Abstract
The hypothalamic-pituitary-adrenal (HPA) axis is the primary endocrine system to respond to stress. The HPA axis may be affected by increased level of corticotrophin-releasing factors under chronic stress and by chronic administration of monosodium glutamate (MSG). The purpose of this study was to investigate whether chronic MSG administration aggravates chronic variable stress (CVS)-induced behavioral and hormonal changes. Twenty-four adult male Sprague-Dawley rats, weighing 200~220 g, were divided into 4 groups as follows: water administration (CON), MSG (3 g/kg) administration (MSG), CVS, and CVS with MSG (3 g/kg) administration (CVS+MSG). In addition, for the purpose of comparing the effect on plasma corticosterone levels between chronic stress and daily care or acute stress, 2 groups were added at the end of the experiment; the 2 new groups were as follows: naïve mice (n=7) and mice exposed to restraint stress for 2 h just before decapitation (A-Str, n=7). In an open field test performed after the experiment, the CVS+MSG group significant decrease in activity. The increase in relative adrenal weights in the CVS and CVS+MSG group was significantly greater than those in the CON and/or MSG groups. In spite of the increase in the relative adrenal weight, there was a significant decrease in the plasma corticosterone levels in the CVS+MSG group as compared to all other groups, except the naïve group. These results suggest that impaired HPA axis function as well as the decrease in the behavioral activity in adult rats can be induced by chronic MSG administration under CVS rather than CVS alone.
Keywords: Chronic variable stress, Corticosterone, Hypothalamic-pituitary-adrenal (HPA) axis, Monosodium glutamate (MSG), Open field test
INTRODUCTION
The activation of the hypothalamic-pituitary-adrenal (HPA) axis is a typical response of the stress process. Many types of stress affect the responsiveness of the HPA axis and stimulate the release of corticotropin-releasing factor (CRF) from the paraventricular nucleus (PVN) in the hypothalamus [1]. The production and release of adrenocorticotropic hormone (ACTH) from the pituitary gland concomitantly increases with the increase in the release of CRF [1]. ACTH, in turn, stimulates the release of glucocorticoids in humans or corticosterone in animals from the adrenal cortex [1]. Excitatory amino acid neurotransmitters as well as brain stimulants have also been implicated in the chronic activation of the HPA axis [2]. Although the precise role of these neurotransmitters in stress response is unclear, there is increasing preclinical evidence that glutamate, which is an excitatory amino acid, plays an important role in the regulation of the HPA axis [2]. Glutamate is the most abundant excitatory amino acid neurotransmitter in the brain and has been known to activate the HPA axis and induce ACTH elevation [3].
Currently, thousands of agents are intentionally added to food, and we daily consume a considerable amount of these agents. Among them, the flavor enhancer monosodium glutamate (MSG), which is a sodium salt of glutamate, is one of the most widely used food additives in our daily diet [4]. Since Lucas and Newhouse first described the harmful effects of MSG [5], MSG treatment has been known to cause hormonal alterations, which in turn affect the physical state and behavior, especially in neonates who have an immature blood-brain barrier [6].
However, there have been reports that the general use of glutamate salts (L-MSG and others) as food additives can be regarded harmless [7]. MSG has not been reported to have any noteworthy adverse effect in adults [7]. Paradoxically, many food production companies claim these days that their products are MSG-free.
In spite of the ongoing efforts to show the neurotoxic effects of MSG in studies in adult animals and human, the neurotoxic effects of MSG have not yet been confirmed. However, previous studies have also proven that alterations in the central nervous system (CNS)-pituitary-adrenal axis (including an increase in serum levels of corticosterone and ACTH) as well as in the nerve growth factor (NGF) and neuropeptide Y (NPY) concentrations in the hypothalamic and pituitary glands can be induced in MSG-treated neonatal rats [8,9]. Furthermore, several researchers have reported that systemic or dietary MSG administration caused prominent increases in the glutamate levels in adult animals, although there were some differences between glutamate levels in the plasma and cerebrospinal fluid (CSF) depending on the administration route [10,11].
All living organisms are continuously exposed to various stresses daily, which if not dealt with well may increase the risk of developing health problems. We inevitably come into contact with food additives known as excitotoxins, such as MSG and aspartame [12], if we do not try to deliberately avoid them.
Currently, both stress and MSG are deeply involved in the everyday life, in that stress is considered to be a reason for many adult diseases, and MSG, which recently became of interest as a food-related substance, is known to affect dietary habits and the endocrine system.
In the present study, we hypothesized that the responsiveness of the HPA axis could be changed by chronic MSG administration. Therefore, the purpose of this study was to investigate whether chronic MSG administration has an aggravating effect on behavioral and hormonal responses under CVS.
METHODS
Animals
Animal use protocols were approved by the Institutional Guidelines of the Committee on Animal Research at the Wonkwang University and all efforts were made to minimize animal suffering, as well as to reduce the number of animals according to the guideline recommendations. A total number of 24 adult male Sprague-Dawley rats purchased from Samtaco (BioKorea, Osan, South Korea), weighing approximately 200 g, were housed in individual cages for 7 days for acclimatization to the study surroundings, and allowed free access to fresh water and chow (Purina Rat Chow no. 5008: Ralston Purina, St. Louis, MO) ad libitum in a temperature-controlled environment of 24℃ with a 12-h light, 12-h dark cycle before initiation of the stress schedule.
The animals were randomly assigned and divided into 4 groups: CON (receiving water freely, n=6), MSG (receiving 3 g/kg MSG solution, n=6), CVS (n=6), and CVS+MSG (receiving 3 g/kg MSG solution under CVS, n=6). Additionally, for the purpose of comparing the effect on the plasma corticosterone levels to chronic stress by daily care or acute stress, 2 groups were added at the end of the main experiment: naïve rats weighing about 250 g which were not exposed to stress or daily care and housed in individual cages for 7 days for acclimatization only (naïve, n=7) and rats exposed to restraint stress for 2 hours just before decapitation (A-Str, n=7). MSG was administered orally by an intubation tube at doses of 3 g/kg which was dissolved in distilled water once every morning for 6 days per week until the end of the experiment. The average administration volume of the MSG solution was around 1.5 ml. From the beginning to the end of the experiment, all rats were weighed twice per week and the amount of food consumed was recorded daily.
Chronic variable stress protocol
The rats in the CVS and CVS+MSG group spent 7 weeks under the CVS protocol and the rats of the other groups spent the same time under normal conditions, except for the administration of MSG (3 g/kg) in the MSG group. The CVS protocol used in this study was modified from that described in a previous study [13]. The weekly protocol consisted of placement in a small cage, sprinkling with ice water, and exposure to white noise, a stroboscope, light at night (illumination), intruding sound, and irregular vibration. Each period of exposure to the stressors lasted 2~16 h each week (Table 1).
Table 1.
Chronic variable stress protocol
Open field test
To compare the behavioral activity before and after the experiment, an open field test was performed using an activity monitor system (MED-Associates, Inc., Georgia, VT) under dim light and in a quiet room between 7:00 p.m. and 10:00 p.m. To eliminate the effect of behavior testing on the plasma corticosterone level, the open field test was completed in all animals at least 1 day before the end of the experiment. The exploring activity was analyzed using the supported program, and test time was divided into 5 sections of a total of 15 minutes. The value of each behavioral response variable such as distance traveled and stereotypic, ambulatory, and vertical counts were compared and analyzed.
To monitor the exploring activity, we used a transparent acrylic box (43.2×43.2×30.5 cm) with 2 sets of 16 laser pointers on each wall. One set consisting of 4 infrared beams was placed in both the X- and Y-axis inside the box to monitor all floor level movements. The other set was positioned 10 cm above floor level to monitor the vertical movements of the rats. Each rat was gently placed in the center of the box. Animal activity was measured via a grid of invisible infrared light beams. A number of equally spaced beams traversed the animal cage from the front to the back, and an equal number of beams traversed the same cage from the left to the right. Some of the beams were interrupted when the body of the rat was placed in the box, thereby revealing its position in the X-Y plane. Horizontal activity was assessed according to the total distance traveled and ambulatory count was recorded as the number of infrared beams interrupted. Vertical activity (rearing) was counted when the animal lifted its forepaws from the ground and stretched itself with or without the support of the wall.
Measurement of corticosterone levels and adrenal weight
The plasma levels of corticosterone were determined by radioimmunoassay (RIA), and the weight of the adrenal glands was also evaluated. Rats were killed by decapitation and blood samples were then obtained for the RIA. All animals were killed between 9:00 a.m. and 12:00 a.m. Blood samples were then centrifuged and the plasma was collected. Plasma corticosterone levels were measured with a commercially available kit (Coat-a-Count rat corticosterone, Diagnostic Product Cooperation, Los Angeles, CA). The Coat-a-Count rat corticosterone antiserum is highly specific for rat corticosterone with extremely low cross-reactivity to other compounds, thus this method is suitable for measuring total corticosterone including protein-bound form. The RIA was performed at the Department of Nuclear Medicine of the Wonkwang University Hospital. The sensitivity of the assay was 5.7 ng/ml. After collection of the blood, both adrenal glands were dissected and fixed in 4% paraformaldehyde, and the wet weight of each gland was measured at the same time after the end of the experiment.
Statistical analyses
All values are represented as mean±S.D. Statistical analyses were carried out with SPSS software (v 11.0 SPSS Inc., Chicago, IL). We performed one-way analysis of variance (ANOVA) and the Bonferroni post-hoc test to analyze changes in all parameters between the groups. A p value <0.05 was considered to indicate significance.
RESULTS
Changes in body weight and food intake
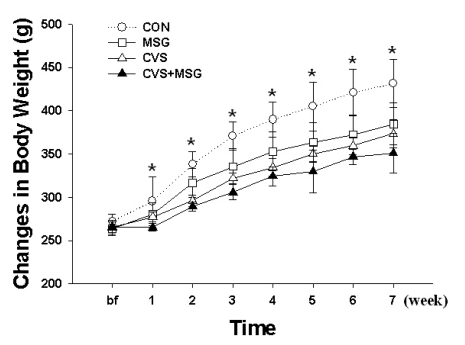
The changes in body weight and food intake through the experimental period in all groups are shown in Fig. 1 and Fig. 2. At the beginning of the stress experiment, the rats weighed approximately 250 g and no significant difference was seen in body weight and food intake between all groups. However, 1 week after beginning of the stress protocol, there was a significant difference in body weight and food intake between the CON and CVS+MSG group. During the following weeks and in contrast to the CON group, considerable differences were observed in the body weight gain in all groups. The average body weight at 7 weeks was 431.33±27.79 g in the CON group, 384.82±23.99 g in the MSG group, 373.47±16.61 g in the CVS group, and 350.99±22.96 g in the CVS+MSG group. In particular, a more noticeable decrease was seen in CVS+MSG group than in the other groups compared with the CON group (Fig. 1, p<0.05).
Fig. 1.
Time-dependent changes of body weight in each group for 7 weeks. *Denotes significant differences among groups based on the one-way ANOVA and Bonferroni test (*p<0.05). There are significant differences in the body weight between CON and other groups; an especially remarkable decrease could be seen in the CVS+MSG group. CON, control; MSG, monosodium glutamate; CVS, chronic variable stress.
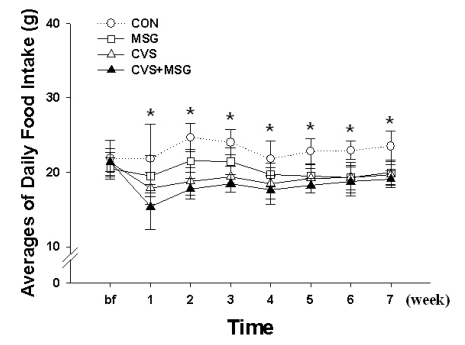
Fig. 2.
Time-dependent changes of food consumption in each group for 7 weeks. *Denotes significant differences among groups based on the one-way ANOVA and Bonferroni test (*p<0.05). There are significant differences in the daily food intake between the control (CON) and other groups. The difference in food intake between the groups gradually decreased during the course of the experiment, except in the CON group. CON, control; MSG, monosodium glutamate; CVS, chronic variable stress.
The largest differences in food intake were observed in the first week after the beginning of the experiment; however, as the experiment progressed, these differences between the groups decreased, except in the CON group. At the end of the experiment, there were significant differences in the food intake between the CON group and other groups (Fig. 2, p<0.05).
Open field test
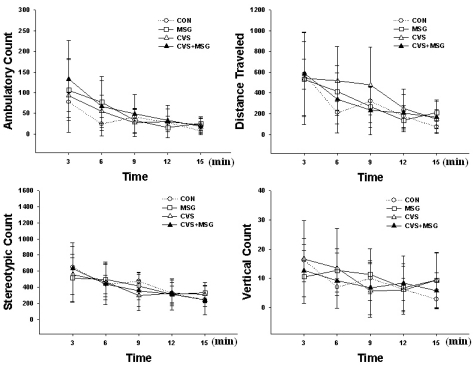
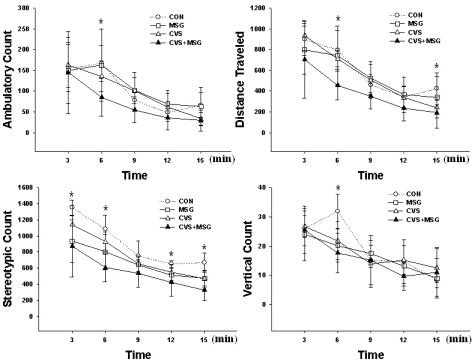
All animals showed a stereotypic movement such as grooming, sniffling and licking, and exploration, including horizontal and vertical movements. Most vertical activity was seen with the support of the wall. The stereotypic and exploring activity gradually decreased as the experiment progressed. Before the beginning of the stress experiment, there were no activity differences between all groups (Fig. 3). However, at the end of the experimental period, there was a decreasing tendency in all parameters, except for the vertical activity in the CVS+MSG group compared to all other groups (Fig. 4, p<0.05). There was no difference in ambulatory count, traveled distance, and vertical count between the CON and MSG or CVS group. However, there was a significant decrease in some sections of the stereotypic count measurement between the CON group and all other groups (Fig. 4, p<0.05). The rats in the CVS+MSG group showed an especially significant decrease in activity by stereotypic count measurement over the whole experimental period of 15 min, except for the third section (Fig. 4, p<0.05).
Fig. 3.
Behavioral response before the stress experiment in the open field test. The stereotypic and exploring activity gradually decreased as time passed in all groups. There is no significant difference in all parameters between the groups. CON, control; MSG, monosodium glutamate; CVS, chronic variable stress.
Fig. 4.
Behavioral response after the stress experiment in the open field test. There are significant differences in all parameters between the groups, especially the rats in the CVS+MSG group showed a remarkable decrease in their activity in the stereotypic count measurement. *Denotes significant differences among the groups based on the one-way ANOVA and Bonferroni test (*p<0.05). CON, control; MSG, monosodium glutamate; CVS, chronic variable stress.
Adrenal gland
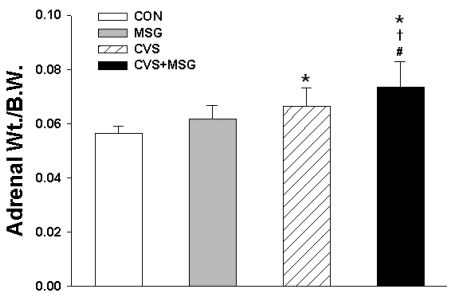
There was no difference in the absolute adrenal weight among the groups. The average adrenal weight was 243.50±16.92 mg in the CON group, 237.67±17.78 mg in the MSG group, 248.00±30.08 mg in the CVS group, and 256.92±25.94 mg in the CVS+MSG group. However, the relative adrenal weight to body weight ratio was significantly higher in the CVS group (0.066±0.007) and CVS+MSG group (0.075±0.009) than in the CON group (0.056±0.002) (Fig. 5, p<0.05). In addition, although there was no significant difference in the relative adrenal weight between the MSG and CVS group, the relative adrenal weight in the CVS+MSG group (0.075±0.009) was significantly higher than in the MSG group (0.062±0.005) (Fig. 5, p<0.05).
Fig. 5.
Relative adrenal weight following administration of MSG for 7 weeks. Statistical analysis was performed by the one-way ANOVA and Bonferroni test. The relative adrenal weight to body weight ratio was significantly higher in the CVS and CVS+MSG group than in the CON group. In addition, although there was no significant difference in the relative adrenal weight between the MSG and CVS group, the relative adrenal weight in the CVS+MSG group (0.75±0.09) was significantly higher than in the MSG group. *Denotes significant differences between CON and other groups (*p<0.05). †Denotes significant difference between the stress and CVS+MSG group (†p<0.05). #Denotes significant difference between the MSG and CVS+MSG group (#p<0.05). CON, control; MSG, monosodium glutamate; CVS, chronic variable stress; A-Str, restraint stress for 2 hours just before decapitation; Wt, weight.
Plasma corticosterone level
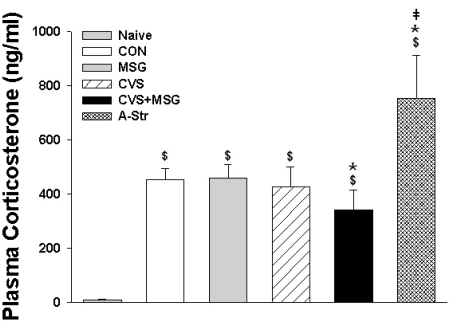
There was a significant difference in the plasma corticosterone levels between the naïve group (8.80±3.47 ng/ml) and all other groups, including the CON group (449.26±75.74 ng/ml). In addition, there was a significant increase in the plasma corticosterone level in the A-Str group (753.31±158.17 ng/ml) compared to all other groups (Fig. 6, p<0.05). Compared to the CON group, there was no difference in the plasma levels of corticosterone in the MSG (458.29±51.98 ng/ml) or CVS group (426.93±72.75 ng/mL). However, the plasma corticosterone level in the CVS+MSG group (340.46±74.79 ng/ml) was significantly lower compared to all other groups, except the naïve group (Fig. 6, p<0.05).
Fig. 6.
Plasma corticosterone levels in each group following 7 weeks experiment. Statistical analysis was performed by the one-way ANOVA and Bonferroni test. There was a significant difference in the plasma corticosterone levels between the naïve group and all other groups, including the CON group. $Denotes significant differences between the naïve and other groups ($p<0.05). *Denotes significant difference between CON and other groups (*p<0.05). ‡Denotes significant difference between CVS+MSG and A-Str group (‡p<0.05). CON, control; MSG, monosodium glutamate; CVS, chronic variable stress; A-Str, restraint stress for 2 hours just before decapitation.
DISCUSSION
The present study was aimed at investigating the influence of MSG and/or chronic stress on body weight, food intake, exploring behavior, and HPA axis in rats using plasma corticosterone levels and the weight of the adrenal glands.
Body weight
There was a gradual increase in body weight in all groups as the experiment progressed, although the rate of the increase was different between each group. One week after the beginning of the experiment, there was a smaller increase in the body weight in all groups than in that of the CON, and an especially significant decrease could be seen in the CVS+MSG group compared to the CON group. Many investigators have shown that stress suppresses food intake and body weight gain in rats [14,15]. However, there were several discrepancies in the changes of the body weight after stress exposure. For example, in a study by Marin et al. on weight changes in rats exposed to chronic restraint stress or CVS for 10 days, a significant weight loss was observed in the latter group [1]. Considering the previous report, the CVS used in the present experiment is known to be difficult to adapt to, which makes this a reasonable result in the CVS or CVS+MSG group. Rats in the MSG group also showed a difference in weight gain compared to the CON group, indicating that MSG administration seems to be a stress at the beginning period of the experiment. However, it has been shown that once the stress has ended and even when adaptation was achieved, the body weight of the rats did not return to that of the CON group [1].
Food Intake
Although the central mechanisms involved in the stress-induced inhibition of food intake have not been fully elucidated, it has been demonstrated that chronic exposure to stress decreases food intake and body weight and induces adrenal hypertrophy [16]. In the present study, the changes in food intake and body weight were also consistent with the results of previous reports when rats were exposed to CVS [14,15,17]. It has been reported that CRF is involved in stress-induced inhibition of food intake [18]. CRF is a key mediator of many aspects related to stress [19], and several investigators have attributed stress-induced anorexia to activation of CRF [20]. In light of the relationship between CRF and food intake, the result of our experiment might be caused by the persistent hypersecretion of CRF due to CVS. Also, the decreased food intake in the MSG group might be caused by increased fasting levels of leptin which is related to the reduced food intake [21].
Open field test
For the purpose of investigating behavioral changes, we conducted an open field test, which is commonly used as a measurement of stress-related behavior [22]. All animals showed a gradual decrease in their locomotor activity during the course of the present experiment. Generally, it has been accepted that animals placed into the same experimental arena show a gradual decrease in the intensity of locomotor and exploratory activity initially shown in that environment [23]. The CVS model (also known as the chronic mild stress model) used in this study has been developed in experimental animals to simulate the number of unpredictable annoying events of the everyday life. It has also been used to investigate behavioral changes associated with depression [24]. As a result induced by CVS, some authors reported decreased [25,26] while others found increased anxiety [27].
In the present study, aggressive or escape behavior such as jumping, digging, climbing, and gnawing was not exhibited by any of the animals during the open field test. Compared to the behavioral results before the initiation of the stress protocol, all animals tended to be more active. It is generally known that the locomotor activity in adult rats is higher than in pups (at about the time of weaning) [28] and handled animals show more exploring activity compared to non-handled animals [29]. Handling is regarded as mild controllable stress rather than severe, uncontrollable stress [30]. Although we tried to prevent the control animals from being stressed, the result of the present study could arise from unintended mild stress that were inflicted on them by daily routines such as intubation for MSG or vehicle administration, cleaning of the cage, or measuring of the food and body weight.
It has been known that normal rats usually show increased rearing and grooming in a novel open field. However, it has been reported that rats exposed to chronic unpredicted mild stress showed a significant decrease in their rearing and grooming activity in a novel open field. This observation may indicate a "refractory loss of interest" which is one of the core symptoms of depression [31,32]. The results of our experiments showed a significant decrease of the stereotypic count in all groups with the exception of the CON group. In the CVS and CVS+MSG group, the results were similar to those of previous studies [31,32] in terms of the decrease in stereotypic movements in all stressed animals. However, there was a difference in the explorative activity. It has been suggested that the changes in behavior depend on species differences, diurnal variation, methodological details, and so on [33-35]. The reason for the difference between our results and those reported by Luo et al. or Wang et al. is due to methodological differences rather than others. That is, showing a depressive behavior in an open field test seems to be more related to severity than chronicity of the stress mode.
Surprisingly, the rats in the MSG group showed unexpected results in regard to depressive behavior in the open field test. Results regarding changes in the exploratory behavior as well as the neurotoxic effects of MSG after neonatal MSG treatment are still controversial. In addition, little attention has been given to the behavioral effects of MSG administration. Although the present study offered an initial contribution to the behavioral effect of MSG in adult rats, more studies are needed in the future.
In addition to the reduction in the stereotypic and exploring activity in the open field by chronic mild stress [35], there is ample evidence that an increase in CRF associated with stress leads to decreased locomotor activity [19,31,32,35] and is related to depression [36]. Therefore, consistent with the well-known relationship between life stress and depression [37], CRF has been assumed to play a role in depressive illnesses.
Thus, the higher decrease in activity seen in the CVS+MSG group compared to the CVS group in this experiment could be explained by persistent overstimulation of the hypothalamus of the animals in the CVS+MSG group not only by unadaptable stress but also by excess glutamate. In addition, excess MSG might induce hypersecretion of CRF, which subsequently might cause the decrease in behavioral activity in the open field test regarded as depressive behavior.
Adrenal response
It has been demonstrated that excitatory amino acids play an important role in the activation of the HPA axis [3], a key component of the stress responses. Glutamate is a well-known excitatory neurotransmitter, and known to be responsible for the fast excitatory input to magnocellular and parvocellular neurons in the PVN, where CRF is released [3]. In rats, glutamate injection into the third ventricle elevated plasma ACTH levels [38], and its agonists had similar effects as endogenous glutamate [39,40]. Excessive accumulation of glutamate in the extracellular spaces may lead to excessive activation of glutamate receptors in the hypothalamic nuclei. In a previous study, although there was a difference between systemic and dietary administration of MSG, glutamate levels in the plasma were apparently increased after administration of MSG. Monno et al. reported that 4 g/kg of MSG given to adult rats by forced gavage induced a 4.2- to 8.9-fold increase in extracellular glutamate levels within the hippocampus and hypothalamus, respectively [10]. Therefore, it is quite possible that glutamate levels in some brain regions, including the hypothalamus or circumventricular organs, which lack a blood-brain-barrier, could increase in proportion to the increase in the plasma glutamate concentration. However, there was a controversial report showing that no changes were observed in the glutamate levels of dialysates of the arcuate nucleus, in spite of increased MSG levels in the plasma [11] that could eventually activate the HPA axis.
To verify the influence of stress and whether the simultaneous administration of MSG has an aggravating effect on the HPA axis, the adrenal gland weight and plasma corticosterone concentration were compared. As reported in previous studies, the relative adrenal gland weight of all stressed animals in this study was significantly increased compared to the CON group. In addition, the relative adrenal gland weight in the CVS+MSG group increased significantly more than in the other groups. It is considered that the adrenal gland in stressed animals, especially in the CVS+MSG group, was more frequently or strongly stimulated than in the other animals. In the CVS group, plasma corticosterone levels were not significantly different compared to the CON group in spite of sustained stress. We demonstrated in a previous study that urinary corticosterone levels were higher from the first to the fifth week, but the urinary and serum corticosterone levels were not statistically different between the groups at 6 week. There are several presumptions to explain the relatively low level of corticosterone and adrenal hypertrophy at the seventh week in the CVS group including the generation of a stronger negative feedback inhibition or down-regulation of the CRF receptor in the hypothalamus due to the high CRF milieu [41,42]. It remains to be elucidated how the HPA axis responds to chronic stress in terms of CRF. In addition, even though the relative adrenal gland weight in the CVS+MSG group was higher than that in the other groups, plasma corticosterone levels were significantly decreased compared to the other groups, including the CVS group.
Stress has been associated with the activation of the HPA axis, ultimately resulting in an increased secretion of glucocorticoids from the adrenal glands. The physiological effects of glucocorticoids help the organism to maintain homeostasis under conditions of stress. It has been accepted that the increase in corticosterone release from the adrenal gland in rats is a response to acute stress. However, there is a discrepancy in the chronic stress responses; that is, contradictory results were found when evaluating the effects of chronic stress on the basal cortisol or corticosterone release. The levels of plasma cortisol in humans or corticosterone in rats that were chronically exposed to physical or emotional stress have been shown to increase, decrease, [43] or not to change. Stress is a frequent precipitating factor in major depression [44]. In the current study, we used a CVS model to investigate the effect of long-term life stress that was sufficient to cause depression. Depression is known to be associated with sustained hyperactivity of the stress axis caused by the stress neurotransmitter CRF, which initiates the change in the HPA axis [36]. In addition to psychological changes that reflect maladaptation to chronic stress, physical changes can also occur that are associated with metabolic changes initiated by hypersecretion of glucocorticoids and hyperactivity of the sympathoadrenal system [45]. In recent years, however, since Yehuda et al. described the phenomenon of hypocortisolism in post-traumatic patients [46], there is increasing evidence for a relatively decreased rather than increased cortisol secretion in individuals [41,42]. The studied individuals have been exposed to severe stress or suffered from stress-related disorders such as chronic fatigue syndrome, fibromyalgia, chronic pelvic pain, depression, and even major life events [41,42]. It has also been reported that stress-related neuropsychiatric disorders may be characterized by insufficient glucocorticoid signaling, as manifested by hypocortisolism or impaired glucocorticoid responsiveness associated with evidence of an increased vulnerability to bodily disorders, immune activation, and hypersecretion of CRF [41,42].
It has been suggested that several mechanisms underlie the development and persistence of hypocortisolism in chronic stress [41,42]. Heim et al. suggested that alterations on several levels of the HPA axis may contribute to the presence of hypocortisolism, and many factors, such as genetics, gender, or early stress experiences among others, may determine the development of hypocortisolism [41]. Among the mechanisms suggested by Heim et al., CRF hypersecretion and adaptive down-regulation of pituitary CRF receptors may be the most feasible explanation of our result. That is, the present result implies that hypocortisolism could be induced by overstimulation of the hypothalamus due to CVS and simultaneous accumulation of excessive glutamate by chronic MSG administration. In other words, when the hypothalamus is stimulated by chronic stress it may become overstimulated by excessive glutamate in the CSF due to chronic MSG administration. Successively, CRF hypersecretion occurs in the hypothalamus and adaptive down-regulation of the pituitary CRF receptor and decreased ACTH release could follow. As a result, the release of corticosterone, the end product of the HPA axis, may have been decreased. Consequently, it is possible to aggravate the response of the HPA axis by chronic stimulation with excitotoxins such as MSG under CVS.
In conclusion, the results showed relatively decreased plasma corticosterone levels as well as decreased sterotypic behavior in the CVS+MSG group as compared to the corresponding levels in the other groups. These results suggest that HPA axis function can be impaired by overstimulation of the hypothalamus and simultaneous accumulation of excessive glutamate due to chronic MSG administration. Further investigations are needed to demonstrate the adverse effects of other excitotoxins, stimulants, or depressants such as aspartame, caffeine, nicotine, and cocaine, and to investigate the effect of changes in the adrenomedullary system on the behavior in rats under CVS and/or those administered MSG.
ACKNOWLEDGEMENTS
This paper was supported by the Wonkwang University in 2008.
ABBREVIATIONS
- HPA axis
hypothalamic-pituitary-adrenal axis
- MSG
L-monosodium glutamate
- CVS
chronic variable stress
- CRF
corticotropin-releasing factor
- PVN
paraventricular nucleus
- ACTH
adrenocorticotropic hormone
- NGF
nerve growth factor
- NPY
neuropeptide Y
- CSF
cerebrospinal fluid
- RIA
radioimmunoassay
- ANOVA
one-way analysis of variance
References
- 1.Marin MT, Cruz FC, Planeta CS. Chronic restraint or variable stresses differently affect the behavior, corticosterone secretion and body weight in rats. Physiol Behav. 2007;90:29–35. doi: 10.1016/j.physbeh.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 2.Mathew SJ, Coplan JD, Schoepp DD, Smith EL, Rosenblum LA, Gorman JM. Glutamate-hypothalamic-pituitary-adrenal axis interactions: implications for mood and anxiety disorders. CNS Spectr. 2001;6:555–556. doi: 10.1017/s1092852900002091. [DOI] [PubMed] [Google Scholar]
- 3.Zelena D, Mergl Z, Makara GB. Glutamate agonists activate the hypothalamic-pituitary-adrenal axis through hypothalamic paraventricular nucleus but not through vasopressinergic neurons. Brain Res. 2005;1031:185–193. doi: 10.1016/j.brainres.2004.10.034. [DOI] [PubMed] [Google Scholar]
- 4.Walker R, Lupien JR. The safety evaluation of monosodium glutamate. J Nutr. 2000;130(Suppl 4):1049S–1052S. doi: 10.1093/jn/130.4.1049S. [DOI] [PubMed] [Google Scholar]
- 5.Lucas DR, Newhouse JP. The toxic effect of MSG on the inner layers of the retina. AMA Arch Ophthalmol. 1957;58:193–201. doi: 10.1001/archopht.1957.00940010205006. [DOI] [PubMed] [Google Scholar]
- 6.Larsen PJ, Mikkelsen JD, Jessop D, Lightman SL, Chowdrey HS. Neonatal monosodium glutamate treatment alters both the activity and the sensitivity of the rat hypothalamo-pituitary-adrenocortical axis. J Endocrinol. 1994;141:497–503. doi: 10.1677/joe.0.1410497. [DOI] [PubMed] [Google Scholar]
- 7.Beyreuther K, Biesalski HK, Fernstrom JD, Grimm P, Hammes WP, Heinemann U, Kempski O, Stehle P, Steinhart H, Walker R. Consensus meeting: monosodium glutamate-an update. Eur J Clin Nutr. 2007;61:304–313. doi: 10.1038/sj.ejcn.1602526. [DOI] [PubMed] [Google Scholar]
- 8.Magariños AM, Estivariz F, Morado MI, De Nicola AF. Regulation of the central nervous system-pituitary-adrenal axis in rats after neonatal treatment with monosodium glutamate. Neuroendocrinology. 1988;48:105–111. doi: 10.1159/000124997. [DOI] [PubMed] [Google Scholar]
- 9.Tirassa P, Lundeberg T, Stenfors C, Bracci-Laudiero L, Theodorsson E, Aloe L. Monosodium glutamate increases NGF and NPY concentrations in rat hypothalamus and pituitary. Neuroreport. 1995;6:2450–2452. doi: 10.1097/00001756-199512150-00003. [DOI] [PubMed] [Google Scholar]
- 10.Monno A, Vezzani A, Bastone A, Salmona M, Garattini S. Extracellular glutamate levels in the hypothalamus and hippocampus of rats after acute or chronic oral intake of monosodium glutamate. Neurosci Lett. 1995;193:45–48. doi: 10.1016/0304-3940(95)11664-i. [DOI] [PubMed] [Google Scholar]
- 11.Bogdanov MB, Tjurmina OA, Wurtman RJ. Consumption of a high dietary dose of monosodium glutamate fails to affect extracellular glutamate levels in the hypothalamic arcuate nucleus of adult rats. Brain Res. 1996;736:76–81. doi: 10.1016/0006-8993(96)00679-8. [DOI] [PubMed] [Google Scholar]
- 12.Russell BL. Excitotoxins, The Taste that kills. 1st ed. Santa Fe, New Mexico: Health Press NA Inc; 1997. pp. 33–57. [Google Scholar]
- 13.Kim YS, Lee MY, Choi CS, Sohn YW, Park BR, Choi MG, Nah YH, Choi SC. The effect of chronic variable stress on bowel habit and adrenal function in rats. J Gastroenterol Hepatol. 2008;23:1840–1846. doi: 10.1111/j.1440-1746.2008.05524.x. [DOI] [PubMed] [Google Scholar]
- 14.Kennett GA, Chaouloff F, Marcou M, Curzon G. Female rats are more vulnerable than males in an animal model of depression: the possible role of serotonin. Brain Res. 1986;382:416–421. doi: 10.1016/0006-8993(86)91355-7. [DOI] [PubMed] [Google Scholar]
- 15.Krahn DD, Gosnell BA, Majchrzak MJ. The anorectic effects of CRH and restraint stress decrease with repeated exposures. Biol Psychiatry. 1990;27:1094–1102. doi: 10.1016/0006-3223(90)90046-5. [DOI] [PubMed] [Google Scholar]
- 16.Mizoguchi K, Yuzurihara M, Ishige A, Sasaki H, Chui D, Tabira T. Chronic stress differentially regulates glucocorticoid negative feedback response in rats. Psychoneuroendocrinology. 2001;26:443–459. doi: 10.1016/s0306-4530(01)00004-x. [DOI] [PubMed] [Google Scholar]
- 17.Martí O, Martí J, Armario A. Effects of chronic stress on food intake in rats: influence of stressor intensity and duration of daily exposure. Physiol Behav. 1994;55:747–753. doi: 10.1016/0031-9384(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 18.Sekino A, Ohata H, Mano-Otagiri A, Arai K, Shibasaki T. Both corticotropin-releasing factor receptor type 1 and type 2 are involved in stress-induced inhibition of food intake in rats. Psychopharmacology (Berl) 2004;176:30–38. doi: 10.1007/s00213-004-1863-1. [DOI] [PubMed] [Google Scholar]
- 19.Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry. 1999;46:1167–1180. doi: 10.1016/s0006-3223(99)00164-x. [DOI] [PubMed] [Google Scholar]
- 20.Krahn DD, Gosnell BA, Grace M, Levine AS. CRF antagonist partially reverses CRF- and stress-induced effects on feeding. Brain Res Bull. 1986;17:285–289. doi: 10.1016/0361-9230(86)90233-9. [DOI] [PubMed] [Google Scholar]
- 21.Diniz YS, Faine LA, Galhardi CM, Rodrigues HG, Ebaid GX, Burneiko RC, Cicogna AC, Novelli EL. Monosodium glutamate in standard and high-fiber diets: metabolic syndrome and oxidative stress in rats. Nutrition. 2005;21:749–755. doi: 10.1016/j.nut.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Swiergiel AH, Leskov IL, Dunn AJ. Effects of chronic and acute stressors and CRF on depression-like behavior in mice. Behav Brain Res. 2008;186:32–40. doi: 10.1016/j.bbr.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 23.Hlinák Z, Gandalovicová D, Krejcí I. Behavioral deficits in adult rats treated neonatally with glutamate. Neurotoxicol Teratol. 2005;27:465–473. doi: 10.1016/j.ntt.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Grippo AJ, Beltz TG, Johnson AK. Behavioral and cardiovascular changes in the chronic mild stress model of depression. Physiol Behav. 2003;78:703–710. doi: 10.1016/s0031-9384(03)00050-7. [DOI] [PubMed] [Google Scholar]
- 25.D'Aquila PS, Brain P, Willner P. Effects of chronic mild stress on performance in behavioural tests relevant to anxiety and depression. Physiol Behav. 1994;56:861–867. doi: 10.1016/0031-9384(94)90316-6. [DOI] [PubMed] [Google Scholar]
- 26.Kopp C, Vogel E, Rettori MC, Delagrange P, Misslin R. The effects of melatonin on the behavioural disturbances induced by chronic mild stress in C3H/He mice. Behav Pharmacol. 1999;10:73–83. doi: 10.1097/00008877-199902000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Griebel G, Simiand J, Steinberg R, Jung M, Gully D, Roger P, Geslin M, Scatton B, Maffrand JP, Soubrié P. 4-(2-Chloro-4-methoxy-5-methylphenyl)-N-[(1S)-2-cyclopropyl-1-(3-fluoro-4-methylphenyl)ethyl]5-methyl-N-(2-propynyl)-1, 3-thiazol-2-amine hydrochloride (SSR125543A), a potent and selective corticotrophin-releasing factor(1) receptor antagonist. II. Characterization in rodent models of stress-related disorders. J Pharmacol Exp Ther. 2002;301:333–345. doi: 10.1124/jpet.301.1.333. [DOI] [PubMed] [Google Scholar]
- 28.Buelke-Sam J, Kimmel CA, Nelson CJ, Sullivan PA. Sex and strain differences in the developmental activity profile of rats prenatally exposed to sodium salicylate. Neurobehav Toxicol Teratol. 1984;6:171–175. [PubMed] [Google Scholar]
- 29.Kim DG, Lee S, Lim JS. Neonatal footshock stress alters adult behavior and hippocampal corticosteroid receptors. Neuroreport. 1999;10:2551–2556. doi: 10.1097/00001756-199908200-00021. [DOI] [PubMed] [Google Scholar]
- 30.Huether G. The central adaptation syndrome: psychosocial stress as a trigger for adaptive modifications of brain structure and brain function. Prog Neurobiol. 1996;48:569–612. doi: 10.1016/0301-0082(96)00003-2. [DOI] [PubMed] [Google Scholar]
- 31.Luo DD, An SC, Zhang X. Involvement of hippocampal serotonin and neuropeptide Y in depression induced by chronic unpredicted mild stress. Brain Res Bull. 2008;77:8–12. doi: 10.1016/j.brainresbull.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 32.Wang D, An SC, Zhang X. Prevention of chronic stress-induced depression-like behavior by inducible nitric oxide inhibitor. Neurosci Lett. 2008;433:59–64. doi: 10.1016/j.neulet.2007.12.041. [DOI] [PubMed] [Google Scholar]
- 33.Bekris S, Antoniou K, Daskas S, Papadopoulou-Daifoti Z. Behavioural and neurochemical effects induced by chronic mild stress applied to two different rat strains. Behav Brain Res. 2005;161:45–59. doi: 10.1016/j.bbr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Kim KS, Han PL. Optimization of chronic stress paradigms using anxiety- and depression-like behavioral parameters. J Neurosci Res. 2006;83:497–507. doi: 10.1002/jnr.20754. [DOI] [PubMed] [Google Scholar]
- 35.D'Aquila PS, Peana AT, Carboni V, Serra G. Exploratory behaviour and grooming after repeated restraint and chronic mild stress: effect of desipramine. Eur J Pharmacol. 2000;399:43–47. doi: 10.1016/s0014-2999(00)00332-0. [DOI] [PubMed] [Google Scholar]
- 36.Holsboer F. The rationale for corticotropin-releasing hormone receptor (CRH-R) antagonists to treat depression and anxiety. J Psychiatr Res. 1999;33:181–214. doi: 10.1016/s0022-3956(98)90056-5. [DOI] [PubMed] [Google Scholar]
- 37.Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- 38.Makara GB, Stark E. Effect of intraventricular glutamate on ACTH release. Neuroendocrinology. 1975;18:213–216. doi: 10.1159/000122400. [DOI] [PubMed] [Google Scholar]
- 39.Chautard T, Boudouresque F, Guillaume V, Oliver C. Effect of excitatory amino acid on the hypothalamo-pituitary-adrenal axis in the rat during the stress-hyporesponsive period. Neuroendocrinology. 1993;57:70–78. doi: 10.1159/000126344. [DOI] [PubMed] [Google Scholar]
- 40.Jezová D, Tokarev D, Rusnák M. Endogenous excitatory amino acids are involved in stress-induced adrenocorticotropin and catecholamine release. Neuroendocrinology. 1995;62:326–332. doi: 10.1159/000127021. [DOI] [PubMed] [Google Scholar]
- 41.Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- 42.Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- 43.Pignatelli D, Maia M, Castro AR, da Conceição Magalhães M, Vivier J, Defaye G. Chronic stress effects on the rat adrenal cortex. Endocr Res. 2000;26:537–544. doi: 10.3109/07435800009048567. [DOI] [PubMed] [Google Scholar]
- 44.Anisman H, Merali Z, Poulter MO, Hayley S. Cytokines as a precipitant of depressive illness: animal and human studies. Curr Pharm Des. 2005;11:963–972. doi: 10.2174/1381612053381701. [DOI] [PubMed] [Google Scholar]
- 45.Habib KE, Gold PW, Chrousos GP. Neuroendocrinology of stress. Endocrinol Metab Clin North Am. 2001;30:695–728. doi: 10.1016/s0889-8529(05)70208-5. [DOI] [PubMed] [Google Scholar]
- 46.Yehuda R. Sensitization of the hypothalamic-pituitary-adrenal axis in posttraumatic stress disorder. Ann N Y Acad Sci. 1997;821:57–75. doi: 10.1111/j.1749-6632.1997.tb48269.x. [DOI] [PubMed] [Google Scholar]