Abstract
Phospholipase C-γ1 (PLC-γ1), a tyrosine kinase substrate, has been implicated in the pathway for the epidermal growth factor receptor (EGFR)-induced cell migration. However, the underlying mechanism by which PLC-γ1 mediates EGFR-induced cell migration remains elusive. In the present study, we sought to determine whether the lipase activity of PLC-γ1 is required for EGFR-induced cell migration. We found that overexpression of PLC-γ1 in squamous cell carcinoma SCC4 cells markedly enhanced EGF-induced PLC-γ1 activation, intracellular calcium rise and cell migration. This enhancement was abolished by mutational inactivation of the catalytic domain of PLC-γ1. Inhibition of the downstream signaling processes mediated by the activity of phospholipase C (PLC) using IP3 receptor inhibitor or intracellular calcium chelator blocked EGF-induced cell migration. These data indicate that EGF-induced cell migration is mediated by the lipase domain of PLC-γ1 and the subsequent IP3 generation and intracellular calcium mobilization.
Keywords: Epidermal growth factor receptor, phospholipase C-γ1, migration, catalytic activity
1. Introduction
Induction of cancer cell migration is a key step in invasion and metastasis. In many types of cancer, upregulation of active migration is stimulated by growth factor receptor signaling, the epidermal growth factor receptor (EGFR) being the most frequently implicated [1]. However, the signaling pathways that regulate EGFR-stimulated migration still poorly understood. Elucidation of these pathways may lead to the development of novel cancer therapeutic targets.
Several pathways are known to be activated by EGFR. These pathways include mitogen-activated protein kinase (MAPK), phosphatidylinositol 3-kinase (PI3K), Signal Transducer and Activator of Transcription (STAT) and phospholipase C-γ1 (PLC-γ1) [1]. PLC-γ1, an isozyme of the phosphoinositide-specific phospholipase C (PLC) family, is a multi-domain protein that contains two pleckstrin homology (PH) domains, two Src homology 2 (SH2) domains, one Src homology 3 (SH3) domain and two catalytic domains [2]. In response to EGFR ligands such as epidermal growth factor (EGF) and transforming growth factor α, the SH2 domain of PLC-γ1 binds to EGFR. This binding allows EGFR to phosphorylate PLC-γ1 at its tyrosine residues and activates the lipase. The activated PLC-γ1 hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) to inositol 1,4,5-triphosphate (IP3) and diacylglycerol (DAG). DAG is a physiological activator of protein kinase C (PKC), and IP3 induces the calcium release from intracellular stores. The intracellular calcium rise and PKC activation are essential for many cellular activities [2].
Overexpression of PLC-γ1 has previously been reported in invasive carcinoma tissues, including breast cancer [3], colorectal cancer [4] and squamous cell carcinoma [5], Several studies have demonstrated that inhibition of PLC-γ1 expression or PLC activity blocks EGF-induced cell migration in a variety of tumor cell types [6–13]}. Although these observations implicate a role for PLC-γ1 and the lipase activity of PLC, they do not pinpoint the role of the activity of PLC-γ1 in EGF-induced migration because the PLC inhibitor used in these studies does not selectively target the γ1 isoform of PLC [14,15]. In the present study, we provide direct evidence that PLC-γ1 requires its catalytic activity to mediate EGF-induced squamous cell carcinoma (SCC) cell migration.
2. Materials and Methods
2.1. Cell Culture
Human tongue squamous cell carcinoma SCC4 cells obtained from ATCC were grown in the SCC medium composed of 1:1 mixture of Ham’s F12 and Dulbecco’s Modified Eagle’s Medium with 10% fetal bovine serum. Cells were serum-starved for 24 hrs before treatment with EGF in subsequent experiments.
2.2. DNA constructs and transfection
SCC4 cells were transiently transfected in suspension with pcDNA 3.1 containing full-length PLC-γ1 (a gift from Dr. John Imboden, San Francisco General Hospital) or the X domain-deleted mutant tagged with the FLAG epitope (Figure 1) using a polybrene/glycerol method [16]. The pcDNA3.1 vector contains the neomycin (G418) resistant gene to allow selection. The transfected cells were selected by 4-day incubation in 300 μM G418 starting 2 days after transfection to enrich transfected cells as we previously described [17,18].
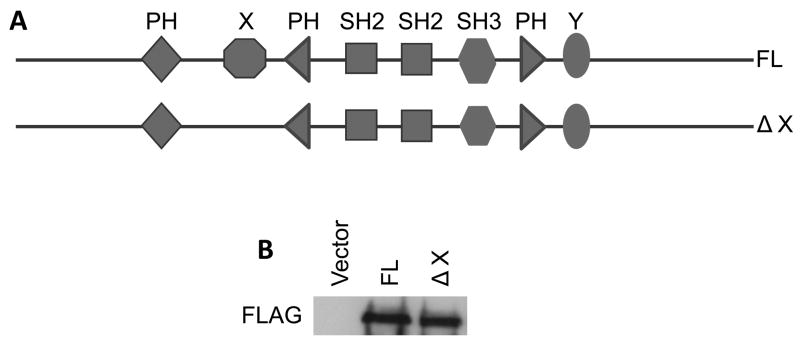
Fig. 1.
Schematic diagram of the full-length and truncated PLC-γ1-FLAG-tagged fusion constructs. A) The constructs of the full-length PLC-γ1 (FL) and PLC-γ1 lacking the X domain (ΔX). (B) SCC4 cells were transfected with DNA constructs as indicated. The total cell lysates were isolated. The level of the FLAG fusion protein was determined by immunoblotting with anti-FLAG antibody.
2.3. Immunoblotting
The total cell lysates were isolated using radioimmunoprecipitation assay lysis buffer containing 50 mM HEPES, pH 7.4, 1% Triton X-100, 0.1% SDS, 150 mM NaCl, 1 mM EDTA, and Complete Protease Inhibitor Cocktail Tablets (Roche Applied Science, Indianapolis, IN). The protein concentration of the lysate was measured by bicinchoninic acid protein assay kits (Thermo Fisher Scientific, Rockford, IL). Equal amounts of protein were electrophoresed by the reducing SDS-polyacrylamide gel electrophoresis and electroblotted onto polyvinylidene fluoride microporous membranes (Immobilon-P, 0.45 μm; Millipore Billerica, MA). After incubation in blocking buffer (100 mM Tris base, 150 mM NaCl, 5% nonfat milk, and 0.5% Tween-20) for 1 h at room temperature, blots were then incubated overnight at 4 °C with a monoclonal antibody against FLAG peptide at a dilution of 1:1000 (Agilent Technologies, Santa Clara, CA).
2.4. PLC-γ1 activity assay
PLC-γ1 activity was determined by measuring accumulation of IP3 according to the experimental procedure described [19–22]. SCC4 cells in 150 mm dishes were washed with PBS containing 0.1% sodium orthovanadate and 0.1% sodium fluoride, and then incubated with 1% NP-40 containing Phosphatase Inhibitor Cocktail (Roche Applied Science, Indianapolis, IN) and Complete Protease Inhibitor Cocktail Tablets (Roche Applied Science) for 5 min. Cells were scraped into microfuge tubes and incubated at 4 °C on a rotator for 1 h. Sixty micrograms of protein from the supernatant collected after centrifugation was incubated with 2 μg polyclonal antibody against the C-terminus of PLC-γ1 (BD Biosciences, San Jose, CA) at 4 °C overnight, and then with 20 μl UltraLink Immobilized Protein G (Thermo Fisher Scientific) at 4 °C for 1 h. The PLC-γ1 antibody reacts with both endogenous and transfected PLC-γ1 including the wildtype and mutants. After centrifugation, the pellet was washed with the reaction buffer (10 mM HEPES, pH 7.0, 10 mM NaCl, 120 mM KCl, 2 mM EGTA, 0.05% deoxycholate, 5 μg/ml bovine serum albumin and 10 μM CaCl2) and resuspended in 200 μl reaction buffer. In triplicates, 50 μl of the suspension was incubated with sonicated vesicles containing [3H]-PIP2 (PerkinElmer Life Science, Waltham, MA), phosphatidylcholine (Sigma Aldrich Corporation, St. Louis, MO) and phosphatidylserine (Sigma Aldrich Corporation) in a molar ratio of 1:3:3 in 100 μl reaction buffer. The reaction was ended by adding 200 μl of 10% trichloroacetic acid at 5 min and 200 μl of 10% bovine serum albumin. The radioactivity of supernatant after centrifugation was determined by a scintillation counter and normalized to the protein content in the immunoprecipitates.
2.5. Intracellular Calcium Determination
Intracellular calcium levels of SCC4 cells attached to glass coverslips were measured using a Dual-wavelength Fluorescence Imaging System. Briefly, the cells were loaded with 1 μM Fura-2 (Molecular Probes, Eugene, OR) in 0.1% Pluronic F127 (Molecular Probes, Eugene, OR) in buffer A (20 mM HEPES buffer, pH 7.4, containing 120 mM sodium chloride, 5 mM potassium chloride, 0.5 mM magnesium chloride, 1 mg/ml pyruvate, and 1 mM/ml glucose) at room temperature for 30 min followed by a 30-min rinse in buffer A. The cells were then alternately illuminated with 200-ms flashes of 340 and 380 nm light every 10 s, monitoring the emission wavelength of 510 nm. The signals from 40 to 60 single cells for each measurement were recorded. Intracellular calcium concentration was calculated based on the ratio of emission at the two excitation wavelengths based on the formula developed by Grynkiewicz et al [23].
2.6. Migration Assay
Cell migration assay was performed using Transwells containing polycarbonate membrane (8–12 μM pore size, Corning Incorporated, Corning, NY). Cells were seeded onto the top of each membrane. Medium containing 10 ng/ml EGF was added to the lower chamber. After incubation for 16 h, non-migrated cells on the upper membrane surface were removed. The attached migrated cells with the membrane were incubated with MTT at a concentration of 1 mg/ml for 2 h. After removing MTT solution, the remaining dark blue crystals were dissolved in dimethyl Sulphoxide. Plates were read on a Dynatech plate reader at a wavelength of 570 nm. The results were normalized to the total cell number. Six wells were harvested for each treatment; three wells were used for the Transwell assay, and the remaining three wells were used to determine the total cell number.
3. Results and Discussion
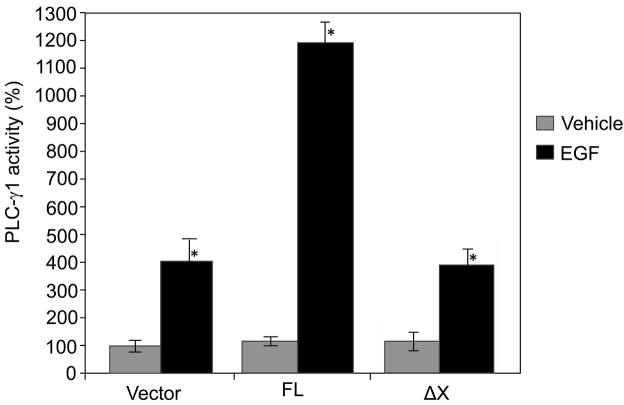
The importance of the catalytic activity of PLC-γ1 in mediating EGF-induced cell migration was investigated by use of the antisense PLC-γ1 constructs or PLC inhibitor U-73122 in previous studies [6–13,24]. However, it remained undetermined that whether the catalytic activity of PLC-γ1 plays a critical role in EGF-induced cell migration because the PLC inhibitor used in these studies is not isoform-specific [14,15]. To pinpoint the role of the catalytic activity of PLC-γ1 in EGF-induced SCC cell migration, we examined the effects of overexpression of PLC-γ1 or the catalytically inactive mutant of PLC-γ1 on EGF-induced PLC-γ1 activity, intracellular calcium rise and cell migration. The cDNA fragments for transfection include a full-length human PLC-γ1 (FL) cDNA and a truncated mutant lacking the X catalytic domain (ΔX). Both the cDNA inserts in the expression vector pcDNA 3.1 were tagged at the N-terminal end with a FLAG epitope which allows observations of transfected PLC-γ1. These constructs were separately transfected into SCC4 cells (Fig. 1). Transfected cells were then stimulated by EGF at a concentration of 50 ng/ml. The expression of the full-length PLC-γ1 or the PLC-γ1 mutant was confirmed by immunoblotting with anti-FLAG antibody (Fig. 1B). Both the constructs were found to express comparable level of FLAG-PLC-γ1 (Fig. 1B). The catalytic activity of PLC-γ1 of these constructs expressed in SCC4 cells was confirmed by PLC-γ1 activity assay. All the constructs showed low and comparable levels of PLC-γ1 activity when transfected into SCC4 cells under serum-starved conditions. EGF treatment for 2 minutes induced activity of endogenous PLC-γ1 by 4-fold (Fig. 2). Overexpression of FL markedly enhanced EGF-induced PLC-γ1 activation (Fig. 2). However, overexpression of ΔX did not enhance EGF-induced PLC-γ1 activation, suggesting that deletion of the X-domain efficiently inactivated the catalytic activity. These results justify the construct validity.
Fig. 2.
Lipase activity of the full-length and X-domain truncated PLC-γ1-FLAG-tagged fusion constructs. SCC4 cells were transfected with the full-length (FL) and X-domain truncated (ΔX) PLC-γ1-FLAG-tagged fusion constructs as indicated. Transfected cells were selected by 4-day incubation in 300 μM G418, and then treated with EGF (50 ng/ml) for 2 minutes. Cells were harvested and total cell lysates were isolated. PLC-γ1 in the total cell lysates was immunoprecipitated using the antibody against the C-terminus of PLCγ1, and PLC-γ1 activity was assayed. The results are expressed as percentages of the values obtained with vector-transfected/vehicle-treated cells. Data are mean ± SD of triplicates within a single representative experiments, *P<0.01 (highly significantly different from the vehicle treated cells). Results shown are representative of three independent experiments.
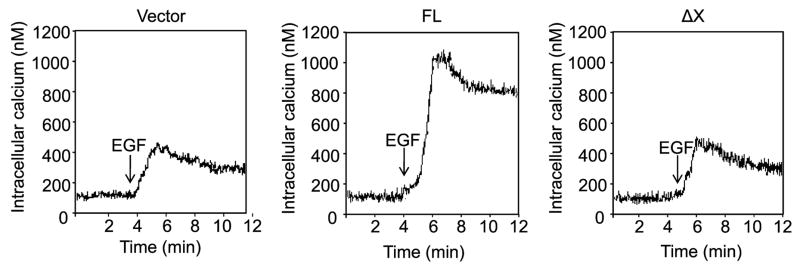
To determine whether the catalytic activity of PLC-γ1 is required for EGF-induced intracellular calcium rise, SCC4 cells were transfected with the FL or ΔX constructs. The empty vector was used as a control. Cells were then treated with G418 to enrich transfected cells, and the response of intracellular calcium level to EGF was determined. The results showed that EGF induced intracellular calcium rise by 4-fold in empty vector-transfected cells. This induction was markedly enhanced by overexpression of FL. However, overexpression of ΔX did not enhance EGF-induced intracellular calcium rise (Fig. 3). These results suggest that PLC-γ1 requires its catalytic activity to mediate EGF-induced intracellular calcium rise.
Fig. 3.
EGF-induced intracellular calcium rise requires the catalytic activity of PLC-γ1. SCC4 cells cultured on glass coverslips were transfected with the full-length (FL) and X-domain truncated (ΔX) PLC-γ1-FLAG-tagged fusion constructs as indicated. Transfected cells were selected by 4-day incubation in 300 μM G418. Intracellular calcium was measured, and the response to EGF (50 ng/ml) was determined. The traces shown in this figure represent the average intracellular calcium of 45–60 individual cells during recording. The results shown are from a representative experiment repeated three times with three separate transfection experiments.
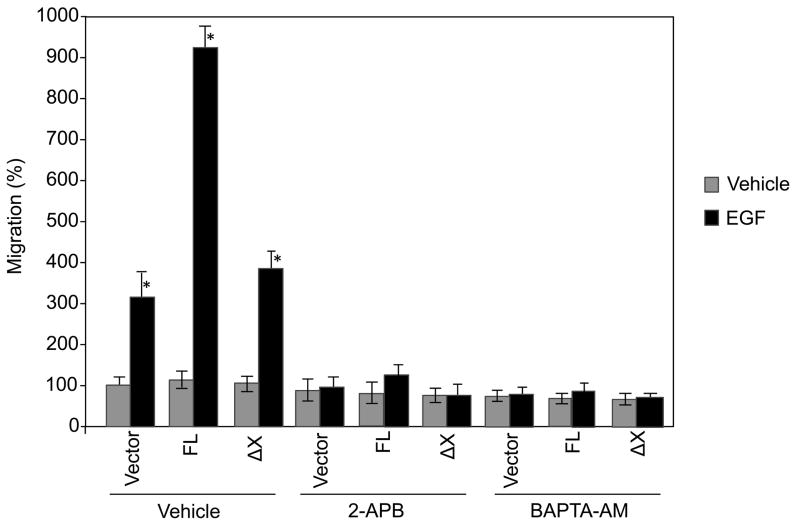
To address whether the catalytic activity of PLC-γ1 play a role in EGF-induced cell migration, SCC4 cells were transfected with FL or ΔX constructs. After treatments with G418, cells were treated with EGF for 16 h and migration was determined. The results showed that EGF induced cell migration by 3-fold. Overexpression of FL dramatically enhanced EGF-induced cell migration. However, overexpression of ΔX did not enhance EGF-induced cell migration (Fig. 4). These data suggest that PLC-γ1 requires its catalytic activity to mediate EGF-induced cell migration.
Fig. 4.
EGF-induced cell migration requires the catalytic activity of PLC-γ1, IP3 and intracellular calcium. SCC4 cells were transfected with the full-length (FL) and X-domain truncated (ΔX) PLC-γ1-FLAG-tagged fusion constructs as indicated. Transfected cells were selected by 4-day incubation in 300 μM G418 and pretreated with 2-APB (50 μM), BAPTA-AM (10 μM) or vehicle, and then treated with EGF (50 ng/ml) for 16 h. Cell migration was determined by Transwell assay. The results are expressed as percentages of the values obtained with vector-transfected/vehicle-treated cells. Data are mean ± SD of triplicates within a single representative experiment, *p<0.01 (highly significantly different from the vehicle treated cells). Results shown are representative of three independent experiments.
To further determine the mechanism by which the catalytic activity of PLC-γ1 mediates EGF-induced cell migration, we examined the effects of the IP3 receptor inhibitor (2-APB) and the intracellular calcium chelator (BAPTA/AM) on EGF-induced SCC4 cell migration. The results showed that treatment with the IP3 receptor inhibitor or intracellular calcium chelator blocked EGF-induced cell migration (Fig. 4). These data suggest a critical role of the IP3 receptor and intracellular calcium in EGF-induced cell migration.
The activation of PLC-γ1 by EGF leads to hydrolysis of PIP2, thus to the generation of DAG and IP3 which leads to the activation of PKC and the release of intracellular stores of calcium, respectively [1]. Application of intracellular chelators of calcium has been reported to produce a decrease in cell migration [25–31]. These observations suggest that cell migration requires intracellular calcium. Our studies determined that EGF-induced intracellular calcium rise and cell migration require the catalytic activity of PLC-γ1. Our results also indicate that EGF-induced migration requires IP3 receptor and intracellular calcium. Thus, EGF requires the catalytic activity of PLC-γ1 to induce migration, perhaps because of the ability of PLC-γ1 to generate second messengers, IP3, and calcium, which stimulate migration.
In summary, this report shows EGF induces SCC4 cell migration by a mechanism that is dependent on the catalytic activity of PLC-γ1, IP3 receptor activation and intracellular calcium mobilization. These findings provide for the first time the direct evidence that the catalytic activity of PLC-γ1 is required for EGF-induced cell migration.
Acknowledgments
This work was supported by Grants 1R03DE018001 and 1R21DE019529-01A2 from the National Institutes of health.
Abbreviations
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- SCC
squamous cell carcinoma
- PLC
phospholipase C
- PLC-γ1
phospholipase C-γ1
- PI3K
phosphoinositide 3-kinases PIP2
- phosphatidylinositol 4
5-bisphosphate
- PIP3
phosphatidylinositol 3,4,5-trisphosphate
- IP3
inositol 1,4,5-trisphosphate
- DAG
diacylglycerol
- PKC
protein kinase C
- SH2
Src homology 2, SH3, Src homology 3, PH, Pleckstrin homology
- BAPTA-AM
1,2-bis(2-aminophenoxy)ethane-N,N,N,N-tetraacetic acid tetrakis (acetoxymethylester)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wells A. EGF receptor. Int J Biochem Cell Biol. 1999;31:637–643. doi: 10.1016/s1357-2725(99)00015-1. [DOI] [PubMed] [Google Scholar]
- 2.Carpenter G, Ji Q. Phospholipase C-gamma as a signal-transducing element. Exp Cell Res. 1999;253:15–24. doi: 10.1006/excr.1999.4671. [DOI] [PubMed] [Google Scholar]
- 3.Arteaga CL, Johnson MD, Todderud G, Coffey RJ, Carpenter G, Page DL. Elevated content of the tyrosine kinase substrate phospholipase C-gamma 1 in primary human breast carcinomas. Proc Natl Acad Sci USA. 1991;88:10435–10439. doi: 10.1073/pnas.88.23.10435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noh DY, Lee YH, Kim SS, Kim YI, Ryu SH, Suh PG, Park JG. Elevated content of phospholipase C-gamma 1 in colorectal cancer tissues. Cancer. 1994;73:36–41. doi: 10.1002/1097-0142(19940101)73:1<36::aid-cncr2820730108>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 5.Xie Z, Chen Y, Liao EY, Jiang Y, Liu FY, Pennypacker SD. Phospholipase C-gamma1 is required for the epidermal growth factor receptor-induced squamous cell carcinoma cell mitogenesis. Biochem Biophys Res Commun. 397:296–300. doi: 10.1016/j.bbrc.2010.05.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen P, Xie H, Sekar MC, Gupta K, Wells A. Epidermal growth factor receptor-mediated cell motility: phospholipase C activity is required, but mitogen-activated protein kinase activity is not sufficient for induced cell movement. J Cell Biol. 1994;127:847–857. doi: 10.1083/jcb.127.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polk DB. Epidermal growth factor receptor-stimulated intestinal epithelial cell migration requires phospholipase C activity. Gastroenterology. 1998;114:493–502. doi: 10.1016/s0016-5085(98)70532-3. [DOI] [PubMed] [Google Scholar]
- 8.Price JT, Tiganis T, Agarwal A, Djakiew D, Thompson EW. Epidermal growth factor promotes MDA-MB-231 breast cancer cell migration through a phosphatidylinositol 3′-kinase and phospholipase C-dependent mechanism. Cancer Res. 1999;59:5475–5478. [PubMed] [Google Scholar]
- 9.Khoshyomn S, Penar PL, Rossi J, Wells A, Abramson DL, Bhushan A. Inhibition of phospholipase C-gamma1 activation blocks glioma cell motility and invasion of fetal rat brain aggregates. Neurosurgery. 1999;44:568–577. doi: 10.1097/00006123-199903000-00073. discussion 577–568. [DOI] [PubMed] [Google Scholar]
- 10.Thomas SM, Coppelli FM, Wells A, Gooding WE, Song J, Kassis J, Drenning SD, Grandis JR. Epidermal growth factor receptor-stimulated activation of phospholipase C-gamma1 promotes invasion of head and neck squamous cell carcinoma. Cancer Res. 2003;63:5629–5635. [PubMed] [Google Scholar]
- 11.Shien T, Doihara H, Hara H, Takahashi H, Yoshitomi S, Taira N, Ishibe Y, Teramoto J, Aoe M, Shimizu N. PLC and PI3K pathways are important in the inhibition of EGF-induced cell migration by gefitinib (‘Iressa’, ZD1839) Breast Cancer. 2004;11:367–373. doi: 10.1007/BF02968044. [DOI] [PubMed] [Google Scholar]
- 12.Nozawa H, Howell G, Suzuki S, Zhang Q, Qi Y, Klein-Seetharaman J, Wells A, Grandis JR, Thomas SM. Combined inhibition of PLC-gamma1 and c-Src abrogates epidermal growth factor receptor-mediated head and neck squamous cell carcinoma invasion. Clin Cancer Res. 2008;14:4336–4344. doi: 10.1158/1078-0432.CCR-07-4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dittmar T, Husemann A, Schewe Y, Nofer JR, Niggemann B, Zanker KS, Brandt BH. Induction of cancer cell migration by epidermal growth factor is initiated by specific phosphorylation of tyrosine 1248 of c-erbB-2 receptor via EGFR. FASEB J. 2002;16:1823–1825. doi: 10.1096/fj.02-0096fje. [DOI] [PubMed] [Google Scholar]
- 14.Bleasdale JE, Thakur NR, Gremban RS, Bundy GL, Fitzpatrick FA, Smith RJ, Bunting S. Selective inhibition of receptor-coupled phospholipase C-dependent processes in human platelets and polymorphonuclear neutrophils. J Pharmacol Exp Ther. 1990;255:756–768. [PubMed] [Google Scholar]
- 15.Smith RJ, Sam LM, Justen JM, Bundy GL, Bala GA, Bleasdale JE. Receptor-coupled signal transduction in human polymorphonuclear neutrophils: effects of a novel inhibitor of phospholipase C-dependent processes on cell responsiveness. J Pharmacol Exp Ther. 1990;253:688–697. [PubMed] [Google Scholar]
- 16.Jiang CK, Connolly D, Blumenberg M. Comparison of methods for transfection of human epidermal keratinocytes. J Invest Dermatol. 1991;97:969–973. doi: 10.1111/1523-1747.ep12491889. [DOI] [PubMed] [Google Scholar]
- 17.Xie Z, Bikle DD. Phospholipase C-gamma1 is required for calcium-induced keratinocyte differentiation. J Biol Chem. 1999;274:20421–20424. doi: 10.1074/jbc.274.29.20421. [DOI] [PubMed] [Google Scholar]
- 18.Xie Z, Bikle DD. Inhibition of 1,25-dihydroxyvitamin-D-induced keratinocyte differentiation by blocking the expression of phospholipase C-gamma1. J Invest Dermatol. 2001;117:1250–1254. doi: 10.1046/j.0022-202x.2001.01526.x. [DOI] [PubMed] [Google Scholar]
- 19.Sun HQ, Lin KM, Yin HL. Gelsolin modulates phospholipase C activity in vivo through phospholipid binding. J Cell Biol. 1997;138:811–820. doi: 10.1083/jcb.138.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie Z, Bikle DD. The recruitment of phosphatidylinositol 3-kinase to the E-cadherin-catenin complex at the plasma membrane is required for calcium-induced phospholipase C-gamma1 activation and human keratinocyte differentiation. J Biol Chem. 2007;282:8695–8703. doi: 10.1074/jbc.M609135200. [DOI] [PubMed] [Google Scholar]
- 21.Xie Z, Chang SM, Pennypacker SD, Liao EY, Bikle DD. Phosphatidylinositol-4-phosphate 5-kinase 1alpha mediates extracellular calcium-induced keratinocyte differentiation. Mol Biol Cell. 2009;20:1695–1704. doi: 10.1091/mbc.E08-07-0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie Z, Singleton PA, Bourguignon LY, Bikle DD. Calcium-induced human keratinocyte differentiation requires src- and fyn-mediated phosphatidylinositol 3-kinase-dependent activation of phospholipase C-gamma1. Mol Biol Cell. 2005;16:3236–3246. doi: 10.1091/mbc.E05-02-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 24.Mouneimne G, Soon L, DesMarais V, Sidani M, Song X, Yip SC, Ghosh M, Eddy R, Backer JM, Condeelis J. Phospholipase C and cofilin are required for carcinoma cell directionality in response to EGF stimulation. J Biol Chem. 2004;166:697–708. doi: 10.1083/jcb.200405156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jung ID, Lee HS, Lee HY, Choi OH. FcepsilonRI-mediated mast cell migration: signaling pathways and dependence on cytosolic free Ca2+ concentration. Cellular Signalling. 2009;21:1698–1705. doi: 10.1016/j.cellsig.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Etienne-Manneville S, Manneville JB, Adamson P, Wilbourn B, Greenwood J, Couraud PO. ICAM-1-coupled cytoskeletal rearrangements and transendothelial lymphocyte migration involve intracellular calcium signaling in brain endothelial cell lines. J Immunol. 2000;165:3375–3383. doi: 10.4049/jimmunol.165.6.3375. [DOI] [PubMed] [Google Scholar]
- 27.Hofman P, d’Andrea L, Guzman E, Selva E, Le Negrate G, Far DF, Lemichez E, Boquet P, Rossi B. Neutrophil F-actin and myosin but not microtubules functionally regulate transepithelial migration induced by interleukin 8 across a cultured intestinal epithelial monolayer. Eur Cytokine Netw. 1999;10:227–236. [PubMed] [Google Scholar]
- 28.Hwang YP, Jeong HG. Metformin blocks migration and invasion of tumour cells by inhibition of matrix metalloproteinase-9 activation through a calcium and protein kinase C-alpha-dependent pathway: phorbol-12-myristate-13-acetate-induced/extracellular signal-regulated kinase/activator protein-1. Br J Pharmacol. 160:1195–1211. doi: 10.1111/j.1476-5381.2010.00762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mertens-Walker I, Bolitho C, Baxter RC, Marsh DJ. Gonadotropin-induced ovarian cancer cell migration and proliferation require extracellular signal-regulated kinase 1/2 activation regulated by calcium and protein kinase C-delta. Endocr Relat Cancer. 17:335–349. doi: 10.1677/ERC-09-0152. [DOI] [PubMed] [Google Scholar]
- 30.Hirai K, Yoshioka H, Kihara M, Hasegawa K, Sakamoto T, Sawada T, Fushiki S. Inhibiting neuronal migration by blocking NMDA receptors in the embryonic rat cerebral cortex: a tissue culture study. Brain Res. 1999;114:63–67. doi: 10.1016/s0165-3806(99)00019-x. [DOI] [PubMed] [Google Scholar]
- 31.Saito H, Minamiya Y, Kitamura M, Saito S, Enomoto K, Terada K, Ogawa J. Endothelial myosin light chain kinase regulates neutrophil migration across human umbilical vein endothelial cell monolayer. J Immunol. 1998;161:1533–1540. [PubMed] [Google Scholar]