Abstract
Summary
Diminished expression of the metastasis suppressor protein RKIP was previously reported in a number of cancers. The underlying mechanism remains unknown. Here we show that the expression of RKIP negatively correlates with that of Snail zinc-transcriptional repressor, a key modulator of normal and neoplastic epithelial-mesenchymal transition (EMT) program. With a combination of loss-of-function and gain-of-function approaches we showed that Snail repressed the expression of RKIP in metastatic prostate cancer cell lines. The effect of Snail on RKIP was on the level of transcriptional initiation and mediated by a proximal E-box on the RKIP promoter. Our results therefore suggest that RKIP is a novel component of the Snail transcriptional regulatory network important for the progression and metastasis of cancer.
RKIP (Raf kinase inhibitor protein) is a member of an evolutionarily conserved group of proteins called PEBP (Phosphatidylethanolamine-binding protein). Recently we and others have identified and characterized RKIP as a metastasis suppresssor protein. On the molecular level, RKIP functions by inhibiting the proliferative and survival Raf-MEK-ERK and NF-κB signaling pathways(Chatterjee, Bai et al., 2004; Park, Yeung et al., 2005). Consistent with its demonstrated inhibitory effect on Raf and NF-κB signaling, we and others have shown that the expression levels of RKIP are downregulated in a number of tumors, including highly metastatic prostate, breast and colon cancer, hepatocellular carcinoma, melanomas and insulinomas(Al-Mulla, Hagan et al., 2006; Chatterjee, Bai et al., 2004; Fu, Smith et al., 2003; Hegan, Al-Mulla et al., 2005; Lee, Tian et al., 2006; Schuierer, Bataille et al., 2004; Schuierer, Bataille et al., 2006; Zhang, Fu et al., 2004). The importance of RKIP in metastases was highlighted by the finding that restoration of RKIP expression inhibits prostate cancer metastasis in a murine model(Fu, Kitagawa et al., 2005; Fu, Smith et al., 2003). More recent studies have shown that RKIP is also a good prognostic marker of the pathogenesis of human prostate cancer(Fu, Kitagawa et al., 2005) and a prognostic indicator for overall survival and disease free survival in colorectal cancer (Al-Mulla, Hagan et al., 2006). Collectively, these studies suggest that RKIP is a novel cancer metastasis suppressor and an effector of signal transduction pathways leading to apoptosis. In spite of the abundance of experimental evidence on the deleterious consequences of reduced RKIP expression in tumors, the mechanisms responsible for the down-regulation of RKIP in cancer are not completely understood.
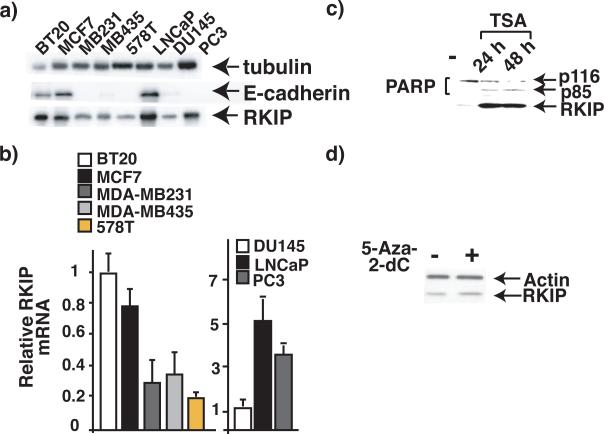
To set up a system to study the transcription regulation of RKIP, we examined RKIP expression levels in cancer cell lines with different metastatic capacity. In accordance with clinical tumor studies, we observed that expression levels of RKIP proteins progressively decrease in breast and prostate cancer cell lines of increasing metastatic potential. The expression of RKIP is low in invasive and metastatic breast (MB231, MB435, and 578T) and prostate (DU145 and PC3) cell lines and high in non-invasive cell lines like MCF7, BT20, and LNCaP (Fig. 1a). Notably, RKIP protein levels correlated well with those of the intercellular adhesion protein E-cadherin (E-cad). E-caderin is a well documented tumor metastasis suppressor protein that is regulated by the Snail and closely related Slug transcription factors (Peinado, Olmeda et al., 2007). Quantitation of RKIP transcript levels in the different cancer cell lines by qRT-PCR demonstrated that they correlated with the levels of the protein, (Fig. 1b), suggesting that RKIP expression is down-regulated at the RNA level, via changes in mRNA stability or transcription initiation.
Figure 1. The expression of RKIP is repressed in highly metastatic cancer cells.
a) Immunoblot analysis of extracts from prostate or breast cancer cells with specific Abs. b) The endogenous levels of RKIP mRNA in prostate cancer cells as measured by qRT-PCR and normalized to the level of GAPDH (left) or mATP6. Each bar represents the mean ± SEM (standard error mean) of the PCR reactions in triplicate. c-d) Immunoblot analysis of extracts from prostate cancer cells DU145 treated with c) TSA or d) 5-Aza-2-dC for 24 or 48 h as indicated. Data shown are representative of three independent experiments.
DNA methylation is an important epigenetic mechanism for gene silencing, commonly used by cancer cells to inactivate tumor suppressor genes(Baylin & Herman, 2000; Herman & Baylin, 2000). Methylation usually occurs at small stretches of DNA containing the CpG dinucleotide or the CpG islands located in the proximal promoter region. Methylated CpG islands are docking sites for the recruitment of histone deacetylatases resulting in stable transcriptional repression. The repressed state of a methylated promoter can be reversed by the methylation inhibitor 5-Aza2dC or by the histone deacetylase inhibitor Trichostatin A (TSA). To determine whether RKIP is repressed by methylation in metastatic prostate cell lines, we examined the effect of TSA on RKIP expression. Immunoblotting against RKIP showed that TSA treatment caused a robust increase in RKIP expression in DU145 cells. Consistent with its role as an important apoptosis regulator, the increase in RKIP expression in TSA-treated cells was also accompanied by extensive apoptosis, as measured by cleavage of PARP, a common apoptosis marker (Fig. 1c). To directly show that RKIP expression is repressed by methylation, DU145 cells were treated with 3 M 5-Aza-2dC for 72 h. Expression of RKIP was monitored by immunoblot with RKIP-specific antibody. However, unlike TSA, 5-Aza-2dC had no effect on RKIP expression (Fig. 1d). As reported(Soengas, Capodieci et al., 2001), treatment of melanoma cells with the same 5-Aza-2dC concentration caused a robust induction of the pro-apoptotic Apaf1 (not shown). These results using the demethylation agent 5-Aza-2dC clearly show that promoter hyper-methylation is not the cause of RKIP downregulation in DU145. Our finding that the expression of RKIP can be induced by TSA implies that RKIP expression may be actively repressed in cancer cells.
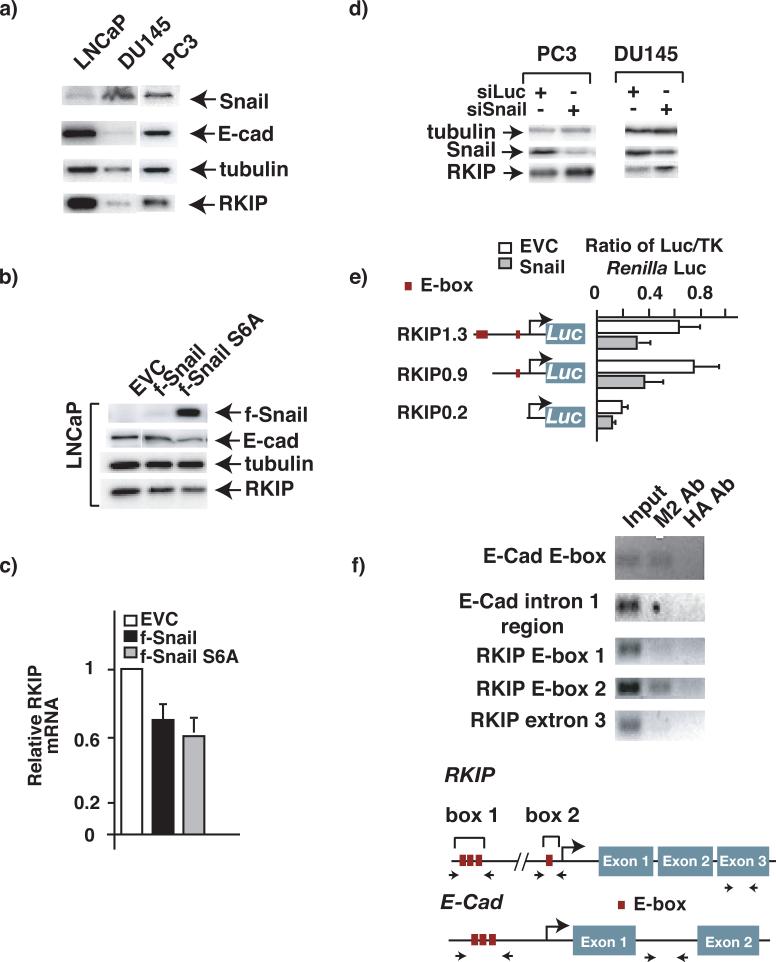
Gene expression studies have identified the transcription repression of E-cad to be the key event in cancer metastasis. Among the transcription factors implicated in this process, Snail has been shown to be the strong repressor of E-cad transcription(Nieto, 2002; Thiery, 2002). In light of the downregulation of RKIP expression in metastatic cancer cell lines, as well as the co-expression of RKIP and E-cad in several cancer cell lines (Fig. 1a), we hypothesized that Snail may act in the same pathway as RKIP. Consistent with this hypothesis, we observed a negative correlation of Snail protein expression with that of RKIP in prostate cancer cell lines (Fig. 2a). To directly examine the causal role of Snail in RKIP expression, we introduced a Snail expression construct by retroviral infection into the non-metastatic cancer cell line LNCaP, which has relatively high RKIP levels. To circumvent the possible effect of the highly unstable Snail on RKIP expression, a mutated stable variant (Snail-6SA), which has all its six phosphorylable Ser in the consensus GSK-3β sites mutated to Ala(Zhou, Deng et al., 2004), was used along with the wildtype Snail. As expected, considerable amounts of Snail-6SA were detected in stably infected LNCaP cells (Fig. 2b), although wildtype Snail was almost undetectable.
Figure 2. Snail is a direct repressor of RKIP expression.
a) Comparison of RKIP, and Snail expression in prostate cancer cell lines by Western blot analysis. Triton x-100 extracted cell lysates (30 μg) were immunoblotted using polyclonal anti-RKIP, or Snail antibodies. The same membrane was re-blotted with an anti-actin serum as a loading control. b-c) Ectopic expression of Snail represses RKIP expression. b) Immunoblot analysis of extracts from cancer cells stably infected with the indicated retroviruses or EVC (empty vector control) with specific Abs. c) Comparison of RKIP mRNA levels in LNCaP cells stably infected with the indicated retroviruses. The endogenous levels of RKIP mRNA in infected cells were measured by qRT-PCR and normalized to the level of GAPDH. Each bar represents the mean ± SEM of the PCR reactions in triplicate. d) Downregulation of Snail in prostate cancer cells enhances RKIP expression. Cells were stably infected with the indicated retroviruses expressing shRNA for luciferase or Snail. The endogenous levels of RKIP, tubulin and Snail proteins in infected cells were measured by immunoblot analysis. e) Snail represses RKIP promoter reporter in cancer cells. MCF7 cells were transfected with the indicated effector and reporter plasmids. 48h after transfection cells were harvested for luciferase assay. Schematic of the RKIP-LUC reporter containing the RKIP promoter region was shown on the left. Positions of the putative E-box are indicated. TK stands for Thymidine Kinase. f) Snail associates with RKIP promoter. ChIP assays were performed with anti Flag or anti-HA Ab on LNCaP cells that stably express Flag-tagged Snail protein. DNAs that were immunoprecipitated down with the Abs were amplified with primer pair by PCR as indicated by arrows in the lower panel.
In accordance with previously published results, ectopic expression of Snail S6A downregulated expression of E-cad in LNCaP cells. In addition, we observed a significant decrease in RKIP expression both at the protein and RNA level in the same cells, while infection with empty virus control (EVC) had no effect (Fig 2b-c). The suppression of RKIP expression correlated with Snail expression levels, as the Snail variant S6A had a stronger effect relative to wildtype Snail (Fig. 2b-c). Conversely, expression levels of RKIP were increased in metastatic PC3 and DU145 prostate cancer cells when expression of Snail was knocked down by specific siRNA (Fig. 2d), implying that Snail is a physiologically relevant repressor of RKIP.
Inspection of the RKIP promoter revealed the presence of at least four potential Snail binding consensus sites (E-box: CANNTG)) clustered in two locations in the proximal RKIP promoter. To determine whether Snail regulates RKIP expression in an E-box-dependent manner, we examined the effect of overexpressing Snail on the activity of RKIP promoter-driven luciferase reporters in MCF7 breast cancer cells, which have a low level of native Snail expression and are comparatively easy to transfect. Three different RKIP promoter luciferase reporters containing all four, one, or no E-box binding sites were used. Consistent with the observation that ectopic expression of Snail downregulated RKIP, forced expression of Snail repressed RKIP luciferase reporters that contained one or all four E-box cis-elements, while the RKIP reporter lacking all E-box elements responded poorly to Snail repression (Fig. 2e).
To determine whether Snail interacts with the RKIP promoter directly we performed a chromatin immunoprecipitation (ChIP) experiment with purified cross-linked chromatin prepared from LNCaP cells stably expressing a flag-tagged SnailS6A. In support of the regulatory role of Snail in RKIP expression, a detectable amount of Snail was found associated with the RKIP promoter at E-box 2, but not at E-box 1 or exon 3 of the RKIP gene locus (Fig. 2f). As expected, our positive control showed that Snail bound to its known direct target gene – the E-box in the E-Cad gene promoter – but not to the intron region. These results confirmed the transient reporter assay, which showed that the proximal E-Box is sufficient for Snail-mediated repression of RKIP promoter.
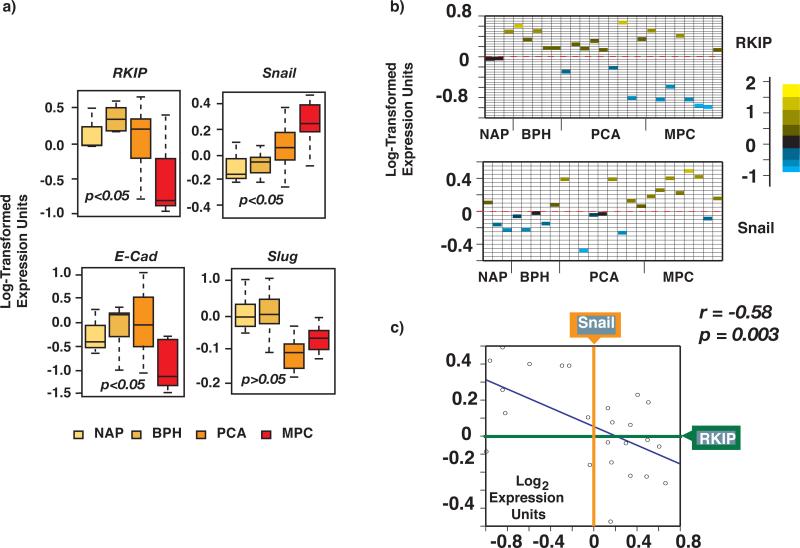
We reasoned that if Snail is a physiologically relevant inhibitor of RKIP expression in prostate cancer, then we ought to observe a negative correlation between their expression patterns in cancer samples. We therefore interrogated publicly available DNA microarray expression datasets derived from human prostate cancers, including Oncomine (www.oncomine.org) and NCBI's Gene Expression Omnibus (GEO) (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=gds), for both RKIP and Snail. We focused initially on datasets studying progressing prostate cancers and metastasis. Ten datasets were identified that included analyses of normal and/or benign prostatic hyperplasia (BPH), localized prostate cancer (PCA), and metastases of prostate cancer (MPC). One of them(Dhanasekaran, Barrette et al., 2001), dataset #1, showed a statistically significant increase of Snail expression from normal to localized to metastatic prostate cancer. As expected, the expression of E-cad, the proven direct target of Snail also decreased significantly between localized cancer samples and metastatic samples. Importantly, the same dataset showed a significant decrease of RKIP expression as the prostate cancers progressed (Fig. 3a). Samples were referenced against their own pool of normal adjacent prostate (NAP) tissue from prostate cancer patients.
Figure 3. The expression of RKIP negatively correlates with Snail in prostate cancer samples.
a) Box plots of RKIP and Snail levels in prostate cancer microarray datasets. The box represents the standard deviation of the distribution and the line through that box represents the mean of that distribution. The horizontal lines above and below the box represent the extreme values of the distribution. Ratios of prostate cancer samples referenced against a pool of normal adjacent prostate (NAP) samples from patients were log-transformed and plotted using the statistical program R. NAP N=3, BPH N=5, PCA N=10, and MET N=7. b) The log transformed expression units of RKIP or Snail were plotted for each sample. Samples were grouped as NAP, BPH, PCA and MPC as in a). c) Scatterplots of Snail versus RKIP of all samples displayed in a)
To study the correlation between RKIP and Snail expression we compared their expression in each prostate cancer sample in dataset #1 (Fig. 3b). We also directly quantified their expression relationship by plotting log-transformed expression units of Snail against RKIP and measured their expression similarity using Pearson correlation coefficients r. As shown in Fig. 3c we observed a significant negative correlation between RKIP and Snail across all samples with r = -0.58 and p value = 0.003. As expected, expression of E-cad was also significantly negatively correlated with Snail across all samples (r = -0.57; p value = 0.003) (Fig. 3c). Similar results were obtained with another dataset (dataset #2), kindly provided by Dr. Arul Chinnaiyan at the University of Michigan (not shown).
Since another member of the Snail superfamily, Slug, shares many physiological functions with Snail and is also implicated in promoting cancer invasiveness, it was of interest to determine whether its expression also negatively correlates with RKIP levels. Unexpectedly, not only did we not observe any increase in the expression levels of Slugs from normal, to BPH, PCA, and MPC samples, but in fact, there was a decrease in both datasets (Fig 3a). Although there was a small increase in Slug between PCA and MPC samples, the difference was not statistically significant.
RKIP was recently reported as being downregulated in metastatic prostate cancer (Fu, Kitagawa et al., 2005; Fu, Smith et al., 2003). However, it is not known how RKIP is downregulated in cancer, or how it is transcriptionally regulated. We showed that by qRT-PCR that the steady-state RKIP mRNA was decreased in high metastatic prostate cancer cell lines PC3 and DU145. Studies with methylation inhibitor 5-Aza2dC and histone deacetylase inhibitor Trichostatin A (TSA) inferred that the transcription initiation of RKIP expression was actively repressed in prostate cancer metastases.
The Snail superfamily of zinc finger transcription factors are essential for the induction of epithelial-to-mesenchymal transition (EMT) during embryogenesis. Abnormalities in the EMT program have been shown to drive cancer cell invasion and metastasis (Thiery, 2002). The process of EMT involves a dramatic phenotypic change including the loss of epithelial markers, the gain of mesenchymal markers, and changes in cell shape. One of the downstream effector targets of Snail leading to EMT is E-cad. Expression of E-cad is central in maintaining the cell-cell adhesion of epithelial cells. Snail directly binds the E-cad promoter to strongly repress transcription (Batlle, Sancho et al., 2000; Cano, Perez-Moreno et al., 2000). An inverse correlation between the expressions of E-cad and Snail has been observed in various cancers including prostate cancer. (Blanco, Moreno-Bueno et al., 2002; Jiao, Miyazaki et al., 2002; Poser, Dominguez et al., 2001; Yokoyama, Kamata et al., 2001). In this study, we presented compelling evidence implicating RKIP is another transcriptional target of Snail in advanced prostate cancer. We observed a statistically significant negative-correlation between the RKIP expression levels with that of transcription repressor Snail in metastatic prostate cancer samples. We observed that RKIP was highly expressed in the low metastatic cell line LNCaP, but reduced in the DU145 and PC3 cell lines. RKIP expression in these cell lines was reduced in a similar fashion to E-cad expression, and inversely related to Snail expression. We found that overexpressing or knocking down Snail could modulate RKIP expression, and that Snail could repress RKIP promoter activity in vitro.
The transcription factor Snail is characterized as having a conserved carboxy terminal region containing four to six C2H2-type zinc finger repeats (Knight & Shimeld, 2001) and an amino Snail/Gfi (SNAG) domain. While the fingers function as sequence specific DNA-binding motifs, the SNAG domain is the effector domain that enhances repressor activity in mammalian cells (Grimes, Chan et al., 1996; Nakayama, Scott et al., 1998). It has been shown that Snail represses transcription initiation by binding to the E-box cis-elements and recruiting chromatin remodeling mSin3A and histone deacetylases containing repressor complexes. By ChIP assay we showed that Snail was physically associated with the putative E-box 2 in RKIP promoter. We also showed that the presence of E-box 2 correlated with the repression of RKIP promoter by Snail in a transient reporter assay. However, at present we do not know whether the E-box 2 is required for the observed repression mediated by Snail. Nor do we know if the zinc fingers or the SNAG domain are essential for repression of RKIP expression.
In addition to E-cad, Snail has many other downstream effector targets. It has been shown that Snail could downregulate the expression of tight-junction components claudins and occludin, and epithelial marker mucin-1. It also increased the expression of the mesenchymal markers vimentin and fibronectin, proteins involved in cancer invasion such as metalloproteinase-2 and -9, and transcriptional factors ZEB-1 and LEF-1 (De Craene, van Roy et al., 2005; Nieto, 2002). The molecular mechanism of how Snail negatively regulates gene expression has been partially delineated and involves the direct binding of Snail to targeted gene promoter. In contrast, very little is known about how Snail activates gene expression. It appears that Snail activates by indirect mechanisms involving another mediators. For instance it has been shown that the induced expression of MMP-9 by Snail is dependent on Raf-MEK-Erk, and PI3K signaling pathways (Jorda, Olmeda et al., 2005). An increase in the binding of NF-κB to the MMP-9 promoter was also observed in Snail-expressing cells. In light of inhibitory functions of RKIP on Raf and NF-κB signaling pathways, it is possible that the observed activation of MMP-9 or other target genes by Snail is caused by the repression of RKIP. In addition to the regulation of genes encoding proteins important for cell-cell and cell-matrix interaction, Snail also plays an important role in promoting resistance to apoptosis and regulating cell cycle progression (Kajita, McClinic et al., 2004; Vega, Morales et al., 2004). The involved repertoire of Snail target genes has not yet been completely elucidated. Because of its demonstrated negative role in cell survival and proliferation, RKIP may represent a novel downstream effector of the Snail transcriptional axis important for the progression and metastasis of cancer.
ACKNOWLEDGEMENTS
We thank Stephen J Weiss and Mien-Chie Hung for plasmid constructs. We also thank Chinnaiyan's lab for help in studying the expression correlation of RKIP and Snail in prostate cancer. This work was supported by NIH grant to KCY (R01 GM64767), AD and WK are supported by Cancer Research UK, and a UTHSC Translational Research Stimulation Award, to KCY.
References
- Al-Mulla F, Hagan S, Behbehani AI, Bitar MS, George SS, Going JJ, Garcia JJ, Scott L, Fyfe N, Murray GI, Kolch W. Raf kinase inhibitor protein expression in a survival analysis of colorectal cancer patients. J Clin Oncol. 2006;24:5672–9. doi: 10.1200/JCO.2006.07.5499. [DOI] [PubMed] [Google Scholar]
- Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–9. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- Baylin SB, Herman JG. DNA hypermethylation in tumorigenesis: epigenetics joins genetics. Trends Genet. 2000;16:168–74. doi: 10.1016/s0168-9525(99)01971-x. [DOI] [PubMed] [Google Scholar]
- Blanco MJ, Moreno-Bueno G, Sarrio D, Locascio A, Cano A, Palacios J, Nieto MA. Correlation of Snail expression with histological grade and lymph node status in breast carcinomas. Oncogene. 2002;21:3241–6. doi: 10.1038/sj.onc.1205416. [DOI] [PubMed] [Google Scholar]
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- Chatterjee D, Bai Y, Wang Z, Beach S, Mott S, Roy R, Braastad C, Sun Y, Mukhopadhyay A, Aggarwal BB, Darnowski J, Pantazis P, Wyche J, Fu Z, Kitagwa Y, Keller ET, Sedivy JM, Yeung KC. RKIP sensitizes prostate and breast cancer cells to drug-induced apoptosis. J Biol Chem. 2004;279:17515–23. doi: 10.1074/jbc.M313816200. [DOI] [PubMed] [Google Scholar]
- De Craene B, van Roy F, Berx G. Unraveling signalling cascades for the Snail family of transcription factors. Cell Signal. 2005;17:535–47. doi: 10.1016/j.cellsig.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Dhanasekaran SM, Barrette TR, Ghosh D, Shah R, Varambally S, Kurachi K, Pienta KJ, Rubin MA, Chinnaiyan AM. Delineation of prognostic biomarkers in prostate cancer. Nature. 2001;412:822–6. doi: 10.1038/35090585. [DOI] [PubMed] [Google Scholar]
- Fu Z, Kitagawa Y, Shen R, Shah R, Mehra R, Rhodes D, Keller PJ, Mizokami A, Dunn R, Chinnaiyan AM, Yao Z, Keller ET. Metastasis suppressor gene Raf kinase inhibitor protein (RKIP) is a novel prognostic marker in prostate cancer. Prostate. 2005 doi: 10.1002/pros.20319. [DOI] [PubMed] [Google Scholar]
- Fu Z, Smith PC, Zhang L, Rubin MA, Dunn RL, Yao Z, Keller ET. Effects of raf kinase inhibitor protein expression on suppression of prostate cancer metastasis. J Natl Cancer Inst. 2003;95:878–89. doi: 10.1093/jnci/95.12.878. [DOI] [PubMed] [Google Scholar]
- Grimes HL, Chan TO, Zweidler-McKay PA, Tong B, Tsichlis PN. The Gfi-1 proto-oncoprotein contains a novel transcriptional repressor domain, SNAG, and inhibits G1 arrest induced by interleukin-2 withdrawal. Mol Cell Biol. 1996;16:6263–72. doi: 10.1128/mcb.16.11.6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegan S, Al-Mulla F, Mallon E, Oien KA, Ferrier R, Gusterson B, Garcia JJC, Kolch W. Reducton of Raf-1 kinase inhibitor protein (RKIP) expression in breast cancer metastases. Clinical cancer research. 2005 doi: 10.1158/1078-0432.CCR-05-0283. [DOI] [PubMed] [Google Scholar]
- Herman JG, Baylin SB. Promoter-region hypermethylation and gene silencing in human cancer. Curr Top Microbiol Immunol. 2000;249:35–54. doi: 10.1007/978-3-642-59696-4_3. [DOI] [PubMed] [Google Scholar]
- Jiao W, Miyazaki K, Kitajima Y. Inverse correlation between E-cadherin and Snail expression in hepatocellular carcinoma cell lines in vitro and in vivo. Br J Cancer. 2002;86:98–101. doi: 10.1038/sj.bjc.6600017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorda M, Olmeda D, Vinyals A, Valero E, Cubillo E, Llorens A, Cano A, Fabra A. Upregulation of MMP-9 in MDCK epithelial cell line in response to expression of the Snail transcription factor. J Cell Sci. 2005;118:3371–85. doi: 10.1242/jcs.02465. [DOI] [PubMed] [Google Scholar]
- Kajita M, McClinic KN, Wade PA. Aberrant expression of the transcription factors snail and slug alters the response to genotoxic stress. Mol Cell Biol. 2004;24:7559–66. doi: 10.1128/MCB.24.17.7559-7566.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight RD, Shimeld SM. Identification of conserved C2H2 zinc-finger gene families in the Bilateria. Genome Biol. 2001;2 doi: 10.1186/gb-2001-2-5-research0016. RESEARCH0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HC, Tian B, Sedivy JM, Wands JR, Kim M. Loss of raf kinase inhibitor protein promotes cell proliferation and migration of human hepatoma cells. Gastroenterology. 2006;131:1208–17. doi: 10.1053/j.gastro.2006.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H, Scott IC, Cross JC. The transition to endoreduplication in trophoblast giant cells is regulated by the mSNA zinc finger transcription factor. Dev Biol. 1998;199:150–63. doi: 10.1006/dbio.1998.8914. [DOI] [PubMed] [Google Scholar]
- Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002;3:155–66. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- Park S, Yeung ML, Beach S, Shields JM, Yeung KC. RKIP downregulates B-Raf kinase activity in melanoma cancer cells. Oncogene. 2005;24:3535–3540. doi: 10.1038/sj.onc.1208435. [DOI] [PubMed] [Google Scholar]
- Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–28. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- Poser I, Dominguez D, de Herreros AG, Varnai A, Buettner R, Bosserhoff AK. Loss of E-cadherin expression in melanoma cells involves up-regulation of the transcriptional repressor Snail. J Biol Chem. 2001;276:24661–6. doi: 10.1074/jbc.M011224200. [DOI] [PubMed] [Google Scholar]
- Schuierer MM, Bataille F, Hagan S, Kolch W, Bosserhoff AK. Reduction in Raf kinase inhibitor protein expression is associated with increased Ras-extracellular signal-regulated kinase signaling in melanoma cell lines. Cancer Res. 2004;64:5186–92. doi: 10.1158/0008-5472.CAN-03-3861. [DOI] [PubMed] [Google Scholar]
- Schuierer MM, Bataille F, Weiss TS, Hellerbrand C, Bosserhoff AK. Raf kinase inhibitor protein is downregulated in hepatocellular carcinoma. Oncol Rep. 2006;16:451–6. [PubMed] [Google Scholar]
- Soengas MS, Capodieci P, Polsky D, Mora J, Esteller M, Opitz-Araya X, McCombie R, Herman JG, Gerald WL, Lazebnik YA, Cordon-Cardo C, Lowe SW. Inactivation of the apoptosis effector Apaf-1 in malignant melanoma. Nature. 2001;409:207–11. doi: 10.1038/35051606. [DOI] [PubMed] [Google Scholar]
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–54. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- Vega S, Morales AV, Ocana OH, Valdes F, Fabregat I, Nieto MA. Snail blocks the cell cycle and confers resistance to cell death. Genes Dev. 2004;18:1131–43. doi: 10.1101/gad.294104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama K, Kamata N, Hayashi E, Hoteiya T, Ueda N, Fujimoto R, Nagayama M. Reverse correlation of E-cadherin and snail expression in oral squamous cell carcinoma cells in vitro. Oral Oncol. 2001;37:65–71. doi: 10.1016/s1368-8375(00)00059-2. [DOI] [PubMed] [Google Scholar]
- Zhang L, Fu Z, Binkley C, Giordano T, Burant CF, Logsdon CD, Simeone DM. Raf kinase inhibitory protein inhibits beta-cell proliferation. Surgery. 2004;136:708–15. doi: 10.1016/j.surg.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, Hung MC. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol. 2004;6:931–40. doi: 10.1038/ncb1173. [DOI] [PubMed] [Google Scholar]