Abstract
Cigarette smoke has been connected to an array of chronic lung diseases and is a major source of morbidity and mortality. Active smoking is responsible for approximately 90% of lung cancer cases. In addition, cigarette smoke is associated with other chronic pulmonary diseases such as pulmonary edema, chronic bronchitis, and pulmonary emphysema, the last two also termed chronic obstructive pulmonary disease (COPD). Lung cancer and COPD are developed very frequently in chronic cigarette smokers. It has been known for some time that lung cancer incidence increases in patients with COPD. Even the existence of some low-grade emphysema without noticeable airflow obstruction is associated with significantly elevated risk of lung cancer. These recent clinical insights demand new thinking and exploration of novel mechanistic studies to fully understand these observations. Lung injury and repair involve cell death and hyperplasia of airway epithelial cells and infiltration of inflammatory cells. All of these occur simultaneously. The mechanisms of cell death and hyperplasia in the lung constitute two sides of the coin of lung injury and repair. However, most molecular studies in airway epithelial cells center on the mechanism(s) of either cell growth and proliferation or cell death and the ceramide-generating machinery that drives aberrant induction of apoptotic cell death. Very few address both sides of the coin as an outcome of cigarette smoke exposure, which is the focus of this review.
Keywords: ceramide machinery, EGFR trafficking, cigarette smoke, lung injury, lung cancer
CIGARETTE SMOKE IN LUNG INJURY AND LUNG CANCER
Cigarette smoke has been connected to an array of chronic lung diseases and is a major source of morbidity and mortality. In the United States over 400,000 deaths per year are attributed to smoking, and the number of deaths since 1964 is estimated at 12 × 106 (1). Active smoking is responsible for approximately 90% of lung cancer cases (2). In addition, cigarette smoke is associated with other chronic pulmonary diseases such as edema, chronic bronchitis, and emphysema, the last two also termed chronic obstructive pulmonary disease (COPD).
COPD and lung cancer are developed very frequently in chronic cigarette smokers and in the United States are the fourth and second major reasons of death, respectively (3). It has been known for some time that lung cancer incidence increases in patients with COPD (4, 5). Moreover, studies by Caballero and coworkers (6) convincingly reported that even the existence of some low-grade emphysema without noticeable airflow obstruction is associated with significantly elevated risk of lung cancer. These recent clinical insights demand new thinking and exploration of novel mechanistic studies to fully understand these observations. One research direction is based on the idea that emphysema starts with cigarette smoke–induced inflammation, which is accompanied by matrix destruction through an increase of oxidative stress and proteinase production. At the same time, the inflammatory cell proteinases (“blamed” for emphysema induction) are also releasing factors required for growth of cancer cells (7). However, cigarette smoke exposure in mice repeatedly causes both inflammation and airspace enlargement typical of human emphysema, but does not usually lead to lung cancer. Therefore, the molecular events may involve inflammatory cells and proteinases but are much less simplistic.
Lung injury and repair involve cell death and hyperplasia of airway epithelial cells and infiltration of inflammatory cells. All of these occur simultaneously, as has been well documented by studies of animal models and cellular alterations in disease processes (8–16). The mechanisms of cell death and hyperplasia in the lung constitute two sides of the coin of lung injury and repair. However, most molecular studies in airway epithelial cells center on the mechanism(s) of either cell growth and proliferation (17–21) or cell death and the ceramide-generating machinery that drives aberrant induction of apoptotic cell death (22–30). Very few address both sides of the coin as an outcome of cigarette smoke exposure, which is the focus of this discussion.
LUNG INJURY
Cigarette Smoke Reactive Oxidants in Lung Injury
Each puff of cigarette smoke contains about 5,000 toxic compounds, with 1015 free radicals in the gas phase and 1018 free radicals per gram of tar, which include potent oxidants such as hydrogen peroxide (H2O2), hydroxyl, and organic radicals (31, 32). These toxic compounds contribute to the adverse effects of cigarette smoke on lung epithelial cells, playing a key role not only in COPD and other pulmonary diseases such as acute respiratory distress syndrome (ARDS), asthma, and interstitial pulmonary fibrosis (15, 33–37), but also in lung cancer (17).
The pathogenesis of emphysema has historically been attributed to the protease–antiprotease imbalance resulting from chronic lung inflammation (36). In this view, chronic inflammation in the lung develops from long-term exposure to inhaled irritants (the most common being cigarette smoke). Inflammatory cells release various proteases, leading to destruction of the extracellular matrix and subsequent loss of the alveolar units (38). However, the concept of inflammation as the initiator of the cascade of events in lung destruction in diseases such as COPD may be in question. Instead, inflammation could represent the result of a long-standing destructive process in the lung and might by itself be a source of additional injury.
Recent studies provide a very strong case for cell death having a major role in lung injury in several pulmonary diseases (8–16, 33, 39–42). In simple terms, loss of cells by augmented apoptosis would be expected to be involved, or perhaps initiate, the overall tissue destruction responsible for lung injury (9, 13–16, 33, 39–42). The role of apoptosis in the pathogenesis of emphysema has become an area of extreme interest. The novel “cell death” hypothesis has proposed that apoptosis of the alveolar cells is the primary initiator of the disease. This concept arose in part due to the observation that smokers with emphysema have increased alveolar apoptotic cells as compared with smokers without emphysema (43). Subsequent studies demonstrated that experimental induction of either vascular endothelial cell apoptosis or pulmonary epithelial cell apoptosis in animal models results in airspace enlargement (8, 40, 44). Loss of cells by elevated apoptosis (pathological cell death) is thus involved in the overall tissue damage.
Ceramide in Pulmonary Diseases
Progress has recently evolved in understanding the underlying mechanisms of these destructive lung processes. For example, the groups of Gulbins and colleagues (45) and Worgall and coworkers (46) have recently reported that ceramide accumulation mediates inflammation, cell death, and infection susceptibility in cystic fibrosis (CF). Other studies implicated sphingomyelin hydrolysis in acute lung injury (40) and pulmonary edema (47). It was also reported that ceramide may be a critical mediator of endothelial and alveolar cell apoptosis in the vascular endothelial growth factor (VEGF) blockade mouse model of COPD (40). Furthermore, superoxide dismutase was shown to protect against apoptosis and alveolar enlargement induced by ceramide (48). Given that cigarette smoke is a predominant cause of COPD, additional animal models using smoke exposure would appear to be very useful for investigation, but it is only relatively recently that such models have been created (49–51).
Ceramide Generation and SMases
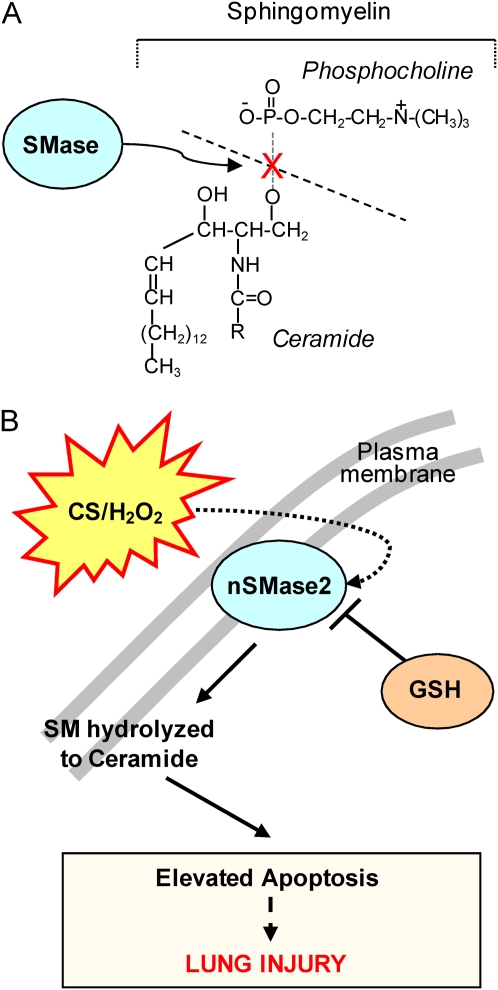
Ceramide is synthesized through either a de novo pathway involving serine palmitoyl-CoA transferase and ceramide synthase, or from breakdown of membrane sphingomyelin (N-acylsphingosine-1-phosphocholine), a phospholipid preferentially concentrated in the plasma membrane of mammalian cells (52). Sphingomyelin catabolism occurs via the action of sphingomyelinases (SMases), which are sphingomyelin-specific forms of phospholipase C that hydrolyze the phosphodiester bond of sphingomyelin, yielding ceramide and phosphocholine (Figure 1A). The main forms of SMases are distinguished by their pH optima (53–58). Alkaline SMase is found predominantly in the digestive system, with considerable differences among species (59, 60). Human and murine acid sphingomyelinase (aSMase; pH optimum 4.5–5.0), which exist in lysosomal or secretory isoforms, have been cloned and determined to be the products of a conserved gene. Furthermore, Mg2+-dependent or -independent neutral SMases (nSMase; pH optimum 7.4) have recently been characterized molecularly (30, 61–63). Interestingly, membrane nSMase does not gain access to the signaling events activated by the lysosomal aSMase and vice versa, indicating that ceramide action may be determined by the subcellular site of its production. Consistent with this observation, an additional neutral SMase, the murine mitochondrial associated SMase (MA-nSMase), has been recently identified (64) as an SMase that displays significant homology to the neutral sphingomyelinase2 (nSMase2), but has a different subcellular localization, being restricted to the mitochondria, where it may be important in regulating sphingolipid metabolism.
Figure 1.
(A) Sphingomyelinase (SMase) hydrolyses sphingomyelin (SM) to ceramide (and phosphocholine). (B) Working hypothesis: lung nSMase2 is upregulated by cigarette smoke (CS), which generates H2O2 and elevates ceramide, thereby enhancing apoptosis and lung injury.
According to current models, sphingomyelin may be constitutively metabolized to ceramide by several SMases, but some SMases may have special pathophysiological significance (65). Levy and colleagues (22, 30), Filosto and coworkers (66), and Rutkute and colleagues (67) have reported that the major SMase isoenzyme that becomes activated by oxidative stress appears to be nSMase2 (SMPD3), which is expressed in the Golgi, but probably also in the plasma membrane (30, 68) (Figure 1B).
Ceramide Generation, Oxidative Stress, and Apoptosis
The literature presents conflicting studies with respect to the placement of ceramide generation relative to caspases in the apoptotic cascade (69–72). However, Ravid and coworkers showed that different stimuli acting at diverse sites to activate ceramide accumulation were able to trigger apoptosis (27). This supported the idea that an increase in ceramide levels, per se, is sufficient to initiate the apoptotic cascade in lung epithelial cells and that ceramide accumulation is the causative signal for apoptosis induction (27) and not just an outcome of epithelial cell death.
Airway epithelial cells are the lung's first line of defense and are thus extensively exposed to reactive oxidants. Over the last few years our group initiated studies to address whether these cells are capable of entering apoptosis when exposed to micromolar concentrations of H2O2, and whether the process is mediated by ceramide as a second messenger (73, 74). The range of 50 to 250 μM H2O2 is considered to be the physiological range in which apoptosis can occur depending on the length of exposure to H2O2. As was shown later (17, 22), exposure to cigarette smoke can generate between 100 and 800 μM H2O2. Any concentration above 400 μM would be considered pathological (22, 26).
Our initial model for oxidative stress and ceramide generation consisted of straightforward exposures of airway epithelial cells to H2O2, glutathione (GSH), or both (19, 26, 27, 75). Even though such studies undertook a reductionist, simplified approach, both scenarios were relevant to the lung epithelium. Indeed, H2O2 is a ubiquitous molecule, freely miscible and able to cross cell membranes readily. It is present in several air pollutants, including the vapor phase of tobacco smoke (17). It is detected in exhaled air of humans (76), and the amounts of exhaled H2O2 appear greater in subjects with pulmonary disease (77) and in cigarette smokers (78). In addition, it was shown that either administration of exogenous H2O2 or enhancement of endogenously generated H2O2 was effective in depleting cellular GSH and initiating ceramide-induced apoptosis (27). Furthermore, cigarette smoke was shown to regulate cell growth and death via its H2O2 component (17, 22).
GSH Modulates Ceramide Generation and Apoptosis
Both lung epithelial cells and the epithelial lining fluid (ELF) contain high concentrations of GSH, the main antioxidant in the lung epithelium (26). GSH is a ubiquitous, essential tripeptide (L-γ-glutamyl-L-cysteinyl-glycine) containing a sulfhydryl group that enables it to be a key intracellular reducing agent, thus providing a fundamental antioxidant defense mechanism in oxidant-induced lung injury (79).
Goldkorn and coworkers demonstrated that low GSH levels were essential for ceramide generation, whereas high GSH levels inhibit the production of ceramide in human airway epithelial (HAE) cells (25, 26). Moreover, it was shown that GSH and N-acetylcysteine (NAC), which is a precursor of GSH, but not other thiol-containing antioxidants or oxidized GSH (GSSG), inhibited H2O2-mediated induction of ceramide and apoptosis. Therefore, GSH plays a critical role in preventing lung epithelial cell death. In this model, inhibitory effects on ceramide production were observed with both extracellular and intracellular GSH. The effects of extracellular GSH are primarily applicable to lung epithelium. It is interesting that even a short 1-hour exposure of cells to 250 μM H2O2, followed by growth in regular medium, was sufficient to induce apoptosis (26). This demonstrated that the events that control the fate of the cells occur within this hour, during which GSH is depleted and ceramide is generated.
SMases and Diseases
aSMase was the first SMase to be shown to have a role in a disease (80), as types A and B Niemann-Pick diseases (NPD) result from the deficient activity of aSMase. Recent reports point toward aSMase as the target responsible for ceramide generation in various pathologies (45, 46, 48, 65). Even though a role for the aSMase has been described in edema (47) and also suggested to play a role in other pulmonary pathologies, such as cystic fibrosis (45, 81) and emphysema (40), recent studies performed by our group suggest that it is nSMase2 that is serving as an exclusive target to generate ceramide under the stress of cigarette smoke exposure (22, 66).
nSMase2 and Ceramide Generation under Oxidative Stress
nSMase2 was previously found in the brain (71), and recent studies indicate a role for nSMase2 in aging (82) and in Alzheimer's disease (83). At the same time, it has been reported by our group that reactive oxidants up-regulate ceramide generation and cause pathological cell death in HAE cells (22–27, 30, 73, 74). This led to the proposal that there must be a specific SMase that is modulated by reactive oxygen species (ROS) in lung epithelial cells, which was followed by the isolation of the novel nSMase2 from monkey lung tissue and from HAE cells (30). It was then demonstrated (22, 30) that upon siRNA-silencing of nSMase2, lung epithelial cells could not undergo cell death in response to ROS or cigarette smoke exposure, suggesting that nSMase2 could be a critical target not only in the brain pathogenesis but also in ROS/cigarette smoke–induced lung injury in respiratory diseases.
nSMase2 Expression in the Lung of Mice, Rats, and Patients with Emphysema
Observations in HAE cells showed that cigarette smoke specifically activates nSMase2 and not aSMase (22, 23, 74). Moreover, it was found that nSMase2 is activated by the H2O2 component of cigarette smoke, thereby increasing ceramide generation via hydrolysis of sphingomyelin and elevating pathological apoptosis in the lung epithelium. GSH blocked these effects of cigarette smoke. Then, Filosto and colleagues investigated whether nSMase2 governs ceramide generation and apoptosis in vivo using rodent and human models of cigarette smoke–induced lung injury (66). It was found that exposure of mice or rats to cigarette smoke led to co-localizing elevations of ceramide levels and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive cells in lung tissues. These increases were nSMase2-dependent and were abrogated by treatment with NAC or anti-nSMase2 siRNA. Moreover, these recent studies in vivo (66) demonstrated that mice heterozygous for nSMase2 had less ceramide accumulation in the lung in comparison to wild-type mice when exposed to cigarette smoke; on the other hand, knockout mice for aSMase could accumulate ceramide under cigarette smoke exposure as much as the wild-type mice, demonstrating that only nSMase2 and not aSMase is modulated by cigarette smoke (66). Finally, it was found that lung tissues from patients with emphysema (smokers) displayed significantly higher levels of nSMase2 expression compared with lung tissues from control subjects. Together, these data establish the central in vivo role of nSMase2 in ceramide generation, aberrant apoptosis, and lung injury under cigarette smoke exposure, underscoring its promise as a novel target for the prevention of cigarette smoke–induced airspace destruction and thus the importance of elucidating the molecular mechanism of nSMase2 activation under oxidative stress (66).
nSMase2 Activation in the Setting of Cigarette Smoke: Molecular Mechanism
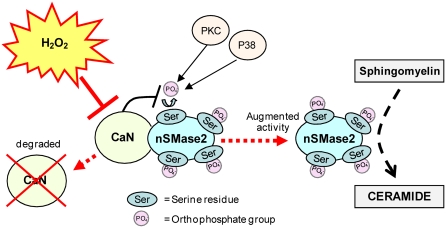
Filosto and colleagues have shown recently that nSMase2 is a phosphoprotein in which the level of phosphorylation is modulated by oxidative stress, which also controls nSMase2 function (84). A critical role for protein phosphatase 2B (PP2B), also known as Calcineurin (CaN) phosphatase, was found in the modulation of the phosphorylation and function of nSMase2. CaN phosphatase interacts directly with nSMase2, but not under exposure to H2O2-induced oxidative stress. CaN is a Ca+2/calmodulin-dependent serine/threonine phosphatase that can be inhibited by H2O2 that modifies 2 Cys residues through the oxidative formation of a disulfide bridge, which eventually leads to CaN degradation (85). A mutant of nSMase2 that does not bind CaN was found to be much more phosphorylated and activated than the WT nSMase2. This validated that the function of nSMase2 is modulated via its de-phosphorylation by CaN. CaN is degraded during exposure to oxidative stress, and thus does not bind nSMase2, allowing it to be fully phosphorylated downstream of p38 mitogen-activated protein kinase (MAPK)/protein kinase C (PKC) (84) (Figure 2).
Figure 2.
Proposed model of nSMase2 mechanism of function under oxidative stress. nSMase2 is a protein constitutively phosphorylated on serine residues downstream of p38 MAPK and PKC; calcineurin (CaN) binds to nSMase2, dephosphorylates it, and reduces its activity. Oxidative stress (H2O2) abolishes the binding of CaN to nSMase2, thus triggering elevated phosphorylation and activation of nSMase2.
LUNG CANCER
ErbB Family in Lung Cancer
In spite of exhaustive preclinical and clinical studies, the prognosis of metastatic lung cancer remains very poor, with only a 5 to 15% 5-year survival rate (86). However, current progress in molecular biology has increased the understanding of key biological pathways in lung carcinogenesis (87, 88). The epidermal growth factor receptor (EGFR) family of receptor tyrosine kinases (TKs) plays a critical role in lung cancer development, and previous reports have shown that cigarette smoking augments EGFR expression in human bronchial epithelium (89–91). This family of receptors is also referred to as the HER or ErbB family, and consists of four members—EGFR (HER1/ErbB1), HER2 (ErbB2), HER3 (ErbB3), and HER4 (ErbB4)—that regulate many developmental, metabolic, and physiological processes. EGFR activation is thought to promote malignancy through its role in proliferation, angiogenesis, metastasis, and inhibition of apoptosis. Although EGFR is expressed in all cells of epithelial origin, a higher level of EGFR expression is seen mostly in squamous cell carcinoma (50–80%) (88, 92), which occurs more frequently in smokers and men (93). EGFR overexpression is observed in tumors from more than 60% of patients with metastatic non–small cell lung carcinoma (NSCLC) and is correlated with poor prognosis (94). These findings have provided a rationale for the development of novel anticancer agents that target EGFR.
The Canonical Mechanism of EGFR Activation
The intracellular TK activity of EGFR is increased as a result of the binding of various cognate ligands, which include EGF, transforming growth factor-α, amphiregulin, and others, leading to the homodimerization of two EGFRs or the heterodimerization of EGFR with other family members, most commonly HER2 (95). Heterodimerization with HER2, which is overexpressed in some tumors, is a more potent activator of EGFR TK than is EGFR homodimerization (96). The activation of receptor TK leads to the autophosphorylation of the intracellular domain of EGFR, and the phosphotyrosine residues that are formed act as docking sites for various adaptor molecules, resulting in the activation of the rat sarcoma (Ras)/MAPK pathway, the phosphoinositide 3-kinases (PI3K)/protein kinases B (PKB/Akt) pathway, and the signal transducers and activators of transcription (STAT) signaling pathways (97). In tumor cells, the TK activity of EGFR may be up-regulated by several oncogenic mechanisms, such as EGFR gene mutation and increased gene copy number and EGFR protein overexpression (98). Aberrant activation of EGFR TK results in increased malignant cell survival, proliferation, invasion, and metastasis (99).
Cigarette Smoke, Lung Cancer, and Aberrant Activation of EGFR
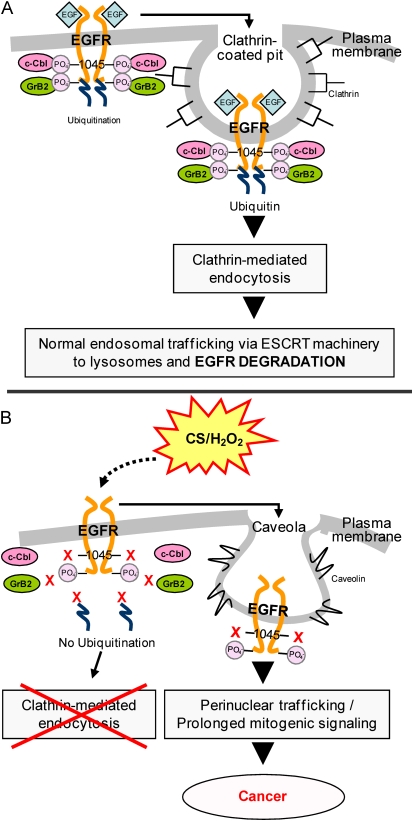
Among the plethora of deleterious chemicals found in cigarette smoke, H2O2 has been reported to be a significant constituent of the gas phase (100). It was thus hypothesized by Khan and coworkers that if mainstream cigarette smoke does indeed contain high amounts of H2O2, the effects of exposure on EGFR activation and stability could parallel those of H2O2 alone (17). As shown by our recent studies, exposure of epithelial cells to H2O2 induced aberrant phosphorylation of the EGFR, resulting in the lack of ubiquitination by the E3 ubiquitin ligase, c-Cbl, and impaired EGFR degradation. EGFR activation without the feedback regulation of normal degradation leads to uncontrolled cell growth and tumor promotion (19–21). Indeed, the pattern of EGFR activation by cigarette smoke was found to be similar to that of H2O2. The exposure of HAE cells to cigarette smoke, as well as to H2O2, not only resulted in an increase in EGFR activation over time, but the EGFR activated by H2O2 or cigarette smoke was neither ubiquitinated nor subsequently degraded due to its inability to bind c-Cbl. Moreover, the stabilized H2O2- and cigarette smoke–activated EGFR remained plasma membrane–bound, while a population of the receptor was trafficked to a perinuclear region via a caveolae-mediated mechanism. Concomitantly, cigarette smoke exposure induced the caveolae-mediated trafficking of the EGFR to the perinuclear region, where it can remain active and contribute to prolonged downstream signaling through the activation of Akt and extracellular signal regulated kinases (ERK1/2)-survival and proliferation pathways (Figure 3). Interestingly, it has been recently demonstrated (101) that ionizing radiation is also able to induce EGFR nuclear localization, where it may be required for repair.
Figure 3.
Proposed model of epidermal growth factor receptor (EGFR) aberrant activation and trafficking in the setting of cigarette smoke (CS). (A) Under EGF exposure, c-Cbl can bind directly and indirectly to the EGFR via phospho-Tyr-1045 and Grb2, respectively, allowing receptor ubiquitination, clathrin-mediated endocytosis, and lysosomal degradation. (B) Under cigarette smoke exposure, EGFR Tyr-1045 is not phosphorylated and c-Cbl can no longer interact with EGFR; therefore, the receptor does not follow the same degradation pathway that is induced by EGF. Instead, the EGFR is stabilized at the plasma membrane and also trafficks to a perinuclear compartment where it remains active and contributes to prolonged signaling.
Somatic Mutations in EGFR and EGFR TK Inhibitors
Since EGFR is often deregulated in NSCLC, it was logical to choose EGFR as a target for therapy. The first targeted strategies used a monoclonal antibody (cetuximab) (93). However, many oncologists found dramatic responses after treating patients with small molecule reversible tyrosine kinase inhibitors (TKIs, gefitinib or erlotinib). At the same time, the repeated remarkable responses were restricted to a subset of patients. The high response rate was frequent in women and in never-smokers with adenocarcinoma and in the Japanese population (93). Then, EGFR mutations were discovered in the TK domain, and these mutations were believed to present a selective growth advantage to the affected lung cells (102–106). Several inhibitors of this pathway are already in clinical studies in patients with lung cancer (88). Of note is that gefitinib and erlotinib inhibit EGFR TK activity via binding reversibly its ADP/ATP binding site.
The specific types of activating mutations that confer sensitivity to EGFR TKIs are present in the TK domain of the EGFR gene. Exon 19 deletion mutations and the single-point substitution mutation L858R in exon 21 are the most frequent in NSCLC and are termed “classical” mutations. The NSCLC tumors insensitive to EGFR TKIs include those driven by the V-Ki-ras2 Kirsten rat sarcoma (KRAS) and N-methyl-N′-nitro-N-nitroso-guanidine transforming (MET) oncogenes. However, most patients who initially respond to gefitinib and erlotinib eventually become resistant and experience progressive disease (99).
Lung Cancer in Nonsmokers
Even though cigarette smoking has been established as the most important cause of lung cancer, approximately 10 to 25% of all patients with lung cancer have no history of smoking (107, 108). Interestingly, the somatic mutations of EGFR in lung cancer, discussed above, are usually found in nonsmoking patients. Recent studies that pay specific attention to lung cancers in never-smokers have suggested that these cancers have characteristics distinct from those in smokers (108). These studies describe the best-characterized signaling pathways implicated in the transduction of proliferative signals and discuss the activity of these pathways in smokers and never-smokers. Of special interest are the recent findings that the mutants' phenotype at the molecular level resembles that of the wild-type EGFR exposed to cigarette smoke (17). Studies performed by the group of Yarden showed (109) that the EGFR mutant L858R presented an impaired association with c-Cbl and ubiquitinilation as had been reported for the wild-type EGFR under oxidative stress (17, 21). Moreover, a recent publication by Band's group (110) demonstrated that the mutant EGFR, but not wild-type EGFR, undergoes perinuclear accumulation and colocalization with recycling endosomal markers such as Rab 11, suggesting that mutant EGFRs display a different pattern of endocytosis, similar to what had been described before by our group for the wild-type EGFR under cigarette smoke–induced oxidative stress exposure (17, 19). This may suggest a unique similarity in conformational changes induced in the EGFR by somatic mutations (111, 112) and by exposure of wild-type EGFR to cigarette smoke.
nSMase2 AND EXOSOME GENERATION: A VEICLE FOR ONCOGENES PROPAGATION AND miRNA SECRETION
Ceramide and Cell Membrane Structure
Studies in recent years demonstrated that lipids in cell membranes do not form a homogenous liquid phase, but are ordered into domains mediated by interactions of sphingolipids and cholesterol (113). The ceramide moiety of sphingomyelin binds to cholesterol via hydrophobic van der Waal interactions (114). The strong hydrophobic interactions and the high local concentration of sphingolipids and cholesterol mediate an association of these lipids in the cell membrane and separation from other phospholipids, thereby forming distinct domains (113–115). These very small, tightly packed sphingolipid- and cholesterol-enriched membrane domains are named lipid rafts. Cholesterol and some cholesterol precursors not only interact with sphingolipids in these rafts, but also stabilize the structure of rafts by filling empty spaces between the massive sphingolipids (116). Since ceramide displaces cholesterol from rafts (117), the generation of ceramide within rafts may also totally change their structure. Ceramide-enriched microdomains have the tendency to fuse and to form ceramide-enriched macrodomains, also named ceramide-enriched membrane platforms, with a diameter from a few hundred nanometers up to several micrometers (118). These ceramide-enriched membrane platforms show altered biophysical properties, and therefore are ideal structures for sorting proteins in cells and for supporting reorganization of receptors. Furthermore, ceramide-enriched membrane platforms were shown to trap and to cluster receptor molecules (118–120).
Exosome Generation
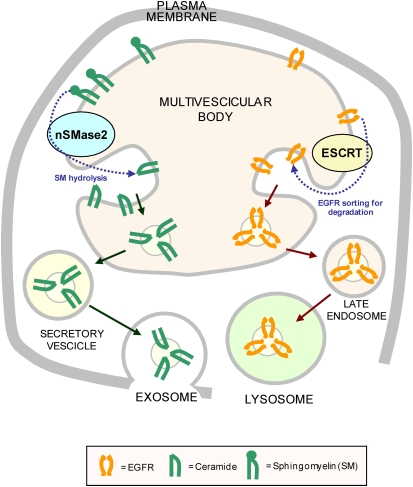
Exosomes were first described as removing excess transferrin receptor from reticulocytes during red blood cell formation (121). They are small (50–100 nm in diameter) membrane-bound vesicles released by various cells (122), and are now known to play important roles in cell-to-cell communication, antigen presentation, and in the pathogenesis of retroviral infections (including HIV) and prion diseases (123, 124). Exosomes are formed by invagination of the membrane of endosomes to produce intraluminal vesicles, thereby altering these organelles into multivesicular bodies (125). Exosomes are then secreted when these multivesicular bodies fuse with the plasma membrane and release their contents. Exosomes can also form directly at the plasma membrane in some cell types (126). The association between exosomes and multivesicular bodies was supported further by the finding of the ESCRT (endosomal sorting complex required for transport) machinery (127). This very conserved set of protein complexes identifies membrane proteins that are ubiquitinated and thereby targeted for sorting to lysosomes. Cells that lack components of the ESCRT machinery frequently fail to convey cargo to lysosomes (122, 125). The presence of ceramide in exosomes may suggest its direct function in the lipid-phase organization of the endosomal membranes, through which the ceramide-enriched phase results in the budding vesicles. This is supported by the presence of a typical membrane raft component in exosomes. Moreover, the molecular mechanism by which a change in lipid composition drives vesicle budding was demonstrated by Trajkovic and coworkers to be regulated via nSMase2 (128). These recent studies provide intriguing insights into exosome formation, making these microvesicles a bit less puzzling. Using mass spectrometric analysis, Trajkovic and colleagues demonstrated that ceramide levels were increased in secreted proteolipid protein-containing exosomes purified from cell culture medium. Moreover, disrupting the expression of nSMase2 by RNA interference or the use of specific inhibitors reduced secretion of these exosomes. This led to their suggestion that ceramide-induced aggregation of lipid microdomains causes inner growing of intraluminal vesicles (Figure 4).
Figure 4.
Proposed model for sorting out exosomes. The ESCRT machinery is largely involved in sorting the ubiquitinated protein (such as EGFR) for degradation in the lysosomes. On the other hand, nSMase2 is essential for the formation of secretory exosomes, underscoring the structural/functional role of ceramide generation in exosomes (122).
Cancer Cells Communicate via Microvesicles
The conventional view is that cellular communications occur mainly through gradients of soluble ligands, identified by the cell-associated receptors. Recent findings, however, suggest the existence of another form of intercellular communication, where the “units” of information are microvesicles containing a mass of biologically active protein and RNA species, including oncogenic receptors such as mutated forms of EGFR or micro RNAs (miRNAs). Importantly, such microvesicles could be recovered from blood samples of patients, thus potentially serving as prognostic biomarkers by revealing the existence of as yet undiagnosed tumor oncogenes (129).
Exosomes and Propagation of Oncogenes
Many tumors have a remarkable ability to shape their stromal vicinity to their own benefit (130). Recent studies showed the importance of interaction between cancer cells and their environment through shedding of membrane exosomes, which can fuse to cells in the surrounding area (131, 132). For example, aggressive human brain tumors (gliomas) express a truncated and oncogenic form of the EGFR, known as EGFRvIII, and it was recently shown that EGFRvIII proteins were transmitted into glioma cells deficient in EGFRvIII via secretory membrane microvesicles (132). This was suggested to initiate transfer of oncogenic activity and supported the new idea that membrane microvesicles or exosomes of cancer cells can play a role in a direct spread of oncogenes and their transforming phenotype between subsets of cancer cells.
miRNA in Lung Cancer and Secretion by Exosomes
miRNAs are short, noncoding RNAs of cellular and viral origin, which control gene expression by repressing the translation of mRNAs into protein (133, 134). In animals they inhibit translation of their target genes, and can lead the mRNAs to degradation through binding to partial complementary sites, usually located in the 3′ untranslated regions (3′-UTR) of the target mRNAs, providing miRNAs the ability to control several biological processes. Over the past several years, it has become clear that deregulation of many kinds of miRNAs are associated with the initiation and progression of human cancer (135).
It was found in the lung that some miRNAs are deregulated in cancers. For example, low expression of let-7a and high expression of miR-155 miRNA were associated with poor clinical outcome (136). At the same time, there are miRNAs overexpressed in lung cancer that have key roles in modulating cancerous cell growth and tumorigenicity (137). A recent study reported that the expression of some specific miRNAs may be linked to EGFR activation (138), and it is remarkable that the amounts of secretory miRNAs were found to be elevated in the plasma of patients with tumors, including patients with lung cancer (139, 140). A recent publication by Kosaka and coworkers reported that exosomes may be used as a means for secretion of miRNAs (141). Interestingly, this group demonstrated that miRNAs secreted from donor cells could be absorbed and remain functional in recipient cells, which suggested an innovative mechanism of intercellular communication by miRNAs release. Since miRNAs are secreted actively through the exosomes, it is clear that they are protected from degradation by RNases, thus suggesting that these miRNAs may function outside the cell in which they were produced. Indeed, Pegtel and colleagues demonstrated recently that miRNAs secreted by Epstein-Barr Virus (EBV)-infected cells were transferred to and acted in uninfected recipient cells (142). Kosaka and coworkers also demonstrated that miRNAs could be incorporated into exosomes and released via the ceramide/nSMase2-dependent pathway, independently of the machinery that requires the endosomal sorting complex for transport (ESCRT) (141), which underscores a novel and critical role of nSMase2 in regulating exosomes-dependent miRNA dispersal.
CONCLUSIONS
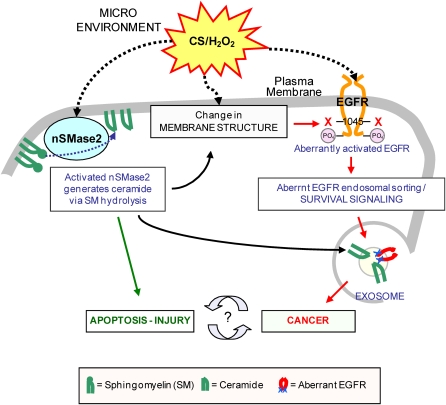
New exciting observations have recently demonstrated that cigarette smoke not only aberrantly stimulates EGFR to induce hyperplasia, but also activates nSMase2 to generate ceramide and induce apoptosis, thereby leading to tissue injury. Furthermore, nSMase2 is overexpressed both in mice chronically exposed to cigarette smoke and in patients with COPD. New findings revealed that nSMase2 is critical for generation of exosomes, which may be instrumental as vehicles spreading activated oncogenes (such as EGFR) and miRNA, thereby leading to tumorigenesis. This could position nSMase2 at a unique crossroads, playing an important role in both lung injury and lung cancer. On one hand, via the generation of ceramide, it clearly enhances apoptosis and injury in the lung. On the other hand, its function may also be crucial for the generation of exosomes, which may turn out to be obligatory in the trafficking and spreading of oncogenes and miRNAs, thereby affecting lung tumorigenesis. In conclusion, nSMase2 functions not only in apoptosis/injury but also indirectly in aberrant proliferation/cancer. Therefore, extensive additional studies investigating both nSMase2 and aberrantly activated oncogenes (such as EGFR) will be required to address the respective potential importance of nSMase2 in apoptosis and lung injury as well as in exosome generation and progression of lung tumorigenesis (Figure 5). Perhaps nSMase2 participates in one of the common signaling pathways, playing a role both in COPD and in the enhanced cancer incidence in patients with COPD.
Figure 5.
Proposed model of nSMase2 dual role in lung injury and in exosomal sorting. nSMase2 is involved in lung injury through enhancing apoptosis, and it also could be involved in lung cancer through its role in exosomal sorting.
This work was supported by grants from the National Institutes of Health (HL-71871 and HL-66189 to T.G.) and from the TRDRP (17RT-0131 to T.G.).
Originally Published in Press as DOI: 10.1165/rcmb.2010-0220RT on June 4, 2010
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Bhalla DK, Hirata F, Rishi AK, Gairola CG. Cigarette smoke, inflammation, and lung injury: a mechanistic perspective. J Toxicol Environ Health B Crit Rev 2009;12:45–64. [DOI] [PubMed] [Google Scholar]
- 2.Alberg AJ, Samet JM. Epidemiology of lung cancer. Chest 2003;123:21S–49S. [DOI] [PubMed] [Google Scholar]
- 3.Kung HC, Hoyert DL, Xu J, Murphy SL. Deaths: Final data for 2005. Natl Vital Stat Rep 2008;56:1–120. [PubMed] [Google Scholar]
- 4.Tockman MS, Anthonisen NR, Wright EC, Donithan MG. Airways obstruction and the risk for lung cancer. Ann Intern Med 1987;106:512–518. [DOI] [PubMed] [Google Scholar]
- 5.Skillrud DM, Offord KP, Miller RD. Higher risk of lung cancer in chronic obstructive pulmonary disease: a prospective, matched, controlled study. Ann Intern Med 1986;105:503–507. [DOI] [PubMed] [Google Scholar]
- 6.Caballero A, Torres-Duque CA, Jaramillo C, Bolivar F, Sanabria F, Osorio P, Orduz C, Guevara DP, Maldonado D. Prevalence of COPD in five Colombian cities situated at low, medium, and high altitude (prepocol study). Chest 2008;133:343–349. [DOI] [PubMed] [Google Scholar]
- 7.Houghton AM, Mouded M, Shapiro SD. Common origins of lung cancer and COPD. Nat Med 2008;14:1023–1024. [DOI] [PubMed] [Google Scholar]
- 8.Demedts IK, Demoor T, Bracke KR, Joos GF, Brusselle GG. Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respir Res 2006;7:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henson PM, Vandivier RW, Douglas IS. Cell death, remodeling, and repair in chronic obstructive pulmonary disease? Proc Am Thorac Soc 2006;3:713–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang HR, Cho SJ, Lee CG, Homer RJ, Elias JA. TGF-beta1 stimulates pulmonary fibrosis and inflammation via a bax-dependent, bid-activated pathway that involves matrix metalloproteinase-12. J Biol Chem 2007;282:7723–7732. [DOI] [PubMed] [Google Scholar]
- 11.Park JW, Ryter SW, Choi AM. Functional significance of apoptosis in chronic obstructive pulmonary disease. COPD 2007;4:347–353. [DOI] [PubMed] [Google Scholar]
- 12.Yoshida T, Tuder RM. Pathobiology of cigarette smoke-induced chronic obstructive pulmonary disease. Physiol Rev 2007;87:1047–1082. [DOI] [PubMed] [Google Scholar]
- 13.Elias J, Kang M, Crothers K, Homer R, Lee C. State of the art. Mechanistic heterogeneity in chronic COPD: insights from transgenic mice. Proc Am Thorac Soc 2006;3:494–498. [DOI] [PubMed] [Google Scholar]
- 14.Melgert BN, Timens W, Kerstjens HA, Geerlings M, Schouten JP, Postma DS, Hylkema MN. Effects of 4 months of smoking in mice with ovalbumin-induced airway inflammation. Clin Exp Allergy 2007;37:1798–1808. [DOI] [PubMed] [Google Scholar]
- 15.Rennard SI, Togo S, Holz O. Cigarette smoke inhibits alveolar repair: a mechanism for the development of emphysema. Proc Am Thorac Soc 2006;3:703–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spurzem JR, Rennard SI. Pathogenesis of COPD. Semin Respir Crit Care Med 2005;26:142–153. [DOI] [PubMed] [Google Scholar]
- 17.Khan EM, Lanir R, Danielson AR, Goldkorn T. EGF receptor exposed to cigarette smoke is aberrantly activated and undergoes perinuclear trafficking. FASEB J 2008;22:910–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldkorn T, Balaban N, Matsukuma K, Last J, Chan C, Chavez C. EGF-receptor phosphorylation and signaling are targeted by H2O2 redox stress. Am J Respir Cell Mol Biol 1998;19:786–798. [DOI] [PubMed] [Google Scholar]
- 19.Khan E, Heidinger J, Levy M, Lisanti M, Ravid T, Goldkorn T. Egf receptor exposed to oxidative stress undergoes src- and caveolin-1-dependent perinuclear trafficking. J Biol Chem 2006;281:14486–14493. [DOI] [PubMed] [Google Scholar]
- 20.Ravid T, Heidinger J, Gee P, Khan E, Goldkorn T. C-cbl-mediated ubiquitinylation is required for EGF receptor exit from the early endosomes. J Biol Chem 2004;279:37153–37162. [DOI] [PubMed] [Google Scholar]
- 21.Ravid T, Sweeney C, Gee P, Carraway KRK, Goldkorn T. EGF receptor activation under oxidative stress fails to promote c–cbl mediated down regulation. J Biol Chem 2002;12:12. [DOI] [PubMed] [Google Scholar]
- 22.Levy M, Khan E, Careaga M, Goldkorn T. Neutral sphingomyelinase 2 is activated by cigarette smoke to augment ceramide-induced apoptosis in lung cell death. Am J Physiol Lung Cell Mol Physiol 2009;297:125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castillo SS, Levy M, Thaikoottathil JV, Goldkorn T. Rns and ROS activate different smases to induce apoptosis in airway epithelial cells. Exp Cell Res 2007;313:2680–2686. [DOI] [PubMed] [Google Scholar]
- 24.Castillo SS, Levy M, Wang C, Thaikoottathil JV, Khan E, Goldkorn T. Nitric oxide-enhanced caspase-3 and acidic sphingomyelinase interaction: a novel mechanism by which airway epithelial cells escape ceramide-induced apoptosis. Exp Cell Res 2007;313:816–823. [DOI] [PubMed] [Google Scholar]
- 25.Goldkorn T, Lavrentiadou S, Chan C, Rassooly R, van der Vliet A, Kawcak T. Glutathione regulation of ceramide pathway in lung epithelial cells. Am J Respir Crit Care Med 2000;161:A242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lavrentiadou SN, Chan C, Ravid T, Tsaba A, van der Vliet A, Rasooly R, Goldkorn T. Ceramide-mediated apoptosis in lung epithelial cells is regulated by GSH. Am J Respir Cell Mol Biol 2001;25:676–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ravid T, Tsaba A, Gee P, Rasooly R, Medina EA, Goldkorn T. Ceramide accumulation precedes caspase-3 activation during apoptosis of a549 human lung adeno. cells. Am J Physiol Lung Cell Mol Physiol 2003;284:L1082–L1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldkorn T, Khan EM. Dual roles of oxidative stress in the lungs. In: Valacchi G, Davis PA, editors. Oxidants in biology: a question of balance. Springer Netherlands; 2008. pp. 231–250.
- 29.Raychaudhuri S, Willgohs E, Nguyen TN, Khan EM, Goldkorn T. Monte Carlo simulation of cell death signaling predicts large cell-to-cell stochastic fluctuations through the type 2 pathway of apoptosis. Biophys J 2008;95:3559–3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levy M, Castillo SS, Goldkorn T. Nsmase2 activation and trafficking are modulated by oxidative stress to induce apoptosis. Biochem Biophys Res Commun 2006;344:900–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Halliwell B, Gutteridge JM. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol 1990;186:1–85. [DOI] [PubMed] [Google Scholar]
- 32.Hoidal JR. Reactive oxygen species and cell signaling. Am J Respir Cell Mol Biol 2001;25:661–663. [DOI] [PubMed] [Google Scholar]
- 33.Elias JA, Lee CG. Lipid let loose in pulmonary emphysema. Nat Med 2005;11:471–472. [DOI] [PubMed] [Google Scholar]
- 34.Rahman I, Swarska E, Henry M, Stolk J, MacNee W. Is there any relationship between plasma antioxidant capacity and lung function in smokers and in patients with chronic obstructive pulmonary disease? Thorax 2000;55:189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sen C, Packer L. Antioxidant and redox regulation of gene transcription. FASEB J 1996;10:709–720. [DOI] [PubMed] [Google Scholar]
- 36.Tuder RM, Yoshida T, Fijalkowka I, Biswal S, Petrache I. Role of lung maintenance program in the heterogeneity of lung destruction in emphysema. Proc Am Thorac Soc 2006;3:673–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Korfei M, Ruppert C, Mahavadi P, Henneke I, Markart P, Koch M, Lang G, Fink L, Bohle RM, Seeger W, et al. Epithelial endoplasmic reticulum stress and apoptosis in sporadic idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2008;178:838–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mouded M, Egea EE, Brown MJ, Hanlon SM, Houghton AM, Tsai LW, Ingenito EP, Shapiro SD. Epithelial cell apoptosis causes acute lung injury masquerading as emphysema. Am J Respir Cell Mol Biol 2009;41:407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, Waltenberger J, Voelkel NF. Inhibition of vegf receptors causes lung cell apoptosis and emphysema. J Clin Invest 2000;106:1311–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petrache I, Natarajan V, Zhen L, Medler TR, Richter AT, Cho C, Hubbard WC, Berdyshev EV, Tuder RM. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat Med 2005;11:491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shapiro SD. Vascular atrophy and vegfr-2 signaling: Old theories of pulmonary emphysema meet new data. J Clin Invest 2000;106:1309–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Segura-Valdez L, Pardo A, Gaxiola M, Uhal BD, Becerril C, Selman M. Upregulation of gelatinases a and b, collagenases 1 and 2, and increased parenchymal cell death in COPD. Chest 2000;117:684–694. [DOI] [PubMed] [Google Scholar]
- 43.Kasahara Y, Tuder R, Cool C, Lynch D, Flores S, Voelkel NF. Endothelial cell death and decreased expression of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 in emphysema. Am J Respir Crit Care Med 2007;163:737–744. [DOI] [PubMed] [Google Scholar]
- 44.Giordano RJ, Lahdenranta J, Zhen L, Chukwueke U, Petrache I, Langley RR, Fidler IJ, Pasqualini R, Tuder RM, Arap W. Targeted induction of lung endothelial cell apoptosis causes emphysema-like changes in the mouse. J Biol Chem 2008;283:29447–29460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teichgraber V, Ulrich M, Endlich N, Riethmuller J, Wilker B, De Oliveira-Munding CC, van Heeckeren AM, Barr ML, von Kurthy G, Schmid KW, et al. Ceramide accumulation mediates inflammation, cell death and infection susceptibility in cystic fibrosis. Nat Med 2008;14:382–391. [DOI] [PubMed] [Google Scholar]
- 46.Hamai H, Keyserman F, Quittell LM, Worgall TS. Defective cftr increases synthesis and mass of sphingolipids that affect membrane composition and lipid signaling. J Lipid Res 2009;50:1101–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goggel R, Winoto-Morbach S, Vielhaber G, Imai Y, Lindner K, Brade L, Brade H, Ehlers S, Slutsky AS, Schutze S, et al. Paf-mediated pulmonary edema: a new role for acid sphingomyelinase and ceramide. Nat Med 2004;10:155–160. [DOI] [PubMed] [Google Scholar]
- 48.Petrache I, Medler TR, Richter AT, Kamocki K, Chukwueke U, Zhen L, Gu Y, Adamowicz J, Schweitzer KS, Hubbard WC, et al. Superoxide dismutase protects against apoptosis and alveolar enlargement induced by ceramide. Am J Physiol Lung Cell Mol Physiol 2008;295:L44–L53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wright JL, Cosio M, Churg A. Animal models of chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol 2008;295:L1–L15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bolton SJ, Pinnion K, Oreffo V, Foster M, Pinkerton KE. Characterisation of the proximal airway squamous metaplasia induced by chronic tobacco smoke exposure in spontaneously hypertensive rats. Respir Res 2009;10:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhong CY, Zhou YM, Douglas GC, Witschi H, Pinkerton KE. MAPK/AP-1 signal pathway in tobacco smoke-induced cell proliferation and squamous metaplasia in the lungs of rats. Carcinogenesis 2005;26:2187–2195. [DOI] [PubMed] [Google Scholar]
- 52.Merrill AH Jr, Jones DD. An update of the enzymology and regulation of sphingomyelin metabolism. Biochim Biophys Acta 1990;1044:1–12. [DOI] [PubMed] [Google Scholar]
- 53.Cifone MG, De Maria R, Roncaioli P, Rippo MR, Azuma M, Lanier LL, Santoni A, Testi R. Apoptotic signaling through cd95 (fas/apo-1) activates an acidic sphingomyelinase. J Exp Med 1994;180:1547–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jayadev S, Liu B, Bielawska AE, Lee JY, Nazaire F, Pushkareva M, Obeid LM, Hannun YA. Role for ceramide in cell cycle arrest. J Biol Chem 1995;270:2047–2052. [DOI] [PubMed] [Google Scholar]
- 55.Lawler JF Jr, Yin M, Diehl AM, Roberts E, Chatterjee S. Tumor necrosis factor-alpha stimulates the maturation of sterol regulatory element binding protein-1 in human hepatocytes through the action of neutral sphingomyelinase. J Biol Chem 1998;273:5053–5059. [DOI] [PubMed] [Google Scholar]
- 56.Okazaki T, Bielawska A, Domae N, Bell RM, Hannun YA. Characteristics and partial purification of a novel cytosolic, magnesium-independent, neutral sphingomyelinase activated in the early signal transduction of 1 alpha,25-dihydroxyvitamin d3-induced hl-60 cell differentiation. J Biol Chem 1994;269:4070–4077. (published erratum appears in j biol chem 1994 jun 10;269(23):16518). [PubMed] [Google Scholar]
- 57.Schutze S, Potthoff K, Machleidt T, Berkovic D, Wiegmann K, Kronke M. TNF activates NF-kappa b by phosphatidylcholine-specific phospholipase c–induced “acidic” sphingomyelin breakdown. Cell 1992;71:765–776. [DOI] [PubMed] [Google Scholar]
- 58.Wiegmann K, Schutze S, Machleidt T, Witte D, Kronke M. Functional dichotomy of neutral and acidic sphingomyelinases in tumor necrosis factor signaling. Cell 1994;78:1005–1015. [DOI] [PubMed] [Google Scholar]
- 59.Feng D, Ohlsson L, Ling W, Nilsson A, Duan RD. Generating ceramide from sphingomyelin by alkaline sphingomyelinase in the gut enhances sphingomyelin-induced inhibition of cholesterol uptake in caco-2 cells. Dig Dis Sci (In press) [DOI] [PubMed]
- 60.Duan RD, Hertervig E, Nyberg L, Hauge T, Sternby B, Lillienau J, Farooqi A, Nilsson A. Distribution of alkaline sphingomyelinase activity in human beings and animals: tissue and species differences. Dig Dis Sci 1996;41:1801–1806. [DOI] [PubMed] [Google Scholar]
- 61.Marchesini N, Hannun YA. Acid and neutral sphingomyelinases: Roles and mechanisms of regulation. Biochem Cell Biol 2004;82:27–44. [DOI] [PubMed] [Google Scholar]
- 62.Mizutani Y, Tamiya-Koizumi K, Nakamura N, Kobayashi M, Hirabayashi Y, Yoshida S. Nuclear localization of neutral sphingomyelinase 1: biochemical and immunocytochemical analyses. J Cell Sci 2001;114:3727–3736. [DOI] [PubMed] [Google Scholar]
- 63.Clarke CJ, Snook CF, Tani M, Matmati N, Marchesini N, Hannun YA. The extended family of neutral sphingomyelinases. Biochemistry 2006;45:11247–11256. [DOI] [PubMed] [Google Scholar]
- 64.Wu BX, Rajagopalan V, Roddy PL, Clarke CJ, Hannun YA. Identification and characterization of murine mitochondrial-associated neutral sphingomyelinase (ma-nsmase), the mammalian sphingomyelin phosphodiesterase 5. J Biol Chem 2010;285:17993–8002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Uhlig S, Gulbins E. Sphingolipids in the lungs. Am J Respir Crit Care Med 2008;178:1100–1114. [DOI] [PubMed] [Google Scholar]
- 66.Filosto S, Castillo S, Danielson A, Franzi L, Khan E, Kenyon N, Last J, Pinkerton K, Tuder R, Goldkorn T. Neutral sphingomyelinase 2: a novel target in cigarette smoke-induced apoptosis and lung injury. Am J Respir Cell Mol Biol (In press) [DOI] [PMC free article] [PubMed]
- 67.Rutkute K, Asmis RH, Nikolova-Karakashian MN. Regulation of neutral sphingomyelinase-2 by GSH: a new insight to the role of oxidative stress in aging-associated inflammation. J Lipid Res 2007;48:2443–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marchesini N, Osta W, Bielawski J, Luberto C, Obeid LM, Hannun YA. Role for mammalian neutral sphingomyelinase 2 in confluence-induced growth arrest of mcf7 cells. J Biol Chem 2004;279:25101–25111. [DOI] [PubMed] [Google Scholar]
- 69.Hannun YA, Luberto C. Ceramide in the eukaryotic stress response. Trends Cell Biol 2000;10:73–80. [DOI] [PubMed] [Google Scholar]
- 70.Dbaibo G, Perry D, Gamard C, Platt R, Poirier G, Obeid L, Hannun YA. Cytokine response modifier a (crma) inhibits ceramide formation in response to TNF-alpha: Crma and bcl-2 target distinct components in the apoptotic pathway. J Exp Med 1997;185:481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hofmann K, Tomiuk S, Wolff G, Stoffel W. Cloning and characterization of the mammalian brain-specific, mg2+- dependent neutral sphingomyelinase. Proc Natl Acad Sci USA 2000;97:5895–5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takeda Y, Tashima M, Takahashi A, Uchiyama T, Okazaki T. Ceramide generation in nitric oxide-induced apoptosis: activation of magnesium-dependent neutral sphingomyelinase via caspase-3. J Biol Chem 1999;274:10654–10660. [DOI] [PubMed] [Google Scholar]
- 73.Goldkorn T, Balaban N, Shannon M, Chea V, Matsukuma K, Gilchrist D, Wang H, Chan C. H2O2 acts on cellular membranes to generate ceramide signaling and initiate apoptosis in tracheobronchial epithelial cells. J Cell Sci 1998;111:3209–3220. [DOI] [PubMed] [Google Scholar]
- 74.Chan C, Goldkorn T. Ceramide path in human lung cell death. Am J Respir Cell Mol Biol 2000;22:460–468. [DOI] [PubMed] [Google Scholar]
- 75.Goldkorn TRT, Medina EA. Ceramide signaling under oxidative stress. Kluwer (Springer Netherlands); 2003.
- 76.Williams MD, Chance B. Spontaneous chemiluminescence of human breath: spectrum, lifetime, temporal distribution, and correlation with peroxide. J Biol Chem 1983;258:3628–3631. [PubMed] [Google Scholar]
- 77.Sznajder JI, Fraiman A, Hall JB, Sanders W, Schmidt G, Crawford G, Nahum A, Factor P, Wood LD. Increased H2O2 in the expired breath of patients with acute hypoxemic respiratory failure. Chest 1989;96:606–612. [DOI] [PubMed] [Google Scholar]
- 78.Nowak D, Antczak A, Krol M, Pietras T, Shariati B, Bialasiewicz P, Jeczkowski K, Kula P. Increased content of H2O2 in the expired breath of cigarette smokers. Eur Respir J 1996;9:652–657. [DOI] [PubMed] [Google Scholar]
- 79.Meister A, Anderson ME. Glutathione. Annu Rev Biochem 1983;52:711–760. [DOI] [PubMed] [Google Scholar]
- 80.Horinouchi K, Erlich S, Perl DP, Ferlinz K, Bisgaier CL, Sandhoff K, Desnick RJ, Stewart CL, Schuchman EH. Acid sphingomyelinase deficient mice: a model of types a and b Niemann-Pick disease. Nat Genet 1995;10:288–293. [DOI] [PubMed] [Google Scholar]
- 81.Smith EL, Schuchman EH. The unexpected role of acid sphingomyelinase in cell death and the pathophysiology of common diseases. FASEB J 2008;22:3419–3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nikolova-Karakashian M, Karakashian A, Rutkute K. Role of neutral sphingomyelinases in aging and inflammation. Subcell Biochem 2008;49:469–486. [DOI] [PubMed] [Google Scholar]
- 83.Satoi H, Tomimoto H, Ohtani R, Kitano T, Kondo T, Watanabe M, Oka N, Akiguchi I, Furuya S, Hirabayashi Y, et al. Astroglial expression of ceramide in Alzheimer's disease brains: a role during neuronal apoptosis. Neuroscience 2005;130:657–666. [DOI] [PubMed] [Google Scholar]
- 84.Filosto S, Fry W, Knowlton AA, Goldkorn T. Neutral sphingomyelinase 2 (nsmase2) is a phosphoprotein regulated by calcineurin (pp2b). J Biol Chem 2010;285:10213–10222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bogumil R, Namgaladze D, Schaarschmidt D, Schmachtel T, Hellstern S, Mutzel R, Ullrich V. Inactivation of calcineurin by hydrogen peroxide and phenylarsine oxide: evidence for a dithiol-disulfide equilibrium and implications for redox regulation. Eur J Biochem 2000;267:1407–1415. [DOI] [PubMed] [Google Scholar]
- 86.Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin 2003;53:5–26. [DOI] [PubMed] [Google Scholar]
- 87.Hirsch FR, Franklin WA, Gazdar AF, Bunn PA Jr. Early detection of lung cancer: clinical perspectives of recent advances in biology and radiology. Clin Cancer Res 2001;7:5–22. [PubMed] [Google Scholar]
- 88.Hirsch FR, Scagliotti GV, Langer CJ, Varella-Garcia M, Franklin WA. Epidermal growth factor family of receptors in preneoplasia and lung cancer: perspectives for targeted therapies. Lung Cancer 2003;41:S29–S42. [DOI] [PubMed] [Google Scholar]
- 89.Polosa R, Prosperini G, Leir SH, Holgate ST, Lackie PM, Davies DE. Expression of c-erbb receptors and ligands in human bronchial mucosa. Am J Respir Cell Mol Biol 1999;20:914–923. [DOI] [PubMed] [Google Scholar]
- 90.Barsky SH, Roth MD, Kleerup EC, Simmons M, Tashkin DP. Histopathologic and molecular alterations in bronchial epithelium in habitual smokers of marijuana, cocaine, and/or tobacco. J Natl Cancer Inst 1998;90:1198–1205. [DOI] [PubMed] [Google Scholar]
- 91.O'Donnell RA, Richter A, Ward J, Angco G, Mehta A, Rousseau K, Swallow DM, Holgate ST, Djukanovic R, Davies DE, et al. Expression of erbb receptors and mucins in the airways of long term current smokers. Thorax 2004;59:1032–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hirsch FR, Bunn PA Jr, Johnson DH, Giaccone G, Rosell R. Lung cancer: introduction. Lung Cancer 2003;41:S1. [DOI] [PubMed] [Google Scholar]
- 93.Yatabe Y. Egfr mutations and the terminal respiratory unit. Cancer Metastasis Rev 2010;29:23–36. [DOI] [PubMed] [Google Scholar]
- 94.Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer 2007;7:169–181. [DOI] [PubMed] [Google Scholar]
- 95.Bazley LA, Gullick WJ. The epidermal growth factor receptor family. Endocr Relat Cancer 2005;12:S17–S27. [DOI] [PubMed] [Google Scholar]
- 96.Hirsch FR, Varella-Garcia M, Cappuzzo F. Predictive value of egfr and her2 overexpression in advanced non-small-cell lung cancer. Oncogene 2009;28:S32–S37. [DOI] [PubMed] [Google Scholar]
- 97.Kumar A, Petri ET, Halmos B, Boggon TJ. Structure and clinical relevance of the epidermal growth factor receptor in human cancer. J Clin Oncol 2008;26:1742–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ciardiello F, Tortora G. Egfr antagonists in cancer treatment. N Engl J Med 2008;358:1160–1174. [DOI] [PubMed] [Google Scholar]
- 99.Gazdar AF. Activating and resistance mutations of egfr in non-small-cell lung cancer: role in clinical response to egfr tyrosine kinase inhibitors. Oncogene 2009;28:S24–S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nakayama T, Kodama M, Nagata C. Generation of hydrogen proxide and superoxide anion radical from cigarette smoke. Gann 1984;75:95–98. [PubMed] [Google Scholar]
- 101.Hsu SC, Miller SA, Wang Y, Hung MC. Nuclear egfr is required for cisplatin resistance and DNA repair. Am J Transl Res 2009;1:249–258. [PMC free article] [PubMed] [Google Scholar]
- 102.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129–2139. [DOI] [PubMed] [Google Scholar]
- 103.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et al. Egfr mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497–1500. [DOI] [PubMed] [Google Scholar]
- 104.Minna JD, Gazdar AF, Sprang SR, Herz J. Cancer: a bull's eye for targeted lung cancer therapy. Science 2004;304:1458–1461. [DOI] [PubMed] [Google Scholar]
- 105.Weinstein IB. Cancer: addiction to oncogenes–the achilles heal of cancer. Science 2002;297:63–64. [DOI] [PubMed] [Google Scholar]
- 106.Shigematsu H, Gazdar AF. Somatic mutations of epidermal growth factor receptor signaling pathway in lung cancers. Int J Cancer 2006;118:257–262. [DOI] [PubMed] [Google Scholar]
- 107.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74–108. [DOI] [PubMed] [Google Scholar]
- 108.Mountzios G, Fouret P, Soria JC. Mechanisms of disease: signal transduction in lung carcinogenesis–a comparison of smokers and never-smokers. Nat Clin Pract Oncol 2008;5:610–618. [DOI] [PubMed] [Google Scholar]
- 109.Shtiegman K, Kochupurakkal BS, Zwang Y, Pines G, Starr A, Vexler A, Citri A, Katz M, Lavi S, Ben-Basat Y, et al. Defective ubiquitinylation of egfr mutants of lung cancer confers prolonged signaling. Oncogene 2007;26:6968–6978. [DOI] [PubMed] [Google Scholar]
- 110.Chung BM, Raja SM, Clubb RJ, Tu C, George M, Band V, Band H. Aberrant trafficking of nsclc-associated egfr mutants through the endocytic recycling pathway promotes interaction with src. BMC Cell Biol 2009;10:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang X, Gureasko J, Shen K, Cole PA, Kuriyan J. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell 2006;125:1137–1149. [DOI] [PubMed] [Google Scholar]
- 112.Jura N, Endres NF, Engel K, Deindl S, Das R, Lamers MH, Wemmer DE, Zhang X, Kuriyan J. Mechanism for activation of the egf receptor catalytic domain by the juxtamembrane segment. Cell 2009;137:1293–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang Y, Li X, Becker KA, Gulbins E. Ceramide-enriched membrane domains–structure and function. Biochim Biophys Acta 2009;1788:178–183. [DOI] [PubMed] [Google Scholar]
- 114.Kolesnick RN, Goni FM, Alonso A. Compartmentalization of ceramide signaling: physical foundations and biological effects. J Cell Physiol 2000;184:285–300. [DOI] [PubMed] [Google Scholar]
- 115.Stancevic B, Kolesnick R. Ceramide-rich platforms in transmembrane signaling. FEBS Lett 2010;584:1728–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xu X, Bittman R, Duportail G, Heissler D, Vilcheze C, London E. Effect of the structure of natural sterols and sphingolipids on the formation of ordered sphingolipid/sterol domains (rafts): comparison of cholesterol to plant, fungal, and disease-associated sterols and comparison of sphingomyelin, cerebrosides, and ceramide. J Biol Chem 2001;276:33540–33546. [DOI] [PubMed] [Google Scholar]
- 117.Megha LE. Ceramide selectively displaces cholesterol from ordered lipid domains (rafts): implications for lipid raft structure and function. J Biol Chem 2004;279:9997–10004. [DOI] [PubMed] [Google Scholar]
- 118.Cremesti A, Paris F, Grassme H, Holler N, Tschopp J, Fuks Z, Gulbins E, Kolesnick R. Ceramide enables fas to cap and kill. J Biol Chem 2001;276:23954–23961. [DOI] [PubMed] [Google Scholar]
- 119.Grassme H, Jekle A, Riehle A, Schwarz H, Berger J, Sandhoff K, Kolesnick R, Gulbins E. Cd95 signaling via ceramide-rich membrane rafts. J Biol Chem 2001;276:20589–20596. [DOI] [PubMed] [Google Scholar]
- 120.Gulbins E, Kolesnick R. Raft ceramide in molecular medicine. Oncogene 2003;22:7070–7077. [DOI] [PubMed] [Google Scholar]
- 121.Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell 1983;33:967–978. [DOI] [PubMed] [Google Scholar]
- 122.Marsh M, van Meer G. Cell biology: no escrts for exosomes. Science 2008;319:1191–1192. [DOI] [PubMed] [Google Scholar]
- 123.Fevrier B, Vilette D, Archer F, Loew D, Faigle W, Vidal M, Laude H, Raposo G. Cells release prions in association with exosomes. Proc Natl Acad Sci USA 2004;101:9683–9688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Stoorvogel W, Kleijmeer MJ, Geuze HJ, Raposo G. The biogenesis and functions of exosomes. Traffic 2002;3:321–330. [DOI] [PubMed] [Google Scholar]
- 125.Urbe S, Sachse M, Row PE, Preisinger C, Barr FA, Strous G, Klumperman J, Clague MJ. The uim domain of hrs couples receptor sorting to vesicle formation. J Cell Sci 2003;116:4169–4179. [DOI] [PubMed] [Google Scholar]
- 126.Booth AM, Fang Y, Fallon JK, Yang JM, Hildreth JE, Gould SJ. Exosomes and HIV gag bud from endosome-like domains of the T cell plasma membrane. J Cell Biol 2006;172:923–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Babst M. A protein's final escrt. Traffic 2005;6:2–9. [DOI] [PubMed] [Google Scholar]
- 128.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brugger B, Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008;319:1244–1247. [DOI] [PubMed] [Google Scholar]
- 129.Al-Nedawi K, Meehan B, Rak J. Microvesicles: messengers and mediators of tumor progression. Cell Cycle 2009;8:2014–2018. [DOI] [PubMed] [Google Scholar]
- 130.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature 2004;432:332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT Jr, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport rna and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 2008;10:1470–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, Rak J. Intercellular transfer of the oncogenic receptor egfrviii by microvesicles derived from tumour cells. Nat Cell Biol 2008;10:619–624. [DOI] [PubMed] [Google Scholar]
- 133.Kim VN, Han J, Siomi MC. Biogenesis of small rnas in animals. Nat Rev Mol Cell Biol 2009;10:126–139. [DOI] [PubMed] [Google Scholar]
- 134.Liu X, Sempere LF, Galimberti F, Freemantle SJ, Black C, Dragnev KH, Ma Y, Fiering S, Memoli V, Li H, et al. Uncovering growth-suppressive micrornas in lung cancer. Clin Cancer Res 2009;15:1177–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Croce CM. Causes and consequences of microrna dysregulation in cancer. Nat Rev Genet 2009;10:704–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yanaihara N, Caplen N, Bowman E, Seike M, Kumamoto K, Yi M, Stephens RM, Okamoto A, Yokota J, Tanaka T, et al. Unique microrna molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell 2006;9:189–198. [DOI] [PubMed] [Google Scholar]
- 137.Liu X, Sempere LF, Ouyang H, Memoli VA, Andrew AS, Luo Y, Demidenko E, Korc M, Shi W, Preis M, et al. Microrna-31 functions as an oncogenic microrna in mouse and human lung cancer cells by repressing specific tumor suppressors. J Clin Invest 2010;120:1298–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Seike M, Goto A, Okano T, Bowman ED, Schetter AJ, Horikawa I, Mathe EA, Jen J, Yang P, Sugimura H, et al. Mir-21 is an egfr-regulated anti-apoptotic factor in lung cancer in never-smokers. Proc Natl Acad Sci USA 2009;106:12085–12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant KC, Allen A, et al. Circulating micrornas as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA 2008;105:10513–10518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo J, Zhang Y, Chen J, Guo X, et al. Characterization of micrornas in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res 2008;18:997–1006. [DOI] [PubMed] [Google Scholar]
- 141.Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of micrornas in living cells. J Biol Chem 2010;285:17442–17452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MA, Hopmans ES, Lindenberg JL, de Gruijl TD, Wurdinger T, Middeldorp JM. Functional delivery of viral mirnas via exosomes. Proc Natl Acad Sci USA 107:6328–6333. [DOI] [PMC free article] [PubMed]