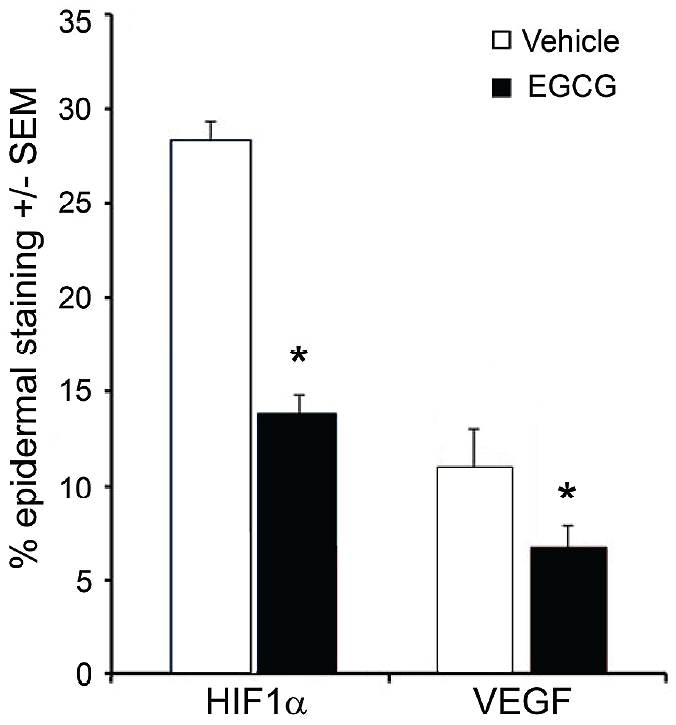
Bar graph showing the mean values ± SEM of positively stained epidermis in skin sections obtained from 4 subjects and analyzed for HIF-1α and VEGF via immunohistochemistry. Six weeks of treatment with EGCG cream (closed bars) resulted in a significantly less expression of the angiogenic factors HIF-1 α and VEGF, relative to treatment with vehicle alone (open bars).
Discussion
Studies have shown the inhibitory effect of EGCG on the angiogenic growth and transcription factors, vascular endothelial growth factor (VEGF) and hypoxia-inducible factor-1 (HIF-1)[4] VEGF plays a critical role in angiogenesis[5]. It is the prototypical vascular growth factor which binds to VEGF receptor 2 (VEGFR2/KDR) on endothelial cells, inducing endothelial cell proliferation, migration, and cell survival. Hypoxia inducible factor-1 (HIF-1) is a transcription factor consisting of two subunits, HIF-1α and HIF-1β. The HIF-1α subunit is tightly regulated by tissue oxygen concentrations[6]. The presence of hypoxic conditions allows HIF-1α to accumulate in the nucleus and form a het-erodimer with HIF-1β. The HIF-1α/HIF-1β complex binds to the Hypoxia Response Element (HRE) in the regulatory portion of target genes. Downstream of HIF-1 activation, numerous other genes including VEGF are promoted, resulting in erythropoiesis, iron metabolism and angiogenesis [4].
The results obtained in this study confirm that the topical application of EGCG results in a decrease in both HIF-1α and VEGF protein expression. The fact that these immunohistochemical findings were not accompanied by a decrease in erythema clinically may be due to the influence of angiogenic factors outside the HIF-1α and VEGF pathways that also play a role in the stimulation of inflammation, erythema and development of telangiectasias. Furthermore, the observed molecular effects of EGCG may be more essential in prevention rather than reversal of already existing telangiectasias. Nonetheless, this is the first report in humans that demonstrates significant suppression of HIF-1α and VEGF expression after in vivo treatment with topically applied EGCG. This data strengthens the evidence for EGCG as an anti-angiogenic compound. Additional studies are needed to optimize its clinical efficacy.
Acknowledgments
Ethical Considerations: The protocol for this study and all procedures were approved by the Institutional Review Board of University Hospitals Case Medical Center. Protocol # 06-05-15. This study was supported in part by Case Western Reserve University Skin Disease Research Center, NIH NIAMS AR-39750. Study materials were provided by Estee Lauder external Research Division. Estee Lauder external Research division provided the compounds used in this study. Daniel Maes and Mary Matsui were given the final copy of the manuscript for review. The final preparation and approval of the manuscript was made by Diana Santo Domingo, Melissa Camouse, Andrew Hsia, Nicole Ward, Kevin Cooper, and El ma Baron.
References
- 1.Elmets CA, Singh D, Tubesing K, Matsui M, Katiyar S, Mukhtar H. Cutaneous photoprotection from ultraviolet injury by green tea polyphenols. J Am Acad Dermatol. 2001;44:425–432. doi: 10.1067/mjd.2001.112919. [DOI] [PubMed] [Google Scholar]
- 2.Katiyar S, Elmets CA, Katiyar SK. Green tea and skin cancer: photoimmunology, angiogenesis and DNA repair. J Nutr Biochem. 2007;18:287–296. doi: 10.1016/j.jnutbio.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Kawai K, Tsuno NH, Kitayama J, Okaji Y, Yazawa K, Asakage M, Hori N, Watanabe T, Takahashi K, Nagawa H. Epigallocatechin gallate attenuates adhesion and migration of CD8+ T cells by binding to CD11b. J Allergy Clin Immunol. 2004;113:1211–1217. doi: 10.1016/j.jaci.2004.02.044. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Q, Tang X, Lu Q, Zhang Z, Rao J, Le AD. Green tea extract and (-)-epigallocatechin-3-gallate inhibit hypoxia- and serum-induced HIF-1alpha protein accumulation and VEGF expression in human cervical carcinoma and hepatoma cells. Mol Cancer Ther. 2006;5:1227–1238. doi: 10.1158/1535-7163.MCT-05-0490. [DOI] [PubMed] [Google Scholar]
- 5.Detmar M. The role of VEGF and thrombospondins in skin angiogenesis. J Dermatol Sci. 2000;24(Suppl 1):S78–84. doi: 10.1016/s0923-1811(00)00145-6. [DOI] [PubMed] [Google Scholar]
- 6.de Vries C, Escobedo JA, Ueno H, Houck K, Ferrara N, Williams LT. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science. 1992;255:989–991. doi: 10.1126/science.1312256. [DOI] [PubMed] [Google Scholar]