Abstract
The HIV-1 regulatory proteins Rev and Tat are expressed early in the virus life cycle and thus may be important targets for the immune control of HIV-1-infection and for effective vaccines. However, the extent to which these proteins are targeted in natural HIV-1 infection as well as precise epitopes targeted by human cytotoxic T lymphocytes (CTL) remain to be defined. In the present study, 57 HIV-1-infected individuals were screened for responses against Tat and Rev by using overlapping peptides spanning the entire Tat and Rev proteins. CD8+ T cell responses against Tat and Rev were found in up to 19 and 37% of HIV-1-infected individuals, respectively, indicating that these regulatory proteins are important targets for HIV-1-specific CTL. Despite the small size of these proteins, multiple CTL epitopes were identified in each. These data indicate that Tat and Rev are frequently targeted by CTL in natural HIV-1 infection and may be important targets for HIV vaccines.
HIV-1 infection is characterized by an early peak of viremia that declines coincident with the development of strong cytotoxic T lymphocyte (CTL) responses (1–3) (reviewed in refs. 4 and 5). Selection for mutations within CTL epitopes demonstrates that CTL exert pressure on virus replication in vivo (6–10), and studies in macaques have provided compelling in vivo data for the role of CD8+ T cells in controlling viremia in both acute and chronic simian immunodeficiency virus (SIV) infection (11, 12). HIV-1-infected individuals who are treated during acute infection show enhancement of both CTL and T helper cell responses against HIV-1 associated with subsequent viral control after treatment interruption (13). Induction of HIV-1-specific cytotoxic T cell responses is therefore considered an important goal for AIDS vaccines (14).
Thus far, the analysis of HIV-1-specific CTL responses has been largely dominated by the study of structural HIV-1 proteins Gag, Pol, and Env, expressed later in the viral life cycle (15). The extent to which the earlier expressed regulatory proteins HIV-1 Tat and Rev are targeted in natural infection as well as precise CTL epitopes within these proteins remains to be defined. Responses directed against these early proteins may be particularly important in viral containment during AIDS-virus infection, because HIV-1 Tat and Rev are the dominant viral proteins produced before Nef down-regulates MHC class I molecules on the cell surface (16).
To identify the determinants of natural and vaccine-induced immune responses directed against Tat and Rev, a detailed analysis of immune responses against these proteins in natural HIV-1 infection is warranted. This is of particular interest because no CTL epitope has yet been identified for HIV-1 Tat and only one for HIV-1 Rev (15, 17). We applied a comprehensive approach of screening for specific CD8+ T cell responses by using overlapping peptides spanning the entire HIV-1 Tat and Rev sequences. This report describes the first CTL epitope within HIV-1 Tat and additional CTL epitopes within HIV-1 Rev and provides evidence that these proteins are frequent targets of cellular host defenses.
Materials and Methods
Subjects.
Fifty-seven HIV-1-infected and 10 HIV-1-negative individuals were studied at the Massachusetts General Hospital (MGH). HIV-1-infected persons included 32 subjects who were diagnosed and treated with highly active antiretroviral therapy (HAART) within 180 days of HIV-1 seroconversion (18), 12 individuals with chronic treated HIV-1 infection, and 13 HIV-1-infected individuals from the HIV-1 Controller Study Cohort, who contained their HIV-1 plasma viremia below 1,000 RNA copies per milliliter in the absence of antiretroviral therapy (referred to as “controllers”) (summary is published as supplemental data on the PNAS web site, www.pnas.org). At the time of first CTL analysis, subjects on HAART had been treated for 6–12 months. HLA class I typing was performed at the MGH Tissue Typing Laboratory by using sequence-specific primer–PCR (19). The study was approved by the MGH Institutional Review Board, and all individuals gave informed consent for participation in the studies.
Generation of CTL Clones.
Specific CTL lines were isolated by limiting dilution and maintained as described (20, 21). HIV-1-specific cytotoxicity was assessed by 51chromium-release assay (22) by using autologous B-lymphoblastoid cell lines pulsed with peptides or infected with recombinant vaccinia virus (rVV) expressing either HIV-1 Tat or Rev (Therion Biologics, Cambridge, MA). HLA restriction of CTL epitopes was determined by using a panel of partially HLA-matched target cells (21).
Synthetic HIV-1 Peptides.
Peptides were synthesized on an automated peptide synthesizer (MBS 396, Advanced ChemTech) by using fluorenylmethoxycarbonyl chemistry. Twenty-one overlapping peptides spanning the HIV-1 BRU-B clade Rev sequence (14–16 mers with 10-aa overlap) and 15 overlapping peptides spanning the HIV-1 BRU-B clade Tat sequence (12–18 mers with 10-aa overlap) were generated (sequences listed in supplemental material). In addition, peptides corresponding to described optimal HIV-1 CTL epitopes and a panel of 259 overlapping peptides (15–20 mers) spanning the entire p17 Gag, p24 Gag, gp41 Env, gp120 Env, RT, and Nef sequence were used.
ELISPOT Assay.
HIV-1-specific CD8+ T cell responses were quantified in duplicate by ELISPOT assay using fresh peripheral blood mononuclear cells (PBMC) (0.5–1 × 105) and peptide (10−5 M), as described previously (23). IFN-γ-producing cells were counted by direct visualization and are expressed as spot-forming cells (SFC) per 106 PBMC. The number of specific IFN-γ-secreting T cells was calculated by subtracting the negative control value from the established SFC count. Negative controls were always <30 SFC per 106 input cells. CD8+ T cell responses were also measured by using rVV-based IFN-γ ELISPOT assay as described elsewhere (24).
Fine Mapping of CTL Epitopes.
Fine mapping of the CD8+ T cell response was achieved by ELISPOT assay by using serial dilutions of truncated peptides as described (23). The optimal peptide was defined as the peptide that induced specific IFN-γ production by T cells at the lowest peptide concentration. ELISPOT results were confirmed by using the same peptide truncations in fine-mapping assays by 51chromium release assay, as described previously (21). All experiments were run in duplicate or triplicate.
Flow-Cytometric Detection of Antigen-Induced Intracellular IFN-γ.
Intracellular cytokine staining assays were performed as described elsewhere with minor modifications (25–27), by using 0.5–1 × 106 PBMC or CTL lines and the following antibodies: anti-CD8-PerCP, anti-CD4-APC, and anti-IFN-γ-FITC. Cells were analyzed on a FACSCalibur flow cytometer (Becton Dickinson).
Statistical Analysis.
Statistical analysis was carried out by using graphpad prism Version 2.00 for Windows 95 (GraphPad, San Diego). Statistical significance (P values) of results was calculated by two-tailed t test and by χ2 analysis.
Results
CD8+ T Cell Responses Against HIV-1 Tat and Rev Peptides.
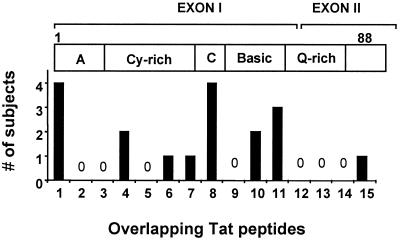
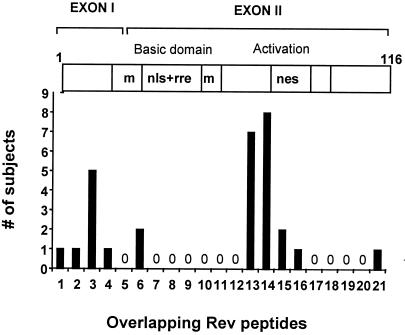
Fifty-seven HIV-1-infected individuals were screened for CD8+ T cell responses against the HIV-1 proteins Tat and Rev by using overlapping peptides in an IFN-γ ELISPOT assay. PBMC from 11/57 (19.3%) HIV-1-positive study subjects recognized at least 1 overlapping Tat peptide (Table 1), and 21/57 (37%) individuals had responses against 1 or more Rev peptides (Table 2). Magnitudes of responses against Tat peptides ranged from 50–920 SFC/106 PBMC (median 250); frequencies for Rev responses showed a range from 40 to 900 SFC/106 PBMC (median 220). All responses were CD8+ T cell mediated as determined by CD4+ T cell depletion and/or flow-based analysis of peptide-specific intracellular IFN-γ production by CD8+ T cells (data not shown). The responses identified through screening with overlapping peptides correlated with the ELISPOT results by using rVV-expressing HIV-1 Tat and Rev in a subset of study subjects (data not shown). No responses against the Tat and Rev peptides were observed in the 10 HIV-1-negative individuals used as controls. For both Tat and Rev, multiple regions were targeted in functional as well as nonfunctional domains of the proteins. These included 8 of the 15 Tat peptides and 10 of 21 Rev peptides (Figs. 1 and 2). However, no single Tat peptide was recognized by more than 7%, and no single Rev peptide was targeted by more than 12% of infected persons.
Table 1.
Magnitude of peptide-specific CD8+ responses directed against Tat overlapping peptides as measured by IFN-γ ELISPOT (SFC/106 PBMC)
| Subject | HIV status | TAT-1 | TAT-4 | TAT-6 | TAT-7 | TAT-8 | Tat-10 | Tat-11 | Tat-15 |
|---|---|---|---|---|---|---|---|---|---|
| ACTD | Acute | 0 | 0 | 0 | 0 | 120 | 0 | 0 | 0 |
| AC07 | Acute | 0 | 0 | 0 | 0 | 0 | 90 | 0 | 0 |
| AC41 | Acute | 0 | 230 | 0 | 0 | 0 | 0 | 130 | 0 |
| AC09 | Acute | 0 | 0 | 0 | 0 | 230 | 0 | 0 | 0 |
| 6002 | Chronic | 50 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6007 | Chronic | 240 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| O13572j | Controller | 700 | 0 | 260 | 600 | 0 | 0 | 0 | 0 |
| MSM 0105 | Controller | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 70 |
| MJR 0515 | Controller | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| F/BN | Controller | 0 | 0 | 0 | 0 | 580 | 850 | 920 | 0 |
| HC/BN | Controller | 0 | 340 | 0 | 0 | 360 | 0 | 400 | 0 |
Table 2.
Magnitude of peptide-specific CD8+ responses directed against Rev overlapping peptides as measured by IFN-γ ELISPOT (SFC/106 PBMC)
| Subject | HIV status | REV-1 | REV-2 | REV-3 | REV-4 | REV-6 | REV-13 | REV-14 | REV-15 | REV-16 | REV-20 | REV-21 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AC04 | Acute | 0 | 0 | 0 | 0 | 0 | 330 | 260 | 0 | 0 | 0 | 0 |
| ACTD | Acute | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 600 | 0 | 0 | 0 |
| AC13 | Acute | 0 | 0 | 0 | 0 | 0 | 470 | 460 | 0 | 0 | 0 | 0 |
| AC41 | Acute | 0 | 0 | 0 | 0 | 0 | 660 | 500 | 0 | 0 | 0 | 0 |
| AC35 | Acute | 0 | 0 | 0 | 0 | 0 | 600 | 730 | 0 | 0 | 0 | 0 |
| AC09 | Acute | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 80 | 340 | 0 | 0 |
| AC42 | Acute | 160 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| AC43 | Acute | 0 | 0 | 0 | 0 | 0 | 300 | 330 | 0 | 0 | 0 | 0 |
| AC39 | Acute | 0 | 0 | 0 | 0 | 0 | 90 | 120 | 0 | 0 | 0 | 0 |
| AC47 | Acute | 0 | 0 | 0 | 0 | 0 | 130 | 0 | 0 | 0 | 100 | |
| 6001 | Chronic | 0 | 0 | 90 | 50 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6003 | Chronic | 0 | 0 | 90 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6007 | Chronic | 0 | 0 | 190 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6010 | Chronic | 0 | 0 | 50 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| AD | Chronic | 0 | 0 | 0 | 0 | 0 | 50 | 0 | 0 | 0 | 0 | 0 |
| 13572j | Controller | 0 | 0 | 0 | 0 | 0 | 0 | 700 | 0 | 0 | 0 | 0 |
| TMH#3 | Controller | 0 | 0 | 340 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 199pg | Controller | 0 | 0 | 0 | 0 | 0 | 40 | 106 | 0 | 0 | 0 | 0 |
| F/BN | Controller | 0 | 0 | 0 | 0 | 900 | 0 | 0 | 0 | 0 | 0 | 300 |
| HC/BN | Controller | 0 | 0 | 0 | 0 | 400 | 0 | 0 | 0 | 0 | 0 | 0 |
| MAL | Controller | 0 | 0 | 0 | 0 | 0 | 0 | 220 | 0 | 0 | 0 | 0 |
Figure 1.
Number of study subjects (dark columns) with specific CD8 responses against the individual overlapping Tat peptides (1–15) as measured by ELISPOT. The clear horizontal bar indicates the HIV-1 Tat protein domains corresponding to the overlapping peptides. A, acidic amino terminal; Cy-rich, cysteine-rich region; C, core domain; Q-rich, glutamine-rich domain; a total of 57 subjects were evaluated.
Figure 2.
Number of study subjects (dark columns) with specific CD8 responses against the individual overlapping Rev peptides (1–21) as measured by ELISPOT. The clear horizontal bar indicates the regions of the HIV-1 Rev protein corresponding to the overlapping peptides. M, multimerization regions; nls, nuclear localization signal; rre, rev responsive element binding; nes, nuclear export signal. A total of 57 subjects were evaluated.
Frequency of Recognition of Tat and Rev.
To address how frequently Tat and Rev were recognized compared with other HIV-1 proteins, we screened a subset of 36 individuals with a total of 259 overlapping peptides spanning the entire p17-Gag, p24-Gag, gp41-Env, gp120-Env, RT, and Nef sequence (Table 3). By dividing frequency of recognition by the number of amino acids per protein (and thus adjusting for protein length), both Rev and Tat were comparable to Nef and p24-Gag in frequency of recognition and receive higher scores than RT, gp41-Env, and gp120-Env.
Table 3.
Percentage of subjects with CTL responses against specific HIV-1 proteins in a subset of 36 individuals who were screened for HIV-1-specific CD8 responses by using overlapping peptides spanning Tat, Rev, Nef, RT, p17-Gag, p24-Gag, gp41-Env, gp120-Env by INF-γ ELISPOT
| Group | n | TAT, % | REV, % | NEF, % | RT, % | p17, % | p24, % | gp41, % | gp120, % |
|---|---|---|---|---|---|---|---|---|---|
| Acute | 21 | 10 | 24 | 43 | 19 | 60 | 70 | 10 | 30 |
| Chronic | 10 | 20 | 30 | 70 | 60 | 29 | 43 | 33 | 24 |
| Controller | 5 | 80 | 40 | 100 | 100 | 100 | 100 | 80 | 80 |
| Total | 36 | 25 | 28 | 58 | 38 | 47 | 58 | 33 | 33 |
| No. amino acids | 88 | 116 | 205 | 560 | 130 | 240 | 350 | 550 | |
| Amino acid adjusted score* | 0.28 | 0.24 | 0.28 | 0.07 | 0.36 | 0.24 | 0.09 | 0.06 |
Frequency of recognition in percent divided by number of amino acids per protein.
Analysis of Differences Among Distinct Groups of HIV-1-Infected Individuals.
We next evaluated the breadth and magnitude of CTL responses directed against Rev and Tat among three different subgroups of infected persons. CTL responses directed against both Tat and Rev were more frequently observed in persons controlling viremia without medications (controllers) (39 and 46%, respectively), compared with treated individuals with chronic (17 and 41%, respectively) or acute (13 and 31%, respectively) infection (Tables 1 and 2). However, these differences did not reach statistical significance. Controllers targeted more CTL epitopes within HIV-1 Tat, compared with the treated individuals (median of 2 epitopes compared with a median of 1 epitope, P < 0.03), and responses directed against these epitopes were of significantly higher magnitude in controllers (471 ± 270 SFC/106 PBMC vs. 156 ± 71 SFC/106 PBMC, P = 0.01). In contrast, differences between the groups did not reach statistical significance for CTL responses directed against Rev.
Functional Characteristics of HIV-1 Rev- and Tat-Specific CD8+ T Cell Lines.
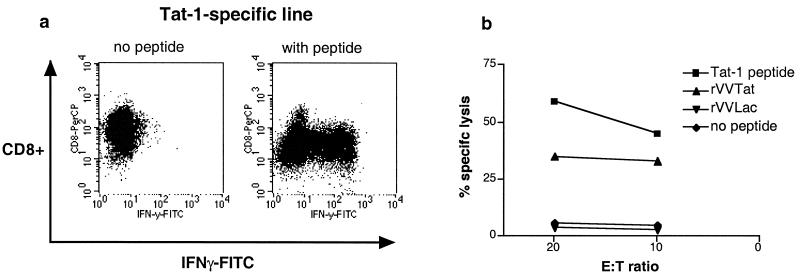
The above data provide evidence that CD8+ T cells are triggered to produce IFN-γ in response to Tat and Rev peptides but do not address their ability to recognize processed viral proteins or to lyse cells. Peptide-specific CD8+ T cell lines for both Tat (peptides Tat-1, Tat-8, Tat-11) and Rev (peptides Rev-3, Rev-14) responses were therefore generated to further address the functional characteristics of these cells. CD8+ T cell lines were first evaluated for their ability to produce IFN-γ on peptide stimulation and then tested for CTL activity in standard 51Cr-release assays. Antigen-specific IFN-γ production and lysis of target cells presenting Tat (Fig. 3 a and b) and Rev (data not shown) peptides was readily evident. 51Cr-release assays performed by using autologous B-lymphoblastoid cell line infected with rVV expressing Tat (Fig. 3) and Rev (data not shown) confirmed that these epitopes were effectively processed intracellularly.
Figure 3.
Functional characteristics exemplified for a Tat-1-specific CD8+ T cell line. (a) Evaluation of ability to produce IFN-γ by flow-based intracellular cytokine staining. (b) Cytotoxic activity for the T cell line as measured by standard 51Cr-release assay at effector-to-target ratios 20:1 and 10:1. Targets included peptide-pulsed autologous B-lymphoblastoid cell line (LCL) and autologous B-LCL infected with rVV expressing Tat rVVTat. Control conditions were established by using unpulsed or rVVLac infected autologous B-cell lines (20).
Identification of Optimal CTL Epitopes Within HIV-1 Tat and Rev.
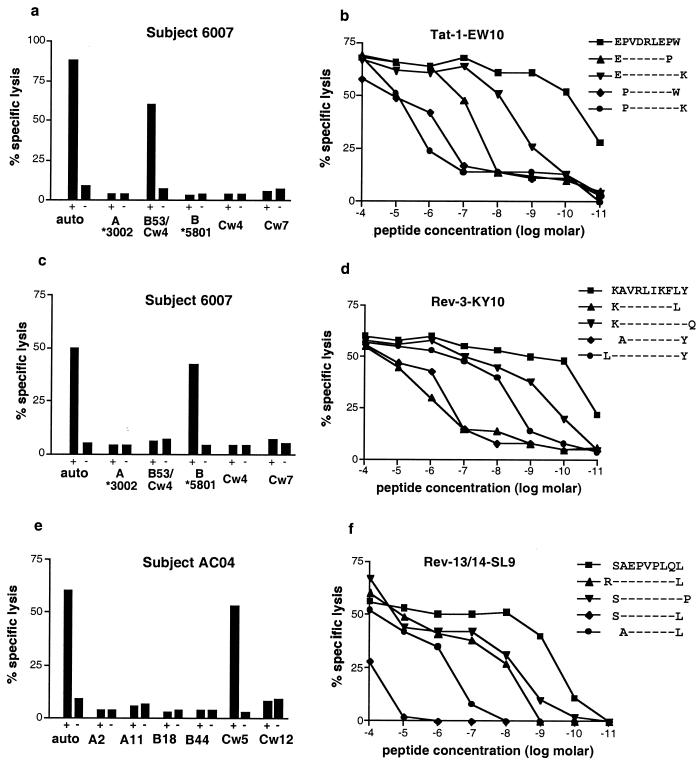
Despite over 130 optimal HIV-1-specific CTL epitopes now defined (15), no Tat epitopes have been mapped, and only one Rev epitope has been reported (17). We therefore determined for the dormant responses the smallest truncated peptide that would afford maximal lysis at the lowest concentration. Fig. 4 a–f summarizes the determination of HLA restriction and the fine mapping of the optimal epitopes. The response to the Tat-1 peptide was found to be restricted by HLA-B53 (Fig. 4a), one of the most frequent HLA class I alleles in the black population worldwide (28). The optimal epitope was identified as EPVDPRLEPW (position Tat 2–11) by both 51Cr-release assay (Fig. 4b) and ELISPOT (data not shown) and exactly fits the published peptide-binding motif for HLA-B53 (29). Of the 4 persons who recognized peptide Tat-1, only 2 expressed HLA-B53 and recognized the Tat epitope restricted by this allele, suggesting that other restricting alleles and possibly other optimal epitopes might be contained within Tat-1.
Figure 4.
Characterization of three CTL epitopes within HIV-1 Tat and Rev. The responses were further investigated in study subjects 6007 (HLA A*3002/-, B53/*5801, Cw4/7) (a–d) and AC04 (HLA A2/11, B18/44, Cw5/Cw12)(e and f). HLA restriction was performed by using peptides presented by autologous and partially HLA-mismatched L-BCL in a 51Cr-release assay (a, c, and e). Fine mapping of the optimal epitope was done by using serial dilutions of truncated peptides in cytotoxicity assays (b, d, and f). Effector-to-target ratio for all assays was 20:1.
The CD8+ T cell response to the Rev-3 peptide was HLA-B*5801-restricted (Fig. 4c) with KAVRIKLFLY as the optimal epitope (Fig. 4d). It was also recognized by another individual in whom it was restricted by HLA*B5701 (data not shown), an HLA class I allele closely related to HLA-B*5801, suggesting crosspresentation between these two HLA alleles, which has been described previously (30).
The most frequently recognized response among all subjects was found to Rev-peptides 13 and 14. HLA restriction identified HLA-Cw5 as the restricting element (Fig. 4e), for which no optimal HIV-specific CTL epitope has been reported thus far (15). Fine mapping revealed the peptide SAEPVPLQL (Rev-SL9 at position Rev 69–77) as the optimal CTL epitope (Fig. 4f). All study individuals expressing HLA-Cw5 were subsequently tested for responses against Rev-SL9 (Table 4). Five of 11 (45%) individuals recognized the epitope at magnitudes ranging from 120 to 730 (median 440) SFC/106 PBMC. Of interest, the epitope was also recognized by 2/5 individuals (AC39, AC43) expressing HLA-Cw8, an HLA molecule differing by only 4 amino acids from HLA-Cw5 (see http://www.anthonynolan.com/HIG/), suggesting promiscuous presentation of SAEPVPLQL between those HLA molecules. This epitope has also been reported as an HLA-B14-restricted CTL epitope; however, HLA-Cw types were not given for the individuals reported in that earlier study (17).
Table 4.
HLA types and CD8+ T cell-mediated responses to SAEPVPLQL (HIV-1-Rev) in study subjects expressing HLA-Cw5 and -Cw8
| Subjects | HLA type | SFC/106PBMC | ||
|---|---|---|---|---|
| AC04 | A2, 11 | B18, 44 | Cw5, 12 | 420 |
| AC35 | A2, 74 | B18, 72 | Cw2, 5 | 780 |
| AC41 | A2, 11 | B35, 44 | Cw4, 5 | 690 |
| AC13 | A2, 68 | B14, 44 | Cw5, 8 | 440 |
| 199PG | A2, 30 | B44, 51 | Cw2, 5 | 120 |
| AC39 | A32, 68 | B60, 64 | Cw3, 8 | 100 |
| AC43 | A24, 31 | B14, 57 | Cw7, 8 | 400 |
Longitudinal Analysis of the Rev-SL9 Response.
To also address the question of longitudinal persistence of responses over time and effects of antiviral therapy, the Rev-SL9 response was chosen because of its high frequency of recognition among the study subjects. Longitudinal samples were available for 6 Rev-SL9 responders, who were treated during acute HIV-1 infection and were analyzed 2 and 12 months after initiation of highly active antiretroviral therapy by using both ELISPOT and flow-based intracellular cytokine staining (ICS). Magnitudes of CTL responses as measured by ICS corresponded well to magnitudes determined by ELISPOT (data not shown). In all individuals, the response was present at both time points without significant changes in magnitude [ICS: 0.18% (range 0.1%–1%) vs. 0.2% (0.1%–0.8%) P = 0.7; ELISPOT: 120 vs. 140SFC]. Two of these individuals (AC04 and AC13) underwent treatment interruption after 12 and 14 months of therapy (13). In both subjects, the response was augmented to higher levels on transient reexposure to antigen during treatment (see supplemental material), as previously demonstrated for CTL responses against structural proteins of HIV-1 (13).
Discussion
Very little detailed data are available on HIV-1-specific responses against the regulatory proteins Tat and Rev in humans (31). We therefore characterized CTL responses directed against HIV-1 Tat and Rev in 57 HIV-1-infected individuals comprehensively by using overlapping peptides spanning the entire Tat and Rev sequence of HIV-1. We observed that CTL responses to epitopes in Tat and Rev, characterized by both IFN-γ production and lysis of target cells, are frequently detectable in HIV-1-infected humans and may contribute importantly to the CD8+ T cell response against HIV-1. Furthermore, to our knowledge this report includes the description of the first optimal CTL epitope within HIV-1 Tat and describes two additional novel CTL epitopes within HIV-1 Rev, one of which represents the first HLA-Cw5-restricted HIV CTL epitope. These results thus indicate that these proteins expressed early in the viral life cycle are important targets for the cellular immune response, and that accurate dissection of CTL responses in natural infection needs to incorporate analysis of Tat and Rev.
CTL responses against regulatory proteins such as Tat and Rev may be of major immunologic importance because of their early expression in the viral life cycle (32) before Nef down-regulates MHC class I molecules on the cell surface (16). The pattern of responses observed by screening with peptides suggests that particular regions of the proteins are preferentially targeted. Most identified responses map to important functional domains of Tat and Rev. It has been suggested that CTL responses directed against functionally important regions within the virus could be more effective, as CTL-induced viral escape mutations within these domains are less likely to occur or may lead to reduced viral competence (33). Longitudinal analysis will have to evaluate whether the virus is less likely to escape from CTL that target epitopes located in these regions of Rev and Tat.
The potential importance of Tat-specific CTL has been suggested in an SIV model of AIDS virus infection showing that Tat-specific CTL select for virus escape variants very early in primary SIV infection (34). The potential for immunization with Tat and Rev immunogens has been addressed in both animal and human studies, with encouraging results. Studies in macaques by using biologically active Tat protein (35), Tat toxoid (36), or recombinant vaccinia vectors expressing Tat and Rev proteins (37) as immunogens showed attenuation of virus replication and disease. Clinical studies in humans assessing safety and immunogenicity of HIV-1 Tat toxoid (38) and an HIV-1 Rev-containing DNA vaccine have been performed.
The use of overlapping peptides in the ELISPOT assays indicates that there are multiple epitopes in Tat and Rev, three of which are optimally defined. Only one of these epitopes (Rev-SAEPVPLQL) had been previously described, albeit as an HLA-B14-restricted CTL epitope (17). However, the HLA-Cw locus was not investigated in the earlier study. In the present study, the response was shown to be HLA-Cw5-restricted and thus represents the first HIV-specific CTL epitope restricted by this HLA class I molecule. The epitope was recognized by several additional individuals expressing HLA-Cw5, as well as 2 individuals expressing HLA-Cw8, but not Cw5. Because the amino acid sequences of HLA-Cw5 and -Cw8 differ only in four amino acid positions, this finding is suggestive of promiscuous presentation between these two restriction elements, as already described for epitopes restricted by HLA-A and -B alleles (23, 30, 39). Our findings indicate that the HLA-C locus is an important component to consider in the determination of HLA restriction, especially in view of linkage disequilibrium between certain HLA-B and -C alleles.
In conclusion, these data indicate that the regulatory proteins HIV-1 Tat and Rev are frequent targets for CTL in natural HIV-1 infection. The identification of epitopes within these proteins should facilitate the testing of CTL responses induced by vaccines expressing Tat and/or Rev and will be important to investigate in studies of HIV pathogenesis. These findings should also encourage investigation of responses to other regulatory proteins such as Vif, Vpr, and Vpu, which may also serve as targets for CTL responses. The epitopes defined herein will allow for a more detailed analysis of CTL responses directed against regulatory proteins and may potentially represent candidates for future multicomponent HIV vaccines.
Supplementary Material
Acknowledgments
We thank all study participants. This work was supported by grants to M.M.A. from the Deutsche Forschungsgemeinschaft (AD 171/1–1), to M.A. from the Deutscher Akademischer Austauschdienst (Grant D/99/08826), to E.S.R. from the Doris Duke Charitable Foundation and the National Institutes of Health (NIH), to P.J.R.G. from the Elizabeth Glaser Pediatric AIDS Foundation, the United Kingdom Medical Research Foundation (Grant G108/274), and the NIH (AI46995), and to B.D.W. through the NIH (AI28568, AI30914) and the Doris Duke Charitable Foundation. P.J.R.G. is an Elizabeth Glaser Scientist of the Elizabeth Glaser Pediatric AIDS Foundation. B.D.W. is a Doris Duke Distinguished Clinical Science Professor.
Abbreviations
- CTL
cytotoxic T lymphocyte
- SIV
simian immunodeficiency virus
- rVV
recombinant vaccinia virus
- SFC
spot-forming cells
- PBMC
peripheral blood mononuclear cells
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Borrow P, Lewicki H, Hahn B H, Shaw G M, Oldstone M B. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koup R A, Safrit J T, Cao Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yasutomi Y, Reimann K A, Lord C I, Miller M D, Letvin N L. J Virol. 1993;67:1707–1711. doi: 10.1128/jvi.67.3.1707-1711.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brander C, Walker B D. Curr Opin Immunol. 1999;11:451–459. doi: 10.1016/S0952-7915(99)80076-4. [DOI] [PubMed] [Google Scholar]
- 5.Goulder P J, Rowland-Jones S L, McMichael A J, Walker B D. AIDS. 1999;13:S121–S136. [PubMed] [Google Scholar]
- 6.Evans D T, O'Connor D H, Jing P, Dzuris J L, Sidney J, da Silva J, Allen T M, Horton H, Venham J E, Rudersdorf R A, et al. Nat Med. 1999;5:1270–1276. doi: 10.1038/15224. [DOI] [PubMed] [Google Scholar]
- 7.Borrow P, Lewicki H, Wei X, Horwitz M S, Peffer N, Meyers H, Nelson J A, Gairin J E, Hahn B H, Oldstone M B, Shaw G M. Nat Med. 1997;3:205–211. doi: 10.1038/nm0297-205. [DOI] [PubMed] [Google Scholar]
- 8.Koenig S, Conley A J, Brewah Y A, Jones G M, Leath S, Boots L J, Davey V, Pantaleo G, Demarest J F, Carter C, et al. Nat Med. 1995;1:330–336. doi: 10.1038/nm0495-330. [DOI] [PubMed] [Google Scholar]
- 9.Price D A, Goulder P J, Klenerman P, Sewell A K, Easterbrook P J, Troop M, Bangham C R, Phillips R E. Proc Natl Acad Sci USA. 1997;94:1890–1895. doi: 10.1073/pnas.94.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goulder P J, Phillips R E, Colbert R A, McAdam S, Ogg G, Nowak M A, Giangrande P, Luzzi G, Morgan B, Edwards A, et al. Nat Med. 1997;3:212–217. doi: 10.1038/nm0297-212. [DOI] [PubMed] [Google Scholar]
- 11.Schmitz J E, Kuroda M J, Santra S, Sasseville V G, Simon M A, Lifton M A, Racz P, Tenner-Racz K, Dalesandro M, Scallon B J, et al. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 12.Jin X, Bauer D E, Tuttleton S E, Lewin S, Gettie A, Blanchard J, Irwin C E, Safrit J T, Mittler J, Weinberger L, et al. J Exp Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenberg E S, Altfeld M, Poon S H, Phillips M N, Wilkes B M, Eldridge R L, Robbins G K, D'Aquila R T, Goulder P J, Walker B D. Nature (London) 2000;407:523–526. doi: 10.1038/35035103. [DOI] [PubMed] [Google Scholar]
- 14.Letvin N L. J Clin Invest. 1998;102:1643–1644. doi: 10.1172/JCI5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brander C, Goulder P J R. In: HIV Molecular Immunology Database 1999. Korber B T M, Brander C, Walker B D, Koup R A, Moore J, Haynes B, Meyers G, editors. Los Alamos, NM: Los Alamos Natl. Lab.; 1999. [Google Scholar]
- 16.Collins K L, Chen B K, Kalams S A, Walker B D, Baltimore D. Nature (London) 1998;391:397–401. doi: 10.1038/34929. [DOI] [PubMed] [Google Scholar]
- 17.Van Baalen C A, Schutten M, Huisman R C, Boers P H, Gruters R A, Osterhaus A D. J Virol. 1998;72:6851–6857. doi: 10.1128/jvi.72.8.6851-6857.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janssen R S, Satten G A, Stramer S L, Rawal B D, O'Brien T R, Weiblen B J, Hecht F M, Jack N, Cleghorn F R, Kahn J O, et al. J Am Med Soc. 1998;280:42–48. doi: 10.1001/jama.280.1.42. [DOI] [PubMed] [Google Scholar]
- 19.Bunce M, Fanning G C, Welsh K I. Tissue Antigens. 1995;45:81–90. doi: 10.1111/j.1399-0039.1995.tb02422.x. [DOI] [PubMed] [Google Scholar]
- 20.Johnson R P, Trocha A, Yang L, Mazzara G P, Panicali D L, Buchanan T M, Walker B D. J Immunol. 1991;147:1512–1521. [PubMed] [Google Scholar]
- 21.Walker B D, Flexner C, Birch-Limberger K, Fisher L, Paradis T J, Aldovini A, Young R, Moss B, Schooley R T. Proc Natl Acad Sci USA. 1989;86:9514–9518. doi: 10.1073/pnas.86.23.9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walker B D, Chakrabarti S, Moss B, Paradis T J, Flynn T, Durno A G, Blumberg R S, Kaplan J C, Hirsch M S, Schooley R T. Nature (London) 1987;328:345–348. doi: 10.1038/328345a0. [DOI] [PubMed] [Google Scholar]
- 23.Altfeld M A, Trocha A, Eldridge R L, Rosenberg E S, Phillips M N, Addo M M, Sekaly R P, Kalams S A, Burchett S A, McIntosh K, et al. J Virol. 2000;74:8541–8549. doi: 10.1128/jvi.74.18.8541-8549.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larsson M, Jin X, Ramratnam B, Ogg G S, Engelmayer J, Demoitie M A, McMichael A J, Cox W I, Steinman R M, Nixon D, Bhardwaj N. AIDS. 1999;13:767–777. doi: 10.1097/00002030-199905070-00005. [DOI] [PubMed] [Google Scholar]
- 25.Pitcher C J, Quittner C, Peterson D M, Connors M, Koup R A, Maino V C, Picker L J. Nat Med. 1999;5:518–525. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]
- 26.Betts M R, Casazza J P, Patterson B A, Waldrop S, Trigona W, Fu T M, Kern F, Picker L J, Koup R A. J Virol. 2000;74:9144–9151. doi: 10.1128/jvi.74.19.9144-9151.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goulder P J R, Tang Y, Brander C, Betts M, Trocha A, He S, Rosenberg E S, Ogg G, O'Callaghan C A, Kalams S A, et al. J Exp Med. 2000;192:1819–1832. doi: 10.1084/jem.192.12.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sidney J, Grey H M, Kubo R T, Sette A. Immunol Today. 1996;17:261–266. doi: 10.1016/0167-5699(96)80542-1. [DOI] [PubMed] [Google Scholar]
- 29.Smith K J, Reid S W, Harlos K, McMichael A J, Stuart D I, Bell J I, Jones E Y. Immunity. 1996;4:215–228. doi: 10.1016/s1074-7613(00)80430-6. [DOI] [PubMed] [Google Scholar]
- 30.Goulder P J R, Bunce M, Krausa P, McIntyre K, Crowley S, Morgan B, Edwards A, Giangrande P, Phillips R E, McMichael A J. AIDS Res Hum Retroviruses. 1996;12:1691–1698. doi: 10.1089/aid.1996.12.1691. [DOI] [PubMed] [Google Scholar]
- 31.van Baalen C A, Pontesilli O, Huisman R C, Geretti A M, Klein M R, de Wolf F, Miedema F, Gruters R A, Osterhaus A D. J Gen Virol. 1997;78:1913–1918. doi: 10.1099/0022-1317-78-8-1913. [DOI] [PubMed] [Google Scholar]
- 32.Cullen B R. FASEB J. 1991;5:2361–2368. doi: 10.1096/fasebj.5.10.1712325. [DOI] [PubMed] [Google Scholar]
- 33.Nietfield W, Bauer M, Fevrier M, Maier R, Holzwarth B, Frank R, Maier B, Riviere Y, Meyerhans A. J Immunol. 1995;154:2189–2197. [PubMed] [Google Scholar]
- 34.Allen T M, O'Connor D H, Jing P, Dzuris J L, Mothe B R, Vogel T U, Dunphy E, Liebl M E, Emerson C, Wilson N, et al. Nature (London) 2000;407:386–390. doi: 10.1038/35030124. [DOI] [PubMed] [Google Scholar]
- 35.Cafaro A, Caputo A, Fracasso C, Maggiorella M T, Goletti D, Baroncelli S, Pace M, Sernicola L, Koanga-Mogtomo M L, Betti M, et al. Nat Med. 1999;5:643–650. doi: 10.1038/9488. [DOI] [PubMed] [Google Scholar]
- 36.Pauza C D, Trivedi P, Wallace M, Ruckwardt T J, Le Buanec H, Lu W, Bizzini B, Burny A, Zagury D, Gallo R C. Proc Natl Acad Sci USA. 2000;97:3515–3519. doi: 10.1073/pnas.070049797. . (First Published March 21, 2000; 10.1073/pnas.070049797) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Osterhaus A D, van Baalen C A, Gruters R A, Schutten M, Siebelink C H, Hulskotte E G, Tijhaar E J, Randall R E, van Amerongen G, Fleuchaus A, et al. Vaccine. 1999;17:2713–2714. doi: 10.1016/s0264-410x(98)00498-8. [DOI] [PubMed] [Google Scholar]
- 38.Gringeri A, Santagostino E, Muca-Perja M, Le Buanec H, Bizzini B, Lachgar A, Zagury J F, Rappaport J, Burny A, Gallo R C, Zagury D. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:371–375. doi: 10.1097/00042560-199904010-00007. [DOI] [PubMed] [Google Scholar]
- 39.Threlkeld S C, Wentworth P A, Kalams S A, Wilkes B M, Ruhl D J, Keogh E, Sidney J, Southwood S, Walker B D, Sette A. J Immunol. 1997;159:1648–1657. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.