Abstract
In 2006, the United States Centers for Disease Control and Prevention (CDC) recommended expanded and routine use of single-session rapid HIV tests in all health care settings to increase the proportion of persons who learn their HIV status. Limited empiric information is available regarding the costs of rapid testing and pre- and posttest counseling in health care settings. We surveyed 45 U.S. hospitals during 2005 through 2006 to assess the costs associated with rapid testing and counseling. Cost analyses were conducted from the provider (hospital) perspective, and results were expressed in year 2006 U.S. dollars. The mean per-test cost of rapid HIV testing and counseling was $48.07 for an HIV-negative test and $64.17 for a preliminary-positive test. Pre- and posttest counseling costs accounted for 38.4% of the total cost of rapid testing for HIV-negative patients. Counseling costs were significantly correlated with overall test costs. Many hospitals contained overall test costs by limiting time spent in pre- and posttest counseling or by using lower-paid personnel for counseling activities or both. Counseling costs constituted a significant proportion of the overall costs of rapid testing and counseling activities at study hospitals. Our data provide useful baseline data before implementation of the CDC's 2006 recommendations. Costs can be reduced by limiting time spent in pre- and posttest counseling or by using lower-paid personnel for counseling activities or both.
Introduction
The Centers for Disease Control and Prevention's (CDC) 2006 guidelines for HIV testing of adults, adolescents, and pregnant women recommend universal, routine HIV screening in all public and private health care settings, including hospital emergency departments, urgent care clinics, inpatient services, occupational health departments, and venues serving pregnant women.1 These recommendations encourage expanded use of single-session rapid HIV antibody tests in health care settings. Unlike non–rapid antibody tests, which require that clients return 1–2 weeks later to receive their test results and posttest counseling, rapid HIV test results are available in as little as 20–40 min, which allows persons who test negative to receive test results and posttest counseling in a single visit.2 Although several estimates of the cost of rapid testing by using the now-discontinued Single Use Diagnostic System (SUDS) assay have been published,3–5 little information is available regarding the cost of hospital-based HIV antibody testing and counseling with currently available rapid HIV assays. To address this gap in the literature, we conducted a cost analysis of rapid HIV testing and counseling at 45 U.S. hospitals. We were interested not only in the average cost of counseling and testing services, but also in how differences in the time spent providing pre- and posttest counseling and associated costs affected the overall cost of rapid HIV testing in study hospitals.
Methods
Study sample
The sample for the cost study was selected from a larger multistage probability sample of hospitals in major U.S. metropolitan areas that participated in a nationwide study of rapid HIV test us.6,7 Ninety-one hospitals that reported offering rapid testing services were eligible for inclusion in the cost analysis. The cost analysis required detailed information from the department providing rapid testing (emergency department, labor and delivery, occupational health, or other department), as well as from the hospital laboratory. In total, 45 department/laboratory pairs were included in the final cost-analysis sample (see Table 1). Excluded hospitals either were not reached despite multiple attempts, provided only laboratory but not departmental data (or vice versa), or were not able to provide cost data in the level of detail needed for analysis (e.g., hospital administrative regulations preventing release of salary or other financial information).
Table 1.
Selected Characteristics of 45 Study Hospitals, U.S., 2006
| Number of hospitals (%) | |
|---|---|
| Hospital sizea | |
| <100 | 4 (8.9) |
| 100–299 | 16 (35.6) |
| 300–499 | 13 (28.9) |
| ≥500 | 12 (26.7) |
| Respondent department type | |
| Emergency department | 18 (40.0) |
| Labor and delivery | 18 (40.0) |
| Occupational health | 8 (17.8) |
| Other | 1 (2.2) |
| Geographic region of U.S. | |
| Midwest | 19 (42.2) |
| Northeast | 15 (33.3) |
| South | 9 (20.0) |
| West | 2 (4.4) |
| Brand of rapid test | |
| OraQuick | 30 (66.7) |
| Reveal | 9 (20.0) |
| Uni-Gold | 5 (11.1) |
| Not specified | 1 (2.2) |
Average daily patient census.
Survey procedures
The cost survey was conducted in 2005 through 2006, before the release of the CDC's revised HIV counseling and testing guidelines, which recommend that providers adopt an “opt out” approach to HIV testing that streamlines consent and counseling procedures, particularly for patients who test HIV negative. Separate telephone interviews were conducted with laboratory directors and persons in charge of HIV testing in hospital departments that provided rapid HIV-testing services. If the interviewee was unable to provide the requested information, additional phone interviews were conducted with the laboratory or departmental personnel that oversee the corresponding budget or with the human resources director at the hospital or both. Interview data for each hospital were reviewed as soon as the initial interviews were completed. When necessary, follow-up interviews were conducted to obtain additional information or to resolve perceived discrepancies or both.
During the interviews, laboratory respondents were asked to provide information about the types of staff who processed rapid-test specimens, the average time required to process a rapid test, the brand(s) of rapid HIV tests used in the hospital, and the amount paid for each rapid-test kit. Study hospital departments were asked about the types of staff and average amount of staff time spent in pretest counseling, specimen collection, and posttest counseling for persons testing negative or preliminary positive. Staff-type categories for both the laboratory and departmental surveys included registered nurse, nurse practitioner, physician's assistant, other medical assistant, physician, medical technician, medical technologist, phlebotomist, and “other” personnel (e.g., HIV counselor or social services worker, as specified by the respondent). Salary or hourly wage rate, together with fringe-benefit information, was collected for each personnel type involved in rapid testing at the study hospitals.
Cost analyses
The cost analyses were conducted from the provider (hospital) perspective. Results are expressed in base-year 2006 U.S. dollars.
The total cost of performing a rapid HIV test included the personnel costs associated with performing counseling and testing-related procedures (described later), the cost of the rapid-test kit, and the cost of miscellaneous disposable materials. The cost of the rapid-test kits was obtained from the study surveys, as described earlier. The cost of miscellaneous disposable items, such as latex gloves, sterile wipes and gauze pads, adhesive bandages, phlebotomy equipment (needles, holders, blood tubes), absorbent workspace covers, biohazard waste-disposal bags, and laboratory supplies (pipettes, tubes) was estimated based on expert opinion at $1.50 per test.
Study hospitals' time estimates and compensation information were combined to estimate the personnel costs associated with each of the five rapid-test procedures (pretest counseling, specimen collection, test processing, and posttest counseling for persons testing negative or preliminary positive). At some study hospitals, a particular procedure could be performed by multiple personnel types. For example, pretest counseling might be provided by either a registered nurse or a physician's assistant. When this situation occurred, the main analysis assumed that all personnel types who sometimes performed the procedure at that hospital were equally likely to perform the procedure. As a check, we conducted sensitivity analyses in which we assumed that each procedure was performed by the lowest-paid (alternately, highest-paid) personnel type who sometimes performed the procedure.
Results
Thirty of the 45 hospitals used OraSure's OraQuick Advance Rapid HIV-1/2 or Rapid HIV-1 test, nine used the Reveal G2 Rapid HIV-1 antibody test, and five used the Uni-Gold Recombigen HIV test (one hospital did not identify the brand of rapid test used). Study hospitals paid between $8.25 and $25.00 (mean, $14.94) for each rapid-test kit. The mean price paid for OraQuick test kits ($14.53) was slightly less than the price paid for Reveal ($15.35) or Uni-Gold test kits ($16.65).
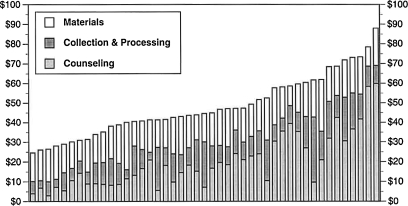
The mean per-test cost of rapid HIV testing and counseling at the 45 study hospitals was $48.07 for an HIV-negative test and $64.17 for a preliminary-positive test. However, these average values mask considerable variability: total costs ranged from $24.64 to $87.94 for negative tests and from $29.07 to $113.74 for preliminary-positive tests, as shown in Table 2. Test costs did not significantly differ by hospital size, region, hospital department, or the brand of rapid test. The distribution of negative test costs across study sites is illustrated in Fig. 1.
Table 2.
Cost of Rapid HIV Testing at 45 Hospitals in the U.S., 2006
| Range | Mean | Median | |
|---|---|---|---|
| Procedure time (min) | |||
| Pre-test counseling | 0–35 | 11.9 | 10 |
| Specimen collection | 1–25 | 4.5 | 4 |
| Rapid test processing | 5–60 | 19.5 | 15 |
| Post-test counseling (neg.) | 1–20 | 8.3 | 8 |
| Post-test counseling (pos.) | 4–60 | 21.1 | 20 |
| Procedure compensation (per h, $) | |||
| Pre-test counseling | 0–113.07 | 57.31 | 54.81 |
| Specimen collection | 14.49–72.20 | 31.90 | 31.68 |
| Rapid test processing | 12.81–53.50 | 28.43 | 28.80 |
| Post-test counseling (neg.) | 17.40–113.08 | 64.67 | 58.92 |
| Post-test counseling (pos.) | 17.40–135.44 | 76.53 | 84.36 |
| Personnel costs ($) | |||
| Pre-test counseling | 0.00–34.20 | 11.05 | 7.49 |
| Specimen collection | 0.43–10.42 | 2.32 | 1.75 |
| Rapid test processing | 2.16–31.59 | 9.22 | 7.80 |
| Post-test counseling (neg.) | 0.50–30.04 | 9.02 | 7.03 |
| Post-test counseling (pos.) | 5.91–59.52 | 25.12 | 19.65 |
| Personnel and materials costs ($) | |||
| Total personnel costs (neg.) | 10.00–68.94 | 31.64 | 28.06 |
| Total personnel costs (pos.) | 14.57–98.78 | 47.73 | 45.41 |
| Rapid test kits | 8.25–25.00 | 14.94 | 15.00 |
| Miscellaneous disposables | — | 1.50 | 1.50 |
| Total cost of counseling and testing ($) | |||
| Negative test result | 24.64–87.94 | 48.07 | 44.56 |
| Preliminary-positive test result | 29.07–113.74 | 64.17 | 64.17 |
FIG. 1.
Distribution of rapid test costs for HIV-negative patients at 45 U.S. hospitals, 2005–2006. “Counseling” includes personnel costs for pre-test and post-test counseling; “Collection and Processing” includes costs associated with specimen collection and performing the test; “Materials” includes the cost of the rapid test kit and the cost of miscellaneous disposable items.
Variability in the time spent performing rapid test–related activities, the types of hospital staff who performed these activities, and compensation levels both between and within particular staff categories resulted in substantial between-site variations in the personnel costs associated with rapid HIV testing. Total personnel costs associated with counseling and test-related procedures ranged from $10.00 to $68.94 for negative tests and from $14.57 to $98.78 for preliminary-positive tests (see Table 2). On average, personnel costs accounted for 62.8% of the total cost of rapid testing and counseling for HIV-negative patients and 72.3% of the cost for preliminary-positive patients.
Counseling costs
Study hospitals spent an average of 11.9 min providing pretest counseling, at a mean cost of $11.05; 8.3 min in posttest counseling for HIV-negative patients (mean cost, $9.02); and 21.1 min counseling preliminary-positive patients (cost, $25.12). On average, personnel costs associated with pre- and posttest counseling accounted for 38.4% of the total cost of rapid testing for HIV-negative patients and 53.7% of the cost for preliminary-positive patients. Higher counseling costs were significantly associated with higher overall costs (Spearman's rho, 0.86; p < 0.01 for negative tests and rho, 0.87; p < .01 for positive tests).
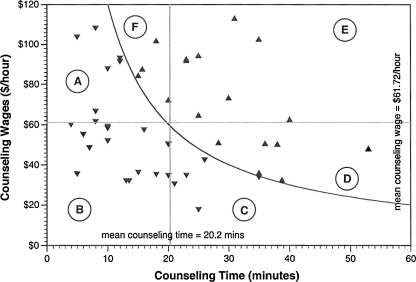
Many hospitals reduced counseling costs (hence, overall costs) by limiting pre- and posttest counseling time, as shown in Fig. 2 for HIV-negative patients. Hospitals that spent less than the mean value of 20.2 min counseling HIV-negative patients were significantly more likely to have below-average counseling costs than were other hospitals (χ2, 15.55; p < 0.0001). Similarly, hospitals that used counseling personnel with compensation rates below the mean compensation rate ($61.72 per hour for counseling HIV-negative patients) were significantly more likely to have below-average counseling costs than were other hospitals (χ2, 8.89; p < 0.005). Similar results were obtained for preliminary-positive patients (χ2, 4.54; p < 0.05 for below-average time and χ2, 8.22; p < 0.005 for below-average compensation).
FIG. 2.
Pre/post-test counseling time and average wages for hospital personnel involved in counseling HIV-negative patients at 45 U.S. hospitals, 2005–2006. Study hospitals spent a mean of 20.2 min in counseling activities, at an average cost of $20.07. The curve separates those hospitals with below-average counseling costs (Regions A, B, and C) from those with above average counseling costs (Regions D, E, and F). Hospitals reduced counseling costs by limiting the amount of time spent in counseling activities (Regions A and B) and/or by utilizing less well compensated personnel in these activities (Regions B and C).
Discussion
This analysis of rapid HIV testing and counseling at 45 U.S. hospitals produced mean cost estimates of $48.07 for a negative test result and $64.17 for a preliminary-positive result. These cost estimates do not include the cost of confirmatory testing or follow-up counseling for preliminary-positive patients, which elsewhere have been estimated at $34.10
Considerable variability in testing and counseling costs was observed across study hospitals. For example, the overall cost per HIV-negative patient ranged from $24.64 to $87.94, or approximately half to twice the mean cost estimates. Similar variability was observed for preliminary-positive test costs.
Differences in the personnel costs associated with pre- and posttest counseling were a main source of variability in overall testing costs. On average, counseling costs accounted for 38.4% of the total cost of rapid testing for HIV-negative patients and 53.7% of the cost for preliminary-positive patients.
As expected, higher counseling costs were associated with higher overall costs. Hospitals that spent less time in counseling activities, or that used less well-compensated personnel (e.g., HIV counselors and social workers) to conduct pre- and posttest counseling sessions, had significantly lower overall counseling and testing costs than did other hospitals.
This analysis is subject to several limitations. First, hospitals were included in the study only if both the departmental and hospital laboratory agreed to participate and provided detailed, somewhat sensitive cost information. The overall study inclusion rate, 49.5%, reflects limitations of this two-stage survey process.
Second, although the survey asked which personnel types performed each of the five rapid test–related procedures, it did not ascertain the proportions with which each personnel type performed each activity. The main analysis assumed that all personnel types who sometimes performed the procedure were equally likely to perform the procedure. If, instead, each procedure was performed by the lowest-paid or highest-paid personnel type who sometimes performed the procedure, the mean cost for a negative test would be ∼12% larger or smaller, respectively.
Third, the study survey did not ask about the type of specimen (e.g., oral fluid or whole blood) collected for the rapid test.
Finally, because of the large number of hospitals involved in the study, it was not practical to conduct time–motion analyses to estimate the amount of time spent performing counseling and testing-related procedures at study hospitals. Instead, the study relied on hospital estimates of procedure times. With one exception, these time estimates were consistent with those of Farnham et al.,8 who used a decision-analytic framework to estimate the cost of rapid testing and counseling at a sexually transmitted disease clinic and rapid antibody screening (testing without pretest counseling) at a hospital emergency department. Hospitals' estimates of the time required to process a rapid-test specimen were problematic, however. Although the survey question that asked about the time to process a test explicitly stipulated that respondents should include “only time spent running the test, including time for set-up, reading results, and recording results,” not “the time that the test is running,” the mean (19.5 min) and median (15 min) values reported by study hospitals suggest that some respondents may have reported the total elapsed time required to run the test, including the time the test was developing before results were available. The CDC estimates that as few as 3 to 5 min of personnel time are required to process a rapid-test specimen.9 We conducted a sensitivity analysis in which processing times exceeding 3.5 min were replaced by a uniform value of 3.5 min. The resultant cost estimates were $40.51 for a negative test and $56.60 for a preliminary-positive test (15.7% and 11.8% smaller than the base-case values, respectively).
The present study was conducted in 2005 through 2006, prior to the September 2006 release of the CDC's revised recommendations for HIV testing in health-care settings.1 Although these guidelines encourage “prevention counseling” for patients at high risk of HIV infection, they further clarify that “in general, prevention counseling should not be required … in health care settings.” Study hospitals spent an average of 11.9 min in pretest counseling activities and 8.3 min providing posttest counseling to patients who tested HIV negative. We conducted a sensitivity analysis to assess the potential cost implications of eliminating pretest counseling and reducing the time spent counseling persons who tested negative to only 1 min, consistent with Farnham et al.8 Streamlining counseling in this manner reduced overall costs to $27.15 for HIV-negative patients (a reduction of 43.5% compared with the base-case cost estimate) and to $51.50 for preliminary-positive patients (a 19.7% reduction).
Conclusion
The mean cost of rapid HIV testing at the 45 study hospitals was $48.07 for a negative test and $64.17 for a preliminary-positive result. Reducing the amount of time spent in counseling activities and increased use of qualified but less well-compensated counselors could substantially reduce the overall cost of hospital-based rapid HIV testing and counseling.
Acknowledgments
This study was supported by grants U65/CCU924523-01 from the Centers for Disease Control and Prevention (CDC) and P30-MH52776 from the National Institute of Mental Health (NIMH). The findings and conclusions presented here are those of the authors and do not necessarily represent the views of the CDC or NIH.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Centers for Disease Control and Prevention. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR. 2006;55:1–17. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Approval of a new rapid test for HIV antibody. MMWR. 2002;51:1051–1052. [PubMed] [Google Scholar]
- 3.Farnham PG. Gorsky RD. Holtgrave DR. Jones WK. Guinan ME. Counseling and testing for HIV prevention: costs, effects, and cost-effectiveness of more rapid screening tests. Public Health Rep. 1996;111(1):44–53. [PMC free article] [PubMed] [Google Scholar]
- 4.Kallenborn JC. Price TG. Carrico R. Davidson AB. Emergency department management of occupational exposures: cost analysis of rapid HIV test. Infect Control Hosp Epidemiol. 2001;22(5):289–293. doi: 10.1086/501902. [DOI] [PubMed] [Google Scholar]
- 5.Kelen GD. Shahan JB. Quinn TC. Emergency department-based HIV screening and counseling: experience with rapid and standard serologic testing. Ann Emerg Med. 1999;33(2):147–155. doi: 10.1016/s0196-0644(99)70387-2. [DOI] [PubMed] [Google Scholar]
- 6.Bogart LM. Howerton D. Lange J. Becker K. Setodji CM. Asch SM. Scope of rapid HIV testing in private nonprofit urban community health settings in the United States. Am J Public Health. 2008;98:736–742. doi: 10.2105/AJPH.2007.111567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bogart LM. Howerton D. Lange J, et al. Provider-related barriers to rapid HIV testing in U.S. urban non-profit community clinics, community-based organizations (CBOs) and hospitals. AIDS Behav. 2010;14:697–707. doi: 10.1007/s10461-008-9456-3. [DOI] [PubMed] [Google Scholar]
- 8.Farnham PG. Hutchinson AB. Sansom SL. Branson BM. Comparing the costs of HIV screening strategies and technologies in health-care settings. Public Health Rep. 2008;123(Suppl 3):51–62. doi: 10.1177/00333549081230S307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Charts comparing rapid HIV antibody screening tests. http://www.hret.org/hret/programs/hivtransmrpd.html. [Apr 7;2009 ]. http://www.hret.org/hret/programs/hivtransmrpd.html
- 10.Paltiel AD. Weinstein MC. Kimmel AD, et al. Expanded screening for HIV in the United States: an analysis of cost-effectiveness. N Engl J Med. 2005;352:586–595. doi: 10.1056/NEJMsa042088. [DOI] [PubMed] [Google Scholar]