Abstract
Our previous studies revealed that, in a murine model of asthma, mice that received Fas-deficient T cells developed a prolonged phase of airway inflammation, mucus production, and airway hyperreactivity that failed to resolve even 6 weeks after the last challenge. To investigate how Fas–Fas ligand (FasL) interaction occurs between T cells and other cells in vivo, Gld mice with abnormalities of the FasL signaling pathway were used. The reconstituted mice were made by transferring T cells from B6 or Gld mice to Rag−/− or FasL-deficient Rag−/− mice. We found that Rag−/− mice that received B6 T cells resolved the airway inflammation, whereas FasL-deficient Rag−/− mice that received Gld T cells developed a prolonged airway inflammation at Day 28, with decreased IFN-γ production. Both FasL-deficient Rag−/− mice that received B6 T cells and Rag−/− mice that received Gld T cells also had completely resolved their airway inflammation by Day 28 after challenge. Interestingly, FasL-deficient Rag−/− mice that received Gld T cells eventually resolved airway inflammation at Day 42, with a similar level of IFN-γ production to that of control group. These results demonstrate that FasL expression on either T cells only or non–T cells only was sufficient for the eventual resolution of airway inflammation, and the prolonged airway inflammation in FasL-deficient Rag−/− mice that received Gld T cells was correlated with decreased IFN-γ production by Gld T cells.
Keywords: T helper cell type 1/T helper cell type 2 cells, eosinophils, apoptosis, lung, inflammation
CLINICAL RELEVANCE.
This article demonstrates the importance of Fas–Fas ligand interaction in the development of a murine model of T helper cell type 2–mediated chronic airway inflammation. Thus, our model represents an altogether new type of animal model for prolonged responses characteristic of asthma.
Persistent airway inflammation is a major contributor to the frequency and severity of asthma exacerbations, and to other characteristics of asthma (1, 2). The failure of subjects with asthma to resolve inflammation in their airways after exacerbations remains one of the most problematic features in both intermittent and persistent asthma (1, 3). Inflammatory cell apoptosis is reduced in the peripheral blood and airways of subjects with asthma (4–6). Duez and colleagues (7) have shown that Fas deficiency delays the resolution of airway hyperresponsiveness (AHR) after allergen sensitization and challenge. Fas-deficient mice have a delayed resolution of airway inflammation, and mice with Fas deficiency only on T cells developed a prolonged phase of airway inflammation, mucus production, and airway hyperreactivity (8). This prolonged airway inflammation phase correlated with decreased IFN-γ production by Fas-deficient T cells. These studies have demonstrated that the Fas signaling pathway plays a critical role in the resolution of airway inflammation in a murine model of asthma.
The Fas receptor (CD95), a type I membrane receptor protein belonging to the tumor necrosis factor (TNF) superfamily, is expressed in various tissues (9). Ligation of the Fas receptor with binding of Fas ligand (FasL) or antibody can lead to induction of apoptosis in inflammatory cells (10–12). FasL is a type II membrane protein of the TNF receptor family (13, 14). It can be expressed on various types of tissues or cells, including activated T cells, testes, intestine, spleen, kidney, lung, corneal epithelium, and endothelium (15–20). It has been suggested that both the Fas receptor and FasL expression in airway epithelium may regulate the inflammatory response in asthmatic lungs (15, 16). FasL expression on bronchial epithelial cells and limited numbers of activated T cells in subjects with asthma has also been reported (6). Although there are many reports regarding the expression of FasL in the airway and lung, the contributory roles of different cell types at different phases of airway inflammation are poorly understood.
To investigate how Fas–FasL interactions occur and regulate the resolution of airway inflammation in vivo, we used a previously described murine model of allergic airway disease and mice with abnormalities in the FasL signaling pathway (8, 21). We found that FasL deficiency (Gld) led to a delayed resolution of airway inflammation similar to our previous observations. To study the specific role of FasL expression on T cells, reconstituted mice with dysfunctional FasL expression on either T cells or non–T cells were generated. When B6 or Gld T cells were transferred to Rag−/− or FasL-deficient Rag−/− mice, airway inflammation resolved normally in the mice with FasL expression on either T cells only or non–T cells only, whereas FasL-deficient Rag−/− mice that received FasL-deficient T cells developed a prolonged airway inflammation. These data suggest that FasL expression on either T cells or non–T cells is sufficient to prevent a prolonged airway inflammation, and FasL expression on T cells and non–T cells contributed to the resolution of airway inflammation.
MATERIALS AND METHODS
Animals
C57BL/6 (B6) mice were purchased from either The Division of Cancer Treatment at the National Cancer Institute (Frederick, MD) or The Jackson Laboratory (Bar Harbor, ME). B6.Smn.C3H.Tnfsf6gld and B6.129S7-Rag1tm1Mom mice were purchased from The Jackson Laboratory and bred and housed in a specific pathogen-free barrier facility maintained by the University of Chicago Animal Resources Center. The two strains were bred together to develop Rag−/− mice that were homozygous for the gld mutation (Gld.Rag−/−). The studies reported here conform to the principles outlined by the Animal Welfare Act and the National Institutes of Health guidelines for the care and use of animals in biomedical research.
Antibodies and Flow Cytometry
Anti-mouse CD3 (clone 17A2) antibody for T lymphocytes was obtained from BD Pharmingen (San Diego, CA). Anti-mouse CCR3 (clone 83,101.111) antibody for eosinophils was obtained from R&D Systems (Minneapolis, MN). Cells were stained and analyzed on either a FACS Calibur or a LSR-II (Becton-Dickinson, San Jose, CA).
Schistosoma mansoni Sensitization and Challenge and BAL Analysis
Sensitization and challenge were described previously (8, 21). Briefly, at Day −14, mice were immunized by intraperitoneal injection of inactivated Schistosoma mansoni eggs, which induce a strong T helper cell type (Th) 2 response in the absence of active infection. At Days −7 and 0, the mice were challenged with 10 μg of soluble egg antigen (SEA) by intranasal and intratracheal aspiration, respectively. The mice were killed between 4 and 28 days after the last challenge. Gld mice at 5–7 weeks of age were used to ensure that they had not yet developed lymphoproliferative disease. Bronchoalveolar lavage (BAL) was performed by delivering approximately 0.8 ml of cold PBS into the cannulated trachea and gently aspirating the fluid. The lavage was repeated a total of four times, and a total volume of 2.5–3.0 ml BAL was collected. The percentage of cell types found within BAL fluid was determined by FACS analysis with cell type–specific markers (anti-CD3 antibody for T lymphocytes and anti-CCR3 antibody for eosinophils).
Adoptive Transfer
T cells were harvested from lymph nodes of B6 and Gld donor mice and enriched by nonadherence to a nylon wool column. The purity as determined by flow cytometry was between 90 and 95% CD3+ T cells. A total of 107 cells were adoptively transferred intravenously into each recipient.
Analysis of Lung Histological Changes
Lungs were removed from mice after BAL and fixed by immersion into 4% paraformaldehyde. Lobes were sectioned sagittally, embedded in paraffin, cut into 5-μm sections, and stained with hematoxylin and eosin (H&E) for analysis. The score of peribronchial and perivascular inflammation and periodic acid Schiff (PAS) staining was measured as previously described (8).
Detection of Th1 and Th2 Cytokines
T cells were incubated at a concentration of 2 × 106 cells/ml in a 48-well plate in the presence or absence of SEA (5 μg/ml), and supernatants were harvested after 48 hours in culture. Cytokine release was analyzed with the Bioplex Protein Array system (Bio-Rad, Hercules, CA) with beads specific for IL-5 and IFN-γ, according to the manufacturer's instructions.
Statistical Analysis
Graph generation and statistical analysis were performed using Prism software (version 4.00; GraphPad, La Jolla, CA). Differences in total cells and eosinophils in the BAL fluid and in lung histological scoring were determined by using an unpaired Student's two-tailed t test. Error bars represent SEM. Statistical significance was claimed with P values of less than 0.05, 0.01, and 0.001 as indicated in the figure legends.
RESULTS
Resolution of Airway and Lung Inflammation Is Delayed in FasL-Deficient Mice
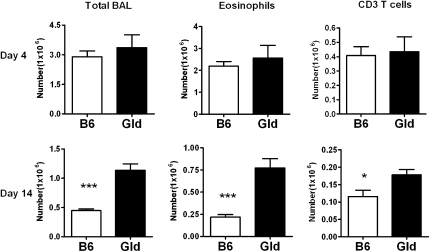
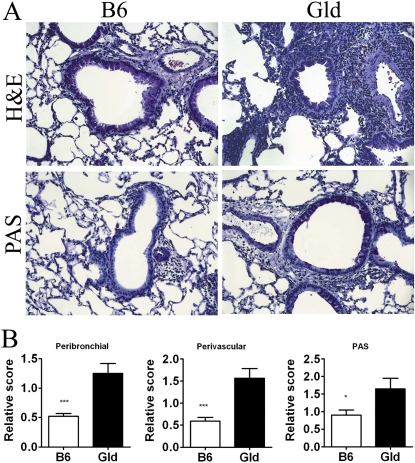
Previously, we have demonstrated that the Fas pathway is involved in the resolution of airway inflammation, and that Fas-deficient (Lpr) mice have a delayed resolution of airway inflammation in a murine model of asthma (8). Because both Lpr and Gld mice are deficient in Fas–FasL interactions, we hypothesized that Gld mice would also have a delayed resolution of airway inflammation. We have previously shown that, in our model, airway eosinophilia is completely dependent on both sensitization and challenge. In the absence of either the sensitization or challenge, the composition of the small number of BAL cells is indistinguishable from BAL of mice without sensitization and challenge (8, 22). After B6 and Gld mice (all strains used in these experiments are on the B6 background) were sensitized and challenged with S. mansoni egg antigen, Gld mice developed levels of airway inflammation similar to those in B6 mice at Day 4 after challenge. At Day 14 after challenge, Gld mice had significantly higher numbers of infiltrating total BAL cells, eosinophils, and T cells (Figure 1) compared with the B6 mice. Histological evaluation of H&E and PAS lung sections revealed dramatically more severe inflammation and mucus production in Gld mice compared with B6 mice at Day 14 after challenge (Figures 2A and 2B). It should be mentioned that the sensitized and challenged Gld mice had much less inflammation at Day 14 than at Day 4 (Figure 1), indicating that factors other than the Fas–FasL pathway alone may also play an important role in the resolution of airway inflammation in this model. Taken together, these data demonstrate that the significant delay in resolution in the Gld strain is partially regulated by Fas–FasL interactions.
Figure 1.
Resolution of airway inflammation is delayed in Fas ligand (FasL)–deficient mice. Gld and B6 mice were sensitized with inactivated Schistosoma mansoni eggs, challenged with soluble egg antigen (SEA) (10 μg/mouse), killed on Days 4 (B6 n = 8, Gld n = 9) and 14 (B6 n = 5, Gld n = 4) after the last challenge, and cells harvested by bronchoalveolar lavage (BAL). The total cell numbers in the BAL of B6 and Gld mice were analyzed by trypan blue staining. The type of BAL cells (eosinophils and CD3 T cells) was determined by calculating the absolute number of each cell type from the FACS profiles and by the total numbers of BAL cells. *P < 0.05, ***P < 0.001. Error bars represent SEM.
Figure 2.
Increased lung inflammation and mucus production in Gld mice. (A) Representative sections of lungs at Day 14. Lung tissues from B6 and Gld mice were fixed in 4% paraformaldehyde and embedded in paraffin. Hematoxylin and eosin (H&E)–stained sections for analysis of airway inflammation and periodic acid Schiff (PAS)–stained sections for analysis of mucus-containing cells are presented. (B) Quantification of perivascular and peribronchial inflammation and mucus production. *P < 0.05, ***P < 0.001. Error bars represent SEM.
FasL Expression on Either T Cells Only or Non–T Cells Only Is Sufficient for the Late, but Not the Early Phase of Resolution of Th2 Airway Inflammation
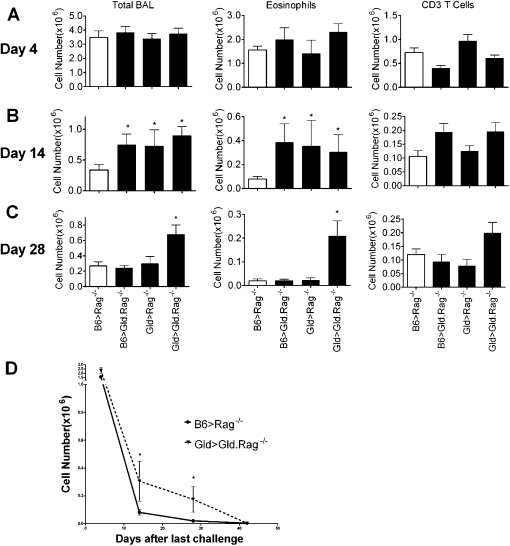
Hamann and colleagues (15) and Gochuico and colleagues (16) have reported that FasL is expressed on the cell surface of human and mouse airway epithelial cells, suggesting that its expression plays an important role in the regulation of the inflammatory response. Because airway epithelial cells are broadly distributed in whole lung tissue, FasL expressed by epithelial cells may be the predominant source of FasL during resolution of airway inflammation. FasL can also be expressed on the surface of activated lymphocytes (23, 24). Its expression on T cells not only helps to terminate T cell immune responses by Fas-mediated cell death, but also kills eosinophils and cells of other types. It has been reported that the level of FasL expression can vary based on different Th cell subsets, such that FasL is expressed highly on Th1 cells, but little or not at all on Th2 cells (23, 24). We hypothesized that FasL expression on T cells in this model plays a minimal role in the resolution of airway inflammation compared with the expression on non–T cells, because this model has a strong Th2-mediated airway inflammation. To test this hypothesis, we used an adoptive transfer model to “design” mice with differential expression of FasL on T cells and non–T cells. Lymph node T cells from either B6 or Gld mice were adoptively transferred into Rag−/− or Gld.Rag−/− mice (B6 > Rag−/−, B6 > Gld.Rag−/−, Gld > Rag−/−, and Gld > Gld.Rag−/−, respectively). Thus, the adoptively transferred B6 > Rag−/− mice had normal expression of FasL on their T cells and non–T cells, whereas Gld > Rag−/− mice had dysfunctional expression of FasL only on T cells, and B6 > Gld.Rag−/− mice had dysfunctional expression of FasL only on non–T cells. Of course, Gld > Gld.Rag−/− mice had no functional FasL expression on any tissue or any type of cell. The mice were then sensitized and challenged as described in the Materials and Methods. As we have previously demonstrated, because eosinophil hematopoiesis occurs in the bone marrow and does not require Rag-1 expression, the resulting mice have eosinophils with the genotype of the recipient, and T cells have the genotype of the donor strain (8). We found that the four groups of reconstituted mice developed similar levels of airway inflammation at Day 4 after challenge, including total BAL cells, eosinophils, and CD3 T cells (Figure 3A). By Day 14, B6 > Rag−/− mice were resolved, but dysfunctional FasL expression on either T cells or non–T cells led to a delayed resolution of airway inflammation (Figure 3B). Thus, surprisingly, functional FasL expression on either T cells or non–T cells was not sufficient for effective resolution of inflammation during the early phase (Day 14).
Figure 3.
FasL expression on either T cells only or non–T cells only was eventually sufficient to prevent chronic airway inflammation. B6 and Gld T cells were adoptively transferred into Rag−/− and Gld.Rag−/− mice 1 day before sensitization (noted as B6 > Rag−/−, B6 > Gld.Rag−/−, Gld > Rag−/−, and Gld > Gld.Rag−/− mice, respectively). The four groups of reconstituted mice were sensitized, challenged, and killed on Days 4 (A), 14 (B), and 28 (C) after the final challenge, and the BAL was analyzed. The total BAL cell numbers, cell types, and absolute numbers were calculated as described in Materials and Methods. (D) BAL eosinophil counts were determined for Days 4, 14, 28, and 42. These eosinophil numbers for Days 4, 14, and 28, shown in A, B, and C, are also included to provide a more complete picture of the entire time course that was tested. A total of 5–14 mice per group per time point were analyzed. *P < 0.05. Error bars represent SEM.
Our previous study found that, in our model, the cellular components of early and chronic airway inflammatory infiltrates are different: there are higher percentages of eosinophils in the acute inflammatory phase and of T lymphocytes in the chronic phase (8). Although FasL expression on T cells or non–T cells alone is not sufficient for early resolution, it is possible that FasL expression on either population will be sufficient to induce the eventual resolution of airway inflammation in these mice. To determine if FasL expression on T cells or non–T cells plays a role in the chronic phase of this model, the four groups of reconstituted mice described previously here were sensitized, challenged, and killed 28 days after the last challenge. Similar to Lpr > Rag−/− mice (8), Gld > Gld.Rag−/− mice developed a prolonged phase of airway inflammation compared with the other three groups (Figure 3C). At this time point, mice with FasL expression on only T cells (B6 > Gld.Rag−/−) or non–T cells (Gld > Rag−/−) had also completely resolved their airway inflammation (Figure 3C). These data demonstrate that, although resolution is slightly delayed, FasL expression on either T cells only or non–T cells only is sufficient to prevent prolonged airway inflammation in this model.
To determine if Gld > Gld.Rag−/− mice have a prolonged airway inflammation similar to Lpr > Rag−/− mice at Day 42 (8), reconstituted B6 > Rag−/− mice and Gld > Gld.Rag−/− mice were sensitized, challenged, and killed at Day 42 after the last challenge. As previously shown, B6 > Rag−/− mice had resolved their airway inflammation by Day 42 (8). Surprisingly, Gld > Gld.Rag−/− mice, which showed a prolonged airway eosinophilia at Day 28 (Figure 3C), had resolved eosinophilia at Day 42 (Figure 3D). The number of total BAL cells and CD3 T cells were very similar between these two groups (data not shown). These data show that, unlike Lpr > Rag−/− mice at Day 42, Gld > Gld.Rag−/− mice had an eventual resolution of airway inflammation by this later time point, suggesting that another signaling pathway could be involved in the resolution of airway inflammation in addition to the Fas–FasL–induced apoptotic signaling pathway.
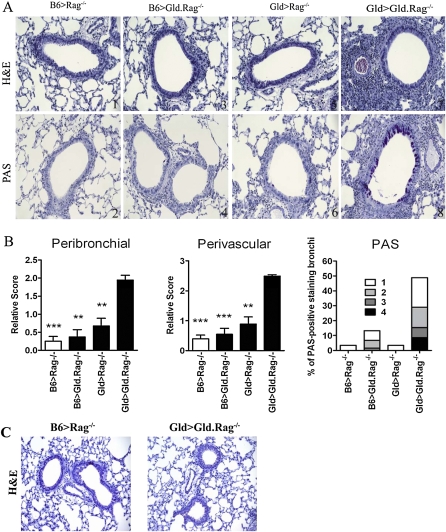
Increased Lung Inflammation and Mucus Production in Gld > Gld.Rag−/− Mice at Day 28
To confirm that lung histological changes are consistent with the BAL cellular infiltration, lung inflammation was further evaluated by scoring H&E and PAS sections for the relative amounts of perivascular and peribronchial inflammation. The lungs from B6 > Gld.Rag−/− mice and Gld > Rag−/− mice showed levels of cellular infiltrates similar to B6 > Rag−/− mice at Day 28 (Figures 4A and 4B). Only the lungs from Gld > Gld.Rag−/− mice showed more severe inflammation by this time point compared with lungs from mice of the other three groups (Figures 4A and 4B). Very low levels of PAS staining were seen in the bronchi of B6 > Rag−/− mice, B6 > Gld.Rag−/− mice, and Gld > Rag−/− mice, but PAS staining in the bronchi of Gld > Gld.Rag−/− mice was significantly greater (Figures 4A and 4B). These findings further demonstrate that FasL expression on either T cells only or non–T cells only is sufficient to prevent a prolonged airway inflammation in a murine model of asthma. As expected from our BAL data, Gld > Gld.Rag−/− mice at Day 42 demonstrated resolved airway and lung inflammation similar to B6 > Rag−/− mice (Figure 4C). Almost no PAS staining was seen in the bronchi of B6 > Rag−/− mice or Gld > Gld.Rag−/− mice at this time point (data not shown). These histological changes are consistent with the findings seen in the BAL, and confirmed the eventual resolution of airway inflammation at Day 42.
Figure 4.
Increased lung inflammation and mucus production in Gld > Gld.Rag−/− mice at Day 28 (A). Representative sections from B6 > Rag−/− (panels 1 and 2), B6 > Gld.Rag−/− (panels 3 and 4), Gld > Rag−/− (panels 5 and 6), and Gld > Gld.Rag−/− (panels 7 and 8) mice killed at Day 28 after the last challenge are shown. The H&E-stained sections (panels 1, 3, 5, and 7) and PAS-stained sections (panels 2, 4, 6, and 8) are shown. (B) Quantification of perivascular and peribronchial inflammation and mucus production from these four groups of mice at Day 28. (C) Representative H&E-stained sections from B6 > Rag−/– and Gld > Gld.Rag−/– mice at Day 42 after the last challenge are shown. **P < 0.01, *** P < 0.001. Error bars represent SEM.
Decreased IFN-γ Production by Gld T Cells Correlates with Prolonged Airway Inflammation in the Gld > Gld.Rag−/− Mice at Day 28
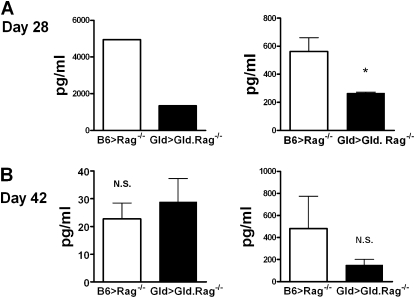
To determine the nature of the T cell response at Days 28 and 42, BAL, spleen T cells, and lung T cells were restimulated with SEA, and the cytokines, IFN-γ and IL-5, were measured. Interestingly, the levels of IFN-γ production by Gld T cells from BAL and spleen at Day 28 were significantly less in the Gld > Gld.Rag−/− mice compared with wild-type T cells (B6 > Rag−/− mice) (Figure 5A), whereas the levels of IFN-γ production by Gld T cells and wild-type T cells in lung and spleen at Day 42 were similar between Gld > Gld. Rag−/− and B6 > Rag−/− mice (Figure 5B). Similar levels of IL-5 production by Gld T cells and wild-type T cells at Days 28 and 42 were detected in the BAL, lung, and spleen between these two groups of mice (data not shown). These findings suggest that IFN-γ production attenuates the airway inflammation in a murine model of asthma, and these data are consistent with our previous findings that the failure of Lpr T cells to produce IFN-γ in the Lpr > Rag−/– mice plays an important role in their inability to resolve Th2-mediated inflammation (8).
Figure 5.
Decreased IFN-γ production by Gld T cells in the Gld > Gld.Rag−/− mice at Day 28. (A) BAL and spleen T cells from B6 > Rag−/− mice and Gld > Gld.Rag−/− mice at Day 28, and (B) lung and spleen T cells from B6 > Rag−/− mice and Gld > Gld.Rag−/− mice at Day 42, were restimulated with SEA, and measured by Bioplex system, as described in Materials and Methods for IFN-γ and IL-5 (data not shown). *P < 0.05. Error bars represent SEM.
DISCUSSION
The contribution of FasL expression by different cells to the resolution of airway inflammation in a strong Th2-mediated murine model of asthma has not been well studied. In this study, we have addressed the question of whether FasL expression by T cells is required to resolve the inflammation, or whether other cell types in the lungs could contribute the FasL signal. We first demonstrated that Gld mice developed a delayed resolution of airway inflammation compared with wild-type mice after sensitization and challenge. By using our adoptive transfer model, we were able to directly address the question of whether FasL expression on T cells is necessary for the resolution of lung inflammation. Because FasL is broadly expressed in the lungs on many cell types, including airway epithelial cells, we hypothesized that FasL expression on the T cells themselves would be unnecessary. Although mice expressing FasL everywhere except on their T cells had resolved their inflammation by Day 28 after challenge (Figure 3C), we were surprised that their lungs were still inflamed at the earlier phase of resolution (Figure 3B). These data suggest that FasL expression on T cells was involved in down-regulating inflammation at an early stage, but played a smaller role later in the resolution. Although FasL expression on T cells was necessary for early, but not late resolution, it was possible that FasL expression on T cells was still sufficient for resolution. To address this possibility, wild-type T cells were transferred to FasL-deficient Rag−/− mice (B6 > Gld.Rag−/−) to produce mice that expressed FasL only on their T cells. Similar to the Gld > Rag−/− mice that had FasL expression everywhere but on their T cells, their lungs were still inflamed at Day 14, but resolved inflammation by Day 28. These results suggest that FasL expression on T cells does play an important role in the resolution of airway inflammation, and FasL expression on T cells is sufficient to prevent prolonged airway inflammation.
That FasL expression on T cells is sufficient for the resolution of airway inflammation by Day 28, but not the early phase of resolution (Day14), could be due to different compositions of inflammatory cell populations at these times. As we have shown, there is a much higher percentage of T cells in the inflammatory infiltrates by Day 28 than at Days 4–14, and thus, perhaps early on, there is less FasL available in the lungs and airways. Furthermore, it is possible that the nature of the T cell population changes such that Fas on T cells plays a larger role at the later stages of resolution to prevent chronic inflammation in the lungs. Finally, because FasL can be secreted from the cell surface, and therefore may transmit a death signal at sites distal to the effector cells (25), it is possible that the overall levels of secreted FasL at early time points require contributions from both T cells and non–T cells. However, by Day 28, the secreted FasL levels may have risen enough for effective resolution, even when some cell types were FasL deficient. Additional studies will be required to examine these potential mechanisms.
Although FasL expression on T cells is contributing to the eventual resolution of airway inflammation in the chronic phase, there is clearly a role of FasL expression on non–T cells, especially the epithelial cells which express high levels of FasL. Furthermore, it has been reported that the administration of FasL-expressing, adenovirus-transfected dendritic cells in Th2-induced allergic mice had significantly decreased AHR, airway inflammation, and cytokine production in a murine model of asthma (26). These data suggest that the role of Fas–FasL interaction from different cells in the resolution of airway inflammation could be complex, and the expression and roles of non–T cells in this model need to be further elucidated.
Our study demonstrates that the Fas–FasL pathway is not the only factor involved in the resolution of airway inflammation in our model, but that other factors also participate in the clearance of airway inflammation. IFN-γ is a proinflammatory cytokine that is known to play a significant role in regulating the proliferation and apoptosis of T lymphocytes (27, 28). It has also been reported that IFN-γ inhibits the proliferation of allergen-stimulated CD4+ T cells by stimulating the surface expression of Fas and FasL, which prompts Fas–FasL–mediated apoptosis (29). Cohn and colleagues (30) have demonstrated that Th1 cells and IFN-γ regulate allergic airway inflammation and mucus production. Our previous study has shown that the failure of Lpr T cells to produce IFN-γ in the Lpr > Rag−/– mice plays an important role in their inability to resolve Th2-mediated inflammation (8). To investigate if IFN-γ also has an effect on the prolonged airway inflammation in Gld > Gld.Rag−/− mice, IFN-γ production was measured. Similar to the Lpr T cells, we found that there was also a decreased IFN-γ production by Gld T cells in Gld > Gld.Rag−/− mice at Day 28. Surprisingly, airway inflammation in Gld > Gld.Rag−/− mice was eventually resolved by Day 42, and, at this time point, similar levels of IFN-γ production were produced from both the GLD and B6 T cells. Thus, these findings further suggest that IFN-γ is involved in the resolution of airway inflammation in this murine model of asthma.
Although both Lpr > Rag−/− mice and Gld > Gld.Rag−/− mice have a defective Fas–FasL–induced apoptotic signaling pathway, the reason why Gld > Gld.Rag−/− mice, but not the Lpr > Rag−/− mice, resolve their inflammation by Day 42 remains unclear. One possibility is that the differences are due to an additional role of FasL in T cell activation. Aside from its death-inducing capacity, FasL has also been implicated in retrograde signal transduction into FasL-expressing cells by so-called “reverse signaling” (31). Because Lpr > Rag−/− mice have Fas expression on non–T cells, these Fas receptors may be able to crosslink FasL on the Lpr T cells. Furthermore, the observation that total CD3 T cells recovered from different groups over time are not significantly different from each other is also hard to explain. We assumed that Fas–FasL interaction is required not only in the induction of apoptosis, but also in the activation of nonapoptotic pathways, such as NF-κB activation (32). It has also previously been reported that, in addition to Fas receptor, other members of the TNF receptor family, such as TNF-R1 and TNF-R2, which are expressed on the surface of many cell types, are implicated in many aspects of airway pathology in asthma (33, 34). Further study will be required to explore the possible mechanisms for these apparently contradictory findings.
In summary, we have demonstrated that FasL deficiency leads to a delayed resolution of airway inflammation. FasL expression on either T cells only or non–T cells only was sufficient for the eventual resolution of airway inflammation, and the prolonged airway inflammation in Gld > Gld.Rag−/− mice at Day 28 was well correlated with decreased IFN-γ production by Gld T cells. However, the combined source of T cells and non–T cells must express FasL in order for resolution to occur in a normal time frame. Our study suggests that the increase in the number of eosinophils and the decrease in the number of apoptotic cells found in individuals with asthma may actually be a direct consequence of decreased expression of FasL in the airway (6, 35).
Acknowledgments
The authors thank the University of Chicago Cancer Research Center Flow Cytometry Facility and staff.
This work was supported by National Institutes of Health (NIH) grants R01 AI67697 and R01 AI50180 (A.I.S.), and American Heart Association 0630292N (J.T.). The University of Chicago Cancer Research Center Flow Cytometry Facility is supported in part by NIH grant P30-CA14599.
Originally Published in Press as DOI: 10.1165/rcmb.2008-0454OC on October 23, 2009
Author Disclosure: H.S.B. has a dependent that received a sponsored grant from the National Institutes of Health (NIH) for $10,001–$50,000. B.S.C. has received a sponsored grant from NIH for $10,001–$50,000. K.J.H. has received a sponsored grant from NIH for more than $100,001. R.A.S. received a grant from Wyeth for $10,001–$50,000, and sponsored grants from NIH for more than $100,001, the American Society of Transplantation for $10,001–$50,000, and the Louis Block Family Fund for $10,001–$50,000. A.I.S. received sponsored grants from NIH for more than $100,001, the Environmental Protection Agency for more than $100,001, and the Blowitz-Ridgeway Foundation for $50,001–$100,000. None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol 2004;22:789–815. [DOI] [PubMed] [Google Scholar]
- 2.Vignola AM, Gagliardo R, Guerrera D, Chiappara G, Chanez P, Bousquet J, Bonsignore G. New evidence of inflammation in asthma. Thorax 2000;55:S59–S60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vignola AM, Chanez P, Campbell AM, Souques F, Lebel B, Enander I, Bousquet J. Airway inflammation in mild intermittent and in persistent asthma. Am J Respir Crit Care Med 1998;157:403–409. [DOI] [PubMed] [Google Scholar]
- 4.Vignola AM, Chanez P, Chiappara G, Siena L, Merendino A, Reina C, Gagliardo R, Profita M, Bousquet J, Bonsignore G. Evaluation of apoptosis of eosinophils, macrophages, and T lymphocytes in mucosal biopsy specimens of patients with asthma and chronic bronchitis. J Allergy Clin Immunol 1999;103:563–573. [DOI] [PubMed] [Google Scholar]
- 5.Jayaraman S, Castro M, O'Sullivan M, Bragdon MJ, Holtzman MJ. Resistance to Fas-mediated T cell apoptosis in asthma. J Immunol 1999;162:1717–1722. [PubMed] [Google Scholar]
- 6.Druilhe A, Wallaert B, Tsicopoulos A, Silva JL, Tillie-Leblond I, Tonnel AB, Pretolani M. Apoptosis, proliferation, and expression of Bcl-2, Fas, and Fas ligand in bronchial biopsies from asthmatics. Am J Respir Cell Mol Biol 1998;19:747–757. [DOI] [PubMed] [Google Scholar]
- 7.Duez C, Tomkinson A, Shultz LD, Bratton DL, Gelfand EW. Fas deficiency delays the resolution of airway hyperresponsiveness after allergen sensitization and challenge. J Allergy Clin Immunol 2001;108:547–556. [DOI] [PubMed] [Google Scholar]
- 8.Tong J, Bandulwala HS, Clay BS, Anders RA, Shilling RA, Balachandran DD, Chen B, Weinstock JV, Solway J, Hamann KJ, et al. Fas-positive T cells regulate the resolution of airway inflammation in a murine model of asthma. J Exp Med 2006;203:1173–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watanabe-Fukunaga R, Brannan CI, Itoh N, Yonehara S, Copeland NG, Jenkins NA, Nagata S. The cDNA structure, expression, and chromosomal assignment of the mouse fas antigen. J Immunol 1992;148:1274–1279. [PubMed] [Google Scholar]
- 10.Yonehara S, Ishii A, Yonehara M. A cell-killing monoclonal antibody (anti-Fas) to a cell surface antigen co-downregulated with the receptor of tumor necrosis factor. J Exp Med 1989;169:1747–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trauth BC, Klas C, Peters AM, Matzku S, Moller P, Falk W, Debatin KM, Krammer PH. Monoclonal antibody–mediated tumor regression by induction of apoptosis. Science 1989;245:301–305. [DOI] [PubMed] [Google Scholar]
- 12.Nagafuji K, Shibuya T, Harada M, Mizuno S, Takenaka K, Miyamoto T, Okamura T, Gondo H, Niho Y. Functional expression of Fas antigen (CD95) on hematopoietic progenitor cells. Blood 1995;86:883–889. [PubMed] [Google Scholar]
- 13.Takahashi T, Tanaka M, Brannan CI, Jenkins NA, Copeland NG, Suda T, Nagata S. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell 1994;76:969–976. [DOI] [PubMed] [Google Scholar]
- 14.Lynch DH, Watson ML, Alderson MR, Baum PR, Miller RE, Tough T, Gibson M, Davis-Smith T, Smith CA, Hunter K, et al. The mouse Fas-ligand gene is mutated in Gld mice and is part of a TNF family gene cluster. Immunity 1994;1:131–136. [DOI] [PubMed] [Google Scholar]
- 15.Hamann KJ, Dorscheid DR, Ko FD, Conforti AE, Sperling AI, Rabe KF, White SR. Expression of Fas (CD95) and FasL (CD95L) in human airway epithelium. Am J Respir Cell Mol Biol 1998;19:537–542. [DOI] [PubMed] [Google Scholar]
- 16.Gochuico BR, Miranda KM, Hessel EM, De Bie JJ, Van Oosterhout AJ, Cruikshank WW, Fine A. Airway epithelial Fas ligand expression: potential role in modulating bronchial inflammation. Am J Physiol 1998;274:L444–L449. [DOI] [PubMed] [Google Scholar]
- 17.Suda T, Takahashi T, Golstein P, Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell 1993;75:1169–1178. [DOI] [PubMed] [Google Scholar]
- 18.French LE, Hahne M, Viard I, Radlgruber G, Zanone R, Becker K, Muller C, Tschopp J. Fas and Fas ligand in embryos and adult mice: ligand expression in several immune-privileged tissues and coexpression in adult tissues characterized by apoptotic cell turnover. J Cell Biol 1996;133:335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas ligand–induced apoptosis as a mechanism of immune privilege. Science 1995;270:1189–1192. (see comments). [DOI] [PubMed] [Google Scholar]
- 20.Wilson SE, Li Q, Weng J, Barry-Lane PA, Jester JV, Liang Q, Wordinger RJ. The Fas–Fas ligand system and other modulators of apoptosis in the cornea. Invest Ophthalmol Vis Sci 1996;37:1582–1592. [PubMed] [Google Scholar]
- 21.Tesciuba AG, Subudhi S, Rother RP, Faas SJ, Frantz AM, Elliot D, Weinstock J, Matis LA, Bluestone JA, Sperling AI. Inducible costimulator regulates Th2-mediated inflammation, but not Th2 differentiation, in a model of allergic airway disease. J Immunol 2001;167:1996–2003. [DOI] [PubMed] [Google Scholar]
- 22.Padrid PA, Mathur M, Li X, Herrmann K, Qin Y, Cattamanchi A, Weinstock J, Elliott D, Sperling AI, Bluestone JA. CTLA4Ig inhibits airway eosinophilia and hyperresponsiveness by regulating the development of Th1/Th2 subsets in a murine model of asthma. Am J Respir Cell Mol Biol 1998;18:453–462. [DOI] [PubMed] [Google Scholar]
- 23.Ju ST, Cui H, Panka DJ, Ettinger R, Marshak-Rothstein A. Participation of target Fas protein in apoptosis pathway induced by CD4+ Th1 and CD8+ cytotoxic T cells. Proc Natl Acad Sci USA 1994;91:4185–4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramsdell F, Seaman MS, Miller RE, Picha KS, Kennedy MK, Lynch DH. Differential ability of Th1 and Th2 T cells to express Fas ligand and to undergo activation-induced cell death. Int Immunol 1994;6:1545–1553. [DOI] [PubMed] [Google Scholar]
- 25.Dhein J, Walczak H, Baumler C, Debatin KM, Krammer PH. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95). Nature 1995;373:438–441. (see comments). [DOI] [PubMed] [Google Scholar]
- 26.Chuang YH, Suen JL, Chiang BL. Fas-ligand–expressing adenovirus-transfected dendritic cells decrease allergen-specific T cells and airway inflammation in a murine model of asthma. J Mol Med 2006;84:595–603. [DOI] [PubMed] [Google Scholar]
- 27.Dalton DK, Haynes L, Chu CQ, Swain SL, Wittmer S. Interferon gamma eliminates responding CD4 T cells during mycobacterial infection by inducing apoptosis of activated CD4 T cells. J Exp Med 2000;192:117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Novelli F, Di Pierro F, Francia di Celle P, Bertini S, Affaticati P, Garotta G, Forni G. Environmental signals influencing expression of the IFN-gamma receptor on human T cells control whether IFN-gamma promotes proliferation or apoptosis. J Immunol 1994;152:496–504. [PubMed] [Google Scholar]
- 29.De Rose V, Cappello P, Sorbello V, Ceccarini B, Gani F, Bosticardo M, Fassio S, Novelli F. IFN-{gamma} inhibits the proliferation of allergen-activated T lymphocytes from atopic, asthmatic patients by inducing Fas/FasL–mediated apoptosis. J Leukoc Biol 2004;76:423–432. [DOI] [PubMed] [Google Scholar]
- 30.Cohn L, Homer RJ, Niu N, Bottomly K. T helper 1 cells and interferon gamma regulate allergic airway inflammation and mucus production. J Exp Med 1999;190:1309–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lettau M, Paulsen M, Kabelitz D, Janssen O. Storage, expression and function of Fas ligand, the key death factor of immune cells. Curr Med Chem 2008;15:1684–1696. [DOI] [PubMed] [Google Scholar]
- 32.Legembre P, Barnhart BC, Zheng L, Vijayan S, Straus SE, Puck J, Dale JK, Lenardo M, Peter ME. Induction of apoptosis and activation of NF-kappaB by CD95 require different signalling thresholds. EMBO Rep 2004;5:1084–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bazzoni F, Beutler B. The tumor necrosis factor ligand and receptor families. N Engl J Med 1996;334:1717–1725. [DOI] [PubMed] [Google Scholar]
- 34.Wajant H, Henkler F, Scheurich P. The TNF-receptor–associated factor family: scaffold molecules for cytokine receptors, kinases and their regulators. Cell Signal 2001;13:389–400. [DOI] [PubMed] [Google Scholar]
- 35.Woolley KL, Gibson PG, Carty K, Wilson AJ, Twaddell SH, Woolley MJ. Eosinophil apoptosis and the resolution of airway inflammation in asthma. Am J Respir Crit Care Med 1996;154:237–243. [DOI] [PubMed] [Google Scholar]