Abstract
Background
We undertook a prospective longitudinal study to examine humoral and cellular immune responses to influenza vaccination in hematopoietic cell transplant (HCT) patients and healthy adults.
Methods
Healthy volunteers and HCT patients had blood samples taken prior to influenza vaccination and 30, 90, and 180 days post-vaccination. Serum from pre and post-vaccination time points were tested for influenza A IgG and IgM by ELISA as well as tested for neutralizing antibody (NAb) titers via hemagluttination inhibition assay. Polychromatic flow cytometry was used to examine CD4+ T cells for levels of IFN-γ, TNF-α, and CD154 (CD40 ligand) expression after stimulation with inactivated flu virus.
Results
In healthy subjects, we found a significant increase in Influenza A IgG and IgM levels as well as an increase in NAb titers pre and post-influenza vaccination. Notably, NAb titers of most HCT patients did not rise to a protective level post-vaccination. CD4+ T cell expression of CD154 and cytokine responses were significantly reduced in HCT recipients compared to healthy adults.
Conclusions
A lack of B cell reconstitution and dysfunctional CD4 T cell co-stimulation (as marked by low CD154 expression) is associated with low NAb levels post-vaccination in HCT patients.
Keywords: CD154, CD40L, Influenza, Vaccination, Stem Cell Transplant
INTRODUCTION
HCT recipients undergo ablative chemotherapy prior to transplantation, losing all T cell and most B cell memory. Some donor immunity is transferred to the recipient, but revaccination is required in the recipient to establish memory responses against common opportunistic infections regardless of the donor’s immune status (1–3). The recipient must wait for immune reconstitution to occur and then build immunity through vaccination or re-exposure(4, 5). The appropriate time for vaccination to generate protective immunity during immune reconstitution has been studied for some pathogens (2, 6, 7), but is less well understood for others, including influenza(8–13). During immune reconstitution following HCT, CD3 positive lymphocytes are developed 6–8 weeks post transplant in autologous HCT and approximately 12 weeks in allogeneic HCT (14). CD8 T cell repopulation occurs faster (by about 4 months) than CD4 T cell repopulation which can take as long as 6–9 months (14). B cell levels are restored quickly and close to normal 1–2 months after transplant (14); however, humoral immune dysfunction remains for 18–24 months (15). While B cells have reconstituted to levels sufficient to support influenza vaccination at 6 months post-transplant (7), a lack of expression of adhesions molecules on B cells (16) and a lack of functional T helper cells after transplant leads to a failure to produce protective levels of NAb post-vaccination.
Important in generating a protective immune response post-vaccination is the activation of CD4 T cells necessary to co-stimulate B cells and generate protective antibodies (17–20). CD154 expression on helper CD4 T cells is necessary for B cells to produce a protective NAb titer (17, 19, 20). Our studies and others show that HCT patients produce non-protective NAb levels, leaving transplant patients susceptible to influenza infection (6, 9, 21–25).
METHODS
Subject Recruitment
Healthy individuals were recruited during the City of Hope (COH) employee influenza vaccination campaign. The COH IRB approved the research protocol and informed consent was obtained from all subjects prior to participation in the study. HCT patients were recruited to participate in the study and informed consent was obtained pre-transplant by the transplant nurse coordinator.
Flu Vaccination
Healthy subjects were vaccinated at City of Hope (COH) with the 2007–2008 seasonal flu vaccine. HCT patients were vaccinated at COH with the 2008–2009 seasonal vaccine according to physician orders. It was our original intent to accrue patients in the same flu season as we accrued healthy volunteers. However, we were only able to follow 2 HCT patients during the 2007–2008 flu season. Accrual was better in the 2008–2009 flu season and represents the data presented here.
Blood draws
Consented individuals had 30–40 ml of blood drawn at the COH General Clinical Research Center prior to flu vaccination (d0) and at days 30, 90 and 180 (d30, d90, D180) post-vaccination. Serum samples were used for IgG, IgM and NAb studies while peripheral blood mononuclear cells (PBMC) were purified from whole blood and used in CD4 T cell assays.
Specimen Preparation
5ml of blood was drawn into a red top BD vacutainer® for serum collection. Serum from HCT patients was collected d0 and at d30 and d180 post-vaccination for HCT patients and d0 and at d180 post-vaccination for healthy adults. The tubes were centrifuged and serum removed. Serum was aliquoted and stored at −80°C until run on ELISA or used in hemagglutination inhibition assays (HAI). 30–40 ml of blood was drawn into BD vacutainers® containing 86 USP units of sodium heparin. Whole blood was then mixed 1:1 with phosphate buffered saline (PBS) and layered over ficoll-paque™ (GE Healthcare). PBMC were isolated by centrifugation over the ficoll for 30 minutes at 1200 RPM. PBMC were removed and washed twice in PBS. PBMC were stored in fetal bovine serum containing 10% dimethyl sulfoxide in liquid nitrogen until assay. Frozen cells were used with no detriment to the detection of activation markers and batching samples together reduces the inter-assay variability.
IgG and IgM Testing
IgG and IgM specific for Influenza A were measured using ELISA (IBL-America). Samples were tested in duplicate according to the manufacturer’s protocol.
Hemaglutination Inhibition Assays (HAI)
Components for the 07–08 and 08–09 flu seasons were obtained from the World Health Organizations (WHO) Influenza reagent program via the Centers for Disease Control (CDC) and Prevention in Atlanta, GA. Turkey erythrocytes were obtained from Bio-Link inc. HAI testing was performed using the WHO protocol for 2007–2008 or 2008–2009 Influenza reagent kit for identification of influenza isolates. The virus strains in the 2007–2008 influenza vaccines were A/SolomonIslands/3/2006 (H1N1), A/Wisconsin/67/2005 (H3N2), and B/Malaysia/2506/2004. The virus strains in the 2008–2009 influenza vaccines were A/Brisbane/59/2007 (H1N1), A/Brisbane/10/2007 (H3N2), and B/Florida/4/2006. Serum samples were incubated with receptor destroying enzyme (Cholera filtrate, Sigma-Aldrich USA) overnight at 37°C. The samples were diluted in physiologic saline to a final concentration of 1:10 and ten titrated down to 1:1280. The samples were incubated with turkey erythrocytes for 1 hour before being assayed for NAb concentrations.
CD4 T cell activation assay
PBMC were thawed and plated at 1 × 106 cells/well in a 96 well plate in RPMI containing 10% FBS. 2μl each of CD28 and CD49b antibodies (Becton-Dickinson) were added to each well. Media containing 0.5% DMSO was used for mock stimulation and 50μl of the matched year influenza vaccine was added to wells for stimulation. CD154 PE (e-bioscience) was added to all wells and plates were incubated at 37°C for one hour. Golgistop™ (Becton-Dickinson) and Golgiplug™ (Becton-Dickinson) were added after 1 hour and incubation continued overnight. The following day cells were washed in PBS+ 0.5% bovine serum albumin (BSA). CD4 PerCP-Cy5 (e-bioscience) antibody was then added and incubated on ice for 30 minutes followed by two washes in PBS + 0.5% BSA. PBMC were then re-suspended in 100 μl of BD Cytofix/Cytoperm™ (Becton-Dickinson) for 20 minutes on ice followed by two washes in BD Perm/Wash™ (Becton-Dickinson). Antibodies to IFN-γ and TNF-α (e-bioscience) were then added at the manufacturer’s recommended concentration for 30 minutes on ice. Cells were washed twice more in PBS + 0.5% BSA and analyzed on a FACSCalibur™ flow cytometer (Becton-Dickinson). Data analysis was performed using FlowJo software (TreeStar).
Statistical analyses
In order to compare data between healthy and HCT subjects nonparametric statistical tests were used. P values were calculated using the Mann-Whitney test or 2 way nonparametric ANOVA analogs as indicated in the text and figure legends. The Kruskal-Wallis test was used to compare the overall levels of CD154, TNF-α and IFN-γ produced in healthy subjects to HCT patients.
Results
Subject Characteristics
Table 1 shows the demographics of both healthy subjects and HCT patients. The ages and genders between the two groups were similar (Table 1). Our study design left the day post-transplant that vaccination occurred at the discretion of the treating physician and thus patients were vaccinated over a large range of time post-transplant. The median number of days between transplant and vaccination was 255 days, indicating that the majority of patients were vaccinated according to CDC recommended guidelines of vaccination >180 days post-transplant (7, 26). It is believed that vaccination 180 days post-transplant should allow for sufficient B and T cell reconstitution to provide protection from influenza sequelae (7). Because reconstitution is delayed in HCT patients who develop chronic graft vs host disease (GVHD) or are treated with Rituximab (15, 27) we removed those patients from the analysis. 16 of 17 alloHCT patients were on prophylaxis to prevent the development of acute GVHD. Prophylaxis consisted of treatment with FK-506, Sirolimus and/or MTX). The mean time from completion of GVHD prophylaxis and vaccination was 189 days. When analyzing the data we found no statistical differences (Mann-Whitney test) in the data obtained from allo vs auto HCT patients (data not shown). Therefore in our cohort we combined data from all HCT patients for analysis. There is no evidence at this time that GVHD prophylaxis impacted vaccination responses. The effects of steroid treatment will be examined in future studies and patients receiving steroid treatment are not included in the study cohort.
Table 1.
Demographics of Participating Subjects
| Healthy Subjects | ||
|---|---|---|
| Sex | Subjects | Percent |
| Females | 43 | 75 |
| Males | 14 | 25 |
| Total | 57 | |
| Age range in years | ||
| Age at Vaccination | (24 – 70) | |
| HCT Patients | ||
| Sex | Subjects | Percent |
| Females | 20 | 57 |
| Males | 15 | 43 |
| Total | 35 | |
| Age range in years | ||
| Age at Transplant | (25 – 65) | |
| Days | Range of Days | |
| Median # Days Post-Transplant at Vaccination | 255 | (20 – 443) |
| Disease Category | Subjects | Percent |
| AML | 5 | 14 |
| Multiple Myeloma | 6 | 17 |
| NHL | 7 | 20 |
| Other | 17 | 49 |
| Autologous | 18 | 51 |
| Allogeneic | 17 | 49 |
| Sib | 2 | 12 |
| MUD | 6 | 35 |
| related match | 9 | 53 |
Influenza A IgG and IgM levels post-vaccination
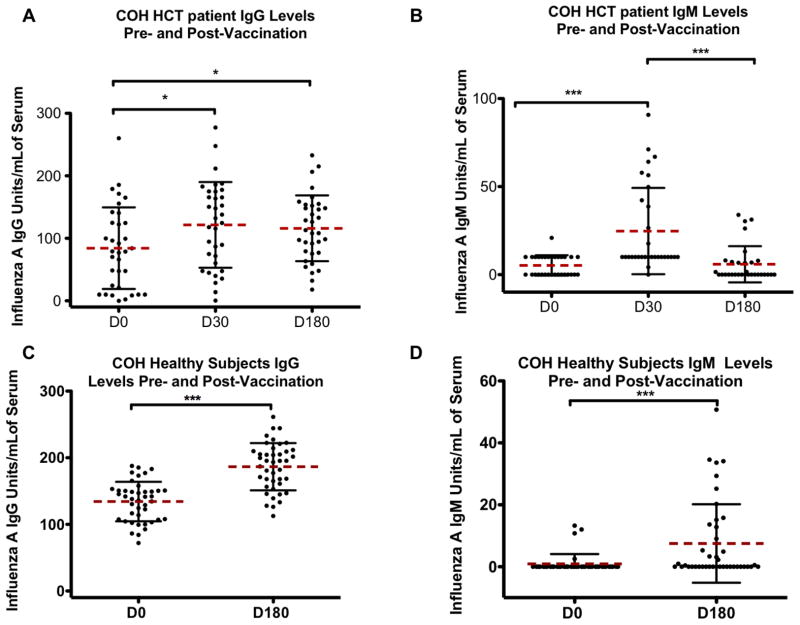
Sera from HCT and healthy subjects taken pre and post-vaccination were tested for influenza A IgG and IgM levels using ELISA. Figure 1 top panels show the IgG and IgM responses for HCT patients and the bottom panels show the results for healthy volunteers. Healthy subjects and HCT patients both showed a significant increase in IgG levels post-vaccination compared to pre-vaccination (Figure 1). Mann-Whitney tests showed a high level of significance in comparing influenza A IgG (p <0.001) and IgM (p<0.001) levels pre and post-vaccination in healthy subjects. There was a significant change in IgG levels of patients at d30 (p<0.05) and d180 (p<0.05) post-vaccination compared to d0 levels. However, the mean level of IgG was significantly decreased in patients compared to healthy controls as measured by a Kruskal-Wallis test (p<0.01). The influenza A IgM levels of HCT patients showed a significant increase in IgM levels (p<0.01) at d30 post-vaccination, but IgM levels had returned to baseline by d180 post-vaccination. This is in direct contrast to the d180 IgM levels seen in healthy subjects.
Figure 1. Influenza A IgG and IgM levels in hematopoietic cell transplant (HCT) patients (A) and healthy adults (B).
Serum from day 0 (d0) pre-vaccination or day 180 (d180) post-vaccination for healthy adults or d0 and day 30 (d30) and d180 post-vaccination for HCT patients was tested by ELISA for IgG and IgM specific for Influenza A. Black error bars represent the standard deviation from the mean (dashed bar). The p values are calculated using a two-tailed Mann-Whitney test. Healthy subjects showed sustained increases in both IgG and IgM levels at d180 post-vaccination. In HCT patients IgG and IgM levels were elevated at d30 post-vaccination, but for IgM the Ab level had returned to baseline by d180 post-vaccination. * = p<.05, ***=p<.001
Influenza HAI assays
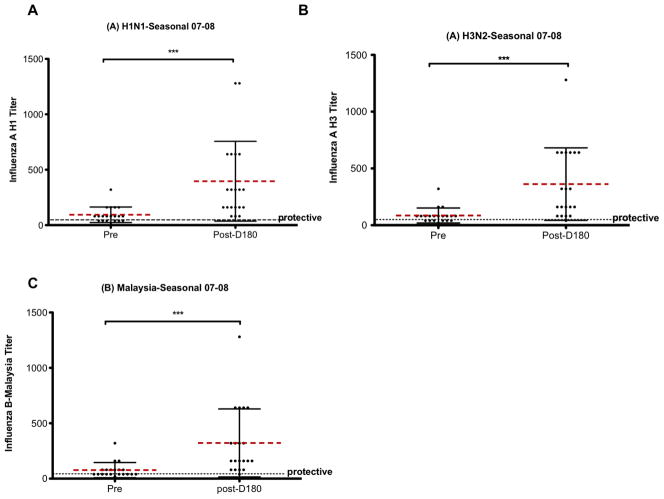
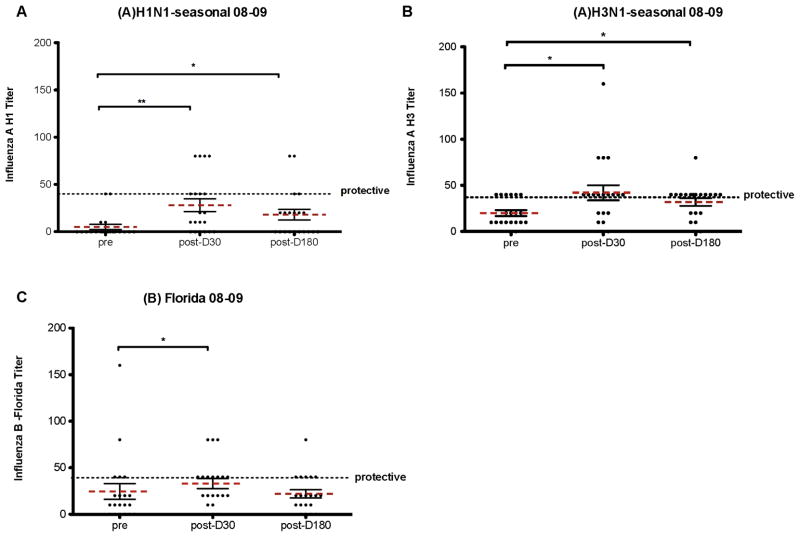
Qualitative antibody data was determined by measuring the titer of NAb produced post-vaccination. HAI assays against each flu strain in the 2007–2008 (healthy subjects) or 2008–2009 (HCT patients) were performed (see methods for full strain identification). Figure 2 shows the HAI results for healthy volunteers against the 2007–2008 influenza (A)H1N1, (A)H3N1, and (B)Malaysia strains. Figure 3 shows the HAI results for HCT patients against the 2008–2009 influenza (A)H1N1, (A)H3N2 and (B)Florida strains. A NAb titer above 40 is considered protective. All healthy adults showed some increase in NAb titers 6 months post-vaccination compared to pre-vaccination levels. The majority of healthy adults had NAb titers above the protective level at 6 months post-vaccination (Figure 2). Contrary to what was seen in healthy adults the NAb titers seen in HCT recipients (Figure 3) even d30 post-vaccination were rarely above the protective level.
Figure 2. Hemagglutination inhibition assay (HAI) measure of neutralizing antibody (NAb) levels in healthy subjects pre- and post-vaccination for the 2007–2008 flu season.
Healthy subject sera was tested for NAb titer at d0 and at d180 post-vaccination. NAb titers measured were against the 2007–2008 flu vaccine antigens (A)H1N1, (A)H3N2 and (B)Malaysia flu strains. Black error bars represent the standard deviation from the mean (dashed bar). The dotted black line represents a titer of 40 which is considered protective. ***=p<.001, p = two-tailed Mann-Whitney test.
Figure 3. HAI assay measure of NAb levels in HCT patients pre- and post- vaccination during the 2008–2009 flu season.
NAb titer from serum was measured at d0 and at d30 and d180 post-vaccination. HCT patient sera was measured against the 08–09 flu vaccine antigens for (A)H1N1, (A)H3N2, and (B)Florida flu strains. Black error bars represent the standard deviation from the mean (dashed bar). The dotted black line represents a titer of 40 which is considered protective. * = p<.05, **=p<.01, p = two tailed Mann-Whitney test.
CD4 T cell assays for CD154 expression
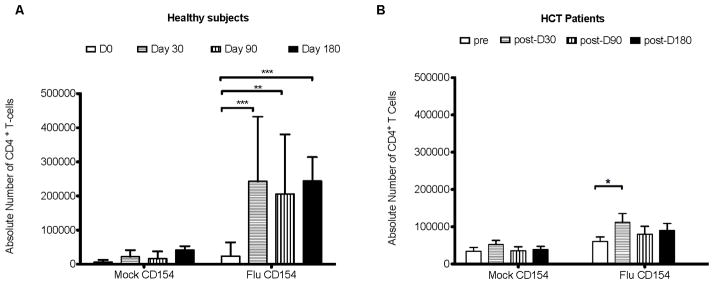
Based on previous studies (6, 9, 28) it is evident that simply measuring antibody responses to flu vaccination does not provide a comprehensive picture of the immune response, especially in immune compromised individuals. One reason for the poor NAb response shown in Figure 3 is the result of weak or no co-stimulation from CD4 helper T cells in HCT patients. CD154 is a marker of newly activated CD4 T cells and is important in B cell co-stimulation (19, 29, 30). CD154 (Figure 4 & 5), TNF-α and IFN-γ (Figure 6) expression were measured on CD4+ T-cells from healthy and HCT subjects. CD154 expression on CD4+ T-cells post-vaccination stimulated with flu vaccine showed a significant increase (using a 2 way ANOVA) compared to pre-vaccination samples in both healthy and HCT subjects 1 month post- vaccination (Figure 4). Figure 4B shows the CD154 response in HCT patients prior to vaccination, and at d30, d90 and d180 post-vaccination. CD154 levels were significantly higher at d30 post-vaccination only compared to d0 in HCT patients. When simultaneously comparing all of the time points in both the mock and flu vaccine treatment groups, the two groups were significantly different from each other using a Kruskal-Wallis test (p<0.0001; data not shown) indicating the measured levels of CD154 were above the background for the assay.
Figure 4. CD154 expression on CD4 T cells after stimulation with inactivated trivalent flu vaccine in healthy (A) and HCT (B) subjects.
CD154 expression was measured by flow cytometry after 18 hours of stimulation with mock diluent or inactivated flu virus (matched annual vaccine). CD154 expression was then calculated in terms of the absolute number of CD4 T cells at each time point. Black error bars represent the standard deviation from the mean. The p values were determined using a 2 way ANOVA. * = p<.05, **=p<.01, ***=p<.001.
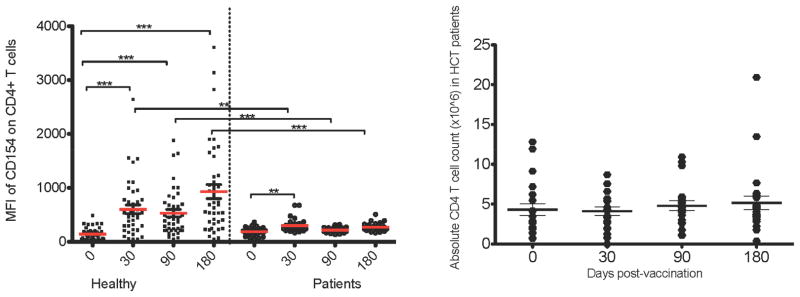
Figure 5. Comparison of Median Fluorescence Intensity (MFI) of CD154 on healthy subjects vs. HCT recipients.
(A) The MFI of CD154 on the cell surface after overnight stimulation with flu vaccine is shown. The p values represent two tailed Mann-Whitney test where **=p<.01, ***=p<.001. HCT patients show an attenuated CD154 expression level. (B) The absolute CD4 T cell count of the patients tested is graphed to show CD4 T cell reconstitution over time. There was no statistically significant difference in the mean absolute CD4 T cell count after vaccination.
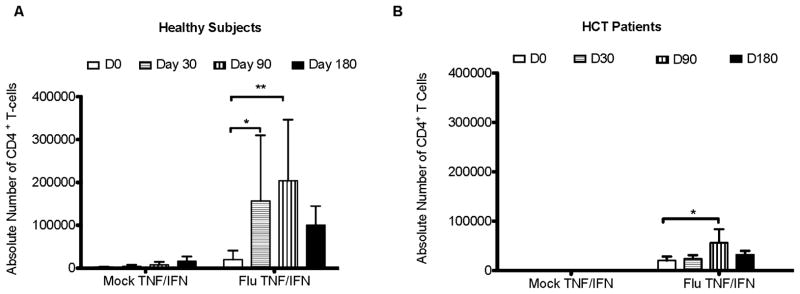
Figure 6. IFN & TNF double expression on CD4 T cells after stimulation with inactivated flu virus in healthy (A) and HCT (B) subjects.
Cytokine expression was measured by flow cytometry after 18 hours of stimulation with mock diluent or inactivated flu virus. Black error bars represent the standard deviation from the mean. The p values were determined using a 2 way ANOVA. * = p<0.05, **=p<0.01.
CD154 expression on CD4 T cells
To determine the expression level of CD154 on a per cell basis, the median fluorescence intensity (MFI) of CD154 on CD4 T cells was graphed (Figure 5A) for both healthy and HCT subjects. The MFI of CD154 did not change post- vaccination in HCT patients, but it did increase in healthy subjects. Using a Kruskal-Wallis test to compare the levels of all mock time points stimulated CD4 T cells to all inactivated flu virus stimulated time points yielded a significant difference in both the healthy and patient populations (p<0.0001; data not shown). This indicates that the CD154 MFI observed on stimulated CD4 T cells is above the background level seen with mock stimulation of CD4 T cells. Comparing the MFI of CD154 between healthy and HCT patients with Mann-Whitney tests showed a significant decrease in CD154 levels of HCT patients at d30, d90 and d180 post-vaccination (Figure 5A). Not only is the magnitude of CD4 T cells responding to flu vaccination attenuated in HCT recipients, but Figure 5A shows that the MFI of CD154 on CD4 T cells from HCT patients is significantly diminished compared to healthy subjects post-vaccination and does not correspond with overall CD4 T cell reconstitution (Figure 5B). The absolute CD4 T cell value for patients is shown in Figure 5B indicating the mean level of CD4 T cell reconstitution was equivalent at all time points. The p values shown in Figure 5A are the result of a two-tailed Mann-Whitney comparison. All time points in HCT patients showed diminished CD154 expression when compared to the same time point in healthy adults (Figure 5).
CD4 T cell assays for cytokine production
Previous studies indicate that the most effective T cells are capable of producing multiple cytokines after antigenic stimulation (18, 29, 31, 32). Similar to our CD154 findings, IFN-γ/TNF-α expressing double positive T cells were significantly attenuated in HCT recipients compared to healthy adults (Figure 6). Measuring IFN-γ and TNF-α levels in T cells after stimulation with killed flu virus from the vaccine provides a measure of the functionality of these CD4 T cells to recognize and respond to subsequent flu infection (29, 33–36). TH2 CD4 T cell responses were evaluated using IL-4 antibody. No changes in intracellular IL-4 levels were found post-vaccination compared to pre-vaccination (data not shown). Others have shown that TH1 CD4 T cell responses are critical for antibody responses that can control influenza virus (37, 38). There is no current evidence that TH2 responses are important in controlling flu infection.
Discussion
Although B cell reconstitution occurs early post-transplant, there is a chronic dysfunction in B cells and antibody responses (15, 16). Others have shown that lagging development of adhesion molecules on B cells can partially explain the longevity of B cell dysfunction seen post-transplant (16). Further, treatment with Rituximab, steroid treatment and incidence of GVHD in allogeneic HCT patients leads to a longer period of recovery for B cells to reach pre-transplant levels (15, 27). Finding ways to overcome B cell dysfunction would increase the efficacy of vaccination post-transplant. The data shows that reconstituted CD4 T cells are also dysfunctional post-transplant (Figures 4–6) and a lack of co-stimulation (CD154) contributes to the longevity of the B cell immune dysfunction. Low CD154 levels also contribute to the lack of memory plasma cells generated post-vaccination, leading to decreased durability of NAbs. The low levels of CD154 expression seen in HCT patients is associated with the failure of HCT patients to generate protective NAb titers to influenza post-vaccination. The data show that CD154 expression and CD4 T cell help is required to promote influenza specific B cells to produce NAb titers capable of protecting from influenza infection post-vaccination in HCT patients.
HCT patients have sufficient B cell reconstitution to produce IgG and IgM after influenza vaccination as illustrated in Figure 1 and as shown by others (3, 15). However, the antibody produced post-transplant is not equivalent to the antibody produced in healthy subjects as indicated by the low NAb levels shown in Figure 3. Taken together Figures 1–3 show that B cells are present and capable of producing antibody post-vaccination, but the antibodies produced are not protective from influenza infection. Routine B lymphocyte counts are not performed on HCT patients in our institution and this information is lacking in our analysis of the patient subset, but could be prospectively monitored in future studies.
Studies of vaccine immunogenicity and efficacy across flu seasons show protective responses in around 65–70% of healthy adults across various flu seasons (39, 40). Studies have shown that slight variations in immunogenicity results from changes in the yearly flu strain composition of the vaccine, larger variability results from differences in adjuvants and manufacturer (41–43). Additionally, live attenuated (LAV) vs. inactivated trivalent (TIV) causes large variation in immune responses (41, 44, 45). Due to viral shedding LAV vaccine is contraindicated for HCT patients (44, 46), thus, all patients and healthy subjects included in our study were vaccinated with TIV from the same manufacturer. While some of the differences we see in HAI and CD154 levels between HCT patients and healthy adults maybe the result of changes in the vaccine components from year to year, we believe the incredibly low levels of NAb and CD154 seen in HCT patients can mostly be contributed to defects in the ability of HCT reconstituting cells to respond to vaccination. Controlling for differences in manufacturer and TIV vs. LAV is more influential to consistent results than comparing across flu seasons. Also of consideration is the importance of previous vaccination on the response to current vaccination.
There is little data available on the impact of previous vaccination on T cell responses but there are reports of the effect of previous vaccination on B cell responses (47). Sasaki et al. performed a direct comparison of HAI response after vaccination with either TIV, LAV or no vaccination in previous years. Sasaki showed that previous vaccination with TIV lead to higher baseline HAI measures, but to lower or decreased changes in HAI after secondary TIV or LAV vaccination (47). The vaccination history of the healthy adults and HCT donors/HCT patients participating in our study is unknown; however, inclusion in our study required the 2008–2009 flu vaccine to be the first flu vaccine received post-transplant for all HCT patients. While allo HCT patients could receive some pre-exisitng immunity from the HCT donor we did not see excessively high pre-vaccination NAb levels to indicate that immune memory was passed on from the donor. The higher pre-vaccination NAb levels shown in Figure 2 could be a result of long term B cell memory attributed to previous TIV flu vaccination.
Further complicating the interpretation of our results is the impact of concurrent vaccinations. Healthy subjects did not receive concurrent vaccinations at the time of flu vaccination. Only 1/35 patients included in our study received a concurrent vaccination on the same day as they received the flu vaccine. This patient received a pneumococcal pneumonia vaccine at the same visit as their flu vaccine. Vaccination to other agents could clearly muddle the results shown here; however, in our cohort patients who have documented vaccinations in their COH record received those vaccinations at subsequent visits more than 6 months after flu vaccination with the exception of the noted pneumococcal vaccine stated above. Because subsequent vaccinations occurred outside the scope of our study we do not believe them to be complicating factors in interpreting the data presented. We acknowledge that some patients could have been vaccinated outside of COH and we would not have knowledge of these vaccinations. Every effort is made by phone and mail to determine a complete vaccination record of participants after enrollment on study.
In a mouse model, Brown et al showed that adaptive transfer of flu specific CD4 T cells along with influenza specific B cells into naïve mice provided protection from subsequent lethal challenge with influenza virus, when transfer of B cells alone did not protect from death (36). Brown et al concluded that when antigen specific CD4 T cells are present to co-stimulate and drive B cells, higher levels of NAb are produced and protection to influenza challenge is established in a mouse model (36). Our data show that the poor quality of influenza antibody response seen after vaccination in HCT patients (Figure 3) is due to a lack of CD4 T cell activation (Figure 4) and low CD154 expression (Figure 5). Also in support of our findings, Lee et al showed that the development of influenza specific IgG in mice lacking functional CD4 T cell help could recover from a challenge with influenza virus within the first 30 days of vaccination, but that long term antibody protection from influenza challenge was lost (17). This study supports our finding that HCT patients were able to develop some NAb (independent of CD4 T cell help) within the first 30 days post-vaccination, but that by 6 months post-vaccination IgG, IgM (Figure 1) and NAb responses (Figure 3) diminish in the absence of functional CD4 T cell help. CD4 T cell co-stimulation via CD154 is critical to developing a long term protective NAb response, and the lack of CD154 expression on CD4 T cells from HCT patients (Figure 5) explains the lack of durability of NAb responses in HCT patients (Figure 3) compared to healthy adults (Figure 2) following influenza vaccination.
Eaton et al conducted an adaptive transfer study between healthy aged and young mice. The authors found that when CD4 T cells from the aged mice were transferred into young healthy adult mice the B cell responses to vaccination were impaired(19). The authors were able to attribute this immune dysfunction to low CD154 expression levels on CD4 T cells from the aged mice (19). This finding on the importance of CD154 expression in the healthy aged mouse population supports our finding that low levels of CD154 expression (Figures 4 & 5) correlate with low NAb titers in HCT patients (Figure 3) post-vaccination. CD154 expression is critical to the efficacy of vaccination in the elderly and in immunosupressed populations such as HCT patients.
Similar to our results (Figure 6), Avetisyan et al looked at IFN-γ responses in HCT patients 4 weeks post- vaccination and found a 10-100 fold decrease in the level of IFN-γ measured by ELISPOT compared to healthy adults (8). Our results confirm and extend this finding and suggest that solely looking at IFN-γ production may provide an incomplete profile of T cell response to vaccination. Although the cytokine responses measured in HCT patients (Figure 6), were significantly increased post-vaccination, the extremely low levels of IFN-γ and TNF-α expressed compared to CD4 T cell expression levels in healthy subjects indicates further immune dysfunction of CD4 T cells in HCT patients. The attenuated cytokine response of CD4 T cells post-transplant contributes to the failure of HCT patients to mount protective immune responses post-vaccination. Using flow cytometry permits examination of multiple parameters (CD154, IFN-γ, TNF-α) of T cell activation and function in a single cell after stimulation compared to ELISPOT. Finding multiple indicators of CD4 T cell dysfunction indicates that CD4 T cells in HCT patients post-transplant are incapable of providing effector function or the co-stimulation necessary for productive B cell responses post-vaccination.
The data show the importance of examining both humoral and cellular immune responses post-transplant in order to determine the appropriate time to provide vaccination and protection against influenza post-transplant. Although it was previously known that HCT recipients do not produce equivalent antibody responses to vaccination as healthy adults (6, 9, 22, 23, 48), little has been written about the mechanism of this defect or about alternate measures of protective immune response post-vaccination. The data show that impaired functionality of CD4 helper T cells generated post-vaccination in HCT patients explains the failed NAb response to flu vaccination. Without sufficient co-stimulation via CD154 (CD40L) on CD4 T cells, B cells do not produce protective levels of NAb following vaccination in HCT patients. Future studies, on a larger patient population, will help to elucidate the important co-factors such as GVHD, age at time of transplant, and steroid and rituximab treatment, that may contribute to the lack of B cell generated NAb response seen in HCT patients post-vaccination. We have demonstrated the importance of CD154 expression on activated CD4 T cells in inducing NAb post-vaccination in HCT patients.
Acknowledgments
We would like to thank the staff at the General Clinical Research Center (GCRC) at City of Hope for collecting blood samples and performing IgG and IgM ELISA testing. We gratefully acknowledge the valuable input from Drs. Eileen Smith, John Zaia and James Ito who contributed to the design of this study. Partial financial support for this work comes from USPHS awards to P01-CA030206 to SJF and DJD and a Research Supplement to Promote Diversity in Health-Related Research granted to AS. This work was also partially supported by grants CA77544 to DJD, MO1 RR00043 in support of the GCRC and CA33572 to support the COH Comprehensive Cancer Center.
Footnotes
The authors do not have a commercial or other association that might pose a conflict of interest
Clinical trials registration URL and number: http://clinicaltrials.gov/ct2/show/NCT00964821 NCT00964821
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parkman R, Weinberg KI. Immunological reconstitution following bone marrow transplantation. Immunol Rev. 1997;157:73–78. doi: 10.1111/j.1600-065x.1997.tb00975.x. [DOI] [PubMed] [Google Scholar]
- 2.Ljungman P, Lewensohn-Fuchs I, Hammarstrom V, et al. Long-term immunity to measles, mumps, and rubella after allogeneic bone marrow transplantation. Blood. 1994;84:657–663. [PubMed] [Google Scholar]
- 3.Blume KG, Forman Stephen J, Appelbaum Frederick R. Thomas’ hematopoietic cell transplantation. Malden, MA: Blackwell Pub; 2004. [Google Scholar]
- 4.Holmberg LA, Boeckh M, Hooper H, et al. Increased incidence of cytomegalovirus disease after autologous CD34-selected peripheral blood stem cell transplantation. Blood. 1999;94:4029–4035. [PubMed] [Google Scholar]
- 5.Crippa F, Holmberg L, Carter RA, et al. Infectious complications after autologous CD34-selected peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2002;8:281–289. doi: 10.1053/bbmt.2002.v8.pm12064366. [DOI] [PubMed] [Google Scholar]
- 6.Gandhi MK, Egner W, Sizer L, et al. Antibody responses to vaccinations given within the first two years after transplant are similar between autologous peripheral blood stem cell and bone marrow transplant recipients. Bone Marrow Transplant. 2001;28:775–781. doi: 10.1038/sj.bmt.1703239. [DOI] [PubMed] [Google Scholar]
- 7.Dykewicz CA, Jaffe Harold W, Kaplan Jonathan E. Guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. MMWR Recomm Rep. 2000;49:1–125. CE121–127. [PubMed] [Google Scholar]
- 8.Avetisyan G, Aschan J, Hassan M, Ljungman P. Evaluation of immune responses to seasonal influenza vaccination in healthy volunteers and in patients after stem cell transplantation. Transplantation. 2008;86:257–263. doi: 10.1097/TP.0b013e3181772a75. [DOI] [PubMed] [Google Scholar]
- 9.Engelhard D, Nagler A, Hardan I, et al. Antibody response to a two-dose regimen of influenza vaccine in allogeneic T cell-depleted and autologous BMT recipients. Bone Marrow Transplant. 1993;11:1–5. [PubMed] [Google Scholar]
- 10.Haining WN, Evans JW, Seth NP, et al. Measuring T cell immunity to influenza vaccination in children after haemopoietic stem cell transplantation. Br J Haematol. 2004;127:322–325. doi: 10.1111/j.1365-2141.2004.05204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hara M, Sakamoto T, Tanaka K. Effectiveness of influenza vaccination in preventing influenza-like illness among community-dwelling elderly: population-based cohort study in Japan. Vaccine. 2006;24:5546–5551. doi: 10.1016/j.vaccine.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 12.Machado CM, Cardoso MR, da Rocha IF, Boas LS, Dulley FL, Pannuti CS. The benefit of influenza vaccination after bone marrow transplantation. Bone Marrow Transplant. 2005;36:897–900. doi: 10.1038/sj.bmt.1705159. [DOI] [PubMed] [Google Scholar]
- 13.Mori S, Kocak U, Shaw JL, Mullen CA. Augmentation of post transplant immunity: antigen encounter at the time of hematopoietic stem cell transplantation enhances antigen-specific donor T-cell responses in the post transplant repertoire. Bone Marrow Transplant. 2005;35:793–801. doi: 10.1038/sj.bmt.1704883. [DOI] [PubMed] [Google Scholar]
- 14.Karl G, Blume SJF, Frederick R. Thomas’ hematopoietic cell transplantation. Malden, MA: Blackwell Pub; 2004. Appelbaum. [Google Scholar]
- 15.Storek J, Ferrara S, Ku N, Giorgi JV, Champlin RE, Saxon A. B cell reconstitution after human bone marrow transplantation: recapitulation of ontogeny? Bone Marrow Transplant. 1993;12:387–398. [PubMed] [Google Scholar]
- 16.Parra C, Roldan E, Brieva JA. Deficient expression of adhesion molecules by human CD5- B lymphocytes both after bone marrow transplantation and during normal ontogeny. Blood. 1996;88:1733–1740. [PubMed] [Google Scholar]
- 17.Lee BO, Rangel-Moreno J, Moyron-Quiroz JE, et al. CD4 T cell-independent antibody response promotes resolution of primary influenza infection and helps to prevent reinfection. J Immunol. 2005;175:5827–5838. doi: 10.4049/jimmunol.175.9.5827. [DOI] [PubMed] [Google Scholar]
- 18.Kannanganat S, Ibegbu C, Chennareddi L, Robinson HL, Amara RR. Multiple-cytokine-producing antiviral CD4 T cells are functionally superior to single-cytokine-producing cells. J Virol. 2007;81:8468–8476. doi: 10.1128/JVI.00228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eaton SM, Burns EM, Kusser K, Randall TD, Haynes L. Age-related defects in CD4 T cell cognate helper function lead to reductions in humoral responses. J Exp Med. 2004;200:1613–1622. doi: 10.1084/jem.20041395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang YC, Thoman M, Linton PJ, Deisseroth A. Use of CD40L immunoconjugates to overcome the defective immune response to vaccines for infections and cancer in the aged. Cancer Immunol Immunother. 2009 doi: 10.1007/s00262-009-0718-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soesman NM, Rimmelzwaan GF, Nieuwkoop NJ, et al. Efficacy of influenza vaccination in adult liver transplant recipients. J Med Virol. 2000;61:85–93. [PubMed] [Google Scholar]
- 22.Duchini A, Hendry RM, Nyberg LM, Viernes ME, Pockros PJ. Immune response to influenza vaccine in adult liver transplant recipients. Liver Transpl. 2001;7:311–313. doi: 10.1053/jlts.2001.23010. [DOI] [PubMed] [Google Scholar]
- 23.Mazzone PJ, Mossad SB, Mawhorter SD, Mehta AC, Schilz RJ, Maurer JR. The humoral immune response to influenza vaccination in lung transplant patients. Eur Respir J. 2001;18:971–976. doi: 10.1183/09031936.01.00215201. [DOI] [PubMed] [Google Scholar]
- 24.Ljungman P, Avetisyan G. Influenza vaccination in hematopoietic SCT recipients. Bone Marrow Transplant. 2008 doi: 10.1038/bmt.2008.264. [DOI] [PubMed] [Google Scholar]
- 25.Gelinck LB, van den Bemt BJ, Marijt WA, et al. Intradermal influenza vaccination in immunocompromized patients is immunogenic and feasible. Vaccine. 2009;27:2469–2474. doi: 10.1016/j.vaccine.2009.02.053. [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention. Recommendations for Influenza vaccination and flu statistics. 2009. Available at. [Google Scholar]
- 27.Buser A, Stern M, Arber C, et al. Impaired B-cell reconstitution in lymphoma patients undergoing allogeneic HSCT: an effect of pretreatment with rituximab? Bone Marrow Transplant. 2008;42:483–487. doi: 10.1038/bmt.2008.229. [DOI] [PubMed] [Google Scholar]
- 28.Machado CM. Influenza infections after hematopoietic stem cell transplantation. Clin Infect Dis. 2005;41:273–274. doi: 10.1086/431304. [DOI] [PubMed] [Google Scholar]
- 29.Chattopadhyay PK, Yu J, Roederer M. A live-cell assay to detect antigen-specific CD4+ T cells with diverse cytokine profiles. Nat Med. 2005;11:1113–1117. doi: 10.1038/nm1293. [DOI] [PubMed] [Google Scholar]
- 30.Ford ML, Koehn BH, Wagener ME, et al. Antigen-specific precursor frequency impacts T cell proliferation, differentiation, and requirement for costimulation. J Exp Med. 2007;204:299–309. doi: 10.1084/jem.20062319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lacey SF, Diamond DJ, Zaia JA. Assessment of cellular immunity to human cytomegalovirus in recipients of allogeneic stem cell transplants. Biol Blood Marrow Transplant. 2004;10:433–447. doi: 10.1016/j.bbmt.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 32.Swain SL, Agrewala JN, Brown DM, et al. CD4+ T-cell memory: generation and multi-faceted roles for CD4+ T cells in protective immunity to influenza. Immunol Rev. 2006;211:8–22. doi: 10.1111/j.0105-2896.2006.00388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avetisyan G, Ragnavolgyi E, Toth GT, Hassan M, Ljungman P. Cell-mediated immune responses to influenza vaccination in healthy volunteers and allogeneic stem cell transplant recipients. Bone Marrow Transplant. 2005;36:411–415. doi: 10.1038/sj.bmt.1705064. [DOI] [PubMed] [Google Scholar]
- 34.Kang I, Hong MS, Nolasco H, et al. Age-associated change in the frequency of memory CD4+ T cells impairs long term CD4+ T cell responses to influenza vaccine. J Immunol. 2004;173:673–681. doi: 10.4049/jimmunol.173.1.673. [DOI] [PubMed] [Google Scholar]
- 35.He XS, Holmes TH, Sasaki S, et al. Baseline levels of influenza-specific CD4 memory T-cells affect T-cell responses to influenza vaccines. PLoS ONE. 2008;3:e2574. doi: 10.1371/journal.pone.0002574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown DM, Dilzer AM, Meents DL, Swain SL. CD4 T cell-mediated protection from lethal influenza: perforin and antibody-mediated mechanisms give a one-two punch. J Immunol. 2006;177:2888–2898. doi: 10.4049/jimmunol.177.5.2888. [DOI] [PubMed] [Google Scholar]
- 37.Palladino G, Scherle PA, Gerhard W. Activity of CD4+ T-cell clones of type 1 and type 2 in generation of influenza virus-specific cytotoxic responses in vitro. J Virol. 1991;65:6071–6076. doi: 10.1128/jvi.65.11.6071-6076.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruat C, Caillet C, Bidaut A, Simon J, Osterhaus AD. Vaccination of macaques with adjuvanted formalin-inactivated influenza A virus (H5N1) vaccines: protection against H5N1 challenge without disease enhancement. J Virol. 2008;82:2565–2569. doi: 10.1128/JVI.01928-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jackson LA, Gaglani MJ, Keyserling HL, et al. Safety, efficacy, and immunogenicity of an inactivated influenza vaccine in healthy adults: a randomized, placebo-controlled trial over two influenza seasons. BMC Infect Dis. 10:71. doi: 10.1186/1471-2334-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.La Montagne JR, Noble GR, Quinnan GV, et al. Summary of clinical trials of inactivated influenza vaccine - 1978. Rev Infect Dis. 1983;5:723–736. doi: 10.1093/clinids/5.4.723. [DOI] [PubMed] [Google Scholar]
- 41.Jefferson T, Smith S, Demicheli V, Harnden A, Rivetti A, Di Pietrantonj C. Assessment of the efficacy and effectiveness of influenza vaccines in healthy children: systematic review. Lancet. 2005;365:773–780. doi: 10.1016/S0140-6736(05)17984-7. [DOI] [PubMed] [Google Scholar]
- 42.Beyer WE, Palache AM, Osterhaus AD. Comparison of Serology and Reactogenicity between Influenza Subunit Vaccines and Whole Virus or Split Vaccines: A Review and Meta-Analysis of the Literature. Clin Drug Investig. 1998;15:1–12. doi: 10.2165/00044011-199815010-00001. [DOI] [PubMed] [Google Scholar]
- 43.Co MD, Orphin L, Cruz J, et al. In vitro evidence that commercial influenza vaccines are not similar in their ability to activate human T cell responses. Vaccine. 2009;27:319–327. doi: 10.1016/j.vaccine.2008.09.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levin MJ, Song LY, Fenton T, et al. Shedding of live vaccine virus, comparative safety, and influenza-specific antibody responses after administration of live attenuated and inactivated trivalent influenza vaccines to HIV-infected children. Vaccine. 2008;26:4210–4217. doi: 10.1016/j.vaccine.2008.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Monto AS, Ohmit SE, Petrie JG, et al. Comparative efficacy of inactivated and live attenuated influenza vaccines. N Engl J Med. 2009;361:1260–1267. doi: 10.1056/NEJMoa0808652. [DOI] [PubMed] [Google Scholar]
- 46.Centers for Disease Control and Prevention. Recommendations for Influenza vaccination and flu statistics. Available at www.cdc.gov/flu.; 12 November 2009.
- 47.Sasaki S, He XS, Holmes TH, et al. Influence of prior influenza vaccination on antibody and B-cell responses. PLoS ONE. 2008;3:e2975. doi: 10.1371/journal.pone.0002975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lausen BF, Hougs L, Schejbel L, Heilmann C, Barington T. Human memory B cells transferred by allogenic bone marrow transplantation contribute significantly to the antibody repertoire of the recipient. J Immunol. 2004;172:3305–3318. doi: 10.4049/jimmunol.172.5.3305. [DOI] [PubMed] [Google Scholar]