Abstract
The potential therapeutic action of shikonin in an experimental model of rheumatoid arthritis (RA) was investigated. As a RA animal model, DBA/1J mice were immunized two times with type II collagen. After the second collagen immunization, mice were orally administered shikonin (2 mg/kg) once a day for 35 days, and the incidence, clinical score, bone mineral density (BMD), bone mineral content (BMC) and joint histopathology were evaluated. BMD in the proximal regions of the tibia largely increased in the shikonin treatment group compared with the control group. We also examined the effect of shikonin on inflammatory cytokines and cartilage protection. Shikonin treatment significantly reduced the incidence and severity of collagen-induced arthritis (CIA), markedly abrogating joint swelling and cartilage destruction. Shikonin also significantly inhibited the production of matrix metalloproteinase (MMP)-1 and up-regulated tissue inhibitors of metalloproteinase (TIMP)-1 in mice with CIA. In conclusion, shikonin exerted therapeutic effects through regulation of MMP/TIMP; these results suggest that shikonin is an outstanding candidate as a cartilage protective medicine for RA.
Keywords: Shikonin, Bone mineral density, Bone mineral contents, MMP-1, TIMP-1
INTRODUCTION
Rheumatoid arthritis (RA) is a systemic autoimmune inflammatory disease involving the breakdown of cartilage and juxta-articular bone that has been shown to be associated with decreased bone mineral density (BMD) and bone mass [1]. The balance between osteoclasts (regulate bone resorption) and osteoblasts (induce bone formation) determines bone mass in adults [2]. Skeletal complications associated with RA consist of focal erosion of marginal and subchondral bone [3]. In RA, tissue destruction is caused by several mechanisms, including the production of cytokines and matrix metalloproteinases (MMP) [4]. Production of these proteases in fibroblasts, macrophages, and chondrocytes is regulated by synovial macrophages and lymphocytes [5].
MMP action is controlled by their natural inhibitors, known as tissue inhibitors of metalloproteinase (TIMP), which under normal conditions neutralize the protease activity [6]. MMP and TIMP are thought to play an important role in the destruction and remodeling of articular tissue in patients with RA [7].
The type II collagen-induced arthritis (CIA) model is utilized extensively to evaluate novel forms of therapy for RA [8]. It can be induced in susceptible strains of mice and rats by immunization with type II collagen, the major component of articular cartilage, and has histopathologic features similar to RA [9]. This model is useful for destruction of cartilage and bone, and is characterized by the increase of some cytokines in synovial fluids [10]. Decreased bone formation and increased bone resorption have been demonstrated during the development of polyarthritis; that is, serum osteocalcin levels and trabecular bone formation rate decreased during the first 2 weeks after injection of Freund's adjuvant [11].
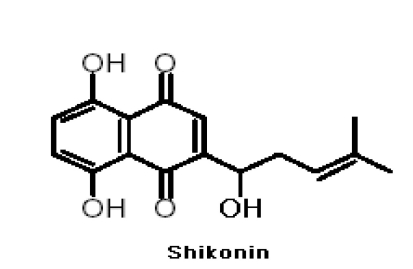
Zicao (purple gromwell), the dried root of Lithospermum erythrorhizon Sieb. et (LE), is a common herbal medicine in China and other countries. LE has long been used in traditional Asian medicine for the treatment of skin measles, chicken pox, hepatitis and skin cancer. It has been reported that extracts from the roots of LE restored immunosuppression induced by cyclophosphamide, an anti-tumor agent [12]. Additionally, LE extracts have been found to suppress LPS- and IFN-γ-induced production of inducible NO synthase (iNOS) and TNF-α by macrophages [13] and to inhibit the mutagenic effects of the carcinogen, N-butyl-N-butanolnitrosamine [14]. We developed zicao-based herbal medicine because of the known functions of herbs described in the literature of traditional Korean and Chinese medicines [15]. Shikonin (MW:288 Fig. 1), major active components of LE, possesses numerous pharmacological properties, including anti-inflammatory and anti-tumor properties and the ability to promote wound healing [16] and inhibits the transcriptional activation of human TNF-α in vivo [17]. However, little is known about the effect of shikonin in CIA. Here, we investigated the role of shikonin in the pathogenesis of RA by evaluating the effects of shikonin in mice with type II CIA.
Fig. 1.
Structure of shikonin.
METHODS
In vivo study
This study was conducted according to the "Guiding Principles for the Care and Use of Laboratory Animals", and all procedures were approved by the Animal Care and Use Committee of Kyung Hee University Medical Center.
Induction of collagen-induced arthritis
Male DBA/1J mice that were 6~8 weeks of age (SLC, Japan) were acclimated for 1 week under standard laboratory conditions at room temperature of 22~26℃ and humidity of 45~65%. The mice had free access to tap water and to a commercial standard mice chow throughout the experimental period.
Mice were given an intradermal injection of 100 µg of bovine type II collagen (CII; Chondrex, Inc., Redmond, WA, USA) emulsified in complete Freund's adjuvant (CFA; Chondrex, Inc.) (1:1, w/v) to the base of the tail. Two weeks later, the mice were given a booster intradermal injection of 100 µg of bovine CII in incomplete Freund's adjuvant (IFA; Difco Laboratories, Detroit, MI, USA) (1:1, v/v). The control mice were treated in the same way except without CII antigen. The next day, mice that had no macroscopic signs of arthritis were selected and divided into three groups of 10.
Shikonin (RA shikonin treated)
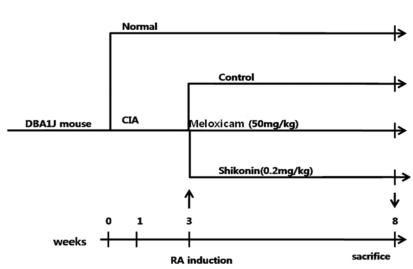
Each arthritic mouse in the group was orally administered 2 mg/kg of shikonin (Santa Cruz Biotechnology; sc-200391) daily for 5 weeks. The control group was treated orally with 300 µl distilled water, and the meloxicam-treated group was orally administered 50 mg/kg meloxicam for 35 days (Fig. 2). The onset of arthritis normally started approximately 2 weeks after initial immunization. Body weight was recorded once per week. Meloxicam, an oxicam derivative that is a member of the enolic acid group of NSAIDs, has recently been approved by the USFDA for use in RA and osteoarthritis [18]. In the UK, U.S., Middle East and Australia, meloxicam is generally marketed under the brand name Meloxicam.
Fig. 2.
Experimental schedule.
Macroscopic scoring of CIA
The progression of CIA was evaluated by macroscopic scoring of the paw every 3 days for the entire experimental period. Paw swelling was measured by water plethysmography as previously described [19]. The edema was defined as the increase in paw volume on the day of experiment compared to day 0.
Each paw was graded with a maximum score of 4: 0, normal, without any macroscopic signs of arthritis; 1, mild, but definite redness and swelling of the ankle, or apparent redness and swelling limited to individual digits, regardless of the number of affected digits; 2, moderate redness and swelling of the ankle; 3, redness and swelling of the entire paw including digits; 4, maximally inflamed limb with involvement of multiple joints. The 4 paw scores for each mouse were summed, and the maximum possible score per mouse was 16. Incidence was expressed as the percentage of mice with an arthritis score=1. The examination was performed by two independent observers who were blinded to the treatment groups.
Measurement of cytokines
Immunoassays were performed using a mouse cytokine immunoassay kit (IL-1β, TNF-α, MMP-1, TIMP-1; R&D Systems, Minneapolis, MN, USA); all measurements were made according to the instructions given by the manufacturers of the ELISA kits. Briefly, for IL-1β and TNF-α, mouse ankles were snapfrozen in liquid nitrogen and ground into powder with a pestle, then lysed with lysis buffer (25 mM Tris HCl, 50 mM NaCl, 0.5% sodium deoxycholate, 2% NP-40, 0.2% sodium dodecyl sulphate, 1 mM phenyl methyl sulfonyl fluoride). For measurements of MMP-1 and TIMP-1, mice were killed on the final day of experimentation and the serum was collected to measure their levels.
Histological processing and analysis of knee joints
Mice were anesthetized with 3.5% chloral hydrate. The knee joints were dissected, fixed in 10% phosphate-buffered formalin, decalcified in 10% ethylene diaminetetraacetate (EDTA) for 7 days, and then embedded in paraffin. Standard frontal sections of 5 µm were prepared and stained with hematoxylin and eosin (Fig. 3) using standard techniques for light microscopic examination as previously described [20]. Photographs of the sections were taken using an Olympus (Tokyo, Japan) I×70 inverted microscope. The histopathological score of arthritis in each joint was classified at four levels based on the following criteria: Bone structure was graded separately on a scale of 0~3 according to the degree of pannus irregularities and clefts to subchondral bone. Cartilage depletion was indicated visually by diminished Safranin O staining of the proteoglycan matrix and was scored as 0 when normal or as 1~3 according to the degree of depletion. Synovium was graded separately on a scale of 0~3, ranging from normal, mild, moderate, and marked inflammation and hyperplasia. All histological evaluation procedures were performed blind.
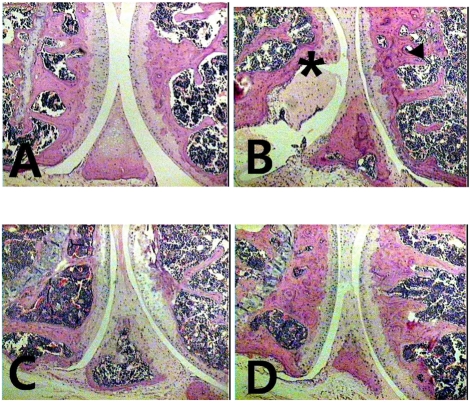
Fig. 3.
Histopathologies of hind paw sections from mice treated with shikonin. (A) Hematoxylin eosin staining of 2 mg/kg shikonin-treated mice, (B) vehicle-treated mice, (C) 50 mg/kg Meloxicam-treated mice, (D) Normal group (non RA). *Pannus formation over articular cartilage.
Bone mineral density and content measurements
Bone mineral density and contents were measured on the expired day at 5 weeks after arthritis induction. Mice were anesthetized with 3.5% chloral hydrate. The right tibia were cleaned of soft tissue and scanned with a DEXA (PIX IMUS™, GE LUNAR Corporation, USA). Throughout the study period, daily quality assurance tests were performed to ensure the effectiveness of lights, beam mechanics, and tissue value of the scanner. The coefficients of variation for the paired measurement of BMC and BMD of standard samples by this technique were 0.8% and 1.0%, respectively. The results are expressed in grams (BMC) and g/cm2 (BMD).
Statistical Analysis
Statistical analysis was performed with SPSS ver 13. The effects of shikonin were evaluated by analysis of variance (ANOVA). Multiple comparisons of treatment groups were performed by Duncan's multiple procedure. Student's t-test was used to assess significance of BMC or BMD changes during shikonin administration. p<0.05 was regarded as significant. Results are expressed as means±SEM.
RESULTS
Effect of shikonin on paw edema
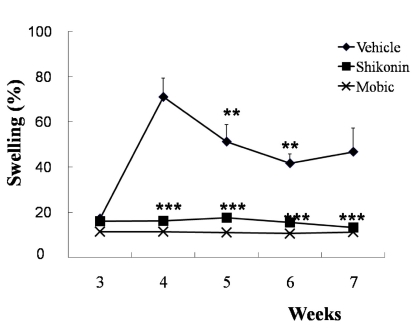
Fig. 4 shows the changes in paw volume. In the CIA group, all animals showed inflammation at 3 weeks as indicated by the increase in paw volume. On day 28, the increase of paw volume peaked at 71% above the age-matched control and persisted until the end of the experiment. The administration of shikonin inhibited the edema. The non-treated mice did not exhibit any edema.
Fig. 4.
Induction of paw edema and effect of shikonin treatment (2 mg/kg) after 2ndimmunization. Paw edema measured in type II collagen-induced arthritic mice (n=10). Treated with vehicle (♦), Meloxicam (50 mg/kg ×), and shikonin (2 mg/kg ▪) after 2ndimmunization. **p<0.005 and ***p<0.001 compared with vehicle-treated mice with CIA.
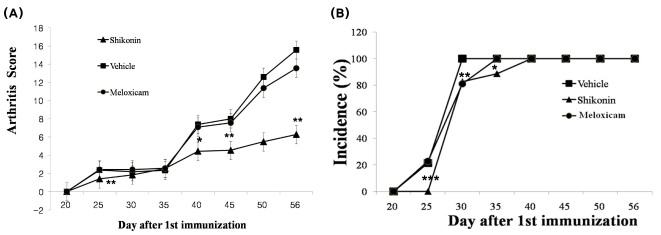
Effects of shikonin on incidence and severity in CIA mice
To clarify the effects of shikonin on disease progression in mice with CIA, we assessed the development of inflammation by scoring the clinical disease activity daily from 20 days after the first immunization. The disease activity in vehicle-treated mice with CIA first appeared on day 25 (Fig. 5A), which was 4 days after the second immunization (Fig. 5A). The severity of the disease gradually increased, and the mean arthritis score reached 15.6 in vehicle-treated mice with CIA on day 56. The severity of disease in shikonin-treated mice was less than that in vehicle-treated mice (Fig. 5A). On day 56, a significant reduction in the arthritis score was still observed in shikonin-treated mice with CIA (mean scores of 3.31 respectively) and in 50 mg/kg Meloxicam-treated mice with CIA (mean score 5.88) compared with that in vehicle-treated mice with CIA (Fig. 5A). The arthritis score in shikonin-treated mice was significantly decreased, showing a similar tendency with that in Meloxicam-treated mice from day 25 to the day the mice were killed (Fig. 5A).
Fig. 5.
Inhibitory effect of shikonin on disease progression in mice with collagen-induced arthritis (CIA). CIA was induced by primary (day 0) and secondary (day 20) immunizations with bovine type II collagen in Freund's complete adjuvant. (A) Arthritis score (clinical severity of arthritis). The maximum possible score is 16, as described in materials and methods. (B) Incidence of arthritis. Values are reported as the mean ± S.E.M. *p<0.05, **p<0.005 and ***p<0.001 compared with vehicle-treated mice with CIA. Treated with vehicle (▪), Meloxicam (50 mg/kg •), and shikonin (2 mg/kg ▴).
Arthritis onset was delayed for 4 days by shikonin treatment, while arthritis in vehicle-treated mice with CIA appeared on day 25 (Fig. 5B). In contrast, the Meloxicam-treated mice exhibited no delay in clinical onset of disease compared with the vehicle-treated mice with CIA (Fig. 5B). Moreover, all mice treated with Meloxicam had developed swelling and/or erythema (C% incidence) by day 40. The clinical score of shikonin was lower than that Meloxicam in mice with CIA.
Effects of shikonin on MMP and TIMP regulation in CIA mice
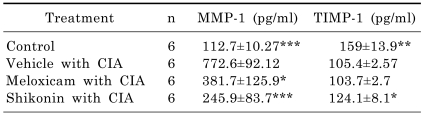
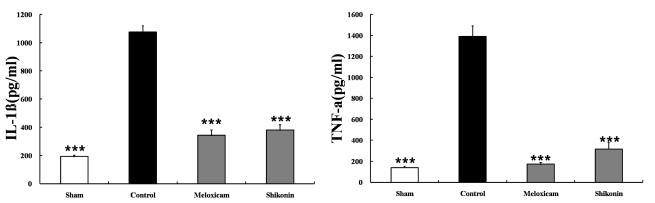
The destructive progression is thought to be mediated by potent enzymes which break down the tissues of the joint. The MMP family is heavily implicated in these processes, as collectively they are able to degrade most components of cartilage. To evaluate whether shikonin affects the activities of the proteolytic enzymes related to cartilage erosion, we determined the serum level of MMP-1 and TIMP-1. The serum level of MMP-1 in shikonin-treated mice was significantly lower than in vehicle-treated mice, whereas the level of TIMP-1 was considerably higher than in vehicle-treated mice (Table 1). In contrast, Meloxicam treatment did not affect the level of TIMP-1 in the serum. This data suggests that shikonin might protect against cartilage erosion by regulating the proteolytic enzymes. Shikonin treatment dramatically reduced the production of inflammatory cytokines (IL-1β, TNF-α) in the joints of arthritic mice (Fig. 6).
Table 1.
Effect of shikonin on serum concentrations of MMP-1 and TIMP-1 in CIA mice
Levels of MMP-1 and TIMP-1 were analyzed by ELISA. Data are reported as the mean±SEM. *p<0.05, **p<0.005, and ***p<0.001 compared with vehicle-treated mice with CIA.
Fig. 6.
Effect of shikonin on IL-1β and TNF-α levels (pg/mg protein, mean±S.E.M) Serum was obtained from four groups of mice: sham group (no arthritis+vehicle treatment, n=10), control group (arthritis+vehicle, n=10), meloxicam group (arthritis+meloxicam treatment at 50 mg/kg/day n=10), and shikonin group (arthritis+shikonin treatment at 2 mg/kg/day n=10). After shikonin treatment, serum IL-1β and TNF-α decreased significantly, ***p<0.001, compared to the vehicle control group.
Effects of shikonin on histologic synovitis and cartilage destruction in the CIA model
To examine the effects of shikonin on cartilage destruction, the knee joint cartilage was histopathologically assessed by H&E staining. In the CIA group, signs of inflammation such as pannus formation and bone erosion were clearly observed. In particular, erosion and fragmentation of the trabecular bone were visible. Shikonin prevented these changes (Fig. 3).
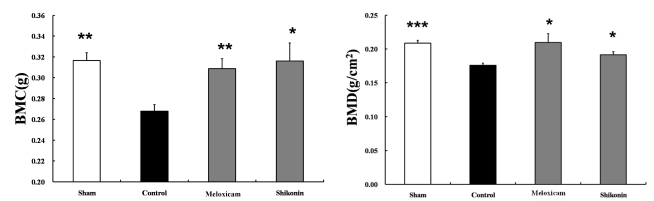
Effect of dietary shikonin on tibial BMD
As shown in Fig. 7, BMD in the proximal bone region of the tibia from shikonin-treated mice remained lower and uniform throughout the study period. Oral administration of shikonin slightly prevented the decrease of BMD (p<0.05). Follow-up the shikonin-treated mice showed a significant (p<0.05) increase in BMC compared with control group.
Fig. 7.
The bone mineral density and bone mineral contents of tibia in vehicle, shikonin, and Meloxicam-treated arthritic mice. Values are reported as the mean±S.E.M. *p<0.05, **p<0.005 and ***p<0.001, compared with vehicle-treated mice with CIA.
DISCUSSION
In this study, we investigated the potential therapeutic action of shikonin in an experimental model of RA. Administration of shikonin greatly inhibited RA at the clinical and pathologic levels; i) has significant protective effects against cartilage destruction in the affected knee joint, presumably by mediating the levels of MMP and TIMP, ii) down-regulated the level of proinflmmatory cytokines (IL-1β, TNF-α). TNF-α, IL-1 and IL-6 and are the key cytokines that drive inflammation in RA and cause joint damage. They are potent stimulators of synovial fibroblasts, osteoclasts, and chondrocytes that release tissue-destroying matrix metalloproteinases (MMP), which contribute to joint damage [21]. The serum and synovial concentrations of IL-1 and TNF-α are high in patients with active RA [22].
MMP and TIMP have been identified as key agents in the remodeling of articular tissue in RA [23]. MMP-1 is expressed not only in the synovia of patients with established erosive RA, but also in that of patients at the early stage of the disease [24]. Recently, it was shown that serum concentrations of MMP-1 correlated with progression of joint destruction not only in patients with long standing RA, but also in patients with early stage disease [25]. Up-regulation of TIMP-1 synthesis in RA seems to be important for the suppression of synovium and cartilage destruction [26]. In our study, shikonin reduced collagenase activity by up-regulating TIMPs and up-regulating MMP-1 in general (Table 1).
At doses of less than 2 mg/kg, the effects of shikonin administration for 5 weeks on bone mass and strength were not pronounced. In contrast, in mice given shikonin at 2 mg/kg for 5 weeks, BMD and BMC increased. This is the first report that shikonin prevents reductions in BMD and trabecular bone volume in adjuvant-induced arthritic mice. In a previous study using a collagen-induced model of arthritis, Enokida et al. [27] measured the time course of changes of BMD and trabecular bone volume in adjuvant-induced arthritic mice. The study showed that juxta-articular trabecular bone was vulnerable to bone loss early in the course of arthritis. Minne et al. [28] reported that generalized bone loss associated with inflammation occurred independent of regulation. Inflammatory arthritis is characterized by joint swelling and destruction. In RA, the immune system attacks intra-articular tissues and the resulting joint inflammation causes pain, heat and swelling [29]. The foot swelling is a result of edema and is an indication of the inflammatory response associated with collagen II injection. Thus, the production of local factors during inflammation plays an important role in the decrease of BMD. Treatment with shikonin in CIA mice suppressed the development of edema like meloxicam.
RA is characterized by the proliferation of the synovial membrane into a pannus, which includes resident fibroblast-like synoviocytes (FLSs) and infiltrating mononuclear cells capable of producing inflammatory cytokines [10]. In particular, the tibia bone loss with the formation of pannus was very clear in this region [11]. Here, we show that shikonin modifies disease progression by abrogating soft tissue and bone lesions and arresting pannus development. In addition, bone remodeling was modulated in the diseased joint area by daily oral administration of shikonin.
In summary, this study investigated shikonin as an immunomodulatory agent with the capacity to deactivate the inflammatory response in vivo at multiple levels and to inhibit cartilage destruction. Our finding provides a powerful rationale for the development of shikonin as a candidate medicine to improve RA treatment.
ACKNOWLEDGEMENTS
This research has been supported by the program (code #PJ007479), Rural Development Administration, Korea.
ABBREVIATIONS
- RA
rheumatoid arthritis
- BMD
bone mineral density
- BMC
bone mineral content
- CIA
collagen-induced arthritis
- TIMP
tissue inhibitors of metalloproteinase
- MMP
matrix metalloproteinase
References
- 1.Hirayama T, Danks L, Sabokbar A, Athanasou NA. Osteoclast formation and activity in the pathogenesis of osteoporosis in rheumatoid arthritis. Rheumatology (Oxford) 2002;41:1232–1239. doi: 10.1093/rheumatology/41.11.1232. [DOI] [PubMed] [Google Scholar]
- 2.Park YR, Eun JS, Choi HJ, Nepal M, Kim DK, Seo SY, Rihua LI, Moon WS, Cho NP, Cho SD, Bae TS, Kim BI, Soh YJ. Hexane-soluble fraction of the common Fig, Ficus carica, inhibits osteoclast differentiation in murine bone marrow-derived macrophages and raw 264.7. Korean J Physiol Pharmacol. 2009;13:417–424. doi: 10.4196/kjpp.2009.13.6.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romas E, Gillespie MT, Martin TJ. Involvement of receptor activator of NF kappa B ligand and tumor necrosis factor-alpha in bone destruction in rheumatoid arthritis. Bone. 2002;30:340–346. doi: 10.1016/s8756-3282(01)00682-2. [DOI] [PubMed] [Google Scholar]
- 4.Garnero P, Geusens P, Landewe R. Biochemical markers of joint tissue turnover in early rheumatoid arthritis. Clin Exp Rheumatol. 2003;21:S54–S58. [PubMed] [Google Scholar]
- 5.Klimiuk PA, Sierakowski S, Domyslawska I, Chwiecko J. Effect of repeated infliximab therapy on serum matrix metalloproteinases and tissue inhibitors of metalloproteinases and tissue inhibitors of metalloproteinases in patients with rheumatoid arthritis. J Rheumatol. 2004;31:238–242. [PubMed] [Google Scholar]
- 6.Matrisian L. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990;6:121–125. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- 7.Goldring SR, Gravallese EM. Pathogenesis of bone erosions in rheumatoid arthritis. Curr Opin Rheumatol. 2000;12:195–199. doi: 10.1097/00002281-200005000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Jones SA, Kennedy AJ, Roberts NA. Assessment of drugs for activity in established type II collagen arthritis. Agents Actions. 1982;12:650–656. doi: 10.1007/BF01965074. [DOI] [PubMed] [Google Scholar]
- 9.Brand DD, Latham KA, Rosloniec EF. Collagen-induced arthritis. Nat Protoc. 2007;2:1269–1275. doi: 10.1038/nprot.2007.173. [DOI] [PubMed] [Google Scholar]
- 10.Hur GM, Hwang YB, Lee JH, Bae SH, Park JS, Lee CJ, Seok JH. Caffeic acid phenethyl ester inhibits the PKC-induced IL-6 gene expression in the synoviocytes of rheumatoid arthritis patients. Korean J Physiol Pharmacol. 2003;7:363–368. [Google Scholar]
- 11.Imaizumi K, Hinoue H, Ueno M, Takata I, Sato T, Minato Y, Takeshita M, Okaniwa A. Histopathological study of arthritic lesions induced by immunization with type II collagen in DBA/1J mouse. Jikken Dobutsu. 1990;39:27–34. doi: 10.1538/expanim1978.39.1_27. [DOI] [PubMed] [Google Scholar]
- 12.Jin R, Wan LL, Mitsuishi T, Kodama K, Kurashige S. Immunomodulative effects of Chinese herbs in mice treated with anti-tumor agent cyclophosphamide. Yakugaku. Zasshi. 1994;114:533–538. doi: 10.1248/yakushi1947.114.7_533. [DOI] [PubMed] [Google Scholar]
- 13.Chung HS, Kang M, Cho C, Park S, Kim H, Yoon YS, Kang J, Shin MK, Hong MC, Bae H. Inhibition of lipopolysaccharide and interferon-gamma-induced expression of inducible nitric oxide synthase and tumor necrosis factor-alpha by Lithospermi radix in mouse peritoneal macrophages. J Ethnopharmacol. 2005;102:412–417. doi: 10.1016/j.jep.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 14.Jin R, Kurashige S. Effects of Chinese herbs on macrophage functions in N-butyl-N-butanolnitrosoamine treated mice. Immunopharmacol. Immunotoxicol. 1996;18:105–114. doi: 10.3109/08923979609007113. [DOI] [PubMed] [Google Scholar]
- 15.Lee HG. Bone Cho Kang Mook [compendium of material medica] Seoul: Yeoil; 2007. (in Korean) [Google Scholar]
- 16.Vassilios PP, Assimopoulou AN, Couladouros EA, Hepworth D, Nicolaou KC. The chemistry and biology of alkannin, shikonin, and related naphthazarin natural products. Angew Chem Int. 1999;38:270–301. doi: 10.1002/(SICI)1521-3773(19990201)38:3<270::AID-ANIE270>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 17.Ishida T, Sakaguchi I. Protection of human keratinocytes from UVB-induced inflammation using root extract of Lithospermum erythrorhizon. Biol Pharm Bull. 2007;30:928–934. doi: 10.1248/bpb.30.928. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed M, Khanna D, Furst DE. Meloxicam in the rheumatoid arthritis. Expert Opin Drug Metab Toxicol. 2005;1:739–751. doi: 10.1517/17425255.1.4.739. [DOI] [PubMed] [Google Scholar]
- 19.Campagnuolo G, Bolon B, Feige U. Kinetics of bone protection by recombinant osteoprotegerin therapy in Lewis rats with adjuvant arthritis. Arthritis Rheum. 2002;46:1926–1936. doi: 10.1002/art.10369. [DOI] [PubMed] [Google Scholar]
- 20.Sun D, Krishnan A, Zaman K, Lawrence R, Bhattacharya A, Fernandes G. Dietary n-3 fatty acids decrease osteoclastogenesis and loss of bone mass in ovariectomized mice. J Bone Miner Res. 2003;18:1206–1216. doi: 10.1359/jbmr.2003.18.7.1206. [DOI] [PubMed] [Google Scholar]
- 21.Shingu M, Nagai Y, Isayama T, Naono T, Nobunaga M, Nagai Y. The effects of cytokines on metalloproteinase inhibitors (TIMP) and collagenase production by human chondrocytes and TIMP production by synovial cells and endothelial cells. Clin Exp Immunol. 1993;94:145–149. doi: 10.1111/j.1365-2249.1993.tb05992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chikanza IC, Kingsley G, Panayi GS. Peripheral blood and synovial fluid monocyte expression of interleukin 1α and TNF-α during active rheumatoid arthritis. J Rheumatol. 1995;22:600–606. [PubMed] [Google Scholar]
- 23.Woessner JF. Matrix metalloproteinase and their inhibitors in connective tissue remodeling. FASEB J. 1998;5:2145–2154. [PubMed] [Google Scholar]
- 24.Jackson CJ, Arkell J, Nguyen M. Rheumatoids synovial endothelial cells secrete decreased levels of tissue inhibitor of MMP (TIMP1) Ann Rheum Dis. 1998;57:158–161. doi: 10.1136/ard.57.3.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Green MJ, Gough AK, Devlin J, Smith J, Astin P, Taylor D. Serum MMP-3 and MMP-1 and progression of joint damage in early rheumatoid arthritis. Rheumatology. 2003;42:82–88. doi: 10.1093/rheumatology/keg037. [DOI] [PubMed] [Google Scholar]
- 26.Tolboom TC, Pieterman E, van der Laan WH, Toes RE, Huidekoper AL, Nelissen RG, Breedveld FC, Huizinga TW. Invasive properties of fibroblast-like synoviocytes: correlation with growth characteristics and expression of MMP-1, MMP-3, and MMP-10. Ann Rheum Dis. 2002;61:975–980. doi: 10.1136/ard.61.11.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Enokida M, Yamasaki D, Okano T, Hagino H, Morio Y, Teshima R. Bone mass changes of tibial and vertebral bones in young and adult rats with collagen-induced arthritis. Bone. 2001;28:87–93. doi: 10.1016/s8756-3282(00)00406-3. [DOI] [PubMed] [Google Scholar]
- 28.Minne HW, Pfeilschifter J, Scharla S, Mutschelknauss S, Schwarz A, Krempien B, Ziegler R. Inflammation-mediated osteopenia in the rat: a new animal model for pathological loss of bone mass. Endocrinology. 1984;115:50–54. doi: 10.1210/endo-115-1-50. [DOI] [PubMed] [Google Scholar]
- 29.Choi S, Lee YA, Hong SJ, Lee GJ, Kang SW, Park JH, Park JH, Park HK. Evaluation of inflammatory change and bone erosion using a murine type II collagen-induced arthritis model. Rheumatol Int. 2010 doi: 10.1007/s00296-009-1333-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]