Abstract
Phosphatidylinositol 3-kinase (PI3K) and phosphatase and tensin homolog deleted on chromosome 10 (PTEN) signaling pathway play an important role in multiple cellular functions such as cell metabolism, proliferation, cell-cycle progression, and survival. PI3K is activated by growth factors and angiogenesis inducers such as vascular endothelial growth factor (VEGF) and angiopoietins. The amplification and mutations of PI3K and the loss of the tumor suppressor PTEN are common in various kinds of human solid tumors. The genetic alterations of upstream and downstream of PI3K signaling molecules such as receptor tyrosine kinases and AKT, respectively, are also frequently altered in human cancer. PI3K signaling regulates tumor growth and angiogenesis by activating AKT and other targets, and by inducing HIF-1 and VEGF expression. Angiogenesis is required for tumor growth and metastasis. In this review, we highlight the recent studies on the roles and mechanisms of PI3K and PTEN in regulating tumorigenesis and angiogenesis, and the roles of the downstream targets of PI3K for transmitting the signals. We also discuss the crosstalk of these signaling molecules and cellular events during tumor growth, metastasis, and tumor angiogenesis. Finally, we summarize the potential applications of PI3K, AKT, and mTOR inhibitors and their outcome in clinical trials for cancer treatment.
I. INTRODUCTION OF PI3K/PTEN SIGNALING PATHWAY
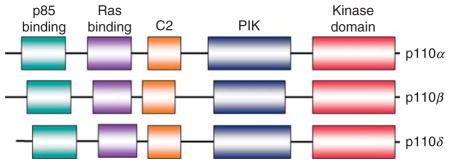
The phosphatidylinositol 3-kinases (PI3Ks) in mammalian cells form a family that can be divided into three classes, class I, II, and III, based on their structure, substrate, distribution, mechanism of activation, and functions (Domin and Waterfield, 1997; Walker et al., 1999). Among these classes, class I PI3Ks are the best understood to play vital roles in regulating cell proliferation, growth, and survival initiated by many growth and survival factors (Cantley, 2002; Fruman et al., 1999; Morita et al., 1999). Based on different associated adaptors, class I PI3Ks are divided into class IA and IB PI3Ks. Class IA PI3Ks are activated by receptor tyrosine kinases (RTKs), while class IB PI3Ks are activated by G-protein-coupled receptors (GPCRs) (Engelman et al., 2006; Vanhaesebroeck et al., 1997). Class IA PI3Ks consist of the heterodimers of a p110 catalytic subunit and a p85 regulatory subunit, and use phosphatidylinositol, phosphatidylinositol-4-phosphate (PIP), and phosphatidylinositol-4,5-bisphosphate (PIP2) as substrates. Three isoforms of p110, p110α, p110β, and p110δ are encoded by PIK3CA, PIK3CB, and PIK3CD, respectively. There are also three isoforms of p85 subunit: p85α, p85β, and p85γ that are encoded by PIK3R1, PIK3R2, and PIK3R3, respectively. Class IB PI3Ks are composed of the heterodimers of a p110γ catalytic subunit and a p101 regulatory subunit or its homologues p84 or p87PIKAP (PI3Kγ adaptor protein of 87 kDa). Class II PI3Ks include PIK3C2α, PIK3C2β, and PIK3C2γ, all of them are characterized by containing a common C2 domain at the C-terminus. Class II PI3Ks can also be activated by RTKs, cytokine recepors and integrins, and use phosphatidylinositol and PIP as substrates (Arcaro et al., 2000; Falasca and Maffucci, 2007; MacDougall et al., 2004; Wheeler and Domin, 2001). But the specific functions of class II PI3Ks in response to these activators are poorly understood. Class III PI3Ks are composed of the heterodimers of catalytic and adaptor subunits. This class of PI3Ks only uses phosphatidylinositol as a substrate (e.g., mammalian PI3K and yeast Vps34p). The structure of PI3K family is shown in Box 1. It has been indicated that class III PI3Ks are involved in the regulation of mammalian target of rapamycin (mTOR) activity in response to amino acid levels, and the regulation of autophagy in response to cellular stress (Gulati et al., 2008; Tassa et al., 2003). The class III PI3K Vps34 is present in all eukaryotic organisms, while both class I and II PI3Ks only exist in multicellular organisms.
The two subfamilies of class IA and IB PI3Ks have evolved in mammals. Class I, especially class IA PI3Ks, are the most extensively investigated in regulating cellular functions such as cell proliferation, growth, and survival. Class I PI3Ks catalyze the conversion of PIP2 at the D-3 position to phosphatidylinositol-3,4,5-trisphosphate (PIP3) via its regulatory subunit p85 linking to upstream receptors that are activated by growth factors or hormones (Cantley, 2002; Luo et al., 2006; Zhao et al., 2006). RTKs, such as epidermal growth factor receptor (EGFR), platelet-derived growth factor receptor (PDGFR), fibroblast growth factor receptor (FGFR), and insulin-like growth factor 1 receptor (IGF-1R), can interact with the p85 regulatory subunit to activate PI3K (Hu et al., 1992; McGlade et al., 1992; Zhu et al., 1992), while Ras protein directly interacts with the p110 catalytic subunit of PI3K in a GTP-dependent manner (Peyssonnaux et al., 2000; Rodriguez-Viciana et al., 1996). In addition, p85 subunit also binds to the intracellular proteins such as protein kinase C, SHP1, Rac, Rho, hormonal receptors, mutated Ras and Src, providing an integration point for p110 activation (Hennessy et al., 2005). It has been demonstrated that PI3K can be regulated by the molecular switch, which is formed by a GTPase-responsive domain and an inhibitory domain on p85 regulatory subunit of PI3K. H-Ras and Rac1 activate PI3K by targeting the GTPase-responsive domain and the stimulatory effects can be blocked by the inhibitory domain, which functions by binding to tyrosine-phosphorylated molecules (Chan et al., 2002).
Phosphatase and tensin homolog deleted on chromosome 10 (PTEN), which is also known as MMAC1 or TEP1, was named due to its sequence homology with phosphatases and the cytoskeletal protein tensin (Dahia et al., 1997; Li et al., 1997b; Maehama and Dixon, 1998). PTEN is a tumor suppressor commonly mutated in many human cancers (Salmena et al., 2008). PTEN locates on 10q23.3, which encodes a 403-residue dual-specificity phosphatase that has protein phosphatase activity, and lipid phosphatase activity that antagonizes PI3K activity (Maehama and Dixon, 1998). Since the product of p110α, PIP3, is a second messenger for promoting cell proliferation, growth, metabolism, and survival, PTEN hydrolyzes the 3-phosphate on PIP3 to generate PIP2, and negatively regulates PIP3-mediated signaling pathways. Thus, PTEN plays an important role in phosphatidylinositol homeostasis (Maehama and Dixon, 1998). It has been demonstrated that PTEN can be upregulated by early growth regulated transcription factor 1 (EGR1) through direct binding to the PTEN promoter. In addition, peroxisome proliferator activated receptor γ (PPARγ), p53, and activating transcription factor 2 (ATF2) can also transcriptionally upregulate PTEN by binding to its promoter (Patel et al., 2001; Shen et al., 2006; Stambolic et al., 2001), while transforming growth factor (TGF)-β, nuclear factor kappaB (NF-κB), and Jun negatively regulate PTEN mRNA expression (Hettinger et al., 2007; Mahimainathan et al., 2006; Xia etal., 2007). Recently, it has been found that some microRNAs such as miR-21, miR-19a, and miR-214 inhibit PTEN through targeting the 3′-untranslated region (UTR) of PTEN, leading to inhibition of PTEN translation (Meng et al., 2007; Pezzolesi et al., 2008; Yang et al., 2008). PTEN activity can also be regulated by the posttranslational regulation including phosphorylation, acetylation, oxidation, and control of its localization (Gericke et al., 2006; Ikenoue et al., 2008; Leslie, 2006; Planchon et al., 2008; Tamguney and Stokoe, 2007).
Serine–threonine protein kinase AKT (also known as protein kinase B) is initially found to be the cellular homolog of AKT8 retroviral oncogene (Bellacosa et al., 1991). AKT is one of the most important downstream targets of PI3K. Human AKT has three isoforms: AKT1, AKT2, and AKT3 (also known as PKBα, PKBβ, and PKBγ, respectively). The product of PI3K, PIP3, binds to AKT and leads to the membrane recruitment of AKT, and also binds to phosphoinositide-dependent kinase 1 (PDK1) via their plekstrin homology (PH) domains (Downward, 1998; Engelman et al., 2006), then PDK1 phosphorylates AKT in the kinase domain (Thr 308 in AKT1). For the full activation of AKT, the phosphorylation within the carboxyl-terminal hydrophobic motif (Ser 473 in AKT1) of AKT by PDK2 is required (Hresko et al., 2003; Sarbassov et al., 2005; Stokoe et al., 1997). Once activated, AKT moves to the cytoplasm and nucleus, where it phosphorylates, activates, or inhibits many downstream targets to regulate various cellular functions including cell metabolism, protein synthesis, cell survival/inhibition of apoptosis, and cell-cycle progression (Box 1). In this review, we will focus on the roles of class IA PI3Ks, PTEN, and AKT in tumor growth and angiogenesis.
II. ANGIOGENESIS REGULATED BY VEGF, ANGIOPOIETINS, AND PI3K ACTIVATION
Angiogenesis is the process by which new blood capillaries are generated from the preexisting vasculature. It is essential for the embryo development, female reproduction, tissue repair, inflammatory diseases, tumor growth, and metastasis. Tumor angiogenesis occurs by sprouting the new vessels from preexisting blood vessels or by inserting interstitial tissue columns into the lumen of preexisting vessels (Carmeliet and Jain, 2000). This process can be triggered by extracellular signals such as growth factors, by genetic alterations such as activation of oncogenes including PI3K, and by mutations of tumor suppressor genes such as PTEN and p53 (Carmeliet and Jain, 2000; Folkman, 1995). Among all the proangiogenic factors, vascular endothelial growth factor (VEGF) and angiopoietins (Ang) and their receptors—VEGF and Tie [tyrosine kinase with immunoglobulin (Ig) and EGF homology domains] receptors play important roles during tumor growth and angiogenesis.
VEGFR family and the Tie receptor family are expressed specifically in endothelium. The VEGF family members are secreted, dimeric glycoproteins. In mammals, VEGF family members consist of VEGF-A, -B, -C, -D, and placenta growth factor (PLGF) (Olsson et al., 2006). VEGF-A plays a key role in vasculogenesis and angiogenesis. Genetic studies have demonstrated that VEGF-A gene knockout mice either homozygotes or heterozygotes die in the embryonic stage due to the defects in vasculature (Carmeliet et al., 1996; Ferrara et al., 1996). There are five human isoforms of VEGF-A: VEGF121, VEGF145, VEGF165, VEGF189, and VEGF206. Among them, VEGF121, VEGF165, and VEGF189 are the dominant subtypes based on the amount and biological activity (Olsson et al., 2006; Shibuya, 2008). VEGF receptors have three family members: VEGFR1 (fms-like tyrosine kinase, Flt-1), VEGFR2 (Flk-1/KDR), and VEGFR3 (Flt-4). All three VEGF receptors contain tyrosine phosphorylation sites with regulatory and signaling functions. These receptors play critical role in promoting vasculogenesis during normal embryogenesis and pathologic angiogenesis. VEGF-A binds to both VEGFR1 and VEGFR2 to regulate tumorigenesis and angiogenesis, while VEGF-B and PLGF bind to VEGFR1. Under pathological conditions, the increased PLGF and VEGF-A can recruit monocytes/macrophages via VEGFR1 to cancer tissues or inflammatory lesions, and significantly induce angiogenesis (Brown et al., 2001; Murakami et al., 2008). VEGF-C and -D mainly bind to VEGFR3, and stimulate lymphangiogenesis.
VEGFR1 binds to the p85 regulatory subunit of PI3K on Tyr1213 and 1333 and has crosstalk with VEGFR2 in controlling cell migration, differentiation, and angiogenesis (Autiero et al., 2003; Cunningham et al., 1995). VEGFR2 is the predominant receptor in angiogenic signaling since it regulates endothelial cell migration, proliferation, differentiation and survival, as well as vessel permeability and dilation (Cebe-Suarez et al., 2006). It has been demonstrated that tyrosines 799 and 1173 of VEGFR2 are binding sites for the p85 subunit, and that activation of PI3K is responsible for endothelial cell proliferation (Dayanir et al., 2001). Previous study showed that VEGFR2 was associated with p85 regulatory subunit of PI3K to phosphorylate p85 subunit, resulting in increased PI3K and AKT activities in vitro (Gerber et al., 1998). Grb2-adapter binder 1 (Gab1) PH domain serves as a primary actor in coupling VEGFR2 to PI3K through an amplification loop involving PIP3 and its PH domain (Dance et al., 2006; Laramee et al., 2007). VEGF-induced endothelial cell survival was blocked by PI3K inhibitors, wortmannin and LY294002, and by overexpression of a dominant-negative form of AKT (AKT-DN) (Gerber et al., 1998). VEGFR3 is expressed in developing veins and lymphatics, in blood vessels in the vicinity of tumors, and in several benign and malignant tumor cells (Cebe-Suarez et al., 2006). VEGFR3 can promote cell migration and survival in lymphatic endothelial cells by activating PI3K and mitogen-activated protein kinase (MAPK) pathways (Lin et al., 2005; Makinen et al., 2001).
The angiopoietins are a family of secreted proteins including three human angiopoietins (Ang-1, Ang-2, and Ang-4), and one mouse angiopoietin, Ang-3. Ang-1 is an angiogenic growth factor with a central role in promotion of structural integrity in the vasculature. Both Ang-1 and Ang-2 can bind to Tie2 receptor. Ang-1 is a Tie2 agonist, while Ang-2 could act as either a context-dependent competitive antagonist or an agonist depending on cell type and microenvironmental conditions (Davis et al., 1996; Maisonpierre et al., 1997). Transgenic overexpression of both Ang-1 and Ang-2 led to vascular defects (Sato et al., 1995). Ang-3 is moderately expressed in multiple mouse tissues, and functions as a Tie2 activator or as a Tie2 antagonist. Ang-4 mRNA is abundantly expressed in human lungs, and functions as a Tie2 agonist (Jones et al., 2001; Makinde and Agrawal, 2008). The Tie receptor family is comprised of Tie1 and Tie2/Tek. Ang-1, 2, 3, and 4 are specific ligands for Tie2. The specific ligand for Tie1 is unknown. The phosphorylation of Tie1 is dependent on Tie2 activation, suggesting that Tie2 tyrosine kinase domain may be responsible for phosphorylating Tie1 as a result of heterodimerization (Yuan et al., 2007). Tie2 is expressed not only in vascular cells, but also in cancer cells. Several tumor cells express high levels of Ang-1, indicating an autocrine/paracrine loop of Ang-1-Tie2 signaling in the tumor. Genetic studies have showed that deletion of Ang-1 or Tie2 genes led to severe defects in the vasculature and subsequent lethality, suggesting that Ang-1/Tie2 signaling pathway is required in microvascular development (Makinde and Agrawal, 2008). There are several lines of evidence suggesting that PI3K/AKT signaling plays a major role in Ang-1-mediated cell migration, survival, and angiogenesis: (1) Ang-1 was shown to induce phosphorylation of Tie2, then recruited and interacted with p85 subunit of PI3K in a phosphotyrosine-dependent manner through their Src homology 2 (SH2) domains, resulting in the induction of PI3K activities and activation of AKT (Jones et al., 1999); (2) Ang-1 induced survival, migration, and sprouting of endothelial cells through PI3K and AKT activation (Jones et al., 1999; Kanda et al., 2005; Kim et al., 2000); (3) In vivo studies also showed that Ang-1 induced angiogenesis through increasing AKT phosphorylation and PI3K-mediated endothelial nitric oxide synthase (eNOS) activation (Babaei et al., 2003; Cho et al., 2004).
III. GENETIC ABERRATIONS OF PI3K, PTEN, AND AKT IN CANCER
PI3K activation is implicated to be involved in oncogenesis by the observation that PI3K is associated with the Src and the middle T oncoproteins (Sugimoto et al., 1984; Whitman et al., 1985). The activation of PI3K is through the interaction with p85 regulatory subunit of PI3K, which contains SH2 domains that bind to phosphotyrosines, and localize PI3K to the plasma membrane (Otsu et al., 1991). The p110α catalytic subunit of PI3K was initially identified as an oncogene from the spontaneous chicken tumor (Chang et al., 1997). The expression of active PI3K by avian retrovirus induced the transformation of chick embryo fibroblasts in vitro, and induced tumor in chicken (Chang et al., 1997). Abnormalities of PI3K upstream molecules are common in cancer and this cascade has a role in tumorigenesis and neoplastic transformation. PI3K is also frequently mutated in various kinds of human cancers such as ovarian, breast, gastric, bowel, brain, colon, and hepatocellular carcinomas (Engelman et al., 2006; Hennessy et al., 2005; Jiang and Liu, 2008). The amplification of PIK3CA, the gene encoding p110α catalytic subunit of PI3K, was observed in ovarian, cervical, gastric, and breast cancers (Engelman et al., 2006; Hennessy et al., 2005; Jiang and Liu, 2008). In addition, the somatic missense mutations of PIK3CA are the most frequently genetic aberrations in breast cancer, especially in HER2-amplified and hormone-receptor-positive breast cancers (Paradiso et al., 2007). The mutations of PIK3CA were also found in colorectal, gastric, lung, ovarian, hepatocellular, thyroid, endometrial cancers, glioblastomas, acute leukemia, as well as in malignancies of the central nervous system (Campbell et al., 2004; Jiang and Liu, 2008; Samuels et al., 2004). The p85 regulatory subunit dimerizes with p110 catalytic subunit, and inhibits PI3K activity in normal cells. The deletion of p85 protein that lacks the inhibitory domain, and loss of the autophosphorylation site at the p85 inhibitory domain, commonly increases PI3K activity. The deletion and somatic mutations of p85α regulatory subunit (PIK3R1) were rare, and occurred in primary human glioblastoma, colon, ovarian cancers, and lymphoma (Jucker et al., 2002; Philp et al., 2001). Recent study has demonstrated that PIK3CA-knockout mouse embryonic fibroblasts are deficient in cellular signaling in response to various growth factors, unable to differentiate into adipocytes, and are resistant to oncogenic transformation induced by RTKs (Zhao et al., 2006). Another genetic study indicated that the kinase activity of p110β was required for GPCR signaling triggered by lysophosphatidic acid and had a function in oncogenic transformation.
PTEN was first discovered as the tumor suppressor on human chromosome 10q23 in 1997 (Li et al., 1997a; Steck et al., 1997). PTEN is highly susceptible to deletion or mutation in many human malignancies including brain, breast, kidney, and prostate cancers (Li et al., 1997a; Steck et al., 1997). A serial of studies have shown that the tumor suppressor PTEN is frequently mutated or lost in many kinds of human primary cancers including glioblastomas, kidney and uterine endometrioid carcinomas, breast cancer, lung cancer, colon cancer, and melanoma (Jiang and Liu, 2008; Salmena et al., 2008; Steck et al., 1997). In addition, the decreasing levels of PTEN expression are correlated with the progressive outcome of solid cancers, including ovarian, prostate, and cervical cancers (Harima et al., 2001; Yoshimoto et al., 2007). PTEN germline mutations lead to a group of autosomal dominant syndromes including Cowden syndrome, Lhermitte–Duclos disease, Bannayan–Riley–Ruvalcaba syndrome, and Proteus and Proteus-like syndromes characterized by developmental disorders, neurological deficits, multiple hamartomas, and an increased risk of breast, thyroid, and endometrial cancers (Liaw et al., 1997; Marsh et al., 1997; Tsou et al., 1997; Tsuchiya et al., 1998). Mice with PTEN deletion and mutation are highly susceptible to tumor induction and conditional knockout of PTEN leads to neoplasia in multiple organs such as the mammary gland, skin, and prostate (Backman et al., 2004; Li et al., 2002; Suzuki et al., 1998). In an animal model of prostate tumor induced by PTEN loss, ablation of p110β impeded tumorigenesis with a concomitant diminution of AKT phosphorylation (Jia et al., 2008), indicating the important role of p110β in cell transformation and tumorigenesis. These studies demonstrate the key roles of PI3K and PTEN in cancer development. The transgenic ablation models of PI3K and PTEN in tumorigenesis are summarized in Table I.
Table I.
Transgenic Ablation Models of PI3K/PTEN/AKT Signaling Pathway Related to Carcinogenesis, Vasculature, and Angiogenesis
| Targeted subunit | Genetic alteration | Comments |
|---|---|---|
| p110α | p110α−/− | Embryonic lethality, multiple vascular defects, lower Tie2 protein levels (Lelievre et al., 2005) |
| Endothelial cell-specific-p110α−/− | Embryonic lethality at mid gestation because of severe defects in angiogenic sprouting and vascular remodeling (Graupera et al., 2008) | |
| p110γ | p110γ−/− | The significantly diminished vascular permeability in response to both Ras and VEGF (Serban et al., 2008) |
| p85α/p55α/p50α (pan-p85α) | pan-p85α−/− | Embryonic lethality, subepidermal blebs flanking the neural tube and bleeding into the blebs during the turning process (Brachmann et al., 2005) |
| p85 subunits | Muscle-specific pan-p85α−/− p85β−/− | Viable, exhibit attenuated AKT signaling in the heart, reduced heart size, and altered cardiac gene expression (Luo et al., 2005) |
| PTEN | PTEN−/− | Early embryonic lethality |
| PTEN+/− | Showed neoplasms in multiple organs including prostate, skin and endometrium, liver, colon, gastrointestinal tract, and thymus, spontaneously developed germ cell, gonadostromal, breast, thyroid tumors, and lymphomas (Di Cristofano et al., 1998; Podsypanina et al., 1999; Stambolic et al., 2000; Suzuki et al., 1998) | |
| PTEN−/− in smooth muscle cells | Died before 6 weeks, increase in phosphorylated AKT in major vessels, hearts, and lungs, pathological vascular remodeling and vascular recruitment of progenitor cells, widespread smooth muscle cell hyperplasia and abdominal leiomyosarcomas (Hernando et al., 2007; Nemenoff et al., 2008) | |
| Bronchioalveolar epithelium-specific PTEN−/− | 90% of SOPtenflox/flox(E10–E16) mice died within 2 h of birth, surviving mice developed spontaneous lung adenocarcinomas with hyperplasia of bronchioalveolar epithelial cells and myofibroblast precursors, enlarged alveolar epithelial cells, and impaired production of surfactant proteins. K-ras was frequently mutated in adeno carcinomas (Yanagi et al., 2007) | |
| Hepatocyte-specific PTEN−/− | Massive hepatomegaly and steatohepatitis with triglyceride accumulation followed by liver fibrosis and hepatocellular carcinoma (Horie et al., 2004; Watanabe et al., 2007) | |
| PTEN−/− in endothelial cells | Embryonic lethality due to endothelial cell hyperproliferation and impaired vascular remodeling (Suzuki et al., 2007) | |
| PTEN−/− in endothelial cells | Enhances postnatal neovascularization, including tumor angiogenesis necessary for tumor growth (Suzuki et al., 2007) | |
| Urothelium-specific PTEN−/− | Exhibited urothelial hyperplasia, 10% of mice spontaneously developed pedicellate papillary transitional cell carcinomas (Tsuruta et al., 2006) | |
| Pancreas-specific PTEN−/− | Progressive replacement of the acinar pancreas with highly proliferative ductal structures, a fraction of these mice develop ductal malignancy (Stanger et al., 2005) | |
| Prostate-targeted PTEN−/− | Hyperproliferation and neoplastic changes in prostate (Backman et al., 2004; Ma et al., 2005; Trotman et al., 2003; Wang et al., 2003, 2006) | |
| Astrocytes-specific PTEN−/− | Hypertrophy and increased proliferation of astrocytes in vivo (Fraser et al., 2004) | |
| Skin-specific PTEN−/− | Hyperproliferation and spontaneous tumorigenesis of the skin keratinocytes (Komazawa et al., 2004) | |
| PTEN+/− in primordial germ cells | Testicular teratoma and enhanced embryonic germ cell production (Kimura et al., 2003) | |
| Mammary-specific PTEN−/− | Precocious development and neoplasia in the mammary gland (Li et al., 2002) | |
| AKT1 | AKT1−/− | Smaller litter sizes, reduced fetal weight, and a higher fetal mortality due to the impaired extraembryonic vascularization and placental hypotrophy (Chen et al., 2001; Cho et al., 2001b; Yang et al., 2003) Impairment of blood vessel maturation and increased vascular permeability, reduced activation of eNOS, and reduced expression of thrombospondins 1 (TSP-1) and TSP-2 (Chen et al., 2005) Defective ischemia-and VEGF-induced angiogenesis and severe peripheral vascular disease (Ackah et al., 2005) Abrogated polarity, migratory directionality, and breast cancer onset of mammary epithelial cells with ErbB2 overexpression (Ju et al., 2007) Resistant to tumors and skin carcinogenesis induced MMTV-v-H-Ras- induced (Skeen et al., 2006) |
| AKT2 | AKT2−/− | Displayed normal cardiac growth in responses to provocative stimulation, and sensitized to cardiomyocyte apoptosis in response to ischemic injury (DeBosch et al., 2006) |
IV. ROLES OF PI3K AND AKT IN REGULATING ANGIOGENESIS
PI3K/AKT signaling pathway also plays an important role in regulating the vasculature and angiogenesis. In zebrafish, K-ras/PI3K/AKT signaling is essential for hematopoiesis and angiogenesis (Liu et al., 2008a). The direct evidence of PI3K and AKT involvement in regulating angiogenesis in vivo was initially observed by the forced expression of PI3K and AKT using RCAS retroviral vector system (Jiang et al., 2000). Overexpression of PI3K or AKT induced angiogenesis, while overexpression of PTEN or of dominant-negative constructs of PI3K inhibited angiogenesis in chicken embryos, suggesting that PI3K signaling is required for normal embryonal angiogenesis (Jiang et al., 2000). Mice deficient in the p110α catalytic subunit of PI3K displayed multiple vascular defects, including dilated vessels in the head, reduced branching morphogenesis in the endocardium, lack of hierarchical order of large and small branches in the yolk sac, impaired development of anterior cardinal veins, and significant decrease of Tie2 protein level (Lelievre et al., 2005). In mice deficient in p110γ, the vascular permeability response to both Ras and VEGF was significantly diminished, suggesting that PI3Kγ is necessary and sufficient for vascular permeability (Serban et al., 2008). Endothelial cell-specific-p110α−/− led to embryonic lethality at mid-gestation due to severe defects in angiogenic sprouting and vascular remodeling (Graupera et al., 2008). Knockout of p85α/p55α/p50α caused perinatal lethality with bleeding into the blebs during the turning process (Brachmann et al., 2005). Muscle-specific pan-p85α−/− p85β−/− mice exhibited reduced heart size and altered cardiac gene expression (Luo et al., 2005). Mutated p110α proteins show a gain of enzymatic function in vitro. Recent studies show that three prevalent mutants of p110α, E542K, E545K, and H1047R, are oncogenic in vivo (Bader et al., 2006). These tumors are marked by increased angiogenesis and the activation of AKT pathway (Bader et al., 2006).
AKT was initially found to be the homolog of a viral oncogene (Bellacosa et al., 1991). In various kinds of tumors, AKT is also overexpressed or amplified, with elevated level of AKT phosphorylation (Hennessy et al., 2005; Jiang and Liu, 2008). There are several reports showing the genetic amplification of AKT isoforms. AKT1 amplification has been observed in gastric adenocarcinoma, glioblastoma, gliosarcoma, and high-grade gliomas (Jiang and Liu, 2008; Liaw et al., 1997; Sasaki et al., 2003; Staal, 1987). AKT2 amplification or mutations are found in head and neck squamous cell carcinoma, pancreatic, ovarian, breast, and colorectal cancers (Hennessy et al., 2005; Jiang and Liu, 2008). Increased AKT3 mRNA level is correlated to breast and prostate cancers (Nakatani et al., 1999). Recent studies have shown that AKT1−/− mice are resistant to ErbB2-or MMTV-v-H-Ras-induced carcinogenesis, indicating the key role of AKT1 in oncogenesis (Ju et al., 2007; Skeen et al., 2006). Among three isoforms of AKT, AKT1 shows closely related with vasculature during animal development and pathological angiogenesis. AKT1−/− mice have defects in both fetal and postnatal growth into adulthood with smaller litter sizes and reduced fetal weight (Chen et al., 2001; Cho et al., 2001b). Since AKT1 is widely expressed in placenta including all types of trophoblast and vascular endothelial cells, AKT1−/− mice exhibited a higher fetal mortality due to the impaired extraembryonic vascularization and placental hypotrophy, indicating the significant role of AKT1 in fetal development and vascularization (Yang et al., 2003). AKT1 is the predominant isoform in vascular cells. AKT1−/− mice showed impaired vascular maturation due to reduced activation of eNOS and the major phenotypic changes in vascular permeability and angiogenesis with decreased expression of thrombospondins 1 and 2 (TSP-1 and TSP-2) (Chen et al., 2005). AKT1 is critical for ischemic-and VEGF-induced angiogenesis. AKT1−/− mice exhibited defective ischemia-and VEGF-induced angiogenesis and showed severe peripheral vascular disease. In response to ischemia, AKT1−/− mice had much less endothelial progenitor cell (EPC) mobilization. Intravenous administration of EPCs from wild-type AKT1 mice, but not EPCs isolated from AKT1−/− mice, into mice improved limb blood flow, increased the migration of fibroblasts and endothelial cells after femoral ligation. These results indicate that AKT1 is sufficient and essential for regulating ischemia-induced angiogenesis (Ackah et al., 2005). AKT2−/− mice displayed normal cardiac growth in response to provocative stimulation, and were sensitized to cardiomyocyte apoptosis in response to ischemic injury (DeBosch et al., 2006). The studies on transgenic models related to vasculature and angiogenesis are summarized in Table I.
V. PI3K/PTEN CONTROLS ANGIOGENESIS THROUGH INCREASING HIF-1 AND VEGF EXPRESSION
Hypoxia is an integral characteristic of the tumor microenvironment, associated with accelerated neoplastic growth. Hypoxia-inducible factor 1 (HIF-1) is a heterodimer consisting of HIF-1α and HIF-1β [also known as the aryl hydrocarbon nuclear translocator (ARNT)] subunits, and acts as a mediator of transcriptional activation in responses to hypoxia (Wang et al., 1995). HIF-1α is rapidly degraded under normoxic conditions by hydroxylation at several proline residues, and acetylation at lysine 5328 (Jeong et al., 2002; Semenza, 2000). The von Hippel-Lindau tumor suppressor gene product, pVHL, functions as the substrate recognition component of an E3-ubiquitin ligase, which targets the oxygen-sensitive HIF alpha-subunit for rapid proteasomal degradation under normoxic conditions and as such plays a central role in oxygen sensing (Maxwell et al., 1999). Hypoxia or lossof pVHL inhibits prolyl-hydroxylation, leading toaccumulationof HIF-1α protein in the cytoplasm (Kapitsinou and Haase, 2008). Growth factors, cytokines, and other signaling molecules stimulate HIF-1α synthesis via activation of PI3K or MAPK pathways (Mazure et al., 1997; Zhong et al., 2000). HIF-1 regulates VEGF expression by binding to the hypoxia responsive element (HRE) of VEGF promoter (Levy et al., 1995; Wang et al., 1995). HIF-1 can activate more than 60 known genes, which are related to cell proliferation, survival, apoptosis, cell mortality, adhesion, erythropoiesis, cytoskeletal structure, pH regulation, epithelial homeostasis, drug resistance, iron, nucleotide, glucose, energy, amino acid, and extracellular-matrix metabolisms, vascular tone, and angiogenesis (Semenza, 2003). HIF1α is upregulated in many human cancers. Among all the angiogenic factors, VEGF is the most potent one in physiological and pathological angiogenesis.
HIF-1α expression is regulated by PI3K activation in response to growth factors. Insulin and EGF induced expression of HIF-1α and VEGF by PI3K signaling pathway (Jiang et al., 2001). Cobalt and hypoxia induced HIF-1α expression through PI3K-dependent mechanism in airway smooth muscle and pulmonary artery smooth muscle cells (Belaiba et al., 2007; Chachami et al., 2004). HIF-1-dependent gene transcription was blocked by AKT-DN or PI3K, and by wild-type PTEN, whereas transcription was stimulated by constitutively active form of AKT. PI3K inhibitor LY294002 and mTOR inhibitor rapamycin also inhibited growth factor-and mitogen-induced secretion of VEGF, which may provide the connection of PI3K/PTEN/AKT to mTOR, HIF-1, and tumor angiogenesis (Jiang et al., 2001; Zhong et al., 2000). On the other hand, overexpression of PI3K or AKT elevated the mRNA levels of VEGF. LY294002 suppressed VEGF mRNA expression, while this inhibition was restored by overexpression of PI3K or AKT (Jiang et al., 2000). These results indicate that PI3K is sufficient to induce angiogenesis, and the effect may be partially through increasing HIF-1 and VEGF expression. Similarly, VEGF transcriptional activation in ovarian cancer cells was regulated by PI3K/AKT through HIF-1α expression (Skinner et al., 2004). A number of studies have demonstrated that PI3K/PTEN/AKT signaling regulates HIF-1 and VEGF expression in different types of cancer cells, Ras-transformed cells, airway smooth muscle cells, pulmonary artery smooth muscle cells, osteoblasts, pulmonary vascular endothelial cells, and mast cells (Belaiba et al., 2007; Carver et al., 2007; Chachami et al., 2004; Jiang et al., 2001; Lee et al., 2008; Mazure et al., 1997; Trisciuoglio et al., 2005; Yen et al., 2005; Zhong et al., 2000). Mast cells mediated VEGF expression by HIF-1α activation through PI3K-HIF-1α pathway in mice with allergic airway disease, resulting in the increase of vascular permeability (Lee et al., 2008). Hypoxia exposure of melanoma cells overexpressing bcl-2 activated phosphorylation of AKT and extracellular signal-regulated kinase (ERK)1/2 proteins, induced VEGF and HIF-1 expression, which can be suppressed by PI3K and MAPK inhibitors, suggesting that bcl-2 synergizes with hypoxia to promote expression of angiogenesis factors in melanoma cells through both PI3K and ERK pathways (Trisciuoglio et al., 2005).
Consistent with those results in vitro, in vivo studies showed that LY294002 significantly decreased the tumor burden of mice and inhibited peritoneal and tumor vascularization, which resulted in numerous leaky, irregular, tortuous vessels in scant, straight, relatively impermeable vessels, demonstrating the role of PI3K in mediating angiogenesis and vascular permeability associated with ovarian carcinoma (Hu et al., 2005). Specific downregulation of p110α expression in ovarian cancer cells using small interfering RNA (siRNA) showed that p110α knockdown greatly decreased ovarian tumor growth and angiogenesis, inhibited VEGF expression through decreasing HIF-1α expression in both ovarian cancer cells and tumor tissues. Moreover, AKT1 is a major downstream mediator for regulating tumor growth, angiogenesis, and VEGF expression, suggesting that p110α and AKT1 play an important role in tumor growth by inducing angiogenesis and by increasing HIF-1αand VEGF expression (Xia et al., 2006). Inhibition of PI3K activity by LY294002 decreased cancer cell-induced angiogenesis (Fang et al., 2007). Reconstitution of PTEN or over-expression of AKT dominant negative also inhibited angiogenesis and tumor growth associated with the decrease of HIF-1α and VEGF expression in the tumor xenographs (Fang et al., 2007). These results suggest that PI3K and AKT may regulate tumorigenesis and angiogenesis through HIF-1 and VEGF expression in cancer cells.
VI. THE DOWNSTREAM SIGNALING MOLECULES MEDIATED BY PI3K/PTEN IN REGULATING TUMOR GROWTH AND ANGIOGENESIS
Overexpression and activation of AKT play an important role in carcinogenesis (Engelman et al., 2006; Hennessy et al., 2005; Jiang and Liu, 2008). The mutations or deletions of PTEN are presented in many kinds of solid tumors. As shown in Fig. 1, upon the stimulation of VEGF and other growth factors, RTKs can activate PI3K which exerts its effect through AKT and other downstream targets (Engelman et al., 2006; Jiang and Liu, 2008). GSK-3β, the downstream target of AKT, together with the adenomatous polyposis coli (APC) protein and axin, forms a multiprotein complex which phosphorylates β-catenin making it for subsequent ubiquitination and degradation (Liu et al., 2005; Rubinfeld et al., 1996). Thus, the reduced expression of GSK-3β can cause the increase of β-catenin activity. On the other hand, PI3K may indirectly activate ERK and p38 MAPK signaling pathways through Rho GTPases (Mizukami et al., 2006; Xue et al., 2006). Recent study has demonstrated that in addition to suppress AKT activation, PTEN also controls the activity of Jun N-terminal kinase (JNK) (Vivanco et al., 2007). Both AKT and ERK can activate NF-κB pathway, performing a complicated network in regulating tumor growth, metastasis, and angiogenesis (Fig. 1). The downstream signaling molecules related to tumorigenesis and angiogenesis are outlined in Fig. 1, and briefly described below.
Fig. 1.
Targets of PI3K and PTEN in regulating tumor growth, metastasis, and angiogenesis. (See Page 1 in Color Section at the back of the book.)
A. Tumor Growth
PI3K may transmit oncogenic signals to AKT for regulating tumorigenesis through several downstream targets. AKT can directly phosphorylate human double minute 2 (HDM2) and regulate HDM2 through p70S6K1 activation (Fang et al., 2005; Mayo and Donner, 2001; Skinner et al., 2004). HDM2 regulates tumor suppressor p53 by promoting its proteasome-mediated degradation (Fang et al., 2006; Skinner et al., 2004). p53 plays a key role in carcinogenesis and cellular apoptosis. AKT activates NF-κB pathway by the phosphorylation of I kappaB kinase (IKK) α/β (Hurt et al., 2002; Lu and Wahl, 2005; Ozes et al., 1999; Tanaka et al., 2005). Activated AKT pathway also exhibits the antiapoptotic effect through the activation of nitric oxide synthase (NOS), the inhibition of FOXO-mediated transcription of proapoptotic proteins, and the inactivation of proapoptotic protein BAD by phosphorylation to activate survival signals. In addition, AKT regulates cell proliferation and tumor growth by increasing the cell-cycle progression. AKT blocks FOXO-mediated transcription of cell-cycle inhibitors, and promotes G1 to S phase transition. AKT stabilizes c-Myc and cyclin D1 through the activation of NF-κB pathway and GSK-3β/β-catenin-signaling axis. Cell proliferation, size, and growth are tightly regulated by the activation of mTOR through PI3K/AKT and MAPK pathways. AKT and MAPK can regulate mTOR to control protein synthesis and cell proliferation, which are associated with carcinogenesis. The regulation of cell survival and cell cycle is associated with the increased cell number in tumors.
B. Tumor Metastasis
The basement membrane forms a cellular support for tumors, and is made up of a complex mix of extracellular matrix (ECM) proteins. The proteolytic enzymes including matrix metalloproteinases (MMPs) can degrade ECM (Orlichenko and Radisky, 2008). PI3K activates MMP-2, MMP-9, and Urokinase-type plasminogen activator (uPA), leading to destruction of ECM (Ispanovic and Haas, 2006; Shukla et al., 2007). PI3K activity is shown to be higher in metastatic cells when compared to non-metastatic cancer cells. Increased levels of MMPs are also due to the activation of AKT/IKK/NF-κB pathway and AKT/GSK-3β/β-catenin axis (Agarwal et al., 2005; Amiri and Richmond, 2005; Ispanovic and Haas, 2006; Kim et al., 2005). PI3K signaling also regulates chemokine (C-X-C motif) ligand 1 (CXCL-1), cyclooxygenase-2 (COX-2), and interleukin-8 (CXCL-8) that enhance tumor metastasis. PI3K and AKT regulate epithelial–mesenchymal transition (EMT), which is a change thought to herald tissue invasion and prophesize metastatic potential (Cheng et al., 2008; Onoue et al., 2006). NF-κB plays a key role in EMT by the activation of mesenchymal program (involving genes such as MMP2/9, VCAM-1, ICAM-1, and Cathepsins B and Z) (Huber et al., 2004) and the repression of E-cadherin, a metastasis suppressor protein, by activating bcl-2 and TWIST (Naugler and Karin, 2008). E-cadherin is a key marker of EMT and loss of E-cadherin disrupts not only cell–cell junctions, but also allows for loss of the normal organ architecture. β-Catenin plays an important role in downregulating E-cadherin expression (Brabletz et al., 2005; Lu et al., 2003). PI3K and AKT also increase invasiveness and downregulate E-cadherin expression (Grille et al., 2003; Larue and Bellacosa, 2005; Schramek et al., 2003; Thiery and Sleeman, 2006). Cell motility is a fundamental process during tumor metastasis. PI3K in combination with the small GTPase Rac and Cdc42 regulates cell motility by controlling actin dynamics in motile cells (Engelman et al., 2006). ERK pathway is also involved in regulating the expression of MMPs, cell migration, and EMT (Reddy et al., 2003).
C. Tumor Angiogenesis
First, PI3K and AKT may regulate tumor angiogenesis by several downstream targets such as mTOR/p70S6K1 signaling axis, the inhibition of FOXO, the induction of NOS (Emerling et al., 2008; Engelman et al., 2006; Quintero et al., 2006; Wang et al., 2004), and/or the inhibition of GSK-3β. These targets commonly increase HIF-1α expression which induces VEGF transcriptional activation. Inhibition of GSK-3β by the activation of PI3K/AKT can upregulate HIF-1α expression, and increases β-catenin activity, which can enhance HIF-1-mediated transcription through the β-catenin-HIF-1α interaction at the promoter region of HIF-1 target genes (Kaidi et al., 2007; Mottet et al., 2003). In addition, hypoxia is a hallmark of the tumor microenvironment in the fast growth tumor. Hypoxia induces HIF-1α production through the increase of its stability and the activation of ERK1/2 pathway. In some kinds of cancer cells, hypoxia stimulates multiple K-ras effectors and PI3K, which induces VEGF expression in a HIF-1-dependent manner or via PI3K/Rho/ROCK/c-myc pathway (Mizukami et al., 2006; Xue et al., 2006). PI3K can induce VEGF expression through HIF-1, ERK1/2, and NF-κB activation to induce tumor angiogenesis. NF-κB can also stimulate tumor necrosis factor (TNF), CXCL-8, IL-1, and IL-6 to induce VEGF (Amiri and Richmond, 2005; Sparmann and Bar-Sagi, 2004). Growing evidence has shown the key roles of PI3K, AKT, mTOR, and their effectors HIF-1α and VEGF in regulating cancer cell-induced angiogenesis (Fang et al., 2007; Hu et al., 2005; Xia et al., 2006).
Next, the angiogenesis and vasculature are regulated though the change of balance between the collective actions of proangiogenic factors (e.g., VEGF) and angiogenic inhibitors (e.g., TSP-1). PI3K/AKT can increase VEGF expression and suppress TSP-1, the endogenous antiangiogenic molecule, in both cancer cells and endothelial cells (Niu et al., 2004; Wen et al., 2001). Furthermore, AKT1−/− mice showed impaired vascular maturation with decreased expression of TSP-1 and TSP-2, while reexpression of TSP-1 and TSP-2 in mice transplanted with wild-type bone marrow is associated with the angiogenic abnormalities in AKT1−/− mice (Chen et al., 2005). Thus, PI3K/AKT signaling pathway induces tumor growth through the overexpression of angiogenic factors and the inhibition of antiangiogenic molecules.
Third, tumor angiogenesis is regulated by the tumor microenvironments composed of tumor cells, vascular endothelial cells, and stromal cells. In addition to cancer cells, the microvascular endothelial cells recruited by the tumor are important for cancer development (Carmeliet and Jain, 2000; Stoeltzing et al., 2006). PI3K/AKT pathway also controls tumor microenvironments, including endothelial cells (Phung et al., 2006; Yuan et al., 2007). PI3K can regulate endothelial migration, proliferation, and survival through the effect of its downstream targets such as NOS, p70S6K1, and FOXO to regulate tumor angiogenesis (Fosbrink et al., 2006; Nakao et al., 2007; Zheng et al., 2008). Class IA PI3Ks regulate vessel integrity during development and tumorigenesis (Yuan et al., 2008). Further analysis of p110 isoforms has demonstrated that p110α is required to control endothelial cell migration and angiogenesis, and p110α−/− endothelial cells lead to embryonic lethality with severe defects in angiogenic sprouting and vascular remodeling (Graupera et al., 2008; Suzuki et al., 2007). PTEN−/− endothelial cells cause embryonic lethality due to endothelial cell hyperproliferation and impaired vascular remodeling; PTEN+/− endothelial cells enhance postnatal neovascularization and tumor angiogenesis to increase tumor growth (Suzuki et al., 2007). Transgenic expression of Myr-AKT1 in endothelial cells is sufficient to recapitulate the abnormal structural and functional features of tumor blood vessels in nontumor tissues, likely due to the induction of VEGF-A (Jiang et al., 2000; Phung et al., 2006). Sustained endothelial AKT activation causes enlarged and hyperpermeable blood vessels and its effect can be completely reversed by AKT inhibition or by rapamycin treatment (Phung et al., 2006). Our studies using chimeric tumor model found that overexpression of p70S6K1 in human dermal microvascular endothelial cells (HDMECs) enhanced tumor growth and angiogenesis, while over-expression of p70S6K1-kinase mutant, or of HIF-1α siRNA significantly inhibited tumor growth and angiogenesis, suggesting that endothelial p70S6K1 controls tumor angiogenesis through HIF-1α and VEGF expression (Liu et al., 2008b).
The interaction of cancer cells and vascular endothelial cells in the tumor microenvironment affects angiogenesis. In cancer cells, stimuli such as growth factors, insulin, and other hormones activate PI3K/AKT/mTOR/HIF-1α axis, and induce the production of VEGF, which switches angiogenic response and causes endothelial cell activation and permeability increased by PI3K pathway (Nyberg et al., 2008; Stoeltzing et al., 2006). Thus, inhibition of PI3K/AKT/mTOR pathway is one of the choices in cancer treatment, which is going on under the preclinical and clinical trials. The signaling pathway of PI3K related to tumor growth, metastasis, and angiogenesis is shown in Fig. 1.
VII. INHIBITION OF PI3K SIGNALING PATHWAY FOR CANCER TREATMENT AND PREVENTION
Given the important role of PI3K signaling pathway in regulating tumor growth and angiogenesis, development of therapeutic drugs using PI3K, AKT, and mTOR inhibitors becomes important for cancer treatment. Here, we introduce the inhibitors of PI3K, AKT, and mTOR.
A. PI3K Inhibitors
PI3K inhibitors, wortmannin, and LY294002, are commonly used to inhibit cancer cell proliferation and tumor growth, and sensitize tumor cells to the treatment of chemotherapeutic drugs and radiation (Granville et al., 2006). Wortmannin is a fungal product isolated from Penicillium wortmanni in 1957, which exerts its effect by the covalent interaction to the conserved Lys802 of the PI3Kα catalytic subunit and Lys833 in PI3Kγ (Walker et al., 2000; Wymann et al., 1996). The pan-PI3K inhibitor LY294002 was synthesized in the early nineties. Both wortmannin and LY294002 also cross-react with PI3K-related kinases such as mTOR and DNA-dependent protein kinases (DNA-PKs). These PI3K inhibitors have poor solubility and high toxicity because they target a broad range of PI3K-related enzymes, which limits their clinical application (Marone et al., 2008). To overcome these shortcomings, many derivatives of wortmannin and LY294002 are being developed (Marone et al., 2008). In addition, inositol(1,3,4,5,6) pentakispho-sphate [Ins(1,3,4,5,6)P5], the PI3K/AKT inhibitor, inhibits tumor growth and angiogenesis in vitro and in vivo (Maffucci et al., 2005). PWT-458, a novel pegylated 17-hydroxywortmannin, is water-soluble and has shown significant improvements in drug stability as well as in vivo pharmacokinetic parameters. It inhibits PI3K signaling and suppresses growth of solid tumors in nude mice (Yu et al., 2005). SF1126, a small molecule conjugate containing a pan-PI3K inhibitor, suppresses PI3K class IA isoforms and other key members of the PI3K superfamily including DNA-PK. In preclinical studies, it has been shown to inhibit tumor growth, dissemination, and angiogenesis (Garlich et al., 2008). The other two pan-PI3K inhibitors, PI-103 and ZSTK474 share the arylmorpholine structure of LY294002. PI-103 is a dual PI3K IA/mTOR inhibitor, while ZSTK474 inhibits the activity of all class I PI3Ks. Both of these drugs exhibit antitumor effect on various kinds of cancers (Chaisuparat et al., 2008; Fan et al., 2006; Kong and Yamori, 2007; Yaguchi et al., 2006; Yuan and Cantley, 2008). IC486068, a p110δ specific inhibitor, enhances radiation-induced tumor vascular destruction (Geng et al., 2004). NVP-BEZ235, an orally administered inhibitor of dual pan-class I PI3K and mTOR kinase, inhibits the growth of breast and prostate cancer cells with active mutations of PI3K, and decreases tumor vasculature (Maira et al., 2008; Schnell et al., 2008; Serra et al., 2008). Recent study has shown that the dual PI3K/PDK-1 inhibitor, BAG956, has inhibitory effect on BCR-ABL-and mutant FLT3-expressing cells both in vitro and in vivo (Weisberg et al., 2008).
Several PI3K inhibitors are used in clinical trials now. For example, XL147 and XL765, the exelixis compounds, are in phase I trials for the treatment of solid tumors. NVP-BEZ235 and another Novartis compound, BGT226, are in ongoing trials for breast and other solid tumors with some promising results (Yuan and Cantley, 2008).
B. AKT Inhibitors
AKT is a major downstream target of PI3K for regulating tumor growth and angiogenesis. The first developed group of AKT inhibitors were lipid-based inhibitors that include perifosine, phosphatidylinositol ether lipid analogs (PIAs), and D-3-deoxy-phosphatidylmyoinositol-1-[(R)-2-methoxy-3-octadecyloxyropyl hydrogen phosphate] (PX-316), which showed antitumor effects in vitro and in vivo (Gills et al., 2006; Granville et al., 2006; Jiang and Liu, 2008; Meuillet et al., 2004). Several other AKT antagonists such as 9-methoxy-2-methylellipticinium acetate (API-59-OMe), indazole-pyridine A-443654, and isoform-specific canthine alkaloid analogs have been identified using high-throughput screening of the chemical libraries and shown to inhibit human cancer cell growth and induce apoptosis (Granville et al., 2006; Liu et al., 2008c; Shi et al., 2005). Other kinds of AKT inhibitors being developed include peptide-based inhibitors of AKT (e. g., KP372-1), pseudopeptide substrates of AKT, a single-chain antibody (scFv) against AKT, an inhibitory form of AKT expressed by adenovirus virus system, and siRNA against AKT (Granville et al., 2006; Jiang and Liu, 2008; Litman et al., 2007; Mandal et al., 2006; Xia et al., 2006).
Perifosine is one of the best-characterized AKT inhibitors, which inhibits the translocation of AKT to the cell membrane. Perifosine inhibits tumor growth in several different kinds of solid tumors. It has been used for clinical trials for the treatment of prostate, breast, gastrointestinal stromal tumors, melanoma, and soft tissue sarcoma, but the clinical outcomes were not satisfied (Table II).
Table II.
Clinical Trials of PI3K/AKT/mTOR Pathway Inhibitors for Cancer Therapy
| Targets | Drug name | Phase | Tumor types | Comments and references |
|---|---|---|---|---|
| AKT | Perifosine | I | Incurable solid malignancies | In order to get suitable dose, pharmacokinetic data, and side effects (Crul et al., 2002; Van Ummersen et al., 2004) |
| I | Advanced solid tumors | Pharmacokinetic study showed that perifosine can be safely combined with fractionated radiotherapy (Vink et al., 2006) | ||
| I/II | Gastrointestinal stromal tumor in combination with imatinib | Ocular toxicity and ulcerative keratitis were associated with Perifosine (Shome et al., 2008) | ||
| II | Advanced breast cancer | No objective responses were seen in this group of pretreated metastatic breast cancer patients (Leighl et al., 2008) | ||
| II | Androgen independent prostate cancer | No significant clinical activity against prostate cancer was observed in this population (Posadas et al., 2005) | ||
| II | Advanced soft tissue sarcoma | Optimism remains for this agent in STS patients (Bailey et al., 2006) No significant response was seen (Knowling et al., 2006) |
||
| II | Recurrent, hormone-sensitive prostate cancer | Modest single-agent clinical activity (Chee et al., 2007) | ||
| II | Pancreatic adenocarcinoma | Perifosine did not appear to be worthy of further study in this group of patients (Marsh et al., 2007) | ||
| II | Recurrent or metastatic head and neck cancer (SCCHN) | Perifosine in the doses and schedule used lacked single-agent activity in SCCHN (Argiris et al., 2006) | ||
| mTOR | Rapamycin (sirolimus) | Hepatocellular and cholangiocellular cancer | A temporary disease-control rate was identified and the toxicity was acceptable (Rizell et al., 2008) | |
| Chronic myeloid leukaemia (CML) | Rapamycin showed antileukemic effects in imatinib-resistant CML (Sillaber et al., 2008) | |||
| I | Nonsmall cell lung cancer (NSCLC) | Combination therapy with sirolimus, radiation, and cisplatin was well tolerated in patients (Sarkaria et al., 2007) | ||
| I | Recurrent PTEN-deficient glioblastoma | Rapamycin had anticancer activity in PTEN-deficient glioblastoma and warrants further clinical study alone or in combination with PI3K pathway inhibitors (Cloughesy et al., 2008) | ||
| I | Recurrent malignant glioma | Gefitinib plus sirolimus was safely coadministered on a continuous, daily dosing schedule (Reardon et al., 2006) | ||
| CCI-779 (temsirolimus) | Solid tumor, recurrent malignant glioma, advanced renal cancer | To establish the safety, tolerability, and pharmacokinetic parameters of CCI-779 (Kuhn et al., 2007; Peralba et al., 2003; Raymond et al., 2004) | ||
| I | Solid tumors or lymphomas | Antitumor efficacy was observed and CCI-779 was generally well tolerated on this intermittent schedule (Hidalgo et al., 2006) | ||
| I | Advanced solid tumors | The administration of CCI-779 and 5-FU/LV at these doses and schedule resulted in unacceptable toxicity and therefore it is not recommended (Punt et al., 2003) | ||
| I | Recurrent malignant glioma | The recommended dose of CCI-779 for patients on enzyme-inducing antiepileptic drugs was 250 mg IV weekly (Chang et al., 2004) | ||
| I/II | Advanced renal-cell carcinoma | The combination of CCI-779 and IFN had an acceptable safety profile and displays antitumor activity in patients with advanced RCC (Motzer et al., 2007) | ||
| II | Advanced breast cancer | CCI-779 showed antitumor activity and a generally tolerable safety profile (Chan et al., 2005) | ||
| II | Recurrent glioblastoma multiforme | CCI-779 was well tolerated in recurrent GBM patients. No response or radiographic improvement was observed in 36% of CCI-779 treated patients (Chang et al., 2005; Galanis et al., 2005) | ||
| II | Advanced neuroendocrine carcinomas | CCI-779 appeared to have little activity and does not warrant further single-agent evaluation in advanced NEC (Duran et al., 2006) | ||
| II | Extensive-stage small-cell lung cancer | CCI-779 seemed not to increase the progression-free survival in this patient population (Pandya et al., 2007) | ||
| II | Metastatic melanoma | CCI-779 was not sufficiently active in this patient population (Margolin et al., 2005) | ||
| II | Advanced refractory renal-cell carcinoma | In patients with advanced RCC, CCI-779 showed antitumor activity and encouraging survival (Atkins et al., 2004) | ||
| III | Advanced renal-cell carcinoma | CCI-779 increased the effect of interferon alpha, improved overall survival among patients with metastatic renal-cell carcinoma and a poor prognosis (Hudes et al., 2007) | ||
| RAD001 (everolimus) | I | Refractory solid tumors in children | Continuous, orally administered RAD001 was well tolerated in children with recurrent or refractory solid tumors and significantly inhibited the mTOR signaling pathway (Fouladi et al., 2007) | |
| I | Advanced solid tumors | RAD001 was satisfactorily tolerated at dosages up to 70 mg/week and 10 mg/day, a dosage of 10 mg/day or 50 mg/week was recommended for further development (O’Donnell et al., 2008; Tabernero et al., 2008) | ||
| I | Advanced NSCLC | A dose of 5 mg daily in combination with daily gefitinib 250 mg was recommended. The two patients with radiographic responses identified were encouraging (Milton et al., 2007) | ||
| I | Advanced breast cancer | Daily therapy with RAD001 plus letrozole was promising and a daily dose of RAD001 10 mg was recommended for further trials (Awada et al., 2008) | ||
| I/II | Relapsed or refractory hematologic malignancies | RAD001 was well tolerated at a daily dose of 10 mg daily and was effective in patients with myelodysplastic syndrome (Yee et al., 2006) | ||
| II | Relapsed chronic lymphocytic leukemia | Although the patient initially responded to therapy, the patient subsequently developed a rapidly fatal Epstein–Barr-virus-associated lymphoproliferative disorder (Gotze et al., 2007) | ||
| II | Low- to intermediate-grade neuroendocrine tumors | RAD001 at 5 or 10 mg/d was well tolerated in combination with octreotide with promising antitumor activity (Yao et al., 2008) | ||
| III | Advanced renal-cell carcinoma | Treatment with everolimus prolonged progression-free survival relative to placebo in patients with metastatic renal-cell carcinoma that had progressed on other targeted therapies (Motzer et al., 2008) | ||
| AP23573 (deforolimus) | I | Advanced malignancies | Deforolimus was well tolerated with encouraging antitumor activity across a broad range of malignancies (Mita et al., 2008) | |
| II | Relapsed or refractory hematologic malignancies | Deforolimus was well tolerated in patients with heavily pretreated hematologic malignancies, and antitumor activity was observed (Rizzieri et al., 2008) |
C. mTOR Inhibitors
The mTOR inhibitor, rapamycin (sirolimus) and its analogs CCI-779 (temsirolimus), RAD001 (everolimus), and AP-23573 (deforolimus) inhibit mTOR activation by binding to FK506-binding protein-12 (Hennessy et al., 2005). These drugs are currently under the clinical trials for cancer treatment. Preclinical studies with these compounds indicated that these compounds have synergistic effects for inhibiting tumor growth when they are used with conventional chemotherapy agent or radiation treatment. In clinical studies, these compounds have been shown to be effective against many types of cancers (Easton and Houghton, 2006; Faivre et al., 2006). In phase I trials, rapamycin has shown anticancer activity in recurrent glioblastoma and gefitinib plus rapamycin can be safely coadministered on a continuous, daily dosing schedule (Cloughesy et al., 2008; Reardon et al., 2006). In phase II and III clinical studies, CCI-779 has been shown to have effects for treating patients with advanced breast cancer and advanced refractory renal-cell carcinoma (Atkins et al., 2004; Chan et al., 2005). Moreover, CCI-779 increased the effect of interferon alpha, improved overall survival among patients with metastatic renal-cell carcinoma, and a poor prognosis (Hudes et al., 2007; Motzer et al., 2007). RAD001 is administered orally for clinical application. The phase II clinical studies have shown that RAD001 treatment enhances the effect of gefitinib in advanced nonsmall cell lung cancer patients, increased the effect of lerozole in advanced breast cancer patients. It is also shown benefits for treating low- to intermediate- grade neuroendocrine tumor combination with octreotide (Awada et al., 2008; Milton et al., 2007; Yao et al., 2008). A recent study has shown that treatment with RAD001 prolongs progression-free patient survival when compared to placebo treated patients with metastatic renal-cell carcinoma that has progressed on other targeted therapies (Motzer et al., 2008). AP-23573 is a phosphorus-containing derivative of rapamycin, and developed in both intravenous and oral formulations for clinical trials. Recent clinical trials have demonstrated that it was well tolerated and showed encouraging activity across a broad range of malignancies, and antitumor activity was observed in patients with heavily hemotologic malignancies (Mita et al., 2008; Rizzieri et al., 2008). The published results in the clinical trials were summarized in Table II.
VIII. CONCLUDING REMARKS
PI3K/PTEN signaling pathway plays a central role in regulating various kinds of cellular functions in response to growth factors, insulin, and other hormones. The intensive interests are on the study of PI3K and PTEN in tumorigenesis. Recent studies have shown that the active form of PI3K is an oncogene, and that amplifications and mutations of PI3K are commonly found in many kinds of human cancers. PTEN, as the tumor suppressor and antagonist of PI3K, is frequently mutated or lost in a number of human cancers. PI3K/PTEN signaling regulates angiogenesis through the interaction of cancer cells and tumor microenvironments, especially endothelial cells. Angiogenesis inducers such as VEGF and angiopoietins activate PI3K signaling for inducing angiogenesis. Forced expression of PI3K alone is sufficient to increase angiogenesis. Genetic alterations of PI3K lead to dysfunction of vasculature and angiogenesis. Mutations of RTKs regulate tumor growth and angiogenesis through PI3K/PTEN signaling. PI3K in turn regulates tumor growth and angiogenesis through downstream targets AKT, mTOR, and p70S6K1; and through effectors, HIF-1 and VEGF. A growing list of evidence shows that PI3K, PTEN, and their upstream and downstream molecules are commonly altered in human cancers; and play an important role in tumorigenesis and angiogenesis. The inhibitors to this signaling pathway, including PI3K, AKT, and mTOR inhibitors, are currently in clinical trials with promising outcomes.
Pan-PI3K inhibitors were initially discovered, and some recently developed versions of pan-PI3K inhibitors broadly target the class IA PI3Ks (p110α, p110β, and p110δ), and the catalytic site of mTOR. Isoform-specific PI3K inhibitors have less toxicity to the cells than those pan-PI3K inhibitors, which could be used to specifically target PI3K activation in certain cancer cells. Clinical data indicates that mTOR inhibitors have stronger effect and more promising results than PI3K and AKT inhibitors. However, there is a feedback loop because p70S6K1 negatively regulates IRS and PDGFR. Rapamycin or its analogs can activate upstream molecules including AKT due to the loss of feedback inhibition. Thus, it is important to exploit the potential benefits of the targeted therapies and optimal treatment with these inhibitors. PI3K pathway inhibitors are likely more effective in patients with active PI3K/AKT pathway, such as PIK3CA mutations or PTEN mutations. In addition, PI3K/AKT signaling is involved in resistance to both chemotherapeutic and radiotherapeutic treatments. Therefore, it would be beneficial to combine these therapeutic agents with PI3K inhibitors. We anticipate that the therapeutic methods targeting PI3K pathway would represent the promising cancer therapy in the near future.
Box 1. PI3K Family and Its Cellular Function.
PI3K composes of three classes based on the substrate, structure, distribution, mechanism of activation, and function. The structure of class I, II, and III PI3Ks is shown as below.
Class IA Regulatory subunits

Catalytic subunits
Class IB Regulatory subunits

Catalytic subunits

Class II

Class III Regulatory subunits

Catalytic subunits

PI3K exerts various cellular functions through its downstream target AKT.
Cell metabolism
AKT promotes glucose uptake in muscle and fat cells by stimulating the glucose transporter, GLUT4, to cell membrane. AKT increases glycogen synthesis by inhibiting glycogen synthase kinase 3 (GSK-3) (Cohen and Frame, 2001). AKT also regulates fattyacid synthesis by activating ATP citrate lyase (Berwick et al., 2002). Moreover, AKT inhibits gluconeogenesis by blocking forkhead (FOXO)-mediated transcription of gluconeogenic enzymes and regulates insulin metabolism in the liver (Engelman et al., 2006). Abnormality of AKT is related with diabetes. AKT2-deficient mice exhibit a diabetes-like syndrome with an elevated fasting plasma glucose level, elevated hepatic glucose output, and peripheral insulin resistance (Cho et al., 2001a; Garofalo et al., 2003).
Initiation of translation and protein synthesis
AKT inhibits the GTPase-activating protein (GAP) activity of the tuberous sclerosis complex 1 (TSC1)-TSC2 complex by phosphorylating TSC2 tuberin protein, leading to the accumulation and activation of the mTOR-raptor kinase complex. mTOR mediates the phosphorylation of the ribosomal protein S6 kinases (p70S6K) and eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1) leading to the release of the translation initiation factor eIF4E (Hennessy et al., 2005; Schmelzle and Hall, 2000). However, there are complicated interactions and feedback loops in this signaling pathway since TSC/mTOR/S6K cascade also inhibits PI3K/AKT pathway by down-regulating insulin receptor substrate (IRS) 1/2 and PDGFR (Harrington et al., 2004; Zhang et al., 2003).
Cell survival/inhibition of apoptosis
One of the important downstream targets of AKT is FOXO family of transcription factors. AKT inactivates FOXO proteins by phosphorylation. Some other important targets of AKT are GSK-3, BAD (Bcl2-antagonist of cell death), IkappaB kinase (IKK), and MDM2. AKT blocks FOXO-mediated transcription of some proapoptotic proteins such as Fas-ligand (FasL) and Bim, directly phosphorylates the proapoptotic protein BAD, thus repressing the prosurvival molecule Bcl-XL. The phosphorylation of IKK results in phosphorylating IκB (inhibitor of NF-κB), leading to its proteasomal degradation and NF-κB nuclear localization. On the other hand, the phosphorylation of MDM2 leads to the degradation of p53, exhibiting the antiapoptotic effect (Brazil et al., 2002). In addition, eIF4E also has antiapoptotic activity in vitro and in vivo (Contreras et al., 2008; Yamaguchi et al., 2008).
Cell cycle
AKT promotes G1-S phase transition by blocking FOXO-mediated transcription of cell-cycle inhibitors including p27Kip1 (Chandramohan et al., 2004; Schmidt et al., 2002). AKT also indirectly stabilizes the cell-cycle protein c-Myc and cyclin D1 by inhibiting GSK-3 (Diehl et al., 1998; Engelman et al., 2006; Gregory et al., 2003).
In addition, PI3K plays a role in regulating cell polarity and motility (Engelman et al., 2006).
Acknowledgments
This work was supported in part by Grants CA109460, ES017237, and HL091456 from National Institutes of Health by the National Basic Research Program of China Grant 2007CB947002.
References
- Ackah E, Yu J, Zoellner S, Iwakiri Y, Skurk C, Shibata R, Ouchi N, Easton RM, Galasso G, Birnbaum MJ, Walsh K, Sessa WC. Akt1/protein kinase Balpha is critical for ischemic and VEGF-mediated angiogenesis. J Clin Invest. 2005;115:2119–2127. doi: 10.1172/JCI24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A, Das K, Lerner N, Sathe S, Cicek M, Casey G, Sizemore N. The AKT/I kappa B kinase pathway promotes angiogenic/metastatic gene expression in colorectal cancer by activating nuclear factor-kappa B and beta-catenin. Oncogene. 2005;24:1021–1031. doi: 10.1038/sj.onc.1208296. [DOI] [PubMed] [Google Scholar]
- Amiri KI, Richmond A. Role of nuclear factor-kappa B in melanoma. Cancer Metastasis Rev. 2005;24:301–313. doi: 10.1007/s10555-005-1579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcaro A, Zvelebil MJ, Wallasch C, Ullrich A, Waterfield MD, Domin J. Class II phosphoinositide 3-kinases are downstream targets of activated polypeptide growth factor receptors. Mol Cell Biol. 2000;20:3817–3830. doi: 10.1128/mcb.20.11.3817-3830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argiris A, Cohen E, Karrison T, Esparaz B, Mauer A, Ansari R, Wong S, Lu Y, Pins M, Dancey J, Vokes E. A phase II trial of perifosine, an oral alkylphospholipid, in recurrent or metastatic head and neck cancer. Cancer Biol Ther. 2006;5:766–770. doi: 10.4161/cbt.5.7.2874. [DOI] [PubMed] [Google Scholar]
- Atkins MB, Hidalgo M, Stadler WM, Logan TF, Dutcher JP, Hudes GR, Park Y, Liou SH, Marshall B, Boni JP, Dukart G, Sherman ML. Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol. 2004;22:909–918. doi: 10.1200/JCO.2004.08.185. [DOI] [PubMed] [Google Scholar]
- Autiero M, Waltenberger J, Communi D, Kranz A, Moons L, Lambrechts D, Kroll J, Plaisance S, De Mol M, Bono F, Kliche S, Fellbrich G, et al. Role of PlGF in the intra- and intermolecular cross talk between the VEGF receptors Flt1 and Flk1. Nat Med. 2003;9:936–943. doi: 10.1038/nm884. [DOI] [PubMed] [Google Scholar]
- Awada A, Cardoso F, Fontaine C, Dirix L, De Greve J, Sotiriou C, Steinseifer J, Wouters C, Tanaka C, Zoellner U, Tang P, Piccart M. The oral mTOR inhibitor RAD001 (everolimus) in combination with letrozole in patients with advanced breast cancer: Results of a phase I study with pharmacokinetics. Eur J Cancer. 2008;44:84–91. doi: 10.1016/j.ejca.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Babaei S, Teichert-Kuliszewska K, Zhang Q, Jones N, Dumont DJ, Stewart DJ. Angiogenic actions of angiopoietin-1 require endothelium-derived nitric oxide. Am J Pathol. 2003;162:1927–1936. doi: 10.1016/S0002-9440(10)64326-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman SA, Ghazarian D, So K, Sanchez O, Wagner KU, Hennighausen L, Suzuki A, Tsao MS, Chapman WB, Stambolic V, Mak TW. Early onset of neoplasia in the prostate and skin of mice with tissue-specific deletion of Pten. Proc Natl Acad Sci USA. 2004;101:1725–1730. doi: 10.1073/pnas.0308217100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader AG, Kang S, Vogt PK. Cancer-specific mutations in PIK3CA are oncogenic in vivo. Proc Natl Acad Sci USA. 2006;103:1475–1479. doi: 10.1073/pnas.0510857103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey HH, Mahoney MR, Ettinger DS, Maples WJ, Fracasso PM, Traynor AM, Erlichman C, Okuno SH. Phase II study of daily oral perifosine in patients with advanced soft tissue sarcoma. Cancer. 2006;107:2462–2467. doi: 10.1002/cncr.22308. [DOI] [PubMed] [Google Scholar]
- Belaiba RS, Bonello S, Zahringer C, Schmidt S, Hess J, Kietzmann T, Gorlach A. Hypoxia up-regulates hypoxia-inducible factor-1alpha transcription by involving phosphatidylinositol 3-kinase and nuclear factor kappaB in pulmonary artery smooth muscle cells. Mol Biol Cell. 2007;18:4691–4697. doi: 10.1091/mbc.E07-04-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellacosa A, Testa JR, Staal SP, Tsichlis PN. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science. 1991;254:274–277. doi: 10.1126/science.254.5029.274. [DOI] [PubMed] [Google Scholar]
- Berwick DC, Hers I, Heesom KJ, Moule SK, Tavare JM. The identification of ATP-citrate lyase as a protein kinase B (Akt) substrate in primary adipocytes. J Biol Chem. 2002;277:33895–33900. doi: 10.1074/jbc.M204681200. [DOI] [PubMed] [Google Scholar]
- Brabletz T, Hlubek F, Spaderna S, Schmalhofer O, Hiendlmeyer E, Jung A, Kirchner T. Invasion and metastasis in colorectal cancer: Epithelial–mesenchymal transition, mesenchymal–epithelial transition, stem cells and beta-catenin. Cells Tissues Organs. 2005;179:56–65. doi: 10.1159/000084509. [DOI] [PubMed] [Google Scholar]
- Brachmann SM, Yballe CM, Innocenti M, Deane JA, Fruman DA, Thomas SM, Cantley LC. Role of phosphoinositide 3-kinase regulatory isoforms in development and actin rearrangement. Mol Cell Biol. 2005;25:2593–2606. doi: 10.1128/MCB.25.7.2593-2606.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazil DP, Park J, Hemmings BA. PKB binding proteins. Getting in on the Akt. Cell. 2002;111:293–303. doi: 10.1016/s0092-8674(02)01083-8. [DOI] [PubMed] [Google Scholar]
- Brown EB, Campbell RB, Tsuzuki Y, Xu L, Carmeliet P, Fukumura D, Jain RK. In vivo measurement of gene expression, angiogenesis and physiological function in tumors using multiphoton laser scanning microscopy. Nat Med. 2001;7:864–868. doi: 10.1038/89997. [DOI] [PubMed] [Google Scholar]
- Campbell IG, Russell SE, Choong DY, Montgomery KG, Ciavarella ML, Hooi CS, Cristiano BE, Pearson RB, Phillips WA. Mutation of the PIK3CA gene in ovarian and breast cancer. Cancer Res. 2004;64:7678–7681. doi: 10.1158/0008-5472.CAN-04-2933. [DOI] [PubMed] [Google Scholar]
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- Carver DJ, Gaston B, Deronde K, Palmer LA. Akt-mediated activation of HIF-1 in pulmonary vascular endothelial cells by S-nitrosoglutathione. Am J Respir Cell Mol Biol. 2007;37:255–263. doi: 10.1165/rcmb.2006-0289SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebe-Suarez S, Zehnder-Fjallman A, Ballmer-Hofer K. The role of VEGF receptors in angiogenesis; complex partnerships. Cell Mol Life Sci. 2006;63:601–615. doi: 10.1007/s00018-005-5426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chachami G, Simos G, Hatziefthimiou A, Bonanou S, Molyvdas PA, Paraskeva E. Cobalt induces hypoxia-inducible factor-1alpha expression in airway smooth muscle cells by a reactive oxygen species- and PI3K-dependent mechanism. Am J Respir Cell Mol Biol. 2004;31:544–551. doi: 10.1165/rcmb.2003-0426OC. [DOI] [PubMed] [Google Scholar]
- Chaisuparat R, Hu J, Jham BC, Knight ZA, Shokat KM, Montaner S. Dual inhibition of PI3Kalpha and mTOR as an alternative treatment for Kaposi’s sarcoma. Cancer Res. 2008;68:8361–8368. doi: 10.1158/0008-5472.CAN-08-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan TO, Rodeck U, Chan AM, Kimmelman AC, Rittenhouse SE, Panayotou G, Tsichlis PN. Small GTPases and tyrosine kinases coregulate a molecular switch in the phosphoinositide 3-kinase regulatory subunit. Cancer Cell. 2002;1:181–191. doi: 10.1016/s1535-6108(02)00033-8. [DOI] [PubMed] [Google Scholar]
- Chan S, Scheulen ME, Johnston S, Mross K, Cardoso F, Dittrich C, Eiermann W, Hess D, Morant R, Semiglazov V, Borner M, Salzberg M, et al. Phase II study of temsirolimus (CCI-779), a novel inhibitor of mTOR, in heavily pretreated patients with locally advanced or metastatic breast cancer. J Clin Oncol. 2005;23:5314–5322. doi: 10.1200/JCO.2005.66.130. [DOI] [PubMed] [Google Scholar]
- Chandramohan V, Jeay S, Pianetti S, Sonenshein GE. Reciprocal control of Forkhead box O 3a and c-Myc via the phosphatidylinositol 3-kinase pathway coordinately regulates p27Kip1 levels. J Immunol. 2004;172:5522–5527. doi: 10.4049/jimmunol.172.9.5522. [DOI] [PubMed] [Google Scholar]
- Chang HW, Aoki M, Fruman D, Auger KR, Bellacosa A, Tsichlis PN, Cantley LC, Roberts TM, Vogt PK. Transformation of chicken cells by the gene encoding the catalytic subunit of PI 3-kinase. Science. 1997;276:1848–1850. doi: 10.1126/science.276.5320.1848. [DOI] [PubMed] [Google Scholar]
- Chang SM, Kuhn J, Wen P, Greenberg H, Schiff D, Conrad C, Fink K, Robins HI, Cloughesy T, De Angelis L, Razier J, Hess K, et al. Phase I/pharmacokinetic study of CCI-779 in patients with recurrent malignant glioma on enzyme-inducing antiepileptic drugs. Invest New Drugs. 2004;22:427–435. doi: 10.1023/B:DRUG.0000036685.72140.03. [DOI] [PubMed] [Google Scholar]
- Chang SM, Wen P, Cloughesy T, Greenberg H, Schiff D, Conrad C, Fink K, Robins HI, De Angelis L, Raizer J, Hess K, Aldape K, et al. Phase II study of CCI-779 in patients with recurrent glioblastoma multiforme. Invest New Drugs. 2005;23:357–361. doi: 10.1007/s10637-005-1444-0. [DOI] [PubMed] [Google Scholar]
- Chee KG, Longmate J, Quinn DI, Chatta G, Pinski J, Twardowski P, Pan CX, Cambio A, Evans CP, Gandara DR, Lara PN., Jr The AKT inhibitor perifosine in biochemically recurrent prostate cancer: A phase II California/Pittsburgh cancer consortium trial. Clin Genitourin Cancer. 2007;5:433–437. doi: 10.3816/CGC.2007.n.031. [DOI] [PubMed] [Google Scholar]
- Chen WS, Xu PZ, Gottlob K, Chen ML, Sokol K, Shiyanova T, Roninson I, Weng W, Suzuki R, Tobe K, Kadowaki T, Hay N. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 2001;15:2203–2208. doi: 10.1101/gad.913901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Somanath PR, Razorenova O, Chen WS, Hay N, Bornstein P, Byzova TV. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat Med. 2005;11:1188–1196. doi: 10.1038/nm1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng GZ, Park S, Shu S, He L, Kong W, Zhang W, Yuan Z, Wang LH, Cheng JQ. Advances of AKT pathway in human oncogenesis and as a target for anti-cancer drug discovery. Curr Cancer Drug Targets. 2008;8:2–6. [PubMed] [Google Scholar]
- Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB, III, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001a;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem. 2001b;276:38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- Cho CH, Kammerer RA, Lee HJ, Steinmetz MO, Ryu YS, Lee SH, Yasunaga K, Kim KT, Kim I, Choi HH, Kim W, Kim SH, et al. COMP-Ang1: A designed angiopoietin-1 variant with nonleaky angiogenic activity. Proc Natl Acad Sci USA. 2004;101:5547–5552. doi: 10.1073/pnas.0307574101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloughesy TF, Yoshimoto K, Nghiemphu P, Brown K, Dang J, Zhu S, Hsueh T, Chen Y, Wang W, Youngkin D, Liau L, Martin N, et al. Antitumor activity of rapamycin in a Phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLoS Med. 2008;5:e8. doi: 10.1371/journal.pmed.0050008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P, Frame S. The renaissance of GSK3. Nat Rev Mol Cell Biol. 2001;2:769–776. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- Contreras V, Richardson MA, Hao E, Keiper BD. Depletion of the cap-associated isoform of translation factor eIF4G induces germline apoptosis in C. elegans. Cell Death Differ. 2008;15:1232–1242. doi: 10.1038/cdd.2008.46. [DOI] [PubMed] [Google Scholar]
- Crul M, Rosing H, de Klerk GJ, Dubbelman R, Traiser M, Reichert S, Knebel NG, Schellens JH, Beijnen JH, Bokkel Huinink WW. Phase I and pharmacological study of daily oral administration of perifosine (D-21266) in patients with advanced solid tumours. Eur J Cancer. 2002;38:1615–1621. doi: 10.1016/s0959-8049(02)00127-2. [DOI] [PubMed] [Google Scholar]
- Cunningham SA, Waxham MN, Arrate PM, Brock TA. Interaction of the Flt-1 tyrosine kinase receptor with the p85 subunit of phosphatidylinositol 3-kinase. Mapping of a novel site involved in binding. J Biol Chem. 1995;270:20254–20257. doi: 10.1074/jbc.270.35.20254. [DOI] [PubMed] [Google Scholar]
- Dahia PL, Marsh DJ, Zheng Z, Zedenius J, Komminoth P, Frisk T, Wallin G, Parsons R, Longy M, Larsson C, Eng C. Somatic deletions and mutations in the Cowden disease gene, PTEN, in sporadic thyroid tumors. Cancer Res. 1997;57:4710–4713. [PubMed] [Google Scholar]
- Dance M, Montagner A, Yart A, Masri B, Audigier Y, Perret B, Salles JP, Raynal P. The adaptor protein Gab1 couples the stimulation of vascular endothelial growth factor receptor-2 to the activation of phosphoinositide 3-kinase. J Biol Chem. 2006;281:23285–23295. doi: 10.1074/jbc.M600987200. [DOI] [PubMed] [Google Scholar]
- Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–1169. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- Dayanir V, Meyer RD, Lashkari K, Rahimi N. Identification of tyrosine residues in vascular endothelial growth factor receptor-2/FLK-1 involved in activation of phosphatidylinositol 3-kinase and cell proliferation. J Biol Chem. 2001;276:17686–17692. doi: 10.1074/jbc.M009128200. [DOI] [PubMed] [Google Scholar]
- DeBosch B, Sambandam N, Weinheimer C, Courtois M, Muslin AJ. Akt2 regulates cardiac metabolism and cardiomyocyte survival. J Biol Chem. 2006;281:32841–32851. doi: 10.1074/jbc.M513087200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP. Pten is essential for embryonic development and tumour suppression. Nat Genet. 1998;19:348–355. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domin J, Waterfield MD. Using structure to define the function of phosphoinositide 3-kinase family members. FEBS Lett. 1997;410:91–95. doi: 10.1016/s0014-5793(97)00617-0. [DOI] [PubMed] [Google Scholar]
- Downward J. Mechanisms and consequences of activation of protein kinase B/Akt. Curr Opin Cell Biol. 1998;10:262–267. doi: 10.1016/s0955-0674(98)80149-x. [DOI] [PubMed] [Google Scholar]
- Duran I, Kortmansky J, Singh D, Hirte H, Kocha W, Goss G, Le L, Oza A, Nicklee T, Ho J, Birle D, Pond GR, et al. A phase II clinical and pharmacodynamic study of temsirolimus in advanced neuroendocrine carcinomas. Br J Cancer. 2006;95:1148–1154. doi: 10.1038/sj.bjc.6603419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton JB, Houghton PJ. mTOR and cancer therapy. Oncogene. 2006;25:6436–6446. doi: 10.1038/sj.onc.1209886. [DOI] [PubMed] [Google Scholar]
- Emerling BM, Weinberg F, Liu JL, Mak TW, Chandel NS. PTEN regulates p300-dependent hypoxia-inducible factor 1 transcriptional activity through Fork-head transcription factor 3a (FOXO3a) Proc Natl Acad Sci USA. 2008;105:2622–2627. doi: 10.1073/pnas.0706790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nat Rev Drug Discov. 2006;5:671–688. doi: 10.1038/nrd2062. [DOI] [PubMed] [Google Scholar]
- Falasca M, Maffucci T. Role of class II phosphoinositide 3-kinase in cell signalling. Biochem Soc Trans. 2007;35:211–214. doi: 10.1042/BST0350211. [DOI] [PubMed] [Google Scholar]
- Fan QW, Knight ZA, Goldenberg DD, Yu W, Mostov KE, Stokoe D, Shokat KM, Weiss WA. A dual PI3 kinase/mTOR inhibitor reveals emergent efficacy in glioma. Cancer Cell. 2006;9:341–349. doi: 10.1016/j.ccr.2006.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Xia C, Cao Z, Zheng JZ, Reed E, Jiang BH. Apigenin inhibits VEGF and HIF-1 expression via PI3K/AKT/p70S6K1 and HDM2/p53 pathways. FASEB J. 2005;19:342–353. doi: 10.1096/fj.04-2175com. [DOI] [PubMed] [Google Scholar]
- Fang J, Meng Q, Vogt PK, Zhang R, Jiang BH. A downstream kinase of the mammalian target of rapamycin, p70S6K1, regulates human double minute 2 protein phosphorylation and stability. J Cell Physiol. 2006;209:261–265. doi: 10.1002/jcp.20749. [DOI] [PubMed] [Google Scholar]
- Fang J, Ding M, Yang L, Liu LZ, Jiang BH. PI3K/PTEN/AKT signaling regulates prostate tumor angiogenesis. Cell Signal. 2007;19:2487–2497. doi: 10.1016/j.cellsig.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- Folkman J. Seminars in Medicine of the Beth Israel Hospital, Boston. Clinical applications of research on angiogenesis. N Engl J Med. 1995;333:1757–1763. doi: 10.1056/NEJM199512283332608. [DOI] [PubMed] [Google Scholar]
- Fosbrink M, Niculescu F, Rus V, Shin ML, Rus H. C5b-9-induced endothelial cell proliferation and migration are dependent on Akt inactivation of forkhead transcription factor FOXO1. J Biol Chem. 2006;281:19009–19018. doi: 10.1074/jbc.M602055200. [DOI] [PubMed] [Google Scholar]
- Fouladi M, Laningham F, Wu J, O’Shaughnessy MA, Molina K, Broniscer A, Spunt SL, Luckett I, Stewart CF, Houghton PJ, Gilbertson RJ, Furman WL. Phase I study of everolimus in pediatric patients with refractory solid tumors. J Clin Oncol. 2007;25:4806–4812. doi: 10.1200/JCO.2007.11.4017. [DOI] [PubMed] [Google Scholar]
- Fraser MM, Zhu X, Kwon CH, Uhlmann EJ, Gutmann DH, Baker SJ. Pten loss causes hypertrophy and increased proliferation of astrocytes in vivo. Cancer Res. 2004;64:7773–7779. doi: 10.1158/0008-5472.CAN-04-2487. [DOI] [PubMed] [Google Scholar]
- Fruman DA, Snapper SB, Yballe CM, Davidson L, Yu JY, Alt FW, Cantley LC. Impaired B cell development and proliferation in absence of phosphoinositide 3-kinase p85alpha. Science. 1999;283:393–397. doi: 10.1126/science.283.5400.393. [DOI] [PubMed] [Google Scholar]
- Galanis E, Buckner JC, Maurer MJ, Kreisberg JI, Ballman K, Boni J, Peralba JM, Jenkins RB, Dakhil SR, Morton RF, Jaeckle KA, Scheithauer BW, et al. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: A North Central Cancer Treatment Group Study. J Clin Oncol. 2005;23:5294–5304. doi: 10.1200/JCO.2005.23.622. [DOI] [PubMed] [Google Scholar]
- Garlich JR, De P, Dey N, Su JD, Peng X, Miller A, Murali R, Lu Y, Mills GB, Kundra V, Shu HK, Peng Q, et al. A vascular targeted pan phosphoinositide 3-kinase inhibitor prodrug, SF1126, with antitumor and antiangiogenic activity. Cancer Res. 2008;68:206–215. doi: 10.1158/0008-5472.CAN-07-0669. [DOI] [PubMed] [Google Scholar]
- Garofalo RS, Orena SJ, Rafidi K, Torchia AJ, Stock JL, Hildebrandt AL, Coskran T, Black SC, Brees DJ, Wicks JR, McNeish JD, Coleman KG. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta. J Clin Invest. 2003;112:197–208. doi: 10.1172/JCI16885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng L, Tan J, Himmelfarb E, Schueneman A, Niermann K, Brousal J, Fu A, Cuneo K, Kesicki EA, Treiberg J, Hayflick JS, Hallahan DE. A specific antagonist of the p110delta catalytic component of phosphatidylinositol 3′-kinase, IC486068, enhances radiation-induced tumor vascular destruction. Cancer Res. 2004;64:4893–4899. doi: 10.1158/0008-5472.CAN-03-3955. [DOI] [PubMed] [Google Scholar]
- Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273:30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- Gericke A, Munson M, Ross AH. Regulation of the PTEN phosphatase. Gene. 2006;374:1–9. doi: 10.1016/j.gene.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Gills JJ, Holbeck S, Hollingshead M, Hewitt SM, Kozikowski AP, Dennis PA. Spectrum of activity and molecular correlates of response to phosphatidylinositol ether lipid analogues, novel lipid-based inhibitors of Akt. Mol Cancer Ther. 2006;5:713–722. doi: 10.1158/1535-7163.MCT-05-0484. [DOI] [PubMed] [Google Scholar]
- Gotze KS, Hoffmann D, Schatzl HM, Peschel C, Fend F, Decker T. Fatal Epstein-Barr virus-associated lymphoproliferative disorder following treatment with a novel mTOR inhibitor for relapsed chronic lymphocytic leukemia leukemia cells. Haematologica. 2007;92:1282–1283. doi: 10.3324/haematol.11155. [DOI] [PubMed] [Google Scholar]
- Granville CA, Memmott RM, Gills JJ, Dennis PA. Handicapping the race to develop inhibitors of the phosphoinositide 3-kinase/Akt/mammalian target of rapamycin pathway. Clin Cancer Res. 2006;12:679–689. doi: 10.1158/1078-0432.CCR-05-1654. [DOI] [PubMed] [Google Scholar]
- Graupera M, Guillermet-Guibert J, Foukas LC, Phng LK, Cain RJ, Salpekar A, Pearce W, Meek S, Millan J, Cutillas PR, Smith AJ, Ridley AJ, et al. Angiogenesis selectively requires the p110alpha isoform of PI3K to control endothelial cell migration. Nature. 2008;453:662–666. doi: 10.1038/nature06892. [DOI] [PubMed] [Google Scholar]
- Gregory MA, Qi Y, Hann SR. Phosphorylation by glycogen synthase kinase-3 controls c-myc proteolysis and subnuclear localization. J Biol Chem. 2003;278:51606–51612. doi: 10.1074/jbc.M310722200. [DOI] [PubMed] [Google Scholar]
- Grille SJ, Bellacosa A, Upson J, Klein-Szanto AJ, van Roy F, Lee-Kwon W, Donowitz M, Tsichlis PN, Larue L. The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Res. 2003;63:2172–2178. [PubMed] [Google Scholar]
- Gulati P, Gaspers LD, Dann SG, Joaquin M, Nobukuni T, Natt F, Kozma SC, Thomas AP, Thomas G. Amino acids activate mTOR complex 1 via Ca2 +/CaM signaling to hVps34. Cell Metab. 2008;7:456–465. doi: 10.1016/j.cmet.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harima Y, Sawada S, Nagata K, Sougawa M, Ostapenko V, Ohnishi T. Mutation of the PTEN gene in advanced cervical cancer correlated with tumor progression and poor outcome after radiotherapy. Int J Oncol. 2001;18:493–497. doi: 10.3892/ijo.18.3.493. [DOI] [PubMed] [Google Scholar]
- Harrington LS, Findlay GM, Gray A, Tolkacheva T, Wigfield S, Rebholz H, Barnett J, Leslie NR, Cheng S, Shepherd PR, Gout I, Downes CP, et al. The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol. 2004;166:213–223. doi: 10.1083/jcb.200403069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- Hernando E, Charytonowicz E, Dudas ME, Menendez S, Matushansky I, Mills J, Socci ND, Behrendt N, Ma L, Maki RG, Pandolfi PP, Cordon-Cardo C. The AKT-mTOR pathway plays a critical role in the development of leiomyosarcomas. Nat Med. 2007;13:748–753. doi: 10.1038/nm1560. [DOI] [PubMed] [Google Scholar]
- Hettinger K, Vikhanskaya F, Poh MK, Lee MK, de BI, Zhang JT, Reddy SA, Sabapathy K. c-Jun promotes cellular survival by suppression of PTEN. Cell Death Differ. 2007;14:218–229. doi: 10.1038/sj.cdd.4401946. [DOI] [PubMed] [Google Scholar]
- Hidalgo M, Buckner JC, Erlichman C, Pollack MS, Boni JP, Dukart G, Marshall B, Speicher L, Moore L, Rowinsky EK. A phase I and pharmacokinetic study of temsirolimus (CCI-779) administered intravenously daily for 5 days every 2 weeks to patients with advanced cancer. Clin Cancer Res. 2006;12:5755–5763. doi: 10.1158/1078-0432.CCR-06-0118. [DOI] [PubMed] [Google Scholar]
- Horie Y, Suzuki A, Kataoka E, Sasaki T, Hamada K, Sasaki J, Mizuno K, Hasegawa G, Kishimoto H, Iizuka M, Naito M, Enomoto K, et al. Hepatocyte-specific Pten deficiency results in steatohepatitis and hepatocellular carcinomas. J Clin Invest. 2004;113:1774–1783. doi: 10.1172/JCI20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hresko RC, Murata H, Mueckler M. Phosphoinositide-dependent kinase-2 is a distinct protein kinase enriched in a novel cytoskeletal fraction associated with adipocyte plasma membranes. J Biol Chem. 2003;278:21615–21622. doi: 10.1074/jbc.M302937200. [DOI] [PubMed] [Google Scholar]
- Hu P, Margolis B, Skolnik EY, Lammers R, Ullrich A, Schlessinger J. Interaction of phosphatidylinositol 3-kinase-associated p85 with epidermal growth factor and platelet-derived growth factor receptors. Mol Cell Biol. 1992;12:981–990. doi: 10.1128/mcb.12.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Hofmann J, Jaffe RB. Phosphatidylinositol 3-kinase mediates angiogenesis and vascular permeability associated with ovarian carcinoma. Clin Cancer Res. 2005;11:8208–8212. doi: 10.1158/1078-0432.CCR-05-0206. [DOI] [PubMed] [Google Scholar]
- Huber MA, Azoitei N, Baumann B, Grunert S, Sommer A, Pehamberger H, Kraut N, Beug H, Wirth T. NF-kappaB is essential for epithelial–mesenchymal transition and metastasis in a model of breast cancer progression. J Clin Invest. 2004;114:569–581. doi: 10.1172/JCI21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, Staroslawska E, Sosman J, McDermott D, Bodrogi I, Kovacevic Z, Lesovoy V, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- Hurt KJ, Musicki B, Palese MA, Crone JK, Becker RE, Moriarity JL, Snyder SH, Burnett AL. Akt-dependent phosphorylation of endothelial nitric-oxide synthase mediates penile erection. Proc Natl Acad Sci USA. 2002;99:4061–4066. doi: 10.1073/pnas.052712499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikenoue T, Inoki K, Zhao B, Guan KL. PTEN acetylation modulates its interaction with PDZ domain. Cancer Res. 2008;68:6908–6912. doi: 10.1158/0008-5472.CAN-08-1107. [DOI] [PubMed] [Google Scholar]
- Ispanovic E, Haas TL. JNK and PI3K differentially regulate MMP-2 and MT1-MMP mRNA and protein in response to actin cytoskeleton reorganization in endothelial cells. Am J Physiol Cell Physiol. 2006;291:C579–C588. doi: 10.1152/ajpcell.00300.2005. [DOI] [PubMed] [Google Scholar]
- Jeong JW, Bae MK, Ahn MY, Kim SH, Sohn TK, Bae MH, Yoo MA, Song EJ, Lee KJ, Kim KW. Regulation and destabilization of HIF-1alpha by ARD1- mediated acetylation. Cell. 2002;111:709–720. doi: 10.1016/s0092-8674(02)01085-1. [DOI] [PubMed] [Google Scholar]
- Jia S, Liu Z, Zhang S, Liu P, Zhang L, Lee SH, Zhang J, Signoretti S, Loda M, Roberts TM, Zhao JJ. Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis. Nature. 2008;454:776–779. doi: 10.1038/nature07091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang BH, Liu LZ. PI3K/PTEN signaling in tumorigenesis and angiogenesis. Biochim Biophys Acta. 2008;1784:150–158. doi: 10.1016/j.bbapap.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Jiang BH, Zheng JZ, Aoki M, Vogt PK. Phosphatidylinositol 3-kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells. Proc Natl Acad Sci USA. 2000;97:1749–1753. doi: 10.1073/pnas.040560897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang BH, Jiang G, Zheng JZ, Lu Z, Hunter T, Vogt PK. Phosphatidylinositol 3-kinase signaling controls levels of hypoxia-inducible factor 1. Cell Growth Differ. 2001;12:363–369. [PubMed] [Google Scholar]
- Jones N, Master Z, Jones J, Bouchard D, Gunji Y, Sasaki H, Daly R, Alitalo K, Dumont DJ. Identification of Tek/Tie2 binding partners. Binding to a multifunctional docking site mediates cell survival and migration. J Biol Chem. 1999;274:30896–30905. doi: 10.1074/jbc.274.43.30896. [DOI] [PubMed] [Google Scholar]
- Jones N, Iljin K, Dumont DJ, Alitalo K. Tie receptors: New modulators of angiogenic and lymphangiogenic responses. Nat Rev Mol Cell Biol. 2001;2:257–267. doi: 10.1038/35067005. [DOI] [PubMed] [Google Scholar]
- Ju X, Katiyar S, Wang C, Liu M, Jiao X, Li S, Zhou J, Turner J, Lisanti MP, Russell RG, Mueller SC, Ojeifo J, et al. Akt1 governs breast cancer progression in vivo. Proc Natl Acad Sci USA. 2007;104:7438–7443. doi: 10.1073/pnas.0605874104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jucker M, Sudel K, Horn S, Sickel M, Wegner W, Fiedler W, Feldman RA. Expression of a mutated form of the p85alpha regulatory subunit of phosphatidylinositol 3-kinase in a Hodgkin’s lymphoma-derived cell line (CO) Leukemia. 2002;16:894–901. doi: 10.1038/sj.leu.2402484. [DOI] [PubMed] [Google Scholar]
- Kaidi A, Williams AC, Paraskeva C. Interaction between beta-catenin and HIF-1 promotes cellular adaptation to hypoxia. Nat Cell Biol. 2007;9:210–217. doi: 10.1038/ncb1534. [DOI] [PubMed] [Google Scholar]
- Kanda S, Miyata Y, Mochizuki Y, Matsuyama T, Kanetake H. Angiopoietin 1 is mitogenic for cultured endothelial cells. Cancer Res. 2005;65:6820–6827. doi: 10.1158/0008-5472.CAN-05-0522. [DOI] [PubMed] [Google Scholar]
- Kapitsinou PP, Haase VH. The VHL tumor suppressor and HIF: Insights from genetic studies in mice. Cell Death Differ. 2008;15:650–659. doi: 10.1038/sj.cdd.4402313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Kim HG, So JN, Kim JH, Kwak HJ, Koh GY. Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3′-Kinase/Akt signal transduction pathway. Circ Res. 2000;86:24–29. doi: 10.1161/01.res.86.1.24. [DOI] [PubMed] [Google Scholar]
- Kim JH, Kim B, Cai L, Choi HJ, Ohgi KA, Tran C, Chen C, Chung CH, Huber O, Rose DW, Sawyers CL, Rosenfeld MG, et al. Transcriptional regulation of a metastasis suppressor gene by Tip60 and beta-catenin complexes. Nature. 2005;434:921–926. doi: 10.1038/nature03452. [DOI] [PubMed] [Google Scholar]
- Kimura T, Suzuki A, Fujita Y, Yomogida K, Lomeli H, Asada N, Ikeuchi M, Nagy A, Mak TW, Nakano T. Conditional loss of PTEN leads to testicular teratoma and enhances embryonic germ cell production. Development. 2003;130:1691–1700. doi: 10.1242/dev.00392. [DOI] [PubMed] [Google Scholar]
- Knowling M, Blackstein M, Tozer R, Bramwell V, Dancey J, Dore N, Matthews S, Eisenhauer E. A phase II study of perifosine (D-21226) in patients with previously untreated metastatic or locally advanced soft tissue sarcoma: A National Cancer Institute of Canada Clinical Trials Group trial. Invest New Drugs. 2006;24:435–439. doi: 10.1007/s10637-006-6406-7. [DOI] [PubMed] [Google Scholar]
- Komazawa N, Suzuki A, Sano S, Horie K, Matsuura N, Mak TW, Nakano T, Takeda J, Kondoh G. Tumorigenesis facilitated by Pten deficiency in the skin: Evidence of p53-Pten complex formation on the initiation phase. Cancer Sci. 2004;95:639–643. doi: 10.1111/j.1349-7006.2004.tb03322.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kong D, Yamori T. ZSTK474 is an ATP-competitive inhibitor of class I phosphatidylinositol 3 kinase isoforms. Cancer Sci. 2007;98:1638–1642. doi: 10.1111/j.1349-7006.2007.00580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn JG, Chang SM, Wen PY, Cloughesy TF, Greenberg H, Schiff D, Conrad C, Fink KL, Robins HI, Mehta M, DeAngelis L, Raizer J, et al. Pharmacokinetic and tumor distribution characteristics of temsirolimus in patients with recurrent malignant glioma. Clin Cancer Res. 2007;13:7401–7406. doi: 10.1158/1078-0432.CCR-07-0781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laramee M, Chabot C, Cloutier M, Stenne R, Holgado-Madruga M, Wong AJ, Royal I. The scaffolding adapter Gab1 mediates vascular endothelial growth factor signaling and is required for endothelial cell migration and capillary formation. J Biol Chem. 2007;282:7758–7769. doi: 10.1074/jbc.M611327200. [DOI] [PubMed] [Google Scholar]
- Larue L, Bellacosa A. Epithelial-mesenchymal transition in development and cancer: Role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene. 2005;24:7443–7454. doi: 10.1038/sj.onc.1209091. [DOI] [PubMed] [Google Scholar]
- Lee KS, Kim SR, Park SJ, Min KH, Lee KY, Choe YH, Park SY, Chai OH, Zhang X, Song CH, Lee YC. Mast cells can mediate vascular permeability through regulation of the PI3K-HIF-1alpha-VEGF axis. Am J Respir Crit Care Med. 2008;178:787–797. doi: 10.1164/rccm.200801-008OC. [DOI] [PubMed] [Google Scholar]
- Leighl NB, Dent S, Clemons M, Vandenberg TA, Tozer R, Warr DG, Crump RM, Hedley D, Pond GR, Dancey JE, Moore MJ. A Phase 2 study of perifosine in advanced or metastatic breast cancer. Breast Cancer Res Treat. 2008;108:87–92. doi: 10.1007/s10549-007-9584-x. [DOI] [PubMed] [Google Scholar]
- Lelievre E, Bourbon PM, Duan LJ, Nussbaum RL, Fong GH. Deficiency in the p110alpha subunit of PI3K results in diminished Tie2 expression and Tie2(−/−)-like vascular defects in mice. Blood. 2005;105:3935–3938. doi: 10.1182/blood-2004-10-3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie NR. The redox regulation of PI 3-kinase-dependent signaling. Antioxid Redox Signal. 2006;8:1765–1774. doi: 10.1089/ars.2006.8.1765. [DOI] [PubMed] [Google Scholar]
- Levy AP, Levy NS, Wegner S, Goldberg MA. Transcriptional regulation of the rat vascular endothelial growth factor gene by hypoxia. J Biol Chem. 1995;270:13333–13340. doi: 10.1074/jbc.270.22.13333. [DOI] [PubMed] [Google Scholar]
- Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner SH, Giovanella BC, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997a;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- Li L, Ernsting BR, Wishart MJ, Lohse DL, Dixon JE. A family of putative tumor suppressors is structurally and functionally conserved in humans and yeast. J Biol Chem. 1997b;272:29403–29406. doi: 10.1074/jbc.272.47.29403. [DOI] [PubMed] [Google Scholar]
- Li G, Robinson GW, Lesche R, Martinez-Diaz H, Jiang Z, Rozengurt N, Wagner KU, Wu DC, Lane TF, Liu X, Hennighausen L, Wu H. Conditional loss of PTEN leads to precocious development and neoplasia in the mammary gland. Development. 2002;129:4159–4170. doi: 10.1242/dev.129.17.4159. [DOI] [PubMed] [Google Scholar]
- Liaw D, Marsh DJ, Li J, Dahia PL, Wang SI, Zheng Z, Bose S, Call KM, Tsou HC, Peacocke M, Eng C, Parsons R. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat Genet. 1997;16:64–67. doi: 10.1038/ng0597-64. [DOI] [PubMed] [Google Scholar]
- Lin J, Lalani AS, Harding TC, Gonzalez M, Wu WW, Luan B, Tu GH, Koprivnikar K, VanRoey MJ, He Y, Alitalo K, Jooss K. Inhibition of lymphogenous metastasis using adeno-associated virus-mediated gene transfer of a soluble VEGFR-3 decoy receptor. Cancer Res. 2005;65:6901–6909. doi: 10.1158/0008-5472.CAN-05-0408. [DOI] [PubMed] [Google Scholar]
- Litman P, Ohne O, Ben Yaakov S, Shemesh-Darvish L, Yechezkel T, Salitra Y, Rubnov S, Cohen I, Senderowitz H, Kidron D, Livnah O, Levitzki A, et al. A novel substrate mimetic inhibitor of PKB/Akt inhibits prostate cancer tumor growth in mice by blocking the PKB pathway. Biochemistry. 2007;46:4716–4724. doi: 10.1021/bi061928s. [DOI] [PubMed] [Google Scholar]
- Liu X, Rubin JS, Kimmel AR. Rapid, Wnt-induced changes in GSK3beta associations that regulate beta-catenin stabilization are mediated by Galpha proteins. Curr Biol. 2005;15:1989–1997. doi: 10.1016/j.cub.2005.10.050. [DOI] [PubMed] [Google Scholar]
- Liu L, Zhu S, Gong Z, Low BC. K-ras/PI3K-Akt signaling is essential for zebrafish hematopoiesis and angiogenesis. PLoS ONE. 2008a;3:e2850. doi: 10.1371/journal.pone.0002850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LZ, Zheng JZ, Wang XR, Jiang BH. Endothelial p70 S6 kinase 1 in regulating tumor angiogenesis. Cancer Res. 2008b;68:8183–8188. doi: 10.1158/0008-5472.CAN-08-0819. [DOI] [PubMed] [Google Scholar]
- Liu X, Shi Y, Woods KW, Hessler P, Kroeger P, Wilsbacher J, Wang J, Wang JY, Li C, Li Q, Rosenberg SH, Giranda VL, et al. Akt inhibitor a-443654 interferes with mitotic progression by regulating aurora a kinase expression. Neoplasia. 2008c;10:828–837. doi: 10.1593/neo.08408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Wahl LM. Production of matrix metalloproteinase-9 by activated human monocytes involves a phosphatidylinositol-3 kinase/Akt/IKKalpha/NF-kappaB pathway. J Leukoc Biol. 2005;78:259–265. doi: 10.1189/jlb.0904498. [DOI] [PubMed] [Google Scholar]
- Lu Z, Ghosh S, Wang Z, Hunter T. Downregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of beta-catenin, and enhanced tumor cell invasion. Cancer Cell. 2003;4:499–515. doi: 10.1016/s1535-6108(03)00304-0. [DOI] [PubMed] [Google Scholar]
- Luo J, McMullen JR, Sobkiw CL, Zhang L, Dorfman AL, Sherwood MC, Logsdon MN, Horner JW, DePinho RA, Izumo S, Cantley LC. Class IA phosphoinositide 3-kinase regulates heart size and physiological cardiac hypertrophy. Mol Cell Biol. 2005;25:9491–9502. doi: 10.1128/MCB.25.21.9491-9502.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Sobkiw CL, Hirshman MF, Logsdon MN, Li TQ, Goodyear LJ, Cantley LC. Loss of class IA PI3K signaling in muscle leads to impaired muscle growth, insulin response, and hyperlipidemia. Cell Metab. 2006;3:355–366. doi: 10.1016/j.cmet.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Ma X, Ziel-van der Made AC, Autar B, van der Korput HA, Vermeij M, van Duijn P, Cleutjens KB, de Krijger R, Krimpenfort P, Berns A, van der Kwast TH, Trapman J. Targeted biallelic inactivation of Pten in the mouse prostate leads to prostate cancer accompanied by increased epithelial cell proliferation but not by reduced apoptosis. Cancer Res. 2005;65:5730–5739. doi: 10.1158/0008-5472.CAN-04-4519. [DOI] [PubMed] [Google Scholar]
- MacDougall LK, Gagou ME, Leevers SJ, Hafen E, Waterfield MD. Targeted expression of the class II phosphoinositide 3-kinase in Drosophila melanogaster reveals lipid kinase-dependent effects on patterning and interactions with receptor signaling pathways. Mol Cell Biol. 2004;24:796–808. doi: 10.1128/MCB.24.2.796-808.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- Maffucci T, Piccolo E, Cumashi A, Iezzi M, Riley AM, Saiardi A, Godage HY, Rossi C, Broggini M, Iacobelli S, Potter BV, Innocenti P, et al. Inhibition of the phosphatidylinositol 3-kinase/Akt pathway by inositol pentakisphosphate results in antiangiogenic and antitumor effects. Cancer Res. 2005;65:8339–8349. doi: 10.1158/0008-5472.CAN-05-0121. [DOI] [PubMed] [Google Scholar]
- Mahimainathan L, Das F, Venkatesan B, Choudhury GG. Mesangial cell hypertrophy by high glucose is mediated by downregulation of the tumor suppressor PTEN. Diabetes. 2006;55:2115–2125. doi: 10.2337/db05-1326. [DOI] [PubMed] [Google Scholar]
- Maira SM, Stauffer F, Brueggen J, Furet P, Schnell C, Fritsch C, Brachmann S, Chene P, De Pover A, Schoemaker K, Fabbro D, Gabriel D, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008;7:1851–1863. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- Makinde T, Agrawal DK. Intra and extravascular transmembrane signalling of angiopoietin-1-Tie2 receptor in health and disease. J Cell Mol Med. 2008;12:810–828. doi: 10.1111/j.1582-4934.2008.00254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinen T, Jussila L, Veikkola T, Karpanen T, Kettunen MI, Pulkkanen KJ, Kauppinen R, Jackson DG, Kubo H, Nishikawa S, Yla-Herttuala S, Alitalo K. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nat Med. 2001;7:199–205. doi: 10.1038/84651. [DOI] [PubMed] [Google Scholar]
- Mandal M, Younes M, Swan EA, Jasser SA, Doan D, Yigitbasi O, McMurphey A, Ludwick J, El Naggar AK, Bucana C, Mills GB, Myers JN. The Akt inhibitor KP372-1 inhibits proliferation and induces apoptosis and anoikis in squamous cell carcinoma of the head and neck. Oral Oncol. 2006;42:430–439. doi: 10.1016/j.oraloncology.2005.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolin K, Longmate J, Baratta T, Synold T, Christensen S, Weber J, Gajewski T, Quirt I, Doroshow JH. CCI-779 in metastatic melanoma: A phase II trial of the California Cancer Consortium. Cancer. 2005;104:1045–1048. doi: 10.1002/cncr.21265. [DOI] [PubMed] [Google Scholar]
- Marone R, Cmiljanovic V, Giese B, Wymann MP. Targeting phosphoinositide 3-kinase: Moving towards therapy. Biochim Biophys Acta. 2008;1784:159–185. doi: 10.1016/j.bbapap.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Marsh DJ, Dahia PL, Zheng Z, Liaw D, Parsons R, Gorlin RJ, Eng C. Germline mutations in PTEN are present in Bannayan-Zonana syndrome. Nat Genet. 1997;16:333–334. doi: 10.1038/ng0897-333. [DOI] [PubMed] [Google Scholar]
- Marsh RW, Rocha Lima CM, Levy DE, Mitchell EP, Rowland KM, Jr, Benson AB., III A phase II trial of perifosine in locally advanced, unresectable, or metastatic pancreatic adenocarcinoma. Am J Clin Oncol. 2007;30:26–31. doi: 10.1097/01.coc.0000251235.46149.43. [DOI] [PubMed] [Google Scholar]
- Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- Mayo LD, Donner DB. A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc Natl Acad Sci USA. 2001;98:11598–11603. doi: 10.1073/pnas.181181198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazure NM, Chen EY, Laderoute KR, Giaccia AJ. Induction of vascular endothelial growth factor by hypoxia is modulated by a phosphatidylinositol 3-kinase/Akt signaling pathway in Ha-ras-transformed cells through a hypoxia inducible factor-1 transcriptional element. Blood. 1997;90:3322–3331. [PubMed] [Google Scholar]
- McGlade CJ, Ellis C, Reedijk M, Anderson D, Mbamalu G, Reith AD, Panayotou G, End P, Bernstein A, Kazlauskas A. SH2 domains of the p85 alpha subunit of phosphatidylinositol 3-kinase regulate binding to growth factor receptors. Mol Cell Biol. 1992;12:991–997. doi: 10.1128/mcb.12.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133:647–658. doi: 10.1053/j.gastro.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuillet EJ, Ihle N, Baker AF, Gard JM, Stamper C, Williams R, Coon A, Mahadevan D, George BL, Kirkpatrick L, Powis G. In vivo molecular pharmacology and antitumor activity of the targeted Akt inhibitor PX-316. Oncol Res. 2004;14:513–527. doi: 10.3727/0965040042380487. [DOI] [PubMed] [Google Scholar]
- Milton DT, Riely GJ, Azzoli CG, Gomez JE, Heelan RT, Kris MG, Krug LM, Pao W, Pizzo B, Rizvi NA, Miller VA. Phase 1 trial of everolimus and gefitinib in patients with advanced nonsmall-cell lung cancer. Cancer. 2007;110:599–605. doi: 10.1002/cncr.22816. [DOI] [PubMed] [Google Scholar]
- Mita MM, Mita AC, Chu QS, Rowinsky EK, Fetterly GJ, Goldston M, Patnaik A, Mathews L, Ricart AD, Mays T, Knowles H, Rivera VM, et al. Phase I trial of the novel mammalian target of rapamycin inhibitor deforolimus (AP23573; MK-8669) administered intravenously daily for 5 days every 2 weeks to patients with advanced malignancies. J Clin Oncol. 2008;26:361–367. doi: 10.1200/JCO.2007.12.0345. [DOI] [PubMed] [Google Scholar]
- Mizukami Y, Fujiki K, Duerr EM, Gala M, Jo WS, Zhang X, Chung DC. Hypoxic regulation of vascular endothelial growth factor through the induction of phosphatidylinositol 3-kinase/Rho/ROCK and c-Myc. J Biol Chem. 2006;281:13957–13963. doi: 10.1074/jbc.M511763200. [DOI] [PubMed] [Google Scholar]
- Morita Y, Manganaro TF, Tao XJ, Martimbeau S, Donahoe PK, Tilly JL. Requirement for phosphatidylinositol-3′-kinase in cytokine-mediated germ cell survival during fetal oogenesis in the mouse. Endocrinology. 1999;140:941–949. doi: 10.1210/endo.140.2.6539. [DOI] [PubMed] [Google Scholar]
- Mottet D, Dumont V, Deccache Y, Demazy C, Ninane N, Raes M, Michiels C. Regulation of hypoxia-inducible factor-1alpha protein level during hypoxic conditions by the phosphatidylinositol 3-kinase/Akt/glycogen synthase kinase 3beta pathway in HepG2 cells. J Biol Chem. 2003;278:31277–31285. doi: 10.1074/jbc.M300763200. [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Hudes GR, Curti BD, McDermott DF, Escudier BJ, Negrier S, Duclos B, Moore L, O’Toole T, Boni JP, Dutcher JP. Phase I/II trial of temsirolimus combined with interferon alfa for advanced renal cell carcinoma. J Clin Oncol. 2007;25:3958–3964. doi: 10.1200/JCO.2006.10.5916. [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, Grunwald V, Thompson JA, Figlin RA, Hollaender N, Urbanowitz G, Berg WJ, et al. Efficacy of everolimus in advanced renal cell carcinoma: A double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- Murakami M, Zheng Y, Hirashima M, Suda T, Morita Y, Ooehara J, Ema H, Fong GH, Shibuya M. VEGFR1 tyrosine kinase signaling promotes lymphangiogenesis as well as angiogenesis indirectly via macrophage recruitment. Arterioscler Thromb Vasc Biol. 2008;28:658–664. doi: 10.1161/ATVBAHA.107.150433. [DOI] [PubMed] [Google Scholar]
- Nakao T, Shiota M, Tatemoto Y, Izumi Y, Iwao H. Pravastatin induces rat aortic endothelial cell proliferation and migration via activation of PI3K/Akt/mTOR/p70 S6 kinase signaling. J Pharmacol Sci. 2007;105:334–341. doi: 10.1254/jphs.fp0070682. [DOI] [PubMed] [Google Scholar]
- Nakatani K, Thompson DA, Barthel A, Sakaue H, Liu W, Weigel RJ, Roth RA. Up-regulation of Akt3 in estrogen receptor-deficient breast cancers and androgen- independent prostate cancer lines. J Biol Chem. 1999;274:21528–21532. doi: 10.1074/jbc.274.31.21528. [DOI] [PubMed] [Google Scholar]
- Naugler WE, Karin M. NF-kappaB and cancer-identifying targets and mechanisms. Curr Opin Genet Dev. 2008;18:19–26. doi: 10.1016/j.gde.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemenoff RA, Simpson PA, Furgeson SB, Kaplan-Albuquerque N, Crossno J, Garl PJ, Cooper J, Weiser-Evans MC. Targeted deletion of PTEN in smooth muscle cells results in vascular remodeling and recruitment of progenitor cells through induction of stromal cell-derived factor-1alpha. Circ Res. 2008;102:1036–1045. doi: 10.1161/CIRCRESAHA.107.169896. [DOI] [PubMed] [Google Scholar]
- Niu Q, Perruzzi C, Voskas D, Lawler J, Dumont DJ, Benjamin LE. Inhibition of Tie-2 signaling induces endothelial cell apoptosis, decreases Akt signaling, and induces endothelial cell expression of the endogenous anti-angiogenic molecule, thrombospondin-1. Cancer Biol Ther. 2004;3:402–405. doi: 10.4161/cbt.3.4.735. [DOI] [PubMed] [Google Scholar]
- Nyberg P, Salo T, Kalluri R. Tumor microenvironment and angiogenesis. Front Biosci. 2008;13:6537–6553. doi: 10.2741/3173. [DOI] [PubMed] [Google Scholar]
- O’Donnell A, Faivre S, Burris HA, III, Rea D, Papadimitrakopoulou V, Shand N, Lane HA, Hazell K, Zoellner U, Kovarik JM, Brock C, Jones S, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumors. J Clin Oncol. 2008;26:1588–1595. doi: 10.1200/JCO.2007.14.0988. [DOI] [PubMed] [Google Scholar]
- Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling—In control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- Onoue T, Uchida D, Begum NM, Tomizuka Y, Yoshida H, Sato M. Epithelial-mesenchymal transition induced by the stromal cell-derived factor-1/CXCR4 system in oral squamous cell carcinoma cells. Int J Oncol. 2006;29:1133–1138. [PubMed] [Google Scholar]
- Orlichenko LS, Radisky DC. Matrix metalloproteinases stimulate epithelial-mesenchymal transition during tumor development. Clin Exp Metastasis. 2008;25:593–600. doi: 10.1007/s10585-008-9143-9. [DOI] [PubMed] [Google Scholar]
- Otsu M, Hiles I, Gout I, Fry MJ, Ruiz-Larrea F, Panayotou G, Thompson A, Dhand R, Hsuan J, Totty N, Smith AD, Morgan SJ, et al. Characterization of two 85 kd proteins that associate with receptor tyrosine kinases, middle-T/pp60c-src complexes, and PI3-kinase. Cell. 1991;65:91–104. doi: 10.1016/0092-8674(91)90411-q. [DOI] [PubMed] [Google Scholar]
- Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine–threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- Pandya KJ, Dahlberg S, Hidalgo M, Cohen RB, Lee MW, Schiller JH, Johnson DH. A randomized, phase II trial of two dose levels of temsirolimus (CCI-779) in patients with extensive-stage small-cell lung cancer who have responding or stable disease after induction chemotherapy: A trial of the Eastern Cooperative Oncology Group (E1500) J Thorac Oncol. 2007;2:1036–1041. doi: 10.1097/JTO.0b013e318155a439. [DOI] [PubMed] [Google Scholar]
- Paradiso A, Mangia A, Azzariti A, Tommasi S. Phosphatidylinositol 3-kinase in breast cancer: Where from here? Clin Cancer Res. 2007;13:5988–5990. doi: 10.1158/1078-0432.CCR-07-1106. [DOI] [PubMed] [Google Scholar]
- Patel L, Pass I, Coxon P, Downes CP, Smith SA, Macphee CH. Tumor suppressor and anti-inflammatory actions of PPARgamma agonists are mediated via upregulation of PTEN. Curr Biol. 2001;11:764–768. doi: 10.1016/s0960-9822(01)00225-1. [DOI] [PubMed] [Google Scholar]
- Peralba JM, DeGraffenried L, Friedrichs W, Fulcher L, Grunwald V, Weiss G, Hidalgo M. Pharmacodynamic evaluation of CCI-779, an inhibitor of mTOR, in cancer patients. Clin Cancer Res. 2003;9:2887–2892. [PubMed] [Google Scholar]
- Peyssonnaux C, Provot S, Felder-Schmittbuhl MP, Calothy G, Eychene A. Induction of postmitotic neuroretina cell proliferation by distinct Ras downstream signaling pathways. Mol Cell Biol. 2000;20:7068–7079. doi: 10.1128/mcb.20.19.7068-7079.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzolesi MG, Platzer P, Waite KA, Eng C. Differential expression of PTEN-targeting microRNAs miR-19a and miR-21 in Cowden syndrome. Am J Hum Genet. 2008;82:1141–1149. doi: 10.1016/j.ajhg.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philp AJ, Campbell IG, Leet C, Vincan E, Rockman SP, Whitehead RH, Thomas RJ, Phillips WA. The phosphatidylinositol 3′-kinase p85alpha gene is an oncogene in human ovarian and colon tumors. Cancer Res. 2001;61:7426–7429. [PubMed] [Google Scholar]
- Phung TL, Ziv K, Dabydeen D, Eyiah-Mensah G, Riveros M, Perruzzi C, Sun J, Monahan-Earley RA, Shiojima I, Nagy JA, Lin MI, Walsh K, et al. Pathological angiogenesis is induced by sustained Akt signaling and inhibited by rapamycin. Cancer Cell. 2006;10:159–170. doi: 10.1016/j.ccr.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planchon SM, Waite KA, Eng C. The nuclear affairs of PTEN. J Cell Sci. 2008;121:249–253. doi: 10.1242/jcs.022459. [DOI] [PubMed] [Google Scholar]
- Podsypanina K, Ellenson LH, Nemes A, Gu J, Tamura M, Yamada KM, Cordon-Cardo C, Catoretti G, Fisher PE, Parsons R. Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. Proc Natl Acad Sci USA. 1999;96:1563–1568. doi: 10.1073/pnas.96.4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posadas EM, Gulley J, Arlen PM, Trout A, Parnes HL, Wright J, Lee MJ, Chung EJ, Trepel JB, Sparreboom A, Chen C, Jones E, et al. A phase II study of perifosine in androgen independent prostate cancer. Cancer Biol Ther. 2005;4:1133–1137. doi: 10.4161/cbt.4.10.2064. [DOI] [PubMed] [Google Scholar]
- Punt CJ, Boni J, Bruntsch U, Peters M, Thielert C. Phase I and pharmacokinetic study of CCI-779, a novel cytostatic cell-cycle inhibitor, in combination with 5-fluorouracil and leucovorin in patients with advanced solid tumors. Ann Oncol. 2003;14:931–937. doi: 10.1093/annonc/mdg248. [DOI] [PubMed] [Google Scholar]
- Quintero M, Brennan PA, Thomas GJ, Moncada S. Nitric oxide is a factor in the stabilization of hypoxia-inducible factor-1alpha in cancer: Role of free radical formation. Cancer Res. 2006;66:770–774. doi: 10.1158/0008-5472.CAN-05-0333. [DOI] [PubMed] [Google Scholar]
- Raymond E, Alexandre J, Faivre S, Vera K, Materman E, Boni J, Leister C, Korth-Bradley J, Hanauske A, Armand JP. Safety and pharmacokinetics of escalated doses of weekly intravenous infusion of CCI-779, a novel mTOR inhibitor, in patients with cancer. J Clin Oncol. 2004;22:2336–2347. doi: 10.1200/JCO.2004.08.116. [DOI] [PubMed] [Google Scholar]
- Reardon DA, Quinn JA, Vredenburgh JJ, Gururangan S, Friedman AH, Desjardins A, Sathornsumetee S, Herndon JE, Dowell JM, McLendon RE, Provenzale JM, Sampson JH, et al. Phase 1 trial of gefitinib plus sirolimus in adults with recurrent malignant glioma. Clin Cancer Res. 2006;12:860–868. doi: 10.1158/1078-0432.CCR-05-2215. [DOI] [PubMed] [Google Scholar]
- Reddy KB, Nabha SM, Atanaskova N. Role of MAP kinase in tumor progression and invasion. Cancer Metastasis Rev. 2003;22:395–403. doi: 10.1023/a:1023781114568. [DOI] [PubMed] [Google Scholar]
- Rizell M, Andersson M, Cahlin C, Hafstrom L, Olausson M, Lindner P. Effects of the mTOR inhibitor sirolimus in patients with hepatocellular and cholangiocellular cancer. Int J Clin Oncol. 2008;13:66–70. doi: 10.1007/s10147-007-0733-3. [DOI] [PubMed] [Google Scholar]
- Rizzieri DA, Feldman E, Dipersio JF, Gabrail N, Stock W, Strair R, Rivera VM, Albitar M, Bedrosian CL, Giles FJ. A phase 2 clinical trial of deforolimus (AP23573, MK-8669), a novel mammalian target of rapamycin inhibitor, in patients with relapsed or refractory hematologic malignancies. Clin Cancer Res. 2008;14:2756–2762. doi: 10.1158/1078-0432.CCR-07-1372. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Viciana P, Marte BM, Warne PH, Downward J. Phosphatidylinositol 3′ kinase: One of the effectors of Ras. Philos Trans R Soc Lond B Biol Sci. 1996;351:225–231. doi: 10.1098/rstb.1996.0020. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science. 1996;272:1023–1026. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- Salmena L, Carracedo A, Pandolfi PP. Tenets of PTEN tumor suppression. Cell. 2008;133:403–414. doi: 10.1016/j.cell.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, Willson JK, Markowitz S, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Sarkaria JN, Schwingler P, Schild SE, Grogan PT, Mladek AC, Mandrekar SJ, Tan AD, Kobayashi T, Marks RS, Kita H, Miller RC, Limper AH, et al. Phase I trial of sirolimus combined with radiation and cisplatin in non-small cell lung cancer. J Thorac Oncol. 2007;2:751–757. doi: 10.1097/JTO.0b013e3180cc2587. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Arai H, Beppu T, Ogasawara K. Detection of gene amplification and deletion in high-grade gliomas using a genome DNA microarray (GenoSensor Array 300) Brain Tumor Pathol. 2003;20:59–63. doi: 10.1007/BF02483448. [DOI] [PubMed] [Google Scholar]
- Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, Qin Y. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- Schmelzle T, Hall MN. TOR, a central controller of cell growth. Cell. 2000;103:253–262. doi: 10.1016/s0092-8674(00)00117-3. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Fernandez de Mattos S, van der Horst A, Klompmaker R, Kops GJ, Lam EW, Burgering BM, Medema RH. Cell cycle inhibition by FoxO forkhead transcription factors involves downregulation of cyclin D. Mol Cell Biol. 2002;22:7842–7852. doi: 10.1128/MCB.22.22.7842-7852.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell CR, Stauffer F, Allegrini PR, O’Reilly T, McSheehy PM, Dartois C, Stumm M, Cozens R, Littlewood-Evans A, Garcia-Echeverria C, Maira SM. Effects of the dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235 on the tumor vasculature: Implications for clinical imaging. Cancer Res. 2008;68:6598–6607. doi: 10.1158/0008-5472.CAN-08-1044. [DOI] [PubMed] [Google Scholar]
- Schramek H, Feifel E, Marschitz I, Golochtchapova N, Gstraunthaler G, Montesano R. Loss of active MEK1-ERK1/2 restores epithelial phenotype and morphogenesis in transdifferentiated MDCK cells. Am J Physiol Cell Physiol. 2003;285:C652–C661. doi: 10.1152/ajpcell.00463.2002. [DOI] [PubMed] [Google Scholar]
- Semenza GL. HIF-1 and human disease: One highly involved factor. Genes Dev. 2000;14:1983–1991. [PubMed] [Google Scholar]
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- Serban D, Leng J, Cheresh D. H-ras regulates angiogenesis and vascular permeability by activation of distinct downstream effectors. Circ Res. 2008;102:1350–1358. doi: 10.1161/CIRCRESAHA.107.169664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra V, Markman B, Scaltriti M, Eichhorn PJ, Valero V, Guzman M, Botero ML, Llonch E, Atzori F, Di Cosimo S, Maira M, Garcia-Echeverria C, et al. NVP- BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res. 2008;68:8022–8030. doi: 10.1158/0008-5472.CAN-08-1385. [DOI] [PubMed] [Google Scholar]
- Shen YH, Zhang L, Gan Y, Wang X, Wang J, LeMaire SA, Coselli JS, Wang XL. Up-regulation of PTEN (phosphatase and tensin homolog deleted on chromosome ten) mediates p38 MAPK stress signal-induced inhibition of insulin signaling. A cross-talk between stress signaling and insulin signaling in resistin-treated human endothelial cells. J Biol Chem. 2006;281:7727–7736. doi: 10.1074/jbc.M511105200. [DOI] [PubMed] [Google Scholar]
- Shi Y, Liu X, Han EK, Guan R, Shoemaker AR, Oleksijew A, Woods KW, Fisher JP, Klinghofer V, Lasko L, McGonigal T, Li Q, et al. Optimal classes of chemotherapeutic agents sensitized by specific small-molecule inhibitors of akt in vitro and in vivo. Neoplasia. 2005;7:992–1000. doi: 10.1593/neo.05355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya M. Vascular endothelial growth factor-dependent and -independent regulation of angiogenesis. BMB Rep. 2008;41:278–286. doi: 10.5483/bmbrep.2008.41.4.278. [DOI] [PubMed] [Google Scholar]
- Shome D, Trent J, Espandar L, Hatef E, Araujo DM, Song CD, Kim SK, Esmaeli B. Ulcerative keratitis in gastrointestinal stromal tumor patients treated with perifosine. Ophthalmology. 2008;115:483–487. doi: 10.1016/j.ophtha.2007.11.016. [DOI] [PubMed] [Google Scholar]
- Shukla S, Maclennan GT, Hartman DJ, Fu P, Resnick MI, Gupta S. Activation of PI3K-Akt signaling pathway promotes prostate cancer cell invasion. Int J Cancer. 2007;121:1424–1432. doi: 10.1002/ijc.22862. [DOI] [PubMed] [Google Scholar]
- Sillaber C, Mayerhofer M, Bohm A, Vales A, Gruze A, Aichberger KJ, Esterbauer H, Pfeilstocker M, Sperr WR, Pickl WF, Haas OA, Valent P. Evaluation of antileukaemic effects of rapamycin in patients with imatinib-resistant chronic myeloid leukaemia. Eur J Clin Invest. 2008;38:43–52. doi: 10.1111/j.1365-2362.2007.01892.x. [DOI] [PubMed] [Google Scholar]
- Skeen JE, Bhaskar PT, Chen CC, Chen WS, Peng XD, Nogueira V, Hahn-Windgassen A, Kiyokawa H, Hay N. Akt deficiency impairs normal cell proliferation and suppresses oncogenesis in a p53-independent and mTORC1-dependent manner. Cancer Cell. 2006;10:269–280. doi: 10.1016/j.ccr.2006.08.022. [DOI] [PubMed] [Google Scholar]
- Skinner HD, Zheng JZ, Fang J, Agani F, Jiang BH. Vascular endothelial growth factor transcriptional activation is mediated by hypoxia-inducible factor 1alpha, HDM2, and p70S6K1 in response to phosphatidylinositol 3-kinase/AKT signaling. J Biol Chem. 2004;279:45643–45651. doi: 10.1074/jbc.M404097200. [DOI] [PubMed] [Google Scholar]
- Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell. 2004;6:447–458. doi: 10.1016/j.ccr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Staal SP. Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: Amplification of AKT1 in a primary human gastric adenocarcinoma. Proc Natl Acad Sci USA. 1987;84:5034–5037. doi: 10.1073/pnas.84.14.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stambolic V, Tsao MS, MacPherson D, Suzuki A, Chapman WB, Mak TW. High incidence of breast and endometrial neoplasia resembling human Cowden syndrome in pten+/− mice. Cancer Res. 2000;60:3605–3611. [PubMed] [Google Scholar]
- Stambolic V, MacPherson D, Sas D, Lin Y, Snow B, Jang Y, Benchimol S, Mak TW. Regulation of PTEN transcription by p53. Mol Cell. 2001;8:317–325. doi: 10.1016/s1097-2765(01)00323-9. [DOI] [PubMed] [Google Scholar]
- Stanger BZ, Stiles B, Lauwers GY, Bardeesy N, Mendoza M, Wang Y, Greenwood A, Cheng KH, McLaughlin M, Brown D, DePinho RA, Wu H, et al. Pten constrains centroacinar cell expansion and malignant transformation in the pancreas. Cancer Cell. 2005;8:185–195. doi: 10.1016/j.ccr.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T, Frye C, Hu R, et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356–362. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- Stoeltzing O, Meric-Bernstam F, Ellis LM. Intracellular signaling in tumor and endothelial cells: The expected and, yet again, the unexpected. Cancer Cell. 2006;10:89–91. doi: 10.1016/j.ccr.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Stokoe D, Stephens LR, Copeland T, Gaffney PR, Reese CB, Painter GF, Holmes AB, McCormick F, Hawkins PT. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science. 1997;277:567–570. doi: 10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Whitman M, Cantley LC, Erikson RL. Evidence that the Rous sarcoma virus transforming gene product phosphorylates phosphatidylinositol and diacylglycerol. Proc Natl Acad Sci USA. 1984;81:2117–2121. doi: 10.1073/pnas.81.7.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, de la Pompa JL, Stambolic V, Elia AJ, Sasaki T, del Barco Barrantes I, Ho A, Wakeham A, Itie A, Khoo W, Fukumoto M, Mak TW. High cancer susceptibility and embryonic lethality associated with mutation of the PTEN tumor suppressor gene in mice. Curr Biol. 1998;8:1169–1178. doi: 10.1016/s0960-9822(07)00488-5. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Hamada K, Sasaki T, Mak TW, Nakano T. Role of PTEN/PI3K pathway in endothelial cells. Biochem Soc Trans. 2007;35:172–176. doi: 10.1042/BST0350172. [DOI] [PubMed] [Google Scholar]
- Tabernero J, Rojo F, Calvo E, Burris H, Judson I, Hazell K, Martinelli E, Cajal S, Jones S, Vidal L, Shand N, Macarulla T, et al. Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: A phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Oncol. 2008;26:1603–1610. doi: 10.1200/JCO.2007.14.5482. [DOI] [PubMed] [Google Scholar]
- Tamguney T, Stokoe D. New insights into PTEN. J Cell Sci. 2007;120:4071–4079. doi: 10.1242/jcs.015230. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Fujita N, Tsuruo T. 3-Phosphoinositide-dependent protein kinase-1- mediated IkappaB kinase beta (IkkB) phosphorylation activates NF-kappaB signaling. J Biol Chem. 2005;280:40965–40973. doi: 10.1074/jbc.M506235200. [DOI] [PubMed] [Google Scholar]
- Tassa A, Roux MP, Attaix D, Bechet DM. Class III phosphoinositide 3-kinase-Beclin1 complex mediates the amino acid-dependent regulation of autophagy in C2C12 myotubes. Biochem J. 2003;376:577–586. doi: 10.1042/BJ20030826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery JP, Sleeman JP. Complex networks orchestrate epithelial–mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- Trisciuoglio D, Iervolino A, Zupi G, Del Bufalo D. Involvement of PI3K and MAPK signaling in bcl-2-induced vascular endothelial growth factor expression in melanoma cells. Mol Biol Cell. 2005;16:4153–4162. doi: 10.1091/mbc.E04-12-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotman LC, Niki M, Dotan ZA, Koutcher JA, Di Cristofano A, Xiao A, Khoo AS, Roy-Burman P, Greenberg NM, Van Dyke T, Cordon-Cardo C, Pandolfi PP. Pten dose dictates cancer progression in the prostate. PLoS Biol. 2003;1:E59. doi: 10.1371/journal.pbio.0000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou HC, Teng DH, Ping XL, Brancolini V, Davis T, Hu R, Xie XX, Gruener AC, Schrager CA, Christiano AM, Eng C, Steck P, et al. The role of MMAC1 mutations in early-onset breast cancer: Causative in association with Cowden syndrome and excluded in BRCA1-negative cases. Am J Hum Genet. 1997;61:1036–1043. doi: 10.1086/301607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya KD, Wiesner G, Cassidy SB, Limwongse C, Boyle JT, Schwartz S. Deletion 10q23.2-q23.33 in a patient with gastrointestinal juvenile polyposis and other features of a Cowden-like syndrome. Genes Chromosomes Cancer. 1998;21:113–118. doi: 10.1002/(sici)1098-2264(199802)21:2<113::aid-gcc6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Tsuruta H, Kishimoto H, Sasaki T, Horie Y, Natsui M, Shibata Y, Hamada K, Yajima N, Kawahara K, Sasaki M, Tsuchiya N, Enomoto K, et al. Hyperplasia and carcinomas in Pten-deficient mice and reduced PTEN protein in human bladder cancer patients. Cancer Res. 2006;66:8389–8396. doi: 10.1158/0008-5472.CAN-05-4627. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Leevers SJ, Panayotou G, Waterfield MD. Phosphoinositide 3-kinases: A conserved family of signal transducers. Trends Biochem Sci. 1997;22:267–272. doi: 10.1016/s0968-0004(97)01061-x. [DOI] [PubMed] [Google Scholar]
- Van Ummersen L, Binger K, Volkman J, Marnocha R, Tutsch K, Kolesar J, Arzoomanian R, Alberti D, Wilding G. A phase I trial of perifosine (NSC 639966) on a loading dose/maintenance dose schedule in patients with advanced cancer. Clin Cancer Res. 2004;10:7450–7456. doi: 10.1158/1078-0432.CCR-03-0406. [DOI] [PubMed] [Google Scholar]
- Vink SR, Schellens JH, Beijnen JH, Sindermann H, Engel J, Dubbelman R, Moppi G, Hillebrand MJ, Bartelink H, Verheij M. Phase I and pharmaco-kinetic study of combined treatment with perifosine and radiation in patients with advanced solid tumours. Radiother Oncol. 2006;80:207–213. doi: 10.1016/j.radonc.2006.07.032. [DOI] [PubMed] [Google Scholar]
- Vivanco I, Palaskas N, Tran C, Finn SP, Getz G, Kennedy NJ, Jiao J, Rose J, Xie W, Loda M, Golub T, Mellinghoff IK, et al. Identification of the JNK signaling pathway as a functional target of the tumor suppressor PTEN. Cancer Cell. 2007;11:555–569. doi: 10.1016/j.ccr.2007.04.021. [DOI] [PubMed] [Google Scholar]
- Walker EH, Perisic O, Ried C, Stephens L, Williams RL. Structural insights into phosphoinositide 3-kinase catalysis and signalling. Nature. 1999;402:313–320. doi: 10.1038/46319. [DOI] [PubMed] [Google Scholar]
- Walker EH, Pacold ME, Perisic O, Stephens L, Hawkins PT, Wymann MP, Williams RL. Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol Cell. 2000;6:909–919. doi: 10.1016/s1097-2765(05)00089-4. [DOI] [PubMed] [Google Scholar]
- Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Gao J, Lei Q, Rozengurt N, Pritchard C, Jiao J, Thomas GV, Li G, Roy-Burman P, Nelson PS, Liu X, Wu H. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4:209–221. doi: 10.1016/s1535-6108(03)00215-0. [DOI] [PubMed] [Google Scholar]
- Wang FS, Kuo YR, Wang CJ, Yang KD, Chang PR, Huang YT, Huang HC, Sun YC, Yang YJ, Chen YJ. Nitric oxide mediates ultrasound-induced hypoxia-inducible factor-1alpha activation and vascular endothelial growth factor-A expression in human osteoblasts. Bone. 2004;35:114–123. doi: 10.1016/j.bone.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Wang S, Garcia AJ, Wu M, Lawson DA, Witte ON, Wu H. Pten deletion leads to the expansion of a prostatic stem/progenitor cell subpopulation and tumor initiation. Proc Natl Acad Sci USA. 2006;103:1480–1485. doi: 10.1073/pnas.0510652103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Horie Y, Kataoka E, Sato W, Dohmen T, Ohshima S, Goto T, Suzuki A. Non-alcoholic steatohepatitis and hepatocellular carcinoma: Lessons from hepatocyte-specific phosphatase and tensin homolog (PTEN)-deficient mice. J Gastroenterol Hepatol. 2007;22(Suppl 1):S96–S100. doi: 10.1111/j.1440-1746.2006.04665.x. [DOI] [PubMed] [Google Scholar]
- Weisberg E, Banerji L, Wright RD, Barrett R, Ray A, Moreno D, Catley L, Jiang J, Hall-Meyers E, Sauveur-Michel M, Stone R, Galinsky I, et al. Potentiation of antileukemic therapies by the dual PI3K/PDK-1 inhibitor, BAG956: Effects on BCR-ABL- and mutant FLT3-expressing cells. Blood. 2008;111:3723–3734. doi: 10.1182/blood-2007-09-114454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen S, Stolarov J, Myers MP, Su JD, Wigler MH, Tonks NK, Durden DL. PTEN controls tumor-induced angiogenesis. Proc Natl Acad Sci USA. 2001;98:4622–4627. doi: 10.1073/pnas.081063798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler M, Domin J. Recruitment of the class II phosphoinositide 3-kinase C2beta to the epidermal growth factor receptor: Role of Grb2. Mol Cell Biol. 2001;21:6660–6667. doi: 10.1128/MCB.21.19.6660-6667.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman M, Kaplan DR, Schaffhausen B, Cantley L, Roberts TM. Association of phosphatidylinositol kinase activity with polyoma middle-T competent for transformation. Nature. 1985;315:239–242. doi: 10.1038/315239a0. [DOI] [PubMed] [Google Scholar]
- Wymann MP, Bulgarelli-Leva G, Zvelebil MJ, Pirola L, Vanhaesebroeck B, Waterfield MD, Panayotou G. Wortmannin inactivates phosphoinositide 3-kinase by covalent modification of Lys-802, a residue involved in the phosphate transfer reaction. Mol Cell Biol. 1996;16:1722–1733. doi: 10.1128/mcb.16.4.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia C, Meng Q, Cao Z, Shi X, Jiang BH. Regulation of angiogenesis and tumor growth by p110 alpha and AKT1 via VEGF expression. J Cell Physiol. 2006;209:56–66. doi: 10.1002/jcp.20707. [DOI] [PubMed] [Google Scholar]
- Xia D, Srinivas H, Ahn YH, Sethi G, Sheng X, Yung WK, Xia Q, Chiao PJ, Kim H, Brown PH, Wistuba II, Aggarwal BB, et al. Mitogen-activated protein kinase kinase-4 promotes cell survival by decreasing PTEN expression through an NF kappa B-dependent pathway. J Biol Chem. 2007;282:3507–3519. doi: 10.1074/jbc.M610141200. [DOI] [PubMed] [Google Scholar]
- Xue Y, Bi F, Zhang X, Zhang S, Pan Y, Liu N, Shi Y, Yao X, Zheng Y, Fan D. Role of Rac1 and Cdc42 in hypoxia induced p53 and von Hippel-Lindau suppression and HIF1alpha activation. Int J Cancer. 2006;118:2965–2972. doi: 10.1002/ijc.21763. [DOI] [PubMed] [Google Scholar]
- Yaguchi S, Fukui Y, Koshimizu I, Yoshimi H, Matsuno T, Gouda H, Hirono S, Yamazaki K, Yamori T. Antitumor activity of ZSTK474, a new phosphatidylinositol 3-kinase inhibitor. J Natl Cancer Inst. 2006;98:545–556. doi: 10.1093/jnci/djj133. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Ishihara H, Yamada T, Tamura A, Usui M, Tominaga R, Munakata Y, Satake C, Katagiri H, Tashiro F, Aburatani H, Tsukiyama-Kohara K, et al. ATF4-mediated induction of 4E-BP1 contributes to pancreatic beta cell survival under endoplasmic reticulum stress. Cell Metab. 2008;7:269–276. doi: 10.1016/j.cmet.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Yanagi S, Kishimoto H, Kawahara K, Sasaki T, Sasaki M, Nishio M, Yajima N, Hamada K, Horie Y, Kubo H, Whitsett JA, Mak TW, et al. Pten controls lung morphogenesis, bronchioalveolar stem cells, and onset of lung adenocarcinomas in mice. J Clin Invest. 2007;117:2929–2940. doi: 10.1172/JCI31854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZZ, Tschopp O, Hemmings-Mieszczak M, Feng J, Brodbeck D, Perentes E, Hemmings BA. Protein kinase B alpha/Akt1 regulates placental development and fetal growth. J Biol Chem. 2003;278:32124–32131. doi: 10.1074/jbc.M302847200. [DOI] [PubMed] [Google Scholar]
- Yang H, Kong W, He L, Zhao JJ, O’Donnell JD, Wang J, Wenham RM, Coppola D, Kruk PA, Nicosia SV, Cheng JQ. MicroRNA expression profiling in human ovarian cancer: MiR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res. 2008;68:425–433. doi: 10.1158/0008-5472.CAN-07-2488. [DOI] [PubMed] [Google Scholar]
- Yao JC, Phan AT, Chang DZ, Wolff RA, Hess K, Gupta S, Jacobs C, Mares JE, Landgraf AN, Rashid A, Meric-Bernstam F. Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: Results of a phase II study. J Clin Oncol. 2008;26:4311–4318. doi: 10.1200/JCO.2008.16.7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee KW, Zeng Z, Konopleva M, Verstovsek S, Ravandi F, Ferrajoli A, Thomas D, Wierda W, Apostolidou E, Albitar M, O’Brien S, Andreeff M, et al. Phase I/II study of the mammalian target of rapamycin inhibitor everolimus (RAD001) in patients with relapsed or refractory hematologic malignancies. Clin Cancer Res. 2006;12:5165–5173. doi: 10.1158/1078-0432.CCR-06-0764. [DOI] [PubMed] [Google Scholar]
- Yen ML, Su JL, Chien CL, Tseng KW, Yang CY, Chen WF, Chang CC, Kuo ML. Diosgenin induces hypoxia-inducible factor-1 activation and angiogenesis through estrogen receptor-related phosphatidylinositol 3-kinase/Akt and p38 mitogen-activated protein kinase pathways in osteoblasts. Mol Pharmacol. 2005;68:1061–1073. doi: 10.1124/mol.104.010082. [DOI] [PubMed] [Google Scholar]
- Yoshimoto M, Cunha IW, Coudry RA, Fonseca FP, Torres CH, Soares FA, Squire JA. FISH analysis of 107 prostate cancers shows that PTEN genomic deletion is associated with poor clinical outcome. Br J Cancer. 2007;97:678–685. doi: 10.1038/sj.bjc.6603924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K, Lucas J, Zhu T, Zask A, Gaydos C, Toral-Barza L, Gu J, Li F, Chaudhary I, Cai P, Lotvin J, Petersen R, et al. PWT-458, a novel pegylated-17-hydroxywortmannin, inhibits phosphatidylinositol 3-kinase signaling and suppresses growth of solid tumors. Cancer Biol Ther. 2005;4:538–545. doi: 10.4161/cbt.4.5.1660. [DOI] [PubMed] [Google Scholar]
- Yuan TL, Cantley LC. PI3K pathway alterations in cancer: Variations on a theme. Oncogene. 2008;27:5497–5510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan HT, Venkatesha S, Chan B, Deutsch U, Mammoto T, Sukhatme VP, Woolf AS, Karumanchi SA. Activation of the orphan endothelial receptor Tie1 modifies Tie2-mediated intracellular signaling and cell survival. FASEB J. 2007;21:3171–3183. doi: 10.1096/fj.07-8487com. [DOI] [PubMed] [Google Scholar]
- Yuan TL, Choi HS, Matsui A, Benes C, Lifshits E, Luo J, Frangioni JV, Cantley LC. Class 1A PI3K regulates vessel integrity during development and tumorigenesis. Proc Natl Acad Sci USA. 2008;105:9739–9744. doi: 10.1073/pnas.0804123105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Cicchetti G, Onda H, Koon HB, Asrican K, Bajraszewski N, Vazquez F, Carpenter CL, Kwiatkowski DJ. Loss of Tsc1/Tsc2 activates mTOR and disrupts PI3K-Akt signaling through downregulation of PDGFR. J Clin Invest. 2003;112:1223–1233. doi: 10.1172/JCI17222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JJ, Cheng H, Jia S, Wang L, Gjoerup OV, Mikami A, Roberts TM. The p110alpha isoform of PI3K is essential for proper growth factor signaling and oncogenic transformation. Proc Natl Acad Sci USA. 2006;103:16296–16300. doi: 10.1073/pnas.0607899103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Dai T, Zhou B, Zhu J, Huang H, Wang M, Fu G. SDF-1alpha/CXCR4 decreases endothelial progenitor cells apoptosis under serum deprivation by PI3K/Akt/eNOS pathway. Atherosclerosis. 2008 doi: 10.1016/j.atherosclerosis.2008.02.011. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu MM, Simons JW, Semenza GL. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: Implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60:1541–1545. [PubMed] [Google Scholar]
- Zhu G, Decker SJ, Saltiel AR. Direct analysis of the binding of Src-homology 2 domains of phospholipase C to the activated epidermal growth factor receptor. Proc Natl Acad Sci USA. 1992;89:9559–9563. doi: 10.1073/pnas.89.20.9559. [DOI] [PMC free article] [PubMed] [Google Scholar]



