Abstract
Background
Postoperative cognitive dysfunction (POCD) is a significant cause of morbidity after noncardiac surgery. Identified risk factors are largely limited to demographic characteristics. We hypothesized that POCD was associated with Apolipoprotein E4 (APOE4) genotype and plasma biomarkers of brain injury and inflammation.
Methods
394 patients over age 55 undergoing major elective noncardiac surgery were enrolled in this prospective observational study. Apolipoprotein E genotyping was performed at baseline. Plasma was collected at baseline, end of surgery, 4.5, 24, and 48-h postoperatively. Six protein biomarkers were assayed (B-type natriuretic peptide, C-reactive protein, D-dimer, matrix metalloproteinase-9, neuron specific enolase, S-100B). Neurocognitive testing was conducted at baseline, 6 weeks, and 1 yr after surgery; scores were subjected to factor analysis. The association of APOE4 and biomarkers with POCD was tested using multivariable regression modeling.
Results
350 patients (89%) completed 6-week neurocognitive testing. POCD occurred in 54.3% of participants at 6 weeks and 46.1% at 1 yr. There was no difference in POCD between patients with or without the APOE4 allele (56.6 vs. 52.6%; p = 0.58). The continuous cognitive change score (mean ± SD) was similar between groups (APOE4: 0.05 ± 0.27 vs. non-APOE4: 0.07 ± 0.28; p = 0.53). 291 subjects (74%) completed testing at 1 yr. POCD occurred in 45.9% of APOE4 subjects versus 46.3% of non-APOE4 subjects (p = 0.95). The cognitive score was again similar (APOE4: 0.08 ± 0.27 vs. non-APOE4: 0.05 ± 0.25; p = 0.39). Biomarker levels were not associated with APOE4 genotype or cognition at 6 weeks or 1 yr.
Conclusion
Cognitive decline after major noncardiac surgery is not associated with APOE4 genotype or plasma biomarker levels.
Introduction
A significant number of elderly patients undergoing major noncardiac surgery will have a decline in cognitive function postoperatively.1,2,3 The etiology of this decline is unclear but likely involves a combination of patient, surgical, and anesthetic factors. Postoperative cognitive dysfunction (POCD) can be a manifestation of transient or permanent cerebral injury. While cognitive function tends to improve over months to years postoperatively in affected individuals, some proportion have seemingly permanent cognitive injury.4,5,6
Apolipoprotein E4 (APOE4) genotype has been shown to be a risk factor for worse cerebral injury and poor neurologic outcome from a variety of insults, including traumatic brain injury7 and intracranial hemorrhage,8 and is a risk factor for Alzheimer’s Disease9 and atherosclerosis.10 It is therefore conceivable that APOE4 genotype may influence susceptibility to POCD. However, clinical evidence over the past decade has been conflicting.11,12,13 APOE4 has also been implicated in the proinflammatory response.14,15,16,17 This link between APOE4 and a heightened inflammatory response along with reports of association between plasma inflammatory markers and POCD,18 suggest that APOE4, inflammation, and POCD may be interlinked. We therefore hypothesized that APOE4 subjects experience greater postoperative cognitive decline as a consequence of a heightened inflammatory response. We aimed to measure the magnitude and nature of this inflammatory response by assaying plasma biomarkers of inflammation and brain injury. The identification of a susceptible genotype could allow preoperative identification of patients likely to develop POCD. Similarly, a plasma biomarker panel assayed perioperatively could identify patients likely to develop POCD, allowing early intervention and management.
Materials and Methods
Study Population
This was a prospective observational study of patients over 55 yr of age undergoing general and/or regional (e.g., spinal, epidural) anesthesia for elective vascular, thoracic or major orthopedic (total hip or knee arthroplasty, or spine) surgery. The study was approved by the Duke University School of Medicine Institutional Review Board (Durham, North Carolina) and written informed consent was obtained from all participants. Patients with a history of symptomatic cerebrovascular disease (e.g., stroke with a residual deficit), uncontrolled hypertension, alcoholism (> 2 drinks/day), psychiatric illness (any clinical diagnoses requiring therapy), renal failure (serum creatinine > 2.0 mg/dl), active liver disease (liver function tests > 1.5 times the upper limit of normal), or undergoing surgery for cancer resection were excluded. Pregnant women and patients who were unable to read or who had less than a fifth grade education were also excluded.
Neurocognitive Testing
Experienced psychometricians examined subjects with a well-validated battery of cognitive tests on the day before surgery and again at 6 weeks and 1 yr after surgery. We used a cognitive test battery comprised of the following five instruments that yielded 10 scores:
The Short Story module of the Randt Memory Test requires subjects to recall the details of a short story immediately after it has been read to them and after a 30-min delay. Verbatim and gist recall are both evaluated; (4 scores)
The Digit Span subtest of the Wechsler Adult Intelligence Scale-Revised Test requires subjects to repeat a series of digits that have been orally presented to them both forward and, in an independent test, in reverse order; (2 scores)
Modified Visual Reproduction Test from the Wechsler Memory Scale measures short-and long-term figural memory and requires subjects to reproduce from memory several geometric shapes both immediately and after a 30-min delay; (2 scores)
The Digit Symbol subtest of the Wechsler Adult Intelligence Scale-Revised is a paper and pencil task that requires subjects to reproduce, within 90 s, as many coded symbols as possible in blank boxes beneath randomly generated digits according to a coding scheme for pairing digits with symbols; (1 score)
The Trail Making Test (part B) requires subjects to connect, by drawing a line, a series of numbers, and letters in sequence (i.e., 1-A-2-B) as quickly as possible. (1 score)
APOE Genotyping
APOE genotype was determined from preoperative whole-blood samples with a polymerase chain reaction-based restriction enzyme genotyping protocol as previously described.15 Genotyping accuracy was assessed in a subset of 75 patients in whom genotyping assays were also conducted by matrix assisted laser desorption/ionization time-of-flight mass spectrometry on a Sequenom MassArray system (Sequenom, San Diego, CA) at Agencourt Bioscience Corporation (Beverly, MA).
Biomarker Analysis
Whole blood was drawn at baseline, end of surgery, 4.5 h, 24 h, and 48 h postoperatively, in EDTA-containing tubes, and centrifuged at 3500 rpm for 15 min. Plasma was removed and stored at −70°C until the time of analysis. During shipment, samples were placed on dry ice in insulated coolers. B-type natriuretic peptide, C-reactive protein (CRP), D-dimer, matrix metalloproteinase-9, neuron specific enolase (NSE), and S-100 levels were assayed by Biosite Diagnostics (an Inverness Medical Company, Waltham, MA) through an interinstitutional agreement. These biomarkers were chosen based on our prior studies, including the BRAIN Study,19 and the existing literature.20,21
Immunoassays utilizing human plasma samples were performed in 384-well microtiter plates using Perkin-Elmer Minitrak for all liquid handling steps. Assays were variations of antibody sandwich assays or competitive assays using biotinylated antigen. All assays were heterogeneous and require multiple plate washes. Plates were washed three times with Borate Buffered Saline containing 0.02% Tween 20 (BBS-Tween). Samples were removed from −70° C, thawed at 37° C, and processed at room temperature. Test samples (10 μL/well) were added to the microtiter plates. In addition to test samples, each plate contained a calibration curve consisting of multiple analyte concentrations and controls. Calibration curves were prepared gravimetrically in plasma from healthy donors. For sandwich assays, one concentration in each set of calibrators included neutralizing antibody for correction of endogenous antigen present in the plasma pool. In situations where the sample must be diluted to fit within the calibration curve, the calibrators were prepared in a CD8 assay buffer (10 mmol/L Tris-HCl (pH 8.0), 150 mmol/L NaCl, 1 mmol/L MgCl2, 0.1 mmol/L ZnCl2, 10 mL/L polyvinyl alcohol (MW 9,000–10,000), 10 g/L bovine serum albumin, and 1 g/L NaN3). CD8 assay buffer was also used for sample dilution. The plates were read fluorometrically (Molecular Devices Spectramax 2). Calibration curves were eight points tested at multiple locations on the assay plate. The calibration curve was calculated using a five-parameter logistic fit and sample concentration was determined.
Statistical Methods
Sample Size Determination
The primary aim of the study was to determine the association of POCD with the presence of an APOE4 allele. It was estimated that the enrolment of 400 patients with an expected APOE4 allelic frequency of 25% would identify 100 patients with at least one APOE4 allele. Using our cognitive index, a change of 0.5 is considered clinically meaningful. With a starting sample size of 400, and a 15% loss to follow-up, a two group analysis of variance (ANOVA) test with a 0.050 two-sided significance level would have 90% power to detect a between-group difference of 0.25 in cognitive change scores (assuming a standard deviation of 0.6, estimated from our previous work in coronary artery bypass graft surgery patients).
Statistical Analysis
To characterize cognitive function over time, while minimizing potential redundancy in the cognitive measures, a factor analysis was performed on the 10 cognitive test scores from baseline.22 The ten scores were incorporated into a principal components analysis with orthogonal rotation (a linear transformation of the data) to produce uncorrelated factors. The factor analysis was conducted on the enrolled subjects in this study, and scoring coefficients for all time points were determined using this sample’s baseline rotated factor scores; thus, cognitive domains remained consistent over time. We chose a four-factor solution, which accounts for 84% of the variability in the original 10 test scores and represents four cognitive domains: 1) verbal memory 2) abstraction and visuo-spatial orientation (executive function) 3) visual memory and 4) attention and concentration. Two summary measures were calculated to represent cognitive function: 1) POCD (the dichotomous outcome) was defined as a decline of one standard deviation or more in at least one of the four domains. 2) To quantify overall cognitive function and the degree of learning (i.e., practice effect from repeated exposure to the testing procedures), a baseline cognitive index was first calculated as the mean of the four preoperative domain scores. The cognitive index score has a mean of zero and standard deviation of 0.5. Thus, any positive score is above the mean, any negative score is below the mean, and a score of 0.5 represents one SD above the mean. A continuous change score (the continuous outcome) was then calculated by subtracting the baseline from the follow-up cognitive index. Negative scores indicate decline and positive scores indicate improvement.
Categorical and continuous demographic characteristics were compared between groups with Pearson Chi-Square, Fisher Exact, and t-tests. Hardy-Weinberg equilibrium was assessed for the APOE polymorphisms using an exact test. The effect of APOE4 genotype on the cognitive change score (continuous outcome) and POCD (dichotomous outcome) at 6 weeks and 1 yr was tested using multivariable linear and logistic regression modeling, respectively. Covariates were chosen a priori and remained consistent across all models. Included in each model were: baseline cognitive index, age, years of education, surgery type (defined as orthopedic vs. other), anesthesia type (general vs. regional), gender, diabetes, and the interaction between age and APOE4. Patients were classified as having general anesthesia if they received any general anesthesia, regardless of other anesthesia administered. Interactions between APOE4 status and each covariate were also assessed.
Associations between APOE4 status and biomarker levels were analyzed using repeated measures analysis of variance (ANOVA) based on log transformation with unstructured covariance and Tukey adjustment for post hoc pairwise comparisons. Furthermore, in post hoc analyses, adjusted with a Bonferroni correction for multiple comparisons, aassociations between biomarker levels and the continuous and dichotomous cognitive outcomes at 6 weeks and 1 yr were analyzed with individual multivariable models. Each biomarker was characterized as change from baseline to maximum value within 48 h of surgery. As in the primary analysis, covariates were chosen a priori and remained consistent across models. APOE4 was also tested in each model, as well as the interaction between APOE4 and the biomarker. Finally, models were constructed using all biomarker levels together in the same analysis in an attempt to identify a potential panel of biomarker predictors of cognitive decline.
Results
From March 2000 to January 2005, a total of 520 patients were consented to participate in the study. Of these, 126 patients withdrew voluntarily leaving 394 patients who completed enrolment. Patient and procedural characteristics are shown in table 1 for all enrolled subjects. 24% of subjects had at least one copy of the APOE4 allele and these subjects were more likely to be younger than those without the APOE4 allele. The APOE polymorphisms were in Hardy-Weinberg equilibrium and genotyping accuracy was 100%.
Table 1.
Demographic Characteristics of the Study Population.*
| All Patients (n=394) | Non-APOE4 (n=276) | APOE4 (n=87) | P value | |
|---|---|---|---|---|
| Female Gender (%) | 50% | 49.3 | 49.4 | 1.00 |
| White race (%) | 83.5 | 85.9 | 78.2 | 0.09 |
| Diabetes (%) | 16.0 | 15.9 | 16.1 | 1.00 |
| History of Hypertension (%) | 66.2 | 67.4 | 64.4 | 0.69 |
| Orthopedic Surgery (%) | 74.1 | 73.9 | 74.7 | 0.89 |
| General Anesthesia (%) | 60.8 | 59.6 | 65.5 | 0.37 |
| Age (years) | 68.1 (8.3) | 68.9 (8.3) | 66.1 (8.2) | 0.005 |
| Education (years) | 14.2 (3.3) | 14.2 (3.4) | 14.1 (3.2) | 0.94 |
| Preoperative Cognitive Index | -0.01 (0.50) | -0.04 (0.49) | 0.02 (0.51) | 0.31 |
31 subjects did not have APOE genotyping data
APOE = apolipoprotein E4
Among the 350 (89%) patients who returned for follow-up testing at 6 weeks, POCD was seen in 56.6% with the APOE4 allele compared to 52.6% in those without the allele (p = 0.58). The continuous cognitive score was also not significantly different between the groups (APOE4 [mean ± SD]: 0.05 ± 0.27 vs. non-APOE4: 0.07 ± 0.28; p = 0.53). Multivariable regression analyses adjusting for baseline cognitive index, age, years of education, surgery type, anesthesia type, gender, and diabetes revealed no significant association between APOE4 status and POCD (p = 0.40, table 2), or between APOE4 status and the continuous cognitive change score (p = 0.47, table 3). At one year after surgery, 291 subjects (74%) underwent cognitive testing. POCD was present in 45.9% of APOE4 subjects compared to 46.3% of non-APOE4 subjects (p = 0.95). The continuous cognitive score (mean ± SD) was 0.08 ± 0.27 in the APOE4 group versus 0.05 ± 0.25 in the non-APOE4 group (p = 0.39). Multivariable regression again revealed no significant association between APOE4 status and cognition at 1 yr after surgery. There was also no interaction between age and APOE4 status at either 6 weeks (p = 0.22) or 1 yr (p = 0.79).
Table 2.
Multivariable Logistic Regression Model Predicting Cognitive Deficit (Dichotomous Outcome; i.e., POCD Present or Absent) at 6-week Follow-up.
| Variable | Odds Ratio [95% confidence limits] | P value |
|---|---|---|
| Age | 1.03 [0.99 – 1.06] | 0.10 |
| Years of Education | 0.98 [0.91 – 1.07] | 0.67 |
| Preoperative cognitive index | 2.42 [1.30 – 4.49] | 0.005 |
| Female sex | 1.10 [0.69 – 1.74] | 0.69 |
| Diabetes | 2.34 [1.22 – 4.51] | 0.01 |
| Orthopedic surgery | 0.99 [0.58 – 1.71] | 0.99 |
| General anesthesia | 1.10 [0.68 – 1.76] | 0.71 |
| APOE4 | 1.26 [0.74 – 2.17] | 0.40 |
APOE4 = Apolipopritein E4; POCD = postoperative cognitive dysfunction
Table 3.
Multivariable Linear Regression Model Predicting the Cognitive Change Score (Continuous Outcome) at 6-week Follow-up.
| Variable | Parameter Estimate (95% confidence limits) | P value |
|---|---|---|
| Age | -0.004 [-0.008 - (-0.0005)] | 0.03 |
| Years of Education | 0.009 [-0.001 - 0.020] | 0.03 |
| Preoperative cognitive index | -0.13 [-0.213 - (-0.054)] | 0.001 |
| Female sex | 0.041 [-0.019 - 0.102] | 0.18 |
| Diabetes | -0.007 [-0.089 - 0.075] | 0.87 |
| Orthopedic surgery | -0.029 [-0.100 - 0.041] | 0.41 |
| General anesthesia | -0.013 [-0.075 - 0.048] | 0.67 |
| APOE4 | -0.026 [-0.096 - 0.044] | 0.47 |
APOE4 = Apolipoprotein E4
At 6 weeks, 25% of the patients lost to follow-up were APOE4+ compared to 21.7% of the returners (p = 0.69). Similarly, at 1 yr, 25.2% of non-returners were APOE4+ compared to 21% of the returners (p = 0.49). Among potential predictors of loss to follow-up, including age, diabetes, gender, orthopedic surgery, general anesthesia, APOE4 status, and baseline cognitive score, only baseline cognitive score was significantly associated with not returning (p < 0.001).
For the dichotomous outcome, POCD, 1-yr mortality was 3.16% in those with POCD and 1.25% in those without POCD (p = 0.23) at 6 weeks. For the continuous outcome, mean change in cognitive index score (mean ± SD) at 6 weeks was -0.15 ± 0.32 for patients who died by 1 yr, and 0.06 ± 0.28 for those alive at 1 yr (p = 0.04).
Plasma levels of B-type natriuretic peptide, CRP, D-dimer, and matrix metalloproteinase-9, unlike levels of NSE and S-100, increased from baseline over time but none of these levels were significantly different between the APOE4 groups (p > 0.15, figs. 1A-F). No significant associations were found, at 6 weeks or 1 yr, between either the continuous or dichotomous (POCD) outcome and any of the biomarkers. Significant interactions were also not detected between biomarker level and APOE4 status after adjustment for multiple comparisons. Finally, in the models which considered the biomarkers together as joint predictors, no combination of biomarkers yielded significant associations with cognitive outcome.
Figure 1.
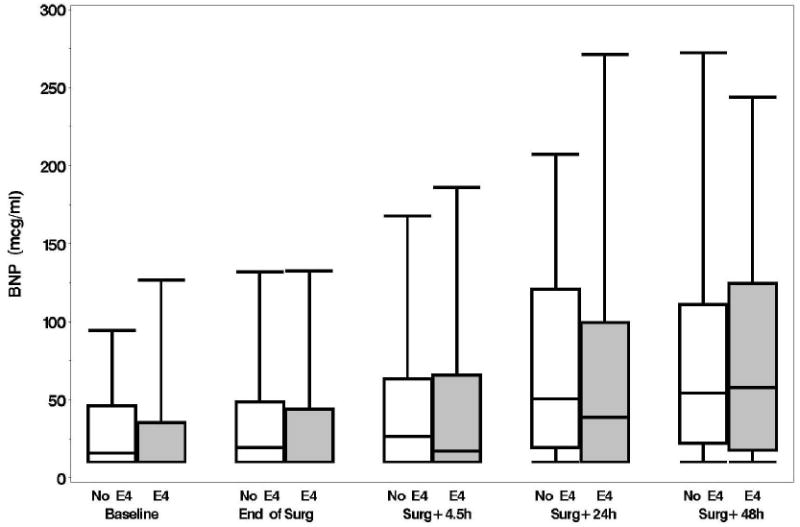
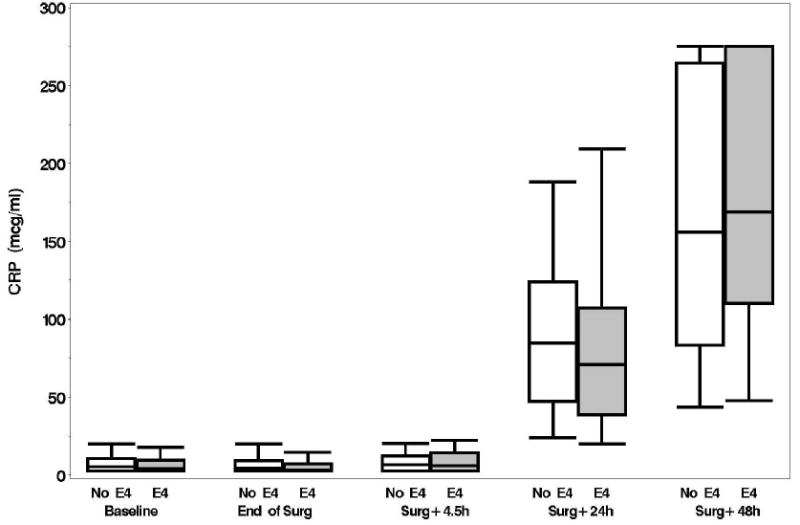
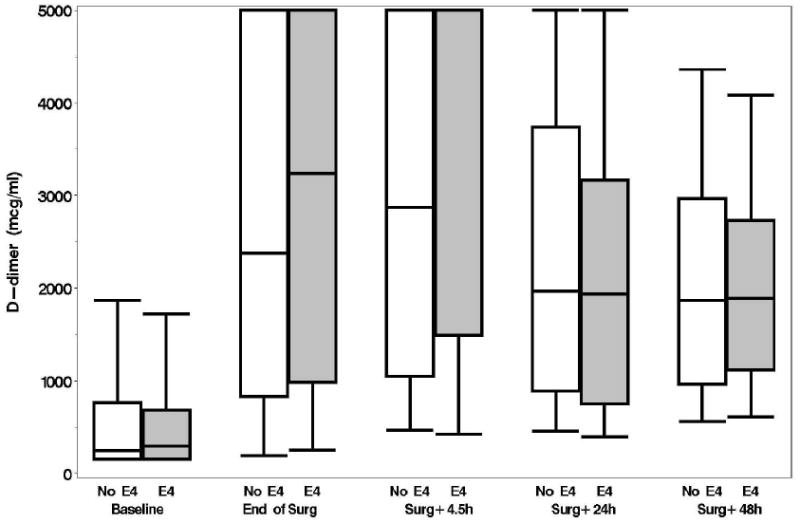
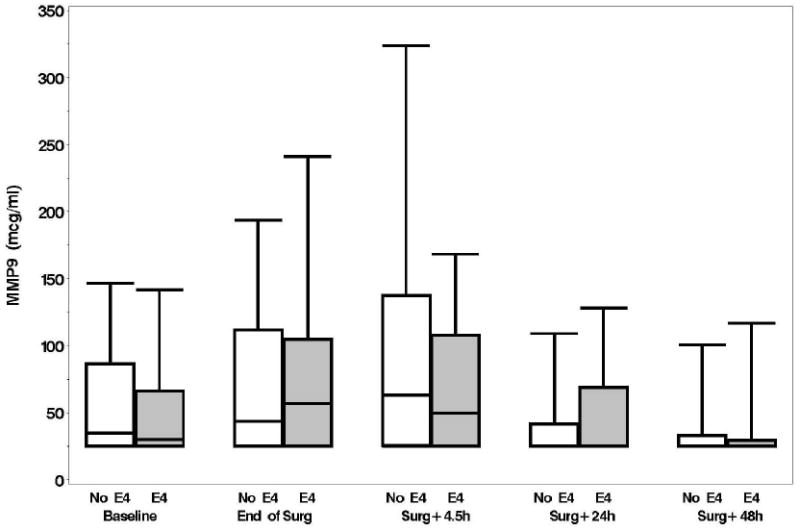
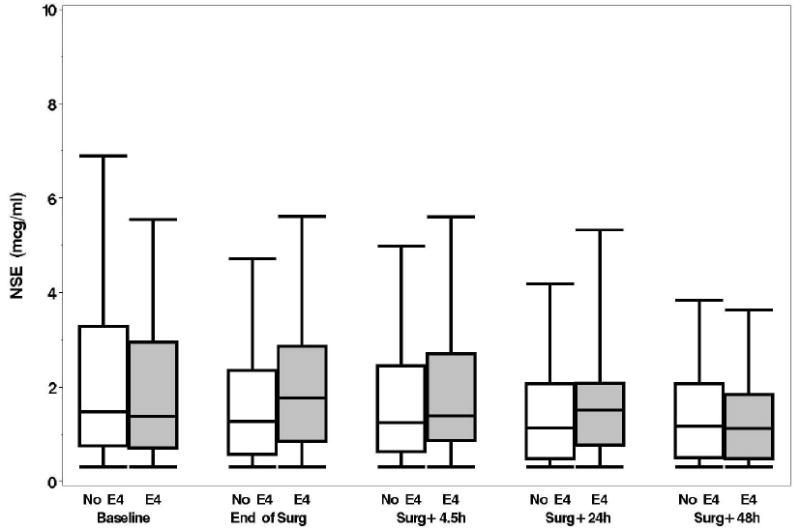
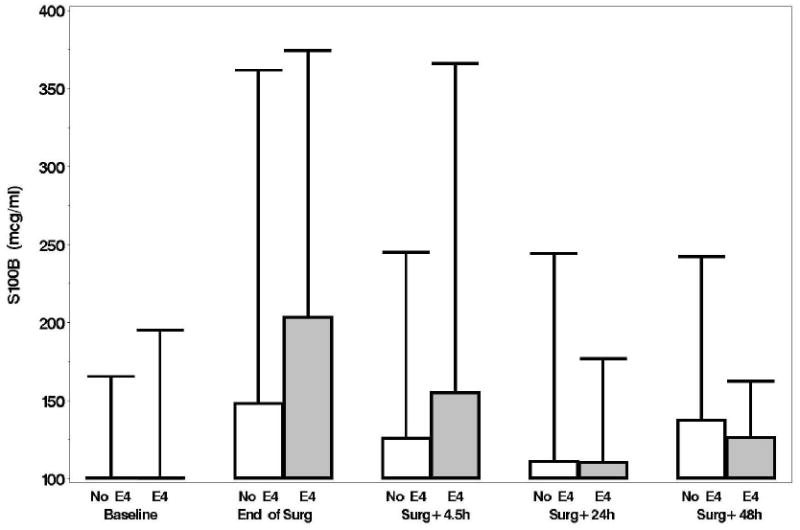
A: Boxplots of biomarker levels in non-APOE4 (white) and APOE4 (shaded) subjects for perioperative time points. Boxes enclose 25th to 75th percentiles; vertical whiskers extend to 10th and 90th percentiles. Median (50th percentile) is marked by the line inside of the box. (Where all five percentile lines are not visible in a plot, the percentiles coincide). BNP
B: CRP
C: D-Dimer
D: MMP-9
E: NSE
F: S100b (100 mcg/ml is the lower limit of the assay)
Discussion
To our knowledge, this study is the first combined analysis of POCD after noncardiac surgery, APOE4 genotype, and plasma biomarkers of brain injury and inflammation. To date, it is also the largest analysis of plasma biomarkers and cognitive decline in noncardiac surgery. Despite a robust sample size, we were unable to find an association between cognitive decline and APOE4 genotype. Similarly, we could find no association between perioperative B-type natriuretic peptide, CRP, D-dimer, matrix metalloproteinase-9, NSE, and S100B levels and postoperative cognitive decline or APOE4 genotype.
Our data illustrate the high incidence of POCD in elderly patients undergoing major noncardiac surgery, 54.3% at 6 weeks and 46.1% at 1 yr. In a comparable patient population, the International Study of Postoperative Cognitive Dysfunction 1 (International Study of Postoperative Cognitive Dysfunction 1) has reported a 25.8% and 9.9% prevalence of POCD at 1 week and 3 months respectively,1 while Monk et al. found a 41.4% and 12.7% prevalence of POCD, at discharge and 3 months respectively.3 The much higher incidence seen in our study may be a consequence of a significantly larger orthopedic cohort (74% of patients) or may be attributable to differences in methodology including the definition of POCD and the use of a control group. Despite the differing rates of POCD, advancing age and baseline cognition or level of education were consistent as predictors of POCD across all 3 studies. As with the International Study of Postoperative Cognitive Dysfunction 2 study,2 we were unable to detect a significant beneficial effect of regional anesthesia. The dominance of baseline cognitive capacity in all studies suggests that cognitive reserve, the increased threshold for neurospsychological impairment in the presence of favorable genetic backgrounds and environments (such as high educational level), may be a significant factor in postoperative cognitive injury.23 In addition to establishing predictors of POCD in the noncardiac population, our data supports the hypothesis that POCD is associated with worse long-term outcome. Similar to the findings of Monk et al.3 we note a trend toward increased 1-yr mortality in patients with cognitive decline at 6 weeks after surgery.
A large number of twin, adoption, and longitudinal family studies suggest that genetic factors account for over 50% of the variance in adult cognitive abilities, mainly by influencing differences in the general cognitive factor, but also by modulating different cognitive domains and specific mental abilities.24 For example, McClearn et al. used a large range of tests in elderly twins and found heritabilities of 0.62 for general cognitive ability, 0.55 for verbal ability, 0.32 for spatial ability, 0.62 for speed and processing, and 0.52 for memory.25 Similarly, measures of executive control were found to show a range of heritabilities from 0.34 to 0.68. Interestingly, all studies converge on the conclusion that genetic factors increase while shared environmental factors decrease in importance with advancing age. Roughly 50% of the phenotypic variance in human cognitive ability is stable from age 11 to age 80.26 Studies of elderly twins found heritabilities of 0.60-0.70 at very old ages (80 and older),27,28 with greater influence at the higher levels of ability.29 Thus, genetic influences in cognitive aging arise from a combination of contributions to the life-long trait of intelligence in general, and to any influences specific to old age.30
The influence of genetics upon POCD, however, is not as clear, particularly in noncardiac surgical patients. Despite the robust connection between APOE4 genotype and outcome after a variety of brain injuries including traumatic brain injury,7 aneurysmal subarachnoid hemorrhage,8 and intracerebral hemorrhage,15 a link to POCD has been elusive. Abildstrom et al. studied the relationship between APOE4 and cognitive dysfunction after major noncardiac surgery in 972 patients from the International Study of Postoperative Cognitive Dysfunction 2 and found no difference in the incidence of POCD (10.3% vs. 9.9%) between patients with and without the APOE4 allele.13 Similarly, we found no association of APOE4 with postoperative cognitive decline in the current study of 350 noncardiac surgery patients as well as in a study of 513 coronary artery bypass graft surgery patients.18 In cardiac surgical patients, however, we did find that minor alleles of the CRP 1059G/C single nulceotide polymorphism (OR: 0.37, 95% CI: 0.16-0.78, p = 0.013) and the SELP 1087G/A single nulceotide polymorphism (OR: 0.51, 95% CI: 0.30-0.85, p = 0.011) were associated with a reduction in cognitive deficit in European Americans.18 The absolute risk reduction in the observed incidence of POCD was 20.6% for carriers of the CRP 1059C allele and 15.2 % for carriers of the SELP 1087A allele. Perioperative serum CRP and degree of platelet activation were also significantly lower in patients with a copy of the minor alleles, providing biological support for the observed allelic association. Thus, age-related postoperative cognitive dysfunction is likely a complex phenotype, influenced by a large number of genetic variants of small effects (polygenic) and their interaction with environmental factors such as the magnitude of surgical-anesthetic injurious stimuli, drugs used in the perioperative period, and postoperative complications.
The mechanism by which APOE4 exerts detrimental effects upon cognition is unclear. Potential etiologies include APOE specific effects on cerebral blood flow (CBF),31,32 altered responses to neuronal injury,7 cerebral metabolic decline,33 and/or increased cerebral microemboli secondary to increased atheroma burden.10 Furthermore, multiple lines of evidence support the involvement of APOE4 in the inflammatory response. APOE modulates the central nervous system inflammatory response to injury by modifying glial activation,34 nitric oxide production,35 inflammatory cytokine production,14 magnitude of cerebral edema,15 and is thought to do so in an isoform specific manner. Effects are seen outside of the central nervous system as well. Carriers of the APOE4 allele have an increased inflammatory response to cardiopulmonary bypass,16,36 and sepsis.37 To test these hypotheses, a synthetic apoE peptide has been shown to cross the blood brain barrier, modulate the central nervous system inflammatory response to injury, and holds potential as a pharmacologic therapeutic.14,17
In the noncardiac surgical population data on inflammatory plasma biomarkers are sparse.38 The evaluation of plasma biomarkers of brain injury, NSE and S-100, has also yielded conflicting results. NSE is generally considered a marker of neuronal injury, while S100B is a marker of astrocytic (glial) injury.20,21 Rasmussen et al. studied 65 elderly patients undergoing abdominal surgery and found no association between NSE or S100B levels and cognitive decline.39 Similarly, Linstedt et al. studied S100 and NSE in 120 patients undergoing noncardiac surgery and found an association of S100 but not NSE with early cognitive dysfunction (less than 1 week postoperatively).40 Overall, NSE did not rise in any of their patients while S100B rose slightly but returned to normal by 18h. Unfortunately, there was no longer term follow-up so it is difficult to exclude the influence of delirium on these results. In the largest study to date, however, we find that there is no association between a pre-specified panel of 6 plasma biomarkers and APOE4 or POCD. Although the majority of the biomarkers increased over time suggesting an active inflammatory response, the flat S100 and NSE profiles suggest an absence of significant neuronal injury or blood brain barrier breakdown in most patients.
There are several limitations to our data and to the conclusions that can be drawn from this study. First, we did not have complete follow-up at 1 yr, missing 26% of the original cohort. Second, the high prevalence of POCD in this prospective observational trial may be related to the lack of a nonsurgical control group. Although a control group was not incorporated into our study design, largely due to funding limitations, our finding of an insignificant effect of APOE4 genotype upon POCD remains valid. Third, although no gold standard exists, defining POCD by establishing a threshold value on cognitive test scores is arbitrary. Our use of a one SD decline in any of four neurocognitive domains is also less conservative than some other study groups. Therefore, we have analyzed the continuous cognitive change score as well and again demonstrate no significant associations between APOE4, cognition, and biomarkers. Fourth, we analyzed only APOE4 and cannot address the broader question of a genetic basis for cognitive decline in this population. A dominant APOE4 genetic model was used in this study based on the available literature.7-11 It is possible that other APOE alleles might correlate with cognitive outcome in a more robust fashion, although post hoc analyses did not suggest that this is the case. A more expansive evaluation of genetic factors, as we had conducted in the cardiac surgical population, may have yielded a significant association.18 Fifth, our biomarker panel was designed to assess brain injury and inflammation but was limited to 6 biomarkers. Clearly, other plasma biomarkers may correlate with cognitive decline. Finally, as presented in figures 1A-B, B-type natriuretic peptide and CRP levels continued to rise through 48 h, suggesting that the inflammatory response had not peaked at 48 h. Plasma sampling beyond 48 h may have identified associations missed in our investigation.
In conclusion, we were unable to find an association between cognitive decline after major noncardiac surgery and APOE4 genotype. Similarly, we found no association between perioperative B-type natriuretic peptide, CRP, D-dimer, matrix metalloproteinase-9, NSE, or S100B levels and postoperative cognitive decline or APOE4 genotype. Future studies of biomarkers in POCD should utilize a combined genomics and proteomics approach, utilize a larger panel of inflammatory biomarkers, and monitor plasma levels beyond 48 h.
Acknowledgments
The authors would like to thank Roger Hall, B.S. (Study Coordinator), Duke University Department of Anesthesiology (Durham, North Carolina), and Julie Maiero, B.S., Director Clinical Operations, & William Arnold, Ph.D., Principal Scientist, Biosite Diagnostics (an Inverness Medical Company, Waltham, Massachusetts).
Sources of financial support: Supported in part by grants #AG016762-05 (Dr. Newman) and M01-RR-30 from the National Institutes of Health, Washington, D.C., and by the Division of Cardiothoracic Anesthesiology and Critical Care Medicine, Department of Anesthesiology, Duke University Medical Center, Durham, North Carolina. Biomarker analysis was performed by Biosite Diagnostics (an Inverness Medical Company, Waltham, MA) through an interinstitutional agreement.
Footnotes
Disclosures: Dr. Laskowitz is a consultant for Biosite Diagnostics.
Meetings at which the work has been presented: American Society of Anesthesiologists Annual Meeting 2009, October 20, 2009, New Orleans, Louisiana.
References
- 1.Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, Rabbitt P, Jolles J, Larsen K, Hanning CD, Langeron O, Johnson T, Lauven PM, Kristensen PA, Biedler A, van Beem H, Fraidakis O, Silverstein JH, Beneken JEW, Gravenstein JS for the International Study of Postoperative Cognitive Dysfunction investigators. Long-term postoperative cognitive dysfunction in the elderly: International Study of Postoperative Cognitive Dysfunction 1 study. Lancet. 1998;351:857–61. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- 2.Rasmussen LS, Johnson T, Kuipers HM, Kristensen D, Siersma VD, Vila P, Jolles J, Papaioannou A, Abildstrom H, Silverstein JH, Bonal JA, Raeder J, Nielsen IK, Korttila K, Munoz L, Dodds C, Hanning CD, Moller JT. International Study of Postoperative Cognitive Dysfunction 2 Investigators: Does anaesthesia cause postoperative cognitive dysfunction? A randomised study of regional versus general anaesthesia in 438 elderly patients. Acta Anaesthesiol Scand. 2003;47:260–6. doi: 10.1034/j.1399-6576.2003.00057.x. [DOI] [PubMed] [Google Scholar]
- 3.Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, Gravenstein JS. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18–30. doi: 10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- 4.Steinmetz J, Christensen KB, Lund T, Lohse N, Rasmussen LS for the International Study of Postoperative Cognitive Dysfunction Group. Long-term consequences of postoperative cognitive dysfunction. Anesthesiology. 2009;110:548–55. doi: 10.1097/ALN.0b013e318195b569. [DOI] [PubMed] [Google Scholar]
- 5.Abildstrom H, Rasmussen LS, Rentowl P, Hanning CD, Rasmussen H, Kristensen PA, Moller JT. Cognitive dysfunction 1-2 years after non-cardiac surgery in the elderly. International Study of Postoperative Cognitive Dysfunction group. International Study of Post-Operative Cognitive Dysfunction. Acta Anaesthesiol Scand. 2000;44:1246–51. doi: 10.1034/j.1399-6576.2000.441010.x. [DOI] [PubMed] [Google Scholar]
- 6.Phillips-Bute B, Mathew JP, Blumenthal JA, Grocott HP, Laskowitz DT, Jones RH, Mark DB, Newman MF. Association of neurocognitive function and quality of life 1 year after coronary artery bypass graft surgery. Psychosom Med. 2006;68:369–75. doi: 10.1097/01.psy.0000221272.77984.e2. [DOI] [PubMed] [Google Scholar]
- 7.Zhou W, Xu D, Peng X, Zhang Q, Jia J, Crutcher KA. Meta-analysis of APOE4 allele and outcome after traumatic brain injury. J Neurotrauma. 2008;25:279–90. doi: 10.1089/neu.2007.0489. [DOI] [PubMed] [Google Scholar]
- 8.Lanterna LA, Ruigrok Y, Alexander S, Tang J, Biroli F, Dunn LT, Poon WS. Meta-analysis of APOE genotype and subarachnoid hemorrhage: clinical outcome and delayed ischemia. Neurology. 2007;69:766–75. doi: 10.1212/01.wnl.0000267640.03300.6b. [DOI] [PubMed] [Google Scholar]
- 9.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–3. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 10.Ti LK, Mackensen GB, Grocott HP, Laskowitz DT, Phillips-Bute BG, Milano CA, Hilton AK, Newman MF, Mathew JP. Neurologic Outcome Research Group: Apolipoprotein E4 increases aortic atheroma burden in cardiac surgical patients. J Thorac Cardiovasc Surg. 2003;125:211–3. doi: 10.1067/mtc.2003.123. [DOI] [PubMed] [Google Scholar]
- 11.Tardiff BE, Newman MF, Saunders AM, Strittmatter WJ, Blumenthal JA, White WD, Croughwell ND, Davis RD, Jr, Roses AD, Reves JG. Preliminary report of a genetic basis for cognitive decline after cardiac operations. The Neurologic Outcome Research Group of the Duke Heart Center. Ann Thorac Surg. 1997;64:715–20. doi: 10.1016/s0003-4975(97)00757-1. [DOI] [PubMed] [Google Scholar]
- 12.Silbert BS, Evered LA, Scott DA, Cowie TF. The apolipoprotein E4 allele is not associated with cgnitive dysfunction in cardiac surgery. Ann Thorac Surg. 2008;86:841–8. doi: 10.1016/j.athoracsur.2008.04.085. [DOI] [PubMed] [Google Scholar]
- 13.Abildstrom H, Christiansen M, Siersma VD, Rasmussen LS. Apolipoprotein E genotype and cognitive dysfunction after noncardiac surgery. Anesthesiology. 2004;101:855–61. doi: 10.1097/00000542-200410000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Lynch JR, Tang W, Wang H, Vitek MP, Bennett ER, Sullivan PM, Warner DS, Laskowitz DT. APOE genotype and an ApoE-mimetic peptide modify the systemic and central nervous system inflammatory response. J Biol Chem. 2003;278:48529–33. doi: 10.1074/jbc.M306923200. [DOI] [PubMed] [Google Scholar]
- 15.James ML, Blessing R, Bennett E, Laskowitz DT. Apolipoprotein E modifies neurological outcome by affecting cerebral edema but not hematoma size after intracerebral hemorrhage in humans. J Stroke Cerebrovasc Dis. 2009;18:144–9. doi: 10.1016/j.jstrokecerebrovasdis.2008.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grocott HP, Newman MF, El-Moalem H, Bainbridge D, Butler A, Laskowitz DT. APOE genotype differentially influences the pro and anti-inflammatory response to cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2001;122:622–3. doi: 10.1067/mtc.2001.115152. [DOI] [PubMed] [Google Scholar]
- 17.James ML, Sullivan PM, Lascola CD, Vitek MP, Laskowitz DT. Pharmacogenomic Effects of Apolipoprotein E on Intracerebral Hemorrhage. Stroke. 2009;40:632–9. doi: 10.1161/STROKEAHA.108.530402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathew JP, Podgoreanu MV, Grocott HP, White WD, Morris RW, Stafford-Smith M, Mackensen GB, Rinder CS, Blumenthal JA, Schwinn DA, Newman MF. PEGASUS Investigative Team: Genetic variants in P-selectin and C-reactive protein influence susceptibility to cognitive decline after cardiac surgery. J Am Coll Cardiol. 2007;49:1934–42. doi: 10.1016/j.jacc.2007.01.080. [DOI] [PubMed] [Google Scholar]
- 19.Laskowitz DT, Kasner SE, Saver J, Remmel KS, Jauch EC for the BRAIN Study Group. Clinical usefulness of a biomarker-based diagnostic test for acute stroke. Stroke. 2009;40:77–85. doi: 10.1161/STROKEAHA.108.516377. [DOI] [PubMed] [Google Scholar]
- 20.Anand N, Stead LG. Neuron-specific enolase as a marker for acute ischemic stroke: A systematic review. Cerebrovasc Dis. 2005;20:213–9. doi: 10.1159/000087701. [DOI] [PubMed] [Google Scholar]
- 21.Kleindienst A, Bullock MR. A critical analysis of the role of the neurotrophic protein S100B in acute brain injury. J Neurotrauma. 2006;23:1185–200. doi: 10.1089/neu.2006.23.1185. [DOI] [PubMed] [Google Scholar]
- 22.Newman MF, Kirchner JL, Phillips-Bute B, Gaver V, Grocott H, Jones RH, Mark DB, Reves JG, Blumenthal JA for the Neurological Outcome Research Group and the Cardiothoracic Anesthesiology Research Endeavors Investigators. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med. 2001;344:395–402. doi: 10.1056/NEJM200102083440601. [DOI] [PubMed] [Google Scholar]
- 23.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–60. [PubMed] [Google Scholar]
- 24.Bouchard TJ, Jr, McGue M. Genetic and environmental influences on human psychological differences. J Neurobiol. 2003;54:4–45. doi: 10.1002/neu.10160. [DOI] [PubMed] [Google Scholar]
- 25.McClearn GE, Johansson B, Berg S, Pedersen NL, Ahern F, Petrill SA, Plomin R. Substantial genetic influence on cognitive abilities in twins 80 or more years old. Science. 1997;276:1560–3. doi: 10.1126/science.276.5318.1560. [DOI] [PubMed] [Google Scholar]
- 26.Deary IJ, Whalley LJ, Lemmon H, Crawford JR, Starr JM. The stability of individual differences in mental ability from childhood to old age: Follow-up of the 1932 Scottish Mental Survey. Intelligence. 2000;28:49–55. [Google Scholar]
- 27.Swan GE, Carmelli D. Evidence for genetic mediation of executive control: A study of aging male twins. J Gerontol B Psychol Sci Soc Sci. 2002;57:133–43. doi: 10.1093/geronb/57.2.p133. [DOI] [PubMed] [Google Scholar]
- 28.Finkel D, Pedersen NL, Plomin R, McClearn GE. Longitudinal and cross-sectional twin data on cognitive abilities in adulthood: the Swedish Adoption/Twin Study of Aging. Dev Psychol. 1998;34:1400–13. doi: 10.1037//0012-1649.34.6.1400. [DOI] [PubMed] [Google Scholar]
- 29.Petrill SA, Johansson B, Pedersen NL, Berg S, Plomin R, Ahern F, McClearn GE. Low cognitive functioning in nondemented 80+-year-old twins is not heritable. Intelligence. 2001;29:75–83. [Google Scholar]
- 30.Deary IJ, Wright AF, Harris SE, Whalley LJ, Starr JM. Searching for genetic influences on normal cognitive ageing. Trends Cogn Sci. 2004;8:178–84. doi: 10.1016/j.tics.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 31.Lehtovirta M, Kuikka J, Helisalmi S, Hartikainen P, Mannermaa A, Ryynänen M, Riekkinen PSr, Soininen H. Longitudinal SPECT study in Alzheimer’s disease: Relation to apolipoprotein E polymorphism. J Neurol Neurosurg Psychiatry. 1998;64:742–6. doi: 10.1136/jnnp.64.6.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kofke WA, Blissitt PA, Rao H, Wang J, Addya K, Detre J. Remifentanil-induced cerebral blood flow effects in normal humans: Dose and ApoE genotype. Anesth Analg. 2007;105:167–75. doi: 10.1213/01.ane.0000266490.64814.ff. [DOI] [PubMed] [Google Scholar]
- 33.Small GW, Ercoli LM, Silverman DH, Huang SC, Komo S, Bookheimer SY, Lavretsky H, Miller K, Siddarth P, Rasgon NL, Mazziotta JC, Saxena S, Wu HM, Mega MS, Cummings JL, Saunders AM, Pericak-Vance MA, Roses AD, Barrio JR, Phelps ME. Cerebral metabolic and cognitive decline in persons at genetic risk for Alzheimer’s disease. Proc Natl Acad Sci USA. 2000;97:6037–42. doi: 10.1073/pnas.090106797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lynch JR, Morgan D, Mance J, Matthew WD, Laskowitz DT. Apolipoprotein E modulates glial activation and the endogenous central nervous system inflammatory response. J Neuroimmunol. 2001;114:107–13. doi: 10.1016/s0165-5728(00)00459-8. [DOI] [PubMed] [Google Scholar]
- 35.Colton CA, Brown CM, Czapiga M, Vitek MP. Apolipoprotein-E allele-specific regulation of nitric oxide production. Ann NY Acad Sci. 2002;962:212–25. doi: 10.1111/j.1749-6632.2002.tb04070.x. [DOI] [PubMed] [Google Scholar]
- 36.Grünenfelder J, Umbehr M, Plass A, Bestmann L, Maly FE, Zünd G, Turina M. Genetic polymorphisms of apolipoprotein E4 and tumor necrosis factor beta as predisposing factors for increased inflammatory cytokines after cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2004;128:92–7. doi: 10.1016/j.jtcvs.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 37.Moretti EW, Morris RW, Podgoreanu M, Schwinn DA, Newman MF, Bennett E, Moulin VG, Mba UU, Laskowitz DT. Perioperative Genetics and Safety Outcomes Study (PEGASUS) Investigative Team: APOE polymorphism is associated with risk of severe sepsis in surgical patients. Crit Care Med. 2005;33:2521–6. doi: 10.1097/01.ccm.0000186368.96146.fb. [DOI] [PubMed] [Google Scholar]
- 38.Rosczyk HA, Sparkman NL, Johnson RW. Neuroinflammation and cognitive function in aged mice following minor surgery. Exp Gerontol. 2008;43:840–6. doi: 10.1016/j.exger.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rasmussen LS, Christiansen M, Rasmussen H, Kristensen PA, Moller JT. Do blood concentrations of neuron specific enolase and S-100 beta protein reflect cognitive dysfunction after abdominal surgery? Br J Anaesth. 2000;84:242–4. doi: 10.1093/oxfordjournals.bja.a013410. [DOI] [PubMed] [Google Scholar]
- 39.Linstedt U, Meyer O, Kropp P, Berkau A, Tapp E, Zenz M. Serum concentration of S-100 protein in assessment of cognitive dysfunction after general anesthesia in different types of surgery. Acta Anaesthesiol Scand. 2002;46:384–9. doi: 10.1034/j.1399-6576.2002.460409.x. [DOI] [PubMed] [Google Scholar]


