Abstract
Ambient GABA modulates firing patterns in adult neural circuits by tonically activating extrasynaptic GABAA receptors. Here, we demonstrate that during a developmental period when activation of GABAA receptors causes membrane depolarization, tonic activation of GABAA receptors blocks all spontaneous activity recorded in retinal ganglion cells (RGCs) and starburst amacrine cells (SACs). Bath application of the GABAA receptor agonist muscimol blocked spontaneous correlated increases in intracellular calcium concentration and compound postsynaptic currents in RGCs associated with retinal waves. In addition, GABAA receptor agonists activated a tonic current in RGCs that significantly reduced their excitability. Using a transgenic mouse in which green fluorescent protein is expressed under the metabotropic glutamate receptor subtype 2 promoter to target recordings from SACs, we found that GABAA receptor agonists blocked compound postsynaptic currents and also activated a tonic current. GABAA receptor antagonists reduced the holding current in SACs but not RGCs, indicating that ambient levels of GABA tonically activate GABAA receptors in SACs. GABAA receptor antagonists did not block retinal waves but did alter the frequency and correlation structure of spontaneous RGC firing. Interestingly, the drug aminophylline, a general adenosine receptor antagonist used to block retinal waves, induced a tonic GABAA receptor antagonist-sensitive current in outside-out patches excised from RGCs, indicating that aminophylline exerts its action on retinal waves by direct activation of GABAA receptors. These findings have implications for how various neuroactive drugs and neurohormones known to modulate extrasynaptic GABAA receptors may influence spontaneous firing patterns that are critical for the establishment of adult neural circuits.
Keywords: spontaneous activity, retinal waves, retinal ganglion cell, starburst amacrine cell, aminophylline, adenosine receptor
Introduction
GABAA receptor-mediated signaling in neural networks can be divided into two modes. The first mode, termed “phasic,” corresponds to fast activation of synaptic GABAA receptors. The second mode, termed “tonic,” corresponds to persistent activation of high-affinity extrasynaptic GABAA receptors by ambient levels of GABA [for review, see Semyanov et al. (2004) and Farrant and Nusser (2005)]. Tonic activation of GABAA receptors is a powerful regulator of network excitability because it causes a persistent increase in the resting conductance of cells (Mitchell and Silver, 2003; Semyanov et al., 2003; Chadderton et al., 2004). Extrasynaptic GABAA receptors are modulated by alcohol, neurosteroids, and anesthetics and therefore represent a clinically relevant modulator of network activity (Farrant and Nusser, 2005; Mody, 2005).
The role of tonic GABAA receptor activation in developing neural circuits is much less well understood. At early stages of neural circuit development, intracellular chloride concentrations in neurons are high, and therefore activation of GABAA receptors leads to chloride efflux, which is depolarizing. The depolarizing action of GABA is a critical component of spontaneous periodic activity in the hippocampus, cortex, and spinal cord (Leinekugel et al., 1997; Garaschuk et al., 1998; O'Donovan, 1999; Ben-Ari, 2001) and is critical for several aspects of circuit development (Rivera et al., 1999; Ben-Ari, 2002; Payne et al., 2003; Ben-Ari et al., 2004; Tozuka et al., 2005). Tonic activation of GABAA receptors precedes phasic activation in early circuit development [for review, see Owens and Kriegstein (2002) and Represa and Ben-Ari (2005)] and may play a critical role in synaptogenesis during development (Demarque et al., 2002; Liu et al., 2006). In developing hippocampus, tonic activation of GABAA receptors increases the excitability of pyramidal cells (Marchionni et al., 2007), potentially contributing to network excitability at early stages of development.
Here, we explore the role of GABA signaling in controlling network excitability in the developing retina, which exhibits highly correlated spontaneous activity called retinal waves. Retinal waves are detected from 1 week before birth to 2 weeks after birth in mice and rats, and they play a critical role in the establishment of normal circuits throughout the developing visual system [for review, see Wong (1999), Torborg and Feller (2005), and Huberman (2007)]. In this study, we focus on retinal waves that occur between birth [postnatal day 0 (P0)] and P7 in mice and rats, which propagate through a network of interconnected cholinergic interneurons, the starburst amacrine cells (SACs) (Zheng et al., 2006). At these ages, retinal ganglion cells (RGCs) (Feller et al., 1996) and starburst amacrine cells (Syed et al., 2004b) receive barrages of GABAergic inputs, and activation of GABAA receptors is depolarizing in RGCs (Fischer et al., 1998; Stellwagen et al., 1999; Johnson et al., 2003; Zhang et al., 2006).
We demonstrate that tonic activation of GABAA receptors blocks retinal waves by reducing the excitability of starburst amacrine cells and RGCs. We also show that endogenous activation of GABAA receptors serves to decorrelate RGC firing. Last, we demonstrate that aminophylline, a drug we previously reported to block retinal waves by blocking adenosine receptors, acts via tonic activation of GABAA receptors.
Materials and Methods
Animals.
The University of California, San Diego Institutional Animal Care and Use Committee approved all procedures. Newborn rats (Sprague Dawley; P1–P7), mice (C57B6/J; P0–P7), and transgenic mice [C57BL/6J-TgN(grm2-IL2RA/GFP)1; P5–P7] in which the fusion protein interleukin 2α subunit (IL2RA)-green fluorescent protein (GFP) is expressed under the metabotropic glutamate receptor subtype 2 (mGluR2) promoter were used (Watanabe et al., 1998; Soda et al., 2003). For these studies, we do not take advantage of the IL2RA, which allows for immunotoxin-mediated selective ablation of starburst amacrine cells (Yoshida et al., 2001).
Retinal preparation.
Retinas were isolated as described previously (Bansal et al., 2000). Briefly, animals were anesthetized with halothane and decapitated. Retinas were isolated in artificial CSF (ACSF) containing the following (in mm): 119.0 NaCl, 26.2 NaHCO3, 11 glucose, 2.5 KCl, 1.0 K2HPO4, 2.5 CaCl2, and 1.3 MgCl2. Retinas were cut into thirds and mounted, ganglion cell side up, onto filter paper. These whole-mount preparations were kept at 32°C in ACSF or culture media bubbled with 95% O2/5% CO2 until use (1–8 h). During experiments, all preparations were superfused continuously with oxygenated ACSF warmed to 32–34°C.
Electrophysiology.
Whole-cell patch-clamp recordings were made from visualized RGCs (40× water-immersion objective; Olympus Optical, Melville, NY). Borosilicate glass pipettes (Garner Glass, Claremont, CA) were pulled (PP-830; Narishige, Tokyo, Japan) to a tip resistance of ∼5 MΩ when filled with a pipette solution containing the following (in mm): 98.3 K-gluconate (or KCl), 40 HEPES, 1.7 KCl, 0.6 EGTA, 5 MgCl2, 2 Na2ATP, and 0.3 Na-GTP; pH was adjusted to 7.25 with KOH. In Figure 5, either the pipette solution or ACSF was modified to yield a specific ion concentration. In these cases, equivalent amounts of KCl replaced K-gluconate in the pipette solution, and K+ replaced Na+ in ACSF, respectively. For whole-cell voltage clamp, the current responses to pharmacological manipulations were recorded at a holding potential of −60 mV or with other protocols as indicated in the figure legends. The holding potentials were off-line corrected for the liquid junction potential of each set of solutions (Neher, 1992). Data were filtered at 1 kHz and digitized at 5 kHz. For whole-cell current clamp, the membrane potential changes were monitored with no current injected unless otherwise indicated. In successful recordings, seals >1 GΩ were obtained in 30 s or less. The ratios of access resistance to input resistance were 5–15% before and after drug application. In some cases, voltage-clamped outside-out patches were excised from the somas of RGCs. Gigaohm seals were obtained by moving the pipette away from the soma immediately after break-in. Recordings were made using Axopatch 200B or Multiclamp 700A patch-clamp amplifiers, and data were acquired to and analyzed on a Pentium-based PC using PClamp software (Molecular Devices, Sunnyvale, CA).
Figure 5.
Aminophylline blocks retinal waves by tonic activation of GABAA receptors. A, Time course of fractional change in fura-2 fluorescence (ΔF/F) in rat retinas in control (top), aminophylline (500 μm; middle), and a combination of aminophylline and the GABAA receptor antagonist gabazine (5 μm; bottom). B, Representative whole-cell voltage-clamp recording from rat RGC in same conditions as A. C, Current responses to pulsed application (200 ms) of aminophylline (500 μm; gray bar) to an outside-out patch excised from rat RGC in absence (top) and presence (bottom) of gabazine (5 μm). D, Representative continuous whole-cell voltage-clamp recording from mouse SAC during application of aminophylline (500 μm). Population data for B and D are summarized in Tables 1 and 2. AMPH, Aminophylline; GBZ, gabazine.
Imaging.
Ca2+ imaging was performed with a video-based intensified SIT camera system as described previously (Bansal et al., 2000). The Ca2+ indicator fura-2 AM (Invitrogen, Eugene, OR) was loaded by a standard protocol (Bansal et al., 2000; Colicos et al., 2004) for 2–8 h before the imaging experiments.
Pharmacology.
All pharmacological agents were purchased from Tocris Biosciences (Ellisville, MO) or Sigma-Aldrich (St. Louis, MO). Aminophylline was dissolved directly in ACSF at its working concentrations; all other drugs were prepared as concentrated stock solutions. Stocks were stored at −20°C and diluted at or above 1:1000 in ACSF on the day of the experiment.
Pulsed applications of aminophylline-containing solutions were delivered through a glass pipette of ∼2 μm tip diameter The pipette was positioned <20 μm from outside-out patches, and the puffing solution was delivered with an ejected pressure of ∼10 psi using a PV830 Pneumatic PicoPump (World Precision Instruments, Sarasota, FL). To maintain a constant pH value in the puffing solution, aminophylline was previously dissolved in an external solution containing the following (in mm): 5 KCl, 123 NaCl, 3 CaCl2, 2 MgCl2, 10 glucose, and 10 HEPES, pH 7.3 with NaOH. Pulsed application of the control external solution did not induce any detectable currents.
Nonstationary noise analysis.
Peak-scaled nonstationary noise analysis was used to estimate the conductance of extrasynaptic GABAA receptors recorded in outside-out patches excised from RGCs. Methods similar to those used for synaptic GABAA receptor responses were used (De Koninck and Mody, 1994). The average binned variance (ς2) was plotted against the amplitude of the current (I) and fit with ς2 = b + iI − I2/N to give estimates of single-channel current (i), the number of channels open at the peak (N), and baseline variance (b).
Immunofluorescence.
We conducted immunofluorescence experiments to determine the expression pattern of GFP in transgenic mice expressing GFP under the mGluR2 promoter (see Fig. 3A). Eyeballs prepared from P7 mice were fixed overnight in 4% paraformaldehyde in PBS at 4°C. Eyecups were cryoprotected in 30% sucrose with 0.1% sodium azide at 4°C, frozen in optimal cutting temperature compound (Ted Pella, Redding, CA), and cut into 30 μm sections with a cryostat. Sections were washed in 1× PBS, blocked for nonspecific binding with 2% normal donkey serum, 2% BSA, and 0.3% Triton X-100 in 1× PBS for 1 h at room temperature, and then incubated overnight with the primary antibody against choline acetyltransferase (1:200; AB144P; Millipore, Billerica, MA). Sections were then incubated for 1 h in block solution at room temperature with the appropriate affinity-purified secondary antibody conjugated to Alexa 488 (1:500; Invitrogen). To measure nonspecific binding of the secondary antibody, the primary antibody was omitted. After processing, sections were rinsed in PBS and mounted in Vectashield (Vector Laboratories, Burlingame, CA). Images were acquired with a CCD camera (Optronics, Goleta, CA) attached to an upright microscope (Axioskop 2; Zeiss, Thornwood, NY) with a 10× objective (numerical aperture, 0.45). Digital images were processed in Adobe Photoshop (Adobe Systems, San Jose, CA) to enhance color and contrast.
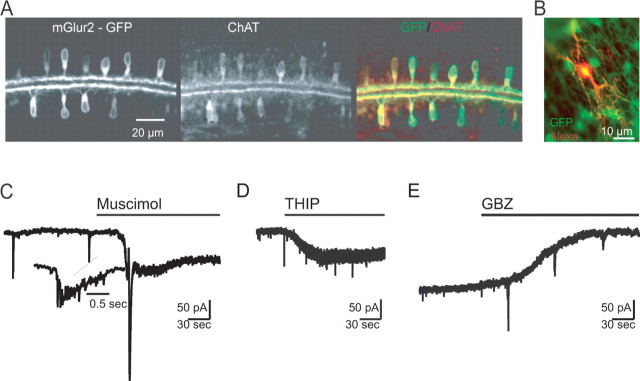
Figure 3.
Tonic activation of GABAA receptors in starburst amacrine cells. A, Immunofluorescence labeling of choline acetyltransferase (ChAT) in developing transgenic mice expressing GFP under the mGluR2 promoter confirms that GFP expression was localized to starburst amacrine cells during stage II waves. B, GFP-positive SACs in live retinal whole mount allow for targeted recording from starburst amacrine cells. The red cell was filled with Alexa 568 via a whole-cell pipette. C, Representative whole-cell voltage-clamp recording from SAC during application of muscimol (25 μm) with the time of application indicated by the black horizontal bar. Inset, Expanded timescale of a wave-associated postsynaptic current. D, Representative whole-cell voltage-clamp recording from SAC during application of THIP (10 μm) with the time of application indicated by the black horizontal bar. THIP induces a tonic conductance change in SACs. E, Representative whole-cell voltage-clamp recording from a starburst amacrine cell during application of gabazine (GBZ; 5 μm). GBZ leads to a significant shift in holding current and a reduction in the resting conductance. Population data for C–E are summarized in Table 2.
Multielectrode array recording.
After enucleation, the eyes were transferred to buffered Ames medium. The lens and vitreous were removed from the eyecup, and the retinal pigment epithelium was detached from the retina. The isolated retinas were placed ganglion cell side down onto a flat, hexagonal array of 61 extracellular electrodes spaced 60 μm apart from each other, with a total diameter of 480 μm (Litke et al., 2003). While on the array, the retinas were superfused with Ames solution bubbled with 95% O2 and 5% CO2 and maintained at 35°C, pH 7.4. Voltage traces from the individual electrodes were bandpass filtered from 80 Hz to 2 kHz, digitized with a temporal resolution of 0.05 ms (Meister et al., 1994), and then stored for off-line analysis. Spikes were segregated into single units using a semiautomated procedure based on principal component analysis of spike waveforms [modified for 61 electrodes from Litke et al. (2004)], and the presence of a refractory period was verified in the spike trains from each unit. Spikes recorded on multiple electrodes were identified by temporal coincidence; only spikes from the electrode with the most clearly defined cluster were analyzed further.
We computed several measures of spiking properties for each single unit recorded as well as for the whole electrode. The average firing rate was calculated by summing the total number of spikes for each 30 min recording and then dividing by the length of the recording. The firing rate as a function of time was computed by counting the spikes in successive time bins and dividing these counts by the bin width. The duration of bursts (defined as a minimum of three spikes firing at 2 Hz) and the interburst interval were computed by averaging over each 30 min recording for an individual electrode or a single unit. The correlation index was calculated as described previously (Wong et al., 1993; Torborg et al., 2005). Briefly, the correlation index measures the factor by which the firing rate of cell B (or the firing rate recorded on electrode B) increases over its mean value within ±100 ms of a spike from a reference cell (or a reference electrode) A: Correlation index = [NAB(−0.1 s, +0.1 s) × T]/[NA(0,T) × NB(0,T) × (0.2 s)], where NAB(−0.1 s, +0.1 s) is the number of spike pairs from cells A and B that are separated by no more than ±100 ms, T is the total recording time, NA(0,T) is the total number of spikes in cell A, and NB(0,T) is the total number of spikes in cell B. Cells were considered to be at the position of the electrode on which they had been recorded.
Statistics.
Differences between means of different groups were evaluated for statistical significance with the Student's test for two groups. For imaging experiments (see Fig. 1Aii,C), statistical significance was assayed with a one-way ANOVA followed by the Newman–Keuls post hoc test to compare each condition to control. Throughout the figures, significance is represented with asterisks with the following notation: *p < 0.05; **p < 0.01; ***p < 0.001.
Figure 1.
GABAA receptor agonists block waves and induce a tonic current in both mice and rats. Ai, Time course of fractional change in fura-2 fluorescence (ΔF/F) associated with waves averaged over a 200 μm2 area before and during bath application of muscimol (25–100 μm). The example shown is from a rat retina, but identical results were found in mice. Inset, Example of transient decrease in fluorescence associated with muscimol wash-in (25 μm). The trace is 100 s long. The bar indicates time of muscimol application. The downward deflection preceding muscimol application is associated with a retinal wave. This transient varied in amplitude and duration across preparations. Aii, Retinal wave frequency as a function of muscimol concentration in mouse retinas. Open circles are averages across all retinas for each concentration. Colored dots are individual retinas; each retina is represented by a single color. Overlapping dots have been offset for clarity. The solid horizontal line represents average wave frequency in control across all retinas, and dotted lines represent SEM for all control retinas. Error bars represent SE. Asterisks denote a significant difference from control. n = 4 retinas for 1 nm; n = 4 for 5 nm; n = 6 for 10 nm; n = 6 for 50 nm; n = 7 for 100 nm; n = 8 for 500 nm; n = 2 for 1 μm. Bi, Representative whole-cell voltage-clamp recording from a rat retinal ganglion cell. The marks (#) show the compound postsynaptic currents associated with retinal waves. Occasional miniature postsynaptic currents occurred in between the waves. The bar shows the time course of bath application of muscimol (100 μm). Bii, Gaussian fits to the all-point histograms from 30 s of baseline current in control and after muscimol application for recording in Bi. Results for all recordings in rats are summarized in Table 1, and recordings from mice are summarized in Table 2. C, Retinal wave frequency as a function of exogenous GABA application in rat retinas. All symbols are as in Aii. n = 3 retinas for 10 nm; n = 3 for 50 nm; n = 4 for 100 nm; n = 3 for 500 nm; n = 4 for 1 μm; n = 6 for 5 μm; n = 4 for 10 μm.
Results
Tonic activation of GABAA receptors blocked spontaneous activity in RGCs and induced a shunting conductance
We first investigated whether tonic activation of GABAA receptors modulated retinal waves as assayed by imaging of the fluorescent calcium indicator fura-2 AM (Fig. 1A) in both rats and mice. We found that bath application of the GABAA receptor agonist muscimol (25–100 μm) completely blocked spontaneous calcium transients (Fig. 1Ai) (n = 7 retinas). To determine the effect of different levels of GABAA receptor activation on retinal waves, we performed calcium imaging while applying different concentrations of muscimol. Wave frequency decreased as the concentration of muscimol increased. A significant decrease in frequency was observed with application of 10 nm muscimol, and waves were completely blocked by 500 nm muscimol (Fig. 1Aii).
Why does tonic activation of GABAA receptors block retinal waves? The reversal potential of GABAA receptor-mediated chloride currents in RGCs is estimated to be −40 mV at P3 and hyperpolarizes to −60 mV by P7 (Zhang et al., 2006). Hence, at ages younger than P6, activation of GABAA receptors causes a chloride efflux that depolarizes retinal neurons (Zhang et al., 2006). Indeed, in contrast to what we have observed in the retina, in developing hippocampus tonic activation of GABAA receptors increases the excitability of the network (Marchionni et al., 2007). Consistent with this depolarizing action, we found that bath application of muscimol caused a variable transient decrease in baseline fluorescence corresponding to an increase in intracellular calcium concentration, suggesting that activation of GABAA receptors led to a tonic depolarization of retinal neurons (example trace shown in Fig. 1Ai, inset).
One possible reason that GABA agonists block retinal waves is that tonic activation of GABAA receptors activates a resting conductance that reduces the excitability of retinal neurons by acting as a shunt. To test this hypothesis, we conducted whole-cell voltage-clamp experiments from RGCs (Fig. 1B). The pipette solution for these recordings contained a high concentration of KCl (ECl = −4 mV), so that GABAA receptor-mediated currents at −60 mV were inward. Bath application of muscimol blocked the compound postsynaptic currents (PSCs) associated with retinal waves, confirming the imaging results. In addition, muscimol activated a tonic current, which we measured as the change in baseline holding current (Fig. 1Bi). Within the first 30 s after muscimol application, there was a large transient increase in holding current that became a sustained inward current. Amplitude distributions of sustained holding current were fit by Gaussians (Fig. 1Bii) to yield the mean amplitude of muscimol-induced tonic current (for summary data, see Tables 1, 2). This increase in the resting conductance was accompanied by an increase in the current variance, consistent with an increase in the number of open GABAA receptor channels (Kaneda et al., 1995; Brickley et al., 1996). Note that we found no significant difference between the magnitudes of tonic currents in rat and mouse RGCs.
Table 1.
Pharmacological effects: rat RGC
| Muscimol (n = 6) | Gabazine (n = 7) | Aminophylline (n = 7) | Aminophylline plus gabazine (n = 6) | |
|---|---|---|---|---|
| Δ Holding current (pA) | −176 ± 123* | −2.3 ± 7 | −104 ± 44* | −22.4 ± 25.2 |
| Noise (% of control) | 231 ± 130* | 100 ± 33 | 370 ± 200* | 140 ± 300 (p = 0.26) |
| Resting conductance (% of control) | 435 ± 126* | 106 ± 33.5 | 279 ± 82* | 119 ± 31 |
Summary of changes in holding current, RMS noise, and resting conductance in rat RGCs under different conditions. Measurements were conducted using whole-cell voltage clamp recording with Vh = −60 mV and using an internal solution with a calculated ECl = −4 mV. Changes in resting conductance and noise are represented as the percentage change of the resting conductance in pharmacological agent compared to control. All data shown as mean ± SD. Asterisks indicate statistical significance (p < 0.05).
Table 2.
Pharmacological effects: mouse
| THIP | Gabazine | Aminophylline | Aminophylline plus gabazine | |
|---|---|---|---|---|
| RGC | (n = 6) | (n = 5) | (n = 8) | (n = 7) |
| Δ Holding current (pA) | −3.1 ± 7.5 | −0.5 ± 4.5 | −96.6 ± 35* | −26.6 ± 29.2 |
| Resting conductance (% of control) | 97.3 ± 29.5 | 83 ± 26 | 230 ± 86* | 138.9 ± 39* |
| SAC | (n = 9) | (n = 8) | (n = 9) | (n = 6) |
| Δ Holding current (pA) | −45.4 ± 34* | 48.7 ± 45* | −129.6 ± 84* | −8.8 ± 21.7 |
| Resting conductance (% of control) | 127.5 ± 74* | 108 ± 48* | 273 ± 120* | 99.5 ± 26 |
Summary of changes in holding current and resting conductance in mouse RGCs and SACs using the same conditions as Table 1. All data are shown as mean ± SD. Asterisks indicate statistical significance (p < 0.05).
To estimate the concentration of ambient GABA capable of modulating wave frequency, we bath applied varying concentrations of GABA (Fig. 1C). Wave frequency decreased as the concentration of exogenous GABA increased, with a significant decrease from control observed at 500 nm and a complete blockade of retinal waves obtained with 10 μm GABA. These results indicate that tonic activation of GABA receptors modulates wave frequency.
To determine how tonic inhibition affects the excitability of RGCs, we counted the number of action potentials fired by RGCs in response to depolarizing current steps of increasing amplitude in the presence and absence of muscimol (Fig. 2A). Muscimol (100 μm) decreased the firing rate at all amplitudes of injected current compared with control (Fig. 2B), suggesting that tonic activation of GABAA receptors reduces the excitability of developing rat RGCs.
Figure 2.
Tonic activation of GABAA receptors reduces RGC excitability. A, Whole-cell current-clamp recordings from rat RGCs in response to depolarizing current steps in the absence and presence of muscimol (100 μm). Stepwise current pulses 250 ms in duration were delivered via a patch pipette to depolarize RGCs. The amplitudes of the current injected in this example were 5, 15, and 25 pA (control) and 80, 120, and 160 pA (muscimol). B, Summary of the relationships between firing rate and injected current steps. Squares, Control; circles, muscimol. n = 5–7 RGCs. Error bars represent SD. *p < 0.05; Student's t test for control versus muscimol. C, Summary of voltage responses to depolarizing current steps in the absence and presence of muscimol. Vm represents the membrane potential at the end of the current step. The slope represents the reciprocal of conductance. Error bars represent SD. *p < 0.05; **p < 0.01; ***p < 0.001; Student's t test for control versus muscimol.
Increasing the resting conductance (i.e., decreasing the input resistance) produces a shunt, which is predicted to cause a slope change in the relationship between the input current and the membrane potential. However, several studies, both theoretical and experimental, have indicated that for several classes of neurons an increase in the resting inhibitory conductance causes a linear shift rather than a slope change in the current–voltage relationship (Brickley et al., 1996; Chance et al., 2002). To determine the effects of tonic inhibitory conductance on RGCs, we plotted membrane potential versus the injected current in control conditions and in the presence of muscimol. We found that activation of a tonic GABAA receptor-mediated conductance significantly altered the current–voltage relationship (Fig. 2C). These findings suggest that the large GABAA receptor-induced conductance change observed in RGCs dramatically reduces the excitability of RGCs by shunting excitatory conductances. This shunt is likely to clamp the membrane at the depolarized reversal potential of GABAA receptors, which is estimated to be −40 mV at this age (Zhang et al., 2006), and therefore may also reduce excitability by inactivating voltage-gated sodium channels.
Endogenous tonic activation of GABAA receptors on SACs but not RGCs
We have demonstrated that tonic activation of GABAA receptors blocks all compound PSCs recorded in RGCs (Fig. 1Bi), indicating that GABAA agonists must also be functioning presynaptic to RGCs. At this stage of development, RGCs receive cholinergic compound PSCs during retinal waves (Feller et al., 1996; Zhou, 1998). Only one class of cells in the retina, the starburst amacrine cell, releases acetylcholine (ACh) (Zhou, 2001). Starburst amacrine cells undergo spontaneous depolarizations (Zheng et al., 2006) and release both ACh and GABA onto neighboring starburst amacrine cells and onto RGCs during retinal waves (Syed et al., 2004b; Zheng et al., 2006).
To determine the effect of GABAA agonists on the cells presynaptic to RGCs, we recorded from SACs. To identify SACs, we used a transgenic mouse in which the expression of a fusion protein of the human interleukin 2α subunit and GFP is driven by the mGluR2 promoter (Watanabe et al., 1998; Soda et al., 2003). Previously, this transgenic mouse line has been used to target an immunotoxin to starburst amacrine cells (Yoshida et al., 2001). Early postnatal mGluR2-GFP mice exhibited GFP expression uniquely in starburst amacrine cells, enabling targeted recording from these cells (Fig. 3A,B).
Whole-cell voltage-clamp recordings from SACs revealed wave-associated compound PSCs, as has been previously reported in ferret (Butts et al., 1999) and rabbit (Zhou, 1998; Zheng et al., 2006) (Fig. 3C). Bath application of muscimol induced a significant shift in holding current, an increase in noise, and a dramatic change in conductance, similar to those observed in RGCs (Fig. 3C).
SACs express the δ subunit of GABAA receptors (Greferath et al., 1993), a subunit found in the extrasynaptic GABAA receptors that mediate tonic inhibition in cerebellum and hippocampus (Mody, 2001; Farrant and Nusser, 2005). To determine whether δ-containing GABAA receptors mediate tonic inhibition in SACs, we bath applied 4,5,6,7-tetrahydroisoxazolo[5,4-c]-pyridin-3-ol (THIP; 10 μm), a GABAA receptor agonist that preferentially activates δ subunit-containing GABAA receptors. THIP induced a tonic current in SACs but not in RGCs (Fig. 3D, Table 2), suggesting a fundamental difference in GABAA receptor composition underlying tonic GABAA receptor-mediated conductances in SACs and RGCs. In contrast to exogenous GABA and muscimol application, which induced both a transient and sustained current, THIP only induced a sustained current, suggesting that SACs may have two distinct types of GABAA receptors.
Retinal waves are modulated by submicromolar concentrations of GABA (Fig. 1C), comparable with the levels of ambient GABA found in the brain, which are estimated to be ∼1 μm (Jensen et al., 2003). (To our knowledge, the ambient level of GABA in the retina is not known.) To determine whether endogenous activation of GABAA receptors modulated spontaneous firing patterns in the acutely isolated retina, we monitored the effects of the GABAA receptor antagonist gabazine on several features of spontaneous activity. We found no effects on the holding current, resting conductance (Tables 1, 2), or excitability of RGCs (data not shown). However, bath application of gabazine did reduce the input conductance of SACs (Fig. 3E, Table 2), suggesting that ambient levels of GABA activate GABAA receptors on SACs, thereby modulating SAC excitability.
Endogenous activation of GABAA receptors alters correlation structure of spontaneous firing patterns
GABAA receptor antagonists do not block retinal waves, nor do they alter the global spatial and temporal properties of waves as assayed with calcium imaging [but see Fischer et al. (1998), Stellwagen et al. (1999), Catsicas and Mobbs (2001), Sernagor et al. (2003), and Syed et al. (2004b)]. To determine the effects of endogenous GABA signaling on the detailed spontaneous firing properties of RGCs, we used a multielectrode array, which allowed us to record extracellularly from many RGCs simultaneously (Meister et al., 1991; Wong et al., 1993; McLaughlin et al., 2003). At P4–P6, retinal neurons fire spontaneous periodic bursts of action potentials that are correlated with action potentials from neighboring cells. We observed similar correlated increases in firing rate in control solutions and in the presence of gabazine, indicating that retinal waves persist when GABAA signaling is blocked (Fig. 4Ai). The smaller-size peaks in the absence of gabazine reflect a smaller fraction of electrodes on which spikes were recorded compared with the larger peaks (Fig. 4Aii), suggesting the presence of waves in control conditions that extend over a smaller part of the array. We found a significant increase in the spatial correlation of spontaneous firing of pairs of RGCs at all distances in the presence of gabazine as assayed by the correlation index (Fig. 4B, Tables 3, 4). In addition, we found a significant increase in the interburst interval (control, 46.57 ± 7.22 s; gabazine, 74.77 ± 15.37 s; p < 0.001; n = 145 and 150 electrodes, from 5 retinas) and a small but significant increase in burst duration (control, 3.60 ± 0.98; gabazine, 5.03 ± 1.80; p < 0.001). Furthermore, an increase in interburst interval measured on a given electrode tended to be correlated with an increase of burst duration on the same electrode (Fig. 4C). We found no difference in the average firing rate or the mean firing rate during bursts (data not shown). Similar results were obtained using isolated single units instead of whole electrodes (data not shown).
Figure 4.
Blockade of endogenous GABAA receptor activity alters the structure of spontaneous firing patterns. Ai, Multielectrode array recordings from a P5 mouse retina in the absence (left) and presence (right) of gabazine. The firing rate of all electrodes was binned into 1 s intervals. Aii, The number of active electrodes (out of 61) for the recordings shown in Ai. An electrode was considered active if its firing rate in a given bin exceeded the average firing rate recorded on the electrode over the course of 30 min by more than two SDs. Bi, Correlation index computed for pairs of spike trains and plotted as a function of the distance between electrodes on which the spikes were recorded. Data are from one P5 mouse retina in the absence and presence of gabazine (5 μm). Error bars are omitted for better readability; the values of SD are summarized in Table 3. Bii, Summary plots combine whole-electrode data from five P5–P6 mice and indicate a significant increase in correlation index at all distances. Error bars represent SD. The statistics summary for these data is included in Table 4. C, Summary of the changes in the interburst interval and burst duration measured on 139 individual electrodes (five mouse retinas) under control conditions and in gabazine. Each data point corresponds to a single electrode, with the ratio of burst duration in gabazine to burst duration in control plotted along the x-axis, and the ratio of interburst intervals plotted along the y-axis. The ratios were computed for the values of interburst interval and burst duration averaged over the course of a 30 min recording. GBZ, Gabazine.
Table 3.
Summary of the correlation index under control conditions, in gabazine, and after washout for the example retina in Figure 4Bi
| Distance (μm) | Correlation index |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Control |
Gabazine |
Washout |
|||||||
| n | Mean | SD | n | Mean | SD | n | Mean | SD | |
| 60 | 37 | 19.64 | 4.35 | 36 | 38.90 | 10.94 | 28 | 21.20 | 5.23 |
| 104 | 31 | 16.63 | 3.79 | 30 | 32.67 | 8.20 | 27 | 18.29 | 4.54 |
| 120 | 28 | 15.85 | 2.83 | 25 | 31.90 | 8.90 | 20 | 17.04 | 2.89 |
| 159 | 53 | 13.50 | 2.49 | 50 | 28.17 | 9.04 | 43 | 15.06 | 3.19 |
| 180 | 23 | 12.50 | 2.41 | 20 | 25.10 | 4.69 | 21 | 13.35 | 2.69 |
| 208 | 18 | 11.00 | 1.75 | 16 | 23.97 | 5.66 | 14 | 12.62 | 2.76 |
| 216 | 38 | 11.10 | 1.47 | 32 | 23.96 | 6.20 | 28 | 12.27 | 2.15 |
| 240 | 20 | 9.80 | 2.00 | 17 | 22.64 | 4.37 | 16 | 10.62 | 2.95 |
| 262 | 25 | 8.99 | 1.33 | 22 | 20.89 | 5.98 | 20 | 10.02 | 2.08 |
| 275 | 24 | 8.37 | 1.89 | 23 | 19.90 | 4.51 | 17 | 9.30 | 3.15 |
| 300 | 9 | 8.00 | 1.45 | 7 | 17.23 | 2.96 | 7 | 7.62 | 1.87 |
| 312 | 11 | 7.83 | 1.22 | 10 | 20.39 | 6.33 | 9 | 8.26 | 1.82 |
| 317 | 13 | 6.89 | 1.52 | 12 | 16.90 | 4.57 | 11 | 9.10 | 1.96 |
| 334 | 14 | 6.74 | 1.24 | 11 | 16.77 | 4.35 | 10 | 7.62 | 1.46 |
| 360 | 4 | 6.88 | 0.89 | 5 | 16.39 | 2.88 | 5 | 6.97 | 1.65 |
| 365 | 11 | 7.15 | 1.19 | 12 | 17.65 | 5.55 | 8 | 7.79 | 0.57 |
| 375 | 7 | 6.36 | 0.70 | 7 | 16.37 | 6.45 | 5 | 6.95 | 2.00 |
| 393 | 7 | 6.31 | 0.56 | 6 | 16.10 | 2.53 | 4 | 7.77 | 1.23 |
| 416 | 1 | 6.41 | — | 2 | 16.62 | 4.39 | 2 | 8.73 | 0.51 |
| 420 | 3 | 6.52 | 0.16 | 4 | 15.82 | 3.90 | 2 | 7.47 | 0.33 |
| 433 | 1 | 5.09 | — | 2 | 17.95 | 4.63 | 1 | 5.93 | — |
| 453 | — | — | — | 2 | 15.08 | 2.23 | 2 | 4.89 | 0.47 |
For each interelectrode distance, the value of n refers to the number of electrode pairs separated by this distance. The set of distances is determined by the geometry of the multielectrode array.
Table 4.
Correlation index under control conditions and in gabazine, averaged over five retinas
| Distance (μm) | Correlation index |
p value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control |
Gabazine |
|||||||||
| n | Mean | SD | n | Mean | SD | |||||
| 60 | 196 | 16.85 | 3.00 | 222 | 30.99 | 8.45 | 1.26 × 10−72 | |||
| 104 | 167 | 14.31 | 2.71 | 194 | 27.71 | 6.54 | 2.62 × 10−79 | |||
| 120 | 153 | 13.32 | 2.65 | 172 | 26.61 | 6.47 | 1.49 × 10−72 | |||
| 159 | 267 | 11.41 | 2.50 | 295 | 23.96 | 6.35 | 1.16 × 10−119 | |||
| 180 | 119 | 10.51 | 2.38 | 129 | 22.41 | 4.96 | 1.57 × 10−65 | |||
| 208 | 99 | 8.84 | 2.16 | 113 | 20.74 | 4.87 | 1.49 × 10−57 | |||
| 216 | 194 | 8.91 | 2.24 | 215 | 20.41 | 5.07 | 2.02 × 10−101 | |||
| 240 | 96 | 8.08 | 2.35 | 107 | 19.71 | 4.96 | 2.73 × 10−52 | |||
| 262 | 159 | 7.12 | 1.85 | 174 | 18.55 | 4.91 | 9.09 × 10−88 | |||
| 275 | 149 | 6.75 | 2.17 | 163 | 17.98 | 4.62 | 1.58 × 10−83 | |||
| 300 | 63 | 5.83 | 1.96 | 64 | 16.50 | 3.83 | 6.19 × 10−40 | |||
| 312 | 72 | 5.71 | 1.86 | 77 | 17.10 | 4.34 | 6.19 × 10−45 | |||
| 317 | 104 | 5.53 | 1.86 | 115 | 16.47 | 4.14 | 4.72 × 10−65 | |||
| 334 | 96 | 5.11 | 1.89 | 104 | 15.87 | 3.81 | 5.51 × 10−63 | |||
| 360 | 27 | 4.88 | 2.059 | 32 | 14.41 | 3.88 | 2.63 × 10−16 | |||
| 365 | 68 | 4.71 | 1.58 | 72 | 15.67 | 4.59 | 1.86 × 10−39 | |||
| 375 | 61 | 4.35 | 1.55 | 63 | 15.18 | 4.46 | 8.05 × 10−36 | |||
| 393 | 48 | 4.07 | 1.65 | 46 | 14.27 | 3.35 | 3.49 × 10−33 | |||
| 416 | 16 | 3.97 | 1.26 | 18 | 15.19 | 3.21 | 3.04 × 10−14 | |||
| 420 | 38 | 3.74 | 1.45 | 44 | 15.51 | 4.46 | 9.34 × 10−26 | |||
| 433 | 15 | 3.59 | 1.41 | 16 | 15.10 | 3.81 | 1.06 × 10−11 | |||
| 453 | 7 | 2.51 | 0.66 | 10 | 12.35 | 3.12 | 9.51 × 10−07 | |||
| 480 | 1 | 1.82 | — | 1 | 8.26 | — | — | |||
For each interelectrode distance, the value of n refers to the number of electrode pairs separated by this distance. The set of distances is determined by the geometry of the multielectrode array. These data are summarized in Figure 4Bii.
From these results, we conclude that activation of GABAA receptors modulates both spatial and temporal properties of retinal waves. Because gabazine blocks both extrasynaptic and synaptic GABAA receptors, these experiments do not distinguish between the effects of activation of GABAA receptors by ambient GABA and synaptically released GABA.
Aminophylline blocks retinal waves by direct activation of GABAA receptors and not by blockade of adenosine receptors
Previously, we and others have reported that bath application of aminophylline blocks retinal waves (Singer et al., 2001; Syed et al., 2004b). The primary action of aminophylline in the nervous system is as an adenosine receptor antagonist (Gulati et al., 2005). Therefore, we concluded from these experiments that adenosine secretion played a critical role in retinal wave generation (Stellwagen et al., 1999; Singer et al., 2001; Syed et al., 2004b). Here, we report that aminophylline blocks retinal waves not by blockade of adenosine receptors, but rather by direct activation of GABAA receptors.
We conducted several experiments that implicate aminophylline as a GABAA receptor agonist. First, using calcium imaging, we found that the blockade of retinal waves by aminophylline (500 μm) was prevented by bath application of the GABAA receptor antagonist gabazine (5 μm) (Fig. 5A) (wave frequency, 0.69 ± 0.2 min−1 in control and 0.58 ± 0.1 min−1 in the presence of aminophylline and gabazine; n = 6; p = 0.55). Second, aminophylline reproduced all of the changes observed in muscimol, including the resting conductance change, increased holding current, increased noise, compound PSC blockade in both RGCs (compare Figs. 5B, 1B) and SACs (compare Figs. 5D, 3C), as well as the reduction in RGC excitability described in Figure 2 (data not shown). The effects of aminophylline on the resting conductance of SACs and RGCs are summarized in Tables 1 and 2. Third, short application of aminophylline (puff duration, 200 ms) induced a gabazine-sensitive inward current in outside-out patches excised from RGC somas (Fig. 5C) (peak current, −36 ± 16 pA; n = 7). Nonstationary noise analysis was used to estimate that a short application of aminophylline activated channels with a mean single-channel conductance of 4.4 ± 0.5 pS (n = 4), consistent with activation of a GABAA receptor-mediated conductance. Fourth, by conducting ion substitution experiments, we confirmed that the increase in resting conductance was mediated by a chloride- but not by a potassium-permeable channel (Fig. 6A) or a sodium-permeable channel (data not shown). Together, these results demonstrate for the first time that aminophylline at high concentration serves as a direct agonist for GABAA receptors.
Figure 6.
Aminophylline effects are not reproduced by specific adenosine receptor antagonists. A, Aminophylline induced a chloride conductance in rat RGCs. Ai, Current–voltage relationships acquired from voltage ramp (protocol shown in the inset). To avoid action potential firing and waves occurring during voltage-ramp experiments, lidocaine (1 mm N-ethyl bromide quaternary salt) was added to the pipette solution to block sodium channels, and the nicotinic ACh receptor antagonist dihydro-β-erythroidine (10 μm) was added to the ACSF to block waves. Current responses in control (black) and in the presence of aminophylline (gray) are shown. The net current (dotted line) is computed as the difference between control and aminophylline. [Cl−]internal = 11.7 mm for this example. Aii, Top, The average reversal potential (Erev) of the net aminophylline (AMPH)-induced current as a function of internal [Cl−] (left) and external [K+] (right). The reversal potential changed with an increase in internal [Cl−]. Solid lines, Left, Fit to the Nernst equation ECl = (RT/F) × ln(internal [Cl−]/external [Cl−]) at 30°C; right, Nernst potential for chloride. This indicates that the reversal potential of the aminophylline-induced conductance did not change with changing extracellular potassium concentration (n = 4–5 per point). Bottom, The resting potential of the cell (RP) as a function of internal [Cl−] (left) and external [K+] (right). Solid lines, Left, Resting potential, which did not change with changing intracellular chloride concentration; right, fit to the Nernst equation EK = (RT/F) × ln(external [K+]/internal [K+]) at 22°C (n = 3–6). Hence, the resting potential was set predominantly by potassium. B, Summary of input conductance changes as a function of changing aminophylline concentration in rat retina. Gray bars indicate currents reversed at ECl; white bars indicate currents reversed at EK. n = 3–5. One micromolar DPCPX, an A1 receptor (A1R) antagonist, was used; 10 μm ZM241385 (ZM), an A2AR antagonist, was used. C, Example current-clamp recording from an RGC used to measure the effect of adenosine receptor antagonists on retinal wave frequency. D, Wave frequency changes (normalized to control) as a function of changing aminophylline concentration. The gray line indicates control wave frequency. Error bars represent SD; n = 4–9 cells per point. E, Changes in wave frequency with individual adenosine receptor subtype antagonists (1 μm A1R antagonist, DPCPX; 10 μm A2AR antagonist, ZM241385; 10 μm A2BR antagonist, alloxazine; 10 μm A3R antagonist, MRS 1523). The gray line indicates wave frequency in control. n = 3–6 cells per condition. Error bars represent SD. *p < 0.05 for ZM241385.
To confirm that aminophylline is not exerting its effect on retinal waves by blocking adenosine receptors, we measured the dose–response relationship of aminophylline on the amplitude of the tonic conductance and wave frequency and compared these results to those obtained with adenosine receptor antagonists (Fig. 6B,C). We found that at low concentrations, aminophylline closed a potassium conductance in RGCs, likely through closing of an A1-adenosine receptor-mediated G-protein-gated inwardly rectifying K+ channel in RGCs (Wetherington and Lambert, 2002; Clark and Newman, 2005). RGC input conductance was not changed in the intermediate concentrations (2–20 μm) of aminophylline. However, at high concentrations (200 or 500 μm) of aminophylline, the input conductance was significantly increased.
We tested the effect of specific adenosine receptor antagonists on conductance in RGCs (Fig. 6B). The adenosine A1 receptor antagonist 1,3-dipropyl-8-cyclopentylxanthine (DPCPX; 1 μm) slightly (but not significantly) decreased the input conductance compared with control. Further reversal potential measurement indicated that the DPCPX-modulated current reversed at EK (data not shown), suggesting that K+ flux was reduced in the presence of DPCPX. The DPCPX-mediated reduction in K+ flux is similar to the effect seen with low concentrations of aminophylline (1 μm), suggesting that aminophylline blocked adenosine A1 receptors at a low concentration. The adenosine A2A receptor antagonist 4-(2-[7-amino-2-(2-furyl)[1,2,4]-triazolo[2,3-a](1,3,5) triazin-5-ylamino]ethyl)phenol (ZM241385; 10 μm) did not alter the input conductance compared with control.
To test the hypothesis that aminophylline blocks waves via its effect on adenosine receptors in addition to its effect on GABAA receptors, we performed current-clamp recordings to measure wave-associated depolarizations in RGCs (Fig. 6C) while applying specific antagonists for different adenosine receptors: A1, A2A, A2B (10 μm alloxazine), and A3 [10 μm 3-propyl-6-ethyl-5-[(ethylthio)carbonyl]-2-phenyl-4-propyl-3-pyridine carboxylate (MRS 1523)]. The A2A antagonist (ZM241385) increased wave frequency over control, whereas none of the other individual adenosine receptor antagonists alone had any effect on retinal waves (Fig. 6E). It is worth noting that aminophylline blocked waves only at high concentrations (Singer et al., 2001; Syed et al., 2004b; this study), whereas a low concentration of aminophylline significantly increased wave frequency (Fig. 6D), the same effect we see with the adenosine A2A receptor antagonist. The increase in wave frequency is therefore likely caused by aminophylline action on adenosine receptors. Applying all the individual adenosine receptor antagonists together does not block waves (Fig. 6B), suggesting that the wave blockade by high concentrations of aminophylline was not caused by blockade of any currently known adenosine receptors, but was the result of tonic activation of GABAA receptors.
Discussion
In this study, our primary results are that (1) tonic activation of GABAA receptors blocks retinal waves; (2) GABAA receptor agonists activate a tonic GABAA conductance on both SACs and RGCs, which significantly reduces the excitability of both cell types; and (3) endogenous activation of GABAA receptors alters the spatial and temporal properties of retinal waves. In addition, we have demonstrated that aminophylline blocks retinal waves not by its previously reported action of blocking adenosine receptors but rather by activating GABAA receptors.
Aminophylline is a GABAA receptor agonist
Aminophylline is one of a short list of pharmacological agents that block retinal waves, and therefore its mode of action is of great significance. In the adult nervous system, aminophylline functions as a general adenosine receptor antagonist, and its use has led to the hypothesis that during development, ambient adenosine plays an important role in promoting wave activity. In support of this hypothesis, application of adenosine deaminase to degrade extracellular adenosine also decreases wave frequency (Stellwagen et al., 1999; Singer et al., 2001; Syed et al., 2004a). In addition, bath application of the general adenosine receptor agonist NECA (5′-N-ethylcarboxamidoadenosine) increases wave frequency (Stellwagen et al., 1999; Syed et al., 2004b). The effect of adenosine receptor agonists on retinal wave frequency is prevented by blockade of protein kinase A, a primary target of cAMP, indicating that its effects are being mediated by an increase in cAMP via A2-type adenosine receptors (Stellwagen et al., 1999).
We present several lines of evidence that aminophylline blocks retinal waves by tonically activating GABAA receptors (Figs. 5, 6). First, aminophylline blockade of spontaneous calcium transients and compound PSCs was reversed in the presence of gabazine. Second, aminophylline induced a chloride conductance in RGCs. Third, short application of aminophylline to outside-out patches excised from RGCs induced a current that was blocked by gabazine. We also conducted an extensive pharmacology study that indicated that specific antagonists of adenosine receptors do not block retinal waves either individually or in combination. Aminophylline is an ethylenediamine salt of the adenosine receptor antagonist theophylline. Several studies have demonstrated that ethylenediamine itself is a GABAA receptor agonist (Davies et al., 1982). Hence, our data suggest that the ethylenediamine component of aminophylline has a potent effect on retinal waves, whereas the adenosine receptor antagonist component, theophylline, does not.
We observed an increase in retinal wave frequency in the presence of an A2A antagonist. A2A adenosine receptors stimulate adenylate cyclase via Gs and therefore elevate levels of cAMP. A2 adenosine receptors in the retina (Stella et al., 2003) have been implicated in modulating transmitter release, primarily through modulation of calcium channels. These results predict that perhaps release of ACh from starburst amacrine cells is regulated by endogenous levels of adenosine. This finding is in contradiction with the result that adenosine deaminase blocks retinal waves (Stellwagen et al., 1999; Singer et al., 2001), which suggests that endogenous adenosine signaling is required for retinal wave propagation. There are two possibilities. First, the high concentration of adenosine deaminase used in our previous studies was blocking waves through an impurity in the adenosine deaminase. Second, the mixture of adenosine receptor antagonists described here did not effectively block all adenosine signaling pathways, suggesting the possible existence of an unexplored adenosine receptor subtype.
The multiple roles of GABAA receptor-mediated signaling in neural circuits
We have demonstrated that GABAA receptor signaling has many sites of action in the developing retina. Previous reports have indicated that retinal waves drive periodic release of GABA that induces phasic GABAA receptor-mediated responses in RGCs (Feller et al., 1996; Zhou, 1998; Zheng et al., 2004) and SACs (Zhou, 1998; Zheng et al., 2004). Here, we demonstrated that both RGCs and SACs had a tonic increase in holding current after application of GABAA receptor agonists (Figs. 1B, 3C,D). Blockade of all GABAA receptors with gabazine did not affect the spatiotemporal properties of retinal waves as assayed with calcium imaging, as reported in several previous studies (Stellwagen et al., 1999; Catsicas and Mobbs, 2001; Sernagor et al., 2003; Syed et al., 2004b). However, by using multielectrode array recordings, we found that blockade of GABAA receptors did change the structure of the spontaneous bursts of action potentials (Fig. 4). The bursts lasted longer and occurred more rarely, and there was an increase in the correlation index across all distances. This change in the firing structure may be attributable to a GABAA receptor antagonist-induced decrease in the resting conductance of SACs (Fig. 3E) or to the blockade of GABAA receptors on RGCs that are synaptically activated during retinal waves.
Our findings are consistent with the multiple modes of GABAA signaling observed in the adult cerebellum, hippocampus, and thalamus, in which the mechanisms underlying the distinct roles of phasic and tonic release have been studied extensively (Semyanov et al., 2004; Farrant and Nusser, 2005). For example, in these brain structures, synaptic and extrasynaptic GABAA signals are mediated by pharmacologically distinct classes of GABAA receptors. These pharmacological distinctions are caused by different subunit compositions of the synaptic versus extrasynaptic GABAA receptors. GABAA receptors are heteromultimeric proteins comprised of five subunits that form a chloride channel. For example, in cerebellum (Mody and Pearce, 2004) and some regions of thalamus (Cope et al., 2005), the δ subunit is found preferentially in extrasynaptic receptors, in which it increases the receptors' affinity for GABA, thereby making the receptors sensitive to ambient levels of GABA (Mody, 2001; Farrant and Nusser, 2005). In contrast, GABAA receptors found in hippocampus mediate a tonic conductance that is not blocked by gabazine but is blocked by picrotoxin (McCartney et al., 2007). It has been proposed that these extrasynaptic GABAA receptors are active in the absence of GABA.
Our results indicate that RGCs and SACs may have distinct GABAA receptor compositions. In the retina, there is a broad distribution of most GABAA receptor subunits that have been analyzed. Interestingly, the δ subunit has a distinct colocalization with starburst amacrine cell processes (Greferath et al., 1993; Wassle et al., 1998; Zucker and Ehinger, 1998; Grunert, 1999). We observed that SACs, but not RGCs, are activated by THIP, consistent with the presence of δ-containing GABAA receptors on starburst cells but not RGCs (Fig. 3D). In addition, gabazine decreased the input conductance of SACs but not RGCs (Fig. 3E, Tables 1, 2), indicating that ambient GABA tonically activates GABAA receptors on SACs. Whether RGCs have a gabazine-insensitive, picrotoxin-sensitive GABAA receptor as recently observed in hippocampus (McCartney et al., 2007) remains to be determined. Furthermore, GABA concentrations in vivo may be higher than in the isolated retina, so we cannot rule out the possibility that GABAA receptors are tonically active on RGCs as well as on SACs.
In the adult nervous system, tonic activation of GABAA receptors profoundly modulates network excitability through a variety of mechanisms, including shunting inhibition, which alters the integrative properties of individual neurons as well as the structure of network oscillations (Cope et al., 2005). We observed that blockade of GABAA receptor signaling increased the correlations in the developing retina. These findings are similar to a recent report that indicates that blockade of GABA signaling in spinal cord increases the correlations between motoneurons (Berg et al., 2007). The observation that GABA signaling decreased correlations is in sharp contrast to its role in adult neocortex and hippocampus, in which it is thought to enhance long-range correlations (Cobb et al., 1995; Whittington and Traub, 2003; Long et al., 2005). One possible advantage of decorrelated bursts in the developing retina is that firing patterns with lower correlations are more appropriate for driving plasticity at developing synapses (Hensch and Fagiolini, 2005). A second possibility is that GABAA receptor-mediated signaling may be critical for differentiating the firing patterns of distinct RGC classes. For example, the GABAA receptor-mediated signaling may facilitate the differential firing patterns of ON and OFF RGCs (Wong and Oakley, 1996; Myhr et al., 2001), which are critical for the refinement of ON and OFF circuits in downstream visual centers (Lee et al., 2002). Whether GABAA receptor-mediated signaling further distinguishes RGC classes remains to be determined.
Implications for fetal development
We found that endogenous activation of GABAA receptors influences the correlation structure of spontaneous firing patterns critical for normal development of the visual system. It is important to note that ambient levels of GABA are likely to be higher in the intact retina than in acutely isolated preparations that are rapidly superfused with GABA-free ACSF, and therefore our results may represent a smaller effect than would be detected in vivo.
Extrasynaptic GABAA receptors are potent sites of modulation by several factors, including alcohol (Weiner and Valenzuela, 2006). This may have profound implications for developing fetuses, in which it has been demonstrated in animal models of fetal alcohol syndrome that exposure to alcohol leads to a variety of neuropathologies, including alterations in the developing visual system (Stromland, 2004) such as a profound loss of plasticity in visual cortex (Medina et al., 2005). Understanding the susceptibility of developing networks to neuromodulatory substances will provide insights into the mechanisms by which exposure to substances such as alcohol impact the prenatal wiring of the nervous system.
Footnotes
This work was supported by National Institutes of Health Grant R01EY13528. J.E. was supported by a National Science Foundation predoctoral fellowship. We thank Papiya Mahapatra and Shiloh Guerrero for breeding transgenic mice.
References
- Bansal A, Singer J, Hwang B, Feller M. Mice lacking specific nAChR subunits exhibit dramatically altered spontaneous activity patterns and reveal a limited role for retinal waves in forming ON/OFF circuits in the inner retina. J Neurosci. 2000;20:7672–7681. doi: 10.1523/JNEUROSCI.20-20-07672.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y. Developing networks play a similar melody. Trends Neurosci. 2001;24:353–360. doi: 10.1016/s0166-2236(00)01813-0. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Khalilov I, Represa A, Gozlan H. Interneurons set the tune of developing networks. Trends Neurosci. 2004;27:422–427. doi: 10.1016/j.tins.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Berg RW, Alaburda A, Hounsgaard J. Balanced inhibition and excitation drive spike activity in spinal half-centers. Science. 2007;315:390–393. doi: 10.1126/science.1134960. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol (Lond) 1996;497:753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts DA, Feller MB, Shatz CJ, Rokhsar DS. Retinal waves are governed by collective network properties. J Neurosci. 1999;19:3580–3593. doi: 10.1523/JNEUROSCI.19-09-03580.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catsicas M, Mobbs P. GABAB receptors regulate chick retinal calcium waves. J Neurosci. 2001;21:897–910. doi: 10.1523/JNEUROSCI.21-03-00897.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadderton P, Margrie TW, Hausser M. Integration of quanta in cerebellar granule cells during sensory processing. Nature. 2004;428:856–860. doi: 10.1038/nature02442. [DOI] [PubMed] [Google Scholar]
- Chance FS, Abbott LF, Reyes AD. Gain modulation from background synaptic input. Neuron. 2002;35:773–782. doi: 10.1016/s0896-6273(02)00820-6. [DOI] [PubMed] [Google Scholar]
- Clark BD, Newman EA. Adenosine-evoked hyperpolarization of retinal ganglion cells is mediated by GIRK channel activation. Soc Neurosci Abstr. 2005;31:375–5. doi: 10.1523/JNEUROSCI.2836-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995;378:75–78. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- Colicos M, Firth S, Bosze J, Goldstein J, Feller M. Emergence of realistic retinal networks in culture promoted by the superior colliculus. Dev Neurosci. 2004;26:406–416. doi: 10.1159/000082283. [DOI] [PubMed] [Google Scholar]
- Cope DW, Hughes SW, Crunelli V. GABAA receptor-mediated tonic inhibition in thalamic neurons. J Neurosci. 2005;25:11553–11563. doi: 10.1523/JNEUROSCI.3362-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies LP, Hambley JW, Johnston GA. Ethylenediamine as a GABA agonist: enhancement of diazepam binding and interaction with GABA receptors and uptake sites. Neurosci Lett. 1982;29:57–61. doi: 10.1016/0304-3940(82)90364-0. [DOI] [PubMed] [Google Scholar]
- De Koninck Y, Mody I. Noise analysis of miniature IPSCs in adult rat brain slices: properties and modulation of synaptic GABAA receptor channels. J Neurophysiol. 1994;71:1318–1335. doi: 10.1152/jn.1994.71.4.1318. [DOI] [PubMed] [Google Scholar]
- Demarque M, Represa A, Becq H, Khalilov I, Ben-Ari Y, Aniksztejn L. Paracrine intercellular communication by a Ca2+- and SNARE-independent release of GABA and glutamate prior to synapse formation. Neuron. 2002;36:1051–1061. doi: 10.1016/s0896-6273(02)01053-x. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Feller MB, Wellis DP, Stellwagen D, Werblin FS, Shatz CJ. Requirement for cholinergic synaptic transmission in the propagation of spontaneous retinal waves. Science. 1996;272:1182–1187. doi: 10.1126/science.272.5265.1182. [DOI] [PubMed] [Google Scholar]
- Fischer K, Lukasiewicz P, Wong R. Age-dependent and cell-class specific modulation of retinal ganglion cell bursting activity by GABA. J Neurosci. 1998;18:3767–3778. doi: 10.1523/JNEUROSCI.18-10-03767.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaschuk O, Hanse E, Konnerth A. Developmental profile and synaptic origin of early network oscillations in the CA1 region of rat neonatal hippocampus. J Physiol (Lond) 1998;507:219–236. doi: 10.1111/j.1469-7793.1998.219bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greferath U, Grunert U, Mohler H, Wassle H. Cholinergic amacrine cells of the rat retina express the delta-subunit of the GABAA-receptor. Neurosci Lett. 1993;163:71–73. doi: 10.1016/0304-3940(93)90231-9. [DOI] [PubMed] [Google Scholar]
- Grunert U. Distribution of GABAA and glycine receptors in the mammalian retina. Clin Exp Pharmacol Physiol. 1999;26:941–944. doi: 10.1046/j.1440-1681.1999.03152.x. [DOI] [PubMed] [Google Scholar]
- Gulati K, Ray A, Pal G, Vijayan VK. Possible role of free radicals in theophylline-induced seizures in mice. Pharmacol Biochem Behav. 2005;82:241–245. doi: 10.1016/j.pbb.2005.06.019. [DOI] [PubMed] [Google Scholar]
- Hensch TK, Fagiolini M. Excitatory-inhibitory balance and critical period plasticity in developing visual cortex. Prog Brain Res. 2005;147:115–124. doi: 10.1016/S0079-6123(04)47009-5. [DOI] [PubMed] [Google Scholar]
- Huberman AD. Mechanisms of eye-specific visual circuit development. Curr Opin Neurobiol. 2007;17:73–80. doi: 10.1016/j.conb.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Jensen K, Chiu CS, Sokolova I, Lester HA, Mody I. GABA transporter-1 (GAT1)-deficient mice: differential tonic activation of GABAA versus GABAB receptors in the hippocampus. J Neurophysiol. 2003;90:2690–2701. doi: 10.1152/jn.00240.2003. [DOI] [PubMed] [Google Scholar]
- Johnson J, Tian N, Caywood MS, Reimer RJ, Edwards RH, Copenhagen DR. Vesicular neurotransmitter transporter expression in developing postnatal rodent retina: GABA and glycine precede glutamate. J Neurosci. 2003;23:518–529. doi: 10.1523/JNEUROSCI.23-02-00518.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda M, Farrant M, Cull-Candy SG. Whole-cell and single-channel currents activated by GABA and glycine in granule cells of the rat cerebellum. J Physiol (Lond) 1995;485:419–435. doi: 10.1113/jphysiol.1995.sp020739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CW, Eglen SJ, Wong RO. Segregation of ON and OFF retinogeniculate connectivity directed by patterned spontaneous activity. J Neurophysiol. 2002;88:2311–2321. doi: 10.1152/jn.00372.2002. [DOI] [PubMed] [Google Scholar]
- Leinekugel X, Medina I, Khalilov I, Ben-Ari Y, Khazipov R. Ca2+ oscillations mediated by the synergistic excitatory actions of GABA(A) and NMDA receptors in the neonatal hippocampus. Neuron. 1997;18:243–255. doi: 10.1016/s0896-6273(00)80265-2. [DOI] [PubMed] [Google Scholar]
- Litke A, Bezayiff N, Chichilnisky E, Cunningham W, Dabrowski W, Grillo A, Grivich M, Grybos P, Hottowy P, Kachiguine S, Kalmar R, Mathieson K, Petrusca D, Rahman M, Sher A. What does the eye tell the brain? Development of a system for the large-scale recording of retinal output activity. IEEE Trans Nucl Sci. 2004;51:1434–1440. [Google Scholar]
- Litke AM, Chichilnisky EJ, Dabrowski W, Grillo AA, Grybos P, Kachiguine S, Rahman M, Taylor G. Large-scale imaging of retinal output activity. Nucl Instrum Methods Phys Res A. 2003;501:298–307. [Google Scholar]
- Liu Z, Neff RA, Berg DK. Sequential interplay of nicotinic and GABAergic signaling guides neuronal development. Science. 2006;314:1610–1613. doi: 10.1126/science.1134246. [DOI] [PubMed] [Google Scholar]
- Long MA, Cruikshank SJ, Jutras MJ, Connors BW. Abrupt maturation of a spike-synchronizing mechanism in neocortex. J Neurosci. 2005;25:7309–7316. doi: 10.1523/JNEUROSCI.0375-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchionni I, Omrani A, Cherubini E. In the developing rat hippocampus a tonic GABAA-mediated conductance selectively enhances the glutamatergic drive of principal cells. J Physiol (Lond) 2007;581:515–528. doi: 10.1113/jphysiol.2006.125609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney MR, Deeb TZ, Henderson TN, Hales TG. Tonically active GABAA receptors in hippocampal pyramidal neurons exhibit constitutive GABA-independent gating. Mol Pharmacol. 2007;71:539–548. doi: 10.1124/mol.106.028597. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Torborg CL, Feller M, O'Leary DDM. Retinotopic map refinement requires spontaneous retinal waves during a brief critical period of development. Neuron. 2003;40:1147–1160. doi: 10.1016/s0896-6273(03)00790-6. [DOI] [PubMed] [Google Scholar]
- Medina AE, Krahe TE, Ramoa AS. Early alcohol exposure induces persistent alteration of cortical columnar organization and reduced orientation selectivity in the visual cortex. J Neurophysiol. 2005;93:1317–1325. doi: 10.1152/jn.00714.2004. [DOI] [PubMed] [Google Scholar]
- Meister M, Wong ROL, Baylor DA, Shatz CJ. Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina. Science. 1991;252:939–943. doi: 10.1126/science.2035024. [DOI] [PubMed] [Google Scholar]
- Meister M, Pine J, Baylor DA. Multi-neuronal signals from the retina: acquisition and analysis. J Neurosci Methods. 1994;51:95–106. doi: 10.1016/0165-0270(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Mitchell SJ, Silver RA. Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron. 2003;38:433–445. doi: 10.1016/s0896-6273(03)00200-9. [DOI] [PubMed] [Google Scholar]
- Mody I. Distinguishing between GABA(A) receptors responsible for tonic and phasic conductances. Neurochem Res. 2001;26:907–913. doi: 10.1023/a:1012376215967. [DOI] [PubMed] [Google Scholar]
- Mody I. Aspects of the homeostaic plasticity of GABAA receptor-mediated inhibition. J Physiol (Lond) 2005;562:37–46. doi: 10.1113/jphysiol.2004.077362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody I, Pearce RA. Diversity of inhibitory neurotransmission through GABA(A) receptors. Trends Neurosci. 2004;27:569–575. doi: 10.1016/j.tins.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Myhr KL, Lukasiewicz PD, Wong RO. Mechanisms underlying developmental changes in the firing patterns of ON and OFF retinal ganglion cells during refinement of their central projections. J Neurosci. 2001;21:8664–8671. doi: 10.1523/JNEUROSCI.21-21-08664.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. Correction for liquid junction potentials in patch clamp experiments. Methods Enzymol. 1992;207:123–131. doi: 10.1016/0076-6879(92)07008-c. [DOI] [PubMed] [Google Scholar]
- O'Donovan MJ. The origin of spontaneous activity in developing networks of the vertebrate nervous system. Curr Opin Neurobiol. 1999;9:94–104. doi: 10.1016/s0959-4388(99)80012-9. [DOI] [PubMed] [Google Scholar]
- Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition? Nat Rev Neurosci. 2002;3:715–727. doi: 10.1038/nrn919. [DOI] [PubMed] [Google Scholar]
- Payne JA, Rivera C, Voipio J, Kaila K. Cation-chloride co-transporters in neuronal communication, development and trauma. Trends Neurosci. 2003;26:199–206. doi: 10.1016/S0166-2236(03)00068-7. [DOI] [PubMed] [Google Scholar]
- Represa A, Ben-Ari Y. Trophic actions of GABA on neuronal development. Trends Neurosci. 2005;28:278–283. doi: 10.1016/j.tins.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl- co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM. GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nat Neurosci. 2003;6:484–490. doi: 10.1038/nn1043. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABA A receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Sernagor E, Young C, Eglen SJ. Developmental modulation of retinal wave dynamics: shedding light on the GABA saga. J Neurosci. 2003;23:7621–7629. doi: 10.1523/JNEUROSCI.23-20-07621.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer J, Mirotznik R, Feller M. Potentiation of L-type calcium channels reveals nonsynaptic mechanisms that correlate spontaneous activity in the developing mammalian retina. J Neurosci. 2001;21:8514–8522. doi: 10.1523/JNEUROSCI.21-21-08514.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soda T, Nakashima R, Watanabe D, Nakajima K, Pastan I, Nakanishi S. Segregation and coactivation of developing neocortical layer 1 neurons. J Neurosci. 2003;23:6272–6279. doi: 10.1523/JNEUROSCI.23-15-06272.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella SL, Jr, Bryson EJ, Cadetti L, Thoreson WB. Endogenous adenosine reduces glutamatergic output from rods through activation of A2-like adenosine receptors. J Neurophysiol. 2003;90:165–174. doi: 10.1152/jn.00671.2002. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Shatz CJ, Feller MB. Dynamics of retinal waves are controlled by cyclic AMP. Neuron. 1999;24:673–685. doi: 10.1016/s0896-6273(00)81121-6. [DOI] [PubMed] [Google Scholar]
- Stromland K. Visual impairment and ocular abnormalities in children with fetal alcohol syndrome. Addict Biol. 2004;9:153–157. doi: 10.1080/13556210410001717024. discussion 159–160. [DOI] [PubMed] [Google Scholar]
- Syed MM, Lee S, He S, Zhou ZJ. Spontaneous waves in the ventricular zone of developing mammalian retina. J Neurophysiol. 2004a;91:1999–2009. doi: 10.1152/jn.01129.2003. [DOI] [PubMed] [Google Scholar]
- Syed MM, Lee S, Zheng J, Zhou ZJ. Stage-dependent dynamics and modulation of spontaneous waves in the developing rabbit retina. J Physiol (Lond) 2004b;560:533–549. doi: 10.1113/jphysiol.2004.066597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torborg CL, Feller MB. Spontaneous patterned retinal activity and the refinement of retinal projections. Prog Neurobiol. 2005;76:213–235. doi: 10.1016/j.pneurobio.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Torborg CL, Hansen KA, Feller MB. High frequency, synchronized bursting drives eye-specific segregation of retinogeniculate projections. Nat Neurosci. 2005;8:72–78. doi: 10.1038/nn1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozuka Y, Fukuda S, Namba T, Seki T, Hisatsune T. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47:803–815. doi: 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Wassle H, Koulen P, Brandstatter JH, Fletcher EL, Becker CM. Glycine and GABA receptors in the mammalian retina. Vision Res. 1998;38:1411–1430. doi: 10.1016/s0042-6989(97)00300-3. [DOI] [PubMed] [Google Scholar]
- Watanabe D, Inokawa H, Hashimoto K, Suzuki N, Kano M, Shigemoto R, Hirano T, Toyama K, Kaneko S, Yokoi M, Moriyoshi K, Suzuki M, Kobayashi K, Nagatsu T, Kreitman RJ, Pastan I, Nakanishi S. Ablation of cerebellar Golgi cells disrupts synaptic integration involving GABA inhibition and NMDA receptor activation in motor coordination. Cell. 1998;95:17–27. doi: 10.1016/s0092-8674(00)81779-1. [DOI] [PubMed] [Google Scholar]
- Weiner JL, Valenzuela CF. Ethanol modulation of GABAergic transmission: the view from the slice. Pharmacol Ther. 2006;111:533–554. doi: 10.1016/j.pharmthera.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Wetherington JP, Lambert NA. Differential desensitization of responses mediated by presynaptic and postsynaptic A1 adenosine receptors. J Neurosci. 2002;22:1248–1255. doi: 10.1523/JNEUROSCI.22-04-01248.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington MA, Traub RD. Interneuron diversity series: inhibitory interneurons and network oscillations in vitro. Trends Neurosci. 2003;26:676–682. doi: 10.1016/j.tins.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Wong R. Retinal waves and visual system development. Annu Rev Neurosci. 1999;22:29–47. doi: 10.1146/annurev.neuro.22.1.29. [DOI] [PubMed] [Google Scholar]
- Wong RO, Oakley DM. Changing patterns of spontaneous bursting activity of on and off retinal ganglion cells during development. Neuron. 1996;16:1087–1095. doi: 10.1016/s0896-6273(00)80135-x. [DOI] [PubMed] [Google Scholar]
- Wong RO, Meister M, Shatz CJ. Transient period of correlated bursting activity during development of the mammalian retina. Neuron. 1993;11:923–938. doi: 10.1016/0896-6273(93)90122-8. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Watanabe D, Ishikane H, Tachibana M, Pastan I, Nakanishi S. A key role of starburst amacrine cells in originating retinal directional selectivity and optokinetic eye movement. Neuron. 2001;30:771–780. doi: 10.1016/s0896-6273(01)00316-6. [DOI] [PubMed] [Google Scholar]
- Zhang LL, Pathak HR, Coulter DA, Freed MA, Vardi N. Shift of intracellular chloride concentration in ganglion and amacrine cells of developing mouse retina. J Neurophysiol. 2006;95:2404–2416. doi: 10.1152/jn.00578.2005. [DOI] [PubMed] [Google Scholar]
- Zheng J, Lee S, Zhou ZJ. A transient network of intrinsically bursting starburst cells underlies the generation of retinal waves. Nat Neurosci. 2006;9:363–371. doi: 10.1038/nn1644. [DOI] [PubMed] [Google Scholar]
- Zheng JJ, Lee S, Zhou ZJ. A developmental switch in the excitability and function of the starburst network in the mammalian retina. Neuron. 2004;44:851–864. doi: 10.1016/j.neuron.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Zhou Z. The function of the cholinergic system in the developing mammalian retina. Prog Brain Res. 2001;131:599–613. doi: 10.1016/s0079-6123(01)31047-6. [DOI] [PubMed] [Google Scholar]
- Zhou ZJ. Direct participation of starburst amacrine cells in spontaneous rhythmic activities in the developing mammalian retina. J Neurosci. 1998;18:4155–4165. doi: 10.1523/JNEUROSCI.18-11-04155.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker CL, Ehinger B. Gamma-aminobutyric acidA receptors on a bistratified amacrine cell type in the rabbit retina. J Comp Neurol. 1998;393:309–319. doi: 10.1002/(sici)1096-9861(19980413)393:3<309::aid-cne4>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]