Abstract
Oxidative stress plays an important role in immune regulation and dendritic cell (DC) maturation. Recent studies indicate that allergens, including ragweed extract (RWE), possess prooxidant activities, but how RWE interacts with DCs is not well understood. Nuclear erythroid 2 p45-related factor 2 (Nrf2) is a key transcription factor that regulates constitutive and coordinated induction of a battery of antioxidant genes. We hypothesized that RWE would activate DCs and that this response would be augmented in the absence of Nrf2. We generated bone marrow–derived DCs (BM-DCs) and isolated lung DCs from Nrf2+/+ and Nrf2−/− mice and studied the effects of RWE on DCs in vitro. Under resting conditions, Nrf2−/− BM-DCs exhibited constitutively greater levels of inflammatory cytokines and costimulatory molecules than Nrf2+/+ BM-DCs. Exposure to RWE impaired endocytic activity, significantly induced oxidative stress, and enhanced the expression of CD80, CD86, and MHCII in Nrf2−/− BM-DCs when compared with Nrf2+/+ BM-DC, in association with reduced expression of Nrf2-regulated antioxidant genes. RWE significantly induced the secretion of inflammatory cytokines IL-6 and TNF-α in BM-DCs and lung DCs from Nrf2−/− mice than Nrf2+/+ mice and significantly inhibited the secretion of IL-12 in Nrf2+/+ BM-DCs and IL-18 in Nrf2+/+ and Nrf2−/− BM-DCs. The stimulatory effects of RWE on DC activation were inhibited to varying degrees by the antioxidant N-acetyl cysteine. Our findings indicate that a defect in Nrf2-mediated signaling mechanisms alters the response of DCs to a common environmental allergen, which may contribute to the susceptibility to allergic diseases.
Keywords: Nrf2, dendritic cells, ragweed extract, antioxidant genes, oxidative stress
CLINICAL RELEVANCE.
The present study demonstrates the importance of Nrf2-mediated antioxidant signaling mechanisms in dendritic cell maturation, cytokine secretion, and endocytic function, and conclude that the inability to combat oxidative stress by host factors such as Nrf2 may enhance the “innate allergic” immune response.
Dendritic cells (DCs) are professional antigen-presenting cells that link the innate and adaptive immune systems (1). The development of DCs is considered to occur in distinct stages. Hematopoietic pluripotent stem cells generate DC progenitors in the bone marrow, which give rise to circulating precursors in the blood (2, 3). These DC precursors home to peripheral tissues, where they reside as sentinel immature cells with high endocytic and phagocytic capacity (4). In response to microbial pathogens or infectious stimuli, immature DCs (iDCs) undergo activation and maturation and subsequently migrate to draining lymph nodes, where they present antigen to naive T lymphocytes (5, 6). DC maturation is associated with coordinated events, such as loss of endocytic/phagocytic receptors, up-regulation of MHC and costimulatory molecules, and secretion of immunomodulatory cytokines and chemokines (7).
Airway inflammation in allergic asthma is thought to reflect an aberrant immune response against otherwise harmless inhaled allergens (8). Although DCs reside in the airway and are richly interdigitated throughout the bronchial epithelium, relatively little is known about how they are affected by allergens (9, 10). Recent studies using extracts of house dust mites have underscored the importance of airway epithelial–derived signals in driving DC maturation (11, 12). Boldogh and colleagues recently showed that most pollens, including ragweed extract (RWE), possess intrinsic NADPH oxidase activity and that this prooxidant signal is required for RWE-induced airway epithelial activation (13). Whether RWE or other allergens activate DCs in an oxidant-dependent manner has not been previously reported.
Oxidative stress plays an important role in immune regulation, and emerging data indicate that antioxidant status affects DC maturation and the ultimate pattern of immune responses. For example, Peterson and colleagues showed that the depletion of glutathione in murine antigen-presenting cells in vivo resulted in lower Th1 activity and higher Th2 activity (14). Other studies confirmed that DCs rely on intracellular redox state for proper development and function and that DC activation and associated immune functions are influenced by oxidative stress (15–21). Oxidative stress occurs when the oxidants overwhelm antioxidant defenses. To counteract oxidative stress, cells have developed an elaborate defense mechanism to maintain redox homeostasis. We (22, 23) and others (24, 25) have shown that nuclear erythroid 2 p45-related factor 2 (Nrf2) is a key transcription factor that positively regulates a battery of antioxidative and electrophile detoxification genes that protect against oxidative stress. Nrf2 is a redox-sensitive, basic-leucine zipper transcription factor, which, in response to oxidative stress, detaches from its cytosolic inhibitor, Keap1, translocates to the nucleus, and binds to the antioxidant response element in the promoters of target genes, leading to their transcriptional induction (26, 27).
Genetic deficiency of Nrf2 renders mice more susceptible to inflammation and hyperresponsiveness driven by exposure to a model allergen or diesel exhaust particles (23, 28), suggesting that Nrf2 normally functions to keep allergen-driven immune responses in check. Little is known about how Nrf2 regulates immune responses. Nrf2-deficient (Nrf2−/−) mice are more susceptible to sepsis, in association with augmented expression of several innate immune response genes (29), suggesting that Nrf2 regulates innate immunity.
In the present study, we exposed bone marrow precursor–derived DCs (BM-DCs) and lung DCs from Nrf2 wild-type (Nrf2+/+) and Nrf2−/− mice to RWE to investigate whether defective antioxidant defenses in innate immune cells would alter responses to a common environmental allergen. Our data demonstrate that the disruption of the Nrf2 gene in BM-DCs leads to increased oxidative stress and dysregulated cytokine secretion with impaired endocytic activity after RWE exposure. RWE-exposed Nrf2+/+ BM-DCs expressed significantly greater levels of key Nrf2-regulated antioxidative genes when compared with Nrf2−/− DCs. Similar to BM-DCs, DCs isolated from the lungs of Nrf2-deficient mice secreted significantly increased amounts of IL-6 and TNF-α than lung DCs from Nrf2 wild-type mice. Furthermore, Nrf2−/− DCs exhibited constitutively higher levels of expression of cell surface molecules and secreted significantly higher amounts of inflammatory cytokines than Nrf2+/+ DCs.
MATERIALS AND METHODS
Antibodies and Reagents
We used the following antibodies and reagents: Fc block, anti-MHC class II-FITC, anti–CD11c-FITC, anti–CD80-FITC, anti–CD86-FITC, and anti–CD40-PE antibodies (BD PharMingen, San Jose, CA); CD11c MicroBeads and MS magnetic columns (Miltenyi Biotec, Auburn, CA); recombinant murine GM-CSF and recombinant murine IL-4 (Peprotech Inc., Rocky Hill, NJ); IL-12 (IL-12 + p40) and TNF-α ELISA kits (Invitrogen-Biosource, Camarillo, CA); IL-6 and vascular endothelial growth factor (VEGF) ELISA kits and antimouse CD8α-PE antibody (eBioscience, Inc., San Diego, CA); IL-18 ELISA kit (MBL, Woburn, MA); fluorescein-conjugated dextran (Molecular Probes, Eugene, OR); ragweed extract (Greer Laboratories, Lenoir, NC); assay on demand primers and probe sets (Applied Biosystems, Foster City, CA); TRIzol reagent, Superscript First-Strand Synthesis System (Invitrogen, Carlsbad, CA); LPS (Escherichia coli–derived endotoxin, serotype 055:B5), FCS, 2-ME, N-acetyl cysteine, NaN3, 5-amino-2,3-dihydro-1,4-phthalazinedione, glucose, and DNaseI (Sigma, St. Louis, MO); RPMI 1640, l-glutamine, gentamycin sulfate, HEPES buffer, and Dispase (GIBCO-Invitrogen, Carlsbad, CA); and Liberase blendzyme (Roche Diagnostics Corp., Indianapolis, IN).
Animals and Care
Nrf2-deficient CD1:ICR mice were generated as previously described (22, 30). Mice were genotyped for Nrf2 status by PCR amplification of genomic DNA extracted from the tail using three different primers: 5′-TGGACGGGACTATTGAAGGCTG-3′ (sense for both genotypes), 5′-CGCCTTTTCAGTAGATGGAGG-3′ (antisense for Nrf2+/+ mice), and 5′-GCGGATTGACCGTAATGGGATAGG-3′ (antisense for LacZ) (23). All animal experiments were approved by the Johns Hopkins University Animal Care and Use Committee and were conducted in accordance with guidelines set by the US Animal Welfare Acts and National Institutes of Health. Mice were fed an AIN-76A diet and water ad libitum and housed in polycarbonate cages with hard wood chips for bedding in a conventional animal facility maintained under controlled conditions (23 ± 2°C; 55 ± 5% humidity; 12-h light/dark cycle).
Preparation and Heat Denaturation of RWE
We purchased a defatted and freeze-dried extract of the short ragweed Ambrosia artemisiifolia from Greer Labs (lot XP56-D18-1320; Lenoir, NC). The procedure used to prepare and for heat denaturation of RWE can be found in the online supplement.
Generation of Bone Marrow Precursor–Derived DCs
Myeloid DCs were generated from bone marrow–derived precursors of Nrf2+/+ and Nrf2−/− mice using well characterized and commonly used protocols (31) with some modifications by us (32). Bone marrow suspensions were collected from the femurs and tibiae of 8-week-old female mice (five mice per pooled group) by flushing the bone marrow cavity with 2 ml of ice-cold complete culture medium. Cells were centrifuged at 400 × g for 10 minutes at 8°C, and the cell pellet was resuspended in complete culture medium (Dutch modification of RPMI-1640 base medium, supplemented with 20 mM HEPES buffer, 2 mM l-glutamine, 2.5 μg/ml gentamycin sulfate, and 8% heat-inactivated FBS [vol/vol]) by gentle pipetting. Erythrocytes were removed by lysis in AKC buffer (0.15 M NH4Cl, 1.0 mM KHCO3, 0.1 mM Na2EDTA; pH 7.4) for 3 minutes at room temperature. The lysis reaction was quenched by adding 25 ml of ice-cold complete culture medium and centrifugation at 400 × g for 10 minutes at 8°C. Cells were resuspended in PBS and centrifuged twice at 200 × g for 10 minutes at 8°C to deplete the platelets. Cells were then seeded into 6-well plates at 2.5 × 105 cells per well in a total volume of 4.0 ml of complete culture medium and cultured at 37°C under 5% CO2 in a fully humidified incubator. Cultures were pulsed every 2 days for 8 days in complete culture medium supplemented with a combination of GM-CSF (25 ng/ml) and IL-4 (10 ng/ml) that we have shown to reproducibly propagate pure populations of myeloid DCs (32). On Day 8, the loosely adherent cells and the free-floating cells containing the iDCs were harvested, washed, and counted. The iDCs (8 × 105 cells/ml) were cultured in 12-well plates (2.0 ml/well) in medium alone or with Coca's buffer (0.085 M NaCl, 0.06 M NaHCO3; pH 8.1) or with different concentrations (final concentration, 0, 25, 50, and 100 μg [protein equivalent]/ml) of RWE in Coca's buffer as indicated previously. In some experiments, the iDCs (8 × 105 cells/ml) were treated with 100 ng/ml LPS (E. coli–derived endotoxin, serotype 055:B5 in endotoxin-free water) for 48 hours at 37°C in a fully humidified 5% CO2/95% air incubator. After this incubation period, reactive oxygen species (ROS) generation, DC maturation and function-associated markers, cytokine secretion, endocytic activity, and antioxidant gene expression were measured.
Isolation of Lung Dendritic Cells
Wild-type and Nrf2−/− mice were killed, and intact lungs were rapidly excised after flushing the right ventricular cavity with sterile PBS. Lungs were minced using forceps into approximately 1-mm fragments and digested with DNase I (100 mg/ml), liberase blendyme (3.5 mg/ml), and dispase (100 mg/ml) for 30 minutes at 37°C. CD11c+ lung DC were purified from single cell suspensions using two rounds of positive immunomagnetic selection with CD11c MicroBeads and a MS magnetic column. Lung DCs were plated at 8 × 105 per ml of complete medium and stimulated with or without RWE (50 μg/ml) for 48 hours. The amounts of secreted IL-6 and TNF-α were measured by ELISA.
Characterization of Dendritic Cell Maturation Markers
The cell surface expression of various maturation markers and costimulatory molecules on DCs were analyzed using flow cytometric analysis (Becton Dickinson, San Jose, CA). Flow cytometric analysis was used to determine the expression of function-associated molecules in iDCs or RWE-treated DCs. Resting or RWE-treated (post 48 h) iDCs were centrifuged, and the cell pellet was resuspended in PBS containing 2% FCS (vol/vol) and 0.02% (wt/vol) sodium azide. To minimize nonspecific binding, DCs were preincubated with Fc block for 10 minutes and then stained with a panel of fluorescently conjugated antibodies. The following antibodies were used: anti-MHC class II-FITC, anti–CD11c-FITC, anti–CD80-FITC, anti–CD86-FITC, anti–CD40-PE, and antimouse CD8α-PE. For each antibody combination, at least 2.5 × 105 DCs were incubated with the appropriate antibody for 30 minutes at 4°C and washed three times with PBS supplemented with 2% FCS and 0.02% sodium azide. Cells were fixed in 1% paraformaldehyde before flow cytometric analysis using a FACSCalibur system and CellQuest 3.1 software (Becton Dickinson). Immature DCs were characterized as being predominantly of the myeloid phenotype by virtue of the presence of moderate expression of the β2-integrin CD11c, high expression of MHC class II (Ia/Ie), and the absence of the lymphoid DC marker CD8-α. In pilot experiments, we optimized the dose of RWE by investigating its effect on the expression of CD80 and MHCII on the cell surface of Nrf2+/+ and Nrf2−/− BM-DCs. RWE at 25 to 50 μg/ml was found to yield optimal expression of CD80 and MHCII in Nrf2+/+ and Nrf2−/− DCs (see Fig. E1 in the online supplement). The RWE at a final concentration of 50 μg/ml was used for all the experimental analysis.
Cytokine and VEGF Measurements
To measure the cytokines released by DCs, iDCs harvested at Day 8 (8 × 105 cells/ml) were pulsed with RWE (50 μg [protein equivalent]/ml) for an additional 48 hours of culture. The levels of IL-6, IL-18, total IL-12 + p40, TNF-α, and VEGF were quantitated in cell-free supernatants by using commercial ELISA kits according to the manufacturer's instructions. In some experiments, we cultured iDCs with LPS (100 ng/ml) for 48 hours, and the level of total IL-12p40 in culture supernatants was quantified by ELISA. The cytokine levels were reported as pg/million cells.
Treatment with N-Acetyl Cysteine
We optimized the concentration of the antioxidant N-acetyl cysteine (NAC) by investigating its effect on the expression of CD80 and MHCII in BM-DCs from Nrf2+/+ mice. The iDCs from Nrf2+/+ mice were cultured (on Day 8) with different concentrations (0, 1, 10, and 100 mM) of NAC for 1 hour and treated with RWE (50 μg/ml) for 48 hours. The cell surface expression of CD80 and MHCII was analyzed using flow cytometry. Optimum inhibitory effect was obtained with as low as 5 mM concentration of NAC. To determine the effect of the antioxidant NAC on cytokine secretion and on the expression of various maturation markers and costimulatory molecules in Nrf2+/+ and Nrf2−/− DCs, we cultured iDCs (Day 8) with NAC (5 mM) for 1 hour and then with or without RWE (50 μg/ml) for 48 hours followed by analysis of costimulatory molecule expression by flow cytometry and cytokine secretion by ELISA. To determine whether the LPS-induced IL-12 secretion is mediated by oxidative stress, we pretreated the iDCs with NAC (5 mM) for 1 hour and then with LPS (100 ng/ml) for an additional 48 hours. The LPS-induced IL-12 secretion in the culture supernatants was quantified using the ELISA kit.
Analysis of Fluid-Phase Endocytosis by Flow Cytometry
Endocytic uptake of fluorescein-conjugated dextran by DCs was measured by following the modified (32) procedure of Sallusto and colleagues (33) as described in detail in the online supplement.
Determination of Oxidative Stress
A recent study (13) showed that the intrinsic NADPH oxidase activity in RWE induces oxidative stress in cultured human epithelial cells. In the present study, we took two approaches to measure RWE-induced oxidative stress. First, we measured the extracellular mitochondria-derived H2O2 by measuring chemiluminescence from 5-amino-2,3-dihydro-1,4-phthalazinedione (luminol) using a Berthold Biolumat LB9505 six-channel luminometer (Perkin Elmer, Boston, MA) (34). To detect extracellular H2O2, 10 μM luminol and 10 μg/ml horseradish peroxidase were added to 1 ml of PBS (containing 2.5 mM MgCl2 and 5 mg glucose) containing 1 × 106 iDCs and RWE (50 μg/50 μl PBS). Resting iDCs without RWE were used as a control. Immediately after adding luminol and horseradish peroxidase, the chemiluminescence was measured continuously at 37°C for 60 minutes.
To determine whether the ability of the RWE to induce oxidative stress is lost upon heat denaturation, we heat inactivated the RWE at 65°C for 30 minutes. The heat-inactivated RWE was dissolved in 3 ml of sterile PBS. The immature BM-DCs (1 × 106) from Nrf2+/+ and Nrf2−/− mice were treated with or without heat-inactivated RWE (final concentration, 50 μg/ml), and the extracellular mitochondria-derived H2O2 was measured using luminol assay. Second, we used the fluorometric probe 2′7′-dichlorofluorescin diacetate (DCFH-DA) to quantify the intracellular ROS in iDCs. On Day 8 of culture, 100 μl (5 mM final concentration) of DCFH-DA (in sterile PBS [pH 7.4] supplemented with 10 mM HEPES, 0.1% wt/vol gelatin, and 10 mM D-glucose) was added to iDCs (2.0 × 105 cells in 200 μl of PBS) and incubated for 15 minutes at 37°C with agitation. The nonfluorescent DCFH-DA rapidly penetrates the cell membrane, where it is deacetylated by intracellular esterases. The nonfluorescent compound dichlorofluorescin (DCFH) is formed and is trapped in the cytosol, where it is preferentially oxidized by hydrogen peroxide and superoxide to the fluorescent dichlorofluorescein. Immediately after incubation, the iDCs were stimulated with RWE (50 μg/ml) or LPS (100 ng/ml) for various time points (0, 20, 40, and 80 min) with constant agitation at 37°C in sterile pyrogen-free tubes. After each time point, the cells were washed twice with FACS buffer and then suspended in 1% paraformaldehyde (vol/vol in FACS buffer). DCFH-DA is excited at 488 nm, and the fluorescein signal was analyzed at 525 nm (FL1) using a FACScalibur flow cytometer using CellQuest 3.1 software (Becton Dickinson).
Determination of Nrf2-Regulated Antioxidant Genes in DCs
We used quantitative real-time RT-PCR to measure the mRNA levels of three classic Nrf2-regulated genes in resting and RWE-treated DCs using a previous published procedure (23). In brief, total RNA was extracted from DCs with TRIzol reagent and reverse transcribed using the Superscript First-Strand Synthesis System following the manufacturer's instructions. Quantitative real-time RT-PCR analyses of murine glutathione cysteine ligase modifier (GCLm) subunit, GCL catalytic (GCLc) subunit, and heme oxygenase 1 (HO-1) were performed by using primers and probe sets from Applied Biosystems and the ABI 7000 Taqman system. GAPDH was used for normalization, and all PCR reactions were assayed in triplicate.
Statistical Analysis
Data are expressed as mean ± SEM. Six independent experiments (at least five mice per genotype) were performed for in vitro DC stimulation, and three independent experiments were performed for oxidative stress assay (n = 6 mice per genotype) and for real time RT-PCR (n = 3 mice per genotype). Comparisons between paired and unpaired data were tested for significant differences using one- and two-way ANOVA, Student's t test, and post hoc correction according to the Bonferroni method. Statistical analysis was performed by using SigmaStat version 2.03 software and Microsoft Excel statistical data analysis software. P values less than 0.05 were considered statistically significant.
RESULTS
Expression of Maturation Markers and Costimulatory Molecules on RWE-Exposed DCs
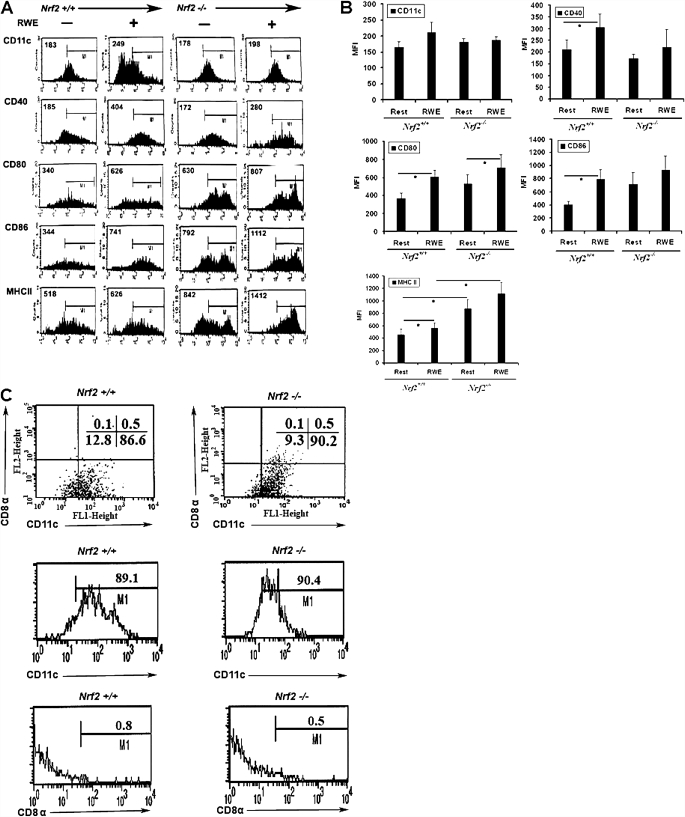
To determine whether the common aeroallergen ragweed would activate dendritic cells, we incubated mouse BM-DCs with RWE and analyzed the expression of a panel of cell surface molecules by flow cytometry. Incubation with RWE led to the significant up-regulation of CD40, CD80, CD86, and MHCII, whereas CD11c expression was not significantly affected (Figures 1A and 1B). The magnitude of induction was greatest on average for CD86 (1.9-fold), followed by CD80 (1.6-fold), CD40 (1.4-fold), and MHCII (1.2-fold). The bone-marrow precursor–derived DCs used in these studies were MHCIIhigh and CD11c+ but did not express the lymphoid marker CD8-α (Figure 1C), indicating that they belong to the myeloid lineage.
Figure 1.
Cell surface expression of maturation markers and costimulatory molecules. Bone marrow precursor–derived dendritic cells from Nrf2+/+ and Nrf2−/− mice were stimulated in vitro with ragweed extract (RWE) (50 μg /ml) for 48 hours, and surface expression of CD11c, CD40, CD80, CD86, and MHCII was measured using two-color flow cytometry. (A) Representative flow histograms from six independent experiments (n = 6 mice per group). Numbers within graph quadrants are percentage of positive cells. (B) Data shown are mean ± SEM of mean fluorescence intensity (MFI) from six independent experiments (n = 6 mice per group). *P < 0.05 when comparing the effect of RWE with medium control (resting). Significant baseline differences between genotype are not indicated in the figure but are contained in Table 1. (C) The purity of immature dendritic cells was further confirmed by staining the cells with anti-CD11c and anti–CD8-α antibodies. The immature dendritic cells from Nrf2+/+ and Nrf2−/−mice are CD11chigh and showed decreased CD8-α expression.
Ragweed and other pollens were recently shown to possess intrinsic NADPH oxidase activity, which in the case of RWE was shown to be required for the induction of airway inflammation in a mouse model (13). We recently showed that mice deficient in the master antioxidant transcription factor Nrf2 developed severe ovalbumin-driven airway inflammation in association with higher oxidative stress (23). We reasoned that BM-DCs derived from Nrf2−/− mice would be more susceptible to activation by RWE, and to test this hypothesis, we incubated BM-DCs from Nrf2−/− mice with RWE and analyzed cell activation as described previously. BM-DCs from Nrf2−/− mice expressed higher levels of CD80 and CD86 than Nrf2+/+ BM-DCs at baseline, which is further augmented by exposure to RWE (Figure 1; Table 1). Nrf2−/− BM-DCs expressed almost twice the cell surface amount of MHCII at baseline, and incubation with RWE increased MHCII expression (∼ 1.2-fold) in Nrf2+/+ and Nrf2−/− DCs, with the highest levels observed in RWE-exposed Nrf2−/− BM-DCs. RWE induced CD80 and MHCII expression at an optimal concentration between 25 and 50 μg/ml, which was used in all subsequent experiments (Figure E1).
TABLE 1.
EXPRESSION OF CELL SURFACE MARKERS IN NRF2 WILD-TYPE AND NRF2 KNOCK OUT BONE MARROW–DERIVED DENDRITIC CELLS AT BASELINE
| Cell Surface Markers |
|||||
|---|---|---|---|---|---|
| DC Phenotypes | CD11c | CD40 | CD80 | CD86 | MHC II |
| Nrf2+/+ | 165 (19) | 211 (42) | 374 (52) | 404 (54) | 449 (95) |
| Nrf2−/− | 181 (12) | 174 (18) | 530 (105) | 718 (176) | 875 (149) |
| P value | NS | NS | 0.08 | 0.06 | 0.03 |
Definition of abbreviations: DC, dendritic cell; NS, not significant.
Values are the mean ± SEM of MIF of six independent experiments. n = 6 mice per genotype. P values were calculated using the paired Student's t test.
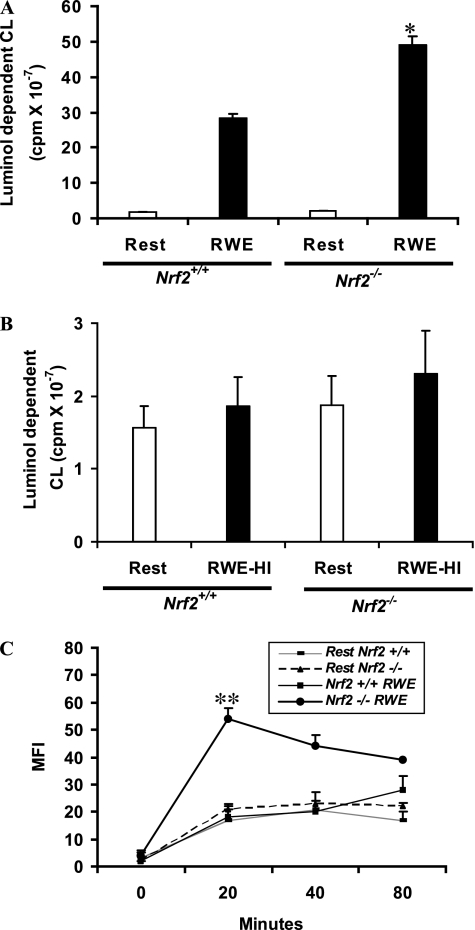
RWE-Induced Oxidative Stress in BM-DCs
We next measured oxidative stress in unstimulated and RWE-exposed DCs using two different methods. We measured extracellular hydrogen peroxide production by the luminol assay. RWE significantly increased extracellular hydrogen peroxide secretion in Nrf2−/− BM-DCs more than wild-type DCs (Figure 2A). The ability of RWE to induce oxidative stress in DCs was lost upon heat inactivation (Figure 2B), suggesting the importance of protein moieties of RWE in inducing oxidative stress. Second, we used the fluorometric probe DCFH-DA to quantify the intracellular ROS levels and found that RWE significantly increased intracellular ROS production in Nrf2−/− DCs (Figure 2C). Unlike RWE-induced extracellular hydrogen peroxide secretion by Nrf2+/+ DCs (Figure 2A), the intracellular ROS produced by RWE-treated Nrf2+/+ DCs was not significantly different from its resting wild-type counterpart or resting Nrf2−/− DCs (Figure 2C).
Figure 2.
Increased oxidative stress in Nrf2−/− dendritic cells (DCs) exposed to ragweed extract (RWE) and LPS. (A) RWE-induced extracellular hydrogen peroxide formation was measured by a peroxidase luminol chemiluminescence method. The chemiluminescence response was initiated by adding 5 μM luminol and 10 μg/ml horseradish peroxidase and continuously monitored at 37°C for 1 hour. Data are mean ± SEM of three independent experiments (n = 6 mice per group). *Significantly higher than the RWE-treated Nrf2+/+ DCs. (B) Heat-inactivated RWE did not induce oxidative stress in Nrf2+/+ and Nrf2−/− DCs. The extracellular hydrogen peroxide formation was measured by a peroxidase luminol chemiluminescence method. Results are mean ± SEM of three independent experiments. RWE-HI = heat-inactivated RWE. (C) RWE-induced intracellular reactive oxygen species was measured using 2′7′-dichlorofluorescin diacetate and flow cytometry. Data are the mean ± SEM of MFI from three independent experiments (n = 5 mice per group). **Significantly higher than the RWE-treated Nrf2+/+ DCs. LPS-induced oxidative stress was significantly higher in Nrf2+/+ DCs (†P < 0.05) and Nrf2−/− DCs (‡P < 0.05) when compared with the respective resting DCs.
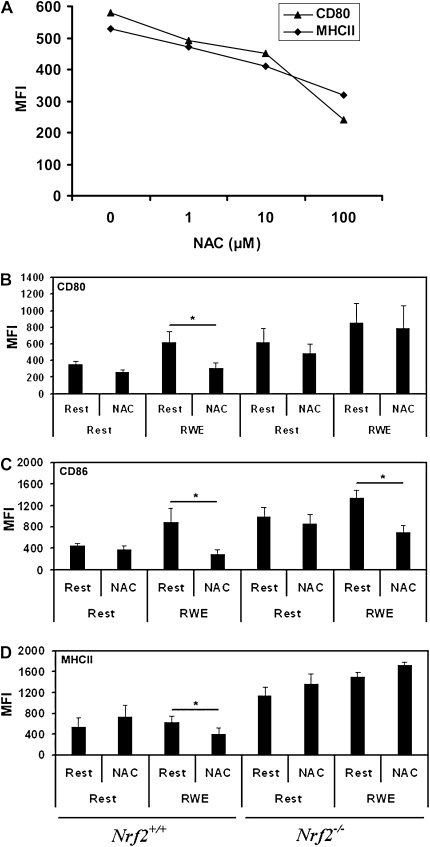
N-acetyl Cysteine Affects RWE-Induced Costimulatory Molecule Expression
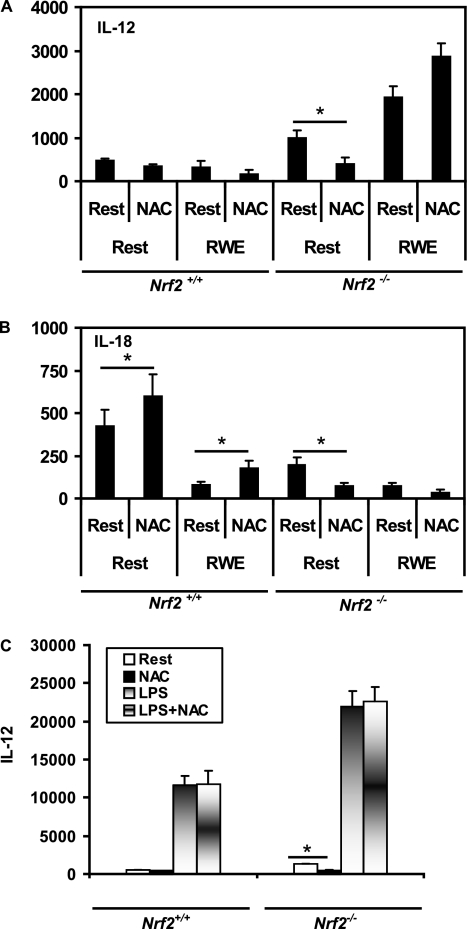
N-acetyl cysteine (NAC) is a widely used antioxidant molecule and has been shown to down-regulate the expression of costimulatory molecules in dendritic cells activated using various agonists. We used NAC to determine if blocking oxidant activity would attenuate RWE-induced costimulatory molecule expression and determined in pilot studies that the optimum concentration of NAC was between 5 and 10 mM in these assays (Figure 3A). These experiments revealed that pretreatment with NAC for 1 hour significantly inhibited the up-regulation of CD80 (Figure 3B), CD86 (Figure 3C), and MHC II (Figure 3D) in RWE-exposed Nrf2+/+ DCs but that NAC did not significantly inhibit RWE-driven CD80 expression in Nrf2−/− DCs (Figure 3B). In contrast to the inhibitory effect of NAC on RWE-driven MHC II expression in Nrf2+/+ DCs, the expression of MHC II was slightly enhanced by NAC in Nrf2−/− DCs (Figure 3D). NAC significantly inhibited RWE-driven CD86 expression in Nrf2−/− DCs (Figure 3C). Incubation with NAC alone did not significantly affect constitutive CD80 or CD86 expression using DCs derived from either genotype. Thus, NAC inhibits RWE-dependent up-regulation of surface molecules, especially CD80 and MHCII, in an Nrf2-dependent manner.
Figure 3.
Effects of N-acetyl cysteine (NAC) on bone marrow precursor–derived DC (BM-DC) surface molecule expression. (A) To determine the inhibitory effect of NAC, immature DCs (Day 8) from Nrf2+/+ mice were preincubated with different concentrations of NAC (0, 1, 10, and 100 mM) for 1 hour and then cultured with RWE (50 μg/ml). After 48 hours, cells were harvested, and the surface expression of CD80 and MHCII were analyzed by flow cytometry after staining with corresponding antibodies. Results of one representative experiment of the three are shown. To determine the effect of NAC on RWE-induced maturation of DCs, BM-DCs from Nrf2+/+ or Nrf2−/− were pretreated with NAC (5 mM) for 1 hour and then exposed to RWE for 48 hours. The expression of (B) CD80, (C) CD86, and (D) MHCII was analyzed by flow cytometry. All data are mean ± SEM of MFI from three independent experiments (n = 5 mice per group). *P < 0.05 for the indicated comparisons.
RWE Affects Fluid-Phase Endocytosis
Endocytosis is an important feature of iDCs, allowing them to efficiently internalize extracellular antigen, which decreases during DC maturation in favor of antigen presentation (35, 36). We determined the endocytotic activity of Nrf2+/+ and Nrf2−/− BM-DCs after treatment with RWE. RWE impaired the endocytic activity of Nrf2−/− DCs (consistent with RWE-induced DC maturation), whereas endocytic activity was enhanced in RWE-exposed Nrf2+/+ DCs (see Methods and Figure E2).
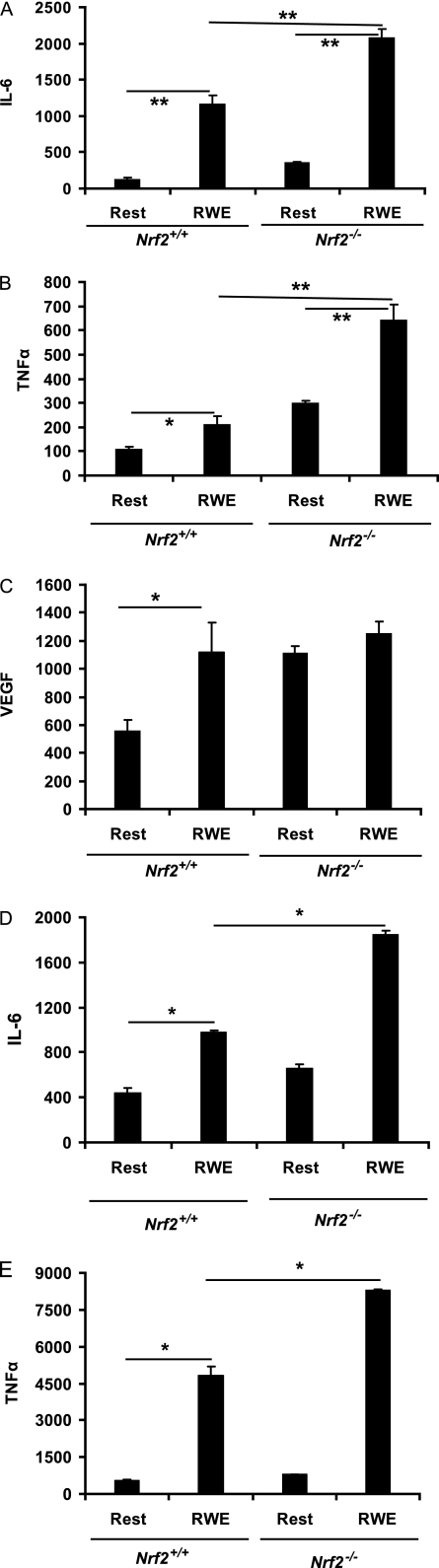
RWE Induces a Complex Pattern of Cytokine Secretion in BM-DCs
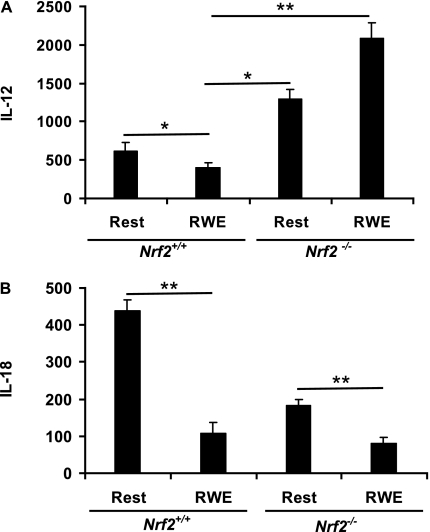
We examined how RWE affected DC secretion of a panel of cytokines implicated in allergic diseases and whether this effect was modified in the absence of Nrf2. The effects of RWE on cytokine secretion were complex and cytokine and genotype dependent. RWE increased the secretion of IL-6 (Figure 4A), TNF-α (Figure 4B), and VEGF (Figure 4C), whereas IL-12 (Figure 5A) and IL-18 (Figure 5B) secretion was inhibited. Figure 4 shows that the greatest effects of RWE were on IL-6 (9-fold increase), whereas TNF-α and VEGF were increased approximately 2-fold in Nrf2+/+ DCs. Similar to the case of costimulatory molecules and MHCII (Table 2), BM-DCs derived from Nrf2−/− mice secreted more IL-6, TNF-α, and VEGF at baseline (Figures 4A and 4B; Table 2). Stimulation with RWE further augmented IL-6 and TNF-α in Nrf2−/− BM-DCs, whereas the level of VEGF was not significantly affected by RWE in Nrf2−/− BM-DCs.
Figure 4.
RWE augments IL-6, TNF-α, and vascular endothelial growth factor secretion by BM-DC and IL-6 and TNF-α secretion in primary lung DCs. Immature BM-DCs from Nrf2+/+ and Nrf2−/− mice were incubated with or without (resting) RWE for 48 hours, followed by analysis of cell-free supernatants for (A) IL-6, (B) TNF-α, and (C) vascular endothelial growth factor by ELISA. Freshly isolated CD11c+ lung DCs (0.8 million cells/ml) from Nrf2+/+ and Nrf2−/− mice were incubated with or without RWE (50 μg/ml) for 48 hours, followed by analysis of cell-free supernatants for (D) IL-6 and (E) TNF-α. Data are expressed as pg/106 cells and are the mean ± SEM (n = 6 mice per group). *P < 0.05; P < 0.05 for the indicated comparisons.
Figure 5.
RWE inhibits IL-12 and IL-18 cytokine secretion from BM-DCs. BM-DCs from Nrf2+/+ and Nrf2−/− mice were incubated with or without (resting) RWE for 48 hours, followed by analysis of cell-free supernatants for (A) IL-12 and (B) IL-18 by ELISA. Data are expressed as pg/106 cells and are the mean ± SEM of six independent experiments (n = 6 mice per group). *P < 0.05; **P < 0.05 for the indicated comparisons.
TABLE 2.
SECRETION OF CYTOKINES AND VEGF BY NRF2 WILD-TYPE AND NRF2-DEFICIENT BONE MARROW–DERIVED DENDRITIC CELLS AT BASELINE
| DC phenotypes | IL-6 | IL-12 | IL-18 | TNF-α | VEGF |
|---|---|---|---|---|---|
| Nrf2+/+ | 128 (17) | 619 (106) | 438 (31) | 104 (15) | 559 (186) |
| Nrf2−/− | 354 (22) | 1,293 (125) | 183 (19) | 296 (13) | 1,109 (54) |
| P value† | <0.001 | 0.003 | 0.001 | <0.001 | <0.001 |
Definition of abbreviation: DC, dendritic cell; VEGF, vascular endothelial growth factor.
Values are in pg/million cells of secreted cytokine. Results are from six independent experiments. n = 6 mice per genotype. P values were calculated using the paired Student's t test.
Although BM-DCs expanded in vitro generate large numbers of cells allowing detailed biochemical studies, these cells do not always reflect those located in peripheral tissues, including the lung. To ensure that primary lung DCs reacted similarly to RWE as BM-DCs, we isolated CD11c+ lung DCs from wild-type and Nrf2−/− mice and studied their response to RWE in vitro. Figures 4D and 4E show that, similar to BM-DC, RWE induces IL-6 and TNF-α secretion from primary lung DCs and that this is significantly augmented using lung DCs obtained from Nrf2−/− mice. Thus, these data indicate that BM-DCs are a reasonable model to study the effects of RWE exposure on DC activation.
We next investigated the effects of NAC on RWE-induced cytokine secretion in Nrf2+/+ and Nrf2−/− BM-DCs. Similar to the case of cell surface molecules, the effects of NAC were cytokine and genotype dependent, although NAC generally had a less pronounced inhibitory effect on RWE-induced cytokine secretion (see Figure E3). In contrast to enhancing IL-6, TNF-α, and VEGF secretion, exposure to RWE instead inhibited BM-DC secretion of IL-12p40 in Nrf2+/+ DCs (Figure 5A) and IL-18 (Figure 5B) in DCs of both genotypes, two cytokines implicated in regulating the Th1/Th2 balance in allergic diseases. The effects of RWE on IL-12 secretion were qualitatively opposite using BM-DCs from Nrf2−/− mice. RWE inhibited IL-12 secretion in Nrf2+/+ BM-DCs (from 619 to 400 pg/million cells; P < 0.05), whereas IL-12 secretion was augmented by RWE in Nrf2−/− BM-DCs (from 1,293 to 2,080 pg/million cells). IL-18 was inhibited by RWE using BM-DCs from Nrf2+/+ mice and Nrf2−/− mice. The effects of NAC on BM-DC IL-12 (Figure 6A) and IL-18 (Figure 6B) secretion were relatively modest, similar to results with IL-6, TNF-α, and VEGF (see Figures E3A–E3C). We did not observe inhibition of IL-12 secretion using LPS as a canonical danger signal, suggesting that this effect in wild-type DCs was specific to RWE (Figure 6C). Instead, LPS augmented IL-12 secretion using DCs from both mouse strains, with significantly more IL-12 produced by Nrf2−/− compared with Nrf2+/+ DCs in a NAC-resistant manner. In contrast, NAC slightly enhanced IL-18 secretion by resting Nrf2+/+ DCs and RWE-induced inhibition of IL-18 in Nrf2+/+ DCs (Figure 6B) and significantly inhibited IL-18 secretion by resting Nrf2−/− DCs (Figure 6B).
Figure 6.
Effects of NAC on BM-DC IL-12 and IL-18 secretion. BM-DCs from Nrf2+/+ and Nrf2−/− mice were preincubated with or without 5 mM NAC followed by RWE and then analyzed for secretion of (A) IL-12 and (B) IL-18. Data are expressed as pg/106 cells and are the mean ± SEM of six independent experiments (n = 5 mice per group). *P < 0.05 for the indicated comparisons. (C) Effect of NAC on LPS-induced IL-12 secretion in DCs. Immature DCs from Nrf2+/+ and Nrf2−/− mice were incubated with NAC (5 mM) for 1 hour and then with RWE for 48 hours. *Significantly inhibited IL-12 secretion in resting Nrf2−/− DCs. Data are the mean ± SEM of three independent experiments (n = 5 mice per group).
Attenuation of Antioxidant Gene Expression in Nrf2-Deficient DCs
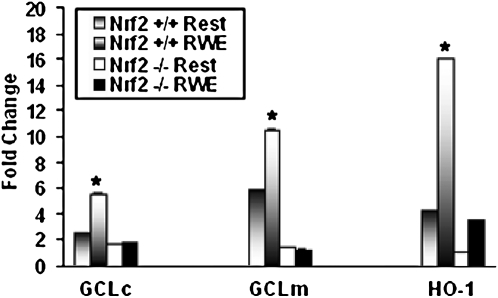
Our data suggest that oxidative stress and Nrf2 are important regulators of DC maturation, but little is known about the expression of antioxidant genes in DCs and how this may be affected by their state of activation. We therefore examined the induction of three classical Nrf2-regulated genes, including GCLc, GCLm, and HO-1, in DCs before or after RWE treatment (Figure 7). RWE significantly induced the expression of GCLc (5.5-fold), GCLm (10.6-fold), and HO-1 (16.1-fold) in Nrf2+/+ DCs. In Nrf2−/− DCs, expression of each of these key antioxidant genes was significantly reduced. The constitutive expression of GCLm and HO-1 was significantly higher in Nrf2+/+ DCs than in Nrf2−/− DCs, and we observed a strikingly lower level of expression of GCLc (1.8-fold), GCLm (1.3-fold), and HO-1 (3.5-fold) mRNAs in Nrf2−/− BM-DCs in response to RWE treatment. Taken together, these data show that RWE is a potent inducer of oxidative stress in DCs, which is enhanced in DCs derived from Nrf2−/− mice, concomitant with reduced expression of three key antioxidative genes.
Figure 7.
Increased transcriptional induction of antioxidant genes in Nrf2+/+ DCs. Quantitative real-time RT-PCR was used to determine the expression of Nrf2-regulated genes in the BM-DCs treated with or without RWE. Real-time RT-PCR analysis showed increased levels of mRNA for genes such as GCLc, GCLm, and HO-1 in the RWE-treated Nrf2+/+ DCs than in Nrf2−/− DCs treated with the pollen allergen. Results are mean ± SEM of three independent experiments (n = 3 mice per genotype). P ≤ 0.05.
DISCUSSION
DCs are thought to contribute to allergic diseases by initiating and propagating T-cell–dependent immune responses and thus provide a link between environmental exposures and allergen-specific immunity. DCs also secrete cytokines that act on many other cell types and cause tissue inflammation. Despite their important role as environmental sensors, relatively little is known about how allergens activate DCs. Pollen allergens including ragweed have been shown to possess intrinsic oxidase activity (13), and oxidative stress has been reported to play a critical role in DC maturation and cytokine secretion. In the present study, we show that a commonly used extract of short ragweed Ambrosia artemisiifolia affected DC surface molecule expression, fluid-phase endocytosis, and cytokine secretion in an oxidant-dependent manner and resulted in an unusual phenotype of DC activation. RWE-dependent DC activation was significantly enhanced in DCs derived from mice deficient in Nrf2, the master antioxidant transcription factor, in association with increased oxidative stress and reduced expression of three key Nrf2-regulated antioxidant genes. Taken together, these data suggest that ragweed is sensed as a “danger signal” by dendritic cells in an oxidative stress- and Nrf2-dependent manner.
During DC maturation, DCs lose their ability to capture and process antigens, increase their expression of MHC II and costimulatory molecules, and up-regulate their production of cytokines (33, 37). Recent studies have revealed that ROS play a critical role in DC activation and maturation (15–21). Boldogh and colleagues demonstrated the critical role of ROS generated by the intrinsic NADPH oxidase activity of RWE in enhancing antigen-induced allergic airway inflammation in mice, possibly by activating airway epithelial cells (13). We used the same extract in our comprehensive in vitro analyses and show that DCs are one key cell type targeted by this common aeroallergen.
RWE significantly induced oxidative stress in DCs, and this was augmented in Nrf2−/− DCs compared with Nrf2+/+ DCs. RWE-induced oxidative stress was lost upon heat inactivation, suggesting that a protein component harbors the oxidative capacity, in keeping with the studies of Boldogh and colleagues (13). We also found that RWE-induced oxidative stress was accompanied by strong up-regulation of CD80, CD86, and HLA-DR cell surface molecules, similar to a recent study by Allakhverdi and colleagues (38) using pollen grain–treated human monocyte–derived DCs. Immature Nrf2−/− DCs expressed higher levels of CD80, CD86, and MHCII when compared with resting Nrf2+/+ DCs at baseline, and this was augmented further by RWE. The antioxidant NAC significantly inhibited RWE-induced up-regulation of each of these molecules in Nrf2+/+ DCs but only CD86 in Nrf2−/− DCs. Taken together, these data suggest that RWE induces surface molecule expression in DCs in an oxidative stress-dependent manner and that the ability of antioxidants to inhibit this process is compromised in the absence of Nrf2.
Dendritic cell maturation is not an all-or-none phenomenon, and subtle differences during this process can affect subsequent immune responses due to soluble and contact-dependent signals that influence T-cell differentiation. In contrast to Th1-promoting DCs, which secrete large amounts of IL-12 and related cytokines, Th2-promoting DCs are generally IL-12lo and express combinations of other cytokines and cell surface receptors (39, 40). We found that RWE induced several features consistent with DC maturation (e.g., enhanced MHCII and costimulatory molecule expression) but inhibited basal IL-12 production in wild-type DCs (Figure 5). Similarly, work by Allakhverdi and colleagues (38) showed that pollen allergen inhibits IL-12 secretion by human monocyte–derived DCs. How exposure to RWE and other allergens influence DC activation and Th2 immune responses is an active area of research. A recent report found that birch pollen inhibited IL-12 production by DCs (41), similar to our findings with RWE. This was attributed to pollen-associated phytoprostanes (41), which, to our knowledge, have not been associated with RWE. We also found that the effects of RWE on BM-DC cytokine production were dependent on Nrf2. In addition to IL-12, RWE induced expression of significantly more TNF-α and IL-6 in BM-DC and primary lung DCs from Nrf2−/− mice compared with their wild-type counterparts. Taken together with the recent observation that Nrf2-deficient mice produced enhanced levels of IL-12, IL-6, and TNF-α after exposure to LPS (29), this suggests that, by keeping proinflammatory cytokine gene expression in check, Nrf2 may play a critical role in regulating activation of innate immune cells in different models. Future studies are needed to determine the precise mechanisms by which Nrf2 regulates cytokine production in dendritic cells and whether Nrf2 deficiency is a risk factor for allergen sensitization in human subjects.
We found the inhibition of IL-12 secretion by RWE in Nrf2+/+ DCs was not restored by the antioxidant NAC and that RWE-induced secretion of IL-12 was enhanced by NAC in Nrf2−/− DCs (see online supplement). The effects of NAC on IL-12 secretion have been shown to be stimulus- and concentration dependent (17, 42), and exactly how Nrf2 deficiency modulates the actions of NAC requires further study. Other products produced by DCs that affect Th2 differentiation include VEGF and IL-18, and we found that secretion of both of these was affected by RWE. Ragweed increased the secretion of VEGF but inhibited IL-18 production by DCs. VEGF has been reported to inhibit IL-12 production and Th1 differentiation by LPS-activated DCs (43), and studies using VEGF overexpressing transgenic mice indicated that VEGF stimulated airway inflammation and remodeling in association with expansion of Th2-promoting lung DCs (44). NAC did not inhibit VEGF secretion in Nrf2+/+ or Nrf2−/− DCs, suggesting that this cytokine may be relatively resistant to the effects of antioxidants. IL-18 was the only cytokine we examined that was produced in higher amounts by resting Nrf2+/+ DCs compared with Nrf2−/− DCs and was inhibited by RWE in DCs of both genotypes. The role of IL-18 has been investigated in murine models of allergic airway inflammation with complex results. In at least one mouse model, IL-18 (together with IL-12) inhibited Th2-driven responses (45). Taken together, our findings suggest that ragweed may promote Th2-domininant allergic immune responses at least in part by inducing IL-12loIL-18loVEGFhi DCs. However, we did not directly investigate the ability of RWE-exposed DCs to stimulate T cells in coculture assays, which requires further study. Future studies should investigate how Nrf2 and antioxidant defenses affect DC maturation and cytokine profiles after RWE exposure in vivo.
We recently reported that Nrf2-deficient BM-DCs were strikingly susceptible to activation by ambient outdoor particulate matter in an oxidative stress-dependent manner (46). Taken together with the data in this report, we conclude that two highly relevant but structurally distinct environmental exposures converge on a common mechanism of DC activation. It will be interesting to study the role of oxidative stress and Nrf2 in innate immune response to other allergens in future studies. Our study revealed increased basal level expression of Nrf2-regulated antioxidant genes GCLc and HO-1 in resting Nrf2+/+ DCs compared with Nrf2−/− DCs (47). Exposure to RWE further augmented the expression of GCLc and GCLm, the two key enzymes involved in glutathione synthesis. Ragweed extract also significantly induced the expression of HO-1, another Nrf2-regulated antioxidant gene, in Nrf2+/+ DCs when compared with Nrf2−/− DCs. HO-1 is a rate-limiting intracellular enzyme that degrades heme to biliverdin, free divalent iron, and CO (48). HO-1 is a stress-responsive gene whose expression has been shown to have a cytoprotective effect against oxidative injury and inflammation (48). In human and rat DCs, induction of HO-1 expression with cobalt protoporphyrin inhibited LPS-induced phenotypic maturation and secretion of proinflammatory cytokines IL-12 p40, TNF-α, and IL-6 (49), suggesting the importance of antioxidant genes in the modulation of DC maturation and cytokine secretion. Thus, decreased expression of HO-1 likely contributes to enhanced activation of Nrf2-deficient BM-DCs in response to RWE.
In summary, our study adds to a growing body of literature indicating that the oxidant activity of allergens is an important determinant of allergic inflammation and suggests a novel link between allergen-induced oxidative stress and activation of DCs. We demonstrate the importance of Nrf2-mediated antioxidant signaling mechanisms in DC maturation, cytokine secretion, and endocytic function and conclude that the inability to combat oxidative stress by host factors such as Nrf2 may enhance the “innate allergic” immune response. Future studies investigating these pathways in human subjects may enhance our understanding of the susceptibility to allergic inflammation and asthma.
Supplementary Material
Acknowledgments
The authors thank Masayuki Yamamoto (Tohoku University Graduate School of Medicine, Japan) for sharing the Nrf2 knock-out mice, Thomas Kensler (Johns Hopkins University) for helpful discussion and critical review of this manuscript, and Kassim Tarore and Rajesh K. Thimmulappa for their assistance on luminol assay.
This work was supported by NIH grant HL081205 (S.B.), NHLBI SCCOR grant P50HL084945 (S.B.), NIEHS Children Asthma Center P50ES015903 (S.B.), NIEHS center grant P30 ES003819 (S.B.), R01 HL073952 (S.G.), R01 HL071933 (S.G.), New Investigator Project from the Center for Childhood Asthma in the Urban Environment (funded by the NIEHS and EPA, P01ES09606 to S.G. and M.A.W.), and a pilot grant (P30 ES03819) from the National Institute of Environmental Health Sciences (M.A.W.).
This article contains an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2008-0438OC on October 5, 2009
Author Disclosure: S.B. received consultancy fees from Merck Frost for $5,001 to $10,000 and lecture fees from Novartis for less than $1,000. He has also received a grant from Quark Pharmaceuticals and the National Institutes of Health for more than $100,001 each. S.G. has received lecture fees from Merck for $1,001 to $5,001. M.W. received a sponsored grant from Health Effects Institute for a contract research program for $50,001 to $100,000 and received a pilot grant from NIEHS for $10,001 to $50,000. He also serves on the program committee of the AII assembly of the ATS (2007 to present). None of the remaining authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Lipscomb MF, Masten BJ. Dendritic cells: immune regulators in health and disease. Physiol Rev 2002;82:97–130. [DOI] [PubMed] [Google Scholar]
- 2.Wu L, Liu YJ. Development of dendritic-cell lineages. Immunity 2007;26:741–750. [DOI] [PubMed] [Google Scholar]
- 3.Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol 2007;7:19–30. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature 1998;392:245–252. [DOI] [PubMed] [Google Scholar]
- 5.Inaba K, Steinman RM. Protein-specific helper T-lymphocyte formation initiated by dendritic cells. Science 1985;229:475–479. [DOI] [PubMed] [Google Scholar]
- 6.Rescigno M, Granucci F, Ricciardi-Castagnoli P. Dendritic cells at the end of the millennium. Immunol Cell Biol 1999;77:404–410. [DOI] [PubMed] [Google Scholar]
- 7.Kleijmeer M, Ramm G, Schuurhuis D, Griffith J, Rescigno M, Ricciardi-Castagnoli P, Rudensky AY, et al. Reorganization of multivesicular bodies regulates MHC class II antigen presentation by dendritic cells. J Cell Biol 2001;155:53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Georas S, Beck L. Dangerous allergens: dendritic cells and innate immunity in allergic asthma. Exp Rev Clin Immunol 2008;4:777–785. [DOI] [PubMed] [Google Scholar]
- 9.Lambrecht BN, Hammad H. Taking our breath away: dendritic cells in the pathogenesis of asthma. Nat Rev Immunol 2003;3:994–1003. [DOI] [PubMed] [Google Scholar]
- 10.Hammad H, Lambrecht BN. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nat Rev Immunol 2008;8:193–204. [DOI] [PubMed] [Google Scholar]
- 11.Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med 2009;15:410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trompette A, Divanovic S, Visintin A, Blanchard C, Hegde RS, Madan M, Thorne PS, Wills-Karp M, Gioannini TL, Weiss JP, et al. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature 2009;457:585–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boldogh I, Bacsi A, Choudhury BK, Dharajiya N, Alam R, Hazra TK, Mitra S, Goldblum RM, Sur S. ROS generated by pollen NADPH oxidase provide a signal that augments antigen-induced allergic airway inflammation. J Clin Invest 2005;115:2169–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peterson JD, Herzenberg LA, Vasquez K, Waltenbaugh C. Glutathione levels in antigen-presenting cells modulate Th1 versus Th2 response patterns. Proc Natl Acad Sci USA 1998;95:3071–3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verhasselt V, Goldman M, Willems F. Oxidative stress up-regulates IL-8 and TNF-alpha synthesis by human dendritic cells. Eur J Immunol 1998;28:3886–3890. [DOI] [PubMed] [Google Scholar]
- 16.Rutault K, Alderman C, Chain BM, Katz DR. Reactive oxygen species activate human peripheral blood dendritic cells. Free Radic Biol Med 1999;26:232–238. [DOI] [PubMed] [Google Scholar]
- 17.Verhasselt V, Vanden Berghe W, Vanderheyde N, Willems F, Haegeman G, Goldman M. N-acetyl-L-cysteine inhibits primary human T cell responses at the dendritic cell level: association with NF-kappaB inhibition. J Immunol 1999;162:2569–2574. [PubMed] [Google Scholar]
- 18.Murata Y, Ohteki T, Koyasu S, Hamuro J. IFN-gamma and pro-inflammatory cytokine production by antigen-presenting cells is dictated by intracellular thiol redox status regulated by oxygen tension. Eur J Immunol 2002;32:2866–2873. [DOI] [PubMed] [Google Scholar]
- 19.Novak N, Kraft S, Haberstok J, Geiger E, Allam A, Bieber T. A reducing microenvironment leads to the generation of FcepsilonRIhigh inflammatory dendritic epidermal cells (IDEC). J Invest Dermatol 2002;119:842–849. [DOI] [PubMed] [Google Scholar]
- 20.Kuppner MC, Scharner A, Milani V, Von Hesler C, Tschop KE, Heinz O, Issels RD. Ifosfamide impairs the allostimulatory capacity of human dendritic cells by intracellular glutathione depletion. Blood 2003;102:3668–3674. [DOI] [PubMed] [Google Scholar]
- 21.Kantengwa S, Jornot L, Devenoges C, Nicod LP. Superoxide anions induce the maturation of human dendritic cells. Am J Respir Crit Care Med 2003;167:431–437. [DOI] [PubMed] [Google Scholar]
- 22.Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest 2004;114:1248–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rangasamy T, Guo J, Mitzner WA, Roman J, Singh A, Fryer AD, et al. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med 2005;202:47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan K, Han XD, Kan YW. An important function of Nrf2 in combating oxidative stress: detoxification of acetaminophen. Proc Natl Acad Sci USA 2001;98:4611–4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanneken A, Lin FF, Johnson J, Maher P. Flavonoids protect human retinal pigment epithelial cells from oxidative-stress-induced death. Invest Ophthalmol Vis Sci 2006;47:3164–3177. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen T, Sherratt PJ, Pickett CB. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol 2003;43:233–260. [DOI] [PubMed] [Google Scholar]
- 27.Cho HY, Reddy SP, Kleeberger SR. Nrf2 defends the lung from oxidative stress. Antioxid Redox Signal 2006;8:76–87. [DOI] [PubMed] [Google Scholar]
- 28.Li YJ, Takizawa H, Azuma A, Kohyama T, Yamauchi Y, Takahashi S, Yamamoto M, Kawada T, Kudoh S, Sugawara I. Disruption of Nrf2 enhances susceptibility to airway inflammatory responses induced by low-dose diesel exhaust particles in mice. Clin Immunol 2008;128:366–373. [DOI] [PubMed] [Google Scholar]
- 29.Thimmulappa RK, Lee H, Rangasamy T, Reddy SP, Yamamoto M, Kensler TW, Biswal S. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J Clin Invest 2006;116:984–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun 1997;236:313–322. [DOI] [PubMed] [Google Scholar]
- 31.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med 1992;176:1693–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams MA, Porter M, Horton M, Guo J, Roman J, Williams D, Breysse P, Georas SN. Ambient particulate matter directs nonclassic dendritic cell activation and a mixed TH1/TH2-like cytokine response by naive CD4+ T cells. J Allergy Clin Immunol 2007;119:488–497. [DOI] [PubMed] [Google Scholar]
- 33.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med 1995;182:389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trush MA, Van Dyke K. Effect of promethazine on human polymorphonuclear chemiluminescence. Pharmacology 1978;16:314–320. [DOI] [PubMed] [Google Scholar]
- 35.Esposito-Farese ME, Sautes C, de la Salle H, Latour S, Bieber T, de la Salle C, et al. Membrane and soluble Fc gamma RII/III modulate the antigen-presenting capacity of murine dendritic epidermal Langerhans cells for IgG-complexed antigens. J Immunol 1995;155:1725–1736. [PubMed] [Google Scholar]
- 36.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell 2001;106:255–258. [DOI] [PubMed] [Google Scholar]
- 37.Caux C, Vanbervliet B, Massacrier C, Dubois B, de Saint Vis, Fayette J, et al. In vitro regulation of development and function of dendritic cells. Hematol Cell Ther 1996;38:463. [DOI] [PubMed] [Google Scholar]
- 38.Allakhverdi Z, Bouguermouh S, Rubio M, Delespesse G. Adjuvant activity of pollen grains. Allergy 2005;60:1157–1164. [DOI] [PubMed] [Google Scholar]
- 39.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol 2003;3:984–993. [DOI] [PubMed] [Google Scholar]
- 40.Williams M, Georas S. Gene expression patterns and susceptibility to allergic responses. Exp Rev Clin Immunol 2006;2:59–73. [DOI] [PubMed] [Google Scholar]
- 41.Traidl-Hoffmann C, Mariani V, Hochrein H, Karg K, Wagner H, Ring J, Mueller MJ, Jakob T, Behrendt H. Pollen-associated phytoprostanes inhibit dendritic cell interleukin-12 production and augment T helper type 2 cell polarization. J Exp Med 2005;201:627–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aihara M, Dobashi K, Akiyama M, Naruse I, Nakazawa T, Mori M. Effects of N-acetylcysteine and ambroxol on the production of IL-12 and IL-10 in human alveolar macrophages. Respiration 2000;67:662–671. [DOI] [PubMed] [Google Scholar]
- 43.Takahashi A, Kono K, Ichihara F, Sugai H, Fujii H, Matsumoto Y. Vascular endothelial growth factor inhibits maturation of dendritic cells induced by lipopolysaccharide, but not by proinflammatory cytokines. Cancer Immunol Immunother 2004;53:543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee CG, Link H, Baluk P, Homer RJ, Chapoval S, Bhandari V, Kang MJ, Cohn L, Kim YK, McDonald DM, et al. Vascular endothelial growth factor (VEGF) induces remodeling and enhances TH2-mediated sensitization and inflammation in the lung. Nat Med 2004;10:1095–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hofstra CL, Van Ark I, Hofman G, Kool M, Nijkamp FP, Van Oosterhout AJ. Prevention of Th2-like cell responses by coadministration of IL-12 and IL-18 is associated with inhibition of antigen-induced airway hyperresponsiveness, eosinophilia, and serum IgE levels. J Immunol 1998;161:5054–5060. [PubMed] [Google Scholar]
- 46.Williams MA, Rangasamy T, Bauer SM, Killedar S, Karp M, Kensler TW, Yamamoto M, Breysse P, Biswal S, Georas SN. Disruption of the transcription factor Nrf2 promotes pro-oxidative dendritic cells that stimulate Th2-like immunoresponsiveness upon activation by ambient particulate matter. J Immunol 2008;181:4545–4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rangasamy T, Williams MA, Kensler TW, Georas SN, Biswal S. Nrf2 modulates the functional maturation of murine dendritic cells by ragweed allergen. American Thoracic Society International Conference, San Francisco, May 18–23, 2007.
- 48.Otterbein LE, Choi AM. Heme oxygenase: colors of defense against cellular stress. Am J Physiol Lung Cell Mol Physiol 2000;279:L1029–L1037. [DOI] [PubMed] [Google Scholar]
- 49.Chauveau C, Remy S, Royer PJ, Hill H, Tanguy-Royer S, Hubert FX, et al. Heme oxygenase-1 expression inhibits dendritic cell maturation and proinflammatory function but conserves IL-10 expression. Blood 2005;106:1694–1702. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.