Abstract
Objectives
We tested the hypothesis that oxidative stress is increased in patients with rheumatoid arthritis (RA) due to increased inflammation and contributes to the pathogenesis of atherosclerosis.
Methods
The independent association between urinary F2-isoprostane excretion, a measure of oxidative stress, and RA was tested using multiple linear regression models in 169 patients with RA and 92 control subjects frequency-matched for age, race and sex. The relationship between F2-isoprostane excretion and coronary calcium, a marker of atherosclerosis, was examined in multivariable proportional odds logistic regression models that also assessed the interactions between oxidative stress and LDL and HDL cholesterol.
Results
F2-isoprostane excretion (median [IQR]) was significantly higher in patients with RA (median 2.75 [interquartile range: 1.60-4.06] ng/mg creatinine (Cr)) than control subjects (1.86 [1.25-2.62] ng/mg Cr, adjusted p=0.006). In patients with RA, F2-isoprostanes were positively correlated with BMI (p<0.001) but not with disease activity or mediators of inflammation, such as DAS28 or serum TNF-α, IL-6 and CRP concentrations in adjusted multivariable models (all P>0.05). In patients with RA, F2-isoprostanes significantly modified the effect of HDL cholesterol on coronary calcification (p-value for interaction=0.02) after adjustment for age, sex and race. As F2-isoprostane levels increased, HDL lost its protective effect against coronary calcification.
Conclusion
Oxidative stress measured as F2-isoprostane excretion was higher in patients with RA than control subjects. Among patients with RA, higher F2-isoprostane excretion and HDL cholesterol concentrations interacted significantly and were positively associated with the severity of coronary calcification.
Keywords: Rheumatoid Arthritis, Oxidative Stress, F2-Isoprostanes, Atherosclerosis, HDL
Introduction
Oxidative stress occurs when there is an imbalance between reactive oxygen species (ROS) relative to antioxidants(1). Consequent free radical-mediated tissue injury is thought to play an important role in the pathogenesis of many inflammatory and degenerative diseases, including atherosclerosis and rheumatoid arthritis (RA)(2,3).
Increased oxidative stress is hypothesized to be important in the pathogenesis of RA, and to both initiate and propagate inflammation(3). Furthermore, inflammation and relative hypoxia in the joints promote additional oxidative stress(3,4). Although oxidative stress is considered important in RA, there are few studies that have addressed this hypothesis directly. These studies were performed in a small number of patients and used a variety of measures of oxidative stress(5-12). One of the problems in this area of research has been that in vivo measures of oxidative stress have lacked sensitivity and specificity(13). The discovery of F2-isoprostanes, prostaglandin-like compounds generated in vivo by non-enzymatic free radical-mediated oxidation of arachidonic acid(14), provided a reliably stable measure of oxidative stress in vivo(1). No large study has examined the relationship between F2-isoprostanes and the clinical characteristics of RA.
In addition to its role in chronic autoimmune inflammatory diseases, oxidative stress may also contribute to the pathogenesis of atherosclerotic cardiovascular disease. In the general population, increased concentrations of F2-isoprostanes are associated with coronary artery calcification and carotid intima-media thickness, non-invasive measures of atherosclerosis that predict long term cardiovascular outcomes(15,16), and also with the presence and severity of coronary artery disease(17).
Patients with RA have increased coronary atherosclerosis(18). Increased oxidative stress is proposed as one of the mechanisms underlying accelerated atherosclerosis in RA(3), but this hypothesis has not been addressed directly. There are several mechanisms by which oxidative stress could accelerate atherosclerosis(2); oxidative modification of LDL and HDL cholesterol is of particular interest in RA and SLE since concentrations of oxidized LDL are elevated and HDL appears to be modified so that it is pro-inflammatory and pro-atherogenic rather than anti-inflammatory(19,20) and is associated with atherosclerosis(21).
Because there is currently limited information about the role of oxidative stress in the pathogenesis of inflammation, and none about its role in atherosclerosis in RA, we examined the hypothesis that oxidative stress, measured by F2-isoprostane excretion, is higher in patients with RA than control subjects, and is associated with inflammation, traditional cardiovascular risk factors and coronary atherosclerosis. We also hypothesized that oxidative stress could interact with LDL and HDL cholesterol and modify their effects on coronary atherosclerosis.
Materials and Methods
Patients and Control Subjects
We enrolled 169 patients with RA and 92 control subjects, frequency-matched for age, race and sex, through advertisements, referral from local rheumatologists, and from a volunteer database maintained by the General Clinical Research Center (GCRC) at Vanderbilt University recruited between 2001 and 2005. Patients were older than 18 years and fulfilled the ACR classification criteria for RA(22); control subjects did not have RA or any inflammatory disease. These subjects have participated in ongoing studies of cardiovascular risk factors in RA and detailed study methods have been described(18). The study was approved by the Vanderbilt University Institutional Review Board and all subjects gave written informed consent.
Clinical and Laboratory Measurements
Clinical information, laboratory data, and coronary calcium scores were obtained as described (18,23). Briefly, urinary F2-isoprostane excretion was quantified using gas chromatography and mass spectroscopy and expressed as ng/mg creatinine (ng/mg Cr)(24). Coronary calcium was measured by electron beam computed tomography (EBCT) scanning with an Imatron C-150 scanner (GE/Imatron) and was quantified as described by Agatston et al.(25). RA disease activity was measured using the disease activity score using 28 joints (DAS28)(26). Radiographic joint damage was measured using the Larsen score in 94 patients as described previously(27). The Framingham risk score, a composite score of traditional cardiovascular risk factors that includes blood pressure, smoking status, serum lipid concentrations, age and sex, but not diabetes, was calculated(28). Obesity was quantified using body mass index (BMI) in kg/m2 and insulin resistance was measured using the homeostasis model assessment (HOMA) index(29) calculated as [serum insulin (uU/ml) × glucose (mmol/l)] / 22.5. Peripheral blood neutrophil and monocyte counts, serum C-reactive protein (CRP), glucose, triglycerides, HDL and LDL cholesterol concentrations were measured by the Vanderbilt University Medical Center Clinical Laboratory. LDL concentrations were calculated using the Friedewald equation(30). Before 2003, the laboratory did not use a high-sensitivity CRP assay, and low concentrations were reported as <3 mg/liter and in 40 patients with RA who had CRP concentrations < 3 mg/liter, concentrations were measured by ELISA (Millipore). For technical reasons HDL and F2-isoprostane measurements were not obtained in 1 patient each. Serum concentrations of tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6) and serum amyloid A (SAA) were measured by multiplex ELISA (Millipore).
Statistical Analysis
Descriptive statistics were calculated as median with the interquartile range [IQR]. The distribution of F2-isoprostane excretion was compared in patients with RA and control subjects using the Wilcoxon rank-sum test. The independent association between disease status (RA vs. controls) and F2-isoprostanes was assessed using a multiple linear regression model with urinary F2-isoprostane excretion as a dependent variable and disease status as a predictor variable, adjusted for traditional cardiovascular risk factors such as age, sex, race, BMI, hypertension, diabetes and current smoking status. Age was assessed for non-linear effects because it had the strongest predictive potential for atherosclerosis(31). F2-isoprostane excretion values were log-transformed to normalize the distribution of regression residuals. The antilog of the regression coefficient for the disease status variable was taken to reflect percent change in F2-isoprostanes with 95% confidence intervals (95%CI).
Among patients with RA we evaluated the association between clinical and disease-associated factors and F2-isoprostane excretion. These factors included 1) traditional cardiovascular risk factors (age, gender, BMI, smoking, hypertension, diabetes, serum HDL and LDL cholesterol, triglycerides, glucose, HOMA index and the Framingham risk score) and 2) disease-associated indices and inflammatory mediators (drug use, DAS28 score, Larsen score, disease duration, peripheral blood neutrophil and monocyte counts, and serum TNF-α, IL-6, CRP and SAA concentrations). Wilcoxon rank-sum test or Spearman's rank-correlation coefficient (rho, ρ) was used to assess unadjusted associations. The multivariate independent relationship was assessed by multiple linear regression using F2-isoprostanes as the outcome variable adjusted for age, race, sex, hypertension, smoking and BMI.
The independent association between F2-isoprostanes, HDL and LDL cholesterol concentrations, and coronary calcium score was examined using proportional odds logistic regression (POR) models. The POR model is also known as the ordinal logistic regression method and is applicable to an ordered response variable(32,33). Ordinal logistic regression is also applicable to skewed continuous dependent variables such as the coronary calcium score by using the ranks of the variable. F2-isoprostane excretion, and serum HDL and LDL cholesterol concentration were each used as the predictor variable, while covariates for adjustment included age, race, sex, hypertension, BMI, diabetes, current smoking and statin use.
To assess the interaction between F2-isoprostanes, HDL and LDL cholesterol on the outcome of coronary calcification, we conducted separate proportional odds models with an interaction term in the model using a cross-product between either HDL or LDL cholesterol and F2-isoprostanes (HDL × F2-isoprostanes or LDL × F2-isoprostanes) adjusted for age, race and sex and then further adjusted for BMI, current smoking, hypertension, diabetes and statin use. Odds ratios (OR) were expressed per IQR difference with 95%CI. All statistical analyses used R 2.7.1 (http://www.r-project.org).
Results
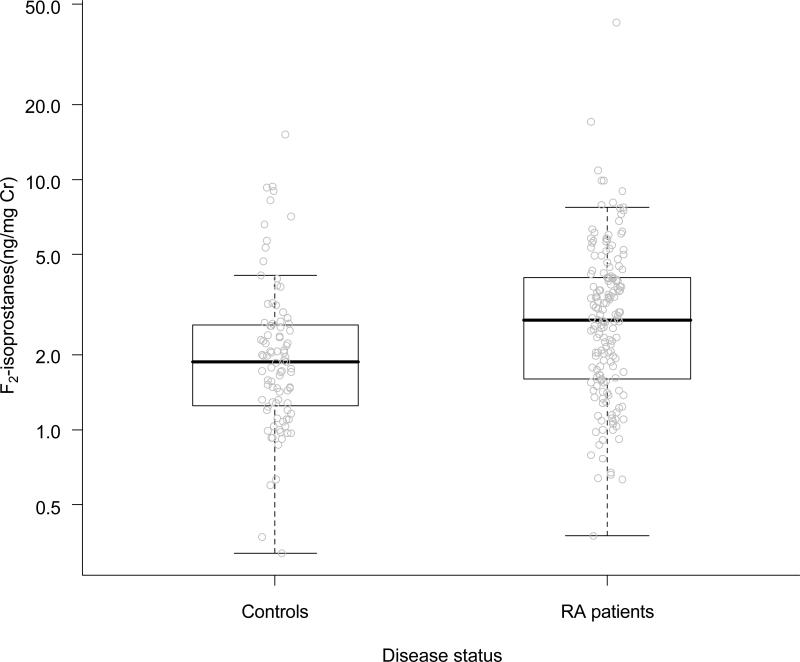
The demographic and clinical characteristics of patients with RA and control subjects are summarized in Table 1. The two groups were similar with regards to age, race, sex and BMI. Patients with RA were more likely to smoke, be insulin resistant and hypertensive. As we have reported previously(18), the coronary calcium score was higher in patients with RA. The distribution of F2-isoprostane excretion in the two groups is depicted in Figure 1. Median F2-isoprostane excretion rates were significantly higher in patients with RA than in control subjects (2.75 [1.60-4.06] ng/mg Cr vs. 1.86 [1.25-2.62] ng/mg Cr, p<0.001), although there was considerable overlap. When adjusted for age, sex, race, hypertension, BMI, diabetes and current smoking status, RA remained significantly associated with higher F2-isoprostane excretion rates (beta=0.22, 95%CI (0.06-0.38), p=0.006). By taking the antilog, the regression coefficient can be interpreted as indicating that having RA leads to a 25% (95%CI 6-46%) adjusted increase in mean F2-isoprostane excretion compared to control subjects.
Table 1.
Clinical Factors and Urinary F2-Isoprostane Excretion in Patients with RA and Control Subjects
| Factor | Controls (N=92) | RA (N=169) | P value |
|---|---|---|---|
| Age (Years) | 53.0 [44.8-59.2] | 54.0 [45.0-63.0] | 0.41 |
| Sex (Male%) | 37.0% | 30.8% | 0.31 |
| Race(Caucasian%) | 84.8% | 88.2% | 0.44 |
| BMI (kg/m2) | 27.0 [24.6-31.8] | 28.3 [24.0-33.2] | 0.44 |
| Current Smokers | 8.7% | 24.3% | 0.002 |
| Hypertension | 39.1% | 53.3% | 0.03 |
| Diabetes | 4.3% | 11.2% | 0.06 |
| Statin Use | 13.0% | 12.4% | 0.89 |
| Disease duration (years) | - | 3 [2-18] | - |
| Current Corticosteroid Use | - | 54.4% | - |
| Current Methotrexate Use | - | 71.0% | - |
| Current Antimalarial Use | - | 24.9% | - |
| Current anti-TNF agent use | - | 20.7% | - |
| HDL cholesterol (mg/dl) | 45.0 [38.5-54.0] | 43.0 [37.0-54.0] | 0.65 |
| LDL cholesterol (mg/dl) | 122.0 [104.0-145.0] | 112.5 [88.8-135.2] | 0.02 |
| Triglycerides (mg/dl) | 103.0 [73.0-135.5] | 110.5 [79.8-158.0] | 0.22 |
| Glucose(mg/dl) | 89.0 [83.0-94.2] | 87.0 [83.0-94.0] | 0.70 |
| HOMA Index | 0.83 [0.54-1.79] | 2.36 [1.19-4.47] | <0.001 |
| Framingham Score | 12.0 [7.0-14.0] | 13.0 [9.0-16.0] | 0.10 |
| Coronary Calcification Score | 0.0 [0.0-18.7] | 1.9 [0.0-150.3] | 0.02 |
| DAS28 Score | - | 3.88 [2.64-4.84] | - |
| F2-isoprostanes (ng/mg Cr) | 1.86 [1.25-2.62] | 2.75 [1.60-4.06] | <0.001 |
Data are expressed as median [interquartile range] or percentage(%).
Figure 1. Distribution of Urinary F2-Isoprostane Excretion in Patients with Rheumatoid Arthritis and Control Subjects.
P value<0.001 (Wilcoxon Rank-Sum test). P=0.006 when adjusted for age, sex, race, hypertension, BMI, diabetes and current smoking status.
The boxplot shows individual data points with median (solid horizontal line) and interquartile range (box).
Among patients with RA, F2-isoprostane excretion was higher in women than men and in current smokers compared to non-smokers (Table 2). There was no significant difference in F2-isoprostane excretion between non-diabetic and diabetic patients, normotensive and hypertensive patients or drug use.
Table 2.
F2-isoprostane Concentrations in Patients with RA According to Categorical Demographic and Clinical Characteristics
| VARIABLE | n | F2- isoprostane | P value* |
|---|---|---|---|
| Gender | 0.006 | ||
| Male | 52 | 2.06 [1.56-3.11] | |
| Female | 117 | 3.23 [1.85-4.54] | |
| Current Smoking | <0.001 | ||
| Yes | 41 | 3.58 [2.85-5.01] | |
| No | 128 | 2.44 [1.52-3.75] | |
| Hypertension | 0.22 | ||
| Yes | 90 | 1.42 [2.58-4.07] | |
| No | 79 | 1.78 [3.02-4.01] | |
| Diabetes Mellitus | 0.72 | ||
| Yes | 19 | 2.28 [1.68-4.00] | |
| No | 150 | 2.81 [1.61-4.06] | |
| Current Corticosteroids | 0.75 | ||
| Yes | 92 | 2.76 [1.71-4.03] | |
| No | 77 | 2.72 [1.50-4.31] | |
| Current Methotrexate | 0.96 | ||
| Yes | 120 | 2.74 [1.60-4.00] | |
| No | 49 | 2.81 [1.63-4.21] | |
| Current Antimalarials | 0.87 | ||
| Yes | 127 | 2.79[1.54-4.07] | |
| No | 42 | 2.75[1.67-4.05] | |
| Current anti-TNF | 0.25 | ||
| Yes | 35 | 3.19 [2.12-3.98] | |
| No | 134 | 2.72 [1.55-4.06] | |
| Current Statin | |||
| Yes | 21 | 2.24 [1.56-3.38] | 0.14 |
| No | 148 | 2.88 [1.62-4.20] |
Wilcoxon rank-sum test.
The correlation between F2-isoprostanes and clinical variables in patients with RA (including after adjustment for age, race, sex, BMI and smoking) are shown in Table 3. F2-isoprostane excretion was negatively correlated with age (rho=-0.33, adjusted p=0.02) and positively correlated with BMI (rho=0.35, adjusted p<0.001). HDL cholesterol (rho=0.04, adjusted p=0.03) and peripheral blood monocyte counts (rho=0.15, adjusted p=0.09) were marginally correlated with F2-isoprostane excretion. Among markers of inflammation and disease activity, Larsen score was not associated with oxidative stress in the univariate correlation (rho=0.025, p=0.75), but after adjustment for potential confounders there was a weak correlation (adjusted p=0.04) (Table 3). Other markers such as TNF-α (p=0.78), IL-6 (p=0.41), CRP (p=0.66) and DAS28 score (p=0.52) were not significantly associated with F2-isoprostane excretion (Table 3).
Table 3.
Relationship between F2-isoprostane Excretion and Clinical Factors in Patients with Rheumatoid Arthritis
| Category | Factor | Rho(ρ)* | Unadjusted p-value | Adjusted p-value† |
|---|---|---|---|---|
| Age | -0.33 | <0.001 | 0.02 | |
| BMI | 0.35 | <0.001 | <0.001 | |
| HDL Cholesterol | 0.04 | 0.61 | 0.03 | |
| Cardiovascular Risk Factors | LDL Cholesterol | 0.04 | 0.56 | 0.34 |
| Triglycerides | 0.01 | 0.94 | 0.17 | |
| HOMA Index | 0.09 | 0.26 | 0.55 | |
| Framingham Score | -0.13 | 0.10 | 0.74 | |
| Disease Duration | -0.03 | 0.68 | 0.08 | |
| Disease-Related Indices and Inflammatory Mediators | DAS28 Index | 0.52 | ||
| 0.02 | 0.75 | |||
|
|
Larsen Score** | 0.025 | 0.75 | 0.04 |
|
|
Neutrophil Count‡ | 0.16 | 0.04 | 0.51 |
|
|
Monocyte Count‡ | 0.15 | 0.06 | 0.09 |
|
|
TNF-α‡ | -0.07 | 0.40 | 0.78 |
|
|
IL-6‡ | 0.02 | 0.82 | 0.41 |
|
|
CRP‡ | 0.06 | 0.41 | 0.66 |
|
|
SAA‡ | -0.06 | 0.47 | 0.43 |
Univariate Spearman correlation coefficient.
n=94.
Adjusted p-values are reported from multiple linear regression models adjusting for age, race, sex, hypertension, BMI and smoking.
log-transformed.
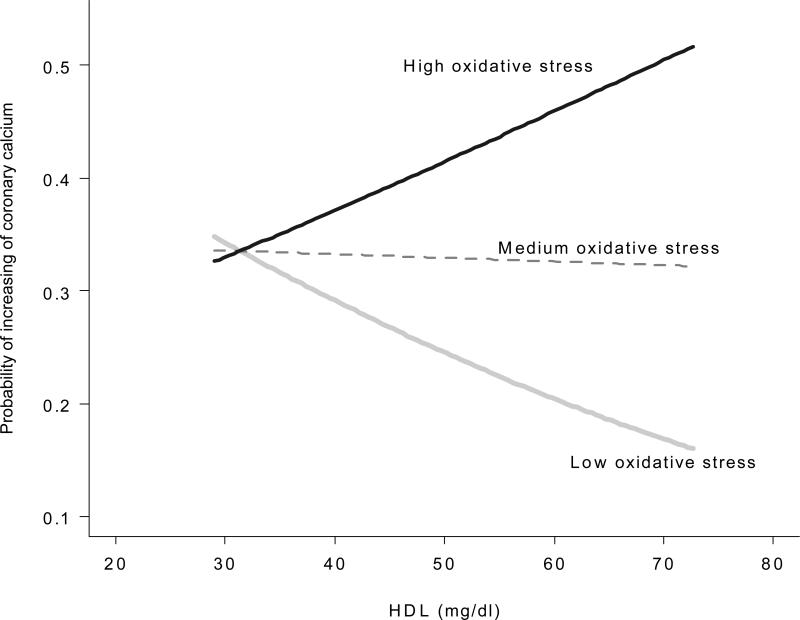
In patients with RA, after adjusting for age, race and sex, there was no significant association between coronary calcium and F2-isoprostane excretion (OR=1.49, 95%CI (0.92-2.43), p=0.11), HDL (OR=0.83, 95%CI (0.55-1.25), p=0.38) or LDL cholesterol (OR=1.12 95%CI (0.72-1.74), p=0.61). A model assessing coronary calcium and the interaction between serum HDL cholesterol concentration and F2-isoprostane excretion was statistically significant (p=0.02, adjusted for age, race and sex). When further adjusted for additional factors including BMI, current smoking, hypertension, statin use and diabetes, the significance was marginally attenuated (P value for interaction=0.07, Figure 2). When F2-isoprostane excretion was low, higher HDL concentrations were associated with a lower risk of coronary calcification. However, as F2-isoprostane excretion increased HDL was no longer protective against coronary calcification. F2-isoprostanes did not interact significantly with serum LDL cholesterol concentrations in relation to coronary calcium scores after adjusting for age, race and sex (p=0.13).
Figure 2. Interaction Plot of F2-Isoprostanes and Serum HDL Concentrations and the Risk of Coronary Calcium.
High, Medium, Low Oxidative Stress refer to as the first, second and third tertile of log F2-isoprostane concentrations. The Figure shows the predicted risk in increasing calcification from the proportional odds model including interaction between log F2-isoprostanes and HDL adjusted for age, sex, race, hypertension, smoking, BMI, diabetes and statin use. Predicted probabilities of HDL on coronary calcium by each level of oxidative stress (high, medium, low) were obtained by using the median values of each tertile of log-F2-isoprostanes. (P value for interaction=0.07). The interaction is significant when adjusted for age, race and sex (p=0.02).
Discussion
The major finding of this study is that oxidative stress is increased in patients with RA, independent of risk factors associated with increased oxidative stress. Furthermore, oxidative stress may have an indirect impact on coronary atherosclerosis in patients with RA through an interaction with HDL cholesterol and modification of the protective effects of HDL on atherosclerosis.
RA is accompanied by activation of neutrophils and macrophages. Activation of such cells leads to the induction of oxidative bursts, which culminate in increased levels of reactive oxygen species, and tilts the balance of the redox system in a pro-oxidant direction(3). Oxidative stress in RA can damage cartilage(34) and modify IgG immunoglobulins to form IgGAGE (advanced glycation end-products) which can induce arthritogenic anti-IgG-AGE autoantibodies(35). Our finding of increased oxidative stress in RA is concordant with other studies(5-12). The strengths of our study included studying a large number of well-characterized patients with RA and using a state-of-the-art measure of oxidative stress. Thus we were able to define the relationships between oxidative stress and mediators of inflammation.
Higher RA disease activity and more inflammation would be expected to be associated with increased oxidative stress; however, we found that disease activity indices such as the DAS28 score, concentrations of inflammatory cytokines such as TNF-α and IL-6, or acute phase reactants such as CRP and SAA, were not associated with increased oxidative stress. In some studies TNF-α and IL-6 have been associated with oxidative stress in patients with RA(36,37). Although disease activity in our patients was relatively low and the majority were receiving DMARD therapy, inflammatory biomarkers such as IL-6 and TNF-α were elevated compared to control subjects(38). Since treatment with a TNF-α inhibitor is reported to have decreased oxidative stress(39,40), it is possible that more severe inflammation may be associated with oxidative stress in RA and that it may improve with treatment. However, in our study F2-isoprostane excretion did not differ between patients receiving anti-TNF agents or corticosteroids and those who were not. Another possibility to account for the lack of association between oxidative stress and systemic measures of inflammation is that most of the oxidative stress occurs locally in the joints, where neutrophils and macrophages play a more important role than lymphocytes. Our results suggest that oxidative stress may be associated with radiographic bone damage, however, the results were from a subgroup of our patients and the significance was marginal, thus the finding requires cautious interpretation.
Increased oxidative stress has been noted to be associated with increased BMI(41), smoking(42), age(43), and blood pressure(17). We found that F2-isoprostane excretion was higher in patients who currently smoked, but was not associated with blood pressure. F2-isoprostane excretion was negatively correlated with age. This finding is not unique to patients with RA; F2-isoprostanes were also inversely associated with age in the Framingham cohort(41) and in a large cohort of healthy subjects from 3 European countries(44). The Framingham study also showed lack of multivariable association between oxidative stress and blood pressure(41). We found a significant association between F2-isoprostane excretion and HDL concentrations only after adjustment for potential confounders; this occurred, in part, because the association between F2-isoprostane excretion and HDL concentrations was negatively confounded by an inverse association between HDL and BMI.
Oxidative stress is thought to play a key role in the pathogenesis of atherosclerosis and has been implicated as an explanation for premature atherosclerosis that occurs in inflammatory rheumatic diseases such as SLE and RA(45). Many traditional cardiovascular risk factors are associated with increased oxidative stress, including hypertension, dyslipidemia, smoking and obesity(1,17). Increased oxidative stress was also associated with increased coronary calcium in young healthy adult populations(15). Since RA is associated with increased cardiovascular mortality(46) and premature coronary atherosclerosis(18,47), it was important to define the relationship between increased oxidative stress and accelerated atherosclerosis. Our findings suggest that F2-isoprostane excretion is not independently associated with coronary calcium score in RA, but rather contributes to increase the risk of coronary calcification by adversely modifying another cardiovascular risk factor, HDL cholesterol.
A key early process in the pathogenesis of atherosclerosis is the oxidation of LDL cholesterol that leads to the accumulation of oxidized LDL in the vessel wall, and consequently an inflammatory response and the formation of atheroma(48). The effects of oxidative processes on HDL cholesterol are less well defined. Recently, inflammatory rheumatic diseases, and the accompanying increased risk of coronary atherosclerosis, have been associated with the presence of a dysfunctional form of HDL, termed pro-inflammatory (or pro-atherogenic) HDL(49). Pro-inflammatory HDL is thought to be formed when anti-atherogenic components of HDL such as apolipoprotein-AI (Apo-AI) or paraoxonase 1 (PON1) are replaced by proatherogenic components such as serum amyloid A (SAA), ceruloplasmin and oxidized lipids(45). Thus, HDL in its pro-inflammatory form fails to protect against atherosclerosis.
Our results showing that increased oxidative stress is associated with a loss of protective effect of HDL cholesterol against coronary calcification are consistent with the finding of increased pro-inflammatory HDL in rheumatic diseases(20,21). Measurement of pro-inflammatory HDL concentrations requires fresh plasma and thus we were unable to determine the relationship between F2-isoprostanes and pro-inflammatory HDL concentrations. Our findings suggest that in patients with RA, high HDL concentrations might not necessarily lead to lower coronary risk and that factors such as oxidative stress may influence the cardioprotective capacity of HDL. Recent studies showing that HDL is the major lipoprotein carrier of F2-isoprostanes(50) are concordant with this observation. Additional studies to define the relationship between increased oxidative stress and pro-inflammatory HDL in patients with RA will be of interest.
Our study had some limitations. The study was cross-sectional and concentrations of HDL and F2-isoprostanes may vary over time. Furthermore, coronary calcification, an excellent measure of the amount of atherosclerosis present in vessels, was the outcome. The outcome of greatest significance is incident cardiovascular events; however, that outcome will require a large prospective study. Also, hs-CRP would have been ideally measured by the same method in all patients. Finally, a larger sample size would have improved statistical power and allowed adjustment for a greater number of potential confounders.
Conclusions
In conclusion, oxidative stress measured as urinary F2-isoprostane excretion was increased in patients with RA compared to control subjects. Oxidative stress modified the relationship between HDL and coronary calcification so that increased oxidative stress at higher HDL concentrations was associated with a greater severity of coronary calcification.
Acknowledgements
The authors thank Dr. Jason D. Morrow, M.D. (deceased) for advice in the planning and performance of the study.
Sources of Funding: Supported by NIH grants HL65082, HL67964, P60 AR056116, GM07569, UL1 RR024975 from NCRR/NIH, and the Dan May Chair in Medicine.
Footnotes
Disclosures: None of the authors has a conflict of interest related to this work.
References
- 1.Montuschi P, Barnes P, Roberts LJ. Insights into oxidative stress: the isoprostanes. Curr Med Chem. 2007;14(6):703–17. doi: 10.2174/092986707780059607. [DOI] [PubMed] [Google Scholar]
- 2.Stocker R, Keaney JF., Jr. Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84(4):1381–478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 3.Hitchon CA, El-Gabalawy HS. Oxidation in rheumatoid arthritis. Arthritis Res Ther. 2004;6(6):265–78. doi: 10.1186/ar1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mapp PI, Grootveld MC, Blake DR. Hypoxia, oxidative stress and rheumatoid arthritis. Br Med Bull. 1995;51(2):419–36. doi: 10.1093/oxfordjournals.bmb.a072970. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Garcia J, Requena JR, Rodriguez-Segade S. Increased concentrations of serum pentosidine in rheumatoid arthritis. Clin Chem. 1998;44(2):250–5. [PubMed] [Google Scholar]
- 6.Ames PR, Alves J, Murat I, Isenberg DA, Nourooz-Zadeh J. Oxidative stress in systemic lupus erythematosus and allied conditions with vascular involvement. Rheumatology (Oxford) 1999;38(6):529–34. doi: 10.1093/rheumatology/38.6.529. [DOI] [PubMed] [Google Scholar]
- 7.Maurice MM, Nakamura H, Gringhuis S, Okamoto T, Yoshida S, Kullmann F, et al. Expression of the thioredoxin-thioredoxin reductase system in the inflamed joints of patients with rheumatoid arthritis. Arthritis Rheum. 1999;42(11):2430–9. doi: 10.1002/1529-0131(199911)42:11<2430::AID-ANR22>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 8.Rall LC, Roubenoff R, Meydani SN, Han SN, Meydani M. Urinary 8-hydroxy-2'-deoxyguanosine (8-OHdG) as a marker of oxidative stress in rheumatoid arthritis and aging: effect of progressive resistance training. J Nutr Biochem. 2000;11(11-12):581–4. doi: 10.1016/s0955-2863(00)00123-6. [DOI] [PubMed] [Google Scholar]
- 9.Basu S, Whiteman M, Mattey DL, Halliwell B. Raised levels of F(2)-isoprostanes and prostaglandin F(2alpha) in different rheumatic diseases. Ann Rheum Dis. 2001;60(6):627–31. doi: 10.1136/ard.60.6.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baskol G, Demir H, Baskol M, Kilic E, Ates F, Karakukcu C, et al. Investigation of protein oxidation and lipid peroxidation in patients with rheumatoid arthritis. Cell Biochem Funct. 2006;24(4):307–11. doi: 10.1002/cbf.1257. [DOI] [PubMed] [Google Scholar]
- 11.Altindag O, Karakoc M, Kocyigit A, Celik H, Soran N. Increased DNA damage and oxidative stress in patients with rheumatoid arthritis. Clin Biochem. 2007;40(3-4):167–71. doi: 10.1016/j.clinbiochem.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Ozkan Y, Yardym-Akaydyn S, Sepici A, Keskin E, Sepici V, Simsek B. Oxidative status in rheumatoid arthritis. Clin Rheumatol. 2007;26(1):64–8. doi: 10.1007/s10067-006-0244-z. [DOI] [PubMed] [Google Scholar]
- 13.Gutteridge JM, Halliwell B. The measurement and mechanism of lipid peroxidation in biological systems. Trends Biochem Sci. 1990;15(4):129–35. doi: 10.1016/0968-0004(90)90206-q. [DOI] [PubMed] [Google Scholar]
- 14.Morrow JD, Awad JA, Boss HJ, Blair IA, Roberts LJ. Non-cyclooxygenase-derived prostanoids (F2-isoprostanes) are formed in situ on phospholipids. Proc Natl Acad Sci U S A. 1992;89(22):10721–5. doi: 10.1073/pnas.89.22.10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gross M, Steffes M, Jacobs DR, Jr., Yu X, Lewis L, Lewis CE, et al. Plasma F2-isoprostanes and coronary artery calcification: the CARDIA Study. Clin Chem. 2005;51(1):125–31. doi: 10.1373/clinchem.2004.037630. [DOI] [PubMed] [Google Scholar]
- 16.Basarici I, Altekin RE, Demir I, Yilmaz H. Associations of isoprostanes-related oxidative stress with surrogate subclinical indices and angiographic measures of atherosclerosis. Coron Artery Dis. 2007;18(8):615–20. doi: 10.1097/MCA.0b013e3282f0efa5. [DOI] [PubMed] [Google Scholar]
- 17.Schwedhelm E, Bartling A, Lenzen H, Tsikas D, Maas R, Brummer J, et al. Urinary 8-isoprostaglandin F2alpha as a risk marker in patients with coronary heart disease: a matched case-control study. Circulation. 2004;109(7):843–8. doi: 10.1161/01.CIR.0000116761.93647.30. [DOI] [PubMed] [Google Scholar]
- 18.Chung CP, Oeser A, Raggi P, Gebretsadik T, Shintani AK, Sokka T, et al. Increased coronary-artery atherosclerosis in rheumatoid arthritis: relationship to disease duration and cardiovascular risk factors. Arthritis Rheum. 2005;52(10):3045–53. doi: 10.1002/art.21288. [DOI] [PubMed] [Google Scholar]
- 19.Van Lenten BJ, Hama SY, de Beer FC, Stafforini DM, McIntyre TM, Prescott SM, et al. Anti-inflammatory HDL becomes pro-inflammatory during the acute phase response. Loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J Clin Invest. 1995;96(6):2758–67. doi: 10.1172/JCI118345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McMahon M, Grossman J, FitzGerald J, hlin-Lee E, Wallace DJ, Thong BY, et al. Proinflammatory high-density lipoprotein as a biomarker for atherosclerosis in patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum. 2006;54(8):2541–9. doi: 10.1002/art.21976. [DOI] [PubMed] [Google Scholar]
- 21.McMahon M, Grossman J, Skaggs B, FitzGerald J, Sahakian L, Ragavendra N, et al. Dysfunctional proinflammatory high-density lipoproteins confer increased risk of atherosclerosis in women with systemic lupus erythematosus. Arthritis Rheum. 2009;60(8):2428–37. doi: 10.1002/art.24677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988. 31(3):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 23.Avalos I, Chung CP, Oeser A, Milne GL, Morrow JD, Gebretsadik T, et al. Oxidative stress in systemic lupus erythematosus: relationship to disease activity and symptoms. Lupus. 2007;16(3):195–200. doi: 10.1177/0961203306075802. [DOI] [PubMed] [Google Scholar]
- 24.Milne GL, Sanchez SC, Musiek ES, Morrow JD. Quantification of F2-isoprostanes as a biomarker of oxidative stress. Nat Protoc. 2007;2(1):221–6. doi: 10.1038/nprot.2006.375. [DOI] [PubMed] [Google Scholar]
- 25.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 26.Prevoo ML, van 't Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38(1):44–8. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 27.Rho YH, Solus J, Sokka T, Oeser A, Chung CP, Gebretsadik T, et al. Adipocytokines are associated with radiographic joint damage in rheumatoid arthritis. Arthritis Rheum. 2009;60(7):1906–14. doi: 10.1002/art.24626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–421. [PubMed] [Google Scholar]
- 29.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 30.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 31.Harrel FE. Regression Modeling Strategies - With Applications to Linear Models, Logistic Regression, and Survival Analysis. Springer; New York: 2001. Multivariable Modeling Strategies. pp. 53–85. [Google Scholar]
- 32.Walker SH, Duncan DB. Estimation of the probability of an event as a function of several independent variables. Biometrika. 1967;54(1):167–79. [PubMed] [Google Scholar]
- 33.Harrel FE. Regression Modeling Strategies - With Applications to Linear Models, Logistic Regression, and Survival Analysis. Springer; New York: 2001. Ordinal Logistic Regression. pp. 331–43. [Google Scholar]
- 34.Henrotin YE, Bruckner P, Pujol JP. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthritis Cartilage. 2003;11(10):747–55. doi: 10.1016/s1063-4584(03)00150-x. [DOI] [PubMed] [Google Scholar]
- 35.Newkirk MM, Goldbach-Mansky R, Lee J, Hoxworth J, McCoy A, Yarboro C, et al. Advanced glycation end-product (AGE)-damaged IgG and IgM autoantibodies to IgG-AGE in patients with early synovitis. Arthritis Res Ther. 2003;5(2):R82–R90. doi: 10.1186/ar622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshida S, Katoh T, Tetsuka T, Uno K, Matsui N, Okamoto T. Involvement of thioredoxin in rheumatoid arthritis: its costimulatory roles in the TNF-alpha-induced production of IL-6 and IL-8 from cultured synovial fibroblasts. J Immunol. 1999;163(1):351–8. [PubMed] [Google Scholar]
- 37.Hein GE, Kohler M, Oelzner P, Stein G, Franke S. The advanced glycation end product pentosidine correlates to IL-6 and other relevant inflammatory markers in rheumatoid arthritis. Rheumatol Int. 2005;26(2):137–41. doi: 10.1007/s00296-004-0518-1. [DOI] [PubMed] [Google Scholar]
- 38.Rho YH, Chung CP, Oeser A, Solus J, Asanuma Y, Sokka T, et al. Inflammatory mediators and premature coronary atherosclerosis in rheumatoid arthritis. Arthritis Rheum. 2009;61(11):1580–5. doi: 10.1002/art.25009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kageyama Y, Takahashi M, Ichikawa T, Torikai E, Nagano A. Reduction of oxidative stress marker levels by anti-TNF-alpha antibody, infliximab, in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2008;26(1):73–80. [PubMed] [Google Scholar]
- 40.Kageyama Y, Takahashi M, Nagafusa T, Torikai E, Nagano A. Etanercept reduces the oxidative stress marker levels in patients with rheumatoid arthritis. Rheumatol Int. 2008;28(3):245–51. doi: 10.1007/s00296-007-0419-1. [DOI] [PubMed] [Google Scholar]
- 41.Keaney JF, Jr., Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, et al. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23(3):434–9. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 42.Morrow JD, Frei B, Longmire AW, Gaziano JM, Lynch SM, Shyr Y, et al. Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. N Engl J Med. 1995;332(18):1198–203. doi: 10.1056/NEJM199505043321804. [DOI] [PubMed] [Google Scholar]
- 43.Hensley K, Floyd RA. Reactive oxygen species and protein oxidation in aging: a look back, a look ahead. Arch Biochem Biophys. 2002;397(2):377–83. doi: 10.1006/abbi.2001.2630. [DOI] [PubMed] [Google Scholar]
- 44.Basu S, Helmersson J, Jarosinska D, Sallsten G, Mazzolai B, Barregard L. Regulatory factors of basal F(2)-isoprostane formation: population, age, gender and smoking habits in humans. Free Radic Res. 2009;43(1):85–91. doi: 10.1080/10715760802610851. [DOI] [PubMed] [Google Scholar]
- 45.Hahn BH, Grossman J, Chen W, McMahon M. The pathogenesis of atherosclerosis in autoimmune rheumatic diseases: roles of inflammation and dyslipidemia. J Autoimmun. 2007;28(2-3):69–75. doi: 10.1016/j.jaut.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 46.Pincus T, Callahan LF. Taking mortality in rheumatoid arthritis seriously--predictive markers, socioeconomic status and comorbidity. J Rheumatol. 1986;13(5):841–5. [PubMed] [Google Scholar]
- 47.Roman MJ, Moeller E, Davis A, Paget SA, Crow MK, Lockshin MD, et al. Preclinical carotid atherosclerosis in patients with rheumatoid arthritis. Ann Intern Med. 2006;144(4):249–56. doi: 10.7326/0003-4819-144-4-200602210-00006. [DOI] [PubMed] [Google Scholar]
- 48.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16):1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 49.Navab M, Anantharamaiah GM, Reddy ST, Van Lenten BJ, Ansell BJ, Fogelman AM. Mechanisms of disease: proatherogenic HDL--an evolving field. Nat Clin Pract Endocrinol Metab. 2006;2(9):504–11. doi: 10.1038/ncpendmet0245. [DOI] [PubMed] [Google Scholar]
- 50.Proudfoot JM, Barden AE, Loke WM, Croft KD, Puddey IB, Mori TA. HDL is the major lipoprotein carrier of plasma F2-isoprostanes. J Lipid Res. 2009;50(4):716–22. doi: 10.1194/jlr.M800607-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]