Abstract
Management of melanoma is a growing and challenging public health issue requiring novel and multidisciplinary approaches to achieve more efficient prevention and therapeutic benefits. The aim of this article is to show the critical role of APE/Ref-1 on melanomagenesis and progression. APE/Ref-1 serves as a redox-sensitive node of convergence of various signals as well as a DNA-repair enzyme, and its activation protects melanocytes and melanoma cells from chronic oxidative stress and promotes cell survival via mediation of downstream pathways. APE/Ref-1 is a strong candidate as a potential drug-treatable target for the prevention and treatment of human melanoma. Lead compounds exhibiting inhibitory effects on APE/Ref-1 are also reviewed. We anticipate potential clinical benefit in the future through inhibition of APE/Ref-1 and/or Ref-1-mediated signaling. Antioxid. Redox Signal. 11, 639–650.
Introduction
Human melanoma is the most serious skin cancer and is among the most drug resistant of all malignancies. Although in recent years, substantial successes in the therapy for other advanced malignancies has been achieved, this has not occurred for metastatic melanoma. This tumor is also one of the few cancers with rapid increases in its incidence rate over the past two decades (75, 98). Approximately 60,000 new cases of invasive melanoma and nearly 8,000 deaths are reported in the United States each year, making this disease an increasing public health concern (46). Ultraviolet radiation (UVR), a well-known initiator and promoter of nonmelanoma skin cancers (14), has been implicated as a major environmental contributor to the development of most cutaneous melanomas, although its mechanistic role in melanocyte carcinogenesis remains poorly understood, and it is likely that other etiologic factors remain to be discovered (99). Basic research has produced some real advances in our biologic understanding of cutaneous malignant melanoma (8, 21, 93); however, this knowledge has not been successfully translated into significant clinical benefit. Currently, limited therapeutic options exist for patients with metastatic melanoma, and novel drug-treatable targets are in great demand.
Abnormal Redox Status and Chronic Oxidative Stress is Important in the Pathogenesis of Human Melanoma
Melanin, a unique product of melanocytes, is the major pigment found in the hair and epidermis, as well as in the brain and other highly nerve-active areas such as the retina and middle ear. The predominant physiologic function of melanin, which is closely linked to its redox potential, is to protect skin from photochemical stress by serving as a disposable buffer by neutralizing reactive oxygen species (ROS) generated by UVR. Melanin synthesis represents a complex series of tightly regulated steps involving the consumption of oxygen and superoxide and the production and utilization of hydrogen peroxide, all carefully orchestrated within a well-organized organelle, the melanosome (37). Melanosomes progressively become more structurally or functionally disordered or both during melanomagenesis, evoking more ROS production and leading to an ongoing peroxide stress within the melanoma cell, especially when bound to Cu (22, 28, 31, 96). Consistently, normal melanocytes efficiently abrogate an exogenous peroxide stress, whereas human melanoma cells are seriously impaired in their ability to do so and generate higher levels of reactive oxygen species (ROS) (74). Contributions of other investigators have also shown that melanoma cells are depleted of cellular antioxidants (85), contain reduced glutathione (GSH) levels (30), and have altered levels of catalase, SOD, and other antioxidant enzymes. These considerations led us to propose a novel model for the etiology and pathogenesis of melanoma (Fig. 1), in which the initial step involved the conversion of melanin from its natural antioxidant/reduced state to that of a superoxide-generating prooxidant compound and the generation of excess (ROS), a process enhanced by the binding to melanin of certain metals found in the environment (28, 75, 76) or represented prominently in the normal melanin-synthesis pathway (i.e., Cu2+ bound to tyrosinase).
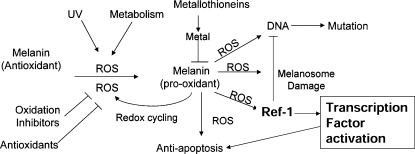
FIG. 1.
A model for chemoprevention of early melanoma progression. Oxidation of melanin leads progressively to generation of a redox-active tautomer (quinone-imine), intracellular redox cycling (enhanced by metals or other substances bound by melanin) with melanosomal and DNA damage, transcription factor activation and enhancement, leading to the development of natural antiapoptotic (drug-resistant) phenotype of the melanocyte. Antioxidants include a number of cellular antioxidants (ascorbic acid, α-tocopherol, and glutathione), whereas upstream inhibitors of oxidation might include such drugs as inhibitors of cholesterol synthesis or inhibitors of mitochondrial activity. Metals come from either xenografted overload or internal release induced by UV radiation and sunburn. The uptake into cells is regulated by metallothioneins, and polymorphisms should contribute to differential uptake and risk. ROS, reactive oxygen species. [Reproduced with permission from Meyskens et al. (75)].
In addition to its beneficial health effects, such as vitamin D3 formation, UVR also produces many acute and chronic detrimental cutaneous changes, which are associated with the development of skin malignancies (107). Elevated ROS (i.e., H2O2) and reactive nitrogen species (RNS) levels were evident after UVR (10, 39, 103), which adds an oxidative burden to melanocytes. ROS induces various oxidative DNA damage such as 8-oxo-dihydro-2'-deoxyguanine (8-oxo-dG), which is a mutagenic lesion that is directly repaired by the DNA base excision–repair pathway (35, 70). It is also well-documented that active dietary antioxidants than can scavenge ROS exhibit promising cancer chemopreventive activities (50). For the process of melanomagenesis, extensive epidemiologic observations support the idea that the effects of UVR on the skin are complex (2, 9). A striking feature in melanoma has been the general inability to detect thymine dimers or other classic UVR-induced mutations in primary or metastatic melanomas, even in genes of interest (9), despite convoluted explanations of why this might be so (110).
Increase of APE/Ref-1 as an Adaptive Response to Oxidative Stress
APE/Ref-1 is a node of convergence for various redox-sensitive signals as well as being important in DNA repair. As the major AP endonuclease in human cells, APE/Ref-1 accounts for >95% of the total AP endonuclease activity and is essential for the protection of cells against the toxic effects of several classes of DNA-damaging agents. Recently, many studies demonstrated that many survival, proliferation, or antiapoptosis signalings are activated by APE/Ref-1–mediated transcription factors such as AP-1, NF-κB, and p53, whose regulation occurs in both a redox-dependent and a redox-independent manner (27, 118). It is well documented that elevated APE/Ref-1 is associated with chemo- and radioresistance in a number of cellular systems (11, 77, 102). Knockdown of APE/Ref-1 efficiently induced apoptosis or sensitization or both to chemical treatments in many cancer cells (57, 115, 116, 120).
APE/Ref-1 is uniquely sensitive to both intracellular and extracellular alterations of redox status. ROS not only can inhibit APE/Ref-1 activities by direct oxidation of amino acid residues (49), but also affects the expression level and subcellular localization of APE/Ref-1 (44, 88, 91, 109). Furthermore, it is well documented that both UVA and UVB cause skin inflammation with release of inflammation mediators like cytokines (IL-1 and IL-6), which further produce more ROS and increase oxidative stress (29, 107). Activation of NF-κB and AP-1 play a critical role in regulating the transcription of numerous genes involved in the immune and inflammatory response (45, 101), in which DNA-binding activity is markedly enhanced by APE/Ref-1 (27). In addition to indirect regulation of inflammation, many studies also revealed distinct induction of APE/Ref-1 in response to inflammatory stresses such as infection and asthma (3, 80, 83). For example, Helicobacter pylori–induced IL-8 gene transcription is also dependent on APE/Ref-1. As two sides of the same coin, APE/Ref-1 has beneficial effects and protects cells from ROS toxicity, but conversely, consistent induction or activation of APE/Ref-1 in response to prolonged oxidative stress switches the cellular signaling to a proliferation/antiapoptosis phenotype.
In melanoma cells, certain metals in combination with UVR, generates low-grade redox cycling with ongoing ROS generation, resulting in progressive and diffuse genetic and other cellular damage. The imbalance between pro- and antioxidants results in the activation of redox-sensitive signal transcriptions, such as AP-1 and NF-κB (15). AP-1 specifically regulates transcription of tetradecanoylphorbol 13-acetate (TPA)-responsive element (TRE)-containing genes by acting on their promoters (25), such as cyclin D1 (104) and p21 (20), which are important regulators of the cell cycle. AP-1 target genes are differentially regulated by distinct AP-1 dimers. In melanoma cells, expression of both c-Jun and JunD is evident (60, 121). Our studies suggest that JunD may be more mitogenic (121), which might be due to its cooperation with NF-κB signaling (92, 111). An activated NF-κB pathway is clearly important in melanomagenesis also (55, 67, 90), not only because this transcription factor regulates many genes (such as PTEN and FLIP) that are critically involved in apoptosis (24, 64), but also because NF-κB can be activated by antiapoptotic Bcl-2 overexpression, which suggests the existence of a forward-feedback loop (97). Our previous extensive studies have shown that in human melanoma cells, abnormal redox status is present compared with normal tissue, and activated ROS-mediated signaling was prominent (i.e., AP-1 and NF-κB) (67, 68, 73, 76, 121).
Our previous studies also demonstrated a remarkable increase of APE/Ref-1 expression levels in all tested melanoma biopsies and cell lines (Fig. 2), which was predominantly localized in the nucleus and contributed to the binding and activation of AP-1 and NF-κB (120). We characterized the APE/Ref-1 response in a series of JB6 cells (122) and found that elevated APE/Ref-1 was associated with decreased intracellular ROS levels as well as reduced oxidative DNA-damage lesions (Fig. 3). Also, depletion of APE/Ref-1 resulted in apoptosis with more ROS production and markedly reduced AP-1 transcription activities (122). Our studies also suggested that, as an adaptive response, induced APE/Ref-1 counteracts ROS stress not only by efficiently repairing oxidative DNA damage, but also through regulating redox-sensitive signaling (such as AP-1 and NF-κB).
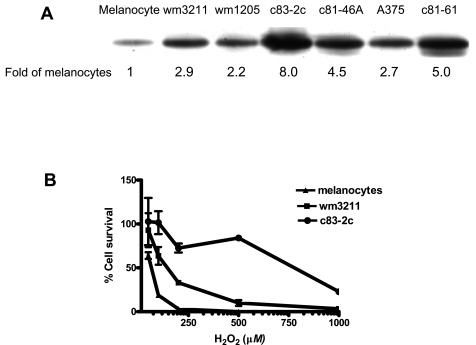
FIG. 2.
(A) Increased expression of APE/Ref-1 protein in nucleus of different human melanoma cell lines compared with cultured normal melanocytes. [Reproduced with slight modification with permission from Yang et al. (120)]. Nuclear proteins were isolated from different melanoma cell lines. All results are presented as folds of the expression levels in normal melanocytes and normalized by tubulin expression levels. (B) Resistance of human melanoma cell lines to H2O2 treatment. After 72-h incubation with H2O2 at different doses, cells were collected. The total number of viable cells was counted by Trypan Blue dye exclusion assay. Values represent the mean of three separate experiments.
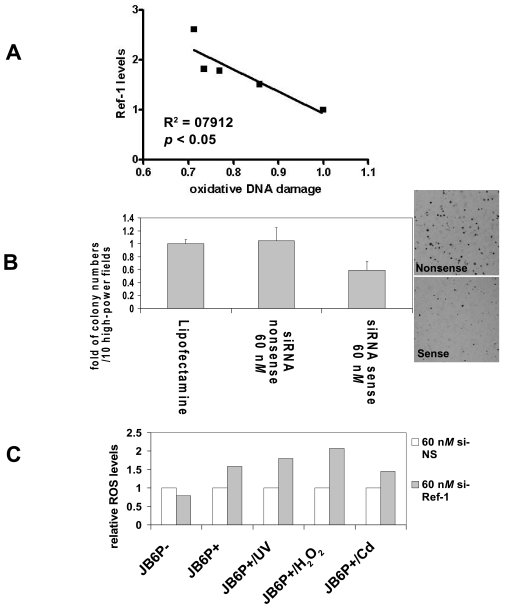
FIG. 3.
(A) Relative intracellular oxidative DNA damage levels in different JB6 series cells. [Reproduced with slight modification with permission from Yang et al. (122)]. Relative oxidative DNA damage product (8-oxo-dG) was detected by Advin-FITC by using flow cytometry; APE/Ref-1 levels were determined by immunoblotting with whole-cell lysates. (B) Tumor promoter TPA-induced anchorage-independent transformation of JB6P+ cells is inhibited by APE/Ref-1 knockdown. The si-NS or si-APE/Ref-1 (60 nM) transfected cells were used to assess colony formation in a cell anchorage–independent assay. For TPA-induced transformation, JB6P+ cells (8 × 103 per ml) were exposed to TPA (20 ng/ml) in 1 ml of 0.33% basal medium Eagle agar containing 10% FBS. (C) Elevation of intracellular ROS levels after APE/Ref-1 depletion. Cells were seeded overnight at the density of 60% for transient transfection of APE/Ref-1 siRNA (60 nM). At 48 h later, cells were collected and stained with DCF fluorescence probe for flow cytometry, as described in Materials and Methods. Data are represented as the fold of si-NS control.
Effects of Metals Involved in Melanomagenesis on APE/Ref-1 and APE/Ref-1–Mediated Signaling
Metals also likely play a crucial role in melanomagenesis (Fig. 4). Natural melanins are associated with a number of metal ions and have the capacity to accumulate metals. As characterized in our previous publications, we postulate that metals play an important role in converting melanin from a normal reducing status to a prooxidant state. Many metals bind to melanin with high affinity, especially certain heavy metals with redox potential, such as Cu(II) and Fe(III) (43). Additional studies also showed that Cu(II)- and Fe(III)-loaded melanin generally caused more DNA damage than Mg(II)-, Ca(II)-, or Zn(II)-loaded melanin (42). The literature indicates that supplemental copper and iron facilitate tumor growth, especially melanoma (52, 86).
FIG. 4.
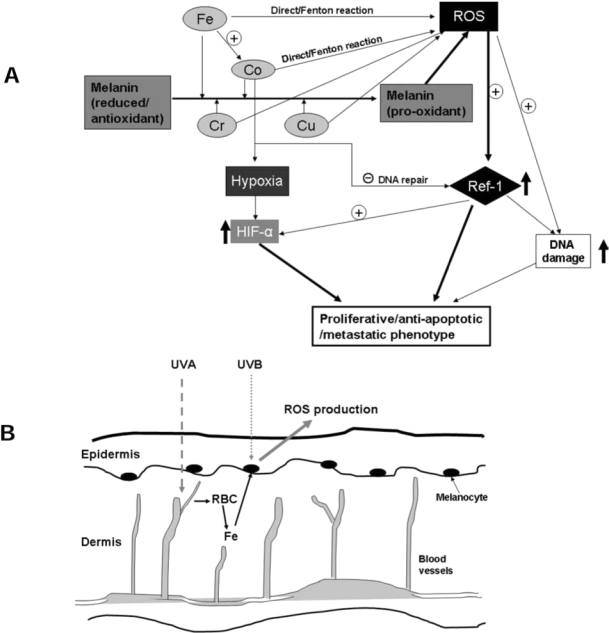
(A) Metal interactions with APE/Ref-1 and potential role in melanomagenesis. (B) Schematic diagram of involvement of iron in melanomagenesis occurring after sunburn. The UVB rays are the most potent rays that reach the earth, whereas UVA, having less energy, can penetrate deepest into the dermis, where blood vessels are located. Exposure to sunburn-dosage of UVR induces remarkable inflammation responses, resulting in cytokine release, dermal edema, and perivascular neutrophilic infiltration. Red blood cells (RBCs) are the major source of iron, which is released to the skin. Subsequently, Fe initiates or facilitates a chronic redox cycling when bound to melanin, and is associated with more ROS generation.
Very interestingly, three large epidemiologic studies of the risk of cancer after hip replacements have been reported (81, 83, 113). Remarkably, patients with prior metal-on-metal hip replacements were identified at increased risk for cutaneous melanoma, prostate, and possibly kidney malignancies in all three studies. However, no increased risk was noted for patients with polyurethane-on-metal hip replacements. Other notable features of these studies were that the risk for melanoma increased with follow-up time in the two cohort studies. Other investigations have shown that serum and urine Co and Cr levels are markedly elevated (threefold to 10-fold) in patients with metal-on-metal hip replacements compared to controls (105). Consistent with these findings, substantial evidence shows that Co and Cr ions accumulate in melanin. Bogacz measured the affinity of various heavy metal ions for melanin in vitro, and ranked Fe3− > Cr3+ > Co2+ > Zn2+ > Mn2+ (13). Interestingly, Co and Cr affect the chemical properties of melanin and cell pigmentation (6, 84).
Depending on the system and experimental condition used, arguments both support and counter the importance of ROS in Cr genotoxicity (16, 112). In welding workers, who are exposed to high amount of vaporized Cr as well as other heavy metals, skin irritations and malignant melanoma were reported (72). Cobalt is a nonessential metal for which sufficient evidence for carcinogenicity exists in animals (IARC), with a high level of DNA-damaging capacity (38). Cobalt also induces ROS production through the Fenton reaction to generate hydroxyl radicals (·OH) in a perinuclear iron-dependent manner. The study of Leonard et al. (1998) showed that cobalt induced a significant increase in the generation of a whole spectrum of ROS (58). Although Co(II) does not react with hydrogen peroxide by the classic Fenton reaction at physiologic pH values, Co (I) mediates a Fenton-like reaction producing ·OH, which was increased remarkably in the presence of Fe2+.
Moreover, Co also mediates ROS generation in an indirect way. CoCl2 treatment is a classic model widely used to mimic the effects of hypoxia (89). Hypoxia-inducible factor-1 is a major oxygen- and metal-responsive transcription factor, which is also a key factor in mediating keratinocytes response to UVB exposure (95). Cobalt efficiently induces the activation of HIF-1 by the production of superoxide (65). Of great interest are studies that show APE/Ref-1 is a critical component of the hypoxia-inducible transcriptional complex that interacts with HIF-1 and p300 (33, 128). In addition, APE/Ref-1 was essential for the full activity of the C-terminal region of HIF-1, which contains its transactivation domain (56). However, no significant changes of APE/Ref-1 expression levels were evident with exposure to CoCl2 (50 μM) in melanoma cells (unpublished data). Whether such an effect occurs in normal melanocytes will be important to determine.
Another very interesting metal that is likely involved in melanomagenesis is iron. It is well documented that on exposure to UVR, an increase in ferric/ferrous iron occurs in the skin, and topical application of iron chelators reduces UVR-induced ROS production and skin damage, indicating a role of iron in photodamage (5, 47, 78, 94, 119). As shown in Fig. 4B, we postulate that photo-induced release of a pool of iron cations in response to blistering sunburn and the binding of Fe2− and/or Fe3+ and binding to at-risk melanin (i.e., pheomelanin and certain types of eumelanin, especially when partially oxidized), initiate low-level oxidative stress. Iron not only directly evokes ROS production by the Fenton reaction, but also potentiates Co-mediated generation of ·OH at physiologic pH values (58). Korytowski et al. (1987) studied the reactive species produced on irradiation of melanin with UVR and visible light (53). Their study showed that the production of ROS increased when melanin was complexed with iron in the presence of EDTA. Notably, melanin in the presence of low-dosage iron is associated with a low level of hydroxyl radical production; however, when melanin is saturated with Fe(III), ROS production occurs in a dose-dependent manner (87, 124). In addition, a recent study also demonstrated the pro-inflammatory activity of iron in the lung injury, at least in part, because of its induction of redox-sensitive factors (for example, TRX, APE/Ref-1, and NF-κB) (32).
The endonuclease activity of APE/Ref-1 has been shown to be affected by many factors, including different metals. The divalent metal ion soaked with the protein crystals (Sm2+ for APE-1) was found specifically to associate with the glutamate residue. Biochemical studies have shown that an APE-1 mutant with E96A change displays a significantly reduced DNA-repair activity in Mg2+ when compared with the wild-type protein (7), further supporting the notion that this amino acid contributes to metal coordination. Of considerable interest is that in in vitro studies with fibroblasts, Co does not enhance UVR-induced cyclobutane pyrimidine dimers (CPD) and 8-oxo-dG lesions formation but rather inhibits their repair (34, 48). However, dimer removal was not affected by Pb(II), Cr(VI), Fe(III), or Sn(III) (106). Controversially, another study demonstrated the inhibitory effects of Pb(II) and Fe(II) on APE/Ref-1 activity but not Co(II) (66).
Interestingly, CoCl2 (500 μM) is essential for AP endonuclease assay. Levin et al. (1991) showed that the activity of Escherichia coli endonuclease IV after exposure to EDTA, a strong nonspecific metal chelator, was restored by incubation with CoCl2 (500 μM) and to a lesser extent by MnCl2 (59). In another study, NiCl2 and CoCl2 at 1 mM concentrations stimulated both NF-κB and AP-1 activities (114), which might be mediated by APE/Ref-1 activation. These studies suggested that the effects of Co on APE/Ref-1 are concentration dependent.
Novel Strategies Targeting APE/Ref-1 to Prevent/Treat Human Melanoma
A variety of observational and experimental studies generated interest in the role of APE/Ref-1–mediated signaling in cancer, especially human melanoma. First, APE/Ref-1 is very sensitive to redox-status alterations. ROS regulates its activity and expression on both transcriptional and posttranscriptional levels. Coupled with the observation that melanoma cells exhibit abnormal redox status, induction of APE/Ref-1 as an adaptive response to prolonged oxidative stress likely plays an important role in human melanoma-genesis. Our previous studies consistently demonstrated abnormally elevated nuclear APE/Ref-1 in human melanoma cells compared with normal melanocytes, also associated with drug resistance and proliferation. Recently, by using a series of JB6 cells, we provided evidence that APE/Ref-1, in combination with ROS, plays a key role in malignant cellular transformation (Fig. 3) (122). In addition, our recent findings strongly suggested that APE/Ref-1 is involved in the regulation of metastatic potential in melanoma cells (unpublished data). Second, as discussed earlier, metals involved in melanomagenesis, especially cobalt and iron, regulate APE/Ref-1 expression and activity directly and indirectly. Third, the NF-κB pathway, regulated by APE/Ref-1, is prominent in mediating cytokine activation of leukocytes in inflammation. The involvement of APE/Ref-1 in the process of inflammation, which also occurs after sunburn, indicates interference with melanomagenesis by targeting APE/Ref-1 as a potential preventive strategy. We propose that excess endogenous ROS in human melanoma cells may also compromise the efficacy of alkylating agents–based or radiation-based therapy by inducing APE/Ref-1 in human melanoma. Accumulating studies on the role of APE/Ref-1 in promotion, progression, and drug resistance in other types of tumors have consistently confirmed its potential as an attractive target for the development of new cancer preventive and therapeutic strategies (36, 108).
In recent years, the rapid increase in the number of high-resolution three-dimensional protein structures (1, 41), and the improvements of docking-and-scoring technology, make virtual screening (VS) an attractive and less-expensive alternative or complementary approach to the traditional methods of lead discovery and optimization (4, 51). Moreover, VS enables diverse compounds that would seldom be tested in a traditional laboratory high-throughput screen to be identified. The number of success stories from using VS in drug discovery keeps growing (69, 80). Recently we applied this docking strategy to the APE/Ref-1 receptor by screening chemical libraries (Fig. 5). Interestingly, resveratrol was found to dock into one of the two drug-treatable pockets located in the redox domain and, in preliminary screens, exhibited promising antimelanoma activities (120). The inhibitory effects of resveratrol on APE/Ref-1 occurred mostly through its redox-regulating functions and might be the major contribution to its pharmacologic activities, which are associated with significantly reduced AP-1 and NF-κB activities in many different human cancers (12, 54). Surprisingly, all other resveratrol analogues that we tested exhibited a lower docking score and lesser toxicity to human melanoma cells. In addition to resveratrol, some active lead compounds specifically targeting these two unique drug-treatable pockets were discovered by our screening. Further confirmation of these findings and chemical modifications are under way, and our early findings are discussed later. It is notable that APE/Ref-1 contains three distinct functional domains: nuclear-localization signal, redox-regulation, and DNA-repair domain. Limited experiments have been reported testing the distinct role of different domains in APE/Ref-1–mediated melanoma malignancies. These data would be critical for developing specific small-molecular inhibitors that interfere with distinct functions.
FIG. 5.
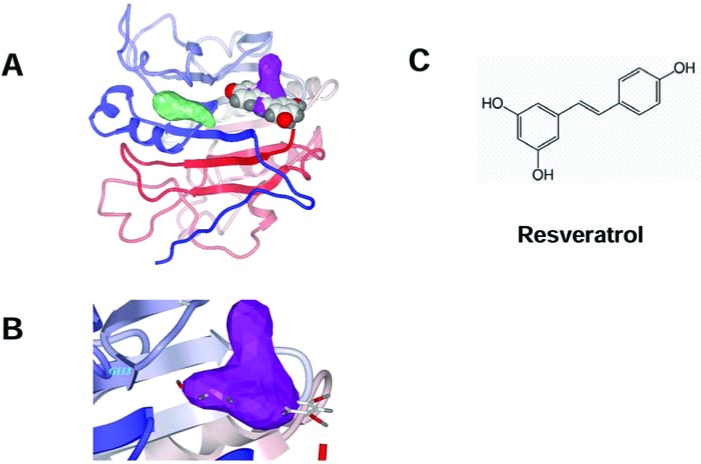
Model of resveratrol docked to one of two drug-treatable pockets of APE/Ref-1. (A) The structure of the human APE/Ref-1 protein is illustrated by a ribbon schematic, by using a color gradient from blue at the N terminus to red at the C terminus. The shapes of each druggable pocket, as identified by ICM in the redox-regulating domain, are shown in green (left) and in purple (right). A CPK space-filling model of resveratrol is docked in the druggable pocket (right). (B) A close-up view of the putative binding site of resveratrol, represented by a stick model, is shown. The resveratrol is mostly confined to the spatial volume of the drug-gable pocket, represented by the purple 3-D mesh. (C) Molecular structure of resveratrol. [Reproduced with slight modification with permission from Yang et al. (120)]. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
As APE/Ref-1 acts hierarchically to regulate many transcription factors (i.e., AP-1 and NF-κB), direct interference with APE/Ref-1 would be expected to result in a more-comprehensive effect than inhibition of just one downstream pathway. A small-molecule PNRI-299 (Fig. 6C) was identified as selective APE/Ref-1 inhibitor and has shown selective inhibition on AP-1 transcription (79). With a generous gift from Dr. Kahn, we tested the effects of PNRI-299 on human melanoma cells; however, at up to 100 μM concentration, no toxicity was evident in our test cell lines. One reason might be its specific inhibition of AP-1, which is markedly reduced in metastatic melanoma cells compared with normal melanocytes (121). However, exposure to intense bursts of sunlight, especially in childhood, is strongly associated with an increased risk for melanoma, whereas thick tumors (4 mm or greater), which have more metastatic potential, have significantly increased in men aged 60 years or older (23, 100). The long latent phase and slow progression of melanomagenesis provide a good opportunity for early intervention or chemoprevention. Specific APE/Ref-1 inhibitors (i.e., resveratrol analogues and PNRI-299), even without any direct melanoma cell–killing potential, might exert antiinflammation activities and be applied as preventive or photoprotective agents and protect skin from UV radiation–induced inflammation, which is mediated by AP-1 (14, 19). More recently, Luo et al. (61) reported that a small-molecule, 3-[5-(2,3-dimethoxy-6-methyl-1,4-ben-zoquinoyl)]-2-nonyl-2-propionic acid (E3330), exhibited remarkable inhibition of the redox function of APE/Ref-1 in vitro, and the IC50 is 6.5 μM, whereas no effects on repair activity of APE/Ref-1 were evident.
FIG. 6.
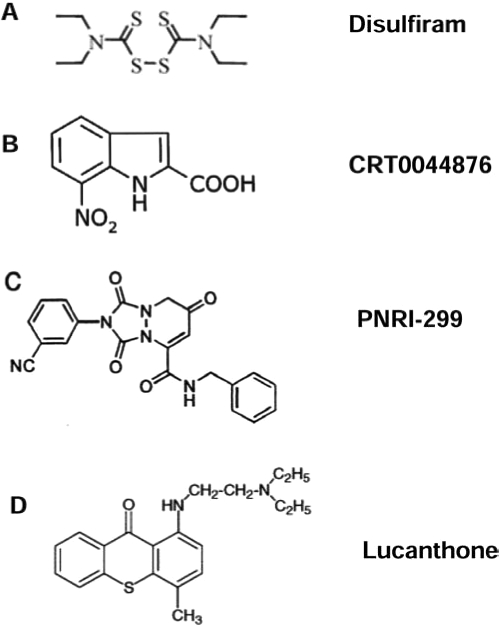
Molecular structure of reported compounds exhibiting APE/Ref-1 inhibitory effects (17, 62, 63, and 79).
Lucanthone (Nilodin, Miracil D) was found to be an inhibitor of postradiation repair and is used as an adjuvant in radiation therapy (Fig. 1). A recent study showed that lucanthone increased the frequency of abasic sites in HeLa cell DNA, reflecting inhibition of APE repair activity, and enhanced the cell-killing effect of alkylating agents (62, 71). Notably, the APE/Ref-1 redox function or exonuclease activity on mismatched nucleotides was not affected by this compound. Additionally, by using fluorescence-based, high-throughput screening, a European group has isolated a small-molecule inhibitor, CRT0044876 (Fig. 6B), that binds to the active site of APE/Ref-1 and effectively inhibits its AP endonuclease, 3'-phosphodiesterase and 3'-phosphatase activities at low micromolar concentrations (63). Studies of in vivo efficacy and further chemical modification will be of great interest.
In addition to these direct APE/Ref-1 inhibitors, possible indirect ways may be found to inhibit APE/Ref-1–mediated signaling. Thioredoxin (TRX), a small cysteine-rich redox-active protein, directly associates with APE/Ref-1 in the nucleus and is essential for APE/Ref-1–mediated potentiation of AP-1 activity (40, 117). Combined with other observations that oxidized APE/Ref-1 lacks endonuclease activity (49), we propose that agents that block TRX/Ref-1 dimerization would decrease APE/Ref-1 nuclear translocation and induce APE/Ref-1 oxidation, resulting in subsequent APE/Ref-1 dysfunction.
Chelation therapy has been of great interest to medicine for several hundred years, although beneficial effects have been elusive in most cases. Adding to the potential mechanistic role of transition metals in melanomagenesis, metal particles are known to induce a vigorous macrophage–cytokine response associated with local osteolysis, which could serve as a promotional event for either melanoma or prostate cancer (105). Theoretically, based on our hypothesis, specific metal chelators could deplete metals and prevent the extra loading to melanin with less ROS production, which alternatively eliminates the stimulation of APE/Ref-1. In previous studies, we demonstrated that the S-based chelator pyrollidine dithiocarbamate (pDTC) strongly induced apoptosis in melanoma but was not toxic to melanocytes up to 10 μg/ml (28). In addition, the metal chelators o-phenanthroline (OP) and deferoxamine (DEF) were selectively toxic to human melanoma cells, whereas normal cultured melanocytes were resistant to OP- and DEF-induced changes (unpublished data). Notably, the alcohol-adversion drug disulfiram (DSF) (Fig. 6A) is a Cu-chelator and induces significant apoptosis in human melanoma at very low concentration (IC50 ~20–50 ng/ml or ~50–125 nM) through a distinct mechanism. It is Cu dependent, and addition of CuCl2 significantly enhances the DSF-induced cell death with a marked increase in intracellular Cu level and rapid ROS production. The cell-killing effect of DSF might be due to the extensive oxidation of protein initiated by Cu (17, 18). Recently, caged-iron chelators that are activated by UV have gained interests as an approach to protect against UV damage with fewer side effects and higher local protection (123).
Conclusions
Over the past decade, we have conducted a series of histologic, biochemical, chemical, and molecular experiments with melanin and human melanoma to explore the molecular mechanisms involved in melanomagenesis and melanoma progression. As summarized in Fig. 7, we proposed that prolonged oxidative stress initiated by UVR and melanin oxidation, a process enhanced by certain metals (cobalt, copper) found in the environment, is an early or primary event. The consequences of this phenomenon include many molecular changes, but as a key regulator, APE/Ref-1 is markedly induced and efficiently protects melanocytes from oxidative damage by inducing the antiapoptotic machinery and stimulating cell survival. Combined with other alterations, such as the depletion of cellular antioxidants and widespread oxidation of macro-molecules, APE/Ref-1 exhibits a critical role in melanoma-genesis and melanoma progression. A number of lead compounds show promising inhibitory effects on APE/Ref-1 and APE/Ref-1–mediated signaling, potentially with a wide range of indications from asthma to cancer therapy. The targeting of APE/Ref-1 may be a useful preventive and therapeutic strategy for the management of human melanoma and perhaps other cancers.
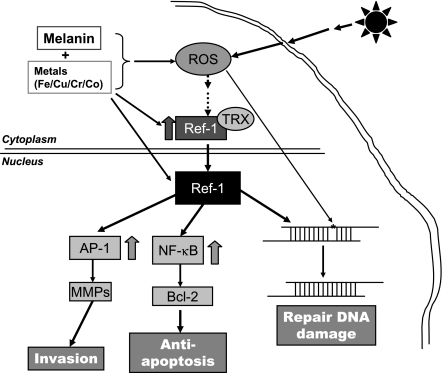
FIG. 7.
Schematic diagram of critical role of APE/Ref-1 in melanomagenesis and melanoma progression. Exposure to UVR, especially at sunburn dosage, induces remarkable production of ROS and leads to DNA damage subsequently. As an adaptive response, APE/Ref-1 is induced and translocated to the nucleus and dimerized with thioredoxin (TRX), creating a reducing environment that facilitates the DNA binding of nuclear transcription factor (i.e., AP-1 and NF-κB) and efficiently repairs damaged DNA. Second, MMPs and Bcl-2 are induced as downstream target genes, leading to a more invasive and proliferative cell phenotype. Possible strategies to inhibit APE/Ref-1–mediated signaling include direct (i.e., APE/Ref-1 inhibitors and APE/Ref-1/TRX binding blockers) and indirect (specific metal chelators, and potential antioxidants and NOS scavengers) interferences. Through targeting APE/Ref-1 signaling, we anticipate identifying new preventive and therapeutic approaches and new agents.
Acknowledgments
This study was supported in part by Chao Family Comprehensive Cancer Center, National Cancer Institute grant P30-CA62203, the Sun Fellowship Award, and the Oxnard and Waltmar Foundations.
Abbreviations
8-oxo-dG, 8-oxo-dihydro-2'-deoxyguanine; AP-1, activating protein-1; apurinic/apyrimidinic endonuclease-1/redox factor-1 (APE/Ref-1); DSF, disulfiram; EDTA, ethylenediaminetetraacetic acid; FLIP, FLICE-like inhibitory protein; HIF-1, hypoxia-inducible factor; NF-κB, nuclear factor kappa B; OP, o-phenanthroline; pDTC, pyrollidine dithiocarbamate; PTEN, phosphatase and tensin homologue deleted on chromosome 10; ROS, reactive oxygen species; SOD, superoxide dismutase; TRX, thioredoxin; UVR, ultraviolet radiation; VS, virtual screening.
References
- 1.Abola E. Kuhn P. Earnest T. Stevens RC. Automation of X-ray crystallography. Nat Struct Biol. 2000;7(suppl):973–977. doi: 10.1038/80754. [DOI] [PubMed] [Google Scholar]
- 2.Agar N. Young AR. Melanogenesis: a photoprotective response to DNA damage? Mutation Res. 2005;571:121–132. doi: 10.1016/j.mrfmmm.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 3.Albrecht C. Knaapen AM. Becker A. Höhr D. Haberzettl P. van Schooten FJ. Borm PJ. Schins RP. The crucial role of particle surface reactivity in respirable quartz-induced reactive oxygen/nitrogen species formation and APE/Ref-1 induction in rat lung. Respir Res. 2005;6:129. doi: 10.1186/1465-9921-6-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amzel LM. Structure-based drug design. Curr Opin Biotechnol. 1998;9:366–369. doi: 10.1016/s0958-1669(98)80009-8. [DOI] [PubMed] [Google Scholar]
- 5.Aubailly M. Santus R. Salmon S. Ferrous ion release from ferritin by ultraviolet-A radiations. Photochem Photobiol. 1991;54:769–773. doi: 10.1111/j.1751-1097.1991.tb02088.x. [DOI] [PubMed] [Google Scholar]
- 6.Bahbouth E. Siwek B. De Pauw-Gillet MC. Sabbioni E. Bassleer R. Effects of trace metals on mouse B16 melanoma cells in culture. Biol Trace Elem Res. 1993;36(2):191–201. doi: 10.1007/BF02783178. [DOI] [PubMed] [Google Scholar]
- 7.Barzilay G. Walker LJ. Robson CN. Hickson ID. Site-directed mutagenesis of the human DNA repair enzyme HAP1: identification of residues important for AP endonuclease and RNase H activity. Nucleic Acids Res. 1995;23:1544–1550. doi: 10.1093/nar/23.9.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennett DC. How to make a melanoma: what do we know of the primary clonal events? Pigment Cell Melanoma Res. 2008;21:27–38. doi: 10.1111/j.1755-148X.2007.00433.x. [DOI] [PubMed] [Google Scholar]
- 9.Berwick M. Pathways to the development of melanoma: a complex issue. J Invest Dermatol. 2006;126:1932–1933. doi: 10.1038/sj.jid.5700419. [DOI] [PubMed] [Google Scholar]
- 10.Bickers DR. Athar M. Oxidative stress in the pathogenesis of skin disease. J Invest Dermatol. 2006;126:2565–2575. doi: 10.1038/sj.jid.5700340. [DOI] [PubMed] [Google Scholar]
- 11.Bobola MS. Finn LS. Ellenbogen RG. Geyer JR. Berger MS. Braga JM. Meade EH. Gross ME. Silber JR. Apurinic/apyrimidinic endonuclease activity is associated with response to radiation and chemotherapy in medulloblastoma and primitive neuroectodermal tumors. Clin Cancer Res. 2005;11:7405–7414. doi: 10.1158/1078-0432.CCR-05-1068. [DOI] [PubMed] [Google Scholar]
- 12.Bode AM. Dong Z. Targeting signal transduction pathways by chemopreventive agents. Mutat Res. 2004;555:33–51. doi: 10.1016/j.mrfmmm.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 13.Bogacz A. Buszman E. Wilczok T. Competition between metal ions for DOPA-melanin. Stud Biophys. 1989;132:189–195. [Google Scholar]
- 14.Bowden GT. Prevention of non-melanoma skin cancer by targeting ultraviolet-B-light signaling. Nat Rev Cancer. 2004;4:23–35. doi: 10.1038/nrc1253. [DOI] [PubMed] [Google Scholar]
- 15.Briganti S. Picardo M. Antioxidant activity, lipid peroxidation and skin diseases: what's new. J Eur Acad Dermatol Venereol. 2003;17:663–669. doi: 10.1046/j.1468-3083.2003.00751.x. [DOI] [PubMed] [Google Scholar]
- 16.Casadevall M. da Cruz Fresco P. Kortenkamp A. Chromium(VI)-mediated DNA damage: oxidative pathways resulting in the formation of DNA breaks and abasic sites. Chem Biol Interact. 1999;123:117–132. doi: 10.1016/s0009-2797(99)00128-3. [DOI] [PubMed] [Google Scholar]
- 17.Cen D. Brayton D. Shahandeh B. Meyskens FL., Jr Farmer PJ. Disulfiram facilitates intracellular Cu uptake and induces apoptosis in human melanoma cells. J Med Chem. 2004;47:6914–6920. doi: 10.1021/jm049568z. [DOI] [PubMed] [Google Scholar]
- 18.Cen D. Gonzalez RI. Buckmeier JA. Kahlon RS. Tohidian NB. Meyskens FL., Jr Disulfiram induces apoptosis in human melanoma cells: a redox-related process. Mol Cancer Ther. 2002;1:197–204. [PubMed] [Google Scholar]
- 19.Cooper SJ. Bowden GT. Ultraviolet B regulation of transcription factor families: roles of nuclear factor-kappa B (NF-kappaB) and activator protein-1 (AP-1) in UVB-induced skin carcinogenesis. Curr Cancer Drug Targets. 2007;7:325–334. doi: 10.2174/156800907780809714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crowe DL. Brown TN. Kim R. Smith SM. Lee MK. A c-fos/estrogen receptor fusion protein promotes cell cycle progression and proliferation of human cancer cell lines. Mol Cell Biol Res Commun. 2000;3:243–248. doi: 10.1006/mcbr.2000.0221. [DOI] [PubMed] [Google Scholar]
- 21.Curiel-Lewandrowski C. Atkins MB. Immunotherapeutic approaches for the treatment of malignant melanoma. Curr Opin Invest Drugs. 2001;2:1553–1563. [PubMed] [Google Scholar]
- 22.Curran RC. McCann BG. The ultrastructure of benign pigmented naevi and melanocarcinomas in man. J Pathol. 1976;119:135–146. doi: 10.1002/path.1711190303. [DOI] [PubMed] [Google Scholar]
- 23.Demierre MF. Sondak VK. Chemoprevention of melanoma: theoretical and practical considerations. Cancer Control. 2005;12:219–222. doi: 10.1177/107327480501200402. [DOI] [PubMed] [Google Scholar]
- 24.Dolcet X. Llobet D. Pallares J. Matias-Guiu X. NF-κB in development and progression of human cancer. Virchows Arch. 2005;446:475–482. doi: 10.1007/s00428-005-1264-9. [DOI] [PubMed] [Google Scholar]
- 25.Eferl R. Wagner EF. AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer. 2003;3:859–868. doi: 10.1038/nrc1209. [DOI] [PubMed] [Google Scholar]
- 26.Erzberger JP. Barsky D. Schärer OD. Colvin ME. Wilson DM., 3rd. Elements in abasic site recognition by the major human and Escherichia coli apurinic/apyrimidinic endonucleases. Nucleic Acids Res. 1998;26:2771–2778. doi: 10.1093/nar/26.11.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evans AR. Limp-Foster M. Kelley MR. Going APE over ref-1. Mutat Res. 2000;461:83–108. doi: 10.1016/s0921-8777(00)00046-x. [DOI] [PubMed] [Google Scholar]
- 28.Farmer PJ. Gidanian S. Shahandeh B. Di Bilio AJ. Tohidian N. Meyskens FL., Jr Melanin as a target for melanoma chemotherapy: pro-oxidant effect of oxygen and metals on melanoma viability. Pigment Cell Res. 2003;16:273–279. doi: 10.1034/j.1600-0749.2003.00046.x. [DOI] [PubMed] [Google Scholar]
- 29.Faustin B. Reed JC. Sunburned skin activates inflammasomes. Trends Cell Biol. 2008;18:4–8. doi: 10.1016/j.tcb.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Fruehauf JP. Zonis S. al-Bassam M. Kyshtoobayeva A. Dasgupta C. Milovanovic T. Parker RJ. Buzaid AC. Melanin content and downregulation of glutathione S-transferase contribute to the action of L-buthionine-S-sulfoximine on human melanoma. Chem Biol Interact. 1998;111–112:277–305. doi: 10.1016/s0009-2797(97)00167-1. [DOI] [PubMed] [Google Scholar]
- 31.Gidanian S. Mentelle M. Meyskens FL., Jr Farmer PJ. Melanosomal damage in normal human melanocytes induced by UVB and metal uptake: a basis for the pro-oxidant state of melanoma. Photochem Photobiol. 2008 doi: 10.1111/j.1751-1097.2008.00309.x. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gorbunov NV. Das DK. Goswami SK. Gurusamy N. Atkins JL. Spatial coordination of cell-adhesion molecules and redox cycling of iron in the microvascular inflammatory response to pulmonary injury. Antioxid Redox Signal. 2007;9:483–495. doi: 10.1089/ars.2006.1296. [DOI] [PubMed] [Google Scholar]
- 33.Gray MJ. Zhang J. Ellis LM. Semenza GL. Evans DB. Watowich SS. Gallick GE. HIF-1alpha, STAT3, CBP/p300 and Ref-1/APE are components of a transcriptional complex that regulates Src-dependent hypoxia-induced expression of VEGF in pancreatic and prostate carcinomas. Oncogene. 2005;24:3110–3120. doi: 10.1038/sj.onc.1208513. [DOI] [PubMed] [Google Scholar]
- 34.Hartwig A. Snyder RD. Schlepegrell R. Beyersmann D. Modulation by Co(II) of UV-induced DNA repair, mutagenesis and sister-chromatid exchanges in mammalian cells. Mutat Res. 1991;248:177–185. doi: 10.1016/0027-5107(91)90099-a. [DOI] [PubMed] [Google Scholar]
- 35.Hazra TK. Das A. Das S. Choudhury S. Kow YW. Roy R. Oxidative DNA damage repair in mammalian cells: a new perspective. DNA Repair (Amst) 2007;6:470–480. doi: 10.1016/j.dnarep.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He T. Weintraub NL. Goswami PC. Chatterjee P. Flaherty DM. Domann FE. Oberley LW. Redox factor-1 contributes to the regulation of progression from G0/G1 to S by PDGF in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2003;285(2):H804–H812. doi: 10.1152/ajpheart.01080.2002. [DOI] [PubMed] [Google Scholar]
- 37.Hearing VJ. Biogenesis of pigment granules: a sensitive way to regulate melanocyte function. J Dermatol Sci. 2005;37:3–14. doi: 10.1016/j.jdermsci.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 38.Hengstler JG. Bolm-Audorff U. Faldum A. Janssen K. Reifenrath M. Götte W. Jung D. Mayer-Popken O. Fuchs J. Gebhard S. Bienfait HG. Schlink K. Dietrich C. Faust D. Epe B. Oesch F. Occupational exposure to heavy metals: DNA damage induction and DNA repair inhibition prove co-exposures to cadmium, cobalt and lead as more dangerous than hitherto expected. Carcinogenesis. 2003;24:63–73. doi: 10.1093/carcin/24.1.63. [DOI] [PubMed] [Google Scholar]
- 39.Herrling T. Jung K. Fuchs J. Measurements of UV-generated free radicals/reactive oxygen species (ROS) in skin. Spectrochim Acta A Mol Biomol Spectrosc. 2006;63:840–845. doi: 10.1016/j.saa.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 40.Hirota K. Matsui M. Iwata S. Nishiyama A. Mori K. Yodoi J. AP-1 transcriptional activity is regulated by a direct association between thioredoxin and Ref-1. Proc Natl Acad Sci U S A. 1997;94:3633–3638. doi: 10.1073/pnas.94.8.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hol WG. Structural genomics for science and society. Nat Struct Biol. 2000;7(suppl):964–966. doi: 10.1038/80744. [DOI] [PubMed] [Google Scholar]
- 42.Hong L. Liu Y. Simon JD. Binding of metal ions to melanin and their effects on the aerobic reactivity. Photochem Photobiol. 2004;80:477–481. doi: 10.1562/0031-8655(2004)080<0477:BOMITM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 43.Hong L. Simon JD. Current understanding of the binding sites, capacity, affinity, and biological significance of metals in melanin. J Phys Chem B. 2007;111:7938–7947. doi: 10.1021/jp071439h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hsieh MM. Hegde V. Kelley MR. Deutsch WA. Activation of APE/Ref-1 redox activity is mediated by reactive oxygen species and PKC phosphorylation. Nucleic Acids Res. 2001;29:3116–3122. doi: 10.1093/nar/29.14.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inoue J. Gohda J. Akiyama T. Semba K. NF-kappaB activation in development and progression of cancer. Cancer Sci. 2007;98:268–274. doi: 10.1111/j.1349-7006.2007.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jemal A. Siegel R. Ward E. Murray T. Xu J. Thun MJ. Cancer statistics 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 47.Juzeniene A. Juzenas P. Iani V. Moan J. Topical applications of iron chelators in photosensitization. Photochem Photobiol Sci. 2007;6:1268–1274. doi: 10.1039/b703861e. [DOI] [PubMed] [Google Scholar]
- 48.Kasten U. Mullenders LH. Hartwig A. Cobalt(II) inhibits the incision and the polymerization step of nucleotide excision repair in human fibroblasts. Mutat Res. 1997;383:81–89. doi: 10.1016/s0921-8777(96)00052-3. [DOI] [PubMed] [Google Scholar]
- 49.Kelley MR. Parsons SH. Redox regulation of the DNA repair function of the human AP endonuclease Ape1/ref-1. Antioxid Redox Signal. 2001;3:671–683. doi: 10.1089/15230860152543014. [DOI] [PubMed] [Google Scholar]
- 50.Khan N. Afaq F. Mukhtar H. Cancer chemoprevention through dietary antioxidants: progress and promise. Antioxid Redox Signal. 2008;10:475–510. doi: 10.1089/ars.2007.1740. [DOI] [PubMed] [Google Scholar]
- 51.Klebe G. Recent developments in structure-based drug design. J Mol Med. 2000;78:269–281. doi: 10.1007/s001090000084. [DOI] [PubMed] [Google Scholar]
- 52.Korohoda W. Michalik M. Pietzkowski Z. Zaporowska-Siwiak E. Addition of iron and zinc complexes to Eagle's minimal essential medium is sufficient to induce and support the proliferation of B16 melanoma cells. Folia Histochem Cytobiol. 1993;31:3–7. [PubMed] [Google Scholar]
- 53.Korytowski W. Pilas B. Sarna T. Kalyanaraman B. Photoinduced generation of hydrogen peroxide and hydroxyl radicals in melanins. Photochem Photobiol. 1987;45:185–190. doi: 10.1111/j.1751-1097.1987.tb05362.x. [DOI] [PubMed] [Google Scholar]
- 54.Kundu JK. Surh YJ. Molecular basis of chemoprevention by resveratrol: NF-kappaB and AP-1 as potential targets. Mutat Res. 2004;555:65–80. doi: 10.1016/j.mrfmmm.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 55.Kuphal S. Poser I. Jobin C. Hellerbrand C. Bosserhoff AK. Loss of E-cadherin leads to upregulation of NFkappaB activity in malignant melanoma. Oncogene. 2004;23:8509–8519. doi: 10.1038/sj.onc.1207831. [DOI] [PubMed] [Google Scholar]
- 56.Lando D. Pongratz I. Poellinger L. Whitelaw ML. A redox mechanism controls differential DNA binding activities of hypoxia-inducible factor (HIF) 1alpha and the HIF-like factor. J Biol Chem. 2000;275:4618–4627. doi: 10.1074/jbc.275.7.4618. [DOI] [PubMed] [Google Scholar]
- 57.Lau JP. Weatherdon KL. Skalski V. Hedley DW. Effects of gemcitabine on APE/ref-1 endonuclease activity in pancreatic cancer cells, and the therapeutic potential of antisense oligonucleotides. Br J Cancer. 2004;91:1166–1173. doi: 10.1038/sj.bjc.6602080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leonard S. Gannett PM. Rojanasakul Y. Schwegler-Berry D. Castranova V. Vallyathan V. Shi X. Cobalt-mediated generation of reactive oxygen species and its possible mechanism. J Inorg Biochem. 1998;70:239–244. doi: 10.1016/s0162-0134(98)10022-3. [DOI] [PubMed] [Google Scholar]
- 59.Levin JD. Shapiro R. Demple B. Metalloenzymes in DNA repair. Escherichia coli endonuclease IV and Saccharomyces cerevisiae Apn1. J Biol Chem. 1991;266:22893–22898. [PubMed] [Google Scholar]
- 60.Lopez-Bergami P. Huang C. Goydos JS. Yip D. Bar-Eli M. Herlyn M. Smalley KS. Mahale A. Eroshkin A. Aaronson S. Ronai Z. Rewired ERK-JNK signaling pathways in melanoma. Cancer Cell. 2007;11:447–460. doi: 10.1016/j.ccr.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luo M. Delaplane S. Jiang A. Reed A. He Y. Fishel M. Nyland RL. Borch RF. Qiao X. Georgiadis MM. Kelley MR. Role of the multifunctional DNA repair and redox signaling protein ape1/Ref-1 in cancer and endothelial cells: small-molecule inhibition of the redox function of ape1. Antioxid Redox Signal. 2008 doi: 10.1089/ars.2008.2120. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luo M. Kelley MR. Inhibition of the human apurinic/apyrimidinic endonuclease (APE1) repair activity and sensitization of breast cancer cells to DNA alkylating agents with lucanthone. Anticancer Res. 2004;24:2127–2134. [PubMed] [Google Scholar]
- 63.Madhusudan S. Smart F. Shrimpton P. Parsons JL. Gardiner L. Houlbrook S. Talbot DC. Hammonds T. Freemont PA. Sternberg MJ. Dianov GL. Hickson ID. Isolation of a small molecule inhibitor of DNA base excision repair. Nucleic Acids Res. 2005;33:4711–4724. doi: 10.1093/nar/gki781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Magnani M. Crinelli R. Bianchi M. Antonelli A. The ubiquitin-dependent proteolytic system and other potential targets for the modulation of nuclear factor-kB (NF-kB) Curr Drug Targets. 2000;1:387–399. doi: 10.2174/1389450003349056. [DOI] [PubMed] [Google Scholar]
- 65.Maxwell P. Salnikow K. HIF-1: an oxygen and metal responsive transcription factor. Cancer Biol Ther. 2004;3:29–35. doi: 10.4161/cbt.3.1.547. [DOI] [PubMed] [Google Scholar]
- 66.McNeill DR. Narayana A. Wong HK. Wilson DM., 3rd. Inhibition of Ape1 nuclease activity by lead, iron, and cadmium. Environ Health Perspect. 2004;112:799–804. doi: 10.1289/ehp.7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McNulty SE. del Rosario R. Cen D. Meyskens FL., Jr Yang S. Comparative expression of NFkappaB proteins in melanocytes of normal skin vs. benign intradermal naevus and human metastatic melanoma biopsies. Pigment Cell Res. 2004;17:173–180. doi: 10.1111/j.1600-0749.2004.00128.x. [DOI] [PubMed] [Google Scholar]
- 68.McNulty SE. Tohidian NB. Meyskens FL., Jr RelA, p50 and inhibitor of kappa B alpha are elevated in human metastatic melanoma cells and respond aberrantly to ultraviolet light B. Pigment Cell Res. 2001;14:456–465. doi: 10.1034/j.1600-0749.2001.140606.x. [DOI] [PubMed] [Google Scholar]
- 69.Melnikova I. Golden J. Apoptosis-targeting therapies. Nat Rev Drug Discov. 2004;3:905–906. doi: 10.1038/nrd1554. [DOI] [PubMed] [Google Scholar]
- 70.Melnikova VO. Ananthaswamy HN. Cellular and molecular events leading to the development of skin cancer. Mutat Res. 2005;571:91–106. doi: 10.1016/j.mrfmmm.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 71.Mendez F. Goldman JD. Bases RE. Abasic sites in DNA of HeLa cells induced by lucanthone. Cancer Invest. 2002;20:983–991. doi: 10.1081/cnv-120005914. [DOI] [PubMed] [Google Scholar]
- 72.Meo SA. Al-Khlaiwi T. Health hazards of welding fumes. Saudi Med J. 2003;24:1176–1182. [PubMed] [Google Scholar]
- 73.Meyskens FL., Jr Buckmeier JA. McNulty SE. Tohidian NB. Activation of nuclear factor-kappa B in human metastatic melanoma cells and the effect of oxidative stress. Clin Cancer Res. 1999;5:1197–1202. [PubMed] [Google Scholar]
- 74.Meyskens FL., Jr Chau HV. Tohidian N. Buckmeier J. Luminol-enhanced chemiluminescent response of human melanocytes and melanoma cells to hydrogen peroxide stress. Pigment Cell Res. 1997;10:184–189. doi: 10.1111/j.1600-0749.1997.tb00482.x. [DOI] [PubMed] [Google Scholar]
- 75.Meyskens FL., Jr Farmer PJ. Anton-Culver H. Etiologic pathogenesis of melanoma: a unifying hypothesis for the missing attributable risk. Clin Cancer Res. 2004;10:2581–2583. doi: 10.1158/1078-0432.ccr-03-0638. [DOI] [PubMed] [Google Scholar]
- 76.Meyskens FL., Jr McNulty SE. Buckmeier JA. Tohidian NB. Spillane TJ. Kahlon RS. Gonzalez RI. Aberrant redox regulation in human metastatic melanoma cells compared to normal melanocytes. Free Radic Biol Med. 2001;31:799–808. doi: 10.1016/s0891-5849(01)00650-5. [DOI] [PubMed] [Google Scholar]
- 77.Minisini AM. Di Loreto C. Mansutti M. Artico D. Pizzolitto S. Piga A. Puglisi F. Topoisomerase IIalpha and APE/ref-1 are associated with pathologic response to primary anthracycline-based chemotherapy for breast cancer. Cancer Lett. 2005;224:133–139. doi: 10.1016/j.canlet.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 78.Mitani H. Koshiishi I. Sumita T. Imanari T. Prevention of the photodamage in the hairless mouse dorsal skin by kojic acid as an iron chelator. Eur J Pharmacol. 2001;411:169–174. doi: 10.1016/s0014-2999(00)00873-6. [DOI] [PubMed] [Google Scholar]
- 79.Nguyen C. Teo JL. Matsuda A. Eguchi M. Chi EY. Henderson WR., Jr Kahn M. Chemogenomic identification of Ref-1/AP-1 as a therapeutic target for asthma. Proc Natl Acad Sci U S A. 2003;100:1169–1173. doi: 10.1073/pnas.0437889100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Noble ME. Endicott JA. Johnson LN. Protein kinase inhibitors: insights into drug design from structure. Science. 2004;303:1800–1805. doi: 10.1126/science.1095920. [DOI] [PubMed] [Google Scholar]
- 81.Nyrén O. McLaughlin JK. Gridley G. Ekbom A. Johnell O. Fraumeni JF., Jr Adami HO. Cancer risk after hip replacement with metal implants: a population-based cohort study in Sweden. J Natl Cancer Inst. 1995;87:28–33. doi: 10.1093/jnci/87.1.28. [DOI] [PubMed] [Google Scholar]
- 82.O'Hara AM. Bhattacharyya A. Mifflin RC. Smith MF. Ryan KA. Scott KG. Naganuma M. Casola A. Izumi T. Mitra S. Ernst PB. Crowe SE. Interleukin-8 induction by Helicobacter pylori in gastric epithelial cells is dependent on apurinic/apyrimidinic endonuclease-1/redox factor-1. J Immunol. 2006;177:7990–7999. doi: 10.4049/jimmunol.177.11.7990. [DOI] [PubMed] [Google Scholar]
- 83.Onega T. Baron J. MacKenzie T. Cancer after total joint arthroplasty: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1532–1537. doi: 10.1158/1055-9965.EPI-06-0127. [DOI] [PubMed] [Google Scholar]
- 84.Palumbo A. d'Ischia M. Misuraca G. Prota G. Schultz TM. Structural modifications in biosynthetic melanins induced by metal ions. Biochim Biophys Acta. 1988;964:193–199. doi: 10.1016/0304-4165(88)90166-3. [DOI] [PubMed] [Google Scholar]
- 85.Picardo M. Grammatico P. Roccella F. Roccella M. Grandinetti M. Del Porto G. Passi S. Imbalance in the antioxidant pool in melanoma cells and normal melanocytes from patients with melanoma. J Invest Dermatol. 1996;107:322–326. doi: 10.1111/1523-1747.ep12363163. [DOI] [PubMed] [Google Scholar]
- 86.Pierson HF. Enhancement of tumorigenicity of B16 melanoma in heterogenetic mice by administration of copper chelates. Cancer Treat Rep. 1985;69:1283–1291. [PubMed] [Google Scholar]
- 87.Pilas B. Sarna T. Kalyanaraman B. Swartz HM. The effect of melanin on iron associated decomposition of hydrogen peroxide. Free Radic Biol Med. 1988;4:285–293. doi: 10.1016/0891-5849(88)90049-4. [DOI] [PubMed] [Google Scholar]
- 88.Pines A. Perrone L. Bivi N. Romanello M. Damante G. Gulisano M. Kelley MR. Quadrifoglio F. Tell G. Activation of APE1/Ref-1 is dependent on reactive oxygen species generated after purinergic receptor stimulation by ATP. Nucleic Acids Res. 2005;33:4379–4394. doi: 10.1093/nar/gki751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Piret JP. Mottet D. Raes M. Michiels C. CoCl2, a chemical inducer of hypoxia-inducible factor-1, and hypoxia reduce apoptotic cell death in hepatoma cell line HepG2. Ann N Y Acad Sci. 2002;973:443–447. doi: 10.1111/j.1749-6632.2002.tb04680.x. [DOI] [PubMed] [Google Scholar]
- 90.Poser I. Bosserhoff AK. Transcription factors involved in development and progression of malignant melanoma. Histol Histopathol. 2004;19:173–188. doi: 10.14670/HH-19.173. [DOI] [PubMed] [Google Scholar]
- 91.Qu J. Liu GH. Huang B. Chen C. Nitric oxide controls nuclear export of APE1/Ref-1 through S-nitrosation of cysteines 93 and 310. Nucleic Acids Res. 2007;35:2522–2532. doi: 10.1093/nar/gkl1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rahmani M. Péron P. Weitzman J. Bakiri L. Lardeux B. Bernuau D. Functional cooperation between JunD and NF-kappaB in rat hepatocytes. Oncogene. 2001;20:5132–5142. doi: 10.1038/sj.onc.1204678. [DOI] [PubMed] [Google Scholar]
- 93.Ralph SJ. An update on malignant melanoma vaccine research: insights into mechanisms for improving the design and potency of melanoma therapeutic vaccines. Am J Clin Dermatol. 2007;8:123–141. doi: 10.2165/00128071-200708030-00001. [DOI] [PubMed] [Google Scholar]
- 94.Reelfs O. Tyrrell RM. Pourzand C. Ultraviolet a radiation-induced immediate iron release is a key modulator of the activation of NF-kappaB in human skin fibroblasts. J Invest Dermatol. 2004;122:1440–1447. doi: 10.1111/j.0022-202X.2004.22620.x. [DOI] [PubMed] [Google Scholar]
- 95.Rezvani HR. Dedieu S. North S. Belloc F. Rossignol R. Letellier T. de Verneuil H. Taïeb A. Mazurier F. Hypoxia-inducible factor-1alpha, a key factor in the keratinocyte response to UVB exposure. J Biol Chem. 2007;282:16413–16422. doi: 10.1074/jbc.M611397200. [DOI] [PubMed] [Google Scholar]
- 96.Rhodes AR. Seki Y. Fitzpatrick TB. Stern RS. Melanosomal alterations in dysplastic melanocytic nevi: a quantitative, ultrastructural investigation. Cancer. 1988;61:358–369. doi: 10.1002/1097-0142(19880115)61:2<358::aid-cncr2820610227>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 97.Ricca A. Biroccio A. Del Bufalo D. Mackay AR. Santoni A. Cippitelli M. bcl-2 Overexpression enhances NF-kappaB activity and induces mmp-9 transcription in human MCF7(ADR) breast-cancer cells. Int J Cancer. 2000;86:188–196. doi: 10.1002/(sici)1097-0215(20000415)86:2<188::aid-ijc7>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 98.Rigel DS. Carucci JA. Malignant melanoma: prevention, early detection, and treatment in the 21st century. CA Cancer J Clin. 2000;50:215–236. doi: 10.3322/canjclin.50.4.215. [DOI] [PubMed] [Google Scholar]
- 99.Rivers JK. Gallagher RP. Public education projects in skin cancer: experience of the Canadian Dermatology Association. Cancer. 1995;75(2 suppl):661–666. doi: 10.1002/1097-0142(19950115)75:2+<661::aid-cncr2820751408>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 100.Rivers JK. Is there more than one road to melanoma? Lancet. 2004;363:728–730. doi: 10.1016/S0140-6736(04)15649-3. [DOI] [PubMed] [Google Scholar]
- 101.Roth M. Black JL. Transcription factors in asthma: are transcription factors a new target for asthma therapy? Curr Drug Targets. 2006;7:589–595. doi: 10.2174/138945006776818638. [DOI] [PubMed] [Google Scholar]
- 102.Sak SC. Harnden P. Johnston CF. Paul AB. Kiltie AE. APE1 and XRCC1 protein expression levels predict cancer-specific survival following radical radiotherapy in bladder cancer. Clin Cancer Res. 2005;11:6205–6211. doi: 10.1158/1078-0432.CCR-05-0045. [DOI] [PubMed] [Google Scholar]
- 103.Sander CS. Chang H. Hamm F. Elsner P. Thiele JJ. Role of oxidative stress and the antioxidant network in cutaneous carcinogenesis. Int J Dermatol. 2004;43:326–335. doi: 10.1111/j.1365-4632.2004.02222.x. [DOI] [PubMed] [Google Scholar]
- 104.Schwabe RF. Bradham CA. Uehara T. Hatano E. Bennett BL. Schoonhoven R. Brenner DA. c-Jun-N-terminal kinase drives cyclin D1 expression and proliferation during liver regeneration. Hepatology. 2003;37:824–832. doi: 10.1053/jhep.2003.50135. [DOI] [PubMed] [Google Scholar]
- 105.Silva M. Heisel C. Schmalzried TP. Metal-on-metal total hip replacement. Clin Orthop Relat Res. 2005;430:53–61. doi: 10.1097/01.blo.0000149995.84350.d7. [DOI] [PubMed] [Google Scholar]
- 106.Snyder RD. Davis GF. Lachmann PJ. Inhibition by metals of x-ray and ultraviolet-induced DNA repair in human cells. Biol Trace Elem Res. 1989;21:389–398. doi: 10.1007/BF02917280. [DOI] [PubMed] [Google Scholar]
- 107.Svobodova A. Walterova D. Vostalova J. Ultraviolet light induced alteration to the skin. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2006;150:25–38. doi: 10.5507/bp.2006.003. [DOI] [PubMed] [Google Scholar]
- 108.Tanner B. Grimme S. Schiffer I. Heimerdinger C. Schmidt M. Dutkowski P. Neubert S. Oesch F. Franzen A. Kölbl H. Fritz G. Kaina B. Hengstler JG. Nuclear expression of apurinic/apyrimidinic endonuclease increases with progression of ovarian carcinomas. Gynecol Oncol. 2004;92:568–577. doi: 10.1016/j.ygyno.2003.10.037. [DOI] [PubMed] [Google Scholar]
- 109.Tell G. Damante G. Caldwell D. Kelley MR. The intracellular localization of APE1/Ref-1: more than a passive phenomenon? Antioxid Redox Signal. 2005;7:367–384. doi: 10.1089/ars.2005.7.367. [DOI] [PubMed] [Google Scholar]
- 110.Thomas NE. Berwick M. Cordeiro-Stone M. Could BRAF mutations in melanocytic lesions arise from DNA damage induced by ultraviolet radiation? J Invest Dermatol. 2006;126:1693–1696. doi: 10.1038/sj.jid.5700458. [DOI] [PubMed] [Google Scholar]
- 111.Toualbi-Abed K. Daniel F. Güller MC. Legrand A. Mauriz JL. Mauviel A. Bernuau D. Jun D cooperates with p65 to activate the proximal kappaB site of the cyclin D1 promoter: role of PI3K/PDK-1. Carcinogenesis. 2008;29:536–543. doi: 10.1093/carcin/bgm293. [DOI] [PubMed] [Google Scholar]
- 112.Tsou TC. Chen CL. Liu TY. Yang JL. Induction of 8-hydroxydeoxyguanosine in DNA by chromium(III) plus hydrogen peroxide and its prevention by scavengers. Carcinogenesis. 1996;17:103–108. doi: 10.1093/carcin/17.1.103. [DOI] [PubMed] [Google Scholar]
- 113.Visuri TI. Pukkala E. Pulkkinen P. Paavolainen P. Cancer incidence and causes of death among total hip replacement patients: a review based on Nordic cohorts with a special emphasis on metal-on-metal bearings. Proc Inst Mech Eng [H] 2006;220:399–407. doi: 10.1243/095441105X63282. [DOI] [PubMed] [Google Scholar]
- 114.Wagner M. Klein CL. Kleinert H. Euchenhofer C. Förstermann U. Kirkpatrick CJ. Heavy metal ion induction of adhesion molecules and cytokines in human endothelial cells: the role of NF-kappaB, I kappaB-alpha and AP-1. Pathobiology. 1997;65:241–252. doi: 10.1159/000164135. [DOI] [PubMed] [Google Scholar]
- 115.Wang D. Luo M. Kelley MR. Human apurinic endonuclease 1 (APE1) expression and prognostic significance in osteosarcoma: enhanced sensitivity of osteosarcoma to DNA damaging agents using silencing RNA APE1 expression inhibition. Mol Cancer Ther. 2004;3:679–686. [PubMed] [Google Scholar]
- 116.Wang D. Zhong ZY. Li MX. Xiang DB. Li ZP. Vector-based Ape1 small interfering RNA enhances the sensitivity of human osteosarcoma cells to endostatin in vivo. Cancer Sci. 2007;98:1993–2001. doi: 10.1111/j.1349-7006.2007.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wei SJ. Botero A. Hirota K. Bradbury CM. Markovina S. Laszlo A. Spitz DR. Goswami PC. Yodoi J. Gius D. Thioredoxin nuclear translocation and interaction with redox factor-1 activates the activator protein-1 transcription factor in response to ionizing radiation. Cancer Res. 2000;60:6688–6695. [PubMed] [Google Scholar]
- 118.Wilson DM., 3rd Barsky D. The major human abasic endonuclease: formation, consequences and repair of abasic lesions in DNA. Mutat Res. 2001;485:283–307. doi: 10.1016/s0921-8777(01)00063-5. [DOI] [PubMed] [Google Scholar]
- 119.Wondrak GT. Let the sun shine in: mechanisms and potential for therapeutics in skin photodamage. Curr Opin Invest Drugs. 2007;8:390–400. [PubMed] [Google Scholar]
- 120.Yang S. Irani K. Heffron SE. Jurnak F. Meyskens FL., Jr Alterations in the expression of the apurinic/apyrimidinic endonuclease-1/redox factor-1 (APE/Ref-1) in human melanoma and identification of the therapeutic potential of resveratrol as an APE/Ref-1 inhibitor. Mol Cancer Ther. 2005;4:1923–1935. doi: 10.1158/1535-7163.MCT-05-0229. [DOI] [PubMed] [Google Scholar]
- 121.Yang S. McNulty S. Meyskens FL., Jr During human melanoma progression AP-1 binding pairs are altered with loss of c-Jun in vitro. Pigment Cell Res. 2004;17:74–83. doi: 10.1046/j.1600-0749.2003.00114.x. [DOI] [PubMed] [Google Scholar]
- 122.Yang S. Misner BJ. Chiu RJ. Meyskens FL., Jr Redox effector factor-1, combined with reactive oxygen species, plays an important role in the transformation of JB6 cells. Carcinogenesis. 2007;28:2382–2390. doi: 10.1093/carcin/bgm128. [DOI] [PubMed] [Google Scholar]
- 123.Yiakouvaki A. Savovië J. Al-Qenaei A. Dowden J. Pourzand C. Caged-iron chelators a novel approach towards protecting skin cells against UVA-induced necrotic cell death. J Invest Dermatol. 2006;126:2287–2295. doi: 10.1038/sj.jid.5700373. [DOI] [PubMed] [Google Scholar]
- 124.Zareba M. Bober A. Korytowski W. Zecca L. Sarna T. The effect of a synthetic neuromelanin on yield of free hydroxyl radicals generated in model systems. Biochim Biophys Acta. 1995;1271:343–348. doi: 10.1016/0925-4439(95)00058-c. [DOI] [PubMed] [Google Scholar]
- 125.Ziel KA. Campbell CC. Wilson GL. Gillespie MN. Ref-1/Ape is critical for formation of the hypoxia-inducible transcriptional complex on the hypoxic response element of the rat pulmonary artery endothelial cell VEGF gene. FASEB J. 2004;18:986–988. doi: 10.1096/fj.03-1160fje. [DOI] [PubMed] [Google Scholar]