Abstract
Acute lung injury (ALI) and severe sepsis are common critical illnesses associated with the mobilization of bone marrow–derived cells into the circulation. By identifying and determining these cells' functional characteristics, unique prognostic biomarkers can be developed to help investigators understand the mechanisms underlying the pathophysiology of these disorders. We previously demonstrated an increased colony-forming unit (CFU) ability of circulating peripheral blood mononuclear cells (PBMCs) in patients with ALI, compared with healthy control subjects, that also correlated with improved survival. Here we hypothesized that the increased CFUs in ALI are associated with lung injury, and therefore ALI will result in an increased number of CFUs compared with patients exhibiting severe sepsis. To test this, blood was collected from 80 patients (63 with ALI, and 17 with severe sepsis) within 72 hours of diagnosis, and from 5 healthy control subjects. A CFU assay was performed on isolated PBMCs. Lung injury scores and the need for mechanical ventilation were greater in patients with ALI than in patients with severe sepsis (P < 0.0001 for each). CFU numbers were highest in patients with ALI compared with patients manifesting severe sepsis or control subjects (median CFU number [25–75% quartiles] of 61 [13–104] versus 17 [3–34] versus 5 [2–13], P < 0.0005). A trend toward improved survival was demonstrated in patients with high (≥ 48) CFUs (P = 0.06). No relationship between CFUs and mechanical ventilation was evident. Our findings suggest that increased colony-forming ability by PBMCs in ALI results from lung injury, independent of sepsis and mechanical ventilation. Factors contributing to colony formation by PBMCs in ALI, and the role PBMCs play in its pathogenesis remain to be fully established.
Keywords: endothelium, critical illness, repair, prognosis, ARDS
CLINICAL RELEVANCE.
The development of prognostic biomarkers in the setting of critical illness may help investigators understand mechanisms underlying the pathophysiology of disorders such as acute lung injury and sepsis. In these investigations, we determined that lung injury was independently associated with increased colony formation by circulating peripheral blood mononuclear cells, independent of sepsis, a pulmonary cause for illness, or mechanical ventilation. The exact role of these cells in lung injury remains to be determined.
Endothelial dysfunction is a hallmark of both acute lung injury (ALI) and severe sepsis, resulting from a variety of host immune mediators operating in concert to activate the endothelium (1, 2). This activation results in the extravasation of intravascular volume to the extravascular space, leading to hypotension and shock, the clinical features of severe sepsis (1, 3). In contrast with severe sepsis, ALI is characterized clinically by the development of noncardiogenic pulmonary edema because of excessive vascular endothelial damage and breakdown in alveolar–capillary barrier function, leading to profound hypoxemia and respiratory failure (2, 4). The extent of endothelial damage may directly affect outcomes in both ALI (5) and severe sepsis (6). Therefore, a more thorough understanding of the mechanisms involved in the endothelial repair of these disorders is important. Exploring the composition and functions of the peripheral blood mononuclear cell (PBMC) subsets mobilized during the course of critical illnesses for their role in the repair of endothelial damage has been a focus of recent investigations in ALI and severe sepsis (7–11). Peripheral blood mononuclear cells play a prominent role in the innate immune response, and can increase during critical illness (12). In the circulation, they may participate in both inflammatory and coagulation cascades, releasing mediators with autocrine and paracrine effects, and activating additional monocytes and endothelial cells (1, 13). Assessing the identity and functional capabilities of PBMCs mobilized during critical illness can lead to the development of unique prognostic biomarkers, thereby advancing our understanding of ALI and the pathophysiology of severe sepsis.
Our group has explored the adhesion and growth properties of PBMCs in the setting of critical illness, using a colony-forming unit (CFU) assay. This CFU assay was determined to be potentially useful as a biomarker for outcomes in ALI (7) where higher CFU numbers are associated with survival. In our previous work, CFU numbers from patients with ALI and ventilated control subjects far exceeded what was observed in healthy control subjects. These findings suggested that lung injury may affect the mobilization of a complement of PBMCs that may be exploited to predict the severity of disease as well as outcomes. Investigations in animal models (14, 15) and human subjects (16) indicate that lung injury results in the release of bone marrow–derived cells into the circulation that may then home to the lungs. This may in part be related to the upregulation of chemotactic factors, such as stromal cell–derived factor-1 (SDF-1, also known as CXCL12) (15). We hypothesized that PBMCs from patients with ALI would demonstrate an increased ability to form CFUs in our culture system when compared with PBMCs from patients with severe sepsis without ALI or healthy control subjects. Secondarily, our group sought to confirm the prognostic utility of enumerating CFUs obtained from cultures of PBMCs in patients with ALI and severe sepsis in relationship to survival. We also wanted to better characterize these colonies, and determine if cells in these CFUs had potential paracrine capabilities relevant to endothelial repair. Some of the research in this study was presented previously in abstract form (17, 18).
MATERIALS AND METHODS
Patient Characteristics
Patients in intensive care were screened from among University of Colorado Hospital and Emory University–affiliated intensive care units. Patients with ALI were enrolled if they met the American-European Consensus Committee criteria (19), and had an at-risk diagnosis for ALI. Patients with severe sepsis met the Society for Critical Care Medicine criteria for this disorder (20). Patients were considered to have a pulmonary cause for their illness (either ALI or sepsis) based on an at-risk diagnosis of pneumonia (from any cause) or aspiration. Patients were considered to have a nonpulmonary cause for their illness based on at-risk conditions that included sepsis from a source other than the lung, pancreatitis, or multiple transfusions. For certain experiments, blood samples were obtained from healthy control subjects without evidence of ALI or sepsis. Our study was approved by the Institutional Review Boards at the University of Colorado and at Emory University.
Colony-Forming Unit Assays
Approximately 16 ml of peripheral blood were obtained from patients with ALI, patients with severe sepsis, and healthy control subjects at a single time point. We isolated PBMCs using density-gradient centrifugation (Becton-Dickinson [BD] Vacutainer CPT Cell Preparation Tube; BD, Franklin Lakes, NJ). Recovered cells were processed as described previously (7). Briefly, cells were washed twice with 1× Dulbecco's PBS (Mediatech, Manassas, VA). Isolated cells were then resuspended in growth medium consisting of Dulbecco's modified Eagle's medium (DMEM) (Invitrogen, Carlsbad, CA), supplemented with 20% heat-inactivated FBS (Atlanta Biologicals, Lawrenceville, GA), 6.5% endothelial cell growth supplement (ECGS) (BD, Franklin Lakes, NJ) resuspended in 5.2 ml of DMEM, and 1% penicillin-streptomycin (Invitrogen), before being plated on Biocoat fibronectin-coated 35-mm dishes (BD). After incubating for 24 hours, nonadherent cells were collected from the supernatant of the 35-mm dish. These nonadherent cells were counted, and then replated onto a fibronectin-coated 24-well plate (BD) at a concentration of 1 × 106 cells/well for the final assessment of number of CFUs per well. A minimum of three wells was plated per subject. Culture media were changed after 3 days. Numbers of CFUs were counted manually after 7 days of incubation by a single observer blinded to the purpose of the experiments, and the average number of CFUs/well was recorded for each subject.
Characterization of CFUs: Staining Techniques
Replicates of CFUs derived from enrolled patients were assessed for low-density lipoprotein (LDL) uptake and lectin binding (7, 21). After removing media from the wells of a patient's 24-well plate, 1 ml of 2.4 μg/ml acetylated LDL, labeled with 1,1'-dioctadecyl–3,3,3',3'-tetramethyl-indocarbocyanine perchlorate (DiI AcLDL) (Invitrogen) was added to each well, and the plate was incubated at 37°C for 1 hour. The DiI AcLDL solution was then removed, and cells were fixed with 1 ml of 2% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) for 10 minutes at room temperature. After removal of the fixative, cells were gently washed twice with PBS. Afterwards, 1 ml of 50 μl per 4 ml of FITC-labeled Ulex europaeus (UEA-1) lectin (Sigma-Aldrich, St. Louis, MO) was added to each well. The plate was then incubated at 37°C for 1 hour. The lectin solution was then removed, and the culture wells were washed once with PBS. Culture wells were stained with 4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich), 10 μl per 10 ml PBS, for 10 minutes at room temperature to stain the nuclei. The DAPI was removed, and the wells were rinsed once with PBS. Stained wells were then stored in 1 ml of PBS at 4°C. Stained colonies were examined microscopically and photographed by an observer blinded to the purpose of the experiments.
Replicates of CFUs derived from enrolled patients with ALI and healthy control subjects were also examined via immunostaining. The PBMCs isolated from whole blood were grown in the media described previously at a concentration of 1 × 106 cells/ml in Biocoat four-well glass culture slides coated with fibronectin (BD), in lieu of plastic fibronectin-coated plates. Human microvascular endothelial cells (HMVEC-LBI) (Lonza, Walkersville, MD), murine mesenchymal stem cells (22), and Jurkat T cells (ATCC, Manassas, VA) were also grown in appropriate media on Biocoat four-well culture slides coated with fibronectin as control samples. Before staining, media were aspirated from culture slides, and cells were washed once with PBST (1 × PBS with 0.1% Tween-20; Fisher Scientific, Pittsburgh, PA). As primary antibodies we used VE-cadherin (also known as CD144; Enzo Life Sciences, Inc., Plymouth Meeting, PA), Flt-1 (also known as vascular endothelial growth factor receptor-1, or VEGFr1; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), E-selectin (Santa Cruz Biotechnology, Inc.), smooth muscle actin (SMA; Dako, Carpinteria, CA), and CD45 (Santa Cruz Biotechnology, Inc.). Primary antibodies were incubated for 1 hour at room temperature, except for the SMA, which was applied after fixation for 30 minutes at room temperature. Cells were washed twice for 5 minutes with PBST, and then fixed with 4% paraformaldehyde in PBS at room temperature for 20 minutes. Cells were again washed twice for 5 minutes in PBS. Secondary antibodies, including Alexa Fluor 594 goat anti-rabbit IgG, Alexa Fluor 488 goat anti-mouse IgG, and Alexa Fluor 488 rabbit anti-goat IgG (all from Invitrogen), were diluted 1:500 in PBST with 5% FBS, applied, and incubated at room temperature for 30 minutes. Cells were again washed with PBS for 20 minutes. The culture wells were then removed, and the slides were mounted with coverslips and Vectashield with DAPI (Vector Laboratories, Inc., Burlingame, CA). After immunofluorescence images were obtained, the presence of collagen on the culture slides was evaluated using Masson's trichrome (Chromaview; Richard-Allan Scientific, Kalamazoo, MI). Photographs were taken using a Nikon Eclipse 90i/DSFi1 camera (Nikon Instruments, Inc., Melville, NY) with NIS Elements AR 3.0 software (Nikon Instruments).
Characterization of CFUs: Real-Time Reverse Transcription Polymerase Chain Reaction
After 7 days of culture, CFUs in wells from patients and control subjects were disrupted and collected using 700 μl of Trizol (Invitrogen). Samples were vortexed for 1 minute to homogenize cells, and were stored at −80°C. After samples were collected, RNA was extracted using QIAshredder columns and the RNeasy Mini Kit (Qiagen, Valencia, CA). The protocol used on-column DNase digestion with the RNase-free DNase Set (Qiagen). Briefly, thawed cells in Trizol were pipetted onto a QIAshredder column and centrifuged for 2 minutes at maximum speed. The homogenate was incubated for 5 minutes at room temperature, and then 140 μl of chloroform were added. The tube was shaken vigorously for 15 seconds, and incubated at room temperature for 3 minutes The sample was centrifuged for 15 minutes at 12,000 × g at 4°C. The aqueous phase was transferred to a new collection tube, and 1.5 volumes of 100% ethanol were added. The sample was mixed thoroughly and transferred to an RNeasy Mini spin column (Qiagen). On-column DNase digestion was also performed according to the manufacturer's instructions. To elute the RNA, 30 μl of the RNase-free water provided were pipetted onto the column, and the column was incubated for 10 minutes at room temperature before centrifugation. The second elution step was performed using the first eluate, also incubated for 10 minutes at room temperature before centrifugation. After elution, RNA quantity and quality was determined using the NanoDrop Spectrophotometer (Thermo Scientific, Wilmington, DE). For each sample, 100 ng of total RNA were reverse-transcribed using the High Capacity RNA-to-cDNA Kit (Applied Biosystems, Foster City, CA). The manufacturer's protocol was followed, and the cDNA was quantified using the NanoDrop Spectrophotometer. For quantitative real-time PCR, 50 ng of cDNA were brought up to 9 μl with nuclease-free water and combined with 1 μl of TaqMan Gene Expression Assay and 10 μl of TaqMan Gene Expression Master Mix (Applied Biosystems). Assays (including a no-template control) were performed in triplicate under standard real-time PCR conditions (50°C for 2 minutes, 95°C for 10 minutes, and 60 cycles of 95°C for 15 seconds, followed by 60° for 1 minute), using a 7300 Real Time PCR System (Applied Biosystems) with sequence-detection software. Inventoried TaqMan Gene Expression Assays (Applied Biosystems) were used to measure levels of mRNA expression for β-actin (assay identification Hs99999903_m1), CD34 (assay identification Hs500156373_m1), CD45 (assay identification Hs00236304_m1), Flt-1/VEGFr1 (assay identification Hs01052937_m1), HGF (assay identification Hs000300159_m1), and CXCL12/SDF-1 (assay identification Hs00171022). We used β-actin as an endogenous control. Relative quantitation of mRNA expression from all samples was performed using the standard curve method. This method uses a set of relative standards from which unknown samples are quantitated. The calibrator for these experiments was the value for listed genes in either HMVEC-LBI (all genes except CD45) or Jurkat T cells (CD45 only) as the basis for comparing results. For all experimental samples, the target quantity was determined by interpolating the value of the experimental sample on the standard curve, and then dividing by the target quantity of the calibrator. Experimental sample quantities are expressed as an n-fold difference relative to the calibrator. As a general example, the experimental (test) sample target mass is normalized by its respective endogenous control results to calculate a normalized experimental value. Then the calibrator sample target mass is normalized by its respective endogenous control result to calculate a normalized calibrator sample value. Finally, the fold difference in the target gene's expression is calculated by dividing the normalized experimental value by the normalized calibrator sample value.
Statistical Analysis
Statistics were performed using JMP software (SAS Institute, Inc., Cary, NC). Non-normally distributed data are reported as median and 25–75% quartiles. Nonparametric analyses (Wilcoxon and Kruskal-Wallis tests) were performed on these data. Normally distributed data were expressed as means with SDs, and assessed using the Student's t test. When three or more groups were compared and found to differ statistically, a post hoc Dunnett test was performed to compare experimental and control groups. Pearson's test or Fisher's exact test was used to compare categorical data. Univariate analyses were initially performed to determine the clinical factors associated with numbers of CFUs. Based on these results, multivariable logistic regression analyses were performed, including those variables determined to be significant in the univariate analyses, along with others that were previously reported to be associated with CFU numbers, including age, sex, and use of tobacco. A backward elimination modeling strategy was used. Individual interaction terms between each individual confounding variable were entered into the initial model to assess for effect modification; no interaction terms were significant (P value > 0.05). Log rank testing was used to assess the survival differences between groups stratified by CFU numbers in a Kaplan-Meier analysis. Reported P values are two-sided, and an α value of 0.05 was used in all analyses.
RESULTS
Patient Characteristics
At the completion of the study, 17 patients with severe sepsis and 66 patients with ALI were enrolled. Data from three patients with ALI were excluded because of culture contamination, leaving 63 patients with ALI for the final analyses, for a total of 80 patients with ALI or severe sepsis. The demographics are listed in Table 1. The two groups were similar in terms of age, sex, and severity of illness (Acute Physiology and Chronic Health Evaluation [APACHE] II scores). The ALI group more commonly had a pulmonary etiology for their illness, required mechanical ventilation more frequently, and had a significantly higher lung injury score compared with those with severe sepsis. Although only five of 63 patients with ALI did not have sepsis at any point during their hospitalization, only 54% met the criteria for septic shock, compared with 76% in the severe sepsis group, entailing a significant difference (P < 0.05). A majority (59%) of our subjects with severe sepsis required mechanical ventilation, likely because 29% of these patients had a pulmonary source for their sepsis (e.g., pneumonia), and because of their severity of illness. Those individuals with a pulmonary source for sepsis tended to have a higher mean APACHE II score (27 in the pulmonary sepsis group, compared with 22 in the nonpulmonary sepsis group; P = 0.13).
TABLE 1.
DEMOGRAPHICS OF ENROLLED SUBJECTS
| Patients with ALI (n = 63) | Patients with Severe Sepsis (n = 17) | P Value | |
|---|---|---|---|
| Age (mean ± SD) | 53 ± 16 | 58 ± 18 | 0.26 |
| Sex (% men) | 59% | 65% | 0.78 |
| Percent smokers | 46% | 35% | 0.58 |
| Percent alcoholic | 27% | 18% | 0.54 |
| WBC (mean) | 17 ± 8 | 17 ± 8 | 0.97 |
| Mortality | 30% | 12% | 0.21 |
| Percent with pulmonary source for illness | 63% | 29% | <0.01 |
| Need for mechanical ventilation (%) | 100% | 59% | <0.0001 |
| Lung injury score (median, with range) | 2.5 (2.0–3.0) | 1.0 (0–2.0) | <0.0001 |
| APACHE II score (mean ± SD) | 25 ± 7 | 23 ± 7 | 0.44 |
| SOFA score (mean ± SD) | 11 ± 4 | 9 ± 4 | 0.06 |
Definition of abbreviations: ALI, acute lung injury; APACHE II, Acute Physiology and Chronic Health Evaluation II score; SOFA, Sequential Organ Failure Assessment score; WBC, white blood cell.
We also enrolled five healthy control subjects, three of whom were women. The mean age of control subjects was 51 ± 12 years, and none had any known comorbidities.
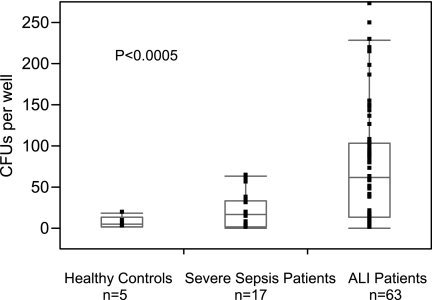
Numbers of CFUs Correlate Positively with a Diagnosis of ALI
The median number of CFUs grown from peripheral blood in the first 72 hours of illness was found to be significantly higher in patients with ALI (median CFU number [25–75% quartiles] of 61 [13–104]) than in patients with severe sepsis (median CFU number [25–75% quartiles] of 17 [3–34]) or in healthy control subjects (median CFU number [25–75% quartiles] of 5 [2–13]), P < 0.0005, Figure 1. A weak association between number of CFUs and peripheral white blood cell (WBC) count on the day the sample was obtained was evident (P < 0.008, R2 = 0.08). Stratifying these 80 subjects (excluding control subjects) into groups by source of illness revealed no significant differences in CFU numbers (pulmonary [n = 45] versus nonpulmonary [n = 35], P = 0.62). To determine the effects of sepsis on number of CFUs independent of lung injury, we examined data from the ALI cohort stratified by sepsis, and found no significant differences in median numbers of CFUs between ALI subjects with sepsis (median CFU number [25–75% quartiles] of 75 [15–112]) and ALI subjects without sepsis (median CFU number [25–75% quartiles] of 47 [7–78]); P = 0.31. To determine the effects of mechanical ventilation on numbers of CFUs independent of ALI, the relationship between mechanical ventilation and numbers of CFUs was examined for the cohort of subjects with severe sepsis. The need for mechanical ventilation among the cohort with severe sepsis was determined not to be associated with higher numbers of CFUs (P = 0.75). No other demographic variables, including mortality, smoking, initial APACHE II and Sequential Organ Failure Assessment (SOFA) scores, lung injury scores, PaO2/FiO2 ratio, history of alcohol abuse, tobacco use, subject's age, or sex, correlated with CFUs in univariate analyses.
Figure 1.
Number of colony-forming units (CFUs) per well in healthy control subjects (n = 5), in patients with severe sepsis (n = 17), and in patients with acute lung injury (ALI) (n = 63). The CFUs were grown from peripheral blood mononuclear cells obtained within 72 hours of diagnosis for each group. Data are presented as medians (line within box) with 25% (top of box) and 75% (bottom of box) quartiles. Whiskers indicate 10% and 90% ranges of data.
Patients were stratified into a group with low CFUs of less than 48 and a group with high CFUs of greater than or equal to 48, based on the median number of CFUs for the entire group of 80 patients (patients with ALI and severe sepsis combined). In a multivariable logistic regression analysis, a diagnosis of ALI was predictive of high CFU numbers (≥48), with an odds ratio of 4.48 (95% confidence interval [CI], 1.87–10.72; P < 0.0007) accounting for the patient's source for illness (nonpulmonary versus pulmonary), SOFA score, and WBC count. Moreover, in this multivariable analysis, a nonpulmonary source of illness also correlated with higher CFU number, with an odds ratio of 2.17 (95% CI, 1.19–3.98; P < 0.01) (Table 2).
TABLE 2.
MULTIVARIABLE LOGISTIC REGRESSION ANALYSIS OF PREDICTORS FOR HIGH (≥ 48) COLONY-FORMING UNIT NUMBERS
| Beta | Odds | 95% CI | P Value | |
|---|---|---|---|---|
| ALI diagnosis | 1.50 | 4.48 | 1.87–10.72 | 0.0007 |
| Nonpulmonary source of illness | 0.78 | 2.17 | 1.19–3.98 | 0.01 |
| SOFA score | −0.12 | 0.89 | 0.77–1.03 | 0.11 |
| WBC count | 0.04 | 1.04 | 0.98–1.11 | 0.18 |
| Intercept | −0.25 | 0.78 | 0.14–4.18 | 0.09 |
Definition of abbreviations: ALI, acute lung injury; SOFA, Sequential Organ Failure Assessment score; WBC, white blood cell.
Higher Numbers of CFUs Correlate with Survival in Those with ALI and Severe Sepsis
To explore the utility of CFUs in disease prognostication, additional analyses were performed to examine the relationship of CFUs to mortality in a Kaplan-Meier survival analysis. We stratified the group of 80 patients with ALI or severe sepsis by their median CFU number into either high (≥48) or low (<48) CFU subsets. Analysis of this data revealed that those with high numbers of CFUs had overall better survival (62% surviving at 28 days) than did those with low numbers of CFUs (30% surviving at 28 days; P = 0.06 by log rank testing; Figure 2). The majority of patients with sepsis (14/17) fell into the low CFU subset, and their mortality was 7% at 28 days (1/14 died), compared with 33% (1/3 died) in the high CFU subset (P = 0.33).
Figure 2.
Entire cohort of patients with ALI and severe sepsis was stratified based on overall median number of CFUs (48 CFUs/well). In a Kaplan-Meier analysis, in-hospital survival tended to be longer among those with high CFUs (≥ 48; blue), compared with those with low CFUs (< 48; red). P = 0.06 by log rank testing.
CFUs Express Endothelial-Like Surface Antigens
CFUs appeared (on average) within 5 days of nonadherent cells being plated. These consisted of rounded clusters of cells, surrounded by individual spindle-shaped cells in a “starburst” pattern. To characterize the cellular composition of our CFUs better, additional staining experiments were performed. CFUs stained robustly for UEA-1 lectin binding, whereas DiI AcLDL uptake was detected primarily at the periphery of CFUs (Figure 3).
Figure 3.
Photomicrographs of a CFU, derived from 7 days' culture of peripheral blood mononuclear cells from a patient with ALI. Green indicates lectin, red indicates LDL, and blue indicates DAPI. (A) Unstained, bright-field microscopy of CFU. (B) CFU stained for lectin binding, LDL uptake, and DAPI nuclear counterstain. (C) CFU stained for LDL uptake with DAPI nuclear counterstain. (D) CFU stained for LDL uptake.
Additional immunostaining assays were performed after 7 days of culture, using fibronectin-coated glass slides with PBMCs from patients with ALI and healthy control subjects. The PBMCs cultured from both the patients with ALI and healthy control subjects expressed the cell surface antigen VE-cadherin (Figure 4), as well as E-selectin and Flt-1/VEGFr-1 (data not shown). Neither group's cells expressed the mesenchymal marker SMA significantly (Figure 4). After 7 days in culture, CD45 was expressed dimly by cells from either group (Figure 5). Additional Masson's trichrome staining detected collagen in both control subjects and patients with ALI (Figure 6) in areas where cell–cell contact was present in culture. However, isolated cells that were not in colonies did not appear to express collagen in either control subjects or patients with ALI.
Figure 4.
Photomicrographs of CFU immunostaining after culturing peripheral blood mononuclear cells for 7 days on fibronectin-coated glass slides. Scale bar = 100 μm. Red indicates VE-cadherin, green indicates α-smooth muscle actin, and blue indicates DAPI nuclear stain. (A–D) Sample from a representative healthy control subject. (E–H) Sample from a representative patient with ALI. (B, F, J ) Enlarged photographs of VE-cadherin staining, to show details. Spindle-shaped cells were evident only in patients with ALI. (I–L) Primary human microvascular pulmonary endothelial cells were used in these experiments as positive controls for VE-cadherin. (M) Murine mesenchymal stem cells were used as positive controls for α-smooth muscle actin.
Figure 5.
Photomicrographs of CFU immunostaining after culturing peripheral blood mononuclear cells for 7 days on fibronectin-coated glass slides. Scale bar = 100 μm. Green indicates CD45, and blue indicates DAPI nuclear stain. (A–C) Sample from a representative healthy control subject. (D–F) Sample from a representative patient with ALI. (G–I) Jurkat T cells were used as positive controls for CD45. (B, E, H) Enlarged photographs of CD45 staining to show details. Dim cell surface staining for CD45 was observed in healthy control subjects and patients with ALI.
Figure 6.
Photomicrographs of CFU trichrome staining for collagen after culturing peripheral blood mononuclear cells for 7 days on fibronectin-coated glass slides. Blue indicates collagen. (A–B) Samples from healthy control subject. (C–D) Samples from a representative patient with ALI. (E) Arterial vessel wall for positive control. Collagen staining was occasionally observed in both control subjects and patients with ALI at sites of cell–cell contact, but not in cells outside of CFUs.
CFUs Express mRNA Consistent with Endothelial-Like and Hematopoietic Cells, as Well as Paracrine Factors Involved in Endothelial Repair
Real-time RT-PCR was performed on CFUs from randomly selected patients with ALI (n = 7) and severe sepsis (n = 5), as well as healthy control subjects (n = 5) (Figure 7). Comparing median gene expression across all three groups for the hematopoietic marker CD45 revealed a 1.5-fold higher degree of gene expression in patients with ALI versus patients with severe sepsis, and a 7.5-fold higher degree than healthy control subjects (P < 0.02 by nonparametric testing). Similarly, expression for the endothelial marker Flt-1 in ALI was 4-fold higher than what was observed in patients with sepsis, and 13-fold higher than in healthy control subjects (P < 0.05 by nonparametric testing). No expression of the progenitor cell marker CD34 was detectable in any patient or control subject. In terms of paracrine factors potentially secreted by these CFUs, we were able to detect SDF-1/CXCL12 and hepatocyte growth factor (HGF) mRNA expression in both patients and control subjects, but these did not differ significantly across groups.
Figure 7.
Gene expression analysis of Flt-1, CD45, hepatocyte growth factor (HGF), and stromal cell–derived factor-1 (SDF-1) using real-time RT-PCR. Data were calculated by the standard curve method and expressed as median-fold difference in expression (line within box), with 25% (top of box), and 75% (bottom of box) quartiles. Whiskers indicate 10% and 90% range of data. Relative quantitation of mRNA expression from all subject samples was performed using the standard curve method, employing the use of a set of relative standards as calibrators for the experimental samples. The calibrator for Flt-1, HGF, and SDF-1 illustrated here was human microvascular endothelial cells. Experimental sample quantities are presented as n-fold difference relative to the calibrator value. Each patient's sample and calibrator samples were assessed in triplicate.
DISCUSSION
In these investigations, we determined that a diagnosis of ALI was independently associated with increased numbers of CFUs formed by culturing PBMCs, accounting for such factors as severity of illness, primary cause of illness (pulmonary versus nonpulmonary), mechanical ventilation, a diagnosis of sepsis, and peripheral WBC count. The CFU-forming ability of PBMCs in severe sepsis was not significantly different from that in healthy control subjects. More numerous CFUs were also associated with improved survival, regardless of whether the underlying diagnosis was ALI or severe sepsis, similar to what we previously observed (9). To help explain these observations and understand the utility of the CFU assay as a biomarker, we determined that the most striking clinical differences between patients with ALI and those with severe sepsis involved the severity of lung injury (as determined by Murray lung injury scores) and the need for mechanical ventilation. Additional analyses focusing on the ALI cohort revealed no association between CFUs in the setting of sepsis. Further, in the severe sepsis cohort, number of CFUs did not correlate with mechanical ventilation. Finally, in a multivariable analysis, a nonpulmonary cause for illness was positively associated with number of CFUs. These observations suggest that lung injury, but not sepsis, mechanical ventilation, or a pulmonary source of illness, is a specific stimulus that leads to the release of a complement of cells from bone marrow with colony-forming ability. The relationship between CFUs and outcomes provides further evidence for a prognostic role of the CFU assay in lung injury. Further experiments to characterize CFUs found that cells within CFUs express endothelial-like cell surface markers, and that mRNA expression differed for Flt-1 and CD45 across groups, suggesting potential functional differences depending on the disease state. To our knowledge, this is the first report of these differences. Additional investigations are necessary to define these mobilized cells' roles and capabilities more precisely.
These experiments were performed to clarify not only the potential of PBMCs mobilized in critical illness to be used as biomarkers, but to better define the characteristics of these CFUs and to understand their pathophysiologic role in disease. Our CFU assay most closely mimics the commercially available CFU assay popularized by Hill and colleagues (21). These assays are known to be affected by the composition of cells within culture, particularly immune cells. They are useful in providing a measurement of cell-to-cell interactions between hematopoietic and/or inflammatory cells, but do not assess structural vasculogenic activity (23–25). The technique we used was not intended to identify true “endothelial progenitor cells,” as was done with PBMCs cultured in endothelial conditions for a period of 7–21 days that will ultimately form tubes and vessels in vitro (23, 25). Culturing PBMCs in a CFU assay can result in the assumption of endothelial features by PBMCs, including the expression of endothelial cell surface markers. We in fact observed endothelial cell surface marker staining by CFUs. A much smaller number exhibited collagen staining, and none stained for SMA. These results suggest that our CFUs do not contain significant numbers of cells with mesenchymal properties. We also used RT-PCR to assess the expression of mRNA by CFUs for paracrine factors that may regulate the angiogenic response (26, 27). The CFUs expressed mRNA not only for the hematopoietic marker CD45, but also for HGF and SDF-1/CXCL12, highlighting a possible paracrine role for circulating PBMCs in the regulation of endothelial repair. The regulation of PBMC mobilization and CFU formation is complex and likely involves a combination of biological and demographic patient factors. Additional mechanistic studies to identify the capabilities of these cells and factors that contribute to their mobilization will help in our understanding of their role in lung injury.
Our CFU culture techniques may be most relevant in terms of prognosis. A similar approach with the use of PBMC culture in prognosis was undertaken in patients with pulmonary arterial hypertension, where investigators enumerated endothelial progenitor cell CFUs derived from PBMCs in an effort to help guide therapy (28). Our work highlights the importance of performing large trials with well-characterized subjects for any prospective biomarker, to conceptualize the effects of comorbidities and other confounders on the biomarker. Additional investigations with PBMCs from subjects in the setting of critical illness, using complementary characterization and functional assays, will be necessary to define clearly the effect of critical illness on these cells and the roles they play in repair.
Although higher CFUs correlated with better in-hospital survival, what effect these cells have on lungs damaged by ALI remains unclear. On the one hand, PBMCs mobilized in injury may exert an adverse effect on pulmonary vascular remodeling, and contribute to the development of pulmonary arterial hypertension (29, 30) observed in a subset of patients who recovered from ALI. On the other hand, the treatment of ALI in an animal model using PBMCs cultured in an endothelial-specific fashion (similar to what we describe) was reported to attenuate some of the abnormalities found in this disorder (8). Related to this, recent clinical trials in adults (31) and children (32) with pulmonary arterial hypertension (a feature of ALI) demonstrated the ability of transplanted autologous PBMCs to improve hemodynamic parameters in these patients. These conflicting observations suggest that further studies are needed to determine whether a specific subset of PBMCs has a therapeutic role in critical illness, and to define cell types that may contribute to abnormal lung remodeling.
The strength of our work involves its careful characterization of patient factors in a sizable number of critically ill patients with ALI and severe sepsis, and their relationship to these types of cells. One limitation was the finite number of PBMCs and serum we were able to obtain from critically ill patients, which prevented us from performing all assays described in every patient. We examined only a limited number of soluble serum factors from these patients, along with the mRNA expression by CFUs for a small number of genes based on published reports of their relevance to endothelial repair. This may partly explain why differences in gene expression between patient groups did not reach statistical significance for all genes examined. However, these data provide novel evidence of these cells' functions in endothelial repair in critical illnesses such as ALI. Measurements of mRNA expression from additional patients for other genes of interest will be necessary for a further clarification of these cells' paracrine role. Other unexamined serum factors may also have had a stronger relationship with CFU number than what we observed for SDF-1/CXCL12. Finally, although our laboratory's CFU assay differed slightly from other CFU assays reported in the literature, we published our methods previously (9) and had reproducible results in two separate cohorts of patients with ALI. We did not observe significant differences in CFU numbers between our septic patients and healthy control subjects. This may be related to a Type II error, attributable to the small sample size of control subjects, and the variability of CFU numbers in our patients with severe sepsis. Overall, these data, along with information gleaned in other experiments, should help provide direction for investigations in human patients and in animal models of critical illness.
In conclusion, this work suggests that lung injury results in the increased mobilization of a subset of PBMCs that can form CFUs in subjects with ALI, and this appears to be related specifically to lung injury. Patients with severe sepsis but without ALI had lower numbers of CFUs, despite an equivalent severity of illness. Further, we demonstrated that cells within CFUs expressed endothelial-like surface markers in vitro and mRNA for factors thought to be important in endothelial repair. Previous investigations of the capabilities of PBMCs in various laboratory and in vivo conditions suggested that these cells perform a primarily paracrine role in endothelial repair. However, the majority of functional studies with human PBMCs used samples from healthy subjects. To clarify more accurately the function and role of PBMCs specifically mobilized in response to injury, investigations need to continue in critically ill subjects, to account for the effects of illness and comorbidities on the composition and function of PBMCs in the milieu of critical illness.
Acknowledgments
The authors thank Diane Sutcliffe, B.S., and Michael Edwards, Ph.D., for their technical expertise in the conduct of these experiments.
This work was supported by a Beginning Grant-in-Aid from the American Heart Association and a Giles Filley Award from the American Physiological Society.
Originally Published in Press as DOI: 10.1165/rcmb.2009-0015OC on October 20, 2009
Author Disclosure: E.L.B. has received sponsored grants from the National Institutes of Health for $50,001–$100,000, the American Heart Association for $50,001–$100,000, and the American Physiological Association for $10,001–$50,000. M.M. has received a sponsored grant from the National Institutes of Health for $50,001–$100,000. S.M. has received a sponsored grant from the National Institutes of Health for more than $100,001. M.M. has received a sponsored grant from the National Institutes of Health for more than $100,001. None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Aird WC. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood 2003;101:3765–3777. [DOI] [PubMed] [Google Scholar]
- 2.Ware LB. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin Respir Crit Care Med 2006;27:337–349. [DOI] [PubMed] [Google Scholar]
- 3.Schouten M, Wiersinga WJ, Levi M, Van der Poll T. Inflammation, endothelium, and coagulation in sepsis. J Leukoc Biol 2008;83:536–545. [DOI] [PubMed] [Google Scholar]
- 4.Tomashefski JF Jr, Davies P, Boggis C, Greene R, Zapol WM, Reid LM. The pulmonary vascular lesions of the adult respiratory distress syndrome. Am J Pathol 1983;112:112–126. [PMC free article] [PubMed] [Google Scholar]
- 5.Nuckton TJ, Alonso JA, Kallet RH, Daniel BM, Pittet JF, Eisner MD, Matthay MA. Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med 2002;346:1281–1286. [DOI] [PubMed] [Google Scholar]
- 6.Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainault JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med 2001;344:699–709. [DOI] [PubMed] [Google Scholar]
- 7.Burnham EL, Taylor WR, Quyyumi AA, Rojas M, Brigham KL, Moss M. Increased circulating endothelial progenitor cells are associated with survival in acute lung injury. Am J Respir Crit Care Med 2005;172:854–860. [DOI] [PubMed] [Google Scholar]
- 8.Lam CF, Liu YC, Hsu JK, Yeh PA, Su TY, Huang CC, Lin MW, Wu PC, Chang PJ, Tsai YC. Autologous transplantation of endothelial progenitor cells attenuates acute lung injury in rabbits. Anesthesiology 2008;108:392–401. [DOI] [PubMed] [Google Scholar]
- 9.Matthay MA. Treatment of acute lung injury: clinical and experimental studies. Proc Am Thorac Soc 2008;5:297–299. [DOI] [PubMed] [Google Scholar]
- 10.Rafat N, Hanusch C, Brinkkoetter PT, Schulte J, Brade J, Zijlstra JG, Van der Woude FJ, Van Ackern K, Yard BA, Beck GC. Increased circulating endothelial progenitor cells in septic patients: correlation with survival. Crit Care Med 2007;35:1677–1684. [DOI] [PubMed] [Google Scholar]
- 11.Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, Brigham KL. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol 2005;33:145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faivre V, Lukaszewicz AC, Alves A, Charron D, Payen D, Haziot A. Accelerated in vitro differentiation of blood monocytes into dendritic cells in human sepsis. Clin Exp Immunol 2007;147:426–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Damas P, Canivet JL, DeGroote D, Vrindts Y, Albert A, Franchimont P, Lamy M. Sepsis and serum cytokine concentrations. Crit Care Med 1997;25:405–412. [DOI] [PubMed] [Google Scholar]
- 14.Abe S, Boyer C, Liu X, Wen FQ, Kobayashi T, Fang Q, Wang X, Hashimoto M, Sharp JG, Rennard SI. Cells derived from the circulation contribute to the repair of lung injury. Am J Respir Crit Care Med 2004;170:1158–1163. [DOI] [PubMed] [Google Scholar]
- 15.Xu J, Mora A, Shim H, Stecenko A, Brigham KL, Rojas M. Role of the SDF-1/CXCR4 axis in the pathogenesis of lung injury and fibrosis. Am J Respir Cell Mol Biol 2007;37:291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamada M, Kubo H, Ishizawa K, Kobayashi S, Shinkawa M, Sasaki H. Increased circulating endothelial progenitor cells in patients with bacterial pneumonia: evidence that bone marrow derived cells contribute to lung repair. Thorax 2005;60:410–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burnham EL, Rojas M, Taylor WR, Moss M. The number of circulating endothelial progenitor cells in ARDS patients is independent of serum growth factor concentration [abstract]. Proc Am Thor Soc 2006;3:A203. [Google Scholar]
- 18.Burnham EL, Edwards M, Sutcliffe D, Taylor WR, Moss M. Variability in circulating progenitor cells between patients with acute lung injury and severe sepsis: impact of a pulmonary etiology [abstract]. Proc Am Thor Soc 2008;177:A233. [Google Scholar]
- 19.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, LeGall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994;149:818–824. [DOI] [PubMed] [Google Scholar]
- 20.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med 2008;36:296–327. [DOI] [PubMed] [Google Scholar]
- 21.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 2003;348:593–600. [DOI] [PubMed] [Google Scholar]
- 22.Martin J, Helm K, Ruegg P, Varella-Garcia M, Burnham E, Majka S. Adult lung side population cells have mesenchymal stem cell potential. Cytotherapy 2008;10:140–151. [DOI] [PubMed] [Google Scholar]
- 23.Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol 2008;28:1584–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rohde E, Bartmann C, Schallmoser K, Reinisch A, Lanzer G, Linkesch W, Guelly C, Strunk D. Immune cells mimic the morphology of endothelial progenitor colonies in vitro. Stem Cells 2007;25:1746–1752. [DOI] [PubMed] [Google Scholar]
- 25.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 2007;109:1801–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell 2006;124:175–189. [DOI] [PubMed] [Google Scholar]
- 27.Jin DK, Shido K, Kopp HG, Petit I, Shmelkov SV, Young LM, Hooper AT, Amano H, Avecilla ST, Heissig B, et al. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat Med 2006;12:557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asosingh K, Aldred MA, Vasanji A, Drazba J, Sharp J, Farver C, Comhair SA, Xu W, Licina L, Huang L, et al. Circulating angiogenic precursors in idiopathic pulmonary arterial hypertension. Am J Pathol 2008;172:615–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sata M. Role of circulating vascular progenitors in angiogenesis, vascular healing, and pulmonary hypertension: lessons from animal models. Arterioscler Thromb Vasc Biol 2006;26:1008–1014. [DOI] [PubMed] [Google Scholar]
- 30.Yao W, Firth AL, Sacks RS, Ogawa A, Auger WR, Fedullo PF, Madani MM, Lin GY, Sakakibara N, Thistlethwaite PA, et al. Identification of putative endothelial progenitor cells (CD34+CD133+Flk-1+) in endarterectomized tissue of patients with chronic thromboembolic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2009;296:L870–L878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang XX, Zhang FR, Shang YP, Zhu JH, Xie XD, Tao QM, Zhu JH, Chen JZ. Transplantation of autologous endothelial progenitor cells may be beneficial in patients with idiopathic pulmonary arterial hypertension: a pilot randomized controlled trial. J Am Coll Cardiol 2007;49:1566–1571. [DOI] [PubMed] [Google Scholar]
- 32.Zhu JH, Wang XX, Zhang FR, Shang YP, Tao QM, Zhu JH, Chen JZ. Safety and efficacy of autologous endothelial progenitor cells transplantation in children with idiopathic pulmonary arterial hypertension: open-label pilot study. Pediatr Transplant 2008;12:650–655. [DOI] [PubMed] [Google Scholar]