Abstract
To benefit maximally from antiretroviral therapy, patients with HIV infection must enter care before their disease is advanced and adhere to care. We sought to determine if and where on this continuum of care racial/ethnic disparities were evident. Data from the Flexible Initial Retrovirus Suppressive Therapies (FIRST) trial, which evaluated three strategies for initial HIV therapy, were compared for White, African American, and Latino subjects. Outcomes included progression of disease and death, HIV viral suppression, and change in CD4+ cell count. Multivariate Cox proportional hazard models adjusted for known predictors of survival. There were 1357 subjects, including 368 non-Latino white, 751 non-Latino African American, and 238 Latino subjects. At baseline, the two latter groups were more likely to have had AIDS and had lower CD4+ cell counts than white subjects. In follow-up, African American subjects had lower self-reported adherence to therapy, lower CD4+ cell count increases, and lower odds of viral suppression. African American and Latino subjects had unadjusted hazard ratios of progression of disease or death of 1.57 (1.17, 2.10; p = 0.0025) and 1.57 (1.09, 2.26; p = 0.02), respectively. Adjusting for baseline differences and differences in adherence, CD4+ cell count change, and viral suppression accounted for the disparities in outcomes. Opportunities to reduce disparities in outcomes for African American and Latino patients exist along the continuum of HIV care. Efforts to promote access to HIV testing and care and to improve adherence have the potential to reduce racial/ethnic disparities in outcomes of patients with HIV infection.
Introduction
Healthy People 2010 calls for the elimination of health related disparities in the United States.1 HIV infection and HIV-related mortality disproportionately burden African American and Latino persons compared to non-Latino white persons. Of the estimated 1.1 million people in the United States living with HIV infection, 46.1% and 17.5% are African American and Latino, despite constituting 12% and 14% of the U.S. population, respectively.2 Of the over 14,000 deaths with AIDS in the United States in 2006, 51% were in African Americans, and 17% were in Latinos.3 Three-year survival after an AIDS diagnosis remains worse for African American patients than for non-Latino white patients well into the highly active antiretroviral therapy (HAART) era.3,4
The causes of these disparities in survival are not entirely known. In order to benefit maximally from HAART, a patient must be diagnosed as HIV infected before his or her immune status becomes too compromised, enter HIV care, be prescribed HAART, and then adhere to HAART and remain in care.5,6 Evidence for disparities along the continuum of care is inconsistent. African Americans are more likely to be diagnosed with HIV infection with more advanced disease,7 although that finding is not universal.8 Studies early in the HAART era found that African Americans had delayed access to HAART,9,10 and more recent data suggest that the differential access to and utilization of HAART may persist.11 However, delayed diagnosis and barriers to care may not completely explain these disparities since research in HIV-infected active military personnel and veterans, populations with few barriers to care, have also found disparities in survival by race/ethnicity.12,13 Other research in military and veteran populations, however, has not found disparities in survival.14,15 Disparities in clinical outcomes, including viral suppression and certain adverse events in response to HAART, have been documented.12,16,17 Emerging data suggest that worse retention in HIV care may partially explain some of these disparities.18,19
African American and Latino patients with HIV infection are underrepresented in research studies, though they are not less willing to participate in research.20,21 Patients voluntarily enrolled in randomized controlled clinical trials presumably have adequate access to care and commitment to remain on treatment. Study subjects receive additional attention and support from physicians and research staff to be recruited, to provide informed consent and data on covariates and outcomes, and to prevent loss to follow-up. Given these differences from routine care, we reasoned that volunteers for a clinical trial would have similar outcomes regardless of race/ethnicity. Finding no disparities would suggest that additional clinical support similar to that offered in randomized clinical trials could help eliminate racial/ethnic disparities.
We examined the laboratory and clinical outcomes of patients with HIV infection who were part of a large clinical trial, the FIRST study (described in the Methods section). The objective of the current study was to determine if there were differences in important surrogate endpoints (adherence to antiretroviral therapy, change in CD4+ cell count, and suppression of HIV viremia) and clinical outcomes (progression of disease and death) for African American and Latino subjects compared to non-Latino white subjects, independent of study arm. If disparities were found, we sought to determine where in the continuum of care the disparities were located. A greater understanding of where in the continuum of care disparities occur could in turn lead to better targeting of clinical resources and focused research on interventions to eliminate disparities.
Methods
Subjects and measurements
The CPCRA (Terry Beirn Community Programs for Clinical Research on AIDS) FIRST (Flexible Initial Retrovirus Suppressive Therapies) trial was a three-arm study comparing an initial treatment strategy of nucleoside reverse transcriptase inhibitors (NRTI) with a protease inhibitor (PI), non-nucleoside reverse transcriptase inhibitor (NNRTI), or both. The methods for the FIRST study have been described in detail.22,23 In brief, antiretroviral-naïve persons at least 13 years of age were randomly allocated, in a ratio of 1:1:1, to one of three starting strategies: PI strategy (PI + NRTI); NNRTI strategy (NNRTI + NRTI); or three-class strategy (PI +NNRTI + NRTI). Treatment could be changed at any time, if necessary, either because of treatment failure or drug intolerance. If treatment was changed because of intolerance, alternate drugs from the same class of medications were to be selected if possible. Between 1999 and 2002, 1397 antiretroviral-treatment-naive subjects, presenting at 18 clinical trial units with 80 research sites in the United States, were enrolled in the study. Subjects had a median of 5 years of follow-up. The primary finding of the study was that the three-class regimen offered no advantages over a two-class strategy for immunological or clinical outcomes and resulted in increased toxic effects. The initiation of antiretroviral treatment with a two-class NNRTI-based regimen or a two-class PI-based regimen resulted in similar clinical and immunological outcomes. Importantly, the efficacy of the three treatment strategies did not differ among the three racial/ethnic groups.
Race and ethnicity were assessed at baseline by self-report, and medical records were reviewed for history of AIDS-defining conditions. Hepatitis C antibody status was assessed at baseline. After randomization, subjects had study visits at months 1 and 4 and then every 4 months thereafter. At screening, randomization, and each follow-up visit, CD4+ cell count and HIV RNA level (Roche Amplicor 1.0, Roche Diagnostics, Indianapolis, IN) were measured. At each study visit, clinical status was assessed and information was obtained about changes in antiretroviral treatment of 30 days or more duration. Self-reported adherence was assessed with the CPCRA Antiretroviral Medication Self-Report instrument, a validated self-report instrument based on 7-day recall.24,25 For each medication prescribed, subjects were asked to record whether they took “all,” “most,” “about half,” “very few,” or “none” of their pills during the preceding 7 days.25 For computation purposes in the present analysis, the responses at each time point were converted to “all” = 100%, “most” = 80%, “about half” = 50%, “very few” = 20%, or “none” = 0%. An adherence score at each visit was calculated as the average of the responses reported for each component of the regimen. Adherence values for fixed dose combination drugs were counted once. A score of 0 was assigned for visits when subjects were off therapy. Missing values were not assigned a value. Cumulative adherence at a visit was calculated as the average adherence from all visits up to that point in time and was used as a time-updated covariate in regression analyses.
The primary end point for the original trial was a composite of progression of disease or death, comprised of an AIDS-defining event, death, or CD4+ cell count decline to less than 200 cells/mm3 (assessed only in subjects with baseline CD4+ cell counts ≥200 cells/mm3). Secondary end points included death, a composite of an AIDS-defining event or death, and viral suppression. Disease progression events were defined as the occurrence of a confirmed or probable AIDS-defining event according to 1993 U.S. Centers for Disease Control (CDC) criteria. A clinical events committee unaware of the assigned treatment group reviewed all disease progression events and deaths. The CD4+ cell count-based component of AIDS was not included in the definition of AIDS used in the present analyses. In addition to these outcomes, we measured HIV viral suppression (HIV RNA level <50 copies per milliliter), change in CD4+ cell count from baseline, and adherence to antiretroviral therapy.
The FIRST study was approved by Institutional Review Boards at all of the participating sites, and all subjects provided written informed consent.
Statistical analysis
Summary statistics of the baseline measures and counts were calculated and compared using the t test or χ2. For the three clinical end points the number of events during follow-up was determined and expressed as a rate (number per 100 person-years). The unadjusted hazard ratios were calculated and compared to 1.0 using the Cox proportional hazards model. This model was also used to obtain the race/ethnicity-specific hazard ratios adjusting for baseline variables and 4 time-dependent covariates: latest CD4+ cell count, whether the subject was still using antiretroviral therapy at that visit, whether the latest HIV RNA level was less than 50 copies per milliliter or not, and cumulative percent adherence score.
Repeated measure analysis assuming compound symmetry correlation structure was used to estimate the race/ethnicity odds ratios for percent reporting use of antiretroviral therapy at each visit, percent with HIV RNA level less than 50 copies per milliliter at each visit, and odds of missed study visits. Mixed linear models with random intercept effects were used to compare average adherence at each visit and the mean changes in CD4+ cell counts by race/ethnicity adjusted for baseline values. All statistical analyses were performed using SAS (version 8.0, SAS Institute, Cary, NC).
Results
Subjects and baseline differences
The FIRST study included 1397 subjects followed for a median of 60 months. The efficacy of the 3 treatment strategies did not differ for white, African American, and Latino patients, as previously reported.22 Forty subjects whose race/ethnicity was other than African American, Latino, and non-Latino white, were excluded from the present analysis. The remaining 1357 subjects included 368 (27.1%) non-Latino white (hereafter, “white”) subjects, 751 (55.3%) non-Latino African American (hereafter, “African American”) subjects, and 238 (17.5%) Latino subjects. The mean age of the subjects was 38.3 years. Women accounted for 20.7% of the study population, 15.1% had used injection drugs, 38.1% had a diagnosis of AIDS, and 19.9% had hepatitis C coinfection. The mean CD4+ cell count was 210.6 cells/mm3, the mean log10 HIV RNA level was 5.0 copies per milliliter, and the mean body mass index was 24.5 kg/m2.
There were a number of differences in baseline characteristics comparing the African American and Latino subjects to the white subjects (Table 1). Women were more heavily represented in the African American group than the white group. African American subjects were more likely to have a history of AIDS and have hepatitis C coinfection compared to white subjects. African American subjects had lower baseline CD4+ cell counts and slightly lower HIV RNA levels than their white counterparts. Latino subjects were younger than the white subjects, a greater proportion was female and had a history of AIDS, and they had lower baseline CD4+ cell counts than white subjects. There were no differences between the Latino and white subjects or the African American and white subjects in randomized strategy in FIRST, history of injection drug use, and body mass index.
Table 1.
Baseline Characteristics of Participants by Race/ethnicity
| |
|
|
|
p Value |
|
|---|---|---|---|---|---|
| Baseline characteristics | White (n = 368) | African American (n = 751) | Latino (n = 238) | African American vs. white | Latino vs. white |
| Age (mean years) | 38.3 | 38.8 | 36.7 | 0.34 | 0.044 |
| Gender (% female) | 6.3 | 27.8 | 20.6 | < 0.0001 | < 0.0001 |
| Randomization strategy | 0.59 | 0.66 | |||
| PI (%) | 31.0 | 34.0 | 34.9 | ||
| NNRTI (%) | 34.5 | 33.2 | 32.4 | ||
| 3-Class (%) | 34.5 | 32.9 | 32.8 | ||
| Prior IDU (%) | 15.3 | 15.3 | 14.3 | 0.97 | 0.74 |
| Prior AIDS (%) | 30.2 | 41.1 | 40.8 | 0.0004 | 0.0074 |
| Hepatitis C (%) | 16.0 | 21.9 | 19.4 | 0.021 | 0.28 |
| CD4 cell counta (mean cells/mm3) | 247.2 | 197.0 | 197.2 | 0.0001 | 0.0022 |
| HIV RNA levela (mean log10 copies/per milliliter) | 5.1 | 5.0 | 5.1 | 0.0093 | 0.80 |
| Body mass index (kg/m2) | 24.1 | 24.6 | 24.7 | 0.077 | 0.16 |
Average of screening and randomization values.
PI, protease inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; IDU, injection drug use.
Adherence, remaining on antiretroviral therapy, and missed study visits
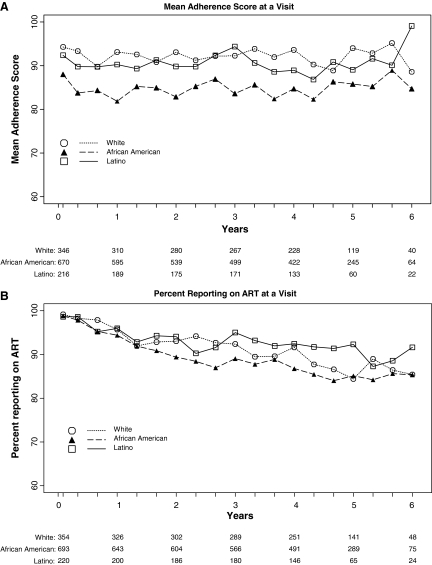
The mean adherence score at each visit by race is displayed in Figure 1A. The means for African American subjects were less than those for white subjects at all visits. The average difference in adherence scores between African American and white subjects over all follow-up time was −8.7% (95% confidence interval [CI] −10.84, −6.54; p < 0.001). The average difference in adherence scores between Latinos and whites was −2.8% (95% CI −5.74, 0.14; p = 0.06). The percent of subjects on antiretroviral therapy at each study visit as displayed in Figure 1B did not differ significantly by race (odds ratio [OR] for African American and Latino subjects compared to white subjects 0.98, 95% CI 0.94, 1.00; p = 0.07, and 1.01, 95% CI 0.99, 1.04; p = 0.30, respectively). White subjects attended 91.3% of study visits, compared to 90.0% for African American and 89.9% for Latino subjects. The odds of attendance did not differ for African American (p = 0.26) or Latino (p = 0.39) subjects compared to white subjects.
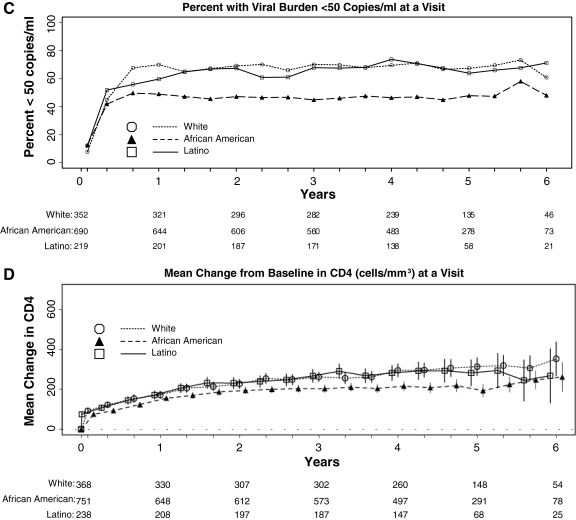
FIG. 1.
(A) Mean adherence score. (B) Percent using antiretroviral therapy. (C) Percent with viral suppression less than 50 copies per milliliter. (D) Mean change in CD4+ cell count, by race/ethnicity at each follow-up visit.
Virologic and CD4+ cell count outcomes
African American subjects were less likely than White subjects to have an HIV RNA level less than 50 copies per milliliter at follow-up visits (Fig. 1C). In a repeated measures analysis, African American subjects had lower odds of viral suppression compared to white subjects (OR 0.48, 95% CI 0.40, 0.58; p < 0.001). The odds of viral suppression were not different for Latino subjects compared to white subjects (OR 0.85, 95% CI 0.67, 1.08; p = 0.19).
The mean change in CD4+ cell count was lower for African American subjects than for white subjects (Fig. 1D). In a repeated measures analysis adjusted for baseline CD4+ cell count, African American subjects had a CD4+ cell count change that was 52.7 cells/mm3 lower than white subjects (95% CI −74.65, −30.75; p < 0.001). The change for Latino subjects was 10.00 cells/mm3 lower than white subjects (95% CI −39.20, 19.20), which was not a statistically significant difference (p = 0.50).
Survival analyses
In unadjusted analyses (Table 2), the hazard ratios for African American and Latino compared to white subjects were significantly greater than 1.0 for all events, including death, AIDS or AIDS-related death, and progression of disease or death, except for the case of deaths comparing Latino and white subjects.
Table 2.
Numbers of Clinical Events (rate per 100 person-years) and Unadjusted Hazard Ratios (95% CI) and p Values for Three End Points by Race
| |
Number of events (rate per 100 person-years) |
Unadjusted hazard ratio (95% CI) p value |
|||
|---|---|---|---|---|---|
| Event | White (n = 368) | African American (n = 751) | Latino (n = 238) | African American vs. white | Latino vs. white |
| Death | 39 (2.3) | 120 (3.5) | 26 (2.4) | 1.56 (1.09, 2.24) 0.02 | 1.09 (0.66, 1.79) 0.74 |
| AIDS or AIDS-related death | 43 (2.7) | 134 (4.4) | 46 (5.0) | 1.61 (1.14, 2.27) 0.01 | 1.82 (1.20, 2.76) 0.0047 |
| Progression of disease or death | 60 (3.7) | 182 (5.9) | 55 (6.0) | 1.57 (1.17, 2.10) 0.0025 | 1.57 (1.09, 2.26) 0.02 |
CI, confidence interval.
To determine whether disparities in outcomes existed after adjusting for baseline differences by race/ethnicity, we created Cox proportional hazard models that included age, gender, prior AIDS, injection drug use, hepatitis C coinfection, and baseline CD4+ cell count, HIV RNA level, and the FIRST randomized strategy group. For African American subjects compared to white subjects, the adjusted hazard ratios for death, AIDS or AIDS-related death, and progression of disease or death were 1.38 (95% CI 0.94, 2.01; p = 0.098), 1.36 (95% CI 0.95, 1.94; p = 0.094), and 1.39 (95% CI 1.03–1.88; p = 0.033), respectively (Table 3A). For Latino subjects compared to white subjects the corresponding adjusted hazard ratios were 1.05 (95% CI 0.64, 1.74; p = 0.84), 1.48 (95% CI 0.97, 2.25; p = 0.07), and 1.41 (95% CI 0.97–2.05; p = 0.068), respectively.
Table 3A.
Hazard Ratios and p Values for Three End Points Adjusted for Baseline Characteristicsa
| |
Hazard ratio (95% CI) p value |
||
|---|---|---|---|
| Variable | Death (n = 1357) | AIDS or AIDS-related death (n = 1357) | Progression of disease or death (n = 1357) |
| African Americanb | 1.38 (0.94, 2.01) 0.098 | 1.36 (0.95, 1.94) 0.094 | 1.39 (1.03, 1.88) 0.033 |
| Latinob | 1.05 (0.64, 1.74) 0.840 | 1.48 (0.97, 2.25) 0.070 | 1.41 (0.97, 2.05) 0.068 |
| Age (per 10 years increase) | 1.42 (1.21, 1.68) < 0.001 | 0.85 (0.73, 1.00) 0.055 | 1.12 (0.98, 1.28) 0.092 |
| Prior AIDS | 1.75 (1.25, 2.45) 0.001 | 2.00 (1.46, 2.74) < 0.001 | 1.76 (1.35, 2.30) < 0.001 |
| Baseline CD4+ cell count (per 100 cells/mm3 higher) | 0.83 (0.74, 0.93) 0.002 | 0.70 (0.61, 0.79) < 0.001 | 0.78 (0.70, 0.86) < 0.001 |
| Baseline HIV RNA level (per log10 copies/per milliliter higher) | 1.18 (0.92, 1.50) 0.187 | 1.26 (1.00, 1.58) 0.049 | 1.31 (1.07, 1.59) 0.007 |
Other baseline variables in the model having nonsignificant hazard ratios: injecting drug use, gender, and hepatitis C coinfection.
Reference group is white.
CI, confidence interval.
We next added time-updated values for the following variables to assess their effects on race/ethnicity-based disparities in survival: whether or not the subject was on antiretroviral therapy at last study visit, cumulative percent adherence, HIV RNA level less than 50 copies per milliliter at last visit, and last CD4+ cell count. These adjustments strongly moderated the disparity of clinical outcomes for the African American subjects (Table 3B). In the fully adjusted model, the highest adjusted hazard ratio (HR), that for progression of disease or death, was only 1.03 (95% CI 0.75, 1.43; p = 0.838). The adjustments similarly reduced the effect of Latino ethnicity. For example, the adjusted HR for progression of disease or death was 1.30 (95% CI 0.87, 1.93; p = 0.203), which is lower than the unadjusted HR of 1.57 (95% CI 1.09–2.26; p = 0.02).
Table 3B.
Hazard Ratios and p Values for Three End Points Adjusted for Baseline and Time-Updated Characteristicsa
| |
Hazard ratio (95% CI) p value |
|
|
|---|---|---|---|
| Variable | Death (n = 1357) | AIDS or AIDS-related death (n = 1357) | Progression of disease or death (n = 1357) |
| African Americanb | 1.02 (0.69, 1.51) 0.919 | 0.99 (0.67, 1.47) 0.968 | 1.03 (0.75, 1.43) 0.838 |
| Latinob | 0.96 (0.57, 1.62) 0.877 | 1.38 (0.87, 2.18) 0.174 | 1.30 (0.87, 1.93) 0.203 |
| Age (per 10 years increase) | 1.73 (1.48, 2.03) < 0.0001 | 1.04 (0.88, 1.23) 0.647 | 1.34 (1.17, 1.53) < 0.0001 |
| Prior AIDS | 1.78 (1.26, 2.51) < 0.0001 | 2.09 (1.49, 2.94) < 0.0001 | 1.81 (1.36, 2.39) < 0.0001 |
| Baseline HIV RNA level (per log10 copies/per milliliter higher) | 1.42 (1.11, 1.82) 0.005 | 1.34 (1.05, 1.71) 0.021 | 1.42 (1.16, 1.75) < 0.0001 |
| CD4 cell count (per 100 cells/mm3 increase)c | 0.66 (0.58, 0.75) < 0.0001 | 0.57 (0.49, 0.66) < 0.0001 | 0.65 (0.58, 0.73) < 0.0001 |
| HIV RNA level < 50 copies/per milliliter (yes vs. no)c | 0.75 (0.50, 1.13) 0.165 | 0.62 (0.41, 0.93) 0.022 | 0.65 (0.47, 0.91) 0.011 |
| ART status (on vs. off )c | 0.57 (0.38, 0.87) 0.009 | 0.92 (0.58, 1.44) 0.708 | 0.84 (0.57, 1.23) 0.368 |
| Mean cumulative adherence score (per 10% decrease)c | 1.14 (1.07, 1.22) < 0.0001 | 1.14 (1.07, 1.20) < 0.0001 | 1.14 (1.08, 1.20) < 0.0001 |
Other baseline variables in the model having nonsignificant hazard ratios: baseline CD4 cell count, two terms for randomization strategy groups, injecting drug use, gender, and hepatitis C coinfection.
Reference group is white.
Time-updated variable.
CI, confidence interval.
Discussion
In these analyses of a large, multisite trial, in unadjusted analyses, we found that African American and Latino subjects experienced less benefit from antiretroviral therapy and had higher rates of important clinical outcomes, including progression of disease and death, than white subjects. For African American subjects, these results were largely explained by more advanced disease at the initiation of antiretroviral therapy and less consistent adherence to antiretroviral therapy, which likely contributed to lower rates of viral suppression and less CD4+ cell count increase, compared to the white subjects (Tables 3A and B). Latino subjects also had more advanced disease at therapy initiation, and their adherence level was somewhat lower than white subjects, reaching borderline statistical significance (p = 0.06). There were no differences in viral suppression or CD4+ cell count change for Latino subjects compared to White subjects. Together, the findings suggest that opportunities to reduce disparities in outcomes for HIV-infected African American and Latino patients exist along the continuum of HIV care, from earlier diagnosis and entry into care to improved adherence to antiretroviral therapy. Importantly, the type of support provided to subjects in randomized controlled trials, while likely helpful and perhaps explaining the high study retention rates in all three groups, is not sufficient to eliminate disparities in adherence and outcomes, even in this self-selected population of patients. To our knowledge, this is the first multisite study to examine the entire continuum of care,26 and the results support recent estimates of the impact of delayed diagnosis and poor retention in care on outcomes.27,28
The significantly lower CD4+ cell counts and higher prevalence of prior AIDS diagnoses among African American and Latino subjects at study entry when compared to white subjects suggest an ongoing disparity in accessing care. This observation is consistent with CDC data showing higher prevalence of delayed diagnosis in minority patients.29 Routine, opt-out testing for HIV infection followed by successful linkage to care for those patients newly diagnosed may reduce the number of minority patients who enter care with advanced HIV disease.30 The success of these programs will depend on access to care for minority patients, which affects HIV testing rates.31 Efforts to increase access to care and promote the adoption of opt-out screening should continue with a sense of urgency, as an earlier diagnosis and linkage to care may be one of the best methods available to reduce disparities in survival for African Americans and Latinos with HIV infection in the United States since it might impact both the CD4+ cell count at initiation of HAART and whether patients have a history of AIDS at the initiation of HAART.
While improved access to HIV testing and antiretroviral therapy may alleviate some race-based disparities in outcomes, earlier diagnosis and access to antiretroviral therapy will not eliminate disparities entirely. We found differences in adherence to antiretroviral therapy by race among subjects in this randomized clinical trial. While it is unlikely that race or ethnicity themselves directly affect adherence, a number of studies have found that African American race is associated with less adherence than White race.32–35 Barriers to adherence are common and include internal barriers, such as stigma, psychiatric and substance use problems, as well as external and system-level barriers, such as difficulty with co-pays for prescriptions and transportation. We could not distinguish among these possibilities. A separate analysis of this dataset found that African American subjects were less likely to have lamivudine in their first regimen than white and Latino subjects, but African Americans had lower rates of discontinuation of initial therapy due to adverse events.16 We examined cumulative drug exposure to all antiretroviral drugs and found that African Americans were less likely to have used lamivudine and efavirenz and more likely to have used abacavir and didanosine during follow-up than white subjects. In models of adherence that adjusted for cumulative exposure to each antiretroviral drug, African American race remained associated with poorer adherence (p < 0.001; data not shown). It is unlikely, therefore, that the differences in adherence we observed are due to differences in adverse event rates. Understanding preferences for adverse events and regimen characteristics may allow better tailoring of regimens to individual patients, thereby improving adherence.36,37 Our results suggest that improving adherence to antiretroviral therapy for African American patients in the United States may mitigate race-based disparities in the United States. Interventions to improve adherence are being actively sought, and culturally appropriate methods should be a special focus of research.38
The FIRST study included a prospective controlled substudy of two interventions to improve adherence, an alarm reminder and a medication manager. The medication manager improved adherence and CD4+ cell counts, while the alarm reminder adversely affected adherence and virologic failure rates.39 The adherence sub-study included 68.4% of the subjects in the present analysis, and African American and Latino subjects were equally represented in the different arms of that study. The proportion of subjects in the FIRST substudy who were African American and Latino was the same as the proportion included in the present analysis (55% and 17%, respectively). The adherence interventions did not affect the number of subjects with viral suppression to less than 50 copies per milliliter. It is therefore unlikely that the substudy intervention explains the differences in adherence we observed.
The use of antiretroviral therapy, viral suppression, change in CD4+ cell count, and adherence during follow-up appeared to mediate event free survival less in Latino subjects than in African American subjects, as evidenced by the still increased hazard ratios for Latino subjects in Table 3B, though the p values were >0.05. This may be random variation. Another possible explanation arises from the observation that the incidences of Kaposi sarcoma and lymphoma varied significantly by race. For Kaposi sarcoma, the incidences were 6 of 238, 6 of 368, and 4 of 751, for Latino, white, and African American subjects, respectively, overall p = 0.04. For lymphoma the incidences were 9 of 238, 6 of 368, and 7 of 751 respectively, overall p = 0.02. Omitting these events from the definition of AIDS or AIDS related death in the survival analyses in Table 3B substantially reduced the adjusted hazards of AIDS or AIDS related death for Latino subjects from 1.38 (95% CI 0.87, 2.18; p = 0.17) to 1.16 (95% CI 0.71, 1.91; p = 0.55).
This study includes data from a large number of subjects recruited throughout the United States, and followed for a median of 5 years. Nonetheless, it has certain limitations that should be acknowledged. Subjects in randomized, controlled clinical trials are not necessarily representative of patients in routine clinical care. They are often younger and healthier than the general population, and historically, racial and ethnic minorities have been underrepresented.20 The analyses could not account for baseline antiretroviral drug resistance because the data are not available for all subjects. However, a subset of 491 subjects did have a baseline genotype result, and 11.6% had at least one major mutation.40 The prevalence of baseline resistance was higher in white subjects than in others, suggesting that underlying resistance does not explain the observed disparities in outcomes. Temporary interruptions (<30 days) in antiretroviral therapy are not well captured in the database and may explain some of the differences found. The study did not consider biologic differences by race/ethnicity, e.g., differences in host genetics that could affect drug metabolism or HIV disease progression. Finally, the antiretroviral regimens used in the study are no longer regarded as optimal first-line therapy.22,41 The simpler dosing schedule of contemporary antiretroviral therapy can result in improved adherence, but that simplification would benefit all racial and ethnic groups equally.
In this analysis of a large randomized clinical trial, we found evidence of disparities in clinical outcomes for African American and Latino subjects, compared to white subjects. These disparities were explained by differences in disease severity at initiation of antiretroviral therapy and differences in adherence to antiretroviral therapy. That the disparities were observed among this self-selected group of research volunteers only serves to heighten the significance of these findings. Efforts to screen for HIV infection, reduce barriers to HIV care, and develop culturally appropriate methods to improve adherence to antiretroviral therapy need the support of the public, health care providers, policy makers, and the research community.
Acknowledgments
The Flexible Initial Retrovirus Suppressive Therapies (FIRST; CPCRA 058) study was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grants 5U01AI042170-10 and 5U01AI046362-03) 058). TPG received support from the National Institute of Mental Health, National Institutes of Health, grant K23MH067505 (TPG), and, in part, from the Houston VA HSR&D Center of Excellence (HFP90-020). The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
An analysis plan was drafted by T.P.G. and revised based on comments from J.A., E.T., S.M., and A.T. The data were analyzed by G.B., Y.Z., and A.T. The manuscript was drafted by T.P.G., and all other authors reviewed and edited the manuscript for important intellectual content and interpretation of the data. Investigators for the FIRST study are listed elsewhere.22
Author Disclosure Statement
No author has any real or potential conflict of interest with people or organizations that could inappropriately influence this work, though some of the authors have received funding from various pharmaceutical companies for research, speaking engagements, travel, and/or consulting. At the time of conduct of the FIRST study and during the initial stages of the present report, J.A. was Assistant Professor of Epidemiology, Mailman School of Public Health, Columbia University, and Attending Physician, Harlem AIDS Treatment Group, Harlem Hospital Center, NY. She is presently Director of Global Clinical Research, Virology, Research and Development, Bristol-Myers Squibb. Her current affiliation had no impact on the conduct of the FIRST study and her participation in the writing of this manuscript.
References
- 1.U.S. Department of Health and Human Services. Healthy People 2010: Understanding and Improving Health. 2nd. Washington, DC: U.S. Government Printing Office; 2000. [Google Scholar]
- 2.Centers for Disease Control and Prevention. HIV Prevalence Estimates—United States, 2006. MMWR. 2008;57:1073–1076. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. HIV/AIDS Surveillance Report, 2006. Vol. 18. Atlanta: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2008. [Google Scholar]
- 4.Blair JM. Fleming PL. Karon JM. Trends in AIDS incidence and survival among racial/ethnic minority men who have sex with men, United States, 1990–1999. J Acquir Immune Defic Syndr. 2002;31:339–347. doi: 10.1097/00126334-200211010-00011. [DOI] [PubMed] [Google Scholar]
- 5.Cheever LW. Engaging HIV-infected patients in care: Their lives depend on it. Clin Infect Dis. 2007;44:1500–1502. doi: 10.1086/517534. [DOI] [PubMed] [Google Scholar]
- 6.Giordano TP. Suarez-Almazor ME. Grimes RM. The population effectiveness of highly active antiretroviral therapy: Are good drugs good enough? Curr HIV/AIDS Rep. 2005;2:177–183. doi: 10.1007/s11904-005-0013-7. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control. Cases of HIV infection and AIDS in the United States, by race/ethnicity, 1998–2002. HIV/AIDS Surveill Suppl Rep. 2004;10:1–38. [Google Scholar]
- 8.Centers for Disease Control and Prevention. Missed opportunities for earlier diagnosis of HIV infection—South Carolina, 1997–2005. MMWR. 2006;55:1269–1272. [PubMed] [Google Scholar]
- 9.Shapiro MF. Morton SC. McCaffrey DF, et al. Variations in the care of HIV-infected adults in the United States: Results from the HIV Cost and Services Utilization Study. JAMA. 1999;281:2305–2315. doi: 10.1001/jama.281.24.2305. [DOI] [PubMed] [Google Scholar]
- 10.Cunningham WE. Markson LE. Andersen RM, et al. Prevalence and predictors of highly active antiretroviral therapy use in patients with HIV infection in the United States. HCSUS Consortium. HIV Cost and Services Utilization. J Acquir Immune Defic Syndr. 2000;25:115–123. doi: 10.1097/00042560-200010010-00005. [DOI] [PubMed] [Google Scholar]
- 11.Gebo KA. Fleishman JA. Conviser R, et al. Racial and gender disparities in receipt of highly active antiretroviral therapy persist in a multistate sample of HIV patients in 2001. J Acquir Immune Defic Syndr. 2005;38:96–103. doi: 10.1097/00126334-200501010-00017. [DOI] [PubMed] [Google Scholar]
- 12.Hartzell JD. Spooner K. Howard R. Wegner S. Wortmann G. Race and mental health diagnosis are risk factors for highly active antiretroviral therapy failure in a military cohort despite equal access to care. J Acquir Immune Defic Syndr. 2007;44:411–416. doi: 10.1097/QAI.0b013e31802f83a6. [DOI] [PubMed] [Google Scholar]
- 13.McGinnis KA. Fine MJ. Sharma RK, et al. Understanding racial disparities in HIV using data from the Veterans Aging Cohort 3-Site Study and VA administrative data. Am J Public Health. 2003;93:1728–1733. doi: 10.2105/ajph.93.10.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giordano TP. Morgan RO. Kramer JR, et al. Is there a race-based disparity in the survival of veterans with HIV? J Gen Intern Med. 2006;21:613–617. doi: 10.1111/j.1525-1497.2006.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silverberg MJ. Wegner SA. Milazzo MJ, et al. Effectiveness of highly-active antiretroviral therapy by race/ethnicity. AIDS. 2006;20:1531–1538. doi: 10.1097/01.aids.0000237369.41617.0f. [DOI] [PubMed] [Google Scholar]
- 16.Tedaldi EM. Absalon J. Thomas AJ. Shlay JC. van den Berg-Wolf M. Ethnicity, race, and gender. Differences in serious adverse events among participants in an antiretroviral initiation trial: Results of CPCRA 058 (FIRST Study) J Acquir Immune Defic Syndr. 2008;47:441–448. doi: 10.1097/QAI.0b013e3181609da8. [DOI] [PubMed] [Google Scholar]
- 17.Mugavero MJ. Lin H-Y. Allison JJ, et al. Racial disparities in HIV virologic failure: Do missed visits matter? J Acquir Immune Defic Syndr. 2009;50:100–108. doi: 10.1097/QAI.0b013e31818d5c37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giordano TP. Gifford AL. White AC, Jr., et al. Retention in care: A challenge to survival with HIV infection. Clin Infect Dis. 2007;44:1493–1499. doi: 10.1086/516778. [DOI] [PubMed] [Google Scholar]
- 19.Mugavero MJ. Lin HY. Allison JJ, et al. Failure to establish HIV care: Characterizing the “no show” phenomenon. Clin Infect Dis. 2007;45:127–130. doi: 10.1086/518587. [DOI] [PubMed] [Google Scholar]
- 20.Gifford AL. Cunningham WE. Heslin KC, et al. Participation in research and access to experimental treatments by HIV-infected patients. N Engl J Med. 2002;346:1373–1382. doi: 10.1056/NEJMsa011565. [DOI] [PubMed] [Google Scholar]
- 21.Adeyemi OF. Evans AT. Bahk M. HIV-infected adults from minority ethnic groups are willing to participate in research if asked. AIDS Patient Care STDs. 2009;23:859–865. doi: 10.1089/apc.2009.0008. [DOI] [PubMed] [Google Scholar]
- 22.MacArthur RD. Novak RM. Peng G, et al. A comparison of three highly active antiretroviral treatment strategies consisting of non-nucleoside reverse transcriptase inhibitors, protease inhibitors, or both in the presence of nucleoside reverse transcriptase inhibitors as initial therapy (CPCRA 058 FIRST Study): A long-term randomised trial. Lancet. 2006;368:2125–2135. doi: 10.1016/S0140-6736(06)69861-9. [DOI] [PubMed] [Google Scholar]
- 23.MacArthur RD. Chen L. Mayers DL, et al. The rationale and design of the CPCRA (Terry Beirn Community Programs for Clinical Research on AIDS) 058 FIRST (Flexible Initial Retrovirus Suppressive Therapies) trial. Control Clin Trials. 2001;22:176–190. doi: 10.1016/s0197-2456(01)00111-8. [DOI] [PubMed] [Google Scholar]
- 24.Mannheimer S. Thackeray L. Huppler Hullsiek K, et al. A randomized comparison of two instruments for measuring self-reported antiretroviral adherence. AIDS Care. 2008;20:161–169. doi: 10.1080/09540120701534699. [DOI] [PubMed] [Google Scholar]
- 25.Mannheimer S. Friedland G. Matts J. Child C. Chesney M. The consistency of adherence to antiretroviral therapy predicts biologic outcomes for human immunodeficiency virus-infected persons in clinical trials. Clin Infect Dis. 2002;34:1115–1121. doi: 10.1086/339074. [DOI] [PubMed] [Google Scholar]
- 26.Ulett KB. Willig JH. Lin HY, et al. The therapeutic implications of timely linkage and early retention in HIV care. AIDS Patient Care STDs. 2009;23:41–49. doi: 10.1089/apc.2008.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Losina E. Schackman BR. Sadownik SN, et al. Racial and sex disparities in life expectancy losses among HIV-infected persons in the united states: Impact of risk behavior, late initiation, and early discontinuation of antiretroviral therapy. Clin Infect Dis. 2009;49:1570–1578. doi: 10.1086/644772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith KY. Paying the price for late starts and early stops: Racial and sex disparities in HIV-related mortality. Clin Infect Dis. 2009;49:1579–1581. doi: 10.1086/644773. [DOI] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. Reported CD4+ T-lymphocyte results for adults and adolescents with HIV/AIDS—33 states, 2005. HIV AIDS Surveill Rep. 2005;11:20. [Google Scholar]
- 30.Branson BM. Handsfield HH. Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR. 2006;55:1–17. quiz CE1–4. [PubMed] [Google Scholar]
- 31.Mimiaga MJ. Reisner SL. Bland S, et al. Health system and personal barriers resulting in decreased utilization of HIV and STD testing services among at-risk black men who have sex with men in Massachusetts. AIDS Patient Care STDs. 2009;23:825–835. doi: 10.1089/apc.2009.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gifford AL. Bormann JE. Shively MJ. Wright BC. Richman DD. Bozzette SA. Predictors of self-reported adherence and plasma HIV concentrations in patients on multidrug antiretroviral regimens. J Acquir Immune Defic Syndr. 2000;23:386–395. doi: 10.1097/00126334-200004150-00005. [DOI] [PubMed] [Google Scholar]
- 33.Golin CE. Liu H. Hays RD, et al. A prospective study of predictors of adherence to combination antiretroviral medication. J Gen Intern Med. 2002;17:756–765. doi: 10.1046/j.1525-1497.2002.11214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schackman BR. Ribaudo HJ. Krambrink A. Hughes V. Kuritzkes DR. Gulick RM. Racial differences in virologic failure associated with adherence and quality of life on efavirenz-containing regimens for initial HIV therapy: Results of ACTG A5095. J Acquir Immune Defic Syndr. 2007;46:547–554. doi: 10.1097/qai.0b013e31815ac499. [DOI] [PubMed] [Google Scholar]
- 35.Li X. Margolick JB. Conover CS, et al. Interruption and discontinuation of highly active antiretroviral therapy in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr. 2005;38:320–328. [PubMed] [Google Scholar]
- 36.Hauber AB. Mohamed AF. Watson ME. Johnson FR. Hernandez JE. Benefits, risk, and uncertainty: Preferences of antiretroviral-naive African Americans for HIV treatments. AIDS Patient Care STDs. 2009;23:29–34. doi: 10.1089/apc.2008.0064. [DOI] [PubMed] [Google Scholar]
- 37.Stone VE. Jordan J. Tolson J. Miller R. Pilon T. Perspectives on adherence and simplicity for HIV-infected patients on antiretroviral therapy: Self-report of the relative importance of multiple attributes of highly active antiretroviral therapy (HAART) regimens in predicting adherence. J Acquir Immune Defic Syndr. 2004;36:808–816. doi: 10.1097/00126334-200407010-00007. [DOI] [PubMed] [Google Scholar]
- 38.Sankar A. Wunderlich T. Neufeld S. Luborsky M. Sero-positive African Americans' beliefs about alcohol and their impact on anti-retroviral adherence. AIDS Behav. 2007;11:195–203. doi: 10.1007/s10461-006-9144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mannheimer SB. Morse E. Matts JP, et al. Sustained benefit from a long-term antiretroviral adherence intervention. Results of a large randomized clinical trial. J Acquir Immune Defic Syndr. 2006;43(Suppl 1):S41–47. doi: 10.1097/01.qai.0000245887.58886.ac. [DOI] [PubMed] [Google Scholar]
- 40.Novak RM. Chen L. MacArthur RD, et al. Prevalence of antiretroviral drug resistance mutations in chronically HIV-infected, treatment-naive patients: implications for routine resistance screening before initiation of antiretroviral therapy. Clin Infect Dis. 2005;40:468–474. doi: 10.1086/427212. [DOI] [PubMed] [Google Scholar]
- 41.DHHS Panel on Clinical Practices for Treatment of HIV Infection. www.aidsinfo.nih.gov. [Jan 21;2010 ];Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents. 2009 1 doi: 10.1310/hct.2000.1.1.008. Updated December. [DOI] [PubMed] [Google Scholar]