Abstract
In very-low-birth weight (VLBW, <1500 gram) infants, late-onset neonatal sepsis and necrotizing enterocolitis prolong the hospital stay, increase the cost of care, and place the infant at greater risk for morbidity and mortality (1). Long-term follow-up studies have demonstrated that these infections significantly increase the risk of neurological disabilities (2). With incidences of ~20% and 5–10% respectively, late-onset sepsis [LOS] and necrotizing enterocolitis [NEC] in VLBW infants need new preventive approaches. A long-held belief is that LOS and NEC result from bacterial translocation [BT]. Bacterial translocation is defined as invasion of indigenous intestinal bacteria through the mucosa into normally sterile tissue (3). This definition has been extended to include bacterial toxins or antigens, which damage intestinal epithelia and enter the circulation resulting in a systemic inflammatory response (4). Local BT through the intestinal mucosa, or toxin-related injury of intestinal epithelia, is associated with NEC (5), while BT beyond the intestine causes sepsis and multi-organ failure (6,7). This chapter describes: 1) development of the intestinal microbiota, 2) how immaturity of the nascent epithelial lining of the gastrointestinal [GI] tract and its sub-mucosal tissues mediate BT, 3) strategies to accelerate barrier functions in the immature GI tract and 4) the effects of nutrition and colonization by commensal bacteria on the susceptibility of the immature intestine to BT.
Keywords: intestinal microbiota, gut epithelia, enterocytes, goblet cells, Paneth cells, human milk, lactoferrin, probiotics, prebiotics
EMERGENCE OF INTESTINAL MICROBIOTA AFTER BIRTH
GENERAL PRINCIPLES
Healthy term infants stay with their mothers, breast feed, and acquire an intestinal microflora from the mother that is genetically compatible. This theory espoused by Hooper et al (8), holds that proper postnatal acquisition of genetically compatible gut microbiota improves nutrition and fortifies the gut’s epithelial barrier. By contrast, VLBW infants are almost always separated from their mothers and are cared for in the NICU containing resistant and invasive pathogens. While early and full human milk feedings reduce LOS (9), this goal is difficult to achieve.
Dysbiosis of the intestine, or abnormal gut flora, increases the risk of LOS and NEC in VLBW infants. Reasons for dysbiosis include: birth by cesarean section, hygiene practices, prolonged antibiotic administration, reduced bowel motility, immature epithelial host defenses, type or mode of nutrition, and parenteral nutrition (10–12). Strategies that alter these variables will reduce the risk of LOS and NEC (12).
DEFINING AN INTESTINAL MICROFLORA IN NEONATES THAT CAUSES BACTERIAL TRANSLOCATION
Standard bacteriologic culture techniques have traditionally been used to define the acquisition and succession of bacteria in the immature intestine. Escherichia coli, staphylococci and enterococci are present in neonatal stools within days of birth, while the genera Bifidobacterium, Clostridium, and Bacteroides can be detected within a week or two (10,13). This is also the time period when NEC becomes prevalent. In 1974, Hill et al (14) showed that BT was associated with an epidemic of sepsis, meningitis and NEC due to Klebsiella. Klebsiella, and the hydrogen sulfide gas this microbe produces during fermentation, were found during micro-puncture of the cysts in infants who had pneumatosis intestinalis. Other studies have shown that delta toxin (δ-toxin) produced by staphylococci can disrupt gut epithelia and initiate bacteremia and NEC (15,16) Numerous studies identifying multiple microorganisms in infants with NEC and LOS highlight the difficulty in defining a disease-specific bacterial pathogen.
Non-culture-based microbial analyses of feces are currently being used to understand the bacterial pathogenesis of NEC (17–19). de la Cochetière et al isolated DNA from stool, amplified microbial 16S rDNA, and then used temporal temperature gradient gel electrophoresis of the PCR amplicons for identification (20). In three infants who developed NEC (but in none of the control infants) Clostridium perfringens was identified. However, causation could not be linked to the presence of Clostridium. Wang et al studied ten preterm infants with NEC (and ten matched controls) using DNA isolated from feces that was amplified using PCR and subjected to terminal restriction fragment length polymorphism analysis (21). A sequence library of 16S rRNA for enteric bacteria was used for identification. Patients with NEC had less bacterial diversity and an increased abundance of γ-proteobacteria [e.g., E. coli] in the stools. In contrast, a recent study by Mshilddazde et al using pyrosequencing technology demonstrated that the overall microbial profiles in patients with NEC were not different than those of control infants (22). Thus to date, molecular methods have not clarified the bacterial pathogenesis in NEC. It is possible that NEC may be similar to Crohn’s disease wherein the “enteric bacterial community”, host genetics and defects in immunity, in combination, contribute to pathogenesis (23). Molecular techniques for microbe identification [metagenomics] will not delineate 1) high numbers of diverse, infectious bacteria attacking gut epithelia [microbial load], 2) multiple mechanisms used by pathogens to gain entry into or around intestinal epithelia [virulence], or 3) bacterial toxins that causes necrotic or apoptotic death of intestinal lining cells [lethality] (24). However, combining molecular microbial identification in patients with NEC with knowledge from the human microbiome project (25) may enable researchers and caregivers to better understand the connection between BT and NEC.
MICROBE AND EPITHELIAL CELL INTERACTIONS THAT MEDIATE BACTERIAL TRANSLOCATION
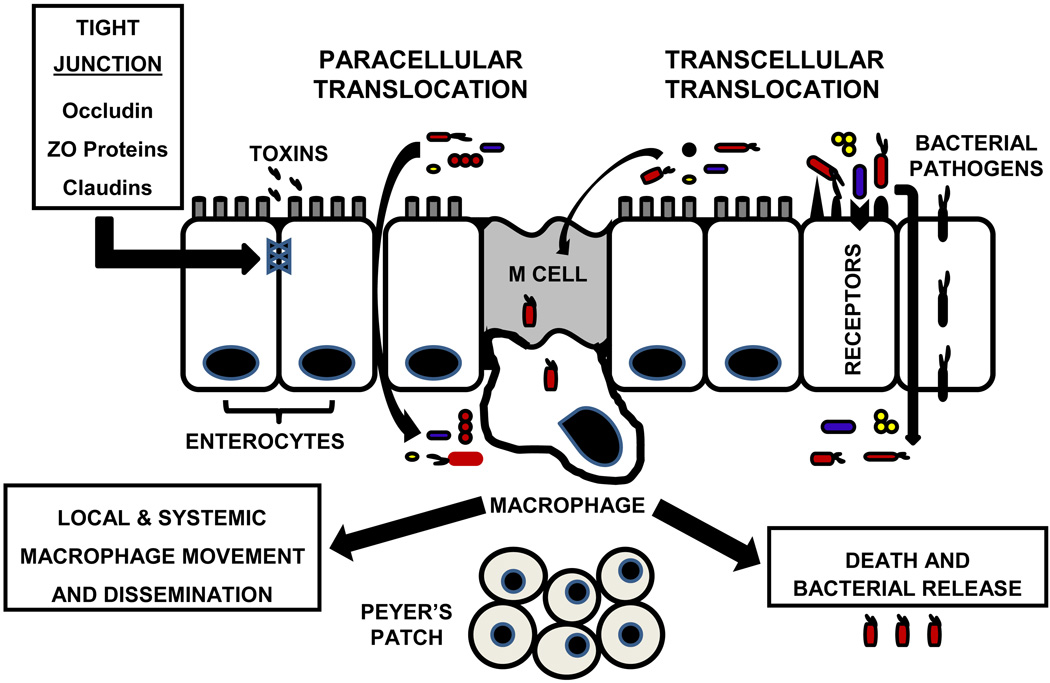
As shown in Figure 1, bacteria reach the sub-mucosa of the intestine via both transcellular and paracellular pathways, while microbial toxins leave the intestinal lumen via the paracellular pathway after loss of tight junctions between enterocytes. Most neonatal studies of BT have been performed in vitro (26), and in vivo information about BT in the immature intestine is limited (27). Our understanding of bacteria and enterocyte interactions that initiate BT is largely derived from studies of enteropathogenic, enteroinvasive, and enterohemorrhagic Escherichia coli, Shigella spp., Salmonella spp., Listeria monocytogenes, and Yersinia spp. (27–29). Several mechanisms of BT have been identified. A zipper mechanism is used by Listeria monocytogenes and Yersinia pseudotuberculosis to enter enterocytes, while Shigella and Salmonella use a trigger mechanism for transcytosis (29). The zipper mechanism exploits transmembrane cell-adhesion proteins as receptors for the bacteria. The trigger mechanism uses the bacterial type III secretory system [TTSS]. TTSS involves a bacterial needle-like probe that injects dedicated bacterial effectors into epithelia and the injected molecules modify the cytoskeleton to facilitate bacterial entry. Other adhesive mechanisms allow invasive Escherichia coli to bind to epithelial surfaces and enter enterocytes while also initiating inflammation (30–32). During inflammation, production of nitric oxide alters expression and localization of the tight junction zonulin proteins ZO-1, ZO-2, ZO-3 and occludin (33). Disruption of tight junctions that surround the upper part and lateral surfaces of enterocytes leads to intestinal hyperpermeability and predisposes to BT. Cronobacter spp. [Enterobacter sakazaki] requires the host cell cytoskeleton for transcytosis, a process enhanced by disruption of tight junctions (34,35). Toll-like receptors [TLR] are present on the luminal surface of enterocytes to sense danger and activate host defenses, but TLRs can also be harmful by mediating phagocytosis and translocation of bacteria across the intestinal barrier (36). Finally, the “enteric bacterial community” plays a role in BT as evidenced by a study showing Campylobacter jejuni assisting commensal bacteria to cross gut epithelia using lipid rafts (37).
Figure 1. Mechanisms of Bacterial Translocation [BT] in the Small Intestine.
Multiple pathways, receptors and cells are involved in BT from the intestinal lumen. Toxins such as flagellin, endotoxins, exotoxins, and other bacterial products can disrupt tight junctions and facilitate paracellular translocation of bacteria between intestinal epithelial cells. Transcellular translocation of bacteria can occur via receptors including Intelectin [also lactoferrin receptor], type III secretory system, Toll-like receptors, LFA-1 [lectin] receptor, β1 integrin, and IgA displayed on M cells. Bacterial uptake through these cells can result in systemic dissemination of the microbe.
Once pathogens pass the mucus and epithelial barriers, sub-mucosal macrophages ingest translocated bacteria. This process occurs without initiation of an inflammatory response (38). The efferent vagus nerve enhances intestinal macrophage phagocytic activity by stimulating the alpha4beta2 nicotinic acetylcholine receptor (39). The vagus nerve also dampens cytokine-driven inflammation via its actions on the alpha7 nicotinic acetylcholine receptor on intestinal macrophages, in a process termed the cholinergic anti-inflammatory pathway (40–42). The physiology of intestinal macrophages and the efferent vagal regulatory system in the intestine of neonates has not been characterized. If intestinal macrophages are dysfunctional in VLBW infants, as they are in adults with Crohn’s disease (43), this dysfunction may contribute to BT. Pathogenic bacteria can readily destroy sub-mucosal macrophages, thereby enhancing BT.
A major task of sub-mucosal macrophages is protection from BT that occurs via microfold epithelium over Peyer’s patches [called M cells] (44). M cells evaluate the intestinal lumen environment and transport bacteria through the epithelial barrier to sub-mucosal macrophages and dendritic cells, which then act as antigen-presenting cells [APCs]. M cells are portals for BT because they have no peptide antibiotic defense akin to enterocytes and they are not covered by a mucin layer. Dendritic cells use secretory immunoglobulin A [sIgA] to take up bacteria from M cells (45). Pathogen-specific sIgA secretion into the neonatal gut lumen occurs over time in relation to antigen exposure, thus at birth, sIgA is not secreted into the bowel to protect against BT. Neonates can, however, passively acquire sIgA from maternal breast milk.
GUT EPITHELIA AND PROTECTION AGAINST BACTERIAL TRANSLOCATION
The mechanisms used by goblet cells, enterocytes and Paneth cells to protect VLBW infants from BT are summarized in Table 1 (46, 51–53, 62–65).
Table 1.
Functional Characteristics of Goblet Cells, Enterocytes, and Paneth Cells
| Immunologic Agent | Cell Type | Regulation | Function |
|---|---|---|---|
| Mucin [MUC2] | Goblet Cells Paneth Cells |
Constitutive; Stimulation via TLR ligands, cytokines, growth factors/hormones |
Hydrophilic mucus, physical barrier, lubrication |
| Trefoil peptides | Goblet Cells | Constitutive | Mucin polymerization, anti- apoptotic, epithelial renewal |
|
Antimicrobial Peptides: α-defensins secretory phospholipase A2 [PLA2] lysozyme |
}Paneth cells |
Constitutive, Stimulation via TLR or NOD ligands; Cholinergic stimulation releases microbicides |
Disrupt microbial walls, Promote inflammation |
| angiogenin 4 cathelicidins β-defensins |
}Enterocytes | ||
| Lectins RegIIIγ, Collectins |
Paneth cells and Enterocytes |
Constitutive, Stimulation via TLR ligands |
Antimicrobial activity |
| Protease Inhibitors: Secretory Leukocyte Protease Inhibitor [SLPI] Elafin |
Paneth cells and Enterocytes |
Constitutive | Antimicrobial activity |
| Phospholipids | Enterocytes | Cortisone | Lubricant in mucus |
| Nucleotide-binding oligomerization domain |
Enterocyte – NOD1 Paneth cell – NOD2 |
Constitutive | Microbial sensors, activate inflammation |
THE NON-EPITHELIAL BARRIER
The first line of defense for preventing BT is the mucous coat overlying gut epithelia, produced by goblet cells. There are misconceptions about the mucus layer because histologic preparations have not delineated its true characteristics (46). The mucus layer is now appreciated as a gel-like diphasic system that exists in a liquid phase near the lumen and a more solid phase near the epithelia. The outer or luminal mucus layer has degraded mucin, diluted antimicrobial peptides, and some bacteria. The epithelium-associated mucus layer is firmly bound, is rich in natural antibiotic peptides, and has a sterile micro-aerobic environment. In the crypts, antimicrobial peptide concentrations are high because of Paneth cell secretions. Paneth cells are in close proximity to and serve a protective role for intestinal stem cells. Goblet cells secrete MUC2 and trefoil factors to facilitate mucin polymerization. MUC 2 is the structural component of the protective mucus layer, while trefoil factors serve important protective roles such as prevention of enterocyte apoptosis and renewal of epithelial cells. MUC2 is synthesized rapidly in preterm infants based on threonine incorporation into its peptide backbone (47). MUC2 mRNA appears at 12 weeks of human gestation in the jejunum, ileum, and colon (48). In newborn animals, the mucus layer is said to be discontinuous, which would promote BT; however there is speculation that this finding could be an artifact. The quality and quantity of mucin in preterm infants is not well characterized.
THE EPITHELIAL BARRIER
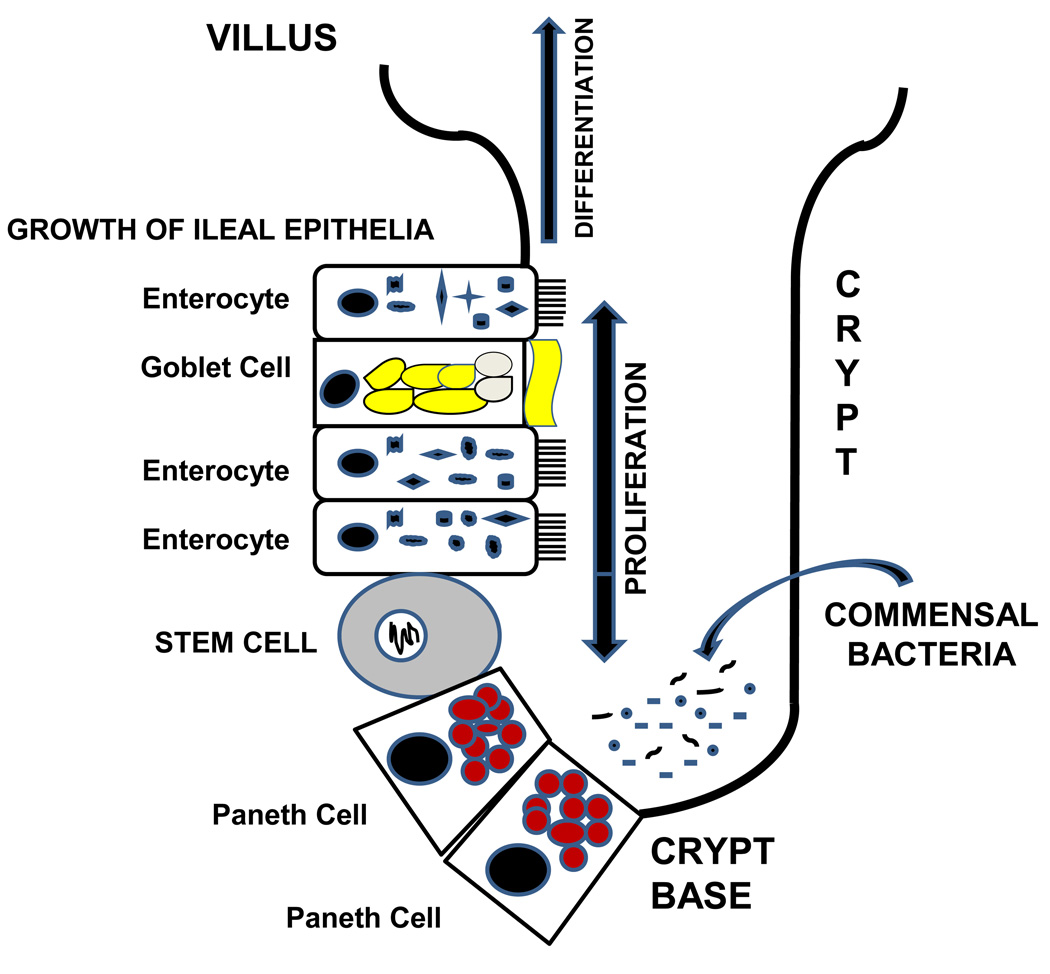
Gut epithelia are essential to host defense in the distal ileum and proximal colon which are the primary areas of involvement in NEC (49). Figure 2 illustrates how Paneth cells sense commensal microflora leading to release of angiogenins that mediate development of the epithelia and villi (50). Goblet cells secrete the mucus barrier, a primary defense against BT. Enterocytes also serve as multi-functional protectors of the gut barrier. Originating from stem cells at the base of the crypts, enterocytes migrate toward the villus tip (51). Human enterocytes contain β-defensins that are secreted into the mucus layer and defend against bacterial transcytosis (52). If enterocytes are invaded, these defensins are secreted and act as chemoattractants for dendritic cells and T cells. Cathelicidins, including LL-37/CAP18 and CRAMP, are a second class of antimicrobial peptides in enterocytes which are microbicidal for Gram-positive and Gram-negative bacteria (53). LL-37 has chemotactic activity for monocytes, macrophages, and T cells and initiates Th1-related cytokine secretion by dendritic cells.
Figure 2. Commensal Bacteria and Paneth Cells Participate in the Development, Maintenance and Repair of an Intestinal Villus.
Stem cells at the base of the crypts give rise to 4 cell lineages (enterocytes, goblet cells, Paneth cells, and intestinal neuroendocrine epithelia. These cells participate in host defense against bacterial translocation (50,63). The role of neuroendocrine epithelia in host defense of the intestinal villus is undefined.
Paneth cells, epithelia localized to the crypts of Lieberkuhn, contribute significantly to gut innate immunity. Mallow et al (54) used in situ hybridization to localize α-defensins to Paneth cells during human gestation. At 19 to 24 wks of gestation, mRNA levels of α-defensins in the small intestine are 40- to 250- fold lower than that seen in adults. A reduced number of Paneth cells per crypt at 24 wks of gestation compared to adults may partly explain the lower mRNA levels of α-defensins. In contrast, Paneth cells in intestinal segments obtained from infants with NEC exhibit increased mRNA transcripts for α-defensins (55). This is a late finding, and it may simply imply that bacterial pathogens induce expression of α-defensins. Coutinho et al demonstrated a depletion of lysozyme in Paneth cells in infants with necrotizing enterocolitis (56). Further support for the importance of Paneth cells in neonatal host defense comes from studies using dithizone to selectively kill Paneth cells and reduce their antimicrobial granules (57). When neonatal rats were given dithizone and then infected with enteroinvasive Escherichia coli, all pups developed bacteremia caused by E. coli and some animals developed ileal necrosis akin to NEC (57). Studies of neonatal mice with a genetic gain or loss of α-defensins [cryptdins] indicate that these Paneth cell-associated antimicrobial peptides play a role in the emergence of the intestinal microbiota after birth (58). The pathogenesis of Crohn’s disease is also linked to a deficiency of α-defensins (59). Finally, cortisone increases the number and complexity of Paneth cells in neonatal rats (60), and antenatal steroids are linked to a reduction in NEC in preterm infants (61). Taken together, these data support the important role of Paneth cells in modulating enteric bacteria and in serving as a major innate defense against BT, LOS and NEC in preterm infants (62).
Paneth cells have additional roles beyond prevention of BT (63–65), such as sensing changes in the microflora of the mature gut and maintaining host-microbial homeostasis at the mucosal surface (66). Paneth cells also stimulate blood vessel growth during development and after intestinal injury, via production of angiogenins (50, 66). Paneth cells produce and respond to inflammation-modulating cytokines. They have abundant mRNA transcripts for tumor necrosis factor-alpha [TNF-α] and there is a marked increase in TNF-α mRNA in Paneth cells in infants with eosinophil- and macrophage-related infiltrates observed during NEC (67). While some evidence suggests that TNF-α from Paneth cells may be responsible for the massive necrosis seen during NEC, other researchers have observed that TNF-α is involved in villus repair after injury. Other cytokines have been reported to regulate Paneth cell number and function. For example, the combination of IL-9 and IL-13 was shown to mediate Paneth cell hyperplasia and up-regulate innate immunity at the gut’s epithelial barrier (68).
THE ROLE OF NUTRITON IN PREVENTING BACTERIAL TRANSLOCATION
GENERAL PRINCIPLES
During the 3rd trimester of pregnancy, the fetus swallows nutrients-, growth factors-, and antibiotic peptide-rich amniotic fluid (69). Human milk is even more complex than amniotic fluid, and it continues to enhance intestinal development after birth (70). In addition to its nutrient composition, human milk contains hormones, growth factors, cytokines, immunomodulators, natural peptide antibiotics, sIgA, and probiotic bacteria (70–76). Secretory IgA in milk is the end result of crosstalk between the mother and her environmental microbiota (76). The continuum of drinking amniotic fluid and then human milk results in NEC being infrequent in breast-fed infants born at term gestation. VLBW infants are born without proper maturation of the intestine or its innate antimicrobial defenses, and mother’s milk is the fallback mechanism to prevent BT, LOS or NEC. A recent report reaffirms that an exclusive human milk diet, rather than partial or full bovine milk-based nutrition, significantly reduces the incidence of NEC (77). VLBW infants cannot consume enough breast milk early in life to achieve an adequate innate host defense in the immature intestine. To address the lack of mother’s milk after birth, lactoferrin, probiotics, and prebiotics are being fed to VLBW infants with the goal of boosting innate host defenses against BT, LOS and NEC.
LACTOFERRIN FOR PREVENTION OF BACTERIAL TRANSLOCATION IN INFANTS
Lactoferrin [LF] is a member of the transferrin family and a multi-functional protein with high concentrations in colostrum and a stable content in mature milk. As summarized in Table 2, LF has antimicrobial, anti-inflammatory, immunoregulatory, and growth-promoting properties which contribute to prevention of BT in VLBW infants (50, 61, 62, 78–94). Recombinant human lactoferrin [rhLF] was studied in preclinical models for its ability to prevent NEC. Feeding of rhLF to neonatal rats before inducing an intestinal infection with enteroinvasive Escherichia coli was shown to enhance survival of the rat pups, reduce infection of the jejunum and ileum (80) and limit E. coli-related translocation to the liver and blood (81). These studies were the basis for a clinical trial of bovine lactoferrin [bLF] prophylaxis in VLBW infants. Manzoni et al observed a significant reduction in LOS among VLBW infants fed bLF (82). When bLF and Lactobacillus rhamnosus GG [LGG] were given enterally to the infants, NEC was significantly lower compared to controls. No adverse events were seen related to feeding bLF to infants.
Table 2.
Mechanisms by Which Lactoferrin [LF] Prevents Bacterial Translocation
| Physiologic or Immune Processes Related to LF or Its Enzymatic Digestion Products |
Type of Action | References |
|---|---|---|
| Fe3+ withholding defense mediates bacteriostasis | Antimicrobial | 63,78,79 |
| Synergy with peptide antibiotics [e.g., lysozyme], antibiotic drugs, IgA to facilitate microbial killing |
Antimicrobial | 83 |
| Low pH & peptic digest of LF kills Gram [+] & Gram [−] bacteria, fungi, viruses, & parasites |
Antimicrobial | 63,78,79 |
| LF-derived peptides kill antibiotic-resistant Staphylococcus aureus & Escherichia coli |
Antimicrobial | 84,85 |
| Binds endotoxins, exotoxins, CpG, flagellin; Blocks toxin-mediated cytokine production |
Anti-inflammatory | 79,86–88 |
| Blocks adhesins on cells for bacteria & biofilms Inhibits type III secretory system, flagella motility |
Antimicrobial | 89 |
| Multiple enzyme activities [e.g., ribonuclease] | Anti-inflammatory | 50,62,78,90 |
| Binds to & promotes growth of probiotic bacteria | Antimicrobial | 91 |
| Binds to Intelectin, promotes proliferation and differentiation of epithelia and immune cells; primes Th1 immunity [Th2 to Th1 switch] |
Antimicrobial Immunoregulatory |
72, 78, 79, 92 |
| Activates macrophages via TLR, other receptors Recruits & activates macrophages, dendritic cells |
Pro-inflammatory, Alarmin |
93, 94 |
PROBIOTIC BACTERIA AND PREVENTION OF BACTERIAL TRANSLOCATION IN INFANTS
In 1907, Nobel laureate Elie Metchnikoff proposed that yoghurt [containing probiotics] prolonged life (95). In 1899, his colleague at the Pasteur Institute, pediatrician Henry Tissier had already discovered bifidobacteria in the feces of breast-fed infants (95). The “bifidogenic effect” of human milk is attributed to its low protein content as well as its lactose, nucleotides, oligosaccharides and lactoferrin (96). There is evidence that emergence of lactic acid bacteria and bifidobacteria in the nascent bowel microbiota hinders BT into the intestinal wall. Streptococcus thermophilus and Lactobacillus acidophilus interact directly with intestinal epithelia to resist E. coli-related invasion (97). Bioactive factors secreted by B. infantis lessen intestinal permeability caused by TNF-α and interferon-γ (98). Prophylaxis with Lactobacillus plantarum likewise prevents changes in tight junction proteins during gut-related infection with enteroinvasive E. coli (99). Cytokine-induced apoptosis of gut epithelia is mitigated by LGG (100). Preclinical studies showed that Bifidobacteria significantly moderates necrotizing enterocolitis in neonatal rats (101).
Beneficial in vivo effects of oral probiotic biotherapy in neonates are reportedly related to more competent gut-related immunity, a less pathogenic intestinal microflora, and diminished intra-luminal microbial toxins (5,101,102). Clinical studies in human newborn infants demonstrated that probiotics reduced the incidence of NEC, although the effect was less evident in extremely preterm infants (103,104). From a safety perspective, no probiotic bacteria were isolated from blood cultures. However, probiotics have not been shown to reduce mortality. A meta-analysis of all probiotic studies to prevent NEC reached similar conclusions (105). More studies with probiotics are needed to show efficacy and safety (106).
PREBIOTICS AND PREVENTION OF BACTERIAL TRANSLOCATION IN INFANTS
When taken orally, prebiotics are non-digestible “foods” that selectively promote growth of one or more bacteria living in the GI tract (107). Prebiotics in human milk include oligosaccharides and other glycans such as glycoproteins, glycolipids, glycoaminoglycans, and mucins (108). These milk components promote gut colonization with bifidobacteria. Some researchers suggest that the “bifidogenic effect” of human milk extends beyond glycans (96). Among many bifidobacteria tested, only Bifidobacterium longum biovar infantis was able to grow during human milk oligosaccharide supplementation alone (109). There appears to be co-evolution of human milk oligosaccharides and bifidobacteria. This finding has guided research towards examining the genetic relationship of milk oligosaccharides in a mother and the emergence of her infant’s intestinal microbiota (109). In addition to the health benefits afforded by bifidobacteria, glycans may: a) inhibit bacterial and viral pathogens from binding to intestinal epithelia, b) detoxify intra-luminal products released by pathogens, c) dampen inflammation initiated by pathogens, and d) assist in development of innate immunity in the gut (108,110). VLBW infants receiving a mixture of neutral and acidic oligosaccharides, however, have not exhibited a significant reduction in serious infectious morbidity (111). Research by German et al has shown that oligosaccharides in mother’s milk confer advantages unique to her infant (109), whereas formula supplemented with oligosaccharides cannot replicate the effect of mother’s-own milk. A worrisome report found newborn rats fed galactooligosaccharides and inulin had increased bacterial translocation (112). No studies have examined the effect of prebiotics alone on the incidence of NEC (114). Probiotic and prebiotic mixtures are fed because they foster development of a gut-related “bifidus flora” akin to that in breast-fed infants (113). If a combined probiotic and prebiotic strategy is used to prevent NEC in VLBW newborns, the risk of BT necessitates frequent assessments of morbidity.
SUMMARY
Bacterial translocation from the GI tract is an important pathway initiating late-onset sepsis and necrotizing enterocolitis in very-low-birth weight infants. The emerging intestinal microbiota, nascent intestinal epithelia, naive immunity, and suboptimal nutrition [lack of breast milk] have roles in facilitating bacterial translocation. Feeding lactoferrin, probiotics or prebiotics have presented exciting possibilities to prevent bacterial translocation in preterm infants, and clinical trials will identify the most safe and efficacious prevention and treatment strategies.
Acknowledgement
This research was supported by NIH grant R44 HD057744.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: The experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110(2):285–291. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 2.Stoll BJ, Hansen NI, Adams-Chapman I, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292(19):2357–2365. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 3.Berg RD, Garlington AW. Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in gnotobiotic mouse model. Infect Immun. 1979;23(2):403–411. doi: 10.1128/iai.23.2.403-411.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gatt M, Reddy BS, MacFie J. Review article: bacterial translocation in the critically ill -- evidence and methods of prevention. Aliment Pharmacol Ther. 2007;25(7):741–757. doi: 10.1111/j.1365-2036.2006.03174.x. [DOI] [PubMed] [Google Scholar]
- 5.Hunter CJ, Upperman JS, Ford HR, et al. Understanding the susceptibility of the premature infant to necrotizing enterocolitis (NEC) Pediatr Res. 2008;63(2):117–123. doi: 10.1203/PDR.0b013e31815ed64c. [DOI] [PubMed] [Google Scholar]
- 6.Sharma R, Tepas JJ, 3rd, Hudak ML, et al. Neonatal gut barrier and multiple organ failure: role of endotoxins and proinflammatory cytokines in sepsis and necrotizing enterocolitis. J Pediatr Surg. 2007;42(3):454–461. doi: 10.1016/j.jpedsurg.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 7.Duffy LC. Symposium: Bioactivity in milk and bacterial interactions in the developing immature intestine. J Nutr. 2000;130(2S Suppl):432S–436S. doi: 10.1093/jn/130.2.432S. [DOI] [PubMed] [Google Scholar]
- 8.Hooper LV, Wong MH, Thelin A, et al. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291(5505):881–884. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- 9.Rønnestad A, Abrahamsen TG, Medbø S, et al. Late-onset septicemia in a Norwegian national cohort of extremely premature infants receiving early full human milk feeding. Pediatrics. 2005;115(3):e269–e276. doi: 10.1542/peds.2004-1833. [DOI] [PubMed] [Google Scholar]
- 10.Adlerberth I, Lindberg E, Åberg N, et al. Reduced enterobacterial and increased staphylococcal colonization of the infantile bowel: An effect of hygienic lifestyle? Pediatr Res. 2005;59(1):96–101. doi: 10.1203/01.pdr.0000191137.12774.b2. [DOI] [PubMed] [Google Scholar]
- 11.Westerbeek EA, van den Berg A, Lafeber HN, et al. The intestinal bacterial colonization in preterm infants: A review of the literature. Clin Nutr. 2006;25(3):361–368. doi: 10.1016/j.clnu.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Neu J, Mshvildadze M, Mai V. A roadmap for understanding and preventing necrotizing enterocolitis. Curr Gastroenterol Rep. 2008;10(5):450–457. doi: 10.1007/s11894-008-0084-x. [DOI] [PubMed] [Google Scholar]
- 13.Björkström MV, Hall L, Söderlund S, et al. Intestinal flora in very-low birth weight infants. Acta Paediatr. 2009;98(11):1762–1767. doi: 10.1111/j.1651-2227.2009.01471.x. [DOI] [PubMed] [Google Scholar]
- 14.Hill HR, Hunt CE, Matsen JM. Nosocomial colonization with Klebsiella, type 26, in a neonatal intensive-care unit associated with an outbreak of sepsis, meningitis, and necrotizing enterocolitis. J Pediatr. 1974;85(3):415–419. doi: 10.1016/s0022-3476(74)80133-2. [DOI] [PubMed] [Google Scholar]
- 15.Scheifele DW, Bjornson GL, Dyer RA, et al. Delta-like toxin produced by coagulase-negative staphylococci is associated with neonatal necrotizing enterocolitis. Infect Immun. 1987;55(9):2268–2273. doi: 10.1128/iai.55.9.2268-2273.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Overturf GD, Sherman MP, Scheifele DW, et al. Neonatal necrotizing enterocolitis associated with delta-toxin producing methicillin-resistant Staphylococcus aureus. Pediatr Infect J. 1990;9(2):88–91. doi: 10.1097/00006454-199002000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Frank DN, Pace NR. Gastrointestinal microbiology enters the metagenomics era. Curr Opin Gastroenterol. 2008;24(1):4–10. doi: 10.1097/MOG.0b013e3282f2b0e8. [DOI] [PubMed] [Google Scholar]
- 18.Brugère J-F, Mihajlovski A, Missaoui M, et al. Tools for stools: the challenge of assessing human intestinal microbiota using molecular diagnostics. Expert Rev Mol Diag. 2009;9(4):353–365. doi: 10.1586/erm.09.16. [DOI] [PubMed] [Google Scholar]
- 19.Morowitz MJ, Poroyko V, Caplan M, et al. Redefining the role of intestinal microbes in the pathogenesis of necrotizing enterocolitis. Pediatrics. 2010;125(4):777–785. doi: 10.1542/peds.2009-3149. [DOI] [PubMed] [Google Scholar]
- 20.de la Cochetiére M-F, Piloquet H, Des Robert C, et al. Early intestinal bacterial colonization and necrotizing enterocolitis in premature infants: The putative role of Clostridium. Pediatr Res. 2004;56(3):366–370. doi: 10.1203/01.PDR.0000134251.45878.D5. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Hoenig JD, Malin KJ, et al. 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J. 2009;3(8):944–954. doi: 10.1038/ismej.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mshvildadze M, Neu J, Shuster J, et al. Intestinal microbial ecology in premature infants assessed with non-culture-based techniques. J Pediatr. 2010;156(2):20–25. doi: 10.1016/j.jpeds.2009.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baker PI, Love DR, Ferguson LR. Role of gut microbiota in Crohn’s disease. Expert Rev Gastroenterol Hepatol. 2009;3(5):535–546. doi: 10.1586/egh.09.47. [DOI] [PubMed] [Google Scholar]
- 24.Phalipon A, Sansonetti PJ. Shigellosis: innate mechanisms of inflammatory destruction of intestinal epithelium, adaptive immune response, and vaccine development. Crit Rev Immunol. 2003;23(5–6):371–401. doi: 10.1615/critrevimmunol.v23.i56.20. [DOI] [PubMed] [Google Scholar]
- 25.Turnbaugh PJ, Ley RE, Hamady M, et al. The human microbiome project. Nature. 2007;449(7164):804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burns JL, Griffith A, Barry JJ, et al. Transcytosis of gastrointestinal epithelial cells by Escherichia coli K1. Pediatr Res. 2001;49(1):30–37. doi: 10.1203/00006450-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Lu L, Walker WA. Pathologic and physiologic interactions of bacteria with the gastrointestinal epithelium. Am J Clin Nutr. 2001;73 suppl:1124S–1130S. doi: 10.1093/ajcn/73.6.1124S. [DOI] [PubMed] [Google Scholar]
- 28.Stebbins CE. Structural microbiology at the pathogen-host interface. Cell Microbiol. 2005;7(9):1227–1236. doi: 10.1111/j.1462-5822.2005.00564.x. [DOI] [PubMed] [Google Scholar]
- 29.Cossart P, Sansonetti PJ. Bacterial invasion: The paradigms of enteroinvasive pathogens. Science. 2004;304(5668):242–248. doi: 10.1126/science.1090124. [DOI] [PubMed] [Google Scholar]
- 30.Luck SN, Bennett-Wood V, Poon R, et al. Invasion of epithelial cells by locus of enterocytes effacement-negative enterohemorrhagic Escherichia coli. Infect Immun. 2005;73(5):3063–3071. doi: 10.1128/IAI.73.5.3063-3071.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berin MC, Dareuille-Michaud A, Egan LJ, et al. Role of EHEC O157:H7 virulence factors in the activation of intestinal epithelial cell NF-kB and MAP kinase pathways and the upregulated expression of interleukin 8. Cell Immunol. 2002;4(10):635–647. doi: 10.1046/j.1462-5822.2002.00218.x. [DOI] [PubMed] [Google Scholar]
- 32.Ruchaud-Sparagano M-H, Maresca M, Kenny B. Enteropathogenic Escherichia coli (EPEC) inactivate innate immune responses prior to compromising epithelial barrier function. Cell Immunol. 2007;9(8):1909–1921. doi: 10.1111/j.1462-5822.2007.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anand RJ, Leaphart CL, Mollen KP, et al. The role of the intestinal barrier in the pathogenesis of necrotizing enterocolitis. Shock. 2007;27(2):124–133. doi: 10.1097/01.shk.0000239774.02904.65. [DOI] [PubMed] [Google Scholar]
- 34.Kim K-P, Loessner MJ. Enterobacter sakazakii invasion in human intestinal Caco-2 cells requires the host cell cytoskeleton and is enhanced by disruption of tight junctions. Infect Immun. 2008;76(2):562–570. doi: 10.1128/IAI.00937-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friedemann M. Epidemiology of invasive neonatal Cronobacter (Enterobacter sakazakii) infections. Eur J Clin Microbiol Infect Dis. 2009;28(11):1297–1304. doi: 10.1007/s10096-009-0779-4. [DOI] [PubMed] [Google Scholar]
- 36.Neal MD, Leaphart C, Levy R, et al. Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J Immunol. 2006;176(5):3070–3079. doi: 10.4049/jimmunol.176.5.3070. [DOI] [PubMed] [Google Scholar]
- 37.Kalischuk LD, Inglis GD, Buret AG. Campylobacter jejuni induces transcellular translocation of commensal bacteria via lipid rafts. Gut Pathog. 2009;1(1):2. doi: 10.1186/1757-4749-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weber B, Saurer L, Mueller C. Intestinal macrophages: differentiation and involvement in intestinal immunopathologies. Semin Immunopathol. 2009;31(2):171–184. doi: 10.1007/s00281-009-0156-5. [DOI] [PubMed] [Google Scholar]
- 39.Van der Zanden EP, Snoek SA, Heinsbroek SE, et al. Vagus nerve activity augments intestinal macrophage phagocytosis via nicotinic acetylcholine receptor alpha4beta2. Gastroenterology. 2009;137(3):1029–1039. 1039.e1–1039.e4. doi: 10.1053/j.gastro.2009.04.057. [DOI] [PubMed] [Google Scholar]
- 40.Kessler W, Traeger T, Westerholt A, et al. The vagal nerve as a link between the nervous and immune system in the instance of polymicrobial sepsis. Langenbecks Arch Surg. 2006;391(2):83–87. doi: 10.1007/s00423-006-0031-y. [DOI] [PubMed] [Google Scholar]
- 41.Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117(2):289–296. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van der Zanden EP, Boeckxstaens GE, de Jonge WJ. The vagus nerve as a modulator of intestinal inflammation. Neurogastroenterol Motil. 2009;21(1):6–17. doi: 10.1111/j.1365-2982.2008.01252.x. [DOI] [PubMed] [Google Scholar]
- 43.Caprilli R, Frieri G. The dyspeptic macrophage 30 years later: an update in the pathogenesis of Crohn’s disease. Dig Liver Dis. 2009;41(2):166–168. doi: 10.1016/j.dld.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 44.Sansonetti PJ, Phalipon A. M cells as ports of entry for enteroinvasive pathogens: mechanisms of interaction, consequences for the disease process. Semin Immunol. 1999;11(3):192–203. doi: 10.1006/smim.1999.0175. [DOI] [PubMed] [Google Scholar]
- 45.Kadaoui KA, Corthésy B. Secretory IgA mediates bacterial translocation to dendritic cells in mouse Peyer’s patches with restriction to mucosal compartment. J Immunol. 2007;179(11):7751–7757. doi: 10.4049/jimmunol.179.11.7751. [DOI] [PubMed] [Google Scholar]
- 46.McGuckin MA, Eri R, Simms LA, et al. Intestinal barrier dysfunction in inflammatory bowel diseases. Inflamm Bowel Dis. 2009;15(1):100–113. doi: 10.1002/ibd.20539. [DOI] [PubMed] [Google Scholar]
- 47.Schaart MW, de Bruijn AC, Sclerbeek H, et al. Small intestinal MUC2 synthesis in human preterm infants. Am J Physiol Gastroenterol Liver Physiol. 2009;296(5):G1085–G1090. doi: 10.1152/ajpgi.90444.2008. [DOI] [PubMed] [Google Scholar]
- 48.Chambers JA, Hollingsworth MA, Trezise AE, et al. Developmental expression of mucin genes MUC1 and MUC2. J Cell Sci. 1994;107(Pt 2):413–424. doi: 10.1242/jcs.107.2.413. [DOI] [PubMed] [Google Scholar]
- 49.Kosloske AM, Musemeche CA. Necrotizing enterocolitis of the neonate. Clin Perinatol. 1989;16(1):97–111. [PubMed] [Google Scholar]
- 50.Stappenbeck TS, Hooper LV, Gordon JI. Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc Natl Acad Sci USA. 2002;99(24):15451–15455. doi: 10.1073/pnas.202604299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Snoeck V, Goddeerie B, Cox E. The role of enterocytes in the intestinal barrier function and antigen uptake. Microbes Infect. 2005;7(7–8):997–1004. doi: 10.1016/j.micinf.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 52.O’Neil DA, Porter EM, Elewaut D, et al. Expression and regulation of the human β-defensins hBD-1 and hBD-2 in intestinal epithelium. J Immunol. 1999;163(12):6718–6724. [PubMed] [Google Scholar]
- 53.Hase K, Eckmann L, Leopard JD, et al. Cell differentiation is a key determinant of cathelicidin LL-37/human cationic antimicrobial protein 18 expression by human colonic epithelium. Infect Immun. 2002;70(2):953–963. doi: 10.1128/iai.70.2.953-963.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mallow EB, Harris A, Salzman N, et al. Human enteric defensins. Gene structure and developmental expression. J Biol Chem. 1996;2719(8):4038–4045. doi: 10.1074/jbc.271.8.4038. [DOI] [PubMed] [Google Scholar]
- 55.Salzman NH, Polin RA, Harris MC, et al. Enteric defensin expression in necrotizing enterocolitis. Pediatr Res. 1998;44(1):20–26. doi: 10.1203/00006450-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 56.Coutinho HB, da Mota HC, Coutinho VB, et al. Absence of lysozyme (muramidase) in the intestinal Paneth cells of newborn infants with necrotising enterocolitis. J Clin Path. 1998;51(7):512–514. doi: 10.1136/jcp.51.7.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sherman MP, Bennett SH, Hwang FFY, et al. Paneth cells and antibacterial host defense in neonatal small intestine. Infect Immun. 2005;73(9):6143–6146. doi: 10.1128/IAI.73.9.6143-6146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salzman NH, Hung K, Haribhai D, et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol. 2010;11(1):76–83. doi: 10.1038/ni.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wehkamp J, Harder J, Weichenthal M, et al. NOD2 (CARD15) mutations in Crohn’s disease are associated with diminished mucosal α-defensin expression. Gut. 2004;53(11):1658–1664. doi: 10.1136/gut.2003.032805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dinsdale D, Biles B. Postnatal changes in the distribution and elemental composition of Paneth cells in normal and corticosteroid-treated rats. Cell Tissue Res. 1986;246(1):183–187. doi: 10.1007/BF00219016. [DOI] [PubMed] [Google Scholar]
- 61.Halac E, Halac J, Bégué EF, et al. Prenatal and postnatal corticosteroid therapy to prevent neonatal necrotizing enterocolitis, a controlled trial. J Pediatr. 1990;117(1 Pt 1):132–138. doi: 10.1016/s0022-3476(05)72461-6. [DOI] [PubMed] [Google Scholar]
- 62.Salzman NH, Underwood MA, Bevins CL. Paneth cells, defensins, and the commensal microbiota: a hypothesis on intimate interplay at the intestinal mucosa. Semin Immunol. 2007;19(2):70–83. doi: 10.1016/j.smim.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 63.Mukherjee S, Vaishnava S, Hooper LV. Multi-layered regulation of intestinal antimicrobial defense. Cell Mol Life Sci. 2008;65(19):3019–3027. doi: 10.1007/s00018-008-8182-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hooper LV, Stappenbeck TS, Hong CV, et al. Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nat Immunol. 2003;4(3):269–273. doi: 10.1038/ni888. [DOI] [PubMed] [Google Scholar]
- 65.Eckmann L. Innate immunity and mucosal bacterial interactions in the intestine. Curr Opin Gastroenterol. 2004;20(2):82–88. doi: 10.1097/00001574-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 66.Vaishnava S, Behrendt C, Ismail AS, et al. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci USA. 2008;105(52):20858–20863. doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tan X, Hsueh W, Gonzalez-Crussi F. Cellular localization of tumor necrosis factor (TNF)-α transcripts in normal bowel and in necrotizing enterocolitis. Am J Pathol. 1993;142(6):1858–1865. [PMC free article] [PubMed] [Google Scholar]
- 68.Steenwinckel V, Louahed J, Lemaire MM, et al. IL-9 promotes IL-13-dependent Paneth cell hyperplasia and up-regulation of innate immunity mediators in intestinal mucosa. J Immunol. 2009;182(8):4737–4743. doi: 10.4049/jimmunol.0801941. [DOI] [PubMed] [Google Scholar]
- 69.Underwood MA, Gilbert WM, Sherman MP. Amniotic fluid: not just fetal urine anymore. J Perinatol. 2005;25(5):341–348. doi: 10.1038/sj.jp.7211290. [DOI] [PubMed] [Google Scholar]
- 70.Wagner CL, Taylor SN, Johnson D. Host factors in amniotic fluid and breast milk that contribute to gut maturation. Clin Rev Allergy Immunol. 2008;34(2):191–204. doi: 10.1007/s12016-007-8032-3. [DOI] [PubMed] [Google Scholar]
- 71.Tunzi CR, Harper PA, Bar-Oz B, et al. Beta-defensin expression in human mammary gland epithelia. Pediatr Res. 2000;48(1):30–35. doi: 10.1203/00006450-200007000-00008. [DOI] [PubMed] [Google Scholar]
- 72.Takahata Y, Takada H, Nomura A, et al. Interleukin-18 in human milk. Pediatr Res. 2001;50(2):268–272. doi: 10.1203/00006450-200108000-00017. [DOI] [PubMed] [Google Scholar]
- 73.Vidal K, Donnel-Hughes A. CD14: a soluble pattern recognition receptor in milk. Adv Exp Med Biol. 2008;606:195–216. doi: 10.1007/978-0-387-74087-4_7. [DOI] [PubMed] [Google Scholar]
- 74.Martin R, Langa S, Reviriego C, et al. Human milk is a source of lactic acid bacteria for the infant gut. J Pediatr. 2003;143(6):754–758. doi: 10.1016/j.jpeds.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 75.Martin R, Jiménez E, Heilig H, et al. Isolation of bifidobacteria from breast milk and assessment of the bifidobacterial population by PCR-denaturing gradient gel electrophoresis and quantitative real-time PCR. Appl Environ Microbiol. 2009;75(4):965–969. doi: 10.1128/AEM.02063-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brandtzaeg P. The mucosal immune system and its integration with the mammary glands. J Pediatr. 2010;156(2):S8–S15. doi: 10.1016/j.jpeds.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 77.Sullivan S, Schanler RJ, Kim JH, et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J Pediatr. 2010;156(4):562–567. doi: 10.1016/j.jpeds.2009.10.040. e1. [DOI] [PubMed] [Google Scholar]
- 78.González-Chávez SA, Arévalo-Gallegos S, Rascón-Cruz Q. Lactoferrin: structure, function, and applications. Int J Antimicrob Agents. 2009;33(4):301.e1–e8. doi: 10.1016/j.ijantimicag.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 79.Legrand D, Mazurier J. A critical review of the roles of host lactoferrin in immunity. Biometals. 2010 Feb 9; doi: 10.1007/s10534-010-9297-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 80.Sherman MP, Bennett SH, Hwang FF, et al. Neonatal small bowel epithelia: enhancing anti-bacterial defense with lactoferrin and Lactobacillus GG. Biometals. 2004;17(3):285–289. doi: 10.1023/b:biom.0000027706.51112.62. [DOI] [PubMed] [Google Scholar]
- 81.Edde L, Hipolito RB, Hwang FF, et al. Lactoferrin protects neonatal rats from gut-related systemic infection. Am J Physiol Gastrointest Liver Physiol. 2001;281(5):1140–1150. doi: 10.1152/ajpgi.2001.281.5.G1140. [DOI] [PubMed] [Google Scholar]
- 82.Manzoni P, Rinaldi M, Cattani S, et al. Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: a randomized trial. JAMA. 2009;302(13):1421–1428. doi: 10.1001/jama.2009.1403. [DOI] [PubMed] [Google Scholar]
- 83.Singh PK, Tack BF, McCray PB, Jr, et al. Synergistic and additive killing by antimicrobial factors found in human airway surface liquid. Am J Physiol Cell Mol Physiol. 2000;279(5):L799–L805. doi: 10.1152/ajplung.2000.279.5.L799. [DOI] [PubMed] [Google Scholar]
- 84.Haney EF, Nazmi K, Lau F, et al. Novel lactoferrampin antimicrobial peptides derived from human lactoferrin. Biochimie. 2009;91(1):141–154. doi: 10.1016/j.biochi.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 85.Flores-Villaseñor H, Canizalez-Román A, Reyes-Lopez M, et al. Bactericidal effect of bovine lactoferrin, LFcin, LFampin, and LFchimera on antibiotic-resistant Staphylococcus aureus and Escherichia coli. Biometals. 2010 Mar 2; doi: 10.1007/s10534-010-9306-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 86.Baveye S, Elass E, Fernig DG, et al. Human lactoferrin interacts with soluble CD14 and inhibits expression of endothelial adhesion molecules, E-selectin, and ICAM-1, induced by the CD14-lipopolysachharide complex. Infect Immun. 2000;68(12):6519–6525. doi: 10.1128/iai.68.12.6519-6525.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mulligan P, White NR, Monteleone G, et al. Breast milk lactoferrin regulates gene expression by binding bacterial DNA CpG motifs but not genomic DNA promoters in model intestinal cells. Pediatr Res. 2006;59(5):656–661. doi: 10.1203/01.pdr.0000214958.80011.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hayworth JL, Kasper KJ, Leon-Ponte M, et al. Attenuation of massive cytokine response to the staphylococcal enterotoxin B superantigen by the innate immunomodulatory protein lactoferrin. Clin Exp Immunol. 2009;157(1):60–70. doi: 10.1111/j.1365-2249.2009.03963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ochoa TJ, Cleary TG. Effect of lactoferrin on enteric pathogens. Biochemie. 2009;91(1):30–34. doi: 10.1016/j.biochi.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kanyshkova TG, Babina SE, Semenov DV, et al. Multiple enzymatic activities of human milk lactoferrin. Eur J Biochem. 2003;270(16):3353–3361. doi: 10.1046/j.1432-1033.2003.03715.x. [DOI] [PubMed] [Google Scholar]
- 91.Rahman MM, Kim W-S, Ito T, et al. Growth promotion and cell binding ability of bovine lactoferrin to Bifidobacterium longum. Anaerobe. 2009;15(4):133–137. doi: 10.1016/j.anaerobe.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 92.Legrand D, Elass E, Carpentier M, et al. Interactions of lactoferrin with cells involved with immune function. Biochem Cell Biol. 2006;84(3):282–290. doi: 10.1139/o06-045. [DOI] [PubMed] [Google Scholar]
- 93.Curren CS, Demick KP, Mansfield JM. Lactoferrin activates macrophages via TLR4-dependent and -independent signaling pathways. Cell Immunol. 2006;242(1):23–30. doi: 10.1016/j.cellimm.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 94.Yang D, de la Rosa G, Tewary P, et al. Alarmins link neutrophils and dendritic cells. Trends Immunol. 2009;30(11):531–537. doi: 10.1016/j.it.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shortt C. The probiotic century: historical and current perspectives. Trends Food Sci Technol. 1999;10:411–417. [Google Scholar]
- 96.Coppa GV, Zampini L, Galeazzi T, et al. Prebiotics in human milk: a review. Dig Liver Dis. 2006;38 Suppl 2:S291–S294. doi: 10.1016/S1590-8658(07)60013-9. [DOI] [PubMed] [Google Scholar]
- 97.Resta-Lenert S, Barrett KE. Live probiotics protect intestinal epithelial cells from the effects of infection with enteroinvasive Escherichia coli (EIEC) Gut. 2003;52(7):988–997. doi: 10.1136/gut.52.7.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ewaschuk JB, Diaz H, Meddings L, et al. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial barrier function. Am J Physiol Gastrointest Liver Physiol. 2008;295(5):G1025–G1034. doi: 10.1152/ajpgi.90227.2008. [DOI] [PubMed] [Google Scholar]
- 99.Qin H, Zhang Z, Hang X, et al. L. plantarum prevents enteroinvasive Escherichia coli-induced tight junction proteins changes in intestinal epithelial cells. BMC Microbiol. 2009;31(9):63. doi: 10.1186/1471-2180-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yan F, Polk DB. Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J Biol Chem. 2002;277(52):50959–50965. doi: 10.1074/jbc.M207050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Caplan MS, Miller-Catchpole R, Kaup S, et al. Bifidobacterial supplementation reduces the incidence of necrotizing enterocolitis in a neonatal rat model. Gastroenterology. 1999;117(3):577–583. doi: 10.1016/s0016-5085(99)70450-6. [DOI] [PubMed] [Google Scholar]
- 102.Urao M, Fujimoto T, Lane GJ, et al. Does probiotics administration decrease serum endotoxins levels in infants? J Pediatr Surg. 1999;34(2):273–276. doi: 10.1016/s0022-3468(99)90189-6. [DOI] [PubMed] [Google Scholar]
- 103.Bin-Nun A, Bromiker R, Wilschanski M, et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight neonates. J Pediatr. 2005;147(2):192–196. doi: 10.1016/j.jpeds.2005.03.054. [DOI] [PubMed] [Google Scholar]
- 104.Lin HC, Hsu CH, Chen HL, et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight preterm infants: a multicenter, randomized, controlled trial. Pediatrics. 2008;122(4):693–700. doi: 10.1542/peds.2007-3007. [DOI] [PubMed] [Google Scholar]
- 105.Alfaleh K, Anabrees J, Bassler D. Probiotics reduce the risk of necrotizing enterocolitis in preterm infants: a meta-analysis. Neonatology. 2010;97(2):93–99. doi: 10.1159/000235684. [DOI] [PubMed] [Google Scholar]
- 106.Liong M-T. Safety of probiotics: translocation and infection. Nutr Rev. 2008;66(4):192–202. doi: 10.1111/j.1753-4887.2008.00024.x. [DOI] [PubMed] [Google Scholar]
- 107.Schrezenmeir J, de Vrese M. Probiotics, prebiotics, and synbiotics—approaching a definition. Am J Clin Nutr. 2001;73 suppl:361S–364S. doi: 10.1093/ajcn/73.2.361s. [DOI] [PubMed] [Google Scholar]
- 108.Newburg DS. Neonatal protection by an innate immune system of human milk consisting of oligosaccharides and glycans. J Anim Sci. 2009;87(13 Suppl):27–34. doi: 10.2527/jas.2008-1347. [DOI] [PubMed] [Google Scholar]
- 109.German JB, Freeman SL, Lebrilla CB, et al. Human milk oligosaccharides: Evolution, structures and bioselectivity as substrates for intestinal bacteria. Nestlé Nutr Workshop Ser Pediatr Program. 2008;62:205–218. doi: 10.1159/000146322. discussion 218–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Boehm G, Moro G. Structural and functional aspects of prebiotics used in infant nutrition. J Nutr. 2008;138(9):1818S–1828S. doi: 10.1093/jn/138.9.1818S. [DOI] [PubMed] [Google Scholar]
- 111.Westerbeek EA, van den Berg JP, Lafeber HN, et al. Neutral and acidic oligosaccharides in preterm infants: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2010;91(3):679–686. doi: 10.3945/ajcn.2009.28625. [DOI] [PubMed] [Google Scholar]
- 112.Barrat E, Michel C, Poupeau G, et al. Supplementation with galactooligosaccharides and inulin increases bacterial translocation in artificially reared newborn rats. Pediatr Res. 2008;64(1):34–39. doi: 10.1203/PDR.0b013e3181732381. [DOI] [PubMed] [Google Scholar]
- 113.Bakker-Zierikee AM, Alles MS, Knol J, et al. Effects of infant formula containing a mixture of galacto- and fructo-oligosaccharides or viable Bifidobacterium animalis on the intestinal microflora during the first 4 months of life. Br J Nutr. 2005;94(5):783–790. doi: 10.1079/bjn20051451. [DOI] [PubMed] [Google Scholar]
- 114.Caplan MS. Probiotic and prebiotic supplementation for the prevention of neonatal necrotizing enterocolitis. J Perinatol. 2009;29 Suppl 2:S2–S6. doi: 10.1038/jp.2009.21. [DOI] [PubMed] [Google Scholar]