Abstract
Fullerenes are a class of closed-cage nanomaterials made exclusively from carbon atoms. A great deal of attention has been focused on developing medical uses of these unique molecules especially when they are derivatized with functional groups to make them soluble and therefore able to interact with biological systems. Due to their extended π-conjugation they absorb visible light, have a high triplet yield and can generate reactive oxygen species upon illumination, suggesting a possible role of fullerenes in photodynamic therapy. Depending on the functional groups introduced into the molecule, fullerenes can effectively photoinactivate either or both pathogenic microbial cells and malignant cancer cells. The mechanism appears to involve superoxide anion as well as singlet oxygen, and under the right conditions fullerenes may have advantages over clinically applied photosensitizers for mediating photodynamic therapy of certain diseases.
1. Introduction
Fullerenes (originally buckminsterfullerenes) are a new class of carbon molecules; the first example discovered in 1985,1 being composed of sixty carbon atoms arranged in a soccer-ball structure (C60). The condensed aromatic rings present in the compound lead to an extended π-conjugated system of molecular orbitals and therefore to significant absorption of visible light. In recent years there has been much interest in studying possible biological activities of fullerenes (and other nanostructures produced in the nanotechnology revolution) with a view to using them in medicine.2-4 Although pristine C60 can form nanocrystalline preparations that have been reported to have biological activity,5-8 most workers have studied chemically modified or functionalized fullerenes that acquire solubility in water or biologically compatible solvents and thereby have increased versatility.9-12
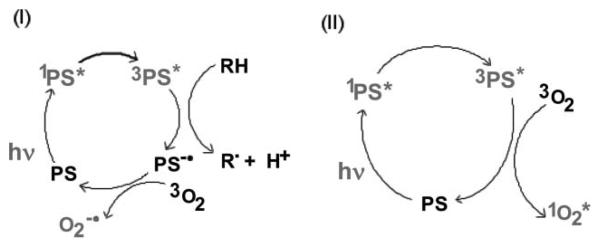
Photodynamic therapy (PDT) is a rapidly advancing treatment for multiple diseases and is based on the administration of a nontoxic drug or dye known as a photosensitizer (PS) either systemically, locally, or topically to a patient bearing a lesion (frequently but not always cancer13), followed after some time by the illumination of the lesion with visible light, which activates the PS and, in the presence of oxygen, leads to the generation of cytotoxic reactive oxygen species and consequently to cell death and tissue destruction.14-16 The light is absorbed by the PS molecule, and an electron is excited to the first excited singlet state. In addition to losing energy by fluorescence or internal conversion, the excited singlet can undergo the process known as intersystem crossing to the long-lived triplet state. The excited triplet state can then interact with ground state molecular oxygen to form reactive oxygen species (ROS). This process may occur by energy transfer from the triplet to produce singlet oxygen or by electron transfer to form superoxide anion17 (Fig. 1). Reaction of singlet oxygen with biological molecules such as proteins, unsaturated lipids and nucleic acids causing oxidative damage, is thought to be responsible for cell death, frequently occurring via the apoptosis pathway initiated by mitochondrial damage.18
Fig. 1.
Schematic representation of the Type I and Type II photochemical mechanisms thought to operate in PDT.
The concept of PDT dates from the early days of the twentieth century19 when workers used dyes such as eosin together with light to treat skin cancer.20 A semi-purified preparation of hematoporphyrin derivative (HPD) known as Photofrin® (PF) was the first PS to gain regulatory approval for treatment of various cancers in many countries throughout the world, including the United States.21,22 After experience of treating tumors with HPD-PDT was accumulated, it was realized that this compound had several disadvantages, including prolonged skin sensitivity necessitating avoidance of sunlight for many weeks,23 sub-optimal tumor selectivity,24 poor light penetration into the tumor due to the relatively short wavelength used (630 nm),25 and the fact that it was a complex mixture of uncertain structure.26 In recent times much work has been done on developing new PS27,28 for PDT, and although the vast majority of these compounds are based on the tetrapyrrole backbone29 found in porphyrins, chlorins and phthalocyanines, other molecular structures are beginning to be studied both preclinically and clinically.30,31
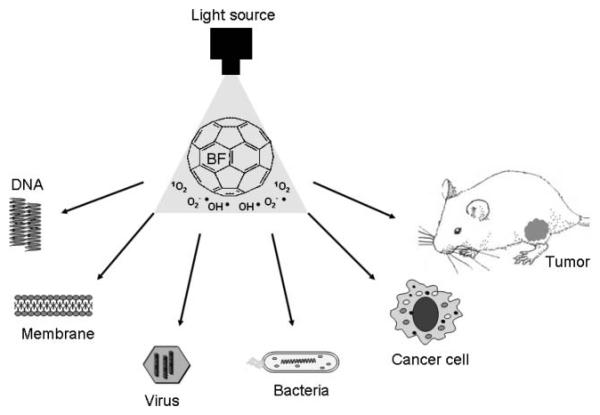
In this review we will cover the existing literature on fullerenes for PDT, summarize results from our laboratory and outline future possibilities concerning applications of fullerenes as PS for PDT. Fig. 2 gives a schematic outline of the PDT applications that have been reported for fullerenes either pristine or functionalized with various solubilizing groups.
Fig. 2.
Schematic outline of the possible applications of fullerenes as PDT sensitizers covered in this review.
2. Photochemical mechanisms
It has been known since shortly after the discovery of fullerenes that these compounds will catalyze the formation of ROS after illumination of both pristine and functionalized C60.32 In a similar fashion to the tetrapyrrole PS used for photodynamic therapy,33,34 illumination of fullerenes dissolved in organic solvents in the presence of oxygen leads to the efficient generation of highly reactive singlet oxygen via energy transfer from the excited triplet state of the fullerene.35 However, some recent reports have shown that in polar solvents, especially those containing reducing agents (such as NADH at concentrations found in cells), illumination of various fullerenes will generate different reactive oxygen derivatives, such as superoxide anion.36 These two pathways (singlet oxygen and superoxide anion) are analogous to the Type II and Type I photochemical mechanisms frequently discussed in PDT with tetrapyrroles.37,38
Fullerenes with their triply degenerate, low lying LUMO are excellent electron acceptors capable of accepting as many as six electrons.39 There is some evidence that fullerene excited states (in particular the triplet) are even better electron acceptors than the ground state.40,41 It is thought that the reduced fullerene triplet or radical anion can transfer an electron to molecular oxygen forming superoxide anion radical O2−•.
Yamakoshi et al.36 carried out a photochemical study comparing energy transfer processes (singlet oxygen 1O2) and electron transfer (reduced active oxygen radicals such as superoxide anion radical O2−• ), using various scavengers of ROS, physicochemical (electron paramagnetic resonance (EPR) radical trapping and near-infrared spectrometry), and chemical methods (nitro blue tetrazolium reaction with superoxide). Whereas 1O2 was generated effectively by photoexcited C60 in nonpolar solvents such as benzene and benzonitrile, they found that O2−• and OH• were produced instead of 1O2 in polar solvents such as water, especially in the presence of a physiological concentration of reductants including NADH.
Miyata et al. solubilized fullerenes into water with polyvinylpyrrolidone (PVP) as a detergent.42 Visible-light irradiation of PVP-solubilized C60 in water in the presence of NADH as a reductant and molecular oxygen resulted in the formation of O2−• , which was detected by the EPR spin-trapping method. Formation of O2−• was also evidenced by the direct observation of a characteristic signal of O2−• by the use of a low-temperature EPR technique at 77 K. On the other hand, no formation of 1O2 was observed by the use of TEMP as a 1O2 trapping agent. No near-IR luminescence of 1O2 was also observed in the aqueous C60/PVP/O2 system. These results suggest that photoinduced bioactivities of the PVP-solubilized fullerene are caused not by 1O2, but by reduced oxygen species such as O2−•, which are generated by the electron-transfer reaction of C60−• with molecular oxygen.
Highly water-soluble hexa(sulfobutyl)fullerenes (FC4S) were synthesized by the treatment of C60 in dimethoxyethane with sodium naphthalide followed by reacting the resulting hexa-anionic fullerene intermediates with an excess of 1,4-butanesulfone.43 Direct detection of 1O2 production after irradiation at 500–600 nm of FC4S self-assembled nanospheres, was obtained by the measurement of its near-infrared luminescence at 1270 nm. Despite having a relatively low optical absorption of FC4S at 600 nm, appreciable 1O2 signal was detected comparable to that of Photofrin at the same molar concentration, but less than sulfonated aluminium phthalocyanine, AlS4Pc. The quantum yield of FC4S for the generation of 1O2 in H2O was roughly estimated to be 0.36 using the relative correlation to that of C60 encapsulated in γ-cyclodextrin. These results demonstrated efficient energy transfer from 3FC4S triplet state to molecular oxygen in the nanosphere structure.
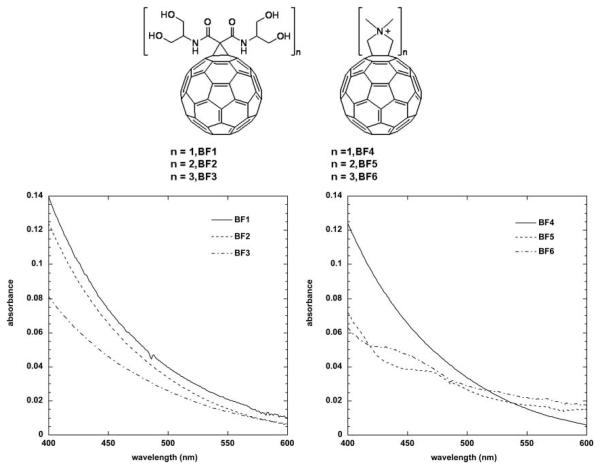
In two recent reports44,45 we studied the photosensitizer properties of two series of three functionalized fullerene compounds, one series with polar diserinol groups (BF1–BF3), and a second series of three compounds with quaternary pyrrolidinium groups (BF4–BF6) (Fig. 3). We asked the question whether the photodynamic effects displayed by these compounds operated primarily by Type I mechanisms (superoxide) or Type 2 mechanisms (singlet oxygen) or a mixture of both and whether there was any difference between a fullerene (BF4) that was highly effective in killing cancer cells and a fullerene (BF6) that was highly effective in killing pathogenic microorganisms (see later)?
Fig. 3.
Structures and visible absorption spectra recorded in DMSO of BF1–BF3 and BF4–BF6.
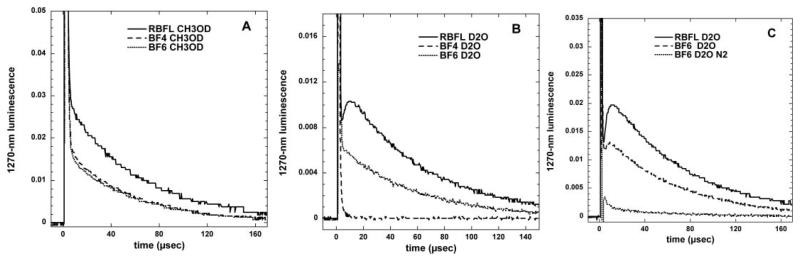
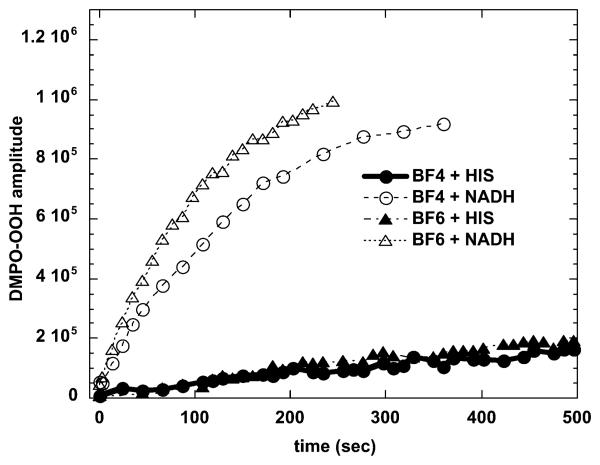
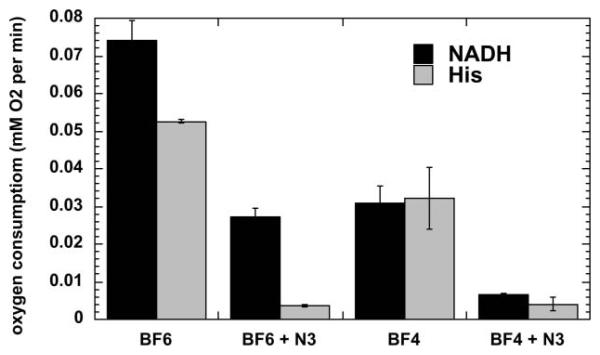
The photochemical mechanism studies confirmed that depending on the precise conditions of the experiment, illuminated fullerenes can produce both superoxide and singlet oxygen. The singlet oxygen production of the more hydrophobic BF4 dropped to almost zero when the solvent was changed from organic to aqueous (compare Fig. 4(A) and 4(B)). This is consistent with the aggregation of the compound in aqueous media, while the more polar BF6 remained completely in solution. The production of superoxide as measured by specific EPR spin-trapping was as expected much higher in the presence of a reducing agent (NADH) than in the presence of a singlet oxygen trap (histidine). In both cases BF6 gave more superoxide than BF4, and the difference was significant in the presence of NADH (Fig. 5). The fact that the reduction of oxygen consumption by both BF4 and BF6 was almost completely inhibited by azide in the presence of histidine confirmed that in the absence of an electron donor and in the presence of a singlet oxygen substrate the mechanism is almost all Type II for both fullerenes (Fig. 6). However in the presence of NADH the relative reduction of oxygen consumption by azide was much less, and proportionately lower for BF6 than for BF4 suggesting that about 40% of the oxygen was transformed into superoxide, and the overall production was higher for BF6 than for BF4.
Fig. 4.
Time decay curves of 1270 nm luminescence from singlet oxygen produced when BF4 (49 μM), BF6 (52 μM) or riboflavin (RBFL, 17 μM) were excited with a 5 ns 449 nm laser pulse;(A) deuterated methanol; (B) deuterated PBS; (C) compare BF6 in airorinnitrogen.
Fig. 5.
Increase with illumination time (broad band white light) in ESR signal from superoxide-specific spin trap (DMPO–OOH) and BF4 or BF6 (35 μM) in presence of 1 mM NADH or 2 mM histidine in 1 : 3 H2O : DMSO.
Fig. 6.
Oxygen consumption rates for BF4 or BF6 (35 μM) in presence of 1 mM NADH or 2 mM histidine with or without 5 mM sodium azide in 1:3 H2O : DMSO determined by ESR oximetry.
3. DNA Cleavage and mutagenicity
One of the first biological applications of photoactivated fullerenes was to produce cleavage of DNA strands after illumination. Cleavage of supercoiled pBR322 DNA was observed after incubation with a fullerene carboxylic acid under visible light irradiation but not in the dark.46 Both nicked circular and linear duplex form DNA were observed and there was considerable selectivity for cleavage at guanine bases. The photoinduced action was more pronounced in D2O in which singlet oxygen has a longer lifetime.
An and coworkers47 prepared a covalent conjugate between an oligodeoxynucleotide and either a fullerene or eosin. Cleavage of target complementary 285-base single stranded DNA was observed at guanosine residues in both cases upon illumination. However the fullerene conjugate was more efficient in cleavage than the eosin conjugate. Moreover cleavage was not quenched by azide or increased by deuterium oxide as was found for the eosin conjugate suggesting the mechanism followed a Type I pathway.
Boutorine et al.48 described a fullerene-oligonucleotide that can bind single- or double-stranded DNA, and which also cleaves the strand(s) proximal to the fullerene moiety upon exposure to light. Nakanishi et al.49 also observed DNA cleavage by functionalized C60. Yamakoshi et al. studied biological activities of fullerenes under illumination including DNA-cleavage, hemolysis, mutagenicity, and cell-toxicity.50 They prepared a conjugate between a fullerene and an acridine molecule as a DNA intercalating agent and compared its DNA photocleavage capacity on pBR322 supercoiled plasmid with pristine fullerene both solubilized in PVP. This compound showed much more effective DNA-cleaving activity in the presence of NADH than pure C60.51 Liu and coworkers52 used a water soluble conjugate between anthrylcyclodextrin and C60 to carry out photocleavage of pGEX5x2 DNA. Ikeda et al.53 used functionalized liposomes encorporating both C60 and C70 fullerenes into the lipid bilayer to carry out photocleavage of ColE1 supercoiled plasmid DNA using λ > 350 nm light. Interestingly C70 was significantly better (3.5 times) than C60 in photocleaving DNA.
It has been also shown that fullerene-mediated-PDT may lead to mutagenic effects. PVP-solubilized fullerene was found to be mutagenic for Salmonella strains TA102, TA104 and YG3003 in the presence of rat liver microsomes when it was irradiated by visible light.54 The mutagenicity was elevated in strain YG3003, a repair enzyme-deficient mutant of TA102. The mutation was reduced in the presence of beta-carotene and parabromophenacyl bromide, a scavenger and an inhibitor, respectively, of phospholipase. The results suggested that singlet oxygen was generated by irradiating the C60 by visible light and that the mutagenicity was due to oxidized phospholipids in rat liver microsomes. The linoleate fraction isolated by high performance liquid chromatography was a major component, and played an important role in mutagenicity. The results of ESR spectrum analysis suggested generation of radicals at the guanine base but not thymine, cytosine and adenine bases and formation of 8-hydroxydeoxyguanosine (8-OH-dG). The mechanism was proposed to involve indirect action of singlet oxygen due to lipid peroxidation of linoleate that causes oxidative DNA damage.
4. Membrane damage by photoactivated fullerenes
A group from India has studied the ability of fullerenes to produce oxidative damage to lipids in microsomal preparations. Cyclodextrin encapsulated C60 added to rat liver microsomes followed by exposure to UV or visible light produced lipid peroxidation as assayed by thiobarbituric acid reactive substances, lipid hydroperoxides, damage to proteins as assessed by protein carbonyls and loss of the membrane-bound enzymes.55 Quenchers of singlet oxygen (beta-carotene and sodium azide) inhibited peroxidation, and deuteration of the buffer enhanced peroxidation, indicating that the photochemical mechanism is predominantly due to Type II (1O2). In a subsequent study56 they compared pristine C60 with a polyhydroxylated derivative C60(OH)18 and found that the latter produced more pronounced peroxidative damage and the mechanism was different and was mediated primarily by radical species. Lipid peroxidation was also shown in sarcoma 180 ascites microsomes.
Yang et al.57 used human erythrocyte membranes (EMs) as a model system, to examine photo-induced lipid peroxidation by a bis-methanophosphonate fullerene (BMPF) and four other fullerene derivatives including a mono-methanophosphonic acid fullerene (MMPF), a dimalonic acid C(60) (DMA C(60)), a trimalonic acid C(60) (TMA C(60)) and a polyhydroxylated fullerene (fullerol). Lipid peroxidation was assessed as the malondialdehyde (MDA) level measured by the thiobarbituric acid assay. It was observed that BMPF increased the MDA level of EMs after irradiation in both time- and dose-dependent manners. The photo-induced activity became very significant (p < 0.01) under the conditions of either the concentration of 10 μM and irradiation time of 30 min or the concentration of 5 μM and irradiation time of 60 min. Involvement of reactive oxygen species (ROS) in the activity was also examined by specific inhibitors of singlet oxygen, superoxide anions and hydroxyl radicals, respectively. While all three kinds were found responsible for the activity, the former two might play more important roles than the last one.
5. Photoinactivation of viruses
Photodynamic reactions induced by photoactivated fullerenes have been shown to inactivate enveloped viruses.58 Buffered solutions containing pristine C60 and Semliki Forest virus (Togaviridae) or vesicular stomatitis virus (VSV) (Rhabdoviridae), when illuminated with visible light for up to 5 h, resulted in up to seven logs of loss of infectivity. Viral inactivation was oxygen-dependent and equally efficient in solutions containing protein.59 Hirayama et al.60 used a methoxy-polyethylene glycol conjugated fullerene at 400 μM in combination with 120 J cm−2 white light to destroy more than five logs of plaque forming units of VSV. VSV inactivation was inhibited by oxygen removal or by the addition of sodium azide, a known singlet oxygen quencher. The substitution of H2O by D2O, which is known to prolong the lifetime of singlet oxygen, promoted the virucidal activity. These results indicate that singlet oxygen may play a major role in VSV photoinactivation by the water-soluble fullerene derivative. The concentration needed for virus inactivation was higher than that of other sensitizers such as methylene blue.
Lin and coworkers61 compared light-dependent and light independent inactivation of dengue-2 and other enveloped viruses by the two regio-isomers of carboxyfullerene, and found that asymmetric isomer had greater dark activity (albeit at much higher concentrations than needed for its PDT effect) due to its interaction with the lipid envelope of the virus.
6. Fullerenes for antimicrobial photoinactivation
The effectiveness of various PS proposed for antimicrobial PDT can be judged on several criteria. These PS should be able to kill multiple classes of microbes at relatively low concentrations and low fluences of light. PS should be reasonably non-toxic in the dark and should demonstrate selectivity for microbial cells over mammalian cells. PS should ideally have large extinction coefficients in the red part of the spectrum and demonstrate high triplet and singlet oxygen quantum yields.
We have shown, in a series of reported experiments that cationic fullerenes fulfill many, but not all of the aforementioned criteria. Our laboratory was the first to demonstrate that the soluble functionalized fullerenes described above, especially the cationic compounds BF4–BF6, were efficient antimicrobial PS and could mediate photodynamic inactivation (PDI) of various classes of microbial cells.45 We used a broadband-pass filter giving an output of the entire visible spectrum (400–700 nm) to excite the fullerenes that maximized the absorption (see Fig. 3).
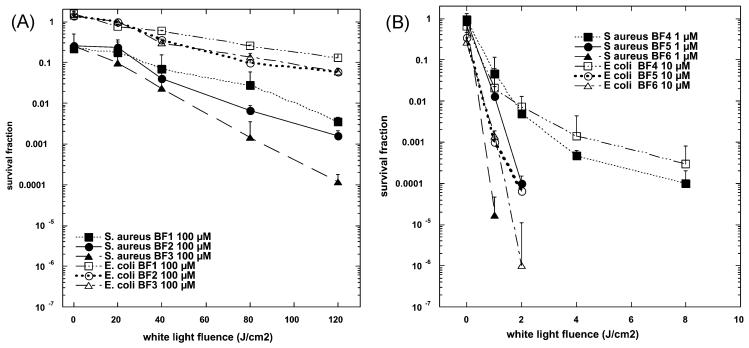
Our initial screening experiment carried out against S. aureus at 100 μM concentrations showed that the C60 substituted with pyrrolidinium groups behaved very differently than the series substituted with di-serinol groups. The cationic fullerenes gave high levels of dark toxicity (except for BF4) while the di-serinol-functionalized C60 had no dark toxicity, but showed a typical light dose-dependent loss of colony-forming ability. However it needed concentrations as high as 100 μM and white-light fluences as high as 120 J cm−2 to achieve significant killing (2–3 logs or up to 99.9%) of the Gram-positive Staphylococcus aureus (Fig. 7(A)). Even with these relatively high doses of both PS and light, the Gram-negative Escherichia coli was only slightly killed (less than 1 log or 90%). In sharp contrast the cationic fullerenes were highly effective PS at much lower concentrations and much lower light doses (Fig. 7(B)). BF4-BF6 were all surprisingly effective in causing light-mediated killing of S. aureus. BF5 and BF6 needed only 1 μM concentration and 1 or 2 J cm−2 of white light to kill 4–5 logs. BF4–6 were tested against E. coli at 10 μM, and BF5 and BF6 showed similar high levels of activity (up to 6 logs). These findings agree with numerous reports in the literature that demonstrate that PS with one (or preferably more cationic groups) are efficient antimicrobial PS.62-66 Quaternary nitrogen based groups are superior to primary, secondary or tertiary amino groups as the positive charge is less dependent on the pH of the surrounding media, or the pKa of the molecules that the PS is interacting with. Microbial cells possess overall negative charges and it is thought that cationic PS bind to these groups on the outer layers of the cell surface. Gram-positive cells have relatively permeable outer layers of peptidoglycan and lipoteichoic acid or beta-glucan respectively. This allows cationic and to a lesser extent non-cationic PS to diffuse inwards to the plasma membrane where the generation of reactive oxygen species under illumination can damage the membrane structure allowing leakage of essential components and cause cell death. Cationic compounds are able to displace divalent cations (Ca2+ and Mg2+) that play a role in the attachment of lipopolysaccharide to the outer membrane.67 This displacement weakens the structure of the outer permeability allowing the PS to penetrate further in a process that has been termed “self promoted uptake”.68 The fact that this mechanism requires cationic compounds explains why BF1–BF3 were relatively non-effective against the Gram-negative E. coli and such findings have been reported with numerous other non-cationic PS.64
Fig. 7.
(A) S. aureus or E. coli (108 cells per mL) were incubated for 10 min with BF1–BF3 at 100 μM concentration in PBS followed by illumination with 400–700 nm light at an irradiance of 200 mW cm−2. Aliquots were removed from the suspensions after the various fluences of light had been delivered and CFU determined. Values are means of six independent experiments and bars are SEM. * p < 0.05 ** p < 0.01; 2-tailed unpaired t-test. (B) S. aureus was incubated with 1 μM concentration of BF4–BF6 and E. coli (both 108 cells per mL) was incubated with BF4–BF6 at 10 μM concentrations for 10 min followed by a wash and illumination with white light. Values are means of six independent experiments and bars are SEM. * p < 0.05, ** p < 0.01, *** p < 0.001; 2-tailed unpaired t-test.
A recent study by Spesia et al.69 reported that a novel N,N-dimethyl-2-(4′-N,N,N-trimethylaminophenyl)fulleropyrrolidinium iodide (DTC(60)(2+)) was synthesized by 1,3-dipolar cycloaddition using 4-(N,N-dimethylamino) benzaldehyde, N-methylglycine and fullerene C(60)and quaternization with methyl iodide. The photodynamic properties of the DTC(60)(2+) were compared with a non-charged N-methyl-2-(4′-acetamidophenyl)-fulleropyrrolidine (MAC60) and a monocationic N,N-dimethyl-2-(4′-acetamidophenyl)fulleropyrrolidinium iodide (DAC(60)(+)). The photodynamic effect was strongly dependent on the medium, and diminished when the sensitizer aggregated and increased in an appropriately surrounded microenvironment. The photodynamic inactivation produced by these fullerene derivatives was investigated in vitro on E. coli. Photosensitized inactivation of E. coli cellular suspensions by DTC(60)(2+) exhibited a approximately 3.5 log decrease of cell survival when the cultures are treated with 1 μM of sensitizer and irradiated for 30 min. This photosensitized inactivation remains high even after one washing step. Also, the photodynamic activity was confirmed by growth delay of E. coli cultures. The growth was arrested when E. coli was exposed to 2 μM of cationic fullerene and irradiated, whereas a negligible effect was found for the non-charged MAC.60
7. Fullerenes and PDT of mammalian cells including cancer cells
It is thought that one requirement for any PS to produce cell killing after illumination, is that the PS should actually be taken up inside the cell, and that the generation of ROS outside the cell (unless in very large quantities) will not be sufficient to produce efficient cell death. Because fullerenes (in contrast to the vast majority of PS) are non-fluorescent, it is impossible to use the common technique of fluorescence microscopy to examine the intracellular uptake and sub-cellular localization of fullerenes. Taking these considerations into account, Scrivens et al.70 were the first to prepare a radiolabeled fullerene and demonstrate its uptake by human keratinocytes in tissue culture. In serum-free medium they found a time (up to 6 h) and concentration dependent uptake so that 50% of added fullerene was taken up. Foley et al.71 used indirect immunofluorescence staining with antibodies that recognize fullerenes and other organelle probes to show that a dicarboxylic acid derivative localized in mitochondria and other intracellular membranes. A recent report5 has in fact used a pristine C60 preparation obtained by sonication in methanol (different from the more commonly used toluene) and giving uniformly sized particles with a mean size of 32.7 nm together, with photoluminescence detection at 750 nm after excitation at 488 -nm, to demonstrate cell uptake in normal and malignant breast cells after culturing them on fullerene coated dishes. Another recent paper72 describes the use of the related techniques of energy-filtered transmission electron microscopy and electron tomography to visualize the cellular uptake of pristine C60 nanoparticulate clusters. When human monocyte derived macrophages were examined C60 was found in the plasma membrane, lysosomes and in the nucleus.
The first demonstration of phototoxicity in cancer cells mediated by fullerenes was in 1993 when Tokuyama et al.46 used carboxylic acid functionalized fullerenes at 6 μM and white light to produce growth inhibition in human HeLa cancer cells. However these same authors later reported that other carboxylic acid derivatives of C60 and C70 were completely without any photoactivity as PDT agents at 50 μM.73
Burlaka et al.74 used pristine C60 at 10 μM with visible light from a mercury lamp to produce some phototoxicity in Ehrlich carcinoma cells or rat thymocytes and used EPR spin-trapping techniques to demonstrate the formation of ROS.
The cytotoxic and photocytotoxic effects of two water-soluble fullerene derivatives, a dendritic C60 mono-adduct and the malonic acid C60 tris-adduct were tested on Jurkat cells when irradiated with UVA or UVB light.75 The cell death was mainly caused by membrane damage and it was UV dose-dependent. Tris-malonic acid fullerene was found to be more phototoxic than the dendritic derivative. This result is in contrast to the singlet oxygen quantum yields determined for the two compounds.
Three C60 derivatives with two to four malonic acid groups (DMA C60,TMA C60 and QMA C60) were prepared and the phototoxicity of these compounds against HeLa cells was determined by MTT assay and cell cycle analysis.76 The relative phototoxicity of these compounds was DMA C60 >TMA C60 > QMA C60. Hydroxyl radical quencher mannitol (10 mM) was not able to prevent cells from the damage induced by irradiated DMA C60. DMA C60, together with irradiation, was found to decrease the number of G(1) cells from 63 to 42% and increase G(2)+M cells from 6 to 26%.
Rancan et al.77 used the following approach to overcome the necessity to use UV or short-wavelength visible light to photoactivate fullerenes. They synthesized two new fullerene-bispyropheophorbide a derivatives: a mono-(FP1) and a hexa-adduct (FHP1). The photophysical characterization of the compounds revealed significantly different parameters related to the number of addends at the fullerene core. In this study, the derivatives were tested with regard to their intracellular uptake and photosensitizing activity towards Jurkat cells in comparison with the free sensitizer, pyropheophorbide a. The C60–hexa-adduct FHP1 had a significant phototoxic activity (58% cell death, after a dose of 400 mJ cm−2 of 688 nm light) but the mono-adduct FP1 had a very low phototoxicity and only at higher light doses. Nevertheless the activity of both adducts was less than that of pure pyropheophorbide a, probably due to the lower cellular uptake of the adducts.
A group from Argentina has also studied the phototoxicity produced by tetrapyrrole-fullerene conjugates. Milanesi et al.78 compared PDT with a porphyrin–C60 dyad (P–C60) and its metal complex with Zn(II) (ZnP–C60) were compared with 5-(4-acetamidophenyl)-10,15,20-tris(4-methoxyphenyl)porphyrin (P), both in homogeneous medium containing photooxidizable substrates and in vitro on the Hep-2 human larynx carcinoma cell line. 1O2 yields (ΦΔ) were determined using 9,10-dimethylanthracene (DMA). The values of ΦΔ were strongly dependent on the solvent polarity. Comparable ΦΔvalues were found for dyads and P in toluene, while 1O2 production was significantly diminished for the dyads in DMF. In more polar solvent, the stabilization of charge-transfer state takes place, decreasing the efficiency of porphyrin triplet-state formation. Also, both dyads photosensitize the decomposition of L-tryptophan in DMF. In biological medium, no dark cytotoxicity was observed using sensitizer concentrations ≤ 1 μM and 24 h of incubation. The uptake of sensitizers into Hep-2 was studied using 1 lM of sensitizer and different times of incubation. Under these conditions, a value of approximately 1.5 nmol/106 cells was found between 4 and 24 h of incubation. The cell survival after irradiation of the cells with visible light was dependent upon light-exposure level. A higher phototoxic effect was observed for P–C60, which inactivates 80% of cells after 15 min of irradiation. Moreover, both dyads keep a high photoactivity even under argon atmosphere. In a subsequent paper79 they showed the cells died by apoptosis by analysis using Hoechst-33258, toluidine blue staining, TUNEL and DNA fragmentation. Changes in cell morphology were analyzed using fluorescence microscopy with Hoechst-33258 under low oxygen concentration. Under this anaerobic condition, necrotic cellular death predominated over the apoptotic pathway. It was found that P–C60 induced apoptosis by a caspase-3-dependent pathway.
Ikeda and coworkers80 used a series of liposomal preparations containing cationic or anionic lipids together with dimyristoylphosphatidylcholine and introduced C60 into the lipid bilayer by exchange from cyclodextrin. By adding a phosopholipid with an additional fluorochrome they were able to use fluorescence microscopy to demonstrate uptake of the liposomes by HeLa cells after 24 h incubation. Illumination with 136 J cm−2 350–500 nm light gave 85% cell killing in the case of cationic liposomes and apoptosis was demonstrated.
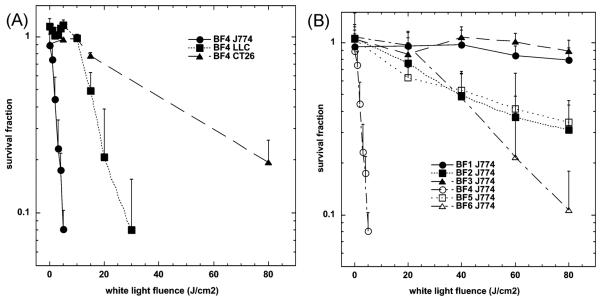
In our laboratory we have tested the hypothesis that fullerenes would be able to kill cancer cells by PDT in vitro. We studied the same group of fullerene derivatives described above BF1–BF3 and BF4–BF6.44 We showed that the C60 molecule mono-substituted with a single pyrrolidinium group (BF4) is a remarkably efficient PS and can mediate killing of a panel of mouse cancer cells at the low concentration of 2 μM with very modest (5 J cm−2) exposure to white light as shown in Fig. 8(A). The cells were all cancer cells; lung (LLC) and colon (CT26) adenocarcinoma and reticulum cell sarcoma (J774) and the latter showed much higher susceptibility perhaps due to having an increased uptake of fullerene because J774 cells behave like macrophages.81 In Fig. 8(B) we show the comparative phototoxicity of all six fullerenes against J774 cells. Besides the exceptionally active BF4, the next group of compounds has only moderate activity (BF2, BF5 and BF6) against J774 cells showing some killing at high fluences. The last two compounds (BF1 and BF3) had no detectable PDT killing up to 80 J cm−2. For the first time we indirectly demonstrated that photoactive fullerenes are taken up into cells by measuring the increase in fluorescence of an intracellular probe (H2DCFDA) that is specific for the formation of ROS (in particular hydrogen peroxide) (Fig. 9). We believe that the superoxide produced from the illuminated fullerene undergoes dismutation either catalyzed by superoxide dismutase, or spontaneously, to produce H2O2 resulting in the increased and diffused fluorescence of the probe.
Fig. 8.
Survival curves of (A) LLC, J774, CT26 cells after 24 h incubation with 2 μM BF4 and (B) J774 cells after 24 h incubation with 2 μM BF1–BF6, in both cases followed by a wash and illumination with white light. A MTT assay was carried out after 24 h incubation. Values are means of 9 separate wells and bars are SD. Experiments were repeated at least twice.
Fig. 9.
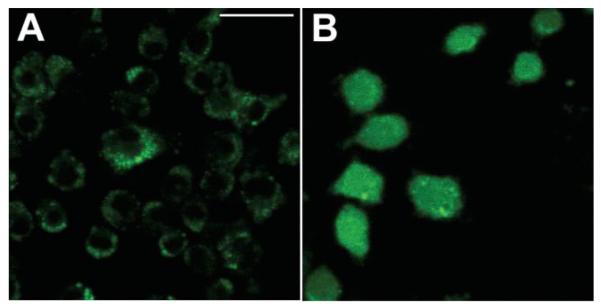
Fluorescence micrographs of J774 cells that had been incubated with intracellular ROS probe H2DCFDA, illuminated with 5 J cm−2 405 nm laser and imaged after 5 min. (A) H2DCFDA without fullerene; (B) BF4 for24hH2DCFDA. Scale bar is 100 μm.
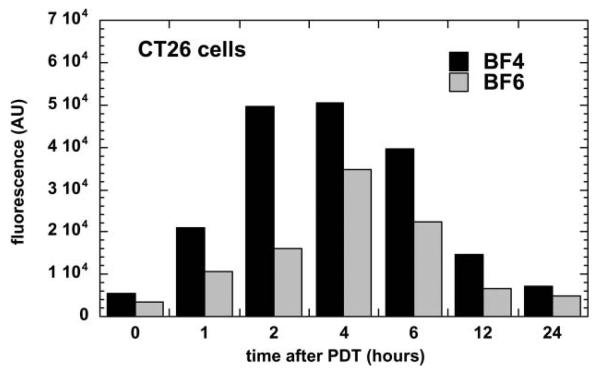
We also demonstrated the induction of apoptosis by PDT mediated by BF4 and by BF6 in CT26 cells at 4–6 h after illumination (Fig. 10). The relatively rapid induction of apoptosis after illumination might suggest the fullerenes are localized in subcellular organelles such as mitochondria, as has been previously shown for PS such as benzoporphyrin derivative.82-84 PS that localize in lysosomes tend to produce apoptosis more slowly after illumination than mitochondrial PS, due to the release of lysosomal enzymes that subsequently activate cytoplasmic caspases.85
Fig. 10.
Time course of apoptosis as measured by a fluorescent caspase assay in CT26 cells receiving BF4-PDT (80% lethal dose) or BF6-PDT (60% lethal dose).
The mono-pyrrolidinium substituted fullerene was the most effective PS by a considerable margin. The explanation for this observation is probably linked to its relative hydrophobicity as demonstrated by its logP value of over 2. The single cationic charge possessed by BF4 is also likely to play an important role in determining its relative phototoxicity. Many lipophilic monocations have been shown to localize fairly specifically in mitochondria86-88 and this property has been proposed as a strategy to target drugs to mitochondria.89
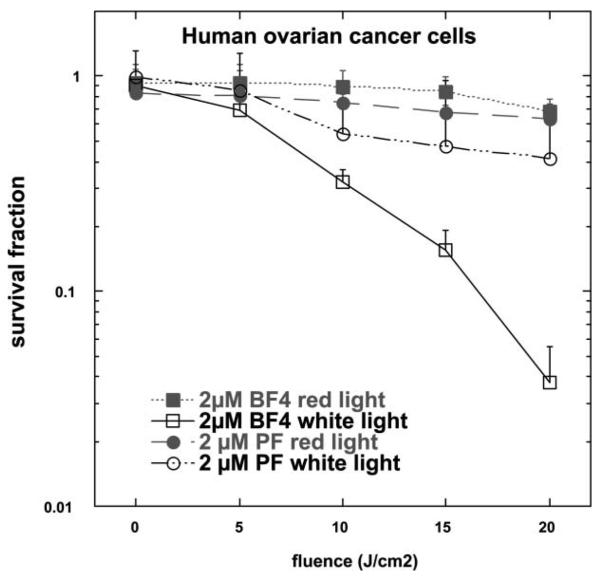
We have also performed preliminary experiments comparing effectiveness of BF4 to Photofrin®, one of the clinically approved photosensitizers for cancer therapy.90 Fig. 11 shows that BF4 was far superior at PDT-mediated killing of human ovarian cancer cells in vitro than Photofrin. Both compounds, BF4 and Photofrin show very little dark toxicity as evidenced by the survival fraction at zero fluence, which was after 24 h incubation of the cells with each PS. Also, both show a light-dose dependent response. However, the response of BF4 at the light fluences used was much more pronounced than that of Photofrin, demonstrating that BF4 is a significantly better photosensitizer than Photofrin against ovarian cancer cells in vitro.
Fig. 11.
Comparison of PDT-induced killing in human ovarian cancer cells by BF4 or Photofrin both incubated for 24 h at 2 μM concentration and illuminated with red (630 nm) or white light (400–700 nm).
8. Photodynamic therapy of tumors
Fullerenes should have a photodynamic effect on tumors, if (a) the compound is accumulated in the tumor tissue, (b) a reasonably efficient way to administer the compound to tumor bearing animals is found, and (c) enough excitation light can be delivered to the photosensitized tumors. The first report of fullerenes being used to carry out PDT of actual tumors was by Tabata et al. in 1997.91 They chemically modified the water-insoluble C60 with polyethylene glycol (PEG), not only to make it soluble in water, but also to enlarge its molecular size. When injected intravenously into mice carrying a subcutaneous tumor on the back, the C60–PEG conjugate exhibited higher accumulation and more prolonged retention in the tumor tissue than in normal tissue. The conjugate was excreted without being accumulated in any specific organ. Following intravenous injection of C60–PEG conjugate or Photofrin (a recognized PS) to tumor-bearing mice, coupled with exposure of the tumor site to visible light, the volume increase of the tumor mass was suppressed and the C60 conjugate exhibited a stronger suppressive effect than Photofrin. Histological examination revealed that conjugate injection plus light irradiation strongly induced tumor necrosis without any damage to the overlying normal skin. The antitumor effect of the conjugate increased with increasing fluence delivered and C60 dose, and cures were achieved by treatment with a low dose of 424 μg kg−1 at a (very high) fluence of 107 Jcm−2.
Liu and others92 conjugated polyethylene glycol (PEG) to C60 (C60–PEG), and diethylenetriaminepentaacetic acid (DTPA) was subsequently introduced to the terminal group of PEG to prepare C60–PEG–DTPA that was mixed with gadolinium acetate solution to obtain Gd3+-chelated C60–PEG–DTPA–Gd. Following intravenous injection of C60–PEG–DTPA–Gd into tumor-bearing mice, the PDT anti-tumor effect and MRI tumor imaging were evaluated. Similar generation of superoxide upon illumination was observed with or without Gd3+ chelation. Intravenous injection of C60–PEG–DTPA–Gd into tumor bearing mice plus light (400–500 nm, 53.5 J cm−2) showed significant anti-tumor PDT effect and the effect depended on the timing of light irradiation that correlated with tumor accumulation as detected by the enhanced intensity of MRI signal.
The FC4S nanostructures described in section 243 have also been tested as PS in vivo.93 The median lethal dose (LD50) of FC4S was defined as approximately 600 mg kg−1 in acute toxicity studies. No adverse effects were noted in the animals when the FC4S was administered orally. ICR mice subcutaneously implanted with sarcoma 180 tumor cells were given FC4S (15 mg kg−1 ) by intraperitoneal injection, and PDT on tumor bearing mice using argon laser irradiation at 514.5 nm with a total light dose of 100 J cm−2 delivered at 200 mW cm−2. Mean tumor size was found to significantly decrease to only 10% of the untreated tumor control at 30 days after laser irradiation. Mice treated with FC4S and no laser irradiation had a slight non-significant reduction in tumor size.
These data summarized above suggest that PDT with fullerenes is not only possible in animal tumor models, but demonstrate the potential use of these compounds as PS for PDT of cancer.
9. Conclusions and future outlook
At the end of this review we must ask ourselves whether in all reality it is likely that fullerenes will ever be accepted as viable PS for PDT of any disease. As discussed previously these compounds have certain unique features that could make them favorable candidates and at the same time other unique features that would argue against them as PS for PDT. The most important favorable property is their rather unusual photochemical mechanism. As shown by us and by others, in aqueous solutions and particularly in the presence of reducing agents, these compounds produce a substantial amount of superoxide anion in a Type I photochemical process involving electron transfer from the excited triplet state to molecular oxygen. Although many workers in the PDT field think that the product of the Type II photochemical process, singlet oxygen, is the major cytotoxic species operating in PDT-induced cell killing, there have been reports that Type I mechanisms may be equally effective or even more effective than Type II. This is because hydroxyl radicals are the most reactive and potentially the most cytotoxic of all ROS. It is assumed that hydroxyl radicals are formed from hydrogen peroxide by Fenton chemistry reactions catalyzed by Fe2+ or Cu+ ions, and that the hydrogen peroxide is produced by dismutation of superoxide anion either by enzyme catalysis or naturally. Another possible mechanism of cytotoxicity is the diffusion-controlled reaction between superoxide and nitric oxide to form the highly toxic species, peroxynitrite. Elevated levels of nitric oxide are present both in cancers and infections thus giving the possibility of additional levels of selectivity for target-specific damage.
The chief disadvantage of fullerenes is likely to be their optical absorption properties. The absorption spectrum of fullerenes is highest in the UVA and blue regions of the spectrum where the tissue penetration depth of illumination is shortest due to a combination of light absorption by cellular chromophores and light scattering by cellular structures. However the molar absorption coefficients of fullerenes are relatively high and the tail of absorption does stretch out into the red regions of the visible spectrum. Fullerenes are not the most amenable molecules for drug delivery and choosing appropriate formulations may be difficult. Nevertheless the polycationic modifications described in this chapter demonstrate that with the correct functionalities present on the fullerene cage these difficulties may be overcome.
Acknowledgements
This work was supported by the US National Institutes of Health (grants R43CA103268 and R44AI68400 to Lynntech Inc, R01CA/AI838801 and R01AI050875 to MRH), and in Poland by Ministry of Science and Higher Education (DS/WBBB/16/06).
Biographies
 Dr Pawel Mroz graduated in 2004 from the Medical University of Warsaw, Poland. While at the University he joined the Student Research Group at the Department of Immunology where under the supervision of Dr Jakob Golab he participated in several research projects involving PDT. After graduation Dr Mroz joined, as the International Union Against Cancer Fellow, the laboratory of Dr Hava Avraham at BIDMC, Harvard Institutes of Medicine where he worked on the molecular biology of human breast cancer. Since January of 2005, he has been working as a Research Fellow at the Wellman Center for Photomedicine, Massachusetts General Hospital. Under the supervision of Dr Michael Hamblin, Dr Mroz has been investigating the variety of anti-tumor immune responses after PDT as well as evaluating the applications of several new photosensitizers. He has received several awards for his research.
Dr Pawel Mroz graduated in 2004 from the Medical University of Warsaw, Poland. While at the University he joined the Student Research Group at the Department of Immunology where under the supervision of Dr Jakob Golab he participated in several research projects involving PDT. After graduation Dr Mroz joined, as the International Union Against Cancer Fellow, the laboratory of Dr Hava Avraham at BIDMC, Harvard Institutes of Medicine where he worked on the molecular biology of human breast cancer. Since January of 2005, he has been working as a Research Fellow at the Wellman Center for Photomedicine, Massachusetts General Hospital. Under the supervision of Dr Michael Hamblin, Dr Mroz has been investigating the variety of anti-tumor immune responses after PDT as well as evaluating the applications of several new photosensitizers. He has received several awards for his research.
 Michael R. Hamblin is a Principal Investigator at the Wellman Center for Photomedicine at Massachusetts General Hospital and an Associate Professor of Dermatology at Harvard Medical School. He was trained as a synthetic organic chemist and received his PhD from Trent University in England. His research interests lie in the areas of photodynamic therapy for infections, cancer, and heart disease and low-level light therapy for wound healing, arthritis and hair regrowth. He has published over 80 peer-reviewed articles, over 100 conference proceedings, book chapters and international abstracts and holds 8 patents. He chairs an annual conference at SPIE entitled “Mechanisms for low level light therapy”.
Michael R. Hamblin is a Principal Investigator at the Wellman Center for Photomedicine at Massachusetts General Hospital and an Associate Professor of Dermatology at Harvard Medical School. He was trained as a synthetic organic chemist and received his PhD from Trent University in England. His research interests lie in the areas of photodynamic therapy for infections, cancer, and heart disease and low-level light therapy for wound healing, arthritis and hair regrowth. He has published over 80 peer-reviewed articles, over 100 conference proceedings, book chapters and international abstracts and holds 8 patents. He chairs an annual conference at SPIE entitled “Mechanisms for low level light therapy”.
Footnotes
The HTML version of this article has been enhanced with additional colour images.
References
- 1.Kroto HW, Heath JR, O'Brien SC, Curl RF, Smalley RE. C60: Buckminsterfullerene. Nature. 1985;318:162–163. [Google Scholar]
- 2.Bosi S, Da Ros T, Spalluto G, Prato M. Fullerene derivatives: an attractive tool for biological applications. Eur. J. Med. Chem. 2003;38:913–923. doi: 10.1016/j.ejmech.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Jensen AW, Wilson SR, Schuster DI. Biological applications of fullerenes. Bioorg. Med. Chem. 1996;4:767–779. doi: 10.1016/0968-0896(96)00081-8. [DOI] [PubMed] [Google Scholar]
- 4.Tagmatarchis N, Shinohara H. Fullerenes in medicinal chemistry and their biological applications. Mini Rev. Med. Chem. 2001;1:339–348. doi: 10.2174/1389557013406684. [DOI] [PubMed] [Google Scholar]
- 5.Levi N, Hantgan RR, Lively MO, Carroll DL, Prasad GL. C60-Fullerenes: detection of intracellular photoluminescence and lack of cytotoxic effects. J. Nanobiotechnol. 2006;4:14. doi: 10.1186/1477-3155-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belgorodsky B, Fadeev L, Kolsenik J, Gozin M. Formation of a soluble stable complex between pristine C60-fullerene and a native blood protein. ChemBioChem. 2006;7:1783–1789. doi: 10.1002/cbic.200600237. [DOI] [PubMed] [Google Scholar]
- 7.Gharbi N, Pressac M, Hadchouel M, Szwarc H, Wilson SR, Moussa F. [60]fullerene is a powerful antioxidant in vivo with no acute or subacute toxicity. Nano Lett. 2005;5:2578–2585. doi: 10.1021/nl051866b. [DOI] [PubMed] [Google Scholar]
- 8.Sayes CM, Gobin AM, Ausman KD, Mendez J, West JL, Colvin VL. Nano-C60 cytotoxicity is due to lipid peroxidation. Biomaterials. 2005;26:7587–7595. doi: 10.1016/j.biomaterials.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura E, Isobe H. Functionalized fullerenes in water. The first 10 years of their chemistry, biology, and nanoscience. Acc. Chem. Res. 2003;36:807–815. doi: 10.1021/ar030027y. [DOI] [PubMed] [Google Scholar]
- 10.Pantarotto D, Tagmatarchis N, Bianco A, Prato M. Synthesis and biological properties of fullerene-containing amino acids and peptides. Mini Rev. Med. Chem. 2004;4:805–814. [PubMed] [Google Scholar]
- 11.Bagno A, Claeson S, Maggini M, Martini ML, Prato M, Scorrano G. 60]Fullerene as a substituent. Chemistry (Weinheim an der Bergstrasse, Germany) 2002;8:1015–1023. doi: 10.1002/1521-3765(20020301)8:5<1015::aid-chem1015>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 12.Martin N, Maggini M, Guldi DM. Fullerenes 2000 - Volume 9: Functionalized Fullerenes; Proceedings of the International Symposium; 2000. [Google Scholar]
- 13.Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. 2003;3:380–387. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 14.Castano AP, Demidova TN, Hamblin MR. Mechanisms in photodynamic therapy: part one-photosensitizers, photochemistry and cellular localization. Photodiagn. Photodyn. Ther. 2004;1:279–293. doi: 10.1016/S1572-1000(05)00007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castano AP, Demidova TN, Hamblin MR. Mechanisms in photodynamic therapy: part two-cellular signalling, cell metabolism and modes of cell death. Photodiagn. Photodyn. Ther. 2005;2 doi: 10.1016/S1572-1000(05)00030-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castano AP, Demidova TN, Hamblin MR. Mechanisms in photodynamic therapy: part three-photosensitizer pharmacokinetics, biodistribution, tumor localization and modes of tumor destruction. Photodiagn. Photodyn. Ther. 2005;2:91–106. doi: 10.1016/S1572-1000(05)00060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castano AP, Demidova TN, Hamblin MR. Mechanisms in photodynamic therapy: part one-photosensitizers, photochemistry and cellular localization. Photodiagn. Photodyn. Ther. 2004;1:279–293. doi: 10.1016/S1572-1000(05)00007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agostinis P, Buytaert E, Breyssens H, Hendrickx N. Regulatory pathways in photodynamic therapy induced apoptosis. Photochem. Photobiol. Sci. 2004;3:721–729. doi: 10.1039/b315237e. [DOI] [PubMed] [Google Scholar]
- 19.Moan J, Peng Q. An outline of the hundred-year history of PDT. Anticancer Res. 2003;23:3591–3600. [PubMed] [Google Scholar]
- 20.Jesionek A, von Tappenier H. Zur behandlung der hautcarcinomit mit fluorescierenden stoffen. Muench. Med. Wochneshr. 1903;47:2042. [Google Scholar]
- 21.Dougherty TJ. A brief history of clinical photodynamic therapy development at Roswell Park Cancer Institute. J. Clin. Laser Med. Surg. 1996;14:219–221. doi: 10.1089/clm.1996.14.219. [DOI] [PubMed] [Google Scholar]
- 22.Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baas P, van Mansom I, van Tinteren H, Stewart FA, van Zandwijk N. Effect of N-acetylcysteine on Photofrin-induced skin photosensitivity in patients. Lasers Surg. Med. 1995;16:359–367. doi: 10.1002/lsm.1900160407. [DOI] [PubMed] [Google Scholar]
- 24.Orenstein A, Kostenich G, Roitman L, Shechtman Y, Kopolovic Y, Ehrenberg B, Malik Z. A comparative study of tissue distribution and photodynamic therapy selectivity of chlorin e6, Photofrin II and ALA-induced protoporphyrin IX in a colon carcinoma model. Br. J. Cancer. 1996;73:937–944. doi: 10.1038/bjc.1996.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spikes JD. Chlorins as photosensitizers in biology and medicine. J. Photochem. Photobiol., B. 1990;6:259–274. doi: 10.1016/1011-1344(90)85096-f. [DOI] [PubMed] [Google Scholar]
- 26.Kessel D, Thompson P. Purification and analysis of hematoporphyrin and hematoporphyrin derivative by gel exclusion and reverse-phase chromatography. Photochem. Photobiol. 1987;46:1023–1025. doi: 10.1111/j.1751-1097.1987.tb04888.x. [DOI] [PubMed] [Google Scholar]
- 27.Boyle RW, Dolphin D. Structure and biodistribution relationships of photodynamic sensitizers. Photochem. Photobiol. 1996;64:469–485. doi: 10.1111/j.1751-1097.1996.tb03093.x. [DOI] [PubMed] [Google Scholar]
- 28.Gomer CJ. Preclinical examination of first and second generation photosensitizers used in photodynamic therapy. Photochem. Photobiol. 1991;54:1093–1107. doi: 10.1111/j.1751-1097.1991.tb02133.x. [DOI] [PubMed] [Google Scholar]
- 29.Nyman ES, Hynninen PH. Research advances in the use of tetrapyrrolic photosensitizers for photodynamic therapy. J. Photochem. Photobiol., B. 2004;73:1–28. doi: 10.1016/j.jphotobiol.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Detty MR, Gibson SL, Wagner SJ. Current clinical and preclinical photosensitizers for use in photodynamic therapy. J. Med. Chem. 2004;47:3897–3915. doi: 10.1021/jm040074b. [DOI] [PubMed] [Google Scholar]
- 31.Agostinis P, Vantieghem A, Merlevede W, de Witte PA. Hypericin in cancer treatment: more light on the way. Int. J. Biochem. Cell Biol. 2002;34:221–241. doi: 10.1016/s1357-2725(01)00126-1. [DOI] [PubMed] [Google Scholar]
- 32.Foote CS. Photophysical and photochemical properties of fullerenes. Top. Curr. Chem. 1994;169:347–363. [Google Scholar]
- 33.Greer A. Christopher Foote's discovery of the role of singlet oxygen [1O2 (1Δg)] in photosensitized oxidation reactions. Acc. Chem. Res. 2006;39:797–804. doi: 10.1021/ar050191g. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt R. Photosensitized generation of singlet oxygen. Photochem. Photobiol. 2006;82:1161–1177. doi: 10.1562/2006-03-03-IR-833. [DOI] [PubMed] [Google Scholar]
- 35.Arbogast JW, Darmanyan AP, Foote CS, Rubin Y, Diederich FN, Alvarez MM, Anz SJ, Whetten RL. Photophysical properties of C60. J. Phys. Chem. A. 1991;95:11–12. [Google Scholar]
- 36.Yamakoshi Y, Umezawa N, Ryu A, Arakane K, Miyata N, Goda Y, Masumizu T, Nagano T. Active oxygen species generated from photoexcited fullerene (C60) as potential medicines: O2−• versus 1O2. J. Am. Chem. Soc. 2003;125:12803–12809. doi: 10.1021/ja0355574. [DOI] [PubMed] [Google Scholar]
- 37.Foote CS. Definition of Type-I and Type-II photosensitized oxidation. Photochem. Photobiol. 1991;54:659–659. doi: 10.1111/j.1751-1097.1991.tb02071.x. [DOI] [PubMed] [Google Scholar]
- 38.Ochsner M. Photophysical and photobiological processes in the photodynamic therapy of tumours. J. Photochem. Photobiol., B. 1997;39:1–18. doi: 10.1016/s1011-1344(96)07428-3. [DOI] [PubMed] [Google Scholar]
- 39.Koeppe R, Sariciftci NS. Photoinduced charge and energy transfer involving fullerene derivatives. Photochem. Photobiol. Sci. 2006;5:1122–1131. doi: 10.1039/b612933c. [DOI] [PubMed] [Google Scholar]
- 40.Guldi DM, Prato M. Excited-state properties of C(60) fullerene derivatives. Acc. Chem. Res. 2000;33:695–703. doi: 10.1021/ar990144m. [DOI] [PubMed] [Google Scholar]
- 41.Arbogast JW, Foote CS, Kao M. Electron-transfer to triplet C-60. J. Am. Chem. Soc. 1992;114:2277–2279. [Google Scholar]
- 42.Miyata N, Yamakoshi Y, Nakanishi I. Reactive species responsible for biological actions of photoexcited fullerenes. J. Pharm. Soc. Jpn. 2000;120:1007–1016. doi: 10.1248/yakushi1947.120.10_1007. [DOI] [PubMed] [Google Scholar]
- 43.Yu C, Canteenwala T, El-Khouly ME, Araki Y, Pritzker K, Ito O, Wilson BC, Chiang LY. Efficiency of singlet oxygen production from self-assembled nanospheres of molecular micelle-like photosensitizers FC4S. J. Mater. Chem. 2005;15:1857–1864. [Google Scholar]
- 44.Mroz P, Pawlak A, Satti M, Lee H, Wharton T, Gali H, Sarna T, Hamblin MR. Functionalized fullerenes mediate photodynamic killing of cancer cells: Type I versus Type II photochemical mechanism. Free Radical Biol. Med. 2007;43:711–719. doi: 10.1016/j.freeradbiomed.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tegos GP, Demidova TN, Arcila-Lopez D, Lee H, Wharton T, Gali H, Hamblin MR. Cationic fullerenes are effective and selective antimicrobial photosensitizers. Chem. Biol. 2005;12:1127–1135. doi: 10.1016/j.chembiol.2005.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tokuyama H, Yamago S, Nakamura E. Photoinduced biochemical activity of fullerene carboxylic acid. J. Am. Chem. Soc. 1993;115:7918–7919. [Google Scholar]
- 47.An YZ, Chen CB, Anderson JL, Sigman DS, Foote CS, Rubin Y. Sequence-specific modification of guanosine in DNA by a C60-linked deoxyoligonucleotide: evidence for a non-singlet oxygen mechanism. Tetrahedron. 1996;52:5179–5189. [Google Scholar]
- 48.Boutorine AS, Tokuyama H, T. M, I. H, N. E, Helene C. Fullerene-oligonucleotide conjugates: photo-induced sequence-specific DNA cleavage. Angew. Chem., Int. Ed. Engl. 1994;33:2462–2465. [Google Scholar]
- 49.Nakanishi I, Fukuzumi S, Konishi T, Ohkubo K, Fujitsuka M, Ito O, Miyata N. DNA cleavage via electron transfer from NADH to molecular oxygen photosensitized by γ-cyclodextrin-bicapped C60. In: Kamat PV, Guldi DM, Kadish DM, editors. Fullerenes for the New Millennium. Vol. 11. The Electrochemical Society; Pennigton, NJ: 2001. pp. 138–151. [Google Scholar]
- 50.Yamakoshi Y, Sueyoshi S, Miyata N. Biological activity of photoexcited fullerene. Kokuritsu Iyakuhin Shokuhin Eisei Kenkyujo hokoku = Bulletin of National Institute of Health Sciences. 1999;117:50–60. [PubMed] [Google Scholar]
- 51.Yamakoshi YN, Yagami T, Sueyoshi S, Miyata N. Acridine Adduct of [60]Fullerene with Enhanced DNA-Cleaving Activity. J. Org. Chem. 1996;61:7236–7237. doi: 10.1021/jo961210q. [DOI] [PubMed] [Google Scholar]
- 52.Liu Y, Zhao YL, Chen Y, Liang P, Li L. A water-soluble beta cyclodextrin derivative possessing a fullerene tether as an efficient photodriven DNA-cleavage reagent. Tetrahedron Lett. 2005;46:2507–2511. [Google Scholar]
- 53.Ikeda A, Doi Y, Hashizume M, Kikuchi J, Konishi T. An extremely effective DNA photocleavage utilizing functionalized liposomes with a fullerene-enriched lipid bilayer. J. Am. Chem. Soc. 2007;129:4140–4141. doi: 10.1021/ja070243s. [DOI] [PubMed] [Google Scholar]
- 54.Sera N, Tokiwa H, Miyata N. Mutagenicity of the fullerene C60-generated singlet oxygen dependent formation of lipid peroxides. Carcinogenesis. 1996;17:2163–2169. doi: 10.1093/carcin/17.10.2163. [DOI] [PubMed] [Google Scholar]
- 55.Kamat JP, Devasagayam TP, Priyadarsini KI, Mohan H, Mittal JP. Oxidative damage induced by the fullerene C60 on photosensitization in rat liver microsomes. Chem.-Biol. Interact. 1998;114:145–159. doi: 10.1016/s0009-2797(98)00047-7. [DOI] [PubMed] [Google Scholar]
- 56.Kamat JP, Devasagayam TP, Priyadarsini KI, Mohan H. Reactive oxygen species mediated membrane damage induced by fullerene derivatives and its possible biological implications. Toxicology. 2000;155:55–61. doi: 10.1016/s0300-483x(00)00277-8. [DOI] [PubMed] [Google Scholar]
- 57.Yang XL, Huang C, Qiao XG, Yao L, Zhao DX, Tan X. Photo-induced lipid peroxidation of erythrocyte membranes by a bis-methanophosphonate fullerene. Toxicol. In Vitro. 2007 doi: 10.1016/j.tiv.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 58.Kasermann F, Kempf C. Photodynamic inactivation of enveloped viruses by buckminsterfullerene. Antiviral Res. 1997;34:65–70. doi: 10.1016/s0166-3542(96)01207-7. [DOI] [PubMed] [Google Scholar]
- 59.Kasermann F, Kempf C. Buckminsterfullerene and photodynamic inactivation of viruses. Rev. Med. Virol. 1998;8:143–151. doi: 10.1002/(sici)1099-1654(199807/09)8:3<143::aid-rmv214>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 60.Hirayama J, Abe H, Kamo N, Shinbo T, Ohnishi-Yamada Y, Kurosawa S, Ikebuchi K, Sekiguchi S. Photoinactivation of vesicular stomatitis virus with fullerene conjugated with methoxy polyethylene glycol amine. Biol. Pharm. Bull. 1999;22:1106–1109. doi: 10.1248/bpb.22.1106. [DOI] [PubMed] [Google Scholar]
- 61.Lin YL, Lei HY, Wen YY, Luh TY, Chou CK, Liu HS. Light-independent inactivation of dengue-2 virus by carboxyfullerene C3 isomer. Virology. 2000;275:258–262. doi: 10.1006/viro.2000.0490. [DOI] [PubMed] [Google Scholar]
- 62.Minnock A, Vernon DI, Schofield J, Griffiths J, Parish JH, Brown SB. Photoinactivation of bacteria. Use of a cationic water-soluble zinc phthalocyanine to photoinactivate both gram-negative and gram-positive bacteria. J. Photochem. Photobiol., B. 1996;32:159–164. doi: 10.1016/1011-1344(95)07148-2. [DOI] [PubMed] [Google Scholar]
- 63.Merchat M, Bertolini G, Giacomini P, Villanueva A, Jori G. Meso-substituted cationic porphyrins as efficient photosensitizers of gram-positive and gram-negative bacteria. J. Photochem. Photobiol., B. 1996;32:153–157. doi: 10.1016/1011-1344(95)07147-4. [DOI] [PubMed] [Google Scholar]
- 64.Hamblin MR, Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 2004;3:436–450. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Demidova TN, Hamblin MR. Photodynamic therapy targeted to pathogens. Int. J. Immunopathol. Pharmacol. 2004;17:245–254. doi: 10.1177/039463200401700304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Demidova TN, Hamblin MR. Effect of cell-photosensitizer binding and cell density on microbial photoinactivation. Antimicrob. Agents Chemother. 2005;49:2329–2335. doi: 10.1128/AAC.49.6.2329-2335.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lambrechts SA, Aalders MC, Langeveld-Klerks DH, Khayali Y, Lagerberg JW. Effect of monovalent and divalent cations on the photoinactivation of bacteria with meso-substituted cationic porphyrins. Photochem. Photobiol. 2004;79:297–302. doi: 10.1562/sa-03-15.1. [DOI] [PubMed] [Google Scholar]
- 68.Hancock RE, Bell A. Antibiotic uptake into gram-negative bacteria. Eur. J. Clin. Microbiol. Infect. Dis. 1988;7:713–720. doi: 10.1007/BF01975036. [DOI] [PubMed] [Google Scholar]
- 69.Spesia MB, Milanesio ME, Durantini EN. Synthesis, properties and photodynamic inactivation of Escherichia coli by novel cationic fullerene C(60) derivatives. Eur. J. Med. Chem. 2007 doi: 10.1016/j.ejmech.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 70.Scrivens WA, Tour JM, Creek KE, Pirisi L. Synthesis of C-14-labeled C-60, its suspension in water, and its uptake by human keratinocytes. J. Am. Chem. Soc. 1994;116:4517–4518. [Google Scholar]
- 71.Foley S, Crowley C, Smaihi M, Bonfils C, Erlanger BF, Seta P, Larroque C. Cellular localisation of a water-soluble fullerene derivative. Biochem. Biophys. Res. Commun. 2002;294:116–119. doi: 10.1016/S0006-291X(02)00445-X. [DOI] [PubMed] [Google Scholar]
- 72.Porter AE, Gass M, Muller K, Skepper JN, Midgley P, Welland M. Visualizing the uptake of C60 to the cytoplasm and nucleus of human monocyte-derived macrophage cells using energy-filtered transmission electron microscopy and electron tomography. Environ. Sci. Technol. 2007;41:3012–3017. doi: 10.1021/es062541f. [DOI] [PubMed] [Google Scholar]
- 73.Irie K, Nakamura Y, Ohigashi H, Tokuyama H, Yamago S, Nakamura E. Photocytotoxicity of water-soluble fullerene derivatives. Biosci., Biotechnol., Biochem. 1996;60:1359–1361. doi: 10.1271/bbb.60.1359. [DOI] [PubMed] [Google Scholar]
- 74.Burlaka AP, Sidorik YP, Prylutska SV, Matyshevska OP, Golub OA, Prylutskyy YI, Scharff P. Catalytic system of the reactive oxygen species on the C60 fullerene basis. Exp. Oncol. 2004;26:326–327. [PubMed] [Google Scholar]
- 75.Rancan F, Rosan S, Boehm F, Cantrell A, Brellreich M, Schoenberger H, Hirsch A, Moussa F. Cytotoxicity and photocytotoxicity of a dendritic C(60) mono-adduct and a malonic acid C(60) tris-adduct on Jurkat cells. J. Photochem. Photobiol., B. 2002;67:157–162. doi: 10.1016/s1011-1344(02)00320-2. [DOI] [PubMed] [Google Scholar]
- 76.Yang XL, Fan CH, Zhu HS. Photo-induced cytotoxicity of malonic acid [C(60)]fullerene derivatives and its mechanism. Toxicol. In Vitro. 2002;16:41–46. doi: 10.1016/s0887-2333(01)00102-3. [DOI] [PubMed] [Google Scholar]
- 77.Rancan F, Helmreich M, Molich A, Jux N, Hirsch A, Roder B, Witt C, Bohm F. Fullerene-pyropheophorbide a complexes as sensitizer for photodynamic therapy: uptake and photo-induced cytotoxicity on Jurkat cells. J. Photochem. Photobiol., B. 2005;80:1–7. doi: 10.1016/j.jphotobiol.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 78.Milanesio ME, Alvarez MG, Rivarola V, Silber JJ, Durantini EN. Porphyrin-fullerene C60 dyads with high ability to form photoinduced charge-separated state as novel sensitizers for photodynamic therapy. Photochem. Photobiol. 2005;81:891–897. doi: 10.1562/2005-01-24-RA-426. [DOI] [PubMed] [Google Scholar]
- 79.Alvarez MG, Prucca C, Milanesio ME, Durantini EN, Rivarola V. Photodynamic activity of a new sensitizer derived from porphyrin-C60 dyad and its biological consequences in a human carcinoma cell line. Int. J. Biochem. Cell Biol. 2006;38:2092–2101. doi: 10.1016/j.biocel.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 80.Ikeda A, Doi Y, Nishiguchi K, Kitamura K, Hashizume M, Kikuchi J, Yogo K, Ogawa T, Takeya T. Induction of cell death by photodynamic therapy with water-soluble lipid-membrane-incorporated [60]fullerene. Org. Biomol. Chem. 2007;5:1158–1160. doi: 10.1039/b701767g. [DOI] [PubMed] [Google Scholar]
- 81.Liang-Takasaki CJ, Makela PH, Leive L. Phagocytosis of bacteria by macrophages: changing the carbohydrate of lipopolysaccharide alters interaction with complement and macrophages. J. Immunol. 1982;128:1229–1235. [PubMed] [Google Scholar]
- 82.Li R, Bounds DJ, Granville D, Ip SH, Jiang H, Margaron P, Hunt DW. Rapid induction of apoptosis in human keratinocytes with the photosensitizer QLT0074 via a direct mitochondrial action. Apoptosis. 2003;8:269–275. doi: 10.1023/a:1023624922787. [DOI] [PubMed] [Google Scholar]
- 83.Granville DJ, Carthy CM, Jiang H, Shore GC, McManus BM, Hunt DW. Rapid cytochrome c release, activation of caspases 3, 6, 7 and 8 followed by Bap31 cleavage in HeLa cells treated with photodynamic therapy. FEBS Lett. 1998;437:5–10. doi: 10.1016/s0014-5793(98)01193-4. [DOI] [PubMed] [Google Scholar]
- 84.Gupta S, Ahmad N, Mukhtar H. Involvement of nitric oxide during phthalocyanine (Pc4) photodynamic therapy-mediated apoptosis. Cancer Res. 1998;58:1785–1788. [PubMed] [Google Scholar]
- 85.Kessel D, Luo Y, Mathieu P, Reiners JJ., Jr. Determinants of the apoptotic response to lysosomal photodamage. Photochem. Photobiol. 2000;71:196–200. doi: 10.1562/0031-8655(2000)071<0196:dotart>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 86.Alvarez MG, Principe F, Milanesio ME, Durantini EN, Rivarola V. Photodynamic damages induced by a monocationic porphyrin derivative in a human carcinoma cell line. Int. J. Biochem. Cell Biol. 2005;37:2504–2512. doi: 10.1016/j.biocel.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 87.Ross MF, Da Ros T, Blaikie FH, Prime TA, Porteous CM, Severina II, Skulachev VP, Kjaergaard HG, Smith RA, Murphy MP. Accumulation of lipophilic dications by mitochondria and cells. Biochem. J. 2006;400:199–208. doi: 10.1042/BJ20060919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rottenberg H. Membrane potential and surface potential in mitochondria: uptake and binding of lipophilic cations. J. Membr. Biol. 1984;81:127–138. doi: 10.1007/BF01868977. [DOI] [PubMed] [Google Scholar]
- 89.Murphy MP, Smith RA. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu. Rev. Pharmacol. Toxicol. 2007;47:629–656. doi: 10.1146/annurev.pharmtox.47.120505.105110. [DOI] [PubMed] [Google Scholar]
- 90.Hahn SM, Putt ME, Metz J, Shin DB, Rickter E, Menon C, Smith D, Glatstein E, Fraker DL, Busch TM. Photofrin uptake in the tumor and normal tissues of patients receiving intraperitoneal photodynamic therapy. Clin. Cancer Res. 2006;12:5464–5470. doi: 10.1158/1078-0432.CCR-06-0953. [DOI] [PubMed] [Google Scholar]
- 91.Tabata Y, Murakami Y, Ikada Y. Photodynamic effect of polyethylene glycol-modified fullerene on tumor. Jpn. J. Cancer Res. 1997;88:1108–1116. doi: 10.1111/j.1349-7006.1997.tb00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu J, Ohta S, Sonoda A, Yamada M, Yamamoto M, Nitta N, Murata K, Tabata Y. Preparation of PEG-conjugated fullerene containing Gd(3+) ions for photodynamic therapy. J. Controlled Release. 2007;117:104–110. doi: 10.1016/j.jconrel.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 93.Yu C, Canteenwala T, Chen HH, Chen BJ, Canteenwala M, Chiang LY. Hexa(sulfobutyl)fullerene-induced photodynamic effect on tumors in vivo and toxicity study in rats. Proc. Electrochem. Soc. 1999;99:234–249. [Google Scholar]