Abstract
Redox effector protein-1 (Ref-1) plays an essential role in DNA repair and redox regulation of several transcription factors. In the present study, we examined the role of Ref-1 in maintaining the redox status and survivability of adult cardiac stem cells challenged with a subtoxic level of H2O2 under inhibition of Ref-1 by RNA interference. Treatment of cardiac stem cells with a low concentration of H2O2 induced Ref-1–mediated survival signaling through phosphorylation of Akt. However, Ref-1 inhibition followed by H2O2 treatment extensively induced the level of intracellular reactive oxygen species (ROS) through activation of the components of NADPH oxidase, like p22phox, p47phox, and Nox4. Cardiac differentiation markers (Nkx2.5, MEF2C, and GATA4), and cell death by apoptosis were significantly elevated in Ref-1 siRNA followed by H2O2-treated stem cells. Further, inhibition of Ref-1 increased the level of p53 but decreased the phosphorylation of Akt, a molecule involved in survival signaling. Treatment with ROS scavenger N-acetyl-L-cysteine attenuated Ref-1 siRNA-mediated activation of NADPH oxidase and cardiac differentiation. Taken together, these results indicate that Ref-1 plays an important role in maintaining the redox status of cardiac stem cells and protects them from oxidative injury–mediated cell death and differentiation. Antioxid. Redox Signal. 11, 589–599.
Introduction
Reactive oxygen species (ROS), including H2O2, superoxide anions, and hydroxyl radicals, are generated intracellularly though various sources including mitochondria and plasma membrane–associated oxidases like nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (41). Several receptor-mediated generations of ROS have been shown to regulate many cellular functions such as growth inhibition, cytotoxicity, apoptosis, mitogenesis, differentiation, and cell hypertrophy (41).
Numerous studies have shown ROS as important chemical mediators in the regulation of cell growth and differentiation (15, 33). Oxidative stress resulting from various ROS-generating agents induces differentiation in a wide variety of cell types that include neuronal (23), monocytic (52), erythroid (9), osteoclast (43), and cardiac cells (39). The cellular redox state and the activity of antioxidant enzyme superoxide dismutase (SOD) are greatly altered during the differentiation of many types of cells (5, 9). Consequently, these changes bring about an increased rate of oxidant production, which cause induction of gene expression, leading to cell differentiation (1). This indicates that the cellular environment becomes more pro-oxidizing during differentiation (1).
The intracellular redox state regulates cellular signaling events by controlling many upstream and downstream molecules (17, 18, 22, 50). Normally, the intracellular redox state in the cell is maintained through many enzymatic and nonenzymatic molecules like SOD, catalase, redox effector factor-1 (Ref-1), thioredoxin, etc. Ref-1 is sensitive to changes in redox status and stimulates a rapid adaptive response to subtoxic level of variety of ROS induced by H2O2, superoxide anion and gamma rays (36). Injuries resulting from ROS generated by UV-radiation or H2O2 treatment remarkably induced the levels of Ref-1, which corresponds with the increase in endonuclease and redox activities of Ref-1 (45). ROS may regulate Ref-1 via activation of several transcription factors involved in oxidative stress such as AP-1, CREP, ATF, and p53 (16). Ref-1 facilitates the DNA binding and activation of transcription factors through reduction of a cysteine residue (16, 28). It also has been shown that ROS affects the functions of Ref-1 by altering the redox status of Ref-1. Oxidative stress or replacement of cysteine in Ref-1 severely affects both DNA repair and the redox regulation activities of Ref-1 (28, 48). Ref-1 deficiency caused many cells to undergo apoptosis induced by ionizing radiation, alkylating agents, and oxidative stress (3). Moreover, Ref-1 was shown to be associated with differentiation in the developing retina (10, 11).
The present study was designed to examine the role of Ref-1 in maintaining the redox status and survivability of adult cardiac stem cells under subtoxic levels of H2O2 treatment. To study the role of Ref-1, Ref-1 expression was blocked in cardiac stem cells by treating them with Ref-1–specific small interference RNA (siRNA). We found that subtoxic levels of hydrogen peroxide under inhibition of Ref-1 caused excessive production of ROS via activation of NADPH oxidase, leading to cardiac differentiation and cell death by apoptosis in adult cardiac stem cells. These results indicate that Ref-1 plays an important role in maintaining the redox status of cardiac stem cells and protects them from oxidative injury–mediated cell death and differentiation.
Materials and Methods
Materials
Control and Ref-1 siRNAs were obtained from Santa Cruz Biotechnology, Santa Cruz, CA. Primary antibodies of α-actinin and p53 were obtained from Cell Signaling Technology (Danvers, MA). Ref-1, Akt, p-Akt, and GAPDH antibodies were obtained from Santa Cruz Biotechnology. Redox-sensitive dye 5-(and −6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester (CM-H2DCFDA), and fluorescent conjugated secondary antibodies such as Alexa Fluor 488 and Alexa Fluor 594, and the nuclear stain To-Pro 1 iodide were obtained from Molecular Probes Inc. (Eugene, OR). N-acetyl-l-cysteine and H2O2 were obtained from Sigma (St. Louis, MO).
Cell culture
Clonogenic adult cardiac stem cells were a generous gift of Dr. Piero Anversa, and the cells were grown in modified neural stem cell medium (mNSCM), as mentioned earlier (6), at 37°C in a humidified chamber with 5% CO2. Cells were not allowed to grow beyond 70% confluency. Cells were treated with H2O2 at a 10 μM concentration for 24 h. Treatment with N-acetyl-L-cysteine at 1 mM concentration was performed 20 min before H2O2 treatment.
In vitro siRNA transfection
To each well of a six-well tissue culture plate, 2 × 105 cells were seeded with 2 ml growth medium. Plates were incubated for 18–24 h until 60–70% confluency was reached. Ref-1 and control siRNAs were used at the final concentration of 25 nM. siRNA transfection was done by using TransPass R2 Transfection Reagent (New England BioLabs), as mentioned in the kit. Cells were grown in complete growth medium for an additional period of 24 h. Follow-up experiments like treatment with H2O2 and N-acetyl-L-cysteine were performed at the end of the 24-h incubation period.
Detection of intracellular ROS
At the end of experimentation, cells were washed with PBS and further treated with CM-H2DCFDA at 5 μM concentration and incubated at 37°C incubator for 30 min. At the end of incubation, cells were washed with PBS and observed immediately under a fluorescent confocal microscope, Zeiss LSM 510 (Thornwood, NY). Fluorescence intensity was measured by using Metamorph 7.2 software (Molecular Devices, Sunnyvale, CA) from 10 random confocal images of each group.
Total RNA isolation and RT-PCR
Total RNA was isolated from cells with TRIzol reagent (Invitrogen, Carlsbad, CA), according to the manufacturer's instructions, and dissolved in 20 μl of DEPC-treated water. Total RNA concentration was determined by measuring the optical density at 260 nm. Reverse transcription and polymerase chain reaction (RT-PCR) was performed with RETROscript (Ambion, Austin, TX), according to the manufacturer's instructions. RNA, 2 μg, was used to prepare cDNA. The following primers were used in our study.
-
MEF2C
Forward, 5′-GGCACACAGAGCACCTTGTA-3′
Reverse, 5′-TGCTTTCTTGGTTCCTGCTT-3′
-
NOX4
Forward, 5′-GGGCCTAGGATTGTGTTTGA-3′
Reverse, 5′-CTGAGAAGTTCAGGGCGTTC-3′
-
NKX2.5
Forward, 5′-ACCGCCCCTACATTTTATCC-3′
Reverse, 5′-GACAGGTACCGCTGTTGCTT-3′
-
p22phox
Forward, 5′-TTGTTGCAGGAGTGCTCATC-3′
Reverse, 5′-CTGCCAGCAGGTAGATCACA-3′
-
p47phox
Forward, 5′-AGCTCCCAGGTGGTATGATG-3′
Reverse, 5′-ATCTTTGGCCGTCAGGTATG-3′
-
GATA4
Forward, 5′-TCTCACTATGGGCACAGCAG-3′
Reverse, 5′-CGAGCAGGAATTTGAAGAGG-3′
-
p53
Forward, 5′-GTCTACGTCCCGCCATAAAA-3′
Reverse, 5′-AGGCAGTGAAGGGACTAGCA-3′
-
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH)
Forward, 5′-AGACAGCCGCATCTTCTTGT-3′
Reverse, 5′-CTTGCCGTGGGTAGAGTCAT-3′
-
PCR reactions were performed in 25-μl reaction volume with the following programs
95°C for 4 min (denaturation)
94°C for 30 sec, AT for 30 sec, 72°C for 1 min, 30 cycle
72°C for 5 min
-
Annealing temperatures were
54°C for NKX2.5, NOX, p22phox, p47phox, GATA4, and MEF2C
55°C for GAPDH
The PCR products were visualized on a UV-transilluminator and digitalized after electrophoresis on 2% agarose gel containing ethidium bromide.
Cell lysate preparation
At the end of experimentation, cells were washed with phosphate-buffered saline and lysed in RIPA buffer (Boston Bioproducts, Boston, MA) supplemented with protease inhibitor cocktail (Sigma) and a phosphatase inhibitor (100 mM sodium orthovanadate) on ice for 1 h. Lysate was centrifuged at 8,000 g for 10 min at 4°C, and the supernatant was mixed with sample buffer and heated at 95°C for 5 min. Thus, prepared cell lysates were used for Western immunoblotting.
Western blot analysis
Proteins were separated in SDS-PAGE and transferred to nitrocellulose filters. Filters were blocked in 5% nonfat dry milk and probed with a primary antibody overnight. Primary antibodies such as α-actinin, p53, and GAPDH were used at 1:500 dilutions. Protein bands were identified with horseradish peroxidase conjugated secondary antibody (1:2,000 dilution) and Western Blotting Luminol Reagent (Santa Cruz Biotechnology). The resulting blots were digitized, subjected to densitometric scanning by using a standard NIH Image program, and normalized against loading control.
Immunofluorescence staining
Cells were washed with phosphate-buffered saline (PBS) and fixed by using 4% paraformaldehyde solution for 20 min. After a wash with PBS, the slides were blocked with Powerblock (BioGenex, San Ramon, CA) for 10 min. Slides were washed with PBS and incubated with primary antibody (1:25 dilution) in PBS containing 1% BSA for 60 min (overnight incubation for α-actinin). Slides were washed and incubated with fluorescein-conjugated secondary antibody (1:500 dilution) in the dark for 45 min. Nuclear staining was done with To-Pro 1 iodide (1:500 dilution) for 45 min in the dark. The slides were washed, covered with mounting medium, and examined under a fluorescence microscope. Confocal microscopic images were obtained by using a Zeiss LSM 510 (Thornwood, NY) confocal laser scanning microscope with ×40 1.3 oil-immersion objective by simultaneous recording in the 488 λ, 530 λ, or 560 λ channels, or a combination of these, as appropriate.
Cell-death assay
Cell-death analysis was performed for the release of the cell-death marker enzyme lactate dehydrogenase (LDH) by using the spent culture medium as the substrate with the LDH Cytotoxicity Assay kit (Cayman Chemical Company, Ann Arbor, MI) as instructed.
TUNEL assay for assessment of apoptotic cell death
Immunohistochemical detection of apoptotic cells was carried out with terminal deoxynucleotidyl transferase nick-end labeling (TUNEL) by using the DeadEnd Fluorometric TUNEL System (Promega, Madison, WI). Cells were fixed by immersion in 4% paraformaldehyde, and then stored in 4°C in PBS. The TUNEL experiment was done according to the manufacturer's instructions. The fluorescence staining was viewed with a fluorescence microscope (Axioplan2 Imaging; Carl Zeiss Microimaging, Inc., New York) at 520 ± 20 nm for the green fluorescence of fluorescein and at >620 nm for the red fluorescence of propidium iodide. The number of apoptotic cells was counted and expressed as a percentage of the total myocyte population.
Statistical analysis
All values were expressed as the mean ± standard error of the mean (SEM). Analysis-of-variance test, followed by Bonferroni's correction, was first carried out to test for any differences between the mean values of all groups. If differences were established, the values of the treated groups were compared with those of the control group with a modified t test. The results were considered significant at p < 0.05.
Results
H2O2 treatment in the absence of Ref-1 induces the production of ROS
A pro-oxidative environment has been shown to induce differentiation in many cell types (15, 33). Treatment with H2O2 alters the cellular redox status and induces differentiation in many cell types (9, 40, 43). Because Ref-1 plays an important role in the regulation of cellular redox status, we examined the role of Ref-1 in the regulation of cellular redox status and its effect on the differentiation of adult cardiac stem cells when the cells were challenged with pro-oxidative treatment with hydrogen peroxide. Adult cardiac stem cells were treated with various concentrations of hydrogen peroxide (10, 25, 50, 100, and 200 μM) for different time periods (30 min, 60 min, 120 min, 24 h, and 48 h). We found that Ref-1 protein expression was induced by 10 μM concentration of H2O2 at two different time points [i.e., 120 min and 24 h (data not shown)]. However, cardiac differentiation markers like GATA4 and MEF2C were found to be induced more during the 24-h time period (data not shown). In subsequent experiments, we examined the role of Ref-1 by challenging cells with a 10 μM concentration of H2O2 for 24 h under the inhibition of Ref-1 by RNA interference.
Our Western immunoblotting results (Fig. 1A) show that treatment with Ref-1 siRNA followed by H2O2 significantly attenuated the H2O2-mediated induction of Ref-1 compared with control siRNA treatment. Ref-1 siRNA treatment alone did not show any significant changes in the alteration of Ref-1 protein expression when compared with normal cells (Fig. 1A). Further, confocal microscopic analysis (Fig. 1B) showed that the expression of Ref-1 is significantly attenuated when the cells are treated with Ref-1 siRNA followed by H2O2, compared with other groups.
FIG. 1.
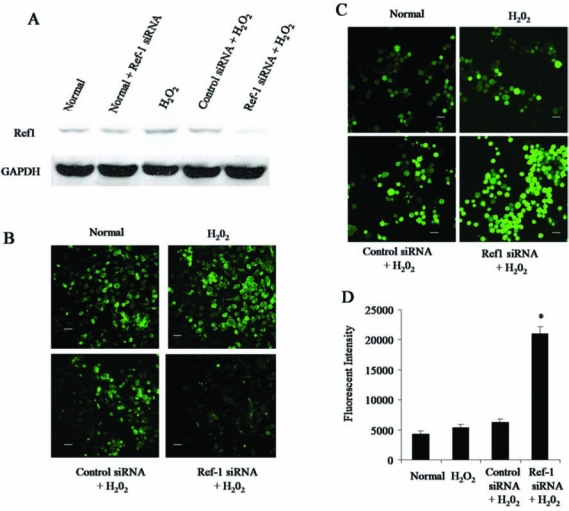
Inhibition of Ref-1 increases the level of intracellular reactive oxygen species (ROS). Adult cardiac stem cells were treated with either control or Ref-1 siRNA followed by H2O2 (10 μM), as mentioned in Methods section. (A) Representative Western immunoblot showing the expression Ref-1 in the total cell lysate. GAPDH was used as loading control. (B) Representative confocal microscopic images showing the staining of Ref-1 in cells. (C) Representative confocal microscopic images showing the staining of cells with a cell-permeable redox-sensitive dye (CM-H2DCFDA) indicating the level of intracellular ROS. (D) Quantification of the average fluorescent intensity of cells stained with CM-H2DCFDA. Scale bar represents 20 μm in B and C. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
To examine the cellular redox status, the cells were treated with cell-permeable dye CM-H2DCFDA, which itself is a nonfluorescent dye, but on entering the cell, it is cleaved by intracellular esterases and oxidized to a fluorescent compound by intracellular pro-oxidants. The fluorescence intensity is directly proportional to the level of ROS present in the cell. Our confocal microscopic analyses showed that the fluorescence intensity was slightly higher on treatment with H2O2 compared with normal cells (Fig. 1C–D). However, Ref-1 siRNA followed by H2O2 treatment massively increased the level of intracellular ROS compared with control siRNA followed by H2O2 treatment (Fig. 1C–D). Ref-1 siRNA treatment alone did not show any significant changes in the alteration of ROS production when compared with normal cells (data not shown). These results indicate that ROS production was significantly increased on challenging the cardiac stem cells with pro-oxidant under inhibition of Ref-1.
Ref-1 inhibition induces the activation of NADPH oxidase
ROS-mediated intracellular signaling events are triggered through the activation of NADPH oxidase components like p22phox, p47phox, and NOX4, leading to the production of superoxide and affecting various cellular functions (8). In the present study, we tested whether Ref-1 inhibition-mediated induction of ROS activates NADPH oxidase. Our RT-PCR analyses show that NOX4, p22phox, and p47phox were slightly but insignificantly induced by hydrogen peroxide treatment alone at the end of the 24-h treatment period (Fig. 2). However, Ref-1 siRNA followed by H2O2 treatment significantly induced the expression of NOX4, p22phox, and p47phox compared with control siRNA followed by H2O2 (Fig. 2). Ref-1 siRNA treatment alone did not show any significant changes in the expression of NOX4, p22phox, and p47phox when compared with normal cells (data not shown). These results show that Ref-1 inhibition–mediated induction of ROS further activates NADPH oxidase components in cardiac stem cells.
FIG. 2.
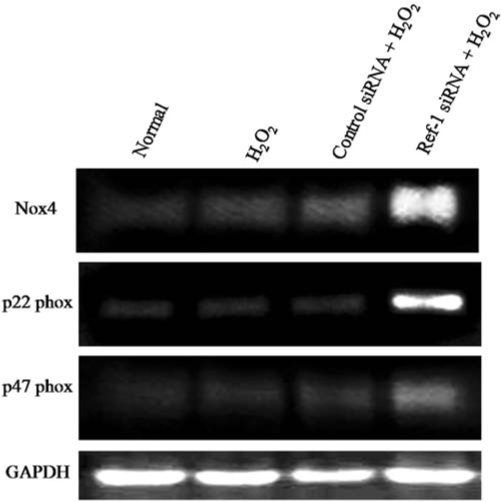
Ref-1 inhibition activates NADPH oxidase components. Adult cardiac stem cells were treated with either control or Ref-1 siRNA followed by H2O2 (10 μM), as mentioned in Methods. At the end of experimentation, cells were lysed in TRIzol reagent, and RNA was isolated. RT-PCR analysis was performed with specific primers against NOX4, p22phox, p47phox, and GAPDH.
Inhibition of Ref-1 induces cardiac differentiation
ROS plays an essential role in the induction of differentiation in many cell types including neuronal (23), monocytic (52), erythroid (9), osteoclast (43), and cardiac cells (39). We tested whether the ROS produced through Ref-1 inhibition mediates cardiac differentiation in adult cardiac stem cells. RT-PCR analyses showed that cardiac differentiation markers like Nkx2.5, MEF2C, and GATA4 were slightly but insignificantly induced on treatment with H2O2 alone (Fig. 3A). However, Ref-1 siRNA followed by H2O2 treatment significantly induced Nkx2.5, MEF2C, and GATA4 compared with control siRNA followed by H2O2 treatment (Fig. 3A). Further, our Western immunoblotting results showed that the expression of another cardiac differentiation marker protein, α-sarcomeric actinin, was slightly but not significantly induced by H2O2 treatment alone, but Ref-1 siRNA followed by H2O2 treatment significantly induced the expression of cardiac sarcomeric actinin compared with control siRNA followed by H2O2 treatment (Fig. 3B). Ref-1 siRNA treatment alone did not show any significant changes in the expression of these differentiation markers when compared with normal cells (data not shown). Moreover, our confocal microscopic images show coexpression of NOX4 and the differentiation marker Nkx2.5 in cells treated with Ref-1 siRNA followed by H2O2 (Fig. 3C). Furthermore, coexpression of Nkx2.5 and α-sarcomeric actinin was found in cardiac stem cells under inhibition of Ref-1 and H2O2 treatment (Fig. 3D). These results clearly indicate the induction of cardiac differentiation on treating cells with Ref-1 siRNA followed by H2O2.
FIG. 3.
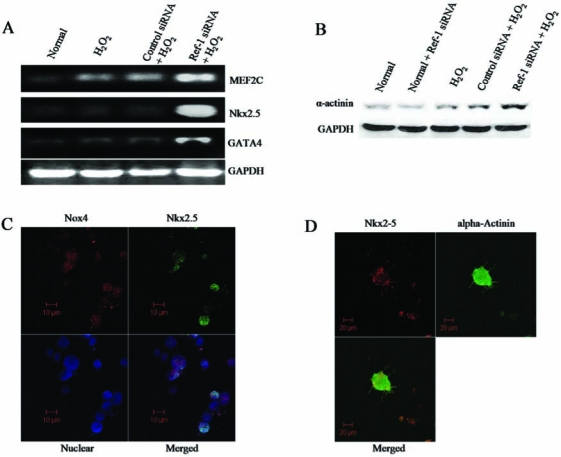
Inhibition of Ref-1 induces differentiation in cardiac stem cells. Adult cardiac stem cells were treated with either control or Ref-1 siRNA followed by H2O2 (10 μM), as mentioned in Methods. (A) RNA was isolated from cells by using TRIzol reagent, and RT-PCR analysis was performed with specific primers against cardiac differentiation markers such as MEF2C, Nkx2.5, and GATA4. GAPDH was used as loading control. (B) Western immunoblotting was performed with total cell lysate by using specific antibodies against α-actinin. GAPDH was used as loading control. (C) Confocal microscopic images showing the Ref-1 inhibition–driven coexpression of NOX4 (red channel, Alexa Fluor 594) and Nkx2.5 (green channel, Alexa Fluor 488) in the nuclear (blue channel, To-Pro 1 iodide) region of the cell. (D) Confocal microscopic images showing the Ref-1 inhibition–mediated coexpression of Nkx2.5 (red channel) and α-actinin (green channel) in a differentiated cell. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Ref-1 inhibition induces cell death
Cell differentiation is mostly accompanied by cell death by apoptosis in many cell types (37, 38). In the present study, the occurrence of cell death is confirmed by the release of cell-death marker enzyme LDH into the culture medium. Figure 4A shows that the release of LDH into the culture medium was significantly increased when the cells were treated with Ref-1 siRNA and H2O2 compared with other groups of cells. Furthermore, TUNEL apoptotic analysis show that Ref-1 siRNA followed by H2O2 treatment significantly increased the number of apoptotic cells compared with other groups of cells (Fig. 4B–C). Ref-1 siRNA treatment alone did not show any significant changes in LDH release and the percentage of apoptotic cells when compared with normal cells (data not shown). These results indicate that the induction of cardiac differentiation and cell death occurred simultaneously when the cells were treated with Ref-1 siRNA followed by H2O2.
FIG. 4.
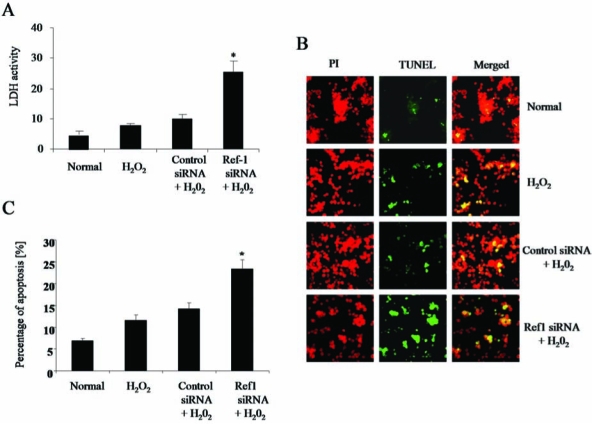
Ref-1 inhibition induces cell death. Adult cardiac stem cells were treated with either control or Ref-1 siRNA followed by H2O2 (10 μM), as mentioned in Methods. (A) The release of lactate dehydrogenase (LDH) enzyme from the cells was measured by using the spent culture medium obtained at the end of experimental period. (B) Fluorescent microscopic images showing the TUNEL staining of apoptotic cells (green channel) and nucleus (red channel, propidium iodide). (C) Quantification of apoptotic cells stained with TUNEL. The results are expressed in percentages. PI, propidium iodide. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
The role of p53 under Ref-1 inhibition
Tumor-suppressor protein p53 plays an inevitable role in cell-cycle arrest, apoptosis, and differentiation (2). Moreover, p53 also is shown to be regulated by Ref-1 (21). In the present study, we examined the role of p53 protein under Ref-1 inhibition with low levels of H2O2 treatment. Our Western immunoblotting and RT-PCR analysis showed that p53 protein was expressed at high levels in normal cardiac stem cells. However, treatment with hydrogen peroxide decreased the level of p53 protein in the total cell lysate compared with normal cells (Fig. 5A and B). Conversely, Ref-1 siRNA treatment followed by H2O2 significantly increased the level of p53 protein compared with normal cell levels (Fig. 5A and B). Akt activation by serum stimulation was shown to inhibit p53; however, proapoptotic stimuli–induced p53 was shown to inhibit the activation of Akt (4, 16). Based on these data, in the present study, we examined the activation of Akt, which is known as a molecule involved in survival signaling. Our Western immunoblotting analysis (Fig. 5A) showed that the phosphorylation of Akt (i.e., activation of Akt) was induced after treating cells with H2O2, but H2O2-induced activation of Akt was almost completely abolished by Ref-1 siRNA treatment as compared with control siRNA treatment (Fig. 5A). Ref-1 siRNA treatment alone did not show any significant changes in the expression of p53 and the phosphorylation of Akt when compared with normal cells (data not shown). These results, in accordance with previous studies (4, 16), show that phosphorylation of Akt is negatively correlated with the level of p53. Our results indicate that a low level of H2O2 treatment–induced survival signaling is attenuated by Ref-1 siRNA treatment, leading to the increased level of p53, which may play an important role in the Ref-1 siRNA-mediated induction of cardiac apoptosis and differentiation.
FIG. 5.
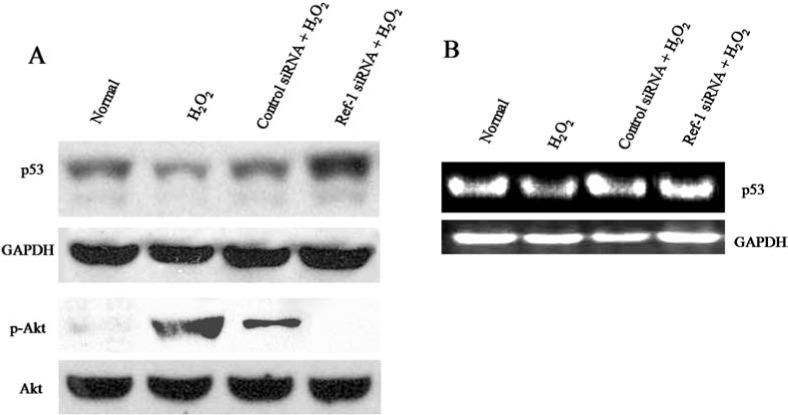
The regulation of p53 under Ref-1 siRNA treatment. Adult cardiac stem cells were treated with either control or Ref-1 siRNA followed by H2O2 (10 μM), as mentioned in Methods. (A) Representative Western immunoblot showing the expression of p53, GAPDH, p-Akt, and Akt in the total cell lysate. (B) RT-PCR analysis showing the expression of p53 and GAPDH.
Treatment with ROS scavenger attenuates cardiac differentiation
In the present study, we found that H2O2 treatment under Ref-1 inhibition increased the level of intracellular ROS, which corroborates the induction of cardiac differentiation and apoptosis. To test the role of ROS in the Ref-1 siRNA-mediated induction of differentiation, the cells were pretreated with ROS scavenger N-acetyl-L-cysteine (1 mM) 20 min before treating the cells with H2O2. Our RT-PCR results show that treatment with N-acetyl-L-cysteine significantly attenuated the Ref-1 siRNA treatment–mediated induction of NADPH oxidase components such as p22phox, p47phox, NOX4, and cardiac transcription factors like Nkx2.5, GATA4, and MEF2C (Fig. 6). These results clearly indicate that ROS produced under Ref-1 inhibition and H2O2 treatment in cardiac stem cells play an essential role in the induction of cardiac differentiation.
FIG. 6.
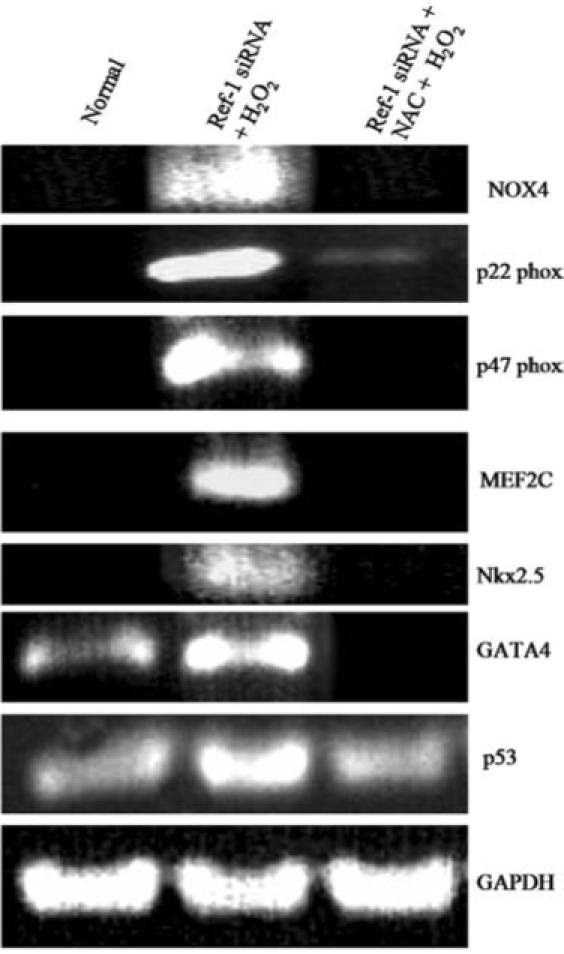
Treatment with ROS scavenger attenuated Ref-1 siRNA–mediated cardiac differentiation. Adult cardiac stem cells were treated with either control or Ref-1 siRNA followed by H2O2 (10 μM), as mentioned in Methods. At the end of experimentation, cells were lysed in TRIzol reagent, and RNA was isolated. RT-PCR analysis was performed with specific primers against NOX4, p22phox, p47phox, MEF2C, Nkx2.5, GATA4, p53, and GAPDH.
Discussion
The salient finding of the present study is that subtoxic level of H2O2 treatment under inhibition of Ref-1 caused excessive production of ROS via activation of NADPH oxidase, leading to cardiac differentiation and cell death by apoptosis in adult cardiac stem cells.
Ref-1 protein has two major functions: (a) repairing DNA base-pair excision produced through either oxidative damage or DNA glycosylases; and (b) redox regulation of many transcription factors like AP1, HIF1α, NF-κB, and p53 (14). In addition to these two major functions, Ref-1 was shown to suppress intracellular oxidative stress and apoptosis through modulation of Rac1-regulated NADPH oxidase (3, 46). Ref-1 promotes survival of neuronal cells from oxidative stress–induced injury (46), and the increased level of Ref-1 is associated with survival of cells during cold injury–induced brain lesions (31). Moreover, depletion of Ref-1 renders cells more sensitive to hyperoxia (47). Loss of Ref-1 was shown to precede DNA fragmentation in apoptotic neurons (49). Furthermore, Ref-1 was found to be associated with differentiation in developing retina (10, 11), and Ref-1 immunoreactivity was decreased with hippocampal development (10).
Stem cells in organs are usually sheltered in specialized structures called niches (i.e., stable microenvironments that control stem cell behavior) (44). A stem cell niche provides a microenvironment that preserves the survival and replication potential of stem cells (42). Recently, it was shown that ROS play a major role in induction of the exit of hematopoietic stem cells from the niche in bone marrow (20). A delicate balance between intracellular oxidizing and reducing equivalents allows ROS to function as second messengers in signaling cascades involved in cell proliferation and differentiation (18, 41). Low concentrations of hydrogen peroxide were shown to induce components of NADPH oxidase and cardiac transcription factors like Nkx2.5, MEF2C, and GATA4 in mouse embryonic stem cells (26). A cardiac-specific gene regulatory network has revealed that MEF2C, Nkx2.5, and GATA4 proteins are vital transcriptional regulators for the development of heart cells (7). The role of NADPH oxidase and NOX4 in the regulation of differentiation of mouse embryonic stem cells has been documented (3, 26). Downregulation of NOX4 caused suppression of cardiogenesis, but this was reversed by treatment with a low concentration of hydrogen peroxide (26). In our study, when the cells were treated with hydrogen peroxide at 10 μM concentration, we found a slight but insignificant activation of NADPH oxidase components like p22phox, p47phox, and Nox4, leading to a slight but insignificant induction of cardiac differentiation markers like Nkx2.5, MEF2C, and GATA4. Moreover, the high proliferative capacity of murine embryonic stem cells was shown to be closely correlated with the high activity of different glycolytic enzymes, elevated glycolytic flux, and low mitochondrial oxygen consumption (25). In leukemic cells, glucose transport is activated by stem cell factor and H2O2, suggesting a potential role for ROS in leukemia proliferation (29). NADPH oxidase plays an important role in promoting the proliferation of skeletal muscle precursor cells (30). Long-term self-renewing hematopoietic stem cells (HSCs) have low levels of intracellular ROS. However, when intracellular ROS levels become excessive, they cause senescence or apoptosis, resulting in a failure of HSC self-renewal (32). Genes that are involved in either DNA-damage responses or longevity-related signaling are shown to play a role in the maintenance of the HSC self-renewal (32).
Adult cardiac stem cells treated only with Ref-1 siRNA showed a slight but not significant increase in the level of intracellular ROS (data not shown), but further treatment with 10 mM H2O2 for 24 h (under Ref-1 inhibition) induced the production of ROS compared with control siRNA-treated cells (Fig. 1C and D). This ROS production in Ref-1 siRNA-treated cells corresponded to the significant increase in the mRNA levels of NADPH oxidase components p22phox, p47phox, and NOX4. Moreover, elevated levels of ROS and NADPH oxidase components in Ref-1 siRNA-treated cells was further accompanied by an enhanced level of cardiac differentiation markers Nkx2.5, MEF2C, GATA4, and a-sarcomeric actinin. To validate the role of ROS in the induction of cardiac differentiation in our study, we treated cardiac stem cells with antioxidant N-acetyl-l-cysteine before treating them with H2O2. N-Acetyl-L-cysteine treatment abolished the Ref-1 siRNA-mediated induction of NOX4, p22phox, p47phox, and cardiac transcription factors Nkx2.5, MEF2C, and GATA4. Our results are in accordance with those of Buggisch et al. (8), who found that ROS and NADPH oxidase–mediated signaling cascades are involved in the proliferation and differentiation of embryonic stem cells. Furthermore, a role for phosphatidylinositol-3-kinase has been identified in ROS-mediated cardiac differentiation of embryonic stem cells (39).
Embryonic stem cell differentiation could recapitulate the in vivo differentiation process, including the occurrence of apoptosis accompanying differentiation (37, 38). Tumor-suppressor protein p53 regulates the cell-cycle checkpoint at G1-S phase transition, differentiation, and induces cell apoptosis. p53 was shown to be involved in the simultaneous induction of apoptosis and differentiation in primary granulose cells (24), neurons, and oligodendrocytes (13). A high level of p53 was shown to induce apoptosis, whereas low levels caused cell-cycle arrest (12). Undifferentiated embryonic stem cells express high levels of p53, followed by a decrease in the level of p53 as differentiation proceeds (2, 37, 38). The addition of retinoic acid, a physiologic regulator of embryonic development, to 8 to 10 days of differentiating murine embryonic stem cells caused an increase the level of p53 followed by accelerated neural differentiation and apoptosis (38). The basal level of p53 functions as an antioxidant, whereas hyperphysiologic levels of p53 behave as a prooxidant (27). p53 was shown to be regulated by both redox-dependent and redox-independent mechanisms (14). Ref-1 was found to be a potent activator of p53 (21). Activation of p53 plays an important role in copper- and zinc-induced generation of ROS, and activation of downstream targets of p53 like PIG3 and Bax leads to increased generation of ROS and apoptosis in epithelial cells (34). Induction of endogenous p53 was found to be associated with differentiation in mouse cultured keratinocytes (51), mouse embryonic stem cells (53), and hematopoietic and muscle cells (19). Taken together, these findings indicate that p53 plays an important role in determining the fine balance between growth, differentiation, and cell death.
In our study, when the cardiac stem cells were treated with low concentrations of H2O2, the level of p53 was decreased more than normal cellular levels. At the same time, H2O2 treatment increased the activation of survival kinase Akt and led to the protection of cells from oxidative injury–mediated apoptosis and differentiation (Fig. 7). Ref-1 siRNA followed by H2O2 treatment almost completely abolished the activation of survival-signaling molecule Akt; and at the same time, the level of p53 was significantly higher than normal levels, leading to enhanced levels of ROS production and ROS-mediated cell death and differentiation (Fig. 7). These results are in accordance with previous findings (4, 16), in which survival signaling–mediated activation of Akt inhibited p53, and proapoptotic stimuli–induced p53 inhibited Akt.
FIG. 7.
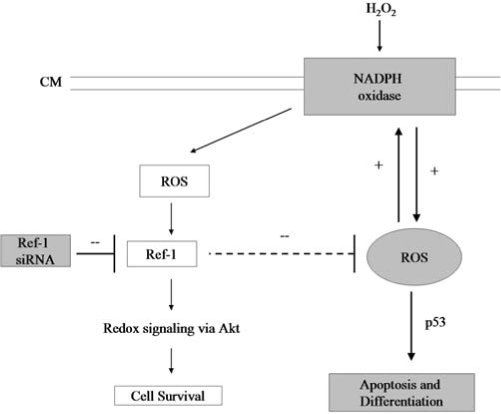
Role of Ref-1 in the redox control of cardiac stem cells. When adult cardiac stem cells are treated with 10 μM H2O2, Ref-1–mediated redox signaling is activated, leading to the protection of cells from injury. However, inhibition of Ref-1 through Ref-1 siRNA treatment resulted in the production of massive amounts of reactive oxygen species (ROS) and activation of NADPH oxidase, leading to p53-mediated apoptosis and differentiation in cardiac stem cells. Discontinuous line, Ref-1 may inhibit the massive production of ROS. Inhibition of Ref-1 with Ref-1 siRNA induces the production of massive amounts of ROS, leading to apoptosis and differentiation in adult cardiac stem cells.
In conclusion, our results show that inhibition of Ref-1 followed by a low level of H2O2 treatment increased the intracellular level of ROS and p53, leading to the induction of cardiac differentiation and cell death by apoptosis in adult cardiac stem cells. Further, these results imply that Ref-1 plays an important role in maintaining the redox status of cardiac stem cells and protects them from oxidative injury–mediated cell death and differentiation.
Acknowledgments
This study was supported in part by NIH HL 34360, HL22559, and HL 33889. The authors have no conflicting financial interests.
Abbreviations
CM-H2DCFDA, 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester RT-PCR, reverse transcription and polymerase chain reaction; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HSC, hematopoietic stem cells; LDH, lactate dehydrogenase; NADPH, nicotinamide adenine dinucleotide phosphate; PBS, phosphate-buffered saline; ROS, reactive oxygen species; SEM, standard error of the mean; siRNA, small interference RNA; SOD, superoxide dismutase; TUNEL, terminal deoxynucleotidyl transferase nick-end labeling.
Disclosure Statement
No competing financial interests exist.
References
- 1.Allen RG. Venkatraj VS. Oxidants and antioxidants in development and differentiation. J Nutr. 1992;122:631–635. doi: 10.1093/jn/122.suppl_3.631. [DOI] [PubMed] [Google Scholar]
- 2.Almog N. Rotter V. Involvement of p53 in cell differentiation and development. Biochim Biophys Acta. 1997;1333:F1–F27. doi: 10.1016/s0304-419x(97)00012-7. [DOI] [PubMed] [Google Scholar]
- 3.Angkeow P. Deshpande SS. Qi B. Liu YX. Park YC. Jeon BH. Ozaki M. Irani K. Redox factor-1: an extra-nuclear role in the regulation of endothelial oxidative stress and apoptosis. Cell Death Differ. 2002;9:717–725. doi: 10.1038/sj.cdd.4401025. [DOI] [PubMed] [Google Scholar]
- 4.Bachelder RE. Ribick MJ. Marchetti A. Falcioni R. Soddu S. Davis KR. Mercurio AM. p53 inhibits alpha 6 beta 4 integrin survival signaling by promoting the caspase 3-dependent cleavage of AKT/PKB. J Cell Biol. 1999;147:1063–1072. doi: 10.1083/jcb.147.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beckman BS. Balin AK. Allen RG. Superoxide dismutase induces differentiation of Friend erythroleukemia cells. J Cell Physiol. 1989;139:370–376. doi: 10.1002/jcp.1041390220. [DOI] [PubMed] [Google Scholar]
- 6.Beltrami AP. Barlucchi L. Torella D. Baker M. Limana F. Chimenti S. Kasahara H. Rota M. Musso E. Urbanek K. Leri A. Kajstura J. Nadal-Ginard B. Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 7.Brand T. Heart development: molecular insights into cardiac specification and early morphogenesis. Dev Biol. 2003;258:1–19. doi: 10.1016/s0012-1606(03)00112-x. [DOI] [PubMed] [Google Scholar]
- 8.Buggisch M. Ateghang B. Ruhe C. Strobel C. Lange S. Wartenberg M. Sauer H. Stimulation of ES-cell-derived cardiomyogenesis and neonatal cardiac cell proliferation by reactive oxygen species and NADPH oxidase. J Cell Sci. 2007;120:885–894. doi: 10.1242/jcs.03386. [DOI] [PubMed] [Google Scholar]
- 9.Chénais B. Andriollo M. Guiraud P. Belhoussine R. Jeannesson P. Oxidative stress involvement in chemically induced differentiation of K562 cells. Free Radic Biol Med. 2000;28:18–27. doi: 10.1016/s0891-5849(99)00195-1. [DOI] [PubMed] [Google Scholar]
- 10.Chiarini LB. Freitas FG. Petrs-Silva H. Linden R. Evidence that the bifunctional redox factor/AP endonuclease Ref-1 is an anti-apoptotic protein associated with differentiation in the developing retina. Cell Death Differ. 2000;7:272–281. doi: 10.1038/sj.cdd.4400639. [DOI] [PubMed] [Google Scholar]
- 11.Chiarini LB. Linden R. Tissue biology of apoptosis: Ref-1 and cell differentiation in the developing retina. Ann N Y Acad Sci. 2000;926:64–78. doi: 10.1111/j.1749-6632.2000.tb05599.x. [DOI] [PubMed] [Google Scholar]
- 12.Decatur AL. Losick R. Three sites of contact between the Bacillus subtilis transcription factor sigmaF its antisigma factor SpoIIAB. Genes Dev. 1996;10:2348–2358. doi: 10.1101/gad.10.18.2348. [DOI] [PubMed] [Google Scholar]
- 13.Eizenberg O. Faber-Elman A. Gottlieb E. Oren M. Rotter V. Schwartz M. p53 plays a regulatory role in differentiation and apoptosis of central nervous system-associated cells. Mol Cell Biol. 1996;16:5178–5185. doi: 10.1128/mcb.16.9.5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans AR. Limp-Foster M. Kelley MR. Going APE over ref-1. Mutat Res. 2000;461:83–108. doi: 10.1016/s0921-8777(00)00046-x. [DOI] [PubMed] [Google Scholar]
- 15.Finkel T. Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 16.Gottlieb TM. Leal JF. Seger R. Taya Y. Oren M. Crosstalk between Akt, p53 and Mdm2: possible implications for the regulation of apoptosis. Oncogene. 2002;21:1299–1303. doi: 10.1038/sj.onc.1205181. [DOI] [PubMed] [Google Scholar]
- 17.Gupta S. Das B. Sen S. Cardiac hypertrophy: mechanisms and therapeutic opportunities. Antioxid Redox Signal. 2007;9:623–652. doi: 10.1089/ars.2007.1474. [DOI] [PubMed] [Google Scholar]
- 18.Haneline LS. Redox regulation of stem and progenitor cells. Antioxid Redox Signal. 2008;11:1849–1852. doi: 10.1089/ars.2008.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henderson S. Allsopp R. Spector D. Wang SS. Harley C. In situ analysis of changes in telomere size during replicative aging and cell transformation. J Cell Biol. 1996;134:1–12. doi: 10.1083/jcb.134.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hosokawa K. Arai F. Yoshihara H. Nakamura Y. Gomei Y. Iwasaki H. Miyamoto K. Shima H. Ito K. Suda T. Function of oxidative stress in the regulation of hematopoietic stem cell-niche interaction. Biochem Biophys Res Commun. 2007;363:578–583. doi: 10.1016/j.bbrc.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 21.Jayaraman L. Murthy KG. Zhu C. Curran T. Xanthoudakis S. Prives C. Identification of redox/repair protein Ref-1 as a potent activator of p53. Genes Dev. 1997;11:558–570. doi: 10.1101/gad.11.5.558. [DOI] [PubMed] [Google Scholar]
- 22.Kamata H. Hirata H. Redox regulation of cellular signalling. Cell Signal. 1999;11:1–14. doi: 10.1016/s0898-6568(98)00037-0. [DOI] [PubMed] [Google Scholar]
- 23.Katoh S. Mitsui Y. Kitani K. Suzuki T. Hyperoxia induces the neuronal differentiated phenotype of PC12 cells via a sustained activity of mitogen-activated protein kinase induced by Bcl-2. Biochem J. 1999;338:465–470. [PMC free article] [PubMed] [Google Scholar]
- 24.Keren-Tal I. Suh BS. Dantes A. Lindner S. Oren M. Amsterdam A. Involvement of p53 expression in cAMP-mediated apoptosis in immortalized granulosa cells. Exp Cell Res. 1995;218:283–295. doi: 10.1006/excr.1995.1157. [DOI] [PubMed] [Google Scholar]
- 25.Kondoh H. Lleonart ME. Nakashima Y. Yokode M. Tanaka M. Bernard D. Gil J. Beach D. A high glycolytic flux supports the proliferative potential of murine embryonic stem cells. Antioxid Redox Signal. 2007;9:293–299. doi: 10.1089/ars.2006.1467. [DOI] [PubMed] [Google Scholar]
- 26.Li J. Stouffs M. Serrander L, et al. The NADPH oxidase NOX4 drives cardiac differentiation: role in regulating cardiac transcription factors and MAP kinase activation. Mol Biol Cell. 2006;17:3978–3988. doi: 10.1091/mbc.E05-06-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu B. Chen Y. St Clair DK. ROS and p53: a versatile partnership. Free Radic Biol Med. 2008;44:1529–1535. doi: 10.1016/j.freeradbiomed.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo M. Delaplane S. Jiang A. Reed A. He Y. Fishel M. Nyland Ii RL. Borch RF. Qiao X. Georgiadis MM. Kelley MR. Role of the multifunctional DNA repair and redox signaling protein Ape1/Ref-1 in cancer and endothelial cells: small-molecule inhibition of the redox function of Ape1. Antioxid Redox Signal. 2008 doi: 10.1089/ars.2008.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maraldi T. Fiorentini D. Prata C. Landi L. Hakim G. Glucose-transport regulation in leukemic cells: how can H2O2 mimic stem cell factor effects? Antioxid Redox Signal. 2007;9:271–279. doi: 10.1089/ars.2007.9.271. [DOI] [PubMed] [Google Scholar]
- 30.Mofarrahi M. Brandes RP. Gorlach A. Hanze J. Terada LS. Quinn MT. Mayaki D. Petrof B. Hussain SN. Regulation of proliferation of skeletal muscle precursor cells by NADPH oxidase. Antioxid Redox Signal. 2008;10:559–574. doi: 10.1089/ars.2007.1792. [DOI] [PubMed] [Google Scholar]
- 31.Morita-Fujimura Y. Fujimura M. Kawase M, et al. Early decrease in apurinic/apyrimidinic endonuclease is followed by DNA fragmentation after cold injury-induced brain trauma in mice. Neuroscience. 1999;93:1465–1473. doi: 10.1016/s0306-4522(99)00231-6. [DOI] [PubMed] [Google Scholar]
- 32.Naka K. Muraguchi T. Hoshii T. Hirao A. Regulation of reactive oxygen species and genomic stability in hematopoietic stem cells. Antioxid Redox Signal. 2008 doi: 10.1089/ars.2008.2114. [DOI] [PubMed] [Google Scholar]
- 33.Nose K. Role of reactive oxygen species in the regulation of physiological functions. Biol Pharm Bull. 2000;23:897–903. doi: 10.1248/bpb.23.897. [DOI] [PubMed] [Google Scholar]
- 34.Ostrakhovitch EA. Cherian MG. Role of p53 and reactive oxygen species in apoptotic response to copper and zinc in epithelial breast cancer cells. Apoptosis. 2005;10:111–121. doi: 10.1007/s10495-005-6066-7. [DOI] [PubMed] [Google Scholar]
- 35.Ozaki M. Suzuki S. Irani K. Redox factor-1/APE suppresses oxidative stress by inhibiting the rac1 GTPase. FASEB J. 2002;16:889–890. doi: 10.1096/fj.01-0664fje. [DOI] [PubMed] [Google Scholar]
- 36.Ramana CV. Boldogh I. Izumi T. Mitra S. Activation of apurinic/apyrimidinic endonuclease in human cells by reactive oxygen species and its correlation with their adaptive response to genotoxicity of free radicals. Proc Natl Acad Sci U S A. 1998;95:5061–5066. doi: 10.1073/pnas.95.9.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sabapathy K. Klemm M. Jaenisch R, et al. Regulation of ES cell differentiation by functional and conformational modulation of p53. EMBO J. 1997;16:6217–6229. doi: 10.1093/emboj/16.20.6217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarkar SA. Sharma RP. All-trans-retinoic acid-mediated modulation of p53 during neural differentiation in murine embryonic stem cells. Cell Biol Toxicol. 2002;18:243–257. doi: 10.1023/a:1016003027850. [DOI] [PubMed] [Google Scholar]
- 39.Sauer H. Rahimi G. Hescheler J. Wartenberg M. Effects of electrical fields on cardiomyocyte differentiation of embryonic stem cells. J Cell Biochem. 1999;75:710–723. doi: 10.1002/(sici)1097-4644(19991215)75:4<710::aid-jcb16>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 40.Sauer H. Rahimi G. Hescheler J. Wartenberg M. Role of reactive oxygen species and phosphatidylinositol 3-kinase in cardiomyocyte differentiation of embryonic stem cells. FEBS Lett. 2000;476:218–223. doi: 10.1016/s0014-5793(00)01747-6. [DOI] [PubMed] [Google Scholar]
- 41.Sauer H. Wartenberg M. Hescheler J. Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell Physiol Biochem. 2001;11:173–186. doi: 10.1159/000047804. [DOI] [PubMed] [Google Scholar]
- 42.Spradling A. Drummond-Barbosa D. Kai T. Stem cells find their niche. Nature. 2001;414:98–104. doi: 10.1038/35102160. [DOI] [PubMed] [Google Scholar]
- 43.Steinbeck MJ. Kim JK. Trudeau MJ. Hauschka PV. Karnovsky MJ. Involvement of hydrogen peroxide in the differentiation of clonal HD-11EM cells into osteoclast-like cells. J Cell Physiol. 1998;176:574–587. doi: 10.1002/(SICI)1097-4652(199809)176:3<574::AID-JCP14>3.0.CO;2-#. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sussman MA. Anversa P. Myocardial aging and senescence: where have the stem cells gone? Annu Rev Physiol. 2004;66:29–48. doi: 10.1146/annurev.physiol.66.032102.140723. [DOI] [PubMed] [Google Scholar]
- 45.Tell G. Damante G. Caldwell D. Kelley MR. The intracellular localization of APE1/Ref-1: more than a passive phenomenon? Antioxid Redox Signal. 2005;7:367–384. doi: 10.1089/ars.2005.7.367. [DOI] [PubMed] [Google Scholar]
- 46.Vasko MR. Guo C. Kelley MR. The multifunctional DNA repair/redox enzyme Ape1/Ref-1 promotes survival of neurons after oxidative stress. DNA Repair (Amst) 2005;4:367–379. doi: 10.1016/j.dnarep.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 47.Walker LJ. Craig RB. Harris AL. Hickson ID. A role for the human DNA repair enzyme HAP1 in cellular protection against DNA damaging agents and hypoxic stress. Nucleic Acids Res. 1994;22:4884–4889. doi: 10.1093/nar/22.23.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walker LJ. Robson CN. Black E. Gillespie D. Hickson ID. Identification of residues in the human DNA repair enzyme HAP1 (Ref-1) that are essential for redox regulation of Jun DNA binding. Mol Cell Biol. 1993;13:5370–5376. doi: 10.1128/mcb.13.9.5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walton M. Lawlor P. Sirimanne E. Williams C. Gluckman P. Dragunow M. Loss of Ref-1 protein expression precedes DNA fragmentation in apoptotic neurons. Brain Res Mol Brain Res. 1997;44:167–170. doi: 10.1016/s0169-328x(96)00291-4. [DOI] [PubMed] [Google Scholar]
- 50.Watson T. Goon PK. Lip GY. Endothelial progenitor cells, endothelial dysfunction, inflammation, and oxidative stress in hypertension. Antioxid Redox Signal. 2008;10:1079–1088. doi: 10.1089/ars.2007.1998. [DOI] [PubMed] [Google Scholar]
- 51.Weinberg WC. Azzoli CG. Chapman K. Levine AJ. Yuspa SH. p53-mediated transcriptional activity increases in differentiating epidermal keratinocytes in association with decreased p53 protein. Oncogene. 1995;10:2271–2279. [PubMed] [Google Scholar]
- 52.Yang KD. Shaio MF. Hydroxyl radicals as an early signal involved in phorbol ester-induced monocytic differentiation of HL60 cells. Biochem Biophys Res Commun. 1994;200:1650–1657. doi: 10.1006/bbrc.1994.1641. [DOI] [PubMed] [Google Scholar]
- 53.Zhu D. Qu L. Zhang X. Lou Y. Icariin-mediated modulation of cell cycle and p53 during cardiomyocyte differentiation in embryonic stem cells. Eur J Pharmacol. 2005;514:99–110. doi: 10.1016/j.ejphar.2005.03.031. [DOI] [PubMed] [Google Scholar]


