Abstract
Sorafenib and vorinostat interact in a synergistic fashion to kill carcinoma cells by activating CD95, and the present studies have determined individually how sorafenib and vorinostat contribute to CD95 activation. Sorafenib (3-6 μM) promoted a dose-dependent increase in Src Y416, ERBB1 Y845 and CD95 Y232/Y291 phosphorylation, and Src Y527 dephosphorylation. Low levels of sorafenib (3 μM) –induced CD95 tyrosine phosphorylation did not promote surface localization whereas sorafenib (6 μM), or sorafenib (3 μM) and vorinostat (500 nM) treatment promoted higher levels of CD95 phosphorylation that correlated with DISC formation, receptor surface localization and autophagy. CD95 (Y232F, Y291F) was not tyrosine phosphorylated and was unable to plasma membrane localize or induce autophagy. Knock down / knock out of Src family kinases abolished sorafenib –induced: CD95 tyrosine phosphorylation; DISC formation; and the induction of cell death and autophagy. Knock down of PDGFRβ enhanced Src Y416 and CD95 tyrosine phosphorylation that correlated with elevated CD95 plasma membrane levels and autophagy, and with a reduced ability of sorafenib to promote CD95 membrane localization. Vorinostat increased ROS levels; and in a delayed NFκB-dependent fashion, those of FAS ligand and CD95. Neutralization of FAS-L did not alter the initial rapid drug-induced activation of CD95 however, neutralization of FAS-L reduced sorafenib + vorinostat toxicity by ~50%. Thus sorafenib contributes to CD95 activation by promoting receptor tyrosine phosphorylation whereas vorinostat contributes to CD95 activation via initial facilitation of ROS generation and subsequently of FAS-L expression.
Keywords: Vorinostat, Sorafenib, CD95, c-FLIP-s, FAS-L, cell death, autophagy
Introduction
In the United States, hepatoma patients have a 5 year survival rates of less than 10% (1). We have recently developed a novel drug therapy combining the multi-kinase inhibitor Sorafenib with the histone deacetylase inhibitor vorinostat, and this combination is entering phase I trial in hepatoma (2-5).
Sorafenib (Bay 43-9006, Nexavar®; a RAF family kinase inhibitor) is a multi-kinase inhibitor that was originally developed as an inhibitor of RAF-1 but which was subsequently shown to inhibit multiple other kinases, including class III tyrosine kinase receptors (6). Anti-tumor effects of sorafenib in renal cell carcinoma and in hepatoma have been ascribed to anti-angiogenic actions of this agent through inhibition of the growth factor receptors (7-9). However, several groups, including ours, have shown in vitro that sorafenib kills human leukemia cells at concentrations below the maximum achievable dose (Cmax) of 15-20 μM, through a mechanism involving down-regulation of MCL-1 (10, 11). Sorafenib-mediated MCL-1 down-regulation occurred through a translational rather than a transcriptional or post-translational process that was mediated by endoplasmic reticulum (ER) stress signaling (12, 13). This suggests that the previously observed anti-tumor effects of sorafenib are mediated by a combination of inhibition of RAF family kinases and the ERK1/2 pathway; receptor tyrosine kinases that signal angiogenesis; and the induction of ER stress signaling.
Histone deacetylase inhibitors (HDACI) represent a class of agents that act by blocking histone de-acetylation, thereby modifying chromatin structure and gene transcription. HDACs, along with histone acetyl-transferases, reciprocally regulate the acetylation status of the positively charged NH2-terminal histone tails of nucleosomes. HDACIs promote histone acetylation and neutralization of positively charged lysine residues on histone tails, allowing chromatin to assume a more open conformation, which favors transcription (14). However, HDACIs also induce acetylation of other non-histone targets, actions that may have plieotropic biological consequences, including inhibition of HSP90 function, induction of oxidative injury and up-regulation of death receptor expression (15-17). With respect to combinatorial drug studies with a multi-kinase inhibitor such as sorafenib, HDACIs are of interest in that they also down-regulate multiple oncogenic kinases by interfering with HSP90 function, leading to proteasomal degradation of these proteins. Vorinostat (suberoylanilide hydroxamic acid, SAHA, Zolinza™) is a hydroxamic acid HDACI that has shown preliminary pre-clinical evidence of activity in hepatoma and other malignancies with a Cmax of ~9 μM (18-28).
We have recently published that sorafenib and vorinostat to interact to kill in a wide range of tumor cell types via activation of the CD95 extrinsic apoptotic pathway, concomitant with drug-induced reduced expression of c-FLIP-s via PERK/eIF2α activation (29, 30). The present studies have extended in greater molecular detail our analyses to understanding how sorafenib and vorinostat individually interact to promote CD95 activation and tumor cell death.
Materials and Methods
Materials
Sorafenib tosylate (Bayer) and vorinostat (Merck) were provided by the Cancer Treatment and Evaluation Program, National Cancer Institute/NIH (Bethesda, MD). Trypsin-EDTA, DMEM, RPMI, penicillin-streptomycin were purchased from GIBCOBRL (GIBCOBRL Life Technologies, Grand Island, NY). HEPG2, HEP3B, HuH7 (hepatoma) cells were purchased from the ATCC. Commercially available validated short hairpin RNA molecules to knock down RNA / protein levels were from Qiagen (Valencia, CA): CD95 (SI02654463; SI03118255); ATG5 (SI02655310); Beclin 1 (SI00055573, SI00055587). We also made use for confirmatory purposes of the short hairpin RNA construct targeting ATG5 (pLVTHM/Atg5) that was a generous gift from Dr. S. Yousefi, Department of Pharmacology, University of Bern, Bern Switzerland. Reagents and performance of experimental procedures were described in refs. 2-5, 21, 29-37.
Methods
Culture and in vitro exposure of cells to drugs
All established cell lines were cultured at 37 °C (5% (v/v CO2) in vitro using RPMI supplemented with 5% (v/v) fetal calf serum and 10% (v/v) Non-essential amino acids. For short term cell killing assays, immunoblotting studies, cells were plated at a density of 3 × 103 per cm2 (~2 × 105 cells per well of a 12 well plate) and 48h after plating treated with various drugs, as indicated. In vitro vorinostat and sorafenib treatments were from 100 mM stock solutions of each drug and the maximal concentration of Vehicle (DMSO) in media was 0.02% (v/v). Cells were not cultured in reduced serum media during any study in this manuscript.
In vitro cell treatments, microscopy, SDS-PAGE and Western blot analysis
For in vitro analyses of short-term cell death effects, cells were treated with Vehicle or vorinostat / sorafenib for the indicated times in the Figure legends. For apoptosis assays where indicated, cells were isolated at the indicated times, and either subjected to trypan blue cell viability assay by counting in a light microscope or fixed to slides, and stained using a commercially available Diff Quick (Geimsa) assay kit. Alternatively, the Annexin V/propidium iodide assay was carried to determine cell viability out as per the manufacturer's instructions (BD PharMingen) using a Becton Dickinson FACScan flow cytometer (Mansfield, MA). Vorinostat / sorafenib lethality, as judged by annexin-PI, was first evident ~24h after drug exposure (data not shown).
For SDS PAGE and immunoblotting, cells were plated at 5 × 105 cells / cm2 and treated with drugs at the indicated concentrations and after the indicated time of treatment, lysed in whole-cell lysis buffer (0.5 M Tris-HCl, pH 6.8, 2%SDS, 10% glycerol, 1% β-mercaptoethanol, 0.02% bromophenol blue), and the samples were boiled for 30 min. The boiled samples were loaded onto 10-14% SDS-PAGE and electrophoresis was run overnight. Proteins were electrophoretically transferred onto 0.22 μm nitrocellulose, and immunoblotted with various primary antibodies against different proteins.
Transfection of cells with siRNA or with plasmids
For Plasmids
Cells were plated as described above and 24h after plating, transfected. For mouse embryonic fibroblasts (2-5μg) or other cell types (0.5μg) plasmids expressing a specific mRNA (or siRNA) or appropriate vector control plasmid DNA was diluted in 50μl serum-free and antibiotic-free medium (1 portion for each sample). Concurrently, 2μl Lipofectamine 2000 (Invitrogen), was diluted into 50μl of serum-free and antibiotic-free medium (1 portion for each sample). Diluted DNA was added to the diluted Lipofectamine 2000 for each sample and incubated at room temperature for 30 min. This mixture was added to each well / dish of cells containing 200μl serum-free and antibiotic-free medium for a total volume of 300 μl, and the cells were incubated for 4 h at 37 °C. An equal volume of 2x medium was then added to each well. Cells were incubated for 48h, then treated with vorinostat / sorafenib.
Transfection with siRNA
Cells were plated in 60 mm dishes from a fresh culture growing in log phase as described above, and 24h after plating transfected. Prior to transfection, the medium was aspirated and 1 ml serum-free medium was added to each plate. For transfection, 10 nM of the annealed siRNA, the positive sense control doubled stranded siRNA targeting GAPDH or the negative control (a “scrambled” sequence with no significant homology to any known gene sequences from mouse, rat or human cell lines) were used. Ten nM siRNA (scrambled or experimental) was diluted in serum-free media. Four μl Hiperfect (Qiagen) was added to this mixture and the solution was mixed by pipetting up and down several times. This solution was incubated at room temp for 10 min, then added dropwise to each dish. The medium in each dish was swirled gently to mix, then incubated at 37 °C for 2h. One ml of 10% (v/v) serum-containing medium was added to each plate, and cells were incubated at 37 °C for 48h before re-plating (50 × 103 cells each) onto 12-well plates. Cells were allowed to attach overnight, then treated with vorinostat / sorafenib (0-48h). Trypan blue exclusion / TUNEL / flow cytometry assays and SDSPAGE/immunoblotting analyses were performed at the indicated time points.
Microscopy for LC3-GFP expression
Cells were transfected with a plasmid to express an LC3-GFP fusion protein, and were then cultured for 24 h. Cells were then treated with drugs, as indicated/ LC3-GFP transfected cells were visualized at the indicated time points on the Zeiss Axiovert 200 microscope using the FITC filter.
Recombinant adenoviral vectors; infection in vitro
We generated and purchased previously noted recombinant adenoviruses to express a wide variety of proteins or to knock down p21 expression (Vector Biolabs, Philadelphia, PA). Cells were infected with these adenoviruses at an approximate m.o.i. of 50. Cells were incubated for 24 h to ensure adequate expression of transduced gene products prior to drug exposures.
Assessment of Reactive Oxygen Species (ROS) Generation
Hepatoma cells were plated in 96 well plates. Cells were pre-incubated with dihydro-DCF (5mM for 30minutes) which is non-fluorescentinitsdi-hydro form but upon reaction with ROS becomes highly fluorescent. Di-hydro-DCFis sensitive to oxidation by hydroxyl radicals and peroxy-nitrite directly and hydrogen peroxide in the presence of oxidases. Fluorescence measurements were obtained 0–30 minutes after drug addition with a Vector 3 plate reader. Data are presented corrected for basal fluorescence of vehicle-treated cells at each time point and expressed as a –Fold increase in ROS levels. Each time point represents the mean of at least six data points per experiment and of a total of three independent experiments.
Data analysis
Comparison of the effects of various treatments was performed using ANOVA and the Student's t test. Differences with a p-value of < 0.05 were considered statistically significant. Experiments shown are the means of multiple individual points (± SEM). Median dose effect isobologram analyses to determine synergism of drug interaction were performed according to the Methods of T.-C. Chou and P. Talalay using the Calcusyn program for Windows (BIOSOFT, Cambridge, UK). Cells are treated with agents at a fixed concentration dose. A combination index (CI) value of less than 1.00 indicates synergy of interaction between the drugs; a value of 1.00 indicates additivity; a value of > 1.00 equates to antagonism of action between the agents.
Results
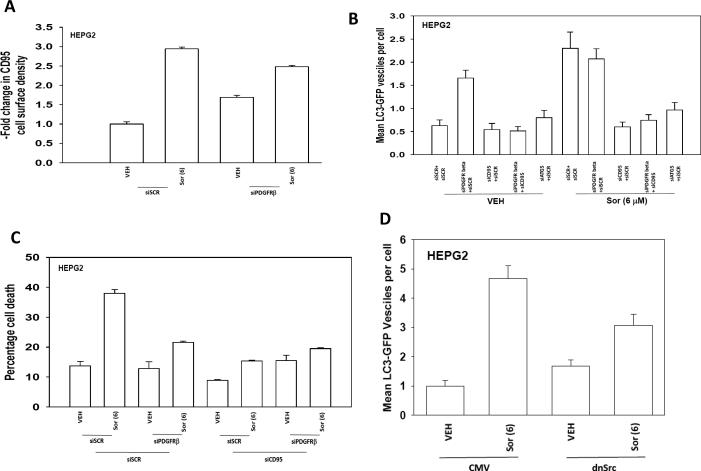
Treatment of GI tumor cells (HEPG2, HEP3B, UOK121LN) with low concentrations of sorafenib (3 μM, 6 μM) increased the tyrosine phosphorylation of Src Y416, ERBB1 Y845 and the basal tyrosine phosphorylation of PDGFRβ and caused Src Y527 dephosphorylation (Figure 1A). Although sorafenib (3 μM) modestly increased CD95 tyrosine phosphorylation, this level of phosphorylation neither promoted CD95 surface localization nor promoted caspase 8 association with CD95 (DISC formation) (Figures 1B, 1C and 1D; Figure S1). Treatment of cells with a higher concentration of sorafenib (6 μM) promoted a further increase in CD95 tyrosine phosphorylation above that induced by sorafenib (3 μM), and that correlated with CD95 surface localization and promoted caspase 8 association with CD95 (Figures 1B, 1C and 1D). Concomitant treatment of cells with vorinostat enhanced sorafenib (3 μM) –induced tyrosine phosphorylation of CD95 that correlated with CD95 surface localization and promoted caspase 8 association with CD95 (Figures 1B, 1C and 1D).
Figure 1. Sorafenib causes a dose-dependent activation of Src, ERBB1 and CD95.
Panel A. HEPG2 cells were treated with vehicle (DMSO), sorafenib (3 μM, 6 μM), as indicated. Thirty minutes after treatment cells were isolated and lysed. Portions of lysates were immunoprecipitated (anti-phospho-tyrosine) and IPs probed for PDGFRβ. After SDS PAGE immunoblotting was performed to determine the phosphorylation of the indicated proteins. Representative blots are shown (n = 3). Panel B. Cells were plated in 8 well chamber slides and were treated with vehicle (DMSO), sorafenib (3 μM, 6 μM) or vorinostat (500 nM), as indicated. Cells were fixed 6h after exposure and surface levels of CD95 determined by IHC. The density of CD95 staining was determined in 40 cells (n = 2, +/- SEM). Panel C. HEPG2 cells were treated with vehicle (DMSO), sorafenib (3 μM, 6 μM) or vorinostat (500 nM), as indicated. Cells were isolated 6h after exposure and CD95 immunoprecipitated. The amount of caspase 8 association with CD95 was determined. Representative blots are shown (n = 3). Panel D. HEPG2 cells were treated with vehicle (DMSO), sorafenib (3 μM, 6 μM) or vorinostat (500 nM), as indicated. Thirty minutes after treatment cells were isolated and lysed. Portions of lysates were immunoprecipitated (anti-CD95). After SDS PAGE immunoblotting was performed to determine the phosphorylation of CD95. Representative blots are shown (n = 3).
HuH7 hepatoma cells lack endogenous CD95 expression (29, 30, 37). Treatment of HuH7 cells with sorafenib (3 μM) and vorinostat resulted in a very modest increase in tumor cell killing which was significantly enhanced when HuH7 cells were transiently transfected with a plasmid to express wild type CD95 (Figure 2A). Transfection of cells with a plasmid to express a CD95 protein lacking the sites of tyrosine phosphorylation (Y232F, Y291F) did not facilitate sorafenib and vorinostat lethality. In general agreement with these data, sorafenib and vorinostat promoted DISC formation in cells transfected with wild type CD95 but not with CD95 (Y232F, Y291F) (Figure 2B). In Figure 1 we noted that Sorafenib promoted activation of Src and enhanced basal levels of PDGFRβ tyrosine phosphorylation, and it has been shown in other cell systems that one of the protein kinases which phosphorylate CD95 is the Src family member c-Yes (37). Knock down of PDGFRβ promoted a compensatory activation of Src within 24h as judged by increased Y416 phosphorylation and decreased Y527 phosphorylation (Figure 2C). Knock down of PDGFRβ enhanced CD95 tyrosine phosphorylation and pro-caspase 8 association with CD95 that whose phosphorylation and association, respectively, in the absence of PDGFRβ expression were not further enhanced by sorafenib (Figure 2D, upper). Enhanced levels of CD95 tyrosine phosphorylation caused by knock down of PDGFRβ were blocked by expression of a dominant negative (kinase inactive) Src protein (Figure 2D, lower).
Figure 2. Sorafenib-induced Src-dependent CD95 tyrosine phosphorylation is due to inhibition of PDGFRβ.
Panel A. HuH7 cells were transfected with empty vector plasmid (CMV), a plasmid to express CD95-YFP or a plasmid to express mutant inactive CD95-YFP (Y232F, Y291F). Twenty four h after transfection cells were treated with vehicle (DMSO) or sorafenib (3 μM) and vorinostat (500 nM). Cells were isolated 48h after exposure and viability determined by trypan blue exclusion (n = 3, +/- SEM). Panel B. HuH7 cells were transfected with a plasmid to express CD95-YFP or a plasmid to express mutant inactive CD95-YFP (Y232F, Y291F) and twenty four h later were treated with vehicle (DMSO) or sorafenib (3 μM) and vorinostat (500 nM). Cells were isolated 6h after exposure and CD95 immunoprecipitated. The amount of caspase 8 association with CD95 was determined. Representative blots are shown (n = 3). Panel C. HEPG2 cells were transfected with scrambled siRNA (siSCR) or an siRNA to knock down PDGFRβ. Thirty six h after transfection cells were treated with vehicle (DMSO) or sorafenib (6 μM). Cells were isolated 30 min later and the phosphorylation of Src determined by immunoblotting. Representative blots are shown (n = 3). Panel D. Upper blots: HEPG2 cells were transfected with scrambled siRNA (siSCR) or an siRNA to knock down PDGFRβ. Thirty six h after transfection cells were treated with vehicle (DMSO) or sorafenib (6 μM). Cells were isolated 6 h later and CD95 immunoprecipitated. The association of caspase 8 with CD95 and the tyrosine phosphorylation of CD95 were determined. Representative blots are shown (n = 3). Lower blots: Cells were transfected with scrambled siRNA (siSCR) or an siRNA to knock down PDGFRβ and in parallel were transfected with an empty vector plasmid (CMV) or a plasmid to express dominant negative c-Src. Thirty six h after transfection cells were isolated and CD95 immunoprecipitated. The tyrosine phosphorylation of CD95 was determined. Representative blots are shown (n = 3).
To confirm our findings in hepatoma cells using other genetic approaches we made use of SV40 Large T antigen transformed mouse embryonic fibroblasts lacking expression of c-Src, c-Fyn and c-Yes. In transformed MEFs: sorafenib (3 μM) and vorinostat treatment, or sorafenib (6 μM) treatment, promoted surface localization of CD95 in a Src family kinase –dependent fashion (Figure 3A). Transfection of wild type MEFs with a plasmid to express wild type CD95, followed by sorafenib (6 μM) exposure resulted in enhanced CD95 tyrosine phosphorylation and enhanced DISC formation (Figure 3B). In MEFs lacking expression of c-Src, c-Fyn and c-Yes, sorafenib (6 μM) exposure did not cause CD95 tyrosine phosphorylation or DISC formation. The toxicity of sorafenib was reduced in fibroblasts lacking expression of c-Src, c-Fyn and c-Yes (Figure 3C). In hepatoma cells expression of dominant negative Src suppressed sorafenib (6 μM) –induced CD95 activation; dominant negative Src increased basal levels of cell death but suppressed drug-induced toxicity (Figure 3D).
Figure 3. Src family kinase signaling is essential for sorafenib-induced CD95 activation and drug toxicity.
Panel A. Wild type or Src / Fyn / Yes null SV40 Large T transformed MEFs plated in 8 well chamber slides and were treated with vehicle (DMSO), sorafenib (3 μM, 6 μM) or vorinostat (500 nM), as indicated. Cells were fixed 6h after exposure and surface levels of CD95 determined by IHC. The density of CD95 staining was determined in 40 cells (n = 2, +/- SEM). Panel B. Wild type or Src / Fyn / Yes null SV40 Large T transformed MEFs were transfected with a plasmid to express CD95-YFP or a plasmid to express mutant inactive CD95-YFP (Y232F, Y291F) and twenty four h later were treated with vehicle (DMSO) or sorafenib (6 μM). Cells were isolated 6 h later and CD95 immunoprecipitated via the YFP tag. The association of caspase 8 with CD95 and the tyrosine phosphorylation of CD95 were determined. Representative blots are shown (n = 3). Panel C. Wild type or Src / Fyn / Yes null SV40 Large T transformed MEFs were treated with vehicle (DMSO) or increasing concentrations of sorafenib (0.1-6.0 μM). Cells were isolated 48 h after drug exposure and viability determined by trypan blue exclusion (n = 3, +/- SEM). Panel D. HEPG2 cells were transfected with empty vector plasmid (CMV) or a plasmid to express dominant negative Src. Twenty four h after transfection cells were treated with sorafenib (3 μM, 6 μM), as indicated. Upper Panel: Cells were isolated 6h after drug exposure, CD95 immunoprecipitated and the levels of CD95 tyrosine phosphorylation and the association of pro-caspase 8 with CD95 determined. Representative blots are shown (n = 3). Lower Panel: Cells were isolated 48h after treatment and viability determined by trypan blue exclusion (n = 3, +/- SEM).
Prior studies by our group had shown that sorafenib and vorinostat –induced activation of CD95 increased the levels of a protective form of autophagy (29, 30, 36). In agreement with data in Figure 2D showing that knock down of PDGFRβ increased CD95 tyrosine phosphorylation; knock down of PDGFRβ increased basal levels of plasma membrane associated CD95 and suppressed the ability of sorafenib (6 μM) to promote CD95 plasma membrane levels (Figure 4A). Knock down of PDGFRβ or treatment with sorafenib (6 μM) increased autophagy in a CD95 and ATG5 –dependent fashion (Figure 4B). The ability of sorafenib to kill hepatoma cells was reduced by knock down of PDGFRβ and sorafenib (6 μM) toxicity was suppressed by knock down of CD95 (Figure 4C). Expression of wild type CD95, but not mutant CD95 (Y232F, Y291F) facilitated sorafenib (6 μM) –induced autophagy in HuH7 cells (Figure S2). In agreement with the above findings, expression of dominant negative Src suppressed Sorafenib (6 μM) –induced autophagy and also tumor cell killing (Figures 3D and 4D). In ATG5 -/- MEFs, that cannot undergo autophagy, sorafenib– and vorinostat- induced toxicities were enhanced (Figure S3). Vorinostat treatment of malignant blood cancer cells strongly and rapidly increases p21Cip-1/WAF1/mda-6 (p21) expression, however in hepatoma cells it more modestly elevated p21 levels and did so in a delayed fashion (Figure S4, upper blot) (20, 21). Increased p21 levels were reduced by co-treatment with sorafenib. Knock down of p21 reduced sorafenib + vorinostat toxicity and reduced drug – induced autophagy (Figure S4, lower graphs). These findings are similar to our prior studies in primary hepatocytes treated with bile acids wherein over-expression of p21 promoted increased CD95 expression and activation and loss of p21 reduced CD95 activation and bile acid –induced autophagy (36). Knock down of p21 suppressed the ability of sorafenib + vorinostat treatment to increased CD95 surface localization and DISC formation (Figure S4, immunohistochemistry).
Figure 4. PDGFRβ regulates CD95-dependent sorafenib –induced autophagy.
Panel A. HEPG2 cells plated in 8 well chamber slides and were transfected with scrambled siRNA (siSCR) or an siRNA to knock down PDGFRβ. Thirty six h after transfection cells were treated with vehicle (DMSO) or sorafenib (6 μM). Cells were fixed 6h after exposure and surface levels of CD95 determined by IHC. The density of CD95 staining was determined in 40 cells (n = 2, +/- SEM). Panel B. HEPG2 cells plated in 8 well chamber slides and were transfected with a plasmid to express LC3-GFP and in parallel with scrambled siRNA (siSCR) or siRNA molecules to knock down PDGFRβ or CD95. Thirty six h after transfection cells were treated with vehicle (DMSO) or sorafenib (6 μM). The number of intense punctate GFP-LC3 staining vesicles was determined in 40 cells (n = 2, +/- SEM). Panel C. HEPG2 cells were transfected with scrambled siRNA (siSCR) or siRNA molecules to knock down PDGFRβ or CD95. Thirty six h after transfection cells were treated with vehicle (DMSO) or sorafenib (6 μM). Forty eight h later cells were isolated and viability determined by trypan blue exclusion (n = 3, +/- SEM). Panel D. HEPG2 cells plated in 8 well chamber slides and were transfected with a plasmid to express LC3-GFP and in parallel with a plasmid to express dominant negative Src. Thirty six h after transfection cells were treated with vehicle (DMSO) or sorafenib (6 μM). The number of intense punctate GFPLC3 staining vesicles was determined in 40 cells (n = 2, +/- SEM).
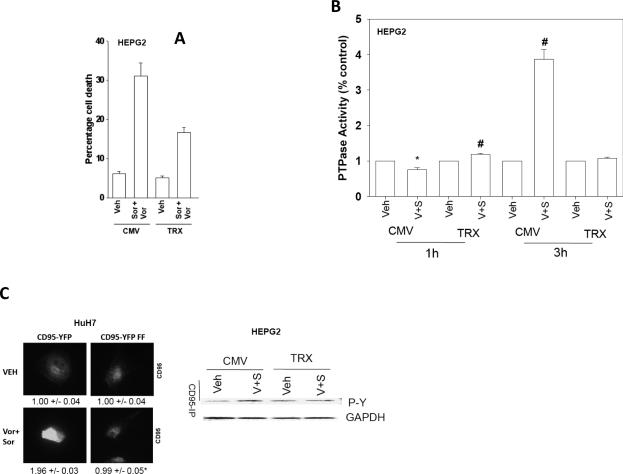
We next determined how vorinostat promoted sorafenib-induced CD95 activation. Sorafenib (3 μM) and vorinostat (500 nM) rapidly promoted the generation of reactive oxygen species (ROS) in HEPG2 cells that was quenched by expression of thioredoxin (TRX) (Figure S5). Similar data were obtained in HEP3B and UOK121LN cells (data not shown). Quenching drug-induced ROS suppressed sorafenib and vorinostat toxicity and blocked CD95 activation (Figure 5A); sorafenib and vorinostat increase cell surface CD95 levels 1.97 +/-0.08 –fold that was reduced by transfection of TRX to a 1.12 +/- 0.06 –fold increase (p < 0.05). In agreement with our CD95 activation data, and the role CD95 plays in stimulating autophagy, quenching of ROS also blocked drug combination –induced autophagy (Figure S6).
Figure 5. Vorinostat –induced ROS plays a key role in promoting PTPase inactivation and CD95 tyrosine phosphorylation.
Panel A. HEPG2 cells were transfected with empty vector plasmid (CMV) or a plasmid to express thioredoxin (TRX). Twenty four h after transfection cells were treated with vehicle (DMSO) or sorafenib (3 μM) and vorinostat (500 nM). Cells were isolated 48h after exposure and viability determined by trypan blue exclusion (n = 2, +/- SEM). Panel B. HEPG2 cells were plated in 96 well plates and 24h after plating were transfected with empty vector plasmid (CMV) or a plasmid to express thioredoxin (TRX). Twenty four h after transfection cells were treated with vehicle (DMSO) or sorafenib (3 μM) and vorinostat (500 nM). PTPase activity was measured using a commercial kit as described in the Methods (n = 2, +/- SEM). Panel C. Left Section: HuH7 cells transfected with plasmids to express CD95-YFP or CD95-YFP FF, 24h after plating in 8 well chamber slides were treated with vehicle (DMSO) or sorafenib (3 μM), and vorinostat (500 nM) in combination. Cells were fixed after 6h and cell surface CD95 levels determined. Right Section: HEPG2 cells were transfected with empty vector (CMV) or to express either wild type Thioredoxin (TRX). Twenty-four h after transfection cells were treated with vehicle (DMSO) or with sorafenib (3.0 μM) and vorinostat (500 nM). Cells were isolated after 6h and CD95 immunoprecipitated to determine DISC formation and CD95 tyrosine phosphorylation (n = 3, +/- SEM).
Treatment of hepatoma cells with sorafenib and vorinostat initially suppressed total cellular protein tyrosine phosphatase activity by ~25% that was blocked by expression of TRX (Figure 5B). However, within 3h vorinostat and sorafenib treatment profoundly enhanced tyrosine phosphatase activity, again, in an ROS dependent fashion. The ability of vorinostat and sorafenib to induce CD95 tyrosine phosphorylation in multiple GI tumor types was blocked by expression of TRX (Figure 5C).
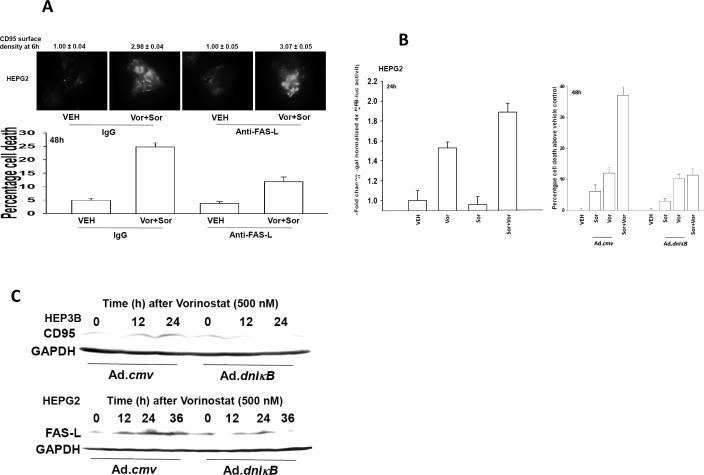
In prior studies we had noted ~6h - ~24h following vorinostat exposure that hepatoma cells increased expression of FAS ligand and CD95 in a cell type dependent fashion (29). Incubation of hepatoma cells with an anti-FAS-L neutralizing antibody did not alter the ability of sorafenib (3 μM) and vorinostat treatment to activate CD95 6h after drug exposure (Figure 6A, upper IHC). However, neutralization of FAS-L suppressed by ~50% the ability of sorafenib (3 μM) and vorinostat treatment to kill tumor cells (Figure 6A, lower graph). Vorinostat activates NFκB, an effect that is modestly enhanced by sorafenib (3 μM), and an effect that was blocked by expression of the dominant negative IκB super-repressor (Figure 6B left graph, data not shown). Expression of the dominant negative IκB super-repressor suppressed drug –induced tumor cell killing (Figure 6B right graph). In other systems it has been shown that HDACIs can, via NFκB, increase expression of death receptors and their cognate ligands (38). We have previously published that vorinostat –induced expression of FAS-L and CD95 in hepatoma cells and we now note that this effect was blocked by expression of the dominant negative IκB (Figure 6C) (29). Collectively our data argue that vorinostat contributes to sorafenib –induced activation of CD95 shortly following drug exposure by elevating ROS levels that inhibit PTPase function and promote CD95 activation whereas at later time points vorinostat promotes CD95 signaling and tumor cell death by increasing FAS-L expression in an NFκB –dependent fashion.
Figure 6. Vorinostat promotes sorafenib toxicity by increasing expression of FAS-L.
Panel A. Upper IHC: HEPG2 cells plated in 8 well chamber slides were pre-treated with control IgG or an IgG to neutralize FAS-L (1 μg/ml) and 30 min later treated with vehicle (DMSO) or sorafenib (3 μM), and vorinostat (500 nM) in combination. Cells were fixed 6h after exposure and surface levels of CD95 determined by IHC. The density of CD95 staining was determined in 40 cells (n = 2, +/- SEM). Lower Graph: HEPG2 cells were pre-treated with control IgG or an IgG to neutralize FAS-L (1 μg/ml) and 30 min later treated with vehicle (DMSO) or sorafenib (3 μM), and vorinostat (500 nM) in combination. Forty eight h later cells were isolated and viability determined by trypan blue exclusion (n = 3, +/- SEM). Panel B. Left Graph: HEPG2 cells 24h after plating in 96 well plates were transfected with NFκB-luciferase and β-galactosidase constitutive reporter constructs. Thirty six hours after transfection cells were treated with vehicle (DMSO), sorafenib (3.0 μM), vorinostat (500 nM) or both sorafenib and vorinostat. Cells were assayed for NFκB-luciferase and β-galactosidase activity 24h after treatment (± SEM, a representative from 2 separate studies). Control studies demonstrated that over-expression of dominant negative IκB blocked vorinostat-induced activation of NFκB-luciferase activity (not shown). Right Graph: HEPG2 cells 24h after plating were infected with control empty vector virus (CMV) or a recombinant virus to express dominant negative IκB S32A S36A (dn IκB). Twenty four hours after infection, cells were treated with vehicle (DMSO), sorafenib (3.0 μM), vorinostat (500 nM) or both sorafenib and vorinostat. Forty eight hours after drug exposure, cells were isolated, spun onto glass slides and stained using established methods for double stranded DNA breaks indicative of apoptosis (TUNEL) as described in the Methods (n = 2, +/- SEM). Panel C. HEPG2 and HEP3B cells 24h after plating were infected with control empty vector virus (CMV) or a recombinant virus to express dominant negative IκB S32A S36A (dn IκB). Twenty four hours after infection, cells were treated with vehicle (DMSO) or vorinostat (500 nM). Cells were isolated 12h and 24h after treatment. SDS PAGE and immunoblotting were performed to determine changes in the expression of CD95 and FAS-L, as indicated. A representative is shown of 3 separate studies.
Discussion
Sorafenib is a small molecule drug that inhibits RAF family serine / threonine kinases and class III receptor tyrosine kinases such as the PDGF and FLT receptors. Previous studies have shown that sorafenib and the histone deacetylase inhibitor vorinostat interact in vitro and in vivo in a greater than additive fashion to kill transformed cells that was mechanistically dependent on activation of CD95 (29, 30). The present studies attempted to determine in much greater detail the molecular mechanisms by which sorafenib and vorinostat, as individual agents, interacted to activate CD95 and promote drug toxicity.
Sorafenib (3-6 μM) caused a dose-dependent increase in CD95 tyrosine phosphorylation and, based on multiple criteria, in parallel activated Src family non-receptor tyrosine kinases. Expression of dominant negative Src or knock out of Src / Fyn / Yes blocked sorafenib –induced CD95 tyrosine phosphorylation. CD95 (Y232F, Y291F) was not activated by either higher sorafenib (6 μM) concentrations or by sorafenib (3 μM) and vorinostat treatment. Knock down of class III receptor tyrosine kinase PDGFRβ expression enhanced Src activity; in a Src-dependent fashion knock down of PDGFRβ enhanced CD95 tyrosine phosphorylation and also suppressed the ability of sorafenib to induce CD95 tyrosine phosphorylation. Vorinostat rapidly enhanced the ability of lower sorafenib concentrations to increase ROS levels and to transiently suppress total cellular PTPase activity within 1h that was due to ROS generation. However, ROS generation also significantly enhanced PTPase activity 3h after drug treatment and correlated with Src Y527 dephosphorylation and with vorinostat-stimulated CD95 tyrosine phosphorylation. Thus molecular one potential model for drug action is that sorafenib-mediated inhibition PDGFRβ causes a compensatory activation of Src family tyrosine kinases that in turn phosphorylate and activate CD95. And, based on the degree to which PDGFRβ is inhibited by sorafenib, CD95 becomes tyrosine phosphorylated in a Src dependent fashion. Vorinostat, by interacting with sorafenib to elevate ROS levels rapidly suppresses cellular PTPase activity that promotes greater levels of CD95 tyrosine phosphorylation and CD95 activation.
Knock down of PDGFRβ increased basal plasma membrane levels of CD95 that was Src dependent and when PDGFRβ was knocked down, the relative ability of sorafenib to cause further activation of CD95 was reduced. This finding was mirrored by changes in autophagy presented in Figure 4B wherein knock down of PDGFRβ increased autophagy in a CD95 dependent fashion and in cells lacking PDGFRβ the ability of sorafenib to cause additional autophagy was reduced. And, although we observed enhanced levels of CD95 activation after PDGFRβ knock down in Figure 4A, this did not translate into increased basal levels of cell killing in Figure 4C; possibly this was because we were observing elevated levels of protective autophagy after knock down as was shown in Figure 4B (29, 30). Thus, logically, based on data in Figures 4A and 4B, the relative reduction in sorafenib-induced cell killing in PDGFRβ knock down cells in Figure 4C is because without expression of the key target, i.e. PDGFRβ, sorafenib cannot instantaneously initiate the series of events that will lead to PDGFRβ inhibition, compensatory Src activation, CD95 tyrosine phosphorylation etc.
Based on our data, if sorafenib is promoting activation of Src family kinases, we reasoned it is probable that other targets of Src kinases are also phosphorylated in response to sorafenib treatment. For example, in a preliminary study we have noted that FAK and IGF1R tyrosine phosphorylation are enhanced in response to sorafenib exposure. Increased FAK signaling has the potential to promote metastatic spread of tumor cells via Src (39). Signaling by Src kinases is known to facilitate ERBB1 Y845 phosphorylation and we observed this in our system following sorafenib treatment. Phosphorylation of Y845 has been linked to ERBB1-induced tumor cell growth, independent of ERK1/2 and PI3K signaling (40). In at least one study, low concentrations of sorafenib have been shown to promote MEK1/2 activation via IGF1 receptor signaling, and inhibition of MEK1/2 signaling enhanced sorafenib toxicity (41). We have recently published similar data in malignant blood cancer cells (42). Thus the practical outcome of sorafenib promoting Src kinase activation is that whilst this drug acts to suppress tumor growth through Src-dependent activation of CD95, the induction of endoplasmic reticulum stress and inhibition of pro-angiogenic growth factor receptors; it also has the capacity to promote tumor cell survival and migration through elevated signaling by Src, FAK, ERBB1, IGF1R and possibly the ERK1/2 pathway. Further studies will be required to define the precise nodal enzymes within the sorafenib-induced signaling network that can promote and suppress sorafenib lethality.
We recently noted using higher concentrations of vorinostat than those used in this manuscript that the drug induced DNA damage, via ROS generation, and this played a key role in the activation of NFκB by the drug (43). Vorinostat facilitated CD95 activation in hepatoma cells by two mechanisms: increased CD95 tyrosine phosphorylation and increased expression of FAS-L. Vorinostat –induced ROS generation, and when in combination with sorafenib, inhibited PTPase activity and then profound activated PTPase activity; effects that were both ROS dependent. These effects also correlated that correlated with increased CD95 phosphorylation, increased Src Y416 phosphorylation and dephosphorylation of Src Y527. PTPases have a ~10-fold higher specific activity than the protein kinases whose actions they reverse meaning that a small reduction in PTPase activity can significantly modify a site of phosphorylation whose phosphorylation is being actively increased due to actions of a kinase (44). The precise PTPase that regulates CD95 tyrosine phosphorylation or the Src Y527 phosphorylation in hepatoma cells has not been identified although it has been shown that both CD45 and SHP1 can proximally modulate CD95 signaling in immune cell types, and that both SHP1 and Src family kinases can associate with CD95 (45, 46). This data, combined with our present findings, argues that specific inhibitors of SHP-1 could be of value in promoting sorafenib toxicity.
Using a neutralizing antibody, inhibition of FAS-L function suppressed sorafenib + vorinostat toxicity by ~50%. This correlated with vorinostat, in an NFκB –dependent fashion, promoting increased expression of FAS-L 12-24h after drug exposure. However, a multitude of studies have shown that NFκB activation can also stimulate expression of anti-apoptotic proteins and promote cell growth. Previously we have shown that the rapid (~6h) –induced activation of CD95 was due, in part, to ceramide generation and our present findings demonstrated that CD95 activation at this time point was insensitive to the neutralizing antibody. Multiple HDACIs have demonstrated to increase protein levels of FAS-L and/or CD95 (e.g. refs. 38, 47), and this has been suggested as one important mechanism for the anti-tumor effects of HDACIs.
One well-described additional action of HDACIs in tumor cells is to increase expression of the cyclin dependent kinase inhibitor p21Cip-1/WAF1/mda-6 (p21) (20, 29, 43). We noted that vorinostat, in contrast to our findings in malignant blood cancer cells, modestly increased p21 levels in hepatoma cells and that this effect was suppressed in cells treated with vorinostat and sorafenib. Increased expression of p21 not only acts to suppress tumor growth but in some cell types suppresses toxic JNK pathway signaling and also facilitates CD95 activation (36, 48). Knock down of p21 expression suppressed vorinostat and sorafenib toxicity and suppressed drug combination-induced autophagy that was associated with reduced CD95 activation. We also noted that loss of Src function blocked sorafenib (6 μM) –induced protective autophagy; autophagy that was CD95 dependent. In contrast, in malignant blood cancer cells we have previously shown that expression of p21 suppressed the lethality of vorinostat and sorafenib treatment (20). Thus vorinostat as a single agent has the potential to kill tumor cells by increasing FAS-L and p21 levels but also could act to promote tumor cell survival through NFκB activation. Thus sorafenib, in part, subverts vorinostat –induced cell killing processes by blocking increased p21 expression. Of note was our finding that sorafenib suppressed vorinostat –induced p21 expression but not the enhancement of FAS-L expression; as p21 protein levels, unlike those of FAS-L, are regulated by both protein and mRNA stabilization as well as for both proteins at the level of transcription, our data suggests that the HDACI –induced expression of p21 most probably occurs at a post-transcriptional level (48, 49).
We have previously demonstrated that sorafenib and vorinostat combination therapy are effective at killing hepatoma cells in vivo, and sorafenib and HDACI combination therapy is entering phase I evaluation in hepatoma, and also in renal carcinoma and non-small cell lung cancer, patients (29, 30). The present studies provide additional mechanistic information as to how these agents interact and, furthermore, predict that inhibitors of survival signaling receptors e.g. ERBB1 and IGF1R, which are activated in response to sorafenib exposure, will enhance the anti-tumor efficacy of this drug combination.
Supplementary Material
Acknowledgements
This work was funded; to P.D. from PHS grants (R01-DK52825, P01-CA104177, R01-CA108520); to S.G. from PHS grants (R01-CA63753; R01-CA77141; R01-CA93738) and a Leukemia Society of America grant 6405-97. These studies were also funded in part by The Jimmy V Foundation. PD is the holder of the Universal Inc. Professorship in Signal Transduction Research.
Abbreviations
- Vor.
Vorinostat
- Sor.
Sorafenib
- ERK
extracellular regulated kinase
- MEK
mitogen activated extracellular regulated kinase
- EGF
epidermal growth factor
- PARP
poly ADP ribosyl polymerase
- PI3K
phosphatidyl inositol 3 kinase
- -/-
null / gene deleted
- ERK
extracellular regulated kinase
- MAPK
mitogen activated protein kinase
- MEK
mitogen activated extracellular regulated kinase
- R
receptor
- JNK
c-Jun NH2-terminal kinase
- dn
dominant negative
- P
phospho-
- ca
constitutively active
- WT
wild type
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics. CA Cancer J Clin 2005. 2002;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Bilimoria KY, Bentrem DJ, Lillemoe KD, Talamonti MS, Ko CY. Pancreatic Cancer Quality Indicator Development Expert Panel, American College of Surgeons. Assessment of pancreatic cancer care in the United States based on formally developed quality indicators. J Natl Cancer Inst. 2009;101:848–59. doi: 10.1093/jnci/djp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dent P. MAP kinase pathways in the control of hepatocyte growth, metabolism and survival. In: Dufour JF, Clavien P-A, editors. Signaling Pathways in Liver Diseases. Springer Press; 2005. pp. 223–238. Chapter 19. [Google Scholar]
- 4.Dent P, Yacoub A, Fisher PB, Hagan MP, Grant S. MAPK pathways in radiation responses. Oncogene. 2003;22:5885–96. doi: 10.1038/sj.onc.1206701. [DOI] [PubMed] [Google Scholar]
- 5.Valerie K, Yacoub A, Hagan MP, et al. Radiation-induced cell signaling: inside-out and outside-in. Mol Cancer Ther. 2007;6:789–801. doi: 10.1158/1535-7163.MCT-06-0596. [DOI] [PubMed] [Google Scholar]
- 6.Grant S, Dent P. Kinase inhibitors and cytotoxic drug resistance. Clin Cancer Res. 2004;10:2205–7. doi: 10.1158/1078-0432.ccr-0001-4. [DOI] [PubMed] [Google Scholar]
- 7.Allan LA, Morrice N, Brady S, Magee G, Pathak S, Clarke PR. Inhibition of caspase-9 through phosphorylation at Thr 125 by ERK MAPK. Nat Cell Biol. 2003;5:647–54. doi: 10.1038/ncb1005. [DOI] [PubMed] [Google Scholar]
- 8.Mori M, Uchida M, Watanabe T, et al. Activation of extracellular signal-regulated kinases ERK1 and ERK2 induces Bcl-xL up-regulation via inhibition of caspase activities in erythropoietin signaling. J Cell Physiol. 2003;195:290–7. doi: 10.1002/jcp.10245. [DOI] [PubMed] [Google Scholar]
- 9.Ley R, Balmanno K, Hadfield K, Weston C, Cook SJ. Activation of the ERK1/2 signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein, Bim. J Biol Chem. 2003;278:18811–6. doi: 10.1074/jbc.M301010200. [DOI] [PubMed] [Google Scholar]
- 10.Wang YF, Jiang CC, Kiejda KA, Gillespie S, Zhang XD, Hersey P. Apoptosis induction in human melanoma cells by inhibition of MEK is caspase-independent and mediated by the Bcl-2 family members PUMA, Bim, and Mcl-1. Clin Cancer Res. 2007;13:4934–42. doi: 10.1158/1078-0432.CCR-07-0665. [DOI] [PubMed] [Google Scholar]
- 11.Qiao L, Han SI, Fang Y, et al. Bile acid regulation of C/EBPbeta, CREB, and c-Jun function, via the extracellular signal-regulated kinase and c-Jun NH2-terminal kinase pathways, modulates the apoptotic response of hepatocytes. Mol Cell Biol. 2003;23:3052–66. doi: 10.1128/MCB.23.9.3052-3066.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li N, Batt D, Warmuth M. B-Raf kinase inhibitors for cancer treatment. Curr Opin Investig Drugs. 2007;8:452–6. [PubMed] [Google Scholar]
- 13.Davies BR, Logie A, McKay JS, et al. AZD6244 (ARRY-142886), a potent inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase 1/2 kinases: mechanism of action in vivo, pharmacokinetic/pharmacodynamic relationship, and potential for combination in preclinical models. Mol Cancer Ther. 2007;6:2209–19. doi: 10.1158/1535-7163.MCT-07-0231. [DOI] [PubMed] [Google Scholar]
- 14.Flaherty KT. Sorafenib: delivering a targeted drug to the right targets. Expert Rev Anticancer Ther. 2007;7:617–26. doi: 10.1586/14737140.7.5.617. [DOI] [PubMed] [Google Scholar]
- 15.Rini BI. Sorafenib. Expert Opin Pharmacother. 2006;7:453–61. doi: 10.1517/14656566.7.4.453. [DOI] [PubMed] [Google Scholar]
- 16.Strumberg D. Preclinical and clinical development of the oral multikinase inhibitor sorafenib in cancer treatment. Drugs Today (Barc) 2005;41:773–84. doi: 10.1358/dot.2005.41.12.937959. [DOI] [PubMed] [Google Scholar]
- 17.Gollob JA. Sorafenib: scientific rationales for single-agent and combination therapy in clear-cell renal cell carcinoma. Clin Genitourin Cancer. 2005;4:167–74. doi: 10.3816/CGC.2005.n.028. [DOI] [PubMed] [Google Scholar]
- 18.Rahmani M, Davis EM, Bauer C, Dent P, Grant S. Apoptosis induced by the kinase inhibitor BAY 43-9006 in human leukemia cells involves down-regulation of Mcl-1 through inhibition of translation. J Biol Chem. 2005;280:35217–27. doi: 10.1074/jbc.M506551200. [DOI] [PubMed] [Google Scholar]
- 19.Rahmani M, Nguyen TK, Dent P, Grant S. The multikinase inhibitor sorafenib induces apoptosis in highly imatinib mesylate-resistant bcr/abl+ human leukemia cells in association with signal transducer and activator of transcription 5 inhibition and myeloid cell leukemia-1 down-regulation. Mol Pharmacol. 2007;72:788–95. doi: 10.1124/mol.106.033308. [DOI] [PubMed] [Google Scholar]
- 20.Dasmahapatra G, Yerram N, Dai Y, Dent P, Grant S. Synergistic interactions between vorinostat and sorafenib in chronic myelogenous leukemia cells involve Mcl-1 and p21CIP1 down-regulation. Clin Cancer Res. 2007;13:4280–90. doi: 10.1158/1078-0432.CCR-07-0835. [DOI] [PubMed] [Google Scholar]
- 21.Rahmani M, Davis EM, Crabtree TR, et al. The kinase inhibitor sorafenib induces cell death through a process involving induction of endoplasmic reticulum stress. Mol Cell Biol. 2007;27:5499–513. doi: 10.1128/MCB.01080-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gregory PD, Wagner K, Horz W. Histone acetylation and chromatin remodeling. Exp. Cell Res. 2001;265:195–202. doi: 10.1006/excr.2001.5187. [DOI] [PubMed] [Google Scholar]
- 23.Marks PA, Miller T, Richon VM. Histone deacetylases. Curr Opin Pharmacol. 2003;3:344–351. doi: 10.1016/s1471-4892(03)00084-5. [DOI] [PubMed] [Google Scholar]
- 24.Bali P, Pranpat M, Swaby R, et al. Activity of suberoylanilide hydroxamic Acid against human breast cancer cells with amplification of her-2. Clin Cancer Res. 2005;11:6382–9. doi: 10.1158/1078-0432.CCR-05-0344. [DOI] [PubMed] [Google Scholar]
- 25.Kwon SH, Ahn SH, Kim YK, et al. Apicidin, a histone deacetylase inhibitor, induces apoptosis and Fas/Fas ligand expression in human acute promyelocytic leukemia cells. J Biol Chem. 2002;277:2073–80. doi: 10.1074/jbc.M106699200. [DOI] [PubMed] [Google Scholar]
- 26.Pang RW, Poon RT. From molecular biology to targeted therapies for hepatocellular carcinoma: the future is now. Oncology. 2007;72(Suppl 1):30–44. doi: 10.1159/000111705. [DOI] [PubMed] [Google Scholar]
- 27.Venturelli S, Armeanu S, Pathil A, et al. Epigenetic combination therapy as a tumor-selective treatment approach for hepatocellular carcinoma. Cancer. 2007;109:2132–41. doi: 10.1002/cncr.22652. [DOI] [PubMed] [Google Scholar]
- 28.Wise LD, Turner KJ, Kerr JS. Assessment of developmental toxicity of vorinostat, a histone deacetylase inhibitor, in Sprague-Dawley rats and Dutch Belted rabbits. Birth Defects Res B Dev Reprod Toxicol. 2007;80:57–68. doi: 10.1002/bdrb.20104. [DOI] [PubMed] [Google Scholar]
- 29.Zhang G, Park MA, Mitchell C, Hamed H, Rahmani M, Martin AP, Curiel DT, Yacoub A, Graf M, Lee R, Roberts JD, Fisher PB, Grant S, Dent P. Vorinostat and sorafenib synergistically kill tumor cells via FLIP suppression and CD95 activation. Clin Cancer Res. 2008;14:5385–99. doi: 10.1158/1078-0432.CCR-08-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park MA, Zhang G, Martin AP, Hamed H, Mitchell C, Hylemon PB, Graf M, Rahmani M, Ryan K, Liu X, Spiegel S, Norris J, Fisher PB, Grant S, Dent P. Vorinostat and sorafenib increase ER stress, autophagy and apoptosis via ceramide-dependent CD95 and PERK activation. Cancer Biol Ther. 2008;7:1648–62. doi: 10.4161/cbt.7.10.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park MA, Zhang G, Mitchell C, Rahmani M, Hamed H, Hagan MP, Yacoub A, Curiel DT, Fisher PB, Grant S, Dent P. Mitogen-activated protein kinase kinase 1/2 inhibitors and 17-allylamino-17-demethoxygeldanamycin synergize to kill human gastrointestinal tumor cells in vitro via suppression of c-FLIP-s levels and activation of CD95. Mol Cancer Ther. 2008;7:2633–48. doi: 10.1158/1535-7163.MCT-08-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yacoub A, Park MA, Gupta P, et al. Caspase-, cathepsin- and PERK-dependent regulation of MDA-7/IL-24-induced cell killing in primary human glioma cells. Mol Cancer Ther. 2008;7:297–313. doi: 10.1158/1535-7163.MCT-07-2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitchell C, Park MA, Zhang G, Han SI, Harada H, Franklin RA, Yacoub A, Li PL, Hylemon PB, Grant S, Dent P. 17-Allylamino-17-demethoxygeldanamycin enhances the lethality of deoxycholic acid in primary rodent hepatocytes and established cell lines. Mol Cancer Ther. 2007;6:618–32. doi: 10.1158/1535-7163.MCT-06-0532. [DOI] [PubMed] [Google Scholar]
- 34.Gupta S, Natarajan R, Payne SG, Studer EJ, Spiegel S, Dent P, Hylemon PB. Deoxycholic acid activates the c-Jun N-terminal kinase pathway via FAS receptor activation in primary hepatocytes. Role of acidic sphingomyelinase-mediated ceramide generation in FAS receptor activation. J Biol Chem. 2001;279:5821–8. doi: 10.1074/jbc.M310979200. [DOI] [PubMed] [Google Scholar]
- 35.Qiao L, Studer E, Leach K, et al. Deoxycholic acid (DCA) causes ligand-independent activation of epidermal growth factor receptor (EGFR) and FAS receptor in primary hepatocytes: inhibition of EGFR/mitogen-activated protein kinase-signaling module enhances DCA-induced apoptosis. Mol Biol Cell. 2001;12:2629–45. doi: 10.1091/mbc.12.9.2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang G, Park MA, Mitchell C, Walker T, Hamed H, Studer E, Graf M, Gupta S, Hylemon PB, Fisher PB, Grant S, Dent P. Multiple cyclin kinase inhibitors promote bile acid –induced apoptosis and autophagy in primary hepatocytes via p53 – CD95 –dependent signaling. J Biol Chem. 2008;283:24343–58. doi: 10.1074/jbc.M803444200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reinehr R, Häussinger D. Hyperosmotic activation of the CD95 system. Methods Enzymol. 2007;428:145–60. doi: 10.1016/S0076-6879(07)28008-5. [DOI] [PubMed] [Google Scholar]
- 38.Emanuele S, Lauricella M, Carlisi D, Vassallo B, D'Anneo A, Di Fazio P, Vento R, Tesoriere G. SAHA induces apoptosis in hepatoma cells and synergistically interacts with the proteasome inhibitor Bortezomib. Apoptosis. 2007;12:1327–38. doi: 10.1007/s10495-007-0063-y. [DOI] [PubMed] [Google Scholar]
- 39.Huang J, Zheng DL, Qin FS, Cheng N, Chen H, Wan BB, Wang YP, Xiao HS, Han ZG. Genetic and epigenetic silencing of SCARA5 may contribute to human hepatocellular carcinoma by activating FAK signaling. J Clin Invest. 2010;120:223–41. doi: 10.1172/JCI38012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chung BM, Dimri M, George M, Reddi AL, Chen G, Band V, Band H. The role of cooperativity with Src in oncogenic transformation mediated by non-small cell lung cancer-associated EGF receptor mutants. Oncogene. 2009;28:1821–32. doi: 10.1038/onc.2009.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huynh H, Ngo VC, Koong HN, Poon D, Choo SP, Toh HC, Thng CH, Chow P, Ong HS, Chung A, Goh BC, Smith PD, Soo KC. AZD6244 enhances the anti-tumor activity of sorafenib in ectopic and orthotopic models of human hepatocellular carcinoma (HCC). J Hepatol. 2009 Oct 28; doi: 10.1016/j.jhep.2009.10.008. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen TK, Jordan N, Friedberg J, Fisher R, Dent P, Grant S. Inhibition of MEK/ERK1/2 sensitizes lymphoma cells to sorafenib –induced apoptosis. Leukemia Research. 2010 doi: 10.1016/j.leukres.2009.07.013. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosato RR, Almenara JA, Maggio SC, Coe S, Atadja P, Dent P, Grant S. Role of histone deacetylase inhibitor-induced reactive oxygen species and DNA damage in LAQ-824/fludarabine antileukemic interactions. Mol Cancer Ther. 2008;7:3285–97. doi: 10.1158/1535-7163.MCT-08-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tonks NK. Redox redux: revisiting PTPs and the control of cell signaling. Cell. 2005;121:667–70. doi: 10.1016/j.cell.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 45.Gupta VA, Hermiston ML, Cassafer G, Daikh DI, Weiss A. B cells drive lymphocyte activation and expansion in mice with the CD45 wedge mutation and Fas deficiency. J Exp Med. 2008;205:2755–61. doi: 10.1084/jem.20081204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Daigle I, Yousefi S, Colonna M, Green DR, Simon HU. Death receptors bind SHP-1 and block cytokine-induced anti-apoptotic signaling in neutrophils. Nat Med. 2002;8:61–7. doi: 10.1038/nm0102-61. [DOI] [PubMed] [Google Scholar]
- 47.Gillenwater AM, Zhong M, Lotan R. Histone deacetylase inhibitor suberoylanilide hydroxamic acid induces apoptosis through both mitochondrial and Fas (Cd95) signaling in head and neck squamous carcinoma cells. Mol Cancer Ther. 2007;6:2967–75. doi: 10.1158/1535-7163.MCT-04-0344. [DOI] [PubMed] [Google Scholar]
- 48.Kim GY, Mercer SE, Ewton DZ, Yan Z, Jin K, Friedman E. The stress-activated protein kinases p38 alpha and JNK1 stabilize p21(Cip1) by phosphorylation. J Biol Chem. 2002;277:29792–802. doi: 10.1074/jbc.M201299200. [DOI] [PubMed] [Google Scholar]
- 49.Park JS, Qiao L, Gilfor D, Yang MY, Hylemon PB, Benz C, Darlington G, Firestone G, Fisher PB, Dent P. A role for both Ets and C/EBP transcription factors and mRNA stabilization in the MAPK-dependent increase in p21 (Cip-1/WAF1/mda6) protein levels in primary hepatocytes. Mol Biol Cell. 2000;11:2915–32. doi: 10.1091/mbc.11.9.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.