Abstract
BACKGROUND
Atrial-, brain- and C-type natriuretic peptides (ANP, BNP, and CNP) are important in regulating a variety of cardiovascular and cellular functions. In cells, these peptides are made as pro-forms that are converted to mature forms. BNP and its related peptides are biomarkers for the diagnosis of heart failure. In this study, we examined glycosylation in pro-ANP, pro-BNP and pro-CNP, which may alter their biochemical and metabolic properties.
METHODS
Human pro-ANP, pro-BNP, and pro-CNP were expressed in human embryonic kidney (HEK) 293 cells and murine HL-1 cardiomyocytes, and analyzed by immunoprecipitation and Western blotting. Deglycosylation enzymes were used to determine the carbohydrate content on these peptides. The effects of inhibiting O-glycosylation on cellular expression and stability of the peptides also were examined.
RESULTS
In HEK 293 and HL-1 cells, pro-BNP, but not pro-ANP and pro-CNP, from the culture medium had a greater molecular mass than that from cell lysate. Digestion with PNGase F, O-glycosidase and sialidase A indicated that pro-BNP contained O-glycans but not N-glycans. The O-glycans on pro-BNP had sialic acids at their termini, protecting it from O-glycosidase digestion. In contrast, pro-ANP and pro-CNP contained no detectable amounts of N- or O-glycans. Inhibition of O-glycosylation on pro-BNP did not prevent its expression in the cells. However, partially O-glycosylated pro-BNP was much less stable than fully O-glycosylated pro-BNP.
CONCLUSIONS
O-glycosylation is not necessary for pro-BNP expression but important for its stability.
The natriuretic peptide family has three structurally related members: atrial natriuretic peptide (ANP) (also called atrial natriuretic factor, ANF), brain- or B-type natriuretic peptide (BNP), and C-type natriuretic peptide (CNP) (1). ANP and BNP are made primarily in cardiomyocytes whereas CNP is produced in many tissue and cell types, such as the brain, kidney, vascular endothelial cells, and chondrocytes. The function of ANP and BNP is to regulate blood pressure and maintain salt-water balance by promoting natriuresis, diuresis, and vasodilation (1). In contrast, the function of CNP is involved mainly in endothelial cell, smooth muscle cell, and chondrocyte growth and differentiation (2, 3). In patients with heart failure (HF), ANP and BNP production and secretion are increased. The up-regulation of the natriuretic peptides serves as a compensatory mechanism to lower blood volume and pressure, thereby improving cardiac function. BNP and its related peptides such as N-terminal (NT)-pro-BNP also are used as biomarkers for HF (4–6). Immunoassays for BNP and NT-pro-BNP are used to identify acute HF in patients with dyspnea in hospital emergency rooms (7–10).
Like many peptide hormones, natriuretic peptides are made as prepropeptides. After the signal peptide is removed, the propeptides undergo additional proteolytic cleavage to become mature peptides (11). Human pro-ANP (126 amino acids), pro-BNP (108 amino acids), and pro-CNP (103 amino acids) have calculated masses of ~14, ~12 and ~11 kDa, respectively. The calculated masses for human mature ANP (28 amino acids), BNP (32 amino acids), and CNP (53 amino acids) are ~3.4, ~3.5 and ~5.8 kDa, respectively. In biochemical analysis, plasma-derived natriuretic peptides and/or fragments may have an apparent molecular mass greater than the calculated one (12, 13). In HPLC profiles, for example, pro-BNP from human plasma was reported to have an apparent mass of 36 kDa (14). The reason for the apparent discrepancy is not clear and may be, at least in part, due to posttranslational modifications. O-glycosylation has been identified in human pro-BNP (15–17). It is not known if similar posttranslational modifications exist in pro-ANP and pro-CNP. In this study, we examined glycosylation in human pro-ANP, pro-BNP and pro-CNP. Our data show that pro-BNP, but not pro-ANP or pro-CNP, contains sialylated O-glycans that are important in maintaining pro-BNP stability.
Materials and Methods
CELL CULTURE
Human embryonic kidney (HEK) 293 cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) with 10% fetal bovine serum (FBS). The murine atrial cardiomyocyte cell line HL-1 (18), a generous gift from Dr. William C. Claycomb (Louisiana State University Medical Center, New Orleans, LA), was cultured in Claycomb medium (Sigma) with 10% FBS, 100 µM norepinephrine, and 4 mM L-glutamine in gelatin/fibronectin-coated plates. All cells were cultured at 37°C in humidified incubators with 5% CO2 and 95% air.
TRANSFECTION, IMMUNOPRECIPITATION AND WESTERN BLOTTING
Plasmids expressing human pro-ANP (pcDNAproANP), pro-BNP (pcDNAproBNP), and pro-CNP (pcDNAproCNP) were reported previously (19–22). Recombinant pro-ANP, pro-BNP, and pro-CNP encoded by these expression plasmids contained a viral V5 tag at their C-termini, which facilitated detection of the proteins by an anti-V5 antibody (Invitrogen). The plasmids were used to transfect HEK 293 and HL-1 cells using FuGENE reagent (Roche Diagnostics) or Lipofectamine 2000 (Invitrogen) according to manufacturers’ instructions. Conditioned medium from the transfected cells was collected and recombinant proteins were immunoprecipitated by an anti-V5 antibody. The cells were washed with phosphate buffer solution and lysed in a buffer containing 50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1% Nonidet P-40 (v/v), and a protease inhibitor cocktail (1:100 dilution, Sigma). Proteins were analyzed by Western blotting using a horseradish peroxidase (HRP)-conjugated anti-V5 antibody (Invitrogen), as described previously (23).
GLYCOSIDASE DIGESTION
To analyze the carbohydrate contents in human pro-ANP, pro-BNP, and pro-CNP, glycosidase digestion experiments were carried out using deglycosylation enzymes including PNGase F from Chryseobacterium meningosepticum, recombinant α-2(3,6,8,9) neuraminidase (also called sialidase A) from Arthrobacter ureafaciens, and recombinant O-glycosidase from Streptococcus pneumonia (Prozyme, San Leandro, CA). Briefly, the conditioned medium containing pro-ANP, pro-BNP, and pro-CNP and their derivatives was collected from cultured HEK 293 and HL-1 cells and concentrated with an ultracentrifugal filtration device (Millipore). Proteins in the concentrated conditioned medium were digested with PNGase F, O-glycosidase, and sialidase A, either individually or in combination at 37°C for 3 hrs according to manufacture’s instructions. Protein samples were analyzed by Western blotting using an anti-V5-HRP antibody.
EFFECTS OF BENZYL 2-ACETAMIDO-2-DEOXY-α-D-GALACTOPYRANOSIDE (BEN-GAL)
To inhibit O-glycosylation in cells, we used Ben-gal (24), which inhibits UDP-GlcNAc:GalNAc-β1,3-N-acetylglucosaminyl-transferase activity in cultured cells. HEK 293 and HL-1 cells expressing pro-ANP, pro-BNP, and pro-CNP were grown in 6-well plates. Ben-gal (Sigma) or vehicle control (DMSO) at different concentrations was added to the cells in separate wells and incubated for 24 h. The conditioned medium was collected and pro-ANP, pro-BNP, and pro-CNP and their derivatives were analyzed by immunoprecipitation and Western blotting.
ROLE OF O-GLYCANS IN PRO-BNP STABILITY
Ben-gal or vehicle control was added to cultured HEK 293 cells (660 µM) or HL-1 cells (4 mM) expressing recombinant human pro-BNP and incubated for overnight. The Ben-gal concentrations used for HEK 293 and HL-1 cells were based on their responses to Ben-gal inhibition and toxicity, as determined in pilot studies. The conditioned medium was collected and divided into series of aliquots, which were incubated at 37°C for various lengths of time. Pro-BNP and its derivatives were analyzed by immunoprecipitaion and Western blotting. The bands on Western blots representing pro-BNP and BNP in each group were quantified using the Quantity One 1-D analysis software (Bio-Rad). The data were used to plot protein concentration curves to determine protein stability.
STATISTICAL ANALYSIS
All data are presented as means ± S.D. Student’s t-test was used for statistical analysis. A p value of <0.05 was considered to be statistically significant.
Results
ANALYSIS OF RECOMBINANT HUMAN PRO-ANP, PRO-BNP AND PRO-CNP IN HEK 293 AND HL-1 CELLS
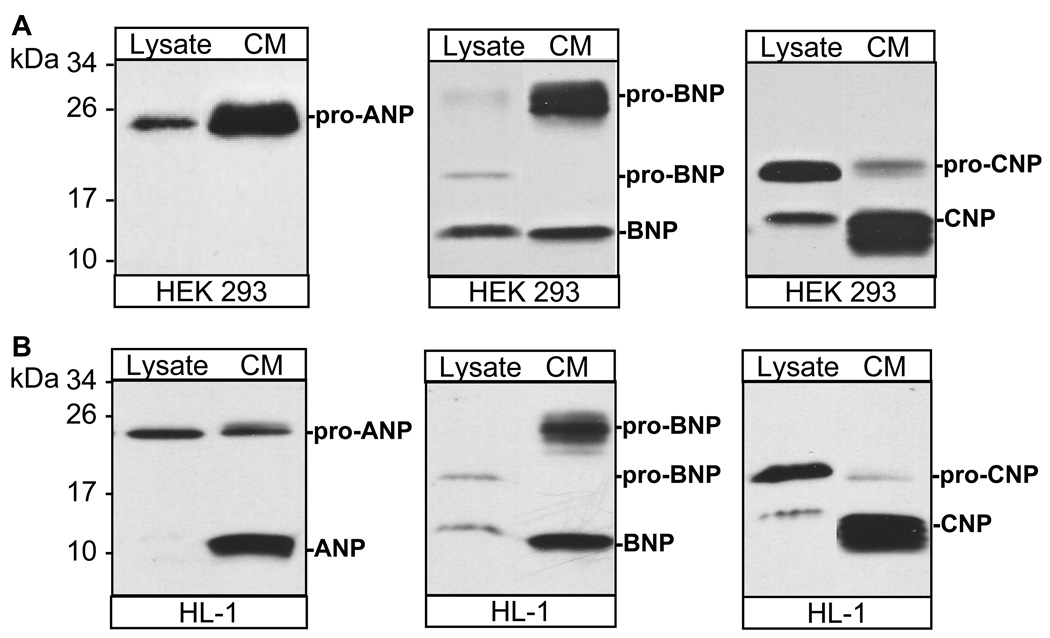
We expressed human pro-ANP, pro-BNP, and pro-CNP in HEK 293 cells and HL-1 cardiomyocytes, and analyzed the recombinant proteins in cell lysate and conditioned medium. In HEK 293 cells, pro-ANP containing a C-terminal V5 tag was expressed as a band of ~25 kDa, which was present in both cell lysate and the conditioned medium (Fig. 1A, left panel). In HL-1 cells (Fig 1B, left panel), a similar pro-ANP band was present in both cell lysate and the conditioned medium. In addition, an ~11-kDa ANP band was detected in the conditioned medium from HL-1 cells, indicating that pro-ANP was processed by corin in HL-1 cardiomyocytes but not in HEK 293 cells that did not express corin. In both HEK 293 and HL-1 cells, pro-CNP was expressed as an ~21-kDa band in cell lysate (Fig. 1A and B, right panels). Another smaller band of ~14 kDa, representing mature CNP, also was present in the cell lysate. In the conditioned medium, only a small fraction of pro-CNP was present but the majority of the detected protein was CNP (Fig. 1A and B, right panels), indicating that pro-CNP was processed intracellularly and that most pro-CNP derivatives secreted in the medium was mature CNP. In HEK 293 and HL-1 cells we detected an ~20-kDa band, representing pro-BNP, and an ~12-kDa band, representing mature BNP (Fig. 1A and B, middle panels). Pro-BNP in the conditioned medium from both HEK 293 and HL-1 cells, however, had a higher molecular mass (~26 kDa) than that in cell lysate (Fig. 1A and B, middle panels), indicating that, unlike pro-ANP and pro-CNP, pro-BNP underwent significant posttranslational modifications when it was secreted from the cells.
Fig. 1. Expression of human pro-ANP, pro-BNP and pro-CNP.
Transfection experiments were performed in HEK 293 (A) or HL-1 (B) cells. Recombinant pro-ANP, pro-BNP, pro-CNP and their derivatives in cell lysate (Lysate) or conditioned medium (CM) were analyzed by immunoprecipitation and Western blotting. Protein bands representing pro-ANP, pro-BNP, pro-CNP and their derivatives are indicated. Pictures were from representative experiments that were repeated at least five times.
ANALYSIS OF GLYCOSYLATION IN PRO-ANP, PRO-BNP AND PRO-CNP
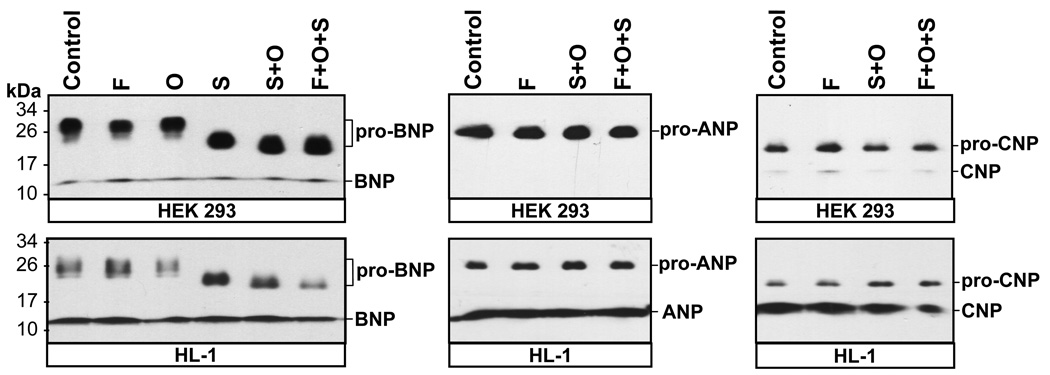
We examined glycosylation in pro-ANP, pro-BNP and pro-CNP. The recombinant proteins in the conditioned medium were treated with PNGase F, O-glycosidase and sialidase A, which cleaved N-linked glycans, O-linked glycans, and terminal sialic acids, respectively. As shown in Fig. 2 (left panels), treatment with PNGase F or O-glycosidase alone did not cause significant changes in pro-BNP mobility on the gel. When pro-BNP was treated with sialidase A alone or together with O-glycosidase, its apparent molecular mass was reduced to ~22 kDa and ~20 kDa, respectively (Fig. 2, left panels). The results were similar for pro-BNP produced in either HEK 293 or HL-1 cells. When PNGase F was used in combination with sialidase A and O-glycosidase, it did not cause further reduction in pro-BNP molecular mass (Fig. 2, left panels). These results indicated that human pro-BNP contained O-linked glycans with sialic acids at their termini, which blocked the action of O-glycosidase. The data also showed that pro-BNP did not contain any detectable N-glycans under our experimental conditions. In contrast to pro-BNP, pro-ANP and pro-CNP did not appear to contain significant amounts of N-glycans or sialylated O-glycans. Treatment with PNGase F, sialidase A, O-glycosidase, alone or in combination, did not cause noticeable changes in pro-ANP and pro-CNP mobility on SDS-PAGE gels (Fig. 2, middle and right panels).
Fig. 2. Glycosidase digestion of human pro-ANP, pro-BNP and pro-CNP.
Recombinant human pro-ANP, pro-BNP and pro-CNP in the conditioned medium from transfected HEK 293 (top) and HL-1 (lower) cells were digested with PNGase F (F), O-glycosidase (O) and sialidase A (S) either individually or in combination. Glycosidase-digested proteins were analyzed by Western blotting. Protein bands representing pro-ANP, pro-BNP, pro-CNP and their derivatives are indicated. Pictures were from representative experiments that were repeated at least four times.
EFFECT OF O-GLYCOSYLATION INHIBITOR ON PRO-BNP EXPRESSION AND SECRETION
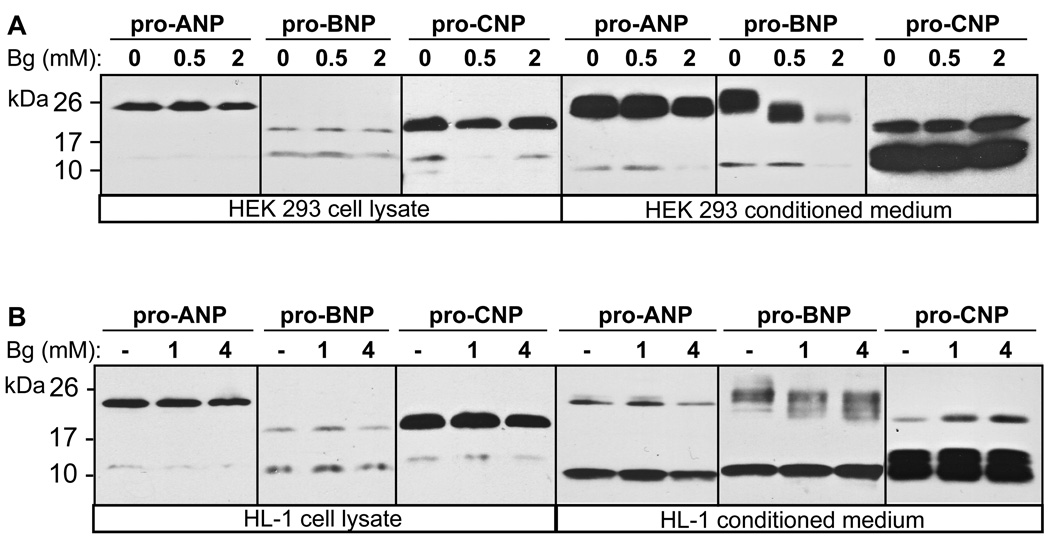
To confirm the observed O-glycosylation on pro-BNP and to examine its potential role in pro-BNP biosynthesis, we tested the effect of an O-glycosylation inhibitor, Ben-gal, on pro-ANP, pro-BNP, and pro-CNP expression and secretion. HEK 293 and HL-1 cells expressing these peptides were incubated with increasing concentrations of Ben-gal. Pro-ANP, pro-BNP, pro-CNP and their derivatives in the conditioned medium and cell lysate were analyzed by immunoprecipitation and Western blotting. As shown in Fig. 3A and B (three left panels), pro-ANP, pro-BNP and pro-CNP expression in HEK 293 and HL-1 cells was not markedly altered in the presence of Ben-gal up to 2–4 mM, concentrations that were sufficient for inhibiting O-glycosylation in living cells. In the conditioned medium from both HEK 293 and HL-1 cells, the mobility of pro-ANP and pro-CNP did not change with or without Ben-gal (Fig. 3A and B, three right panels). In contrast, the molecular mass of pro-BNP was progressively reduced with increasing concentrations of Ben-gal, indicating that Ben-gal inhibited pro-BNP O-glycosylation in these cells (Fig. 3A and B, three right panels). In these experiments, HL-1 cells were less sensitive to Ben-gal inhibition than HEK 293 cells, suggesting that HL-1 cells may contain more UDP-GlcNAc:GalNAc-β1,3-N-acetylglucosaminyl transferase activity. In addition to the molecular mass changes, pro-BNP concentrations also were reduced with increasing concentrations of Ben-gal (Fig. 3A and B, second panels from right). Because pro-BNP concentrations in cell lysate were similar with or without Ben-gal (Fig. 3A and B, second panels from left), it was unlikely that the inhibitor prevented pro-BNP secretion from the cell.
Fig. 3. Effect of O-glycosylation inhibitor on pro-ANP, pro-BNP and pro-CNP expression.
HEK 293 (A) or HL-1 (B) cells expressing pro-ANP, pro-BNP and pro-CNP were treated with increasing concentrations of Ben-gal (Bg), an O-glycosylation inhibitor. Pro-ANP, pro-BNP, pro-CNP and their derivatives in cell lysate and conditioned medium were analyzed by Western blotting. Pictures were from representative experiments that were repeated at least four times.
EFFECT OF O-GLYCOSYLATION INHIBITION ON PRO-BNP AND BNP STABILITY
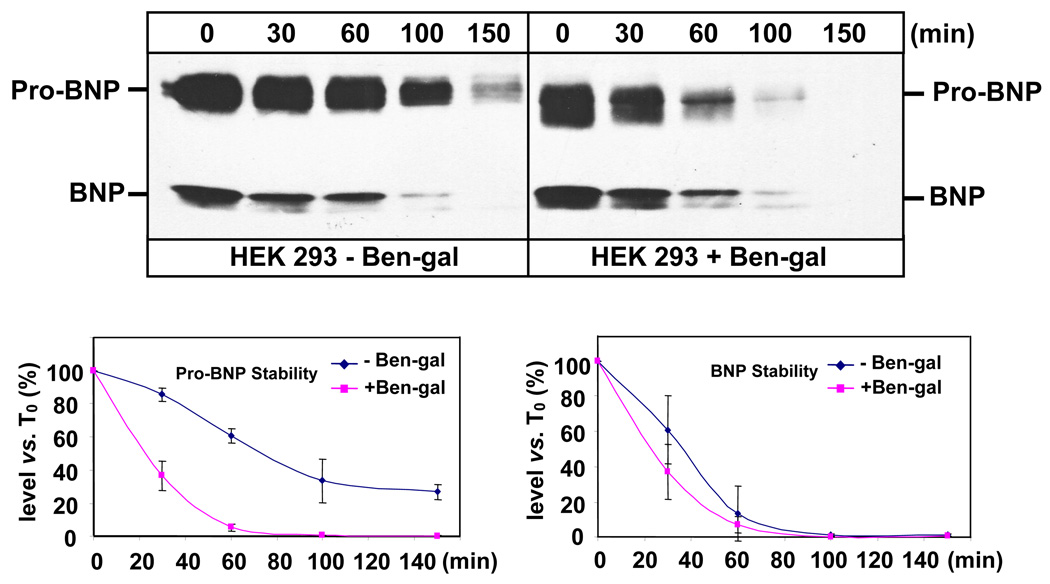
To examine the effect of O-glycosylation on pro-BNP stability, HEK 293 and HL-1 cells were treated with or without Ben-gal. The conditioned medium was collected and incubated at 37°C over time. Pro-BNP and BNP were analyzed by immunoprecipitation and Western blotting. As shown in Fig. 4 (top panels), O-glycosylation in pro-BNP from HEK 293 cells was partially inhibited in the presence of 660 µM of Ben-gal, as indicated by a smaller molecular mass of pro-BNP. Partial inhibition of O-glycosylation allowed us to obtain enough pro-BNP from the conditioned medium for pro-BNP stability experiments. The partially O-glycosylated pro-BNP (Fig. 4, top right panel) appeared less stable compared to the fully O-glycosylated pro-BNP (Fig. 4, top left panel). We quantified pro-BNP and BNP concentrations using densitometry and determined their half-lives in the medium. The results showed that pro-BNP from HEK 293 cells treated with Ben-gal had a much shorter half-life than that from the control cells (23.3 ± 3.6 vs. 79.2 ± 14.4 min, p<0.003) (Fig. 4. lower left panel). In contrast, BNP molecules from HEK 293 cells with or without Ben-gal treatment had similar stability. Quantitative analysis showed that BNP half-lives from the cells with and without Ben-gal treatment were 24.2 ± 6.9 and 35.9 ± 10.5 min, respectively, (p=0.18) (Fig. 4. lower right panel).
Fig. 4. Effect of O-glycosylation inhibition on pro-BNP and BNP stability in HEK 293 cells.
HEK 293 cells expressing pro-BNP were cultured with or without Ben-gal (660 µM). The conditioned medium was collected and incubated at 37°C over time. Pro-BNP and BNP in the conditioned medium were analyzed by immunoprecipitation and Western blotting (top panels). Protein bands on Western blots were quantified by densitometry. Pro-BNP (lower left) and BNP (lower right) concentrations (conc.) at various time points compared with that at time 0 (T0) were plotted to determine protein stability. The data were derived from three independent experiments. Vertical bars represent SD values of each data point.
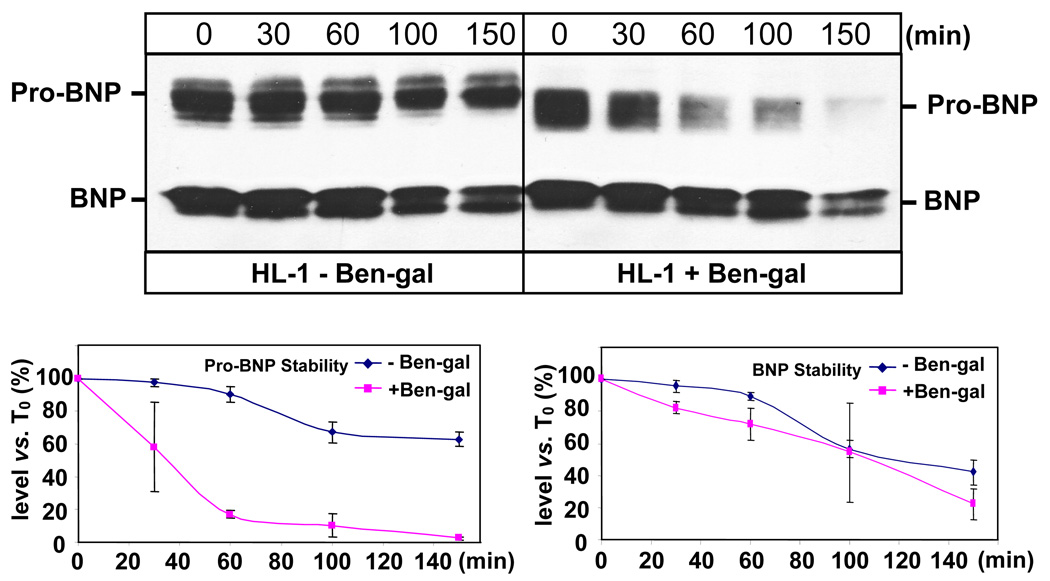
We also examined the effect of O-glycosylation on pro-BNP stability in HL-1 cardiomyocytes. As indicated in Fig. 3, HL-1 cells appeared to be more resistant to Ben-gal inhibition. We used a higher Ben-gal concentration (4 mM) to achieve partial inhibition of O-glycosylation. Similar to the results from HEK 293 cells, pro-BNP from HL-1 cells treated with Ben-gal has a shorter half-life than that from the control cells (32 ± 12 vs. >150 min, p<0.0001) (Fig. 5, top panels and lower left panel). In contrast, BNP molecules in the conditioned medium from HL-1 cells with or without Ben-gal treatment had similar half-lives (108 ± 32 vs. 128 ± 24 min, p=0.57) (Fig. 5, top panels and lower right panel).
Fig. 5. Effect of O-glycosylation inhibition on pro-BNP and BNP stability in HL-1 cells.
HL-1 cells expressing pro-BNP were cultured with or without Ben-gal (4 mM). The conditioned medium was collected and incubated at 37°C over time. Pro-BNP and BNP in the conditioned medium were analyzed by immunoprecipitation and Western blotting (top panels). Protein bands on Western blots were quantified by densitometry. Pro-BNP (lower left) and BNP (lower right) concentrations (conc.) at various time points compared with that at time 0 (T0) were plotted to determine protein stability. The data were derived from three independent experiments. Vertical bars represent SD values of each data point.
Discussion
Posttranslational modifications play an important role in protein biosynthesis, stability and biological activity. In this study, we examined glycosylation in pro-ANP, pro-BNP and pro-CNP in HEK 293 cells and HL-1 cardiomyocytes. Our results indicated that human pro-ANP and pro-CNP did not contain any detectable amounts of either N- or O-glycans under our experimental conditions. In contrast, human pro-BNP contained substantial amounts of O-glycans but no detectable amounts of N-glycans, consistent with previous reports of O-glycans present in native human pro-BNP (15–17, 25). We extended these findings and showed that O-glycans on pro-BNP were terminally sialylated and that the presence of sialic acids protected O-glycans from O-glycosidase digestion.
Glycosylation is one of the most common and diverse protein posttranslational modifications (26–28). In a genome-wide analysis, approximately two thirds of predicted proteins are potential glycoproteins (29). Carbohydrate moieties in proteins are known to be important for normal cellular function, enzyme activation and protein-protein interactions (26–28, 30). Deficiencies in protein glycosylation have been reported in a variety of human diseases (26–28). The consensus sequence for N-linked glycosylation is Asn-X-Ser or Asn-X-Thr, where X can be any amino acid but Pro (31). In contrast, correctly predicting O-glycosylation sites in protein sequences is more challenging. Several web-based programs exist but results from these programs may vary considerably. Schellenberger et al. demonstrated by mass spectrometry that human recombinant pro-BNP expressed in Chinese hamster ovary cells contains O-glycans at a number of residues including Thr36, Ser37, Ser44, Thr48, Ser53, Thr58 and Thr71 (16). Similar analysis of glycans on human native pro-BNP by mass spectrometry has not yet been done, mostly due to technical challenges. Pro-BNP residue Thr71 is close to the activation cleavage site Arg76. O-glycans on this residue has been shown to inhibit furin-mediated activation cleavage (17). To date, however, the biological significance of O-glycans on the other residues, which are away from the activation site, remains unclear. A possible role of glycosylation in pro-BNP to prevent its oligomerization has been proposed (15, 25).
In our study, O-glycans were found to be important in pro-BNP stability. In cultured medium from HEK 293 cells, inhibition of O-glycosylation reduced pro-BNP stability, as indicated by a shorter half-life. Pro-BNP is made primarily in cardiomyocytes (1). We confirmed our findings in HL-1 cells, which retain all structural and functional characteristics of cardiomyocytes (18, 32). In contrast, the half-life of BNP, which does not contain O-glycans (12, 16), was not altered in HEK 293 or HL-1 cell conditioned medium treated with the O-glycosylation inhibitor, Ben-gal. Our data did not distinguish the specific role of O-glycans on each individual residue in pro-BNP stability. Most likely, however, O-glycans on most of, if not all, the identified residues contributed to this effect. Interestingly, pro-BNP and BNP half-lives were shorter in the conditioned medium from HEK 293 cells than that from HL-1 cells, suggesting the presence of higher amounts of pro-BNP/BNP degrading enzyme(s) in HEK 293 cells. Indeed, substantial activities of neutral endopeptidase, which degrades pro-BNP/BNP, were reported in HEK 293 cells (33). Previous studied have shown that both furin and corin processed human pro-BNP (17, 22, 34, 35). Consistent with this, we showed that pro-BNP in HEK 293 cells, which contain furin but not corin, was partially processed. In HL-1 cardiomyocytes, which contain both furin and corin, the ratio of BNP/pro-BNP in the medium was much greater than that in HEK 293 cell medium (Figs. 1–5). The results indicated that in the presence of both corin and furin, pro-BNP was processed more efficiently. As discussed above, O-glycosylation at Thr71 has been shown to inhibit furin-mediated pro-BNP processing (17). Using the general O-glycosylation inhibitor Ben-gal, our current experiments did not allow assessment of the effect of O-glycosylation at Thr71 on furin- vs. corin-mediated pro-BNP processing.
ANP, BNP, and CNP are members of the same family based on their sequence similarities (1, 11, 36). These peptides, however, differ markedly in their biochemical properties. Here we showed that pro-ANP and pro-CNP did not contain detectable amounts of O-glycans. Apparently, O-glycosylation is a unique posttranslational modification for pro-BNP in this family. Our studies were mainly based on glycosidase digestion followed with SDS-PAGE and Western analysis, a classical approach to analyze protein glycosylation. More sensitive methods such as mass spectrometry will help to verify our results. In addition, we used recombinant pro-ANP, pro-BNP and pro-CNP with a C-terminal tag in our studies. This study design allowed us to examine all three peptides with the same antibody under similar experimental conditions. In principle, the presence of such a C-terminal tag may alter protein synthesis and posttranslational modifications. Like native human plasma pro-BNP, however, recombinant pro-BNP with the C-terminal tag from both HEK 293 and HL-1 cells contained O-glycans. It is unlikely, therefore, that the lack of O-glycans in pro-ANP and pro-CNP in our experiments was due to the presence of the C-terminal tag or due to protein overexpression in HEK 293 and HL-1 cells. Further studies with native pro-ANP and pro-CNP will be important to confirm our findings.
Currently, BNP and NT-pro-BNP are biomarkers frequently used in HF diagnosis (4, 6). The use of these biomarkers has greatly improved the management of HF, a serious disease with major health and socioeconomic consequences. Efforts are ongoing to characterize pro-BNP, BNP, and related peptides present in normal and patient blood samples (12, 37–40). The importance of glycosylation in antibody cross-reactivity in commercial BNP and NT-pro-BNP assays has been recognized (40). Our finding of a potential role of sialylated O-glycans in pro-BNP stability should help to better understand the biochemistry and metabolism of pro-BNP and its derivatives and the value of these peptides in the clinical diagnosis of HF.
Acknowledgments
We thank Drs. Shenghan Chen and Xiaofei Qi for helpful discussions. This work was supported in part by grants from the Ralph Wilson Medical Research Foundation, Bakken Heart-Brain Institute, and the NIH (R01HL089298, R01HL089298-S1 to Q.W.).
Abbreviations used
- ANP
atrial natriuretic peptide
- BNP
brain or B-type natriuretic peptide
- CNP
C-type natriuretic peptide
- HEK
human embryonic kidney
- HF
heart failure
- NT
N-terminal
- DMEM
Dulbecco’s Modified Eagle’s Medium
- FBS
fetal bovine serum
- HRP
horseradish peroxidase
- Ben-gal
Benzyl 2-acetamido-2-deoxy-α-D-galactopyranoside
References
- 1.Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med. 1998;339:321–328. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- 2.Chen HH, Burnett JC., Jr C-type natriuretic peptide: the endothelial component of the natriuretic peptide system. J Cardiovasc Pharmacol. 1998;32:S22–S28. [PubMed] [Google Scholar]
- 3.Chusho H, Tamura N, Ogawa Y, Yasoda A, Suda M, Miyazawa T, et al. Dwarfism and early death in mice lacking C-type natriuretic peptide. Proc Natl Acad Sci U S A. 2001;98:4016–4021. doi: 10.1073/pnas.071389098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braunwald E. Biomarkers in heart failure. N Engl J Med. 2008;358:2148–2159. doi: 10.1056/NEJMra0800239. [DOI] [PubMed] [Google Scholar]
- 5.Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol. 2007;50:2357–2368. doi: 10.1016/j.jacc.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 6.Vuolteenaho O, Ala-Kopsala M, Ruskoaho H. BNP as a biomarker in heart disease. Adv Clin Chem. 2005;40:1–36. [PubMed] [Google Scholar]
- 7.Januzzi JL, Jr, Camargo CA, Anwaruddin S, Baggish AL, Chen AA, Krauser DG, et al. The N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Am J Cardiol. 2005;95:948–954. doi: 10.1016/j.amjcard.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 8.Lainchbury JG, Campbell E, Frampton CM, Yandle TG, Nicholls MG, Richards AM. Brain natriuretic peptide and n-terminal brain natriuretic peptide in the diagnosis of heart failure in patients with acute shortness of breath. J Am Coll Cardiol. 2003;42:728–735. doi: 10.1016/s0735-1097(03)00787-3. [DOI] [PubMed] [Google Scholar]
- 9.Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347:161–167. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- 10.Wright SP, Doughty RN, Pearl A, Gamble GD, Whalley GA, Walsh HJ, et al. Plasma amino-terminal pro-brain natriuretic peptide and accuracy of heart-failure diagnosis in primary care: a randomized, controlled trial. J Am Coll Cardiol. 2003;42:1793–1800. doi: 10.1016/j.jacc.2003.05.011. [DOI] [PubMed] [Google Scholar]
- 11.Wu Q, Xu-Cai YO, Chen S, Wang W. Corin: new insights into the natriuretic peptide system. Kidney Int. 2009;75:142–146. doi: 10.1038/ki.2008.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammerer-Lercher A, Halfinger B, Sarg B, Mair J, Puschendorf B, Griesmacher A, et al. Analysis of circulating forms of proBNP and NT-proBNP in patients with severe heart failure. Clin Chem. 2008;54:858–865. doi: 10.1373/clinchem.2007.090266. [DOI] [PubMed] [Google Scholar]
- 13.Mair J. Biochemistry of B-type natriuretic peptide--where are we now? Clin Chem Lab Med. 2008;46:1507–1514. doi: 10.1515/CCLM.2008.295. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu H, Masuta K, Aono K, Asada H, Sasakura K, Tamaki M, et al. Molecular forms of human brain natriuretic peptide in plasma. Clin Chim Acta. 2002;316:129–135. doi: 10.1016/s0009-8981(01)00745-8. [DOI] [PubMed] [Google Scholar]
- 15.Mair J. Clinical significance of pro-B-type natriuretic peptide glycosylation and processing. Clin Chem. 2009;55:394–397. doi: 10.1373/clinchem.2008.119271. [DOI] [PubMed] [Google Scholar]
- 16.Schellenberger U, O'Rear J, Guzzetta A, Jue RA, Protter AA, Pollitt NS. The precursor to B-type natriuretic peptide is an O-linked glycoprotein. Arch Biochem Biophys. 2006;451:160–166. doi: 10.1016/j.abb.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 17.Semenov AG, Postnikov AB, Tamm NN, Seferian KR, Karpova NS, Bloshchitsyna MN, et al. Processing of pro-brain natriuretic peptide is suppressed by O-glycosylation in the region close to the cleavage site. Clin Chem. 2009;55:489–498. doi: 10.1373/clinchem.2008.113373. [DOI] [PubMed] [Google Scholar]
- 18.Claycomb WC, Lanson NA, Jr, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, Izzo NJ., Jr HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci U S A. 1998;95:2979–2984. doi: 10.1073/pnas.95.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knappe S, Wu F, Madlansacay MR, Wu Q. Identification of domain structures in the propeptide of corin essential for the processing of proatrial natriuretic peptide. J Biol Chem. 2004;279:34464–34471. doi: 10.1074/jbc.M405041200. [DOI] [PubMed] [Google Scholar]
- 20.Knappe S, Wu F, Masikat MR, Morser J, Wu Q. Functional analysis of the transmembrane domain and activation cleavage of human corin. J Biol Chem. 2003;278:52363–52370. doi: 10.1074/jbc.M309991200. [DOI] [PubMed] [Google Scholar]
- 21.Wu C, Wu F, Pan J, Morser J, Wu Q. Furin-mediated processing of Pro-C-type natriuretic peptide. J Biol Chem. 2003;278:25847–25852. doi: 10.1074/jbc.M301223200. [DOI] [PubMed] [Google Scholar]
- 22.Yan W, Wu F, Morser J, Wu Q. Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme. Proc Natl Acad Sci USA. 2000;97:8525–8529. doi: 10.1073/pnas.150149097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang W, Liao X, Fukuda K, Knappe S, Wu F, Dries DL, et al. Corin variant associated with hypertension and cardiac hypertrophy exhibits impaired zymogen activation and natriuretic peptide processing activity. Circ Res. 2008;103:502–508. doi: 10.1161/CIRCRESAHA.108.177352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuan SF, Byrd JC, Basbaum C, Kim YS. Inhibition of mucin glycosylation by aryl-N-acetyl-alpha-galactosaminides in human colon cancer cells. J Biol Chem. 1989;264:19271–19277. [PubMed] [Google Scholar]
- 25.Crimmins DL, Kao JL. A glycosylated form of the human cardiac hormone pro B-type natriuretic peptide is an intrinsically unstructured monomeric protein. Arch Biochem Biophys. 2008;475:36–41. doi: 10.1016/j.abb.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eklund EA, Freeze HH. Essentials of glycosylation. Semin Pediatr Neurol. 2005;12:134–143. doi: 10.1016/j.spen.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Patterson MC. Metabolic mimics: the disorders of N-linked glycosylation. Semin Pediatr Neurol. 2005;12:144–151. doi: 10.1016/j.spen.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993;3:97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Apweiler R, Hermjakob H, Sharon N. On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim Biophys Acta. 1999;1473:4–8. doi: 10.1016/s0304-4165(99)00165-8. [DOI] [PubMed] [Google Scholar]
- 30.Liao X, Wang W, Chen S, Wu Q. Role of glycosylation in corin zymogen activation. J Biol Chem. 2007;282:27728–27735. doi: 10.1074/jbc.M703687200. [DOI] [PubMed] [Google Scholar]
- 31.Shakin-Eshleman SH, Spitalnik SL, Kasturi L. The amino acid at the X position of an Asn-X-Ser sequon is an important determinant of N-linked core-glycosylation efficiency. J Biol Chem. 1996;271:6363–6366. doi: 10.1074/jbc.271.11.6363. [DOI] [PubMed] [Google Scholar]
- 32.White SM, Constantin PE, Claycomb WC. Cardiac physiology at the cellular level: use of cultured HL-1 cardiomyocytes for studies of cardiac muscle cell structure and function. Am J Physiol Heart Circ Physiol. 2004;286:H823–H829. doi: 10.1152/ajpheart.00986.2003. [DOI] [PubMed] [Google Scholar]
- 33.Fan D, Bryan PM, Antos LK, Potthast RJ, Potter LR. Down-regulation does not mediate natriuretic peptide-dependent desensitization of natriuretic peptide receptor (NPR)-A or NPR-B: guanylyl cyclase-linked natriuretic peptide receptors do not internalize. Mol Pharmacol. 2005;67:174–183. doi: 10.1124/mol.104.002436. [DOI] [PubMed] [Google Scholar]
- 34.Sawada Y, Suda M, Yokoyama H, Kanda T, Sakamaki T, Tanaka S, et al. Stretch-induced hypertrophic growth of cardiocytes and processing of brain-type natriuretic peptide are controlled by proprotein-processing endoprotease furin. J Biol Chem. 1997;272:20545–20554. doi: 10.1074/jbc.272.33.20545. [DOI] [PubMed] [Google Scholar]
- 35.Wu Q. The serine protease corin in cardiovascular biology and disease. Front Biosci. 2007;12:4179–4190. doi: 10.2741/2379. [DOI] [PubMed] [Google Scholar]
- 36.Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev. 2006;27:47–72. doi: 10.1210/er.2005-0014. [DOI] [PubMed] [Google Scholar]
- 37.Ala-Kopsala M, Magga J, Peuhkurinen K, Leipala J, Ruskoaho H, Leppaluoto J, Vuolteenaho O. Molecular heterogeneity has a major impact on the measurement of circulating N-terminal fragments of A- and B-type natriuretic peptides. Clin Chem. 2004;50:1576–1588. doi: 10.1373/clinchem.2004.032490. [DOI] [PubMed] [Google Scholar]
- 38.Giuliani I, Rieunier F, Larue C, Delagneau JF, Granier C, Pau B, et al. Assay for measurement of intact B-type natriuretic peptide prohormone in blood. Clin Chem. 2006;52:1054–1061. doi: 10.1373/clinchem.2005.061770. [DOI] [PubMed] [Google Scholar]
- 39.Goetze JP. Biochemistry of pro-B-type natriuretic peptide-derived peptides: the endocrine heart revisited. Clin Chem. 2004;50:1503–1510. doi: 10.1373/clinchem.2004.034272. [DOI] [PubMed] [Google Scholar]
- 40.Luckenbill KN, Christenson RH, Jaffe AS, Mair J, Ordonez-Llanos J, Pagani F, et al. Cross-reactivity of BNP, NT-proBNP, and proBNP in commercial BNP and NT-proBNP assays: preliminary observations from the IFCC Committee for Standardization of Markers of Cardiac Damage. Clin Chem. 2008;54:619–621. doi: 10.1373/clinchem.2007.097998. [DOI] [PubMed] [Google Scholar]