Abstract
Two of the proteins found in significant quantity in the extracellular matrix (ECM) of dentin are dentin phosphoprotein (DPP) and dentin sialoprotein (DSP). DPP, the most abundant of the non-collagenous proteins in dentin is an unusually polyanionic protein, containing a large number of aspartic acids (Asp) and phosphoserines (Pse) in the repeating sequences of (Asp-Pse)n. and (Asp-Pse-Pse)n. The many negatively charged regions of DPP are thought to promote mineralization by binding calcium and presenting it to collagen fibers at the mineralization front during the formation of dentin. This purported role of DPP is supported by a sizeable pool of in vitro mineralization data showing that DPP is an important initiator and modulator for the formation and growth of hydroxyapatite crystals. Quite differently, DSP is a glycoprotein, with little or no phosphate. DPP and DSP are the cleavage products of dentin sialophosphoprotein (DSPP). Human and mouse genetic studies have demonstrated that mutations in, or knockout of, the Dspp gene result in mineralization defects in dentin and/or bone. The discoveries in the past 40 years with regard to DPP, DSP and DSPP have greatly enhanced our understanding of biomineralization and set a new stage for future studies. In this review, we summarize the important and new developments made in the past four decades regarding the structure and regulation of the DSPP gene, the biochemical characteristics of DSPP, DPP and DSP, as well as the cell/tissue localizations and functions of these molecules.
Keywords: Dentin sialophosphoprotein, Dentin sialoprotein, Dentin phosphoprotein, Biomineralization, Dentin, Bone
INTRODUCTION
Dentin, bone and cementum are mineralized connective tissues. Dentin, the most voluminous mineralized tissue of the tooth, is formed by odontoblasts; bone that forms the skeleton is made by osteoblasts, while cementum that covers the root dentin of the tooth is the product of cementoblasts. These three mineralized connective tissues develop through similar mechanisms and closely resemble one another in composition. During their formation, the odontoblasts, osteoblasts and cementoblasts secrete unmineralized, type I collagen-rich matrices termed “predentin, osteoid and cementoid”, respectively. As precursors of the corresponding mineralized tissues, these unmineralized organic phases lie between the mineralization front and their forming cells, and are transformed to the mineralized phase after hydroxyapatite (HA) crystals are deposited. This biomineralization process is dynamic and involves active interactions among a number of molecules, including type I collagen and numerous non-collagenous proteins (NCPs). The NCPs are believed to actively promote and control the mineralization of collagen fibers and crystal growth within predentin, osteoid and cementoid when these tissues are converted to dentin, bone and cementum, respectively. Discovered by cDNA cloning using a mouse odontoblast cDNA library [1], dentin sialophosphoprotein (DSPP) is a member of one category of NCPs termed the SIBLING (Small Integrin-Binding Ligand, N-linked Glycoprotein) family [2]. DSPP was originally thought to be dentin-specific. Later on, studies in several research groups demonstrated the expression of DSPP in bone [3, 4], cementum [4], and certain non-mineralized tissues [5, 6]. The importance of DSPP in biomineralization has been illustrated by human and mouse genetic studies, which showed the association of DSPP gene mutations or ablations with mineralization defects in the dentin [7-9] and bone [10].
DSPP was first described in 1997 [1], but its cleaved fragments, dentin phosphoprotein (DPP) and dentin sialoprotein (DSP), had been discovered much earlier. DPP was first reported in 1967 by Veis and Perry [11], while DSP was discovered by Butler et al. in 1981 [12]. Several excellent reviews [13-15] on dentin matrix proteins, including DPP and DSP, were published both before and after the initial report of DSPP. However, no specific review article on DSPP has ever been published.
The overall objectives of this review are to summarize the remarkable progress made during the past four decades in our understanding of DSPP and its cleaved products, DPP and DSP, in the gene structure and regulation, protein structure and metabolism, tissue/cell expression, and the biological functions of DSPP in biomineralization. Finally, we will conclude by providing a summary of the major discoveries made in the last 40 years and the outlook for future research on DSPP.
GENE STRUCTURE AND REGULATION
As stated above, DPP and DSP were discovered much earlier than the cDNA that encodes the parent protein DSPP. Although derived from the same mRNA, the biochemical features of DPP are remarkably different from those of DSP. Based on the belief that DPP and DSP were unrelated, several research groups in the early 1990's were engaged in cloning the “two genes” that would encode DPP and DSP. Using anti-DSP antibodies, Ritchie et al. [16] first screened a rat odontoblast cDNA library and obtained two positive clones that contained coding sequences corresponding to the DSP NH2-terminal amino acid sequence determined by Edman degradation [17]. Using a 750-base pair clone labeled with 32P to rescreen the library, Ritchie et al. obtained a cDNA sequence that apparently corresponded to the coding sequence of DSP. In 1994, they reported that this cDNA coded for the complete sequence of DSP [16]. Later on, it was discovered that there was an error in the sequence of this rat DSP cDNA, which created a frame shift in reading the sequence and generated an artificial stop codon.
In 1996, George et al. [18] cloned a cDNA from a rat odontoblast cDNA library and reported sequences representing the COOH-terminal region of DPP that predicted a 245-amino acid residue sequence. Also in 1996, Ritchie and Wang [19] reported a rat odontoblast cDNA that encoded a supposed 27-residue leading sequence followed by a very acidic 240-residue protein sequence corresponding to DPP. Until that point, DPP and DSP were considered to be encoded by different genes. However, MacDougall et al. [1] reported in 1997 that the coding for the mouse DSP and DPP sequences was on one continuous sequence (i.e., there was a single large gene coding for both DSP and DPP), with the sequence corresponding to DSP on the 5'end of the gene and that for DPP on the 3’ end. The full-length translation product containing both DSP and DPP amino acid sequences was referred to as DSPP by MacDougall [1]. A year after the discovery of DSPP, the genomic organization of mouse DSPP was reported by the same research group [20], which further confirmed that the Dspp gene encodes both DSP and DPP. Subsequently, similar evidence that a single gene encoded both DSP and DPP was published by George et al. [21, 22] and Ritchie et al. [23, 24]. George referred to the full-length translation product that includes both DSP and DPP sequences as “dentin matrix protein 3” (the third dentin matrix protein that her group had ever cloned), while Ritchie named this product “DSP-PP.” Ritchie and colleagues reported that there were multiple DSP-PP transcripts in varying sizes of DPP sequences in the rat [24, 25]. The longest cDNA that they referred to as “DSP-PP523” [25] corresponded to the full-length mouse DSPP, as reported by MacDougall [1], while all of the other DSP-PP transcripts reported by Ritchie's group were considerably shorter than DSP-PP523.
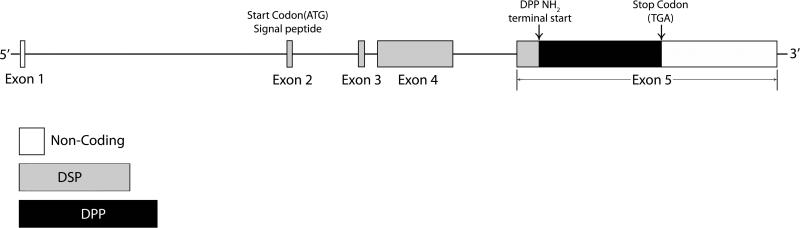
The complete cDNA sequences of DSPP have been determined for mice [1], rats [25] and humans [26], all of which showed that the DSP sequence is on the 5’ end of the DSPP mRNA, and DPP is on the 3’ end. The genomic organization of the DSPP gene is very similar among mice, rats and humans, containing 5 exons and 4 introns: exons 1–4 code for DSP, while exon 5 codes for the COOH-terminus of DSP and the whole sequence of DPP [20, 25, and 26]. Fig. 1 illustrates the genomic structure of mouse DSPP as reported by Feng et al. [20]. In the mouse DSPP, exon 1 contains a non-coding 5’ sequence; exon 2 contains the transcriptional start site, signal peptide, and first two amino acids of the NH2-terminus of DSPP. Exons 3 and 4 contain coding information for 29 and 314 amino acids, respectively, and the remainder of the coding information and the untranslated 3’ region are contained in exon 5.
Fig. 1. Gene Structure of mouse DSPP.
[20]. In the mouse DSPP gene, exon 1 contains a non-coding 5’ sequence; exon 2 contains the transcriptional start site, signal peptide, and first two amino acids of the NH2 -terminus of DSPP; exons 3 and 4 contain coding information for 29 and 314 amino acids, respectively; the remainder of the coding information and the untranslated 3’ region are contained in exon 5. The DSP sequence located at the NH2 terminus is found in exons 1–4 and the 5’ region of exon 5. The DPP sequence is located in the remainder of exon 5.
Two studies reported the isolation of cDNA clones that coded only for DSP in rat [27] and porcine [28]. No studies have ever shown convincing evidence that a DSPP transcript undergoes alternative splicing. Results from DSPP knockout studies [9], human genetic studies [7, 8], and human and mouse genome analyses support the conclusion that there is a single DSPP gene in the human and rodent genomes. Thus, the presence of the DSP-only mRNA cannot be satisfactorily explained. In one of the two reports describing the DSP-only cDNA clones, it was suggested that the DNA sequence redundancies in the DSPP coding region make it prone to cloning artifacts [28]. The multiple DSP-PP transcripts in rats that were shorter than DSP-PP523 were not found in other species. Although recent studies have shown a considerable level of polymorphisms in mouse and porcine DSPP genes (particularly in the DPP region) [29, 30], these harmless allelic variations did not make the DSPP transcripts as short as any of the DSP-PP transcripts that are smaller than DSP-PP523.
DSPP is predominantly expressed in dentin and moderately or slightly expressed in bone (see below), suggesting that the transcription of DSPP is highly regulated. Promoter deletion analysis by Feng et al. [20] revealed that several enhancer and silencer elements within 1447 bp upstream of the transcription initiation site control the expression of the mouse DSPP gene in the mouse odontoblast cell line MO6-G3. Strong promoter activity was found in the region flanking nt -95; two potential enhancer regions were identified between nt -1447 and -791 while two potential suppresser regions were located between nt-791 and -95 bp [20]. These earlier findings are similar to those from a later investigation [31], which showed that the strongest promoter activity of the mouse DSPP gene existed between nt -97 and -54 and that two potential transcriptional enhancers were present between nt-1243 and -792 and between nt -791 and -242, whereas one suppressor element was found between the nt -241 and -98 base pairs. The same study also showed that the CCAAT-binding factor (CBF, also known as NF-Y and CP1), acting as an enhancer, bound to DSPP element 1 (DE1) located between nt -97 and -74. Also identified was a novel 72 kDa nuclear factor that bound to DSPP element 2 (DE2) between nt -127 and -95, serving as a negative regulator of DSPP expression [31].
Analyses of the mouse genomic DSPP revealed three binding sites in the promoter region for Runx2, a transcription factor known to be critical for osteoblast and odontoblast differentiation [32]. DNA-protein assay and antibody supershift experiments demonstrated interactions between these binding sites and the nuclear extracts isolated from the MO6-G3 cells. Later on, the same group reported that two isoforms of Runx2 were expressed in the mouse preodontoblast cell line MD10-F2 as well as in the mature odontoblast cell line MO6-G3. An in situ hybridization assay by this group showed that DSPP expression increased, whereas Runx2 was downregulated during odontoblast differentiation and maturation [33]. They also demonstrated by electrophoretic mobility shift assay and supershift experiments that Runx2 binds to the three Runx2 sites located in the promoter region of mouse DSPP genes. Mutations of the Runx2 sites in the mouse DSPP promoter resulted in a decline of promoter activity in MD10-F2 cells (preodontoblasts) in contrast to an increase of its activity in the MO6-G3 cells (mature odontoblasts). On the other hand, the forced overexpression of Runx2 induced an increase of endogenous DSPP protein levels in the MD10-F2 cells but reduced its expression in the MO6-G3 cells, which is consistent with the DSPP promoter analyses [33]. Based on these observations, Chen et al. concluded that Runx2 upregulates DSPP in the early stage but downregulates this gene in the later (mature) stage of odontoblast differentiation. Recently, we observed that mice overexpressing TRPS1, a transcription factor whose mutations cause Tricho-rhino-phalangeal syndrome (TRPS) which affects the craniofacial complex and the skeleton, have thin dentin and a very low level of DSPP in the incisors (unpublished data recently obtained in collaboration with Dr. Dobrawa Napierala of Baylor College of Medicine). Since TRPS1 represses Runx2-mediated trans-activation [34], it is likely that the repression of Runx2 may downregulate DSPP and thus results in the low level of DSPP. Although the specific roles of Runx2 in dentinogenesis are not clear, these observations strongly suggest that Runx2 may be involved in the regulation of DSPP. It is also possible that TRPS1 binds to the DSPP promoter and directly regulates this gene.
The bone morphogenetic protein (BMP) family has diverse biological functions during embryonic development. Recently, Chen et al. [35] reported that BMP2 upregulates DSPP mRNA and protein expression in mouse preodontoblasts; by sequentially deleting regions of the mouse DSPP promoter, they showed that a BMP2-response element is located between nucleotides -97 and -72 within the region in which the strongest promoter activity was observed previously and to which the heterotrimeric transcription factor Y (NF-Y) binds (also see above). Using antibody and oligonucleotide competition assays in electrophoretic mobility shift analysis and chromatin immunoprecipitation experiments, Chen et al. showed that BMP2 induces NF-Y accumulation into the nucleus. The NF-Y complex physically interacts with the inverted CCAAT box within the BMP2-response element in the proximal promoter region of mouse DSPP, which, in turn, stimulates DSPP expression. This group also showed that Noggin, a BMP2 antagonist, could significantly inhibit the expression of DSPP in the preodontoblasts.
Dentin matrix protein 1 (DMP1), another member of the SIBLING family, is also primarily expressed in dentin and bone [36]. A decrease in the DSPP level in the dentin of Dmp 1-null mice was observed, suggesting that DSPP may be upregulated by DMP1 during dentinogenesis [37]. This speculation is further supported by a later study [38] showing that the COOH-terminal end of DMP1, which is localized in the nucleus during early differentiation of the odontoblasts, is able to bind specifically with the rat DSPP promoter in the region between nt -450 and +80 and to activate the transcription of DSPP.
Although human and mouse genetic studies have shown that DSPP is critical for the mineralization of dentin, the molecular pathways governing the expression of this gene are poorly understood. The currently available pool of data suggests that BMP2, Runx2, NF-Y and DMP1 may be involved in the regulation of DSPP. Clearly, further studies are warranted to delineate the detailed mechanisms governing the expression of DSPP, which is mainly restricted to the mineralized connective tissues, including dentin, bone and cementum.
PROTEIN STRUCTURE AND METABOLISM
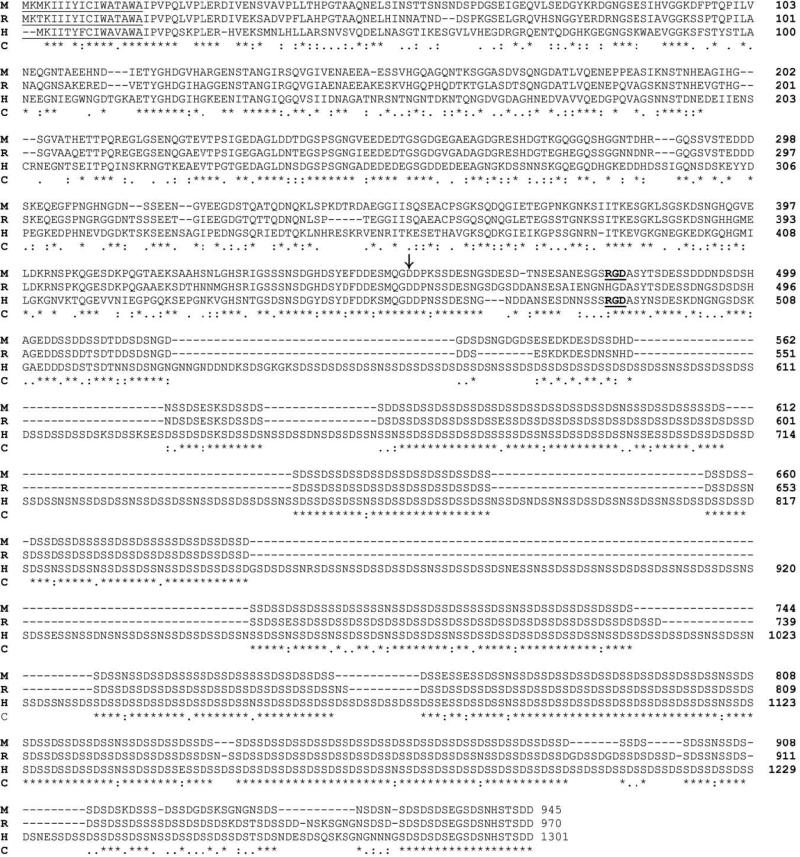
The full-length forms of DSPP in mice [1], rats [25] and humans [26] have 945, 970 and 1301 amino acid residues, respectively (Fig. 2). The hydrophobic signal peptide of mouse and rat DSPP that is present at the NH2-terminus and is responsible for guiding the entry of DSPP into the endoplasmic reticulum comprises 17 amino acid residues, while that of humans contains 15 residues (Fig. 2, underlined). Following the signal peptide is the DSP amino acid sequence. In mouse and human DSPP, an Arg-Gly-Asp (RGD) tripeptide is present in the DPP portion approximately 25-27 amino acid residues away from the start of DPP (Fig. 2, the NH2-terminus of DPP is marked by a vertical arrow). There is no RGD tripeptide in rat DSPP; the Arg in the mouse and human RGD motif is replaced by His in rat DSPP. The absence of the RGD motif in rat DSPP suggests that this tripeptide, which is thought to be responsible for mediating the attachment of the SIBLING family proteins to the cell surface via the integrin receptors [2], may not be essential to the biological functions of DSPP and/or DPP.
Fig. 2. Comparison of amino acid sequences among mouse, rat and human DSPP.
The amino acid sequences from mice [1], rats [25] and humans [26] have been aligned with the aid of the computer program Clustal W2 [88]. M, mouse; R, rat; H, human; C, consensus. The signal peptide region is underlined and the start of DPP (NH2-terminus) is denoted by a vertical arrow. The RGD motifs are underlined and bolded. “*” indicates that the residues in that column are identical in all sequences in the alignment. “:” indicates conserved substitutions. “.” indicates semi-conserved substitutions. “-” indicates that a deletion is observed compared with the other species. Blank spaces denote the absence of any substitution, indicating that these regions are not conserved.
The DPP portion, which contains many repetitive DS and DSS motifs, is very similar among mouse, rat and human DSPP. Excluding the DPP portion, four regions show a high level of homology among mouse, rat and human DSPP. The first region of conservation is between residue 1 and residue 28 (number is based on mouse DSPP sequence), which includes the signal peptide and NH2-terminus of the secreted protein; we refer to this region as the “signal peptide domain.” The second region lies between residue 117 and residue 147, and its importance is unclear. The third region with a high level of conservation is found between residues 222 and 257; the serine residues in the two Ser-Gly (SG) motifs are the attachment sites for glycosaminoglycan (GAG) side chains (Qin et al., unpublished data). This region is designated as the GAG domain. Finally, the fourth region, having a high level of conservation, is between residue 426 and residue 462, which covers the COOH-terminus of DSP and the NH2-terminus of DPP; we call this portion the “linker domain.” The substitution of Asp452 by Ala452 in this domain completely blocked the proteolytic processing of mouse DSPP in eukaryotic cells, suggesting that the peptide bond at the NH2-terminus of Asp452 is critical for the initiation of mouse DSPP processing [39]. As shown in Fig. 2, human DSPP is longer than that of mouse or rat, but the DSP portion of these three species is approximately the same length, containing 451 residues in mice, 447 residues in rats, and 462 residues in humans. It is the different length of the DPP portion that makes the human DSPP longer than the mouse or rat DSPP.
DSPP is a large protein that is proteolytically processed, giving rise to DSP and DPP, with the DSP sequence at the 5’ end and the DPP at the 3’ end of the DSPP transcript [1, 25, 26]. The chemical features of DPP and DSP are remarkably different from each other, and the amount is also dramatically different between the two proteins (the ratio to DPP to DSP is approximately 10:1). Thus, the discovery that these two distinct proteins are encoded by a single mRNA came as a complete surprise to researchers and has brought about new questions and hypotheses.
Earlier protein chemistry work [40] demonstrated that the major NH2-terminus (primary starting point) of rat DPP was Asp448, which corresponds to mouse Asp452 in the DSPP amino acid sequence [1, 25]. The first four amino acid residues of rat DPP are Asp448-Asp-Pro-Asn451, which are identical to those of bovine DPP obtained from sequencing analyses using Edman degradation and mass spectrometry [41, 42]. This NH2-terminal sequence (Asp-Asp-Pro-Asn) is also completely conserved in the amino acid sequences of porcine [28] and human [26] DSPP. These observations indicate that the primary NH2-terminus of rat DPP is Asp448, and the equivalent starts (NH2-termini) of mouse, porcine, and human DPP are Asp452, Asp473, and Asp463, respectively. The observation that the substitution of Asp452 by Ala452 in mouse DSPP blocks the proteolytic processing of this protein in transfected cells [39] further strengthens this conclusion and suggests that cleavage of the peptide bond at the NH2-terminus of Asp452 is essential for releasing the DSP and DPP fragments.
DPP, also called phosphophoryn (PP), was discovered by Veis and colleagues [11, 43, and 44]. It is readily extractable from dentin ECM by acid or ethylenediaminetetraacetic acid (EDTA) solution without guanidine-hydrochloride (Gdm-HCl) [43]. As the most abundant non-collagenous protein in the ECM of dentin, DPP accounts for as much as 50% of the NCPs [45]. The unusual feature of DPP is the presence of large amounts of aspartic acid (Asp) and serine (Ser) residues, with the majority of Ser being phosphorylated [40, 46]. The high levels of Asp and phosphoserine (Pse) produce a very polyanionic macromolecule with an isoelectric point estimated to be 1.1 for rat DPP [47]. Studies showed that rat DPP is heterogeneous in phosphorylation, giving rise to at least three distinct forms of DPP that are either highly phosphorylated (HP), moderately phosphorylated (MP) or low phosphorylated (LP). The highly phosphorylated form of DPP (i.e., HP) has approximately 200 phosphates attached to each protein [40]. The Asp and Pse are mostly present in the repeating sequences of (Asp-Pse-Pse)n and (Asp-Pse)n [1, 15, 18, 19, 25, 26]. Computer-generated predictions portray the repeating sequences of Asp-Pse-Pse and Asp-Pse as extended backbone structures with relatively long ridges of carboxylate and phosphate groups on each side of the peptide backbone [18]. These structures fit well with the purported function of DPP in the nucleation and modulation of apatite crystal formation since they would multiply the calcium molecules in a highly oriented fashion (see below).
While investigating DPP, Butler and colleagues discovered another protein in the dentin extracts that is not as acidic as DPP and can be extracted from dentin by 4 M Gdm-HCl containing 0.5 M EDTA [12, 17]. Purification of this protein showed that it is a glycoprotein with little or no phosphate, which gave a single protein band of ~95 kDa on 5-15% SDS-PAGE [17]. Since this protein contained an unusually high level of carbohydrate, including an unusual amount of sialic acid, it was named “dentin sialoprotein” (DSP). In the ECM of rat dentin, DSP accounts for 5-8% of NCPS and is considerably less abundant than DPP in the dentin ECM [14].
Recently, a high molecular-weight form of DSP [48] was discovered, which was later proven to be the proteoglycan (PG) form of DSP [49, 50]. The GAG chain of the PG form of DSP isolated from porcine dentin is made of chondroitin-6-sulfate [49]. The identification of the PG form of DSP (more abundant than DSP) could partially explain why there is much less DSP than DPP in dentin ECM, even though these two individual proteins originate from the same mRNA.
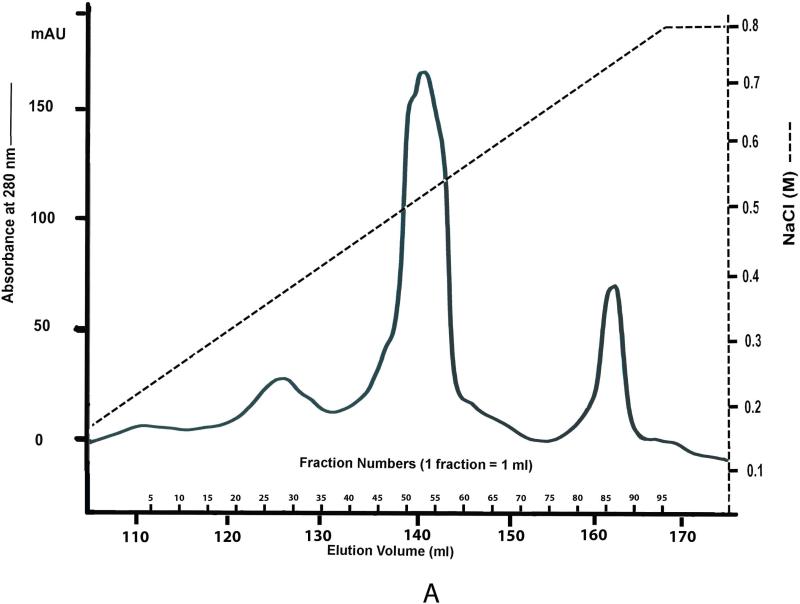
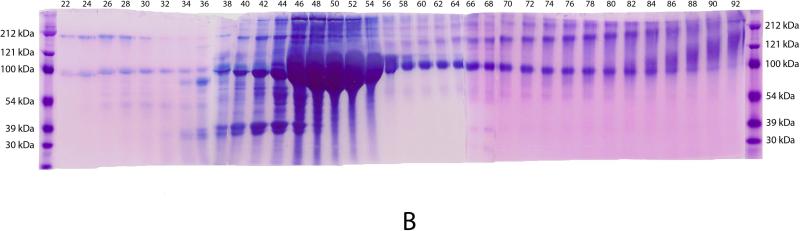
Fig. 3B shows the Stains-All staining profile for the Q-Sepharose chromatographic fractions of NCPs extracted from rat incisor dentin by 4 M Gdm-HCl containing 0.5 M EDTA. DSP is mainly present in fractions 24-36, appearing as a ~100 kDa blue band. DPP is primarily eluted in fractions 46-54, occurring as very broad bands migrating around 100 kDa. The proteoglycan form of DSP is eluted in fractions 66-92, appearing as smears that are stained purple-blue.
Fig. 3. Q-Sepharose separation and Stains-All staining for NCPs extracted from rat dentin by 4 M Gdm-HCl containing 0.5 M EDTA.
Fig. 3A: NCPs were extracted from incisors of 12-week-old rats by 4 M Gdm-HCl containing 0.5 M EDTA and protease inhibitors (0.78 mg ml–1 of benzamidine-HCl, 0.18 mg ml–1 of sodium iodoacetate, 1.8 μg ml–1 of soybean trypsin inhibitor, 0.17 mg ml–1 of phenylmethylsufonyl fluoride, and 5 μg ml–1 of pepstatin). The extracts were separated by Q-Sepharose (Amersham Biosciences, Uppsala, Sweden) chromatography with a gradient ranging from 0.1–0.8 M NaCl in 6 M urea solution. This ion-exchange chromatography separated the NCPs into 95 fractions, each containing 1 ml of 6 M urea.
Fig. 3B: Stains-All staining of fractions 22-92 from the Q-Sepharose chromatography. Numbers on the top of the figure represent fraction numbers. DSP is mainly present in fractions 24-36, appearing as a ~100 kDa blue band. DPP is primarily eluted in fractions 46-54, occurring as a very broad band, migrating around 100 kDa. The proteoglycan form of DSP is eluted in fractions 66-92, occurring as smears that are stained purple-blue.
Porcine dentin seems to contain another molecular variant, designated as dentin glycoprotein (DGP) [51], which is derived from the DSPP sequence. DGP isolated from the extract of porcine tooth dentin has 81 amino acid residues, which are NH2-terminal to the start of porcine DPP. DGP can be visualized by Stains-All and has an apparent molecular mass of 19 kDa on SDS-PAGE, which can be reduced by glycopeptidase A digestion to 16 kDa. DGP has not been reported in other species.
The action of one gene transcribing a single mRNA encoding DSP and DPP indicates that the translated product (DSPP) must be cleaved by a proteinase, giving rise to the individual NH2-terminal and COOH-terminal fragments. Consistent with this conclusion, DSP and DPP are found in significant quantity in dentin ECM, but not DSPP (the protein representing the entire sequence). Studies revealed that at least three peptide bonds in the linker domain are cleaved during the processing of rat DSPP [52, 53]. A recent in vitro study showed that matrix metalloproteinases (MMP-2 and MMP-20) cleaved the NH2-terminal portion of porcine DSPP (i.e., DSP and DGP) at multiple sites, yielding a number of low molecular-weight fragments [54]. Bone morphogenetic protein 1 (BMP-1)/tolloid-like metalloproteinases have been shown to cleave mouse dentin matrix protein 1 (DMP1) at the Ser212-Asp213 bond [55]. Although DSPP and DMP1 belong to the same SIBLING family, the level of sequence homology between the two proteins is low. Nevertheless, the residues surrounding the two cleavage sites, Gly451-Asp452 in mouse DSPP and Ser212-Asp213 in mouse DMP1, are similar and highly conserved across species. Moreover, the amino acid sequences surrounding these two cleavage sites demonstrate similarities to the residues around the cleavage sites within a number of other known physiological substrates of BMP-1/Tolloid-like proteinases [55]. Based on these findings, it is tempting to believe that DSPP is cleaved by BMP-1/Tolloid-like proteinases at Gly451-Asp452, thus releasing DSP and DPP. It is likely that after DSPP is cleaved at this location, the released DSP (corresponding to DSP and DGP in porcine) is further processed by MMP-2 and MMP-20 and perhaps also by other proteinases. We further envision that the initial cleavage of DSPP at Gly451-Asp452 by the BMP-1/Tolloid-like proteinases is an activation step, which converts a precursor from an inactive form to fragments (DSP and DPP) that are fully active at the correct time and site to control the mineralization of dentin. The further fragmentation of DSP by matrix metalloproteinases such as MMP-2 and MMP-20 is likely to be a degradation process.
TISSUE EXPRESSION
For over a decade, DSPP and/or its cleaved products DSP and DPP were believed to be tooth-specific [56]. Recent studies have shown its expression in bone [3, 57], cementum [4] and some non-mineralized tissues [5, 6, 58], although the expression of DSPP in non-dental tissues is lower than in dentin.
DPP can be readily extracted from dentin ECM by acid or EDTA solution, indicating that it is present in the dentin matrix associated with the mineral phase of dentin (i.e., HA crystals). Investigations of the tissue localization of DPP began more than three decades ago. However, since high-titer, specific anti-DPP antibodies have not been available, information about the tissue localization of DPP is still limited. Weinstock and Leblond [59] used [32P] phosphate and [3H] serine in autoradiographic studies to localize rat phosphoproteins and suggested that DPP synthesized by odontoblasts was secreted at the mineralization front (predentin-dentin border), in contrast to collagen, which is secreted in predentin. Since these radioisotopes could also label other phosphoproteins such as DMP1, the autoradiographic imaging could be partially due to the secretion of phosphoproteins in addition to DPP. Nevertheless, the early autoradiographic work performed by Weinstock and Leblond did show that phosphorylated proteins, among which DPP is the most abundant and most highly phosphorylated, are secreted at the mineralization front, not in the predentin. In another study using polyclonal antibodies raised against DPP isolated from mouse dentin, MacDougall et al. showed that mouse DPP was localized in odontoblasts, odontoblastic processes and newly formed mineralized dentin, but not in predentin [45]. Later on, Rabie and Veis [60] confirmed these earlier conclusions by showing that rat DPP was transported through odontoblastic processes directly to the mineralization front.
Using a sequential, three-step extraction approach to extract NCPs from the organic and inorganic phases of the predentin/dentin complex, Huang et al. [61] reported that DSP and DPP are located in the different phases of the predentin/dentin complex. In this sequential extraction protocol, the predentin/dentin complex from the rat incisors was first extracted using 4 M Gdm-HCl without EDTA; this initial step extracted the NCPs present in the unmineralized matrix (predentin) and odontoblast processes. Subsequently, NCPs were extracted with 0.5 M EDTA without Gdm-HCl; this second step extracted NCPs embedded in the mineralized ECM of dentin and tightly bound to the HA crystals. These experiments showed that DSP is mainly present in the first extract (i.e., extract from predentin/odontoblast processes), while DPP exists almost exclusively in the second extract (i.e., extract of the inorganic phase). These results are consistent with previous findings showing that DPP is secreted during mineralization, not in the predentin. More recently, we observed the presence of DPP and DSP in the Gdm-HCl extracts from the dental pulp and odontoblasts that were mechanically dissected from rat teeth (Qin et al. unpublished data). Based on these data, we speculate that DSPP is cleaved in the cells, and then DSP is secreted in the predentin, whereas DPP is secreted at the mineralization front and retained in the mineralized dentin.
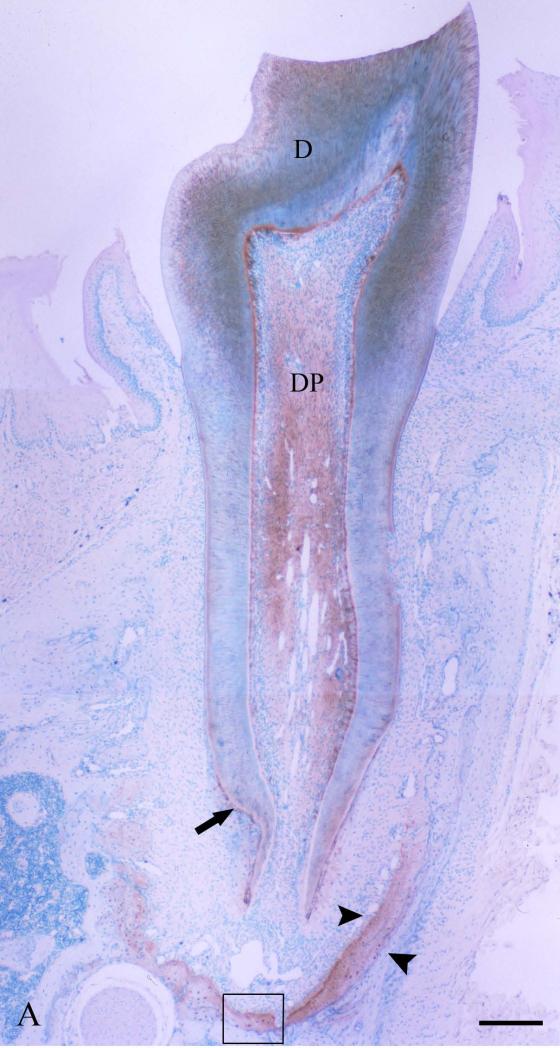
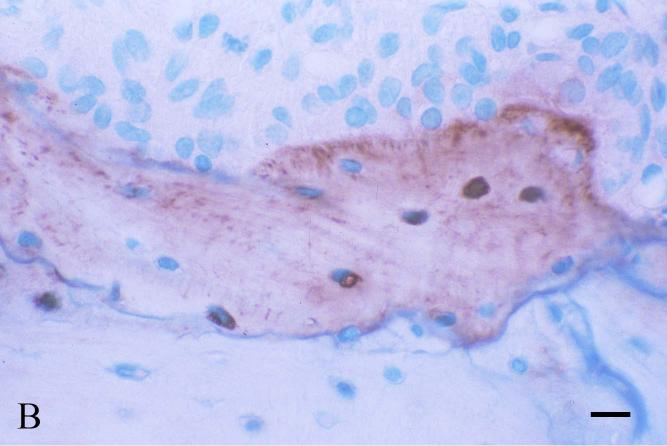
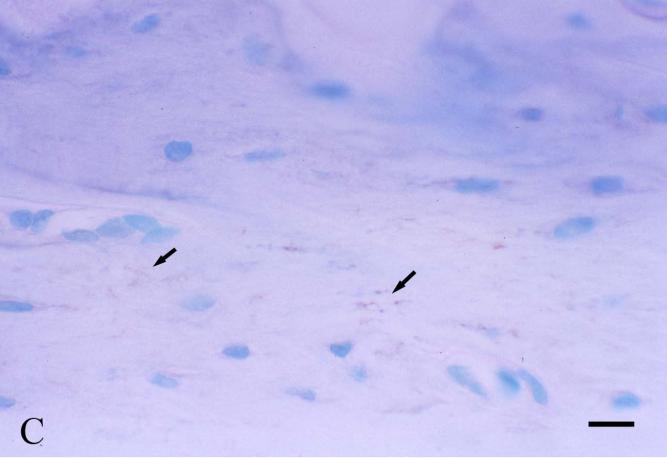
A number of immunohistochemical studies using anti-DSP antibodies showed that DSP is localized in odontoblasts, predentin and dentin [4, 17, 30, 62-64]. Some of these immunolocalization studies indicated that DSP is more abundant in the predentin than in the mineralized dentin [63, 64]. In the mineralized dentin, DSP appears to be localized in the vicinity of the odontoblast processes along the dentinal tubules [64, 65]. Such immunohistochemical findings are consistent with the protein chemistry analyses showing that DSP is mainly present in the predentin and/or associated with odontoblastic processes, and thus can be extracted by Gdm-HCl alone (without EDTA). Immunohistochemistry using anti-DSP antibodies and in situ hybridization analyses using RNA probes matching the DSP and DPP sequences revealed that, in addition to its presence in odontoblasts, DSPP is transiently expressed in the preameloblasts [64, 66, 67]. Because of this expression profile, it appears that DSPP/DSP contributes to the early events of amelogenesis, and in particular to those events resulting in the formation of the dentino-enamel junction and the adjacent “aprismatic” enamel [68]. Studies also detected DSP in cementum, alveolar bone and long bone [4]. Western immunoblotting indicates the quantity of DSP in the rat long bone is approximately 1/400 of that in the dentin. The level of DSP/DSPP in the alveolar bone, particularly at the bottom of the alveolar socket close to the root apex, is much higher than in the long bone, but not as high as in dentin [Fig. 4].
Fig. 4. Immunohistochemical staining of DSP in the first mandibular molar, alveolar bone and tibia of 5-week-old rats.
Immunohistochemical staining was carried out using an ABC kit (Vector Laboratories Inc, Burlingame, CA, USA) following the manufacturer's instructions. The monoclonal anti-DSP antibody clone 2C12.3 [4] was used at a dilution of 1:1000 (approximately 1.9 μg ml-1 of IgG), and the same concentration of normal mouse IgG was used as a negative control. The sections were counterstained with methyl green (Fisher Scientific, Fair Lawn, NJ, USA) solution. Bar in Fig.4A: 100 μm; bars in Figs. 4B, 4C and 4D: 20 μm.
Fig. 4A: Light microscopic images of the anti-DSP immunohistochemistry showing the buccolingual section of first mandibular molar, along with its surrounding periodontal tissues from a postnatal 5-week-old rat. D denotes dentin; DP indicates dental pulp. Arrow indicates cellular cementum (positive to the anti-DSP antibody). Arrowheads indicate alveolar bone. Note that intense reactions are detected in the alveolar bone at the bottom of the socket.
Fig. 4B: High magnification of the boxed area in Fig. 4A. Note the presence of DSP in the matrix as well as in the osteocytes.
Fig. 4C: Very weak positive reaction (arrows) to the anti-DSP antibody is observed in the cortical bone of the rat tibia. Please note that the immunostaining intensity of DSP is much weaker in the tibia than in the alveolar bone, suggesting that the expression level of DSP (DSPP) in the long bone is much lower than in the alveolar bone.
Fig. 4D: Negative control. This section was serial to that used in Fig. 4C. Normal mouse IgG (1.9 μg ml-1) was used to replace the anti-DSP monoclonal antibody in the immunohistochemical staining protocol.
In addition to tooth and bone, DSPP is also detected in some non-mineralized tissues including salivary glands [58], the ureteric bud branches of embryonic metanephric kidney [5], and sweat gland ducts [6]. As this review is focused on DSPP in biomineralization, a detailed discussion about its expression and potential roles in non-mineralized tissues is beyond the scope of this article.
FUNCTIONS OF DSPP, DPP AND DSP
Human genetic studies have shown the association of mutations in the DSPP gene with dentinogenesis imperfecta (DGI), an autosomal dominant disorder causing dentin hypomineralization and significant tooth decay [7, 8, 69-73]. Dspp gene knockout experiments showed that DSPP-deficient mice have defects in the mineralization of dentin [9] and bone [10]. The predentin in the Dspp gene knockout mouse is hypomineralized (widening of predentin), giving rise to a phenotype similar to the manifestations of DGI in humans. All of this information indicates that DSPP is critical for dentin mineralization. Although the exact mechanisms by which DSPP participates in biomineralization are unclear, it has been hypothesized that the proteolytic processing of DSPP into DPP and DSP is an activation step that converts a large precursor into active fragments directly involved in biomineralization [53]; i.e., the roles of DSPP in biomineralization reside in its cleaved products, DPP and DSP. The distinct features of DPP and DSP suggest that these two proteins play different roles in biomineralization. In the following section, we will discuss the potential biological functions of DPP and DSP in dentin mineralization.
Except for type I collagen, DPP is the most abundant ECM component in dentin. Based on its chemical composition and abundance in dentin ECM, DPP was believed to be directly involved in controlling the rate and/or the site of dentin mineralization when it was initially discovered [11, 43, 44]. The high levels of Asp and Pse make DPP a very polyanionic protein that binds large amounts of calcium with a relatively high affinity [74, 75]. DPP forms insoluble aggregates in the presence of Mg++ and Ca++ [76, 77]. Such a special affinity for calcium is thought to be related to the biological role of DPP in the nucleation and growth of HA crystals during dentin mineralization. Subsequently, several in vitro mineralization studies have indicated that DPP is an important initiator and modulator of the formation and growth of HA crystals [78-80]. Binding studies with reconstituted collagen fibrils indicate that DPP has a high affinity for type I collagen [81]. Studies by Traub et al. [82] showed that DPP interacted with turkey tendon fibrillar collagen in the “e” band region, corresponding to the gap region. A number of observations showed that DPP is transported to the mineralization front following its synthesis and secretion by mature odontoblasts [45, 59, 60]. At the mineralization front, DPP binds to collagen fibrils and assumes a structure promoting the formation of initial apatite crystals. As the mineralization process proceeds and predentin is converted to dentin, these mineral crystals grow in an oriented fashion. DPP and other proteins bind to the growing HA faces and inhibit or slow crystal growth, which influences the size and shape of the apatite crystals. Although this putative function for DPP and its purported steps in dentinogenesis remain questionable, they represent the hypotheses that best fit all the data [15, 56].
A recent report has implicated DPP as a coactivator in bone morphogenetic protein-2 (BMP2) signaling [83]. More recently, DPP has been shown to activate the Smad pathway [84]. DPP caused an increase in the phosphorylation of Smad1, which was then translocated to the nucleus, resulting in the upregulation of the Smad1 target genes, Smad6, Dlx5, and Runx2 [5, 84]. Another interesting study suggested that the overexpression of DSPP promoted the mineralization of adipose-derived stem cells (ADSc) along with the expression of early odontogenic marker genes, implying that these cells may differentiate into functional odontoblast-like cells. [85]. Unless new evidence emerges, such purported roles of DPP, other than its direct involvement in biomineralization, are still in question.
The functions of DSP are presently undefined; it has little or no effect on in vitro mineralization and does not promote cell attachment [86]. A recent in vivo study indicates that DSP may be involved in the initiation of dentin mineralization but not in the maturation of this tissue [87]. Recently, a proteoglycan form of DSP has been isolated and characterized [48-50]; this proteoglycan form appears to be more abundant than the core protein DSP in the porcine and rat dentin ECM. However, information on the biological roles of the proteoglycan form of DSP is lacking. It is possible that the proteoglycan form is the functional species of the NH2-terminal fragment of DSPP, while the core protein DSP may be an intermediate product in the degradation process of the proteoglycan form. Clearly, further investigation is needed to study the roles of this proteoglycan form before a conclusion about the functions of the NH2-terminal fragment of DSPP can be drawn.
SUMMARY AND FUTURE WORK
Since the discovery of DPP approximately 40 years ago, much information has been obtained about DPP, DSP and their parent precursor DSPP. These findings have greatly enhanced our understanding of biomineralization. The major discoveries to date include: 1) the discovery and characterization of DPP from different species; 2) the discovery that DPP binds to collagen and calcium, and promotes the nucleation and growth of HA crystals; 3) the demonstration that DPP is secreted at the mineralization front of dentin; 4) the discovery and characterization of DSP; 5) the discovery that DSPP is proteolytically cleaved to form DPP and DSP, which possess distinct biochemical features and are likely to play different biological roles; 6) the finding that mutations in the DSPP gene cause DGI, providing solid in vivo evidence that DSPP is critical to dentin mineralization; 7) the discovery and characterization of the proteoglycan form of DSP; 8) the finding that DSPP is also expressed in bone, cementum and some non-mineralized tissues; and 9) the potential roles of DPP in regulating signal pathways.
While the advances in research on DPP, DSP and DSPP in the past have enriched our knowledge about biomineralization, they have also pointed to new paths of investigation. First, in vivo studies need to be carried out to answer the question of whether proteolytic processing of DSPP is an activation step; one way of testing this hypothesis is to generate and analyze transgenic or knock-in mice expressing mutant DSPP that is not cleaved. Second, the unique similarities between DMP1 and DSPP [65] indicate that the two proteins may function synergistically and that the biologically active cleavage products of DSPP may interact physically with those of DMP1 within the complex milieu of dentin and/or bone ECM; thus, there is a need to examine the potential physical and biochemical interactions between the processed fragments of DSPP and those of DMP1. Third, there is also a need to test if the processed fragments of DSPP interact physically with other NCPs such as bone sialoprotein, osteopontin and osteocalcin. Fourth, further studies are needed to determine the exact pathways controlling the expression of DSPP. Such studies will provide invaluable information about the precise mechanisms by which DSPP is involved in biomineralization.
Other remaining questions about DSPP include: What are the biological functions of the proteoglycan form of DSP? Which proteinase cleaves DSPP, thus releasing the initial DPP and DSP fragments? Are DPP and/or DSP involved in signal transduction? If so, what kind of signaling roles do they play? What are the roles of DSPP in non-mineralized tissues and tumor tissues? Is DSPP cleaved into DPP and DSP in non-mineralized tissues? Answers to these questions are likely to lead us closer to a full delineation of the biological functions of DSPP.
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health Grant DE 005092 (to CQ).
Footnotes
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- 1.MacDougall M, Simmons D, Luan X, Nydegger J, Feng J, Gu TT. Dentin phosphoprotein and dentin sialoprotein are cleavage products expressed from a single transcript coded by a gene on human chromosome 4. Dentin phosphoprotein DNA sequence determination. J Biol Chem. 1997;272:835–42. doi: 10.1074/jbc.272.2.835. [DOI] [PubMed] [Google Scholar]
- 2.Fisher LW, Torchia DA, Fohr B, Young MF, Fedarko NS. Flexible structures of SIBLING proteins, bone sialoprotein, and osteopontin. Biochem Biophys Res Commun. 2001;280:460–5. doi: 10.1006/bbrc.2000.4146. [DOI] [PubMed] [Google Scholar]
- 3.Qin C, Brunn JC, Cadena E, Ridall A, Tsujigiwa H, Nagatsuka H, Nagai N, Butler WT. The expression of dentin sialophosphoprotein gene in bone. J Dent Res. 2002;81:392–4. doi: 10.1177/154405910208100607. [DOI] [PubMed] [Google Scholar]
- 4.Baba O, Qin C, Brunn JC, Jones JE, Wygant JN, McIntyre BW, Butler WT. Detection of dentin sialoprotein in rat periodontium. Eur J Oral Sci. 2004;112:163–70. doi: 10.1111/j.0909-8836.2004.00110.x. [DOI] [PubMed] [Google Scholar]
- 5.Alvares K, Kanwar YS, Veis A. Expression and potential role of dentin phosphophoryn (DPP) in mouse embryonic tissues involved in epithelial-mesenchymal interactions and branching morphogenesis. Dev Dyn. 2006;235:2980–90. doi: 10.1002/dvdy.20935. [DOI] [PubMed] [Google Scholar]
- 6.Ogbureke KU, Fisher LW. SIBLING expression patterns in duct epithelia reflect the degree of metabolic activity. J Histochem Cytochem. 2007;55:403–9. doi: 10.1369/jhc.6A7075.2007. [DOI] [PubMed] [Google Scholar]
- 7.Xiao S, Yu C, Chou X, Yuan W, Wang Y, Bu L, Fu G, Qian M, Yang J, Shi Y, Hu L, Han B, Wang Z, Huang W, Liu J, Chen Z, Zhao G, Kong X. Dentinogenesis imperfecta 1 with or without progressive hearing loss is associated with distinct mutations in DSPP. Nat Genet. 2001;27:201–4. doi: 10.1038/84848. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X, Zhao J, Li C, Gao S, Qiu C, Liu P, Wu G, Qiang B, Lo WH, Shen Y. DSPP mutation in dentinogenesis imperfecta Shields type II. Nat Genet. 2001;27:151–2. doi: 10.1038/84765. [DOI] [PubMed] [Google Scholar]
- 9.Sreenath T, Thyagarajan T, Hall B, Longenecker G, D'Souza R, Hong S, Wright JT, MacDougall M, Sauk J, Kulkarni AB. Dentin sialophosphoprotein knockout mouse teeth display widened predentin zone and develop defective dentin mineralization similar to human dentinogenesis imperfecta type III. J Biol Chem. 2003;278:24874–80. doi: 10.1074/jbc.M303908200. [DOI] [PubMed] [Google Scholar]
- 10.Verdelis K, Ling Y, Sreenath T, Haruyama N, MacDougall M, van der Meulen MC, Lukashova L, Spevak L, Kulkarni AB, Boskey AL. DSPP effects on in vivo bone mineralization. Bone. 2008;43:983–90. doi: 10.1016/j.bone.2008.08.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veis A, Perry A. The phosphoprotein of the dentin matrix. Biochemistry. 1967;6:2409–16. doi: 10.1021/bi00860a017. [DOI] [PubMed] [Google Scholar]
- 12.Butler WT, Bhown M, Dimuzio MT, Linde A. Nonocollagenous proteins of dentin. Isolation and partial characterization of rat dentin proteins and proteoglycans using a three-step preparative method. Coll Relat Res. 1981;1:187–99. doi: 10.1016/s0174-173x(81)80019-2. [DOI] [PubMed] [Google Scholar]
- 13.Linde A, Goldberg M. Dentinogenesis. Crit Rev Oral Biol Med. 1993;4:679–728. doi: 10.1177/10454411930040050301. [DOI] [PubMed] [Google Scholar]
- 14.Butler WT. Dentin matrix proteins and dentinogenesis. Connect Tissue Res. 1995;33:59–65. doi: 10.3109/03008209509016983. [DOI] [PubMed] [Google Scholar]
- 15.Butler WT, Brunn JC, Qin C. Dentin extracellular matrix (ECM) proteins: comparison to bone ECM and contribution to dynamics of dentinogenesis. Connect Tissue Res. 2003;44(Suppl 1):171–8. [PubMed] [Google Scholar]
- 16.Ritchie HH, Hou H, Veis A, Butler WT. Cloning and sequence determination of rat dentin sialoprotein, a novel dentin protein. J Biol Chem. 1994;269:3698–702. [PubMed] [Google Scholar]
- 17.Butler WT, Bhown M, Brunn JC, D'Souza RN, Farach-Carson MC, Happonen RP, Schrohenloher RE, Seyer JM, Somerman MJ, Foster RA, et al. Isolation, characterization and immunolocalization of a 53-kDal dentin sialoprotein (DSP) Matrix. 1992;12:343–51. doi: 10.1016/s0934-8832(11)80030-2. [DOI] [PubMed] [Google Scholar]
- 18.George A, Bannon L, Sabsay B, Dillon JW, Malone J, Veis A, Jenkins NA, Gilbert DJ, Copeland NG. The carboxyl-terminal domain of phosphophoryn contains unique extended triplet amino acid repeat sequences forming ordered carboxyl-phosphate interaction ridges that may be essential in the biomineralization process. J Biol Chem. 1996;271:32869–73. doi: 10.1074/jbc.271.51.32869. [DOI] [PubMed] [Google Scholar]
- 19.Ritchie HH, Wang LH. Sequence determination of an extremely acidic rat dentin phosphoprotein. J Biol Chem. 1996;271:21695–8. doi: 10.1074/jbc.271.36.21695. [DOI] [PubMed] [Google Scholar]
- 20.Feng JQ, Luan X, Wallace J, Jing D, Ohshima T, Kulkarni AB, D'Souza RN, Kozak CA, MacDougall M. Genomic organization, chromosomal mapping, and promoter analysis of the mouse dentin sialophosphoprotein (Dspp) gene, which codes for both dentin sialoprotein and dentin phosphoprotein. J Biol Chem. 1998;273:9457–64. doi: 10.1074/jbc.273.16.9457. [DOI] [PubMed] [Google Scholar]
- 21.George A, Srinivasan R, Thotakura S, Veis A. The phosphophoryn gene family: identical domain structures at the carboxyl end. Eur J Oral Sci. 1998;106(Suppl 1):221–6. doi: 10.1111/j.1600-0722.1998.tb02179.x. [DOI] [PubMed] [Google Scholar]
- 22.George A, Srinivasan RSR, Liu K, Veis A. Rat dentin matrix protein 3 is a compound protein of rat dentin sialoprotein and phosphophoryn. Connect Tissue Res. 1999;40:49–57. doi: 10.3109/03008209909005277. [DOI] [PubMed] [Google Scholar]
- 23.Ritchie HH, Ritchie DG, Wang LH. Six decades of dentinogenesis research. Historical and prospective views on phosphophoryn and dentin sialoprotein. Eur J Oral Sci. 1998;106(Suppl 1):211–20. doi: 10.1111/j.1600-0722.1998.tb02178.x. [DOI] [PubMed] [Google Scholar]
- 24.Ritchie HH, Wang L. The presence of multiple rat DSP-PP transcripts. Biochim Biophys Acta. 2000;1493:27–32. doi: 10.1016/s0167-4781(00)00150-0. [DOI] [PubMed] [Google Scholar]
- 25.Ritchie HH, Wang LH, Knudtson K. A novel rat 523 amino acid phosphophoryn: nucleotide sequence and genomic organization. Biochim Biophys Acta. 2001;1520:212–22. doi: 10.1016/s0167-4781(01)00274-3. [DOI] [PubMed] [Google Scholar]
- 26.Gu K, Chang S, Ritchie HH, Clarkson BH, Rutherford RB. Molecular cloning of a human dentin sialophosphoprotein gene. Eur J Oral Sci. 2000;108:35–42. doi: 10.1034/j.1600-0722.2000.00765.x. [DOI] [PubMed] [Google Scholar]
- 27.Ritchie HH, Li X. A novel rat dentin mRNA coding only for dentin sialoprotein. Eur J Oral Sci. 2001;109:342–7. doi: 10.1034/j.1600-0722.2001.00093.x. [DOI] [PubMed] [Google Scholar]
- 28.Yamakoshi Y, Hu JC, Liu S, Zhang C, Oida S, Fukae M, Simmer JP. Characterization of porcine dentin sialoprotein (DSP) and dentin sialophosphoprotein (DSPP) cDNA clones. Eur J Oral Sci. 2003;111:60–7. doi: 10.1034/j.1600-0722.2003.00009.x. [DOI] [PubMed] [Google Scholar]
- 29.Yamakoshi Y, Lu Y, Hu JC, Kim JW, Iwata T, Kobayashi K, Nagano T, Yamakoshi F, Hu Y, Fukae M, Simmer JP. Porcine dentin sialophosphoprotein: length polymorphisms, glycosylation, phosphorylation, and stability. J Biol Chem. 2008;283:14835–44. doi: 10.1074/jbc.M800633200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan G, Wang Y, Gluhak-Heinrich J, Yang G, Chen L, Li T, Wu LA, Chen Z, Macdougall M, Chen S. Tissue-specific expression of dentin sialophosphoprotein (DSPP) and its polymorphisms in mouse tissues. Cell Biol Int. 2009 doi: 10.1016/j.cellbi.2009.05.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen S, Unterbrink A, Kadapakkam S, Dong J, Gu TT, Dickson J, Chuang HH, MacDougall M. Regulation of the Cell Type-specific dentin sialophosphoprotein gene expression in mouse odontoblasts by a novel transcription repressor and an activator CCAAT-binding factor. J Biol Chem. 2004;279:42182–91. doi: 10.1074/jbc.M402476200. [DOI] [PubMed] [Google Scholar]
- 32.Chen S, Gu TT, Sreenath T, Kulkarni AB, Karsenty G, MacDougall M. Spatial expression of Cbfa1/Runx2 isoforms in teeth and characterization of binding sites in the DSPP gene. Connect Tissue Res. 2002;43:338–44. doi: 10.1080/03008200290000691. [DOI] [PubMed] [Google Scholar]
- 33.Chen S, Rani S, Wu Y, Unterbrink A, Gu TT, Gluhak-Heinrich J, Chuang HH, Macdougall M. Differential regulation of dentin sialophosphoprotein expression by Runx2 during odontoblast cytodifferentiation. J Biol Chem. 2005;280:29717–27. doi: 10.1074/jbc.M502929200. [DOI] [PubMed] [Google Scholar]
- 34.Napierala D, Sam K, Morello R, Zheng Q, Munivez E, Shivdasani RA, Lee B. Uncoupling of chondrocyte differentiation and perichondrial mineralization underlies the skeletal dysplasia in tricho-rhino-phalangeal syndrome. Hum Mol Genet. 2008;17:2244–54. doi: 10.1093/hmg/ddn125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen S, Gluhak-Heinrich J, Martinez M, Li T, Wu Y, Chuang HH, Chen L, Dong J, Gay I, MacDougall M. Bone morphogenetic protein 2 mediates dentin sialophosphoprotein expression and odontoblast differentiation via NF-Y signaling. J Biol Chem. 2008;283:19359–70. doi: 10.1074/jbc.M709492200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qin C, D'Souza R, Feng JQ. Dentin matrix protein 1 (DMP1): new and important roles for biomineralization and phosphate homeostasis. J Dent Res. 2007;86:1134–41. doi: 10.1177/154405910708601202. [DOI] [PubMed] [Google Scholar]
- 37.Ye L, MacDougall M, Zhang S, Xie Y, Zhang J, Li Z, Lu Y, Mishina Y, Feng JQ. Deletion of dentin matrix protein-1 leads to a partial failure of maturation of predentin into dentin, hypomineralization, and expanded cavities of pulp and root canal during postnatal tooth development. J Biol Chem. 2004;279:19141–8. doi: 10.1074/jbc.M400490200. [DOI] [PubMed] [Google Scholar]
- 38.Narayanan K, Gajjeraman S, Ramachandran A, Hao J, George A. Dentin matrix protein 1 regulates dentin sialophosphoprotein gene transcription during early odontoblast differentiation. J Biol Chem. 2006;281:19064–71. doi: 10.1074/jbc.M600714200. [DOI] [PubMed] [Google Scholar]
- 39.Sun Y, Lu Y, Prasad M, Wang X, Butler WT, Qin C. Detection of Full-length Dentin Sialophosphoprotein in Rat Dentin-Pulp Complex. J Dent Res. 2009;88(Special Issue) Abstract # 1690. [Google Scholar]
- 40.Butler WT, Bhown M, DiMuzio MT, Cothran WC, Linde A. Multiple forms of rat dentin phosphoproteins. Arch Biochem Biophys. 1983;225:178–86. doi: 10.1016/0003-9861(83)90021-8. [DOI] [PubMed] [Google Scholar]
- 41.Huq NL, Cross KJ, Talbo GH, Riley PF, Loganathan A, Crossley MA, Perich JW, Reynolds EC. N-terminal sequence analysis of bovine dentin phosphophoryn after conversion of phosphoseryl to S-propylcysteinyl residues. J Dent Res. 2000;79:1914–9. doi: 10.1177/00220345000790111701. [DOI] [PubMed] [Google Scholar]
- 42.Huq NL, Loganathan A, Cross KJ, Chen YY, Johnson NI, Willetts M, Veith PD, Reynolds EC. Association of bovine dentine phosphophoryn with collagen fragments. Arch Oral Biol. 2005;50:807–19. doi: 10.1016/j.archoralbio.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 43.Dickson IR, Dimuzio MT, Volpin D, Ananthanarayanan S, Veis A. The extraction of phosphoproteins from bovine dentin. Calcif Tissue Res. 1975;19:51–61. doi: 10.1007/BF02563990. [DOI] [PubMed] [Google Scholar]
- 44.Dimuzio MT, Veis A. The biosynthesis of phosphophoryns and dentin collagen in the continuously erupting rat incisor. J Biol Chem. 1978;253:6845–52. [PubMed] [Google Scholar]
- 45.MacDougall M, Zeichner-David M, Slavkin HC. Production and characterization of antibodies against murine dentine phosphoprotein. Biochem J. 1985;232:493–500. doi: 10.1042/bj2320493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee SL, Veis A, Glonek T. Dentin phosphoprotein: an extracellular calcium-binding protein. Biochemistry. 1977;16:2971–9. doi: 10.1021/bi00632a026. [DOI] [PubMed] [Google Scholar]
- 47.Jonsson M, Fredriksson S. Isoelectric focusing of the phosphoprotein of ratincisor dentin in ampholine and acid pH gradients. Evidence for carrier ampholyte-protein complexes. J Chromatogr. 1978;157:234–42. doi: 10.1016/s0021-9673(00)92338-0. [DOI] [PubMed] [Google Scholar]
- 48.Qin C, Brunn JC, Baba O, Wygant JN, McIntyre BW, Butler WT. Dentin sialoprotein isoforms: detection and characterization of a high molecular weight dentin sialoprotein. Eur J Oral Sci. 2003;111:235–42. doi: 10.1034/j.1600-0722.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- 49.Yamakoshi Y, Hu JC, Fukae M, Iwata T, Kim JW, Zhang H, Simmer JP. Porcine dentin sialoprotein is a proteoglycan with glycosaminoglycan chains containing chondroitin 6-sulfate. J Biol Chem. 2005;280:1552–60. doi: 10.1074/jbc.M409606200. [DOI] [PubMed] [Google Scholar]
- 50.Sugars RV, Olsson ML, Waddington R, Wendel M. Substitution of bovine dentine sialoprotein with chondroitin sulfate glycosaminoglycan chains. Eur J Oral Sci. 2006;114:89–92. doi: 10.1111/j.1600-0722.2006.00271.x. [DOI] [PubMed] [Google Scholar]
- 51.Yamakoshi Y, Hu JC, Fukae M, Zhang H, Simmer JP. Dentin glycoprotein: the protein in the middle of the dentin sialophosphoprotein chimera. J Biol Chem. 2005;280:17472–9. doi: 10.1074/jbc.M413220200. [DOI] [PubMed] [Google Scholar]
- 52.Qin C, Cook RG, Orkiszewski RS, Butler WT. Identification and characterization of the carboxyl-terminal region of rat dentin sialoprotein. J Biol Chem. 2001;276:904–9. doi: 10.1074/jbc.M006271200. [DOI] [PubMed] [Google Scholar]
- 53.Qin C, Baba O, Butler WT. Post-translational modifications of sibling proteins and their roles in osteogenesis and dentinogenesis. Crit Rev Oral Biol Med. 2004;15:126–36. doi: 10.1177/154411130401500302. [DOI] [PubMed] [Google Scholar]
- 54.Yamakoshi Y, Hu JC, Iwata T, Kobayashi K, Fukae M, Simmer JP. Dentin sialophosphoprotein is processed by MMP-2 and MMP-20 in vitro and in vivo. J Biol Chem. 2006;281:38235–43. doi: 10.1074/jbc.M607767200. [DOI] [PubMed] [Google Scholar]
- 55.Steiglitz BM, Ayala M, Narayanan K, George A, Greenspan DS. Bone morphogenetic protein-1/Tolloid-like proteinases process dentin matrix protein-1. J Biol Chem. 2004;279:980–6. doi: 10.1074/jbc.M310179200. [DOI] [PubMed] [Google Scholar]
- 56.Butler WT. Dentin matrix proteins. Eur J Oral Sci. 1998;106(Suppl 1):204–10. doi: 10.1111/j.1600-0722.1998.tb02177.x. [DOI] [PubMed] [Google Scholar]
- 57.Qin C, Brunn JC, Cadena E, Ridall A, Butler WT. Dentin sialoprotein in bone and dentin sialophosphoprotein gene expressed by osteoblasts. Connect Tissue Res. 2003;44(Suppl 1):179–83. [PubMed] [Google Scholar]
- 58.Ogbureke KU, Fisher LW. Expression of SIBLINGs and their partner MMPs in salivary glands. J Dent Res. 2004;83:664–70. doi: 10.1177/154405910408300902. [DOI] [PubMed] [Google Scholar]
- 59.Weinstock M, Leblond CP. Radioautographic visualization of the deposition of a phosphoprotein at the mineralization front in the dentin of the rat incisor. J Cell Biol. 1973;56:838–45. doi: 10.1083/jcb.56.3.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rabie AM, Veis A. An immunocytochemical study of the routes of secretion of collagen and phosphophoryn from odontoblasts into dentin. Connect Tissue Res. 1995;31:197–209. doi: 10.3109/03008209509010811. [DOI] [PubMed] [Google Scholar]
- 61.Huang B, Sun Y, Maciejewska I, Qin D, Peng T, McIntyre B, Wygant J, Butler WT, Qin C. Distribution of SIBLING proteins in the organic and inorganic phases of rat dentin and bone. Eur J Oral Sci. 2008;116:104–12. doi: 10.1111/j.1600-0722.2008.00522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.D'Souza RN, Bronckers AL, Happonen RP, Doga DA, Farach-Carson MC, Butler WT. Developmental expression of a 53 KD dentin sialoprotein in rat tooth organs. J Histochem Cytochem. 1992;40:359–66. doi: 10.1177/40.3.1552175. [DOI] [PubMed] [Google Scholar]
- 63.Bronckers AL, D'Souza RN, Butler WT, Lyaruu DM, van Dijk S, Gay S, Woltgens JH. Dentin sialoprotein: biosynthesis and developmental appearance in rat tooth germs in comparison with amelogenins, osteocalcin and collagen type-I. Cell Tissue Res. 1993;272:237–47. doi: 10.1007/BF00302729. [DOI] [PubMed] [Google Scholar]
- 64.Baba O, Qin C, Brunn JC, Wygant JN, McIntyre BW, Butler WT. Colocalization of dentin matrix protein 1 and dentin sialoprotein at late stages of rat molar development. Matrix Biol. 2004;23:371–9. doi: 10.1016/j.matbio.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 65.Qin C, Baba O, Brunn JC, McKee MD, Bonewald L, Butler WT. Dentin matrix protein 1 (DMP1) and dentin sialophosphoprotein (DSPP) share unique properties including tissue localization, proteolytic processing, and high molecular weight forms. In: SODEK J, LANDIS W, editors. Proceedings of the 8th ICCBMT. University of Toronto Press, Toronto, Canada; Banff, Alberta, Canada: 2005. pp. 174–177. [Google Scholar]
- 66.Begue-Kirn C, Ruch JV, Ridall AL, Butler WT. Comparative analysis of mouse DSP and DPP expression in odontoblasts, preameloblasts, and experimentally induced odontoblast-like cells. Eur J Oral Sci. 1998;106(Suppl 1):254–9. doi: 10.1111/j.1600-0722.1998.tb02184.x. [DOI] [PubMed] [Google Scholar]
- 67.Begue-Kirn C, Krebsbach PH, Bartlett JD, Butler WT. Dentin sialoprotein, dentin phosphoprotein, enamelysin and ameloblastin: tooth-specific molecules that are distinctively expressed during murine dental differentiation. Eur J Oral Sci. 1998;106:963–70. doi: 10.1046/j.0909-8836.1998.eos106510.x. [DOI] [PubMed] [Google Scholar]
- 68.Paine ML, Luo W, Wang HJ, Bringas P, Jr., Ngan AY, Miklus VG, Zhu DH, MacDougall M, White SN, Snead ML. Dentin Sialoprotein and dentin phosphoprotein overexpression during amelogenesis. J Biol Chem. 2005;280:31991–8. doi: 10.1074/jbc.M502991200. [DOI] [PubMed] [Google Scholar]
- 69.Holappa H, Nieminen P, Tolva L, Lukinmaa PL, Alaluusua S. Splicing site mutations in dentin sialophosphoprotein causing dentinogenesis imperfecta type II. Eur J Oral Sci. 2006;114:381–4. doi: 10.1111/j.1600-0722.2006.00391.x. [DOI] [PubMed] [Google Scholar]
- 70.Malmgren B, Lindskog S, Elgadi A, Norgren S. Clinical, histopathologic, and genetic investigation in two large families with dentinogenesis imperfecta type II. Hum Genet. 2004;114:491–8. doi: 10.1007/s00439-004-1084-z. [DOI] [PubMed] [Google Scholar]
- 71.Rajpar MH, Koch MJ, Davies RM, Mellody KT, Kielty CM, Dixon MJ. Mutation of the signal peptide region of the bicistronic gene DSPP affects translocation to the endoplasmic reticulum and results in defective dentine biomineralization. Hum Mol Genet. 2002;11:2559–65. doi: 10.1093/hmg/11.21.2559. [DOI] [PubMed] [Google Scholar]
- 72.Kim JW, Nam SH, Jang KT, Lee SH, Kim CC, Hahn SH, Hu JC, Simmer JP. A novel splice acceptor mutation in the DSPP gene causing dentinogenesis imperfecta type II. Hum Genet. 2004;115:248–54. doi: 10.1007/s00439-004-1143-5. [DOI] [PubMed] [Google Scholar]
- 73.Dong J, Gu T, Jeffords L, MacDougall M. Dentin phosphoprotein compound mutation in dentin sialophosphoprotein causes dentinogenesis imperfecta type III. Am J Med Genet A. 2005;132A:305–9. doi: 10.1002/ajmg.a.30460. [DOI] [PubMed] [Google Scholar]
- 74.Zanetti M, de Bernard B, Jontell M, Linde A. Ca2+-binding studies of the phosphoprotein from rat-incisor dentine. Eur J Biochem. 1981;113:541–5. doi: 10.1111/j.1432-1033.1981.tb05096.x. [DOI] [PubMed] [Google Scholar]
- 75.Marsh ME. Binding of calcium and phosphate ions to dentin phosphophoryn. Biochemistry. 1989;28:346–52. doi: 10.1021/bi00427a047. [DOI] [PubMed] [Google Scholar]
- 76.Kuboki Y, Fujisawa R, Aoyama K, Sasaki S. Calcium-specific precipitation of dentin phosphoprotein: a new method of purification and the significance for the mechanism of calcification. J Dent Res. 1979;58:1926–32. doi: 10.1177/00220345790580092001. [DOI] [PubMed] [Google Scholar]
- 77.Marsh ME. Self-association of calcium and magnesium complexes of dentin phosphophoryn. Biochemistry. 1989;28:339–45. doi: 10.1021/bi00427a046. [DOI] [PubMed] [Google Scholar]
- 78.Linde A. Dentin matrix proteins: composition and possible functions in calcification. Anat Rec. 1989;224:154–66. doi: 10.1002/ar.1092240206. [DOI] [PubMed] [Google Scholar]
- 79.Boskey AL, Maresca M, Doty S, Sabsay B, Veis A. Concentration-dependent effects of dentin phosphophoryn in the regulation of in vitro hydroxyapatite formation and growth. Bone Miner. 1990;11:55–65. doi: 10.1016/0169-6009(90)90015-8. [DOI] [PubMed] [Google Scholar]
- 80.Saito T, Arsenault AL, Yamauchi M, Kuboki Y, Crenshaw MA. Mineral induction by immobilized phosphoproteins. Bone. 1997;21:305–11. doi: 10.1016/s8756-3282(97)00149-x. [DOI] [PubMed] [Google Scholar]
- 81.Stetler-Stevenson WG, Veis A. Type I collagen shows a specific binding affinity for bovine dentin phosphophoryn. Calcif Tissue Int. 1986;38:135–41. doi: 10.1007/BF02556873. [DOI] [PubMed] [Google Scholar]
- 82.Traub W, Jodaikin A, Arad T, Veis A, Sabsay B. Dentin phosphophoryn binding to collagen fibrils. Matrix. 1992;12:197–201. doi: 10.1016/s0934-8832(11)80062-4. [DOI] [PubMed] [Google Scholar]
- 83.Saito T, Ogawa M, Hata Y, Bessho K. Acceleration effect of human recombinant bone morphogenetic protein-2 on differentiation of human pulp cells into odontoblasts. J Endod. 2004;30:205–8. doi: 10.1097/00004770-200404000-00005. [DOI] [PubMed] [Google Scholar]
- 84.Jadlowiec JA, Zhang X, Li J, Campbell PG, Sfeir C. Extracellular matrix-mediated signaling by dentin phosphophoryn involves activation of the Smad pathway independent of bone morphogenetic protein. J Biol Chem. 2006;281:5341–7. doi: 10.1074/jbc.M506158200. [DOI] [PubMed] [Google Scholar]
- 85.Wu L, Zhu F, Wu Y, Lin Y, Nie X, Jing W, Qiao J, Liu L, Tang W, Zheng X, Tian W. Dentin sialophosphoprotein-promoted mineralization and expression of odontogenic genes in adipose-derived stromal cells. Cells Tissues Organs. 2008;187:103–12. doi: 10.1159/000110079. [DOI] [PubMed] [Google Scholar]
- 86.Boskey A, Spevak L, Tan M, Doty SB, Butler WT. Dentin sialoprotein (DSP) has limited effects on in vitro apatite formation and growth. Calcif Tissue Int. 2000;67:472–8. doi: 10.1007/s002230001169. [DOI] [PubMed] [Google Scholar]
- 87.Suzuki S, Sreenath T, Haruyama N, Honeycutt C, Terse A, Cho A, Kohler T, Muller R, Goldberg M, Kulkarni AB. Dentin sialoprotein and dentin phosphoprotein have distinct roles in dentin mineralization. Matrix Biol. 2009;28:221–9. doi: 10.1016/j.matbio.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. ClustalW and ClustalX version 2. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]