Abstract
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of unknown cause that afflicts the central nervous system. MS is typified by a highly clonally restricted antigen-driven antibody response that is confined largely to the central nervous system. The major antigenic targets of this response and the role of antibody in disease pathogenesis remain unclear. To help resolve these issues, we cloned the IgG repertoire directly from active plaque and periplaque regions in MS brain and from B cells recovered from the cerebrospinal fluid of a patient with MS with subacute disease. We found that high-affinity anti-DNA antibodies are a major component of the intrathecal IgG response in the patients with MS that we studied. Furthermore, we show DNA-specific monoclonal antibodies rescued from two subjects with MS as well as a DNA-specific antibody rescued from an individual suffering from systemic lupus erythematosus bound efficiently to the surface of neuronal cells and oligodendrocytes. For two of these antibodies, cell-surface recognition was DNA dependent. Our findings indicate that anti-DNA antibodies may promote important neuropathologic mechanisms in chronic inflammatory disorders, such as MS and systemic lupus erythematosus.
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of the central nervous system (CNS) and the most common disabling neurological disorder in young adults. Its cause is unknown. Evidence collected from epidemiological and experimental studies suggests a complex interaction between environmental (possibly infectious) and genetic factors confers susceptibility to the disease (1). Interplay between these factors may account for the extremely heterogeneous clinical course found in MS (2).
The chief pathological features in MS brain and spinal cord are focal areas of demyelination that manifest as plaques and an inflammatory response consisting of perivascular infiltrates of B cells, T cells, and macrophages within and immediately surrounding the plaque borders (2, 3). To what degree individual components of this inflammatory response promote demyelination and plaque formation is understood poorly. However, substantive evidence collected from histological and molecular studies of MS suggests antibodies may contribute to plaque development (4). First, MS is typified by intrathecal IgG synthesis as evidenced by large quantities of IgG mRNA in brain plaques but not in normal brain white matter (5). Second, B cells are more abundant in acute lesions in which there is ongoing demyelination than in older, inactive lesions (6). Third, there is direct evidence of the induction of antibody-mediated effector mechanisms in MS lesions. IgG deposition around the borders of actively demyelinating MS plaques has been shown to correlate with the presence of activated complement fragments and complexes (7–9).
Elevated IgG production within the CNS and cerebrospinal fluid (CSF) may be visualized via isoelectric focusing as oligoclonal bands in CSF, but not sera, in more than 90% of patients with MS (10). Oligoclonal bands also are encountered routinely in infectious diseases of the CNS, such as subacute sclerosing panencephalitis (SSPE), neurosyphilis, mumps meningitis, progressive rubella panencephalitis, and cryptococcal meningitis. In each of these conditions, a substantial proportion of the antibody response in the CNS is directed against the causative agent (11). For example, in SSPE, as much as 75% of the antibody in CSF and brain extracts is directed against measles virus (12, 13). Extensive efforts directed toward identifying consistent targets, either infectious or otherwise, of the oligoclonal antibody bands in MS have proven fruitless (14).
Detailed studies examining the clonality of B cell populations in CSF and brain plaques in MS have indicated consistently that the IgG response is of restricted diversity and that the B cell populations fuelling the response are compartmentalized within the CNS and not well represented in the peripheral circulation (5, 15–17). IgG sequences recovered from MS plaques and CSF feature extensive somatic mutations, suggesting active, antigen-driven B cell selection and clonal expansion.
Significantly, no single autoantigenic target of either humoral or cellular immunity has been linked directly with the development of MS, and no antibody specificity is diagnostic for disease. The central role of demyelination in MS has engendered the assumption that the antigens driving the autoreactive immune response and subsequent tissue damage would be likely components of myelin or oligodendrocytes. Studies of experimental autoimmune encephalomyelitis (EAE), a nonviral prototypic immunopathology of the CNS, have supported the potential importance of such antigens in triggering autoimmune disease of the CNS (18–20). EAE also has emphasized the role of myelin-reactive T cells in demyelination, although similar levels of these cells have been found not only in patients with MS, but also in healthy individuals (21). Additionally, in patients with MS, the occurrence of autoantibodies reactive with a number of myelin antigens, most notably myelin basic protein (MBP), myelin oligodendrocyte glycoprotein (MOG), and 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNP-1), have been described in serologic and histologic studies (22–26). However, no direct role in MS pathogenesis has been proven for antibody recognizing any of these proteins. It is conceivable that these antibodies may be elicited as a secondary phenomenon, ensuing from myelin damage caused by another pathogenic mechanism. Indeed, elevated levels of anti-MBP and anti-MOG antibodies are found in individuals with various unrelated nondemyelinating neurologic conditions (27).
Defining the dominant specificities of antibodies found in MS plaques and CSF is likely to yield valuable insights into the molecular events underlying this enigmatic condition. To address this problem, we have examined systematically the intrathecal antibody response in MS by cloning IgG produced by B cell populations resident in acute brain lesions and CSF of patients with subacute disease. Our approach has been first to characterize the clonal composition of the antibody repertoire found in situ and then to determine the antigenic targets of the most prevalent clones. In this report, we identify DNA as a major antigenic target of CNS IgG in MS.
Materials and Methods
Clinical Samples.
Acute plaques were identified by B. K. Demasters (University of Colorado Health Science Center) by the presence of cellular infiltrates, axonal pathology, and demyelination from flash-frozen brain obtained at autopsy from a 39-year-old woman (subject 95-2) with confirmed MS. CSF cells were obtained from a 35-year-old woman with subacute MS (subject 99-1). Subject 99-1 experienced blurred vision of the left eye at age 17 that resolved spontaneously. At age 35, she developed a progressive spastic paraparesis and ataxia. MRI scans revealed multiple periventricular lesions in the brain and cervical spinal cord. The CSF IgG was estimated to represent 15% of the total protein (normal 3–13%) and contained four to five oligoclonal bands.
Antibody Cloning and Sequence Analysis.
Total RNA was prepared from either CSF cells or acute plaques. Approximately 200,000 mononuclear cells were recovered from 2 ml of CSF, yielding 1 μg of total RNA. First-strand cDNA was synthesized by using an oligo(dT) primer and avian myeloblastosis virus reverse transcriptase per the manufacturer's instructions (Roche Molecular Biochemicals). Genes encoding antibody heavy chain (Fd) of the IgG1 subclass and κ-chain were PCR amplified from cDNA as described (28) and cloned sequentially into the pComb3H vector, creating a combinatorial recombinant antibody Fab library. Light-chain and heavy-chain variable region sequences, chosen at random from the library, were determined by dideoxy sequencing as described (29). To align antibody sequences to their closest germ-line segment, we used the pcgene database as described (5).
Generation of Recombinant IgGs.
Selected Fabs were converted to whole IgG molecules (mAbs). The light-chain gene and variable gene fragment of the heavy-chain sequence of each clone were inserted into a eukaryotic expression vector containing the human IgG1 constant region gene. The resulting constructs were expressed in Chinese hamster ovary cells as described (30, 31). IgG was purified from culture supernatants over protein A Sepharose (Amersham Pharmacia).
Immunocytochemistry.
Recombinant IgG antibodies were measured for binding to a human oligodendrocyte cell line (kindly provided by I. Lipkin, University of California, Irvine), monkey kidney (Vero or BSC-1) cells, or human T (JJhan) cells. Cells were grown to near confluence on 11 × 22-mm glass coverslips, rinsed in PBS, and fixed in acetone for 1 min. All staining reactions were performed at room temperature. The fixed cells were blocked for 1 h with a 10% (vol/vol) solution of goat serum in PBS and then incubated for an additional 2 h with antibody diluted to 5 μg/ml in 5% (vol/vol) goat serum/PBS. Cells were then washed five times in PBS and incubated for an additional 1 h with either fluorescein isothiocyanate-conjugated or alkaline phosphatase-conjugated goat anti-human IgG polyclonal antibody diluted 1:200 in 5% (vol/vol) goat serum/PBS. The cells were washed five times in PBS, and bound antibody was visualized under a UV light or with New Fuschin substrate (Dako) followed by counterstaining with hematoxylin.
Flow Cytometry.
Cloned antibodies were assessed for their reactivity with the surfaces of living cells. The rat neuroblastoma line ND7 and a human oligodendrocyte line were detached from culture flasks by exposure to 5 mM EDTA in PBS. Detached cells were washed twice in PBS and resuspended in fluorescence-activated cell sorter (FACS) buffer [PBS containing 1% (mass/vol) BSA and 0.02% (mass/vol) sodium azide]. Cells were then incubated for an additional 20 min at 37°C in either PBS/1% (mass/vol) BSA containing 1 mM EDTA or PBS/1% (mass/vol) BSA containing 2 mM MgCl2 and 10 μg/ml DNaseI and then both were washed twice in PBS/1% (mass/vol) BSA. Next, the cells were incubated for 30 min on ice with the appropriate primary antibody at a concentration of 20 μg/ml in FACS buffer and then washed an additional three times in FACS buffer. Cell-surface-bound antibody was detected by incubation on ice for 30 min with a 1:200 dilution of goat anti-human IgG Fab fragment-fluorescein isothiocyanate conjugate (Jackson ImmunoResearch). The cells were washed three times in PBS, resuspended in 1 ml of PBS, and analyzed with a FACScan (Becton Dickinson). Data were plotted by using WINMDI2.8 (http://facs.scripps.edu).
ELISA.
Double-stranded human placental DNA (dsDNA; Sigma), single- stranded oligonucleotide (5′-GAGAGAGAAGGGCCGGCCTGGCCACTAGTTTTGTCACAAGATTT-3′), and RNA derived from calf liver (Sigma), each resuspended in PBS, together with cardiolipin and ganglioside GD2 in 96% (vol/vol) ethanol, were dried at 37°C overnight onto ELISA wells. Tetanus toxoid, Fc fragment of human IgG, BSA, ovalbumin, rat MBP, MOG, proteolipid protein (PLP), CNP-1, and chicken myelin-associated glycoprotein (MAG) were resuspended in PBS and coated overnight at 4°C. All antigens were coated at a level of 50 ng per well. The wells were then washed twice with water and blocked for 1 h at 37°C with 3% (mass/vol) BSA. Primary antibody, diluted to 3 μg/ml in 1% (mass/vol) BSA/PBS, was then added to the antigen-coated ELISA wells for 1 h at 37°C, followed by washing 10 times with PBS/0.05% (vol/vol) Tween 20 (Sigma), with the exception of GD2 and cardiolipin-coated wells that were washed with PBS/1% (mass/vol) BSA. Bound antibody was detected by using an alkaline phosphatase-conjugated goat anti-human IgG F(ab′)2 antibody (Pierce) diluted 1:1,000 in 1% (mass/vol) BSA/PBS, visualized with p-nitrophenol phosphate substrate (Sigma), and monitored at 405 nm. The specificity of the recombinant antibodies was studied further by treating DNA-coated wells with RNase-free DNaseI (Roche Molecular Biochemicals) at a concentration of 500 units/ml for 30 min at 37°C.
Affinity Measurements.
Kinetic constants for the binding of DNA-specific antibodies were measured via surface plasmon resonance by using the BIAcore instrument. dsDNA in the form of (i) a 700-bp DNA fragment that was PCR amplified by using oligonucleotide primers biotinylated at their 5′ termini and (ii) a synthetic oligonucleotide comprised of the sequence 5′-CCC CCC CCT GCG TGG GCG CCC TTT TGG GCG CCC ACG CAG G- 3′, which forms a hairpin structure linked by the TTTT loop, was coupled to a BIAcore sensor chip as described (32).
Results and Discussion
In MS, antibody production within the CNS is a hallmark of disease, but the antigenic targets and relevance of this response to the disease process remain unclear. To investigate the role of antibody in MS further, we independently cloned IgG1κ sequences from a pathologically confirmed active MS brain plaque/periplaque region and from cells collected from the CSF of an individual with subacute disease.
Genes encoding IgG1κ antibody Fab sequences were amplified by PCR by using an established panel of oligonucleotide primers and introduced into a phagemid vector. Our original strategy was to express the cloned antibody libraries on the surface of filamentous phage and then employ a variety of antigenic preparations generated from MS plaques to select antibody clones reacting specifically with MS-related antigens. However, before selection, the composition of the two antibody libraries was determined by examining the DNA sequences of antibody heavy- and light-chain variable regions in a representative number of randomly selected clones (Table 1). We found, in good agreement with other descriptions of the IgG heavy-chain repertoires deposited in brain and CSF of patients with MS (5, 15, 16), that antibody sequence diversity within the two antibody libraries was restricted remarkably. Of the 26 heavy-chain sequences cloned from MS brain (95-2 library), 11 were identical. More strikingly, all 16 of the heavy-chain sequences cloned from CSF (99-1 library) were identical. These findings could be explained by our use of a limited PCR primer set that may have failed to amplify the full complement of VH gene families represented in the tissue samples. However, these same primers have been used extensively to successfully recover highly diverse collections of mAbs from human donors. Moreover, these recombinant antibody panels have been shown to reproduce many of the characteristics of the commensurate in vivo response against both pathogens and autoantigens (29, 33–37). Comparing the dominant antibody sequences we recovered from the patients with MS to the nearest germ-line VH or VL segment revealed that one of the two dominant heavy chains and both of the dominant light chains were heavily somatically mutated, possessing homologies of 90 to 96% to the closest germ-line gene. Overall, these data suggest that, in the two patients with MS that we studied, the intrathecal IgG repertoire is of restricted diversity and that the bulk of the antibody response was driven by a limited antigen set.
Table 1.
Amino acid sequences and genetic characteristics of antibody light-chain and heavy-chain genes amplified from MS donors 95-2 and 99-1
| Antibody library tissue source | Clone designation | HCDR3 | LCDR3 | Frequency of representation in the library | Family | Germ line | Homology to germ line, % |
|---|---|---|---|---|---|---|---|
| Acute brain plaque/periplaque (donor 95-2) | A | GDKTSTDY | 11/26 | VH4 | DP63 | 90.0 | |
| HPHLLDLSWFDP | 2/26 | ||||||
| TRYSSGFHSFYY | 2/26 | ||||||
| CSF (donor 99-1) | B | DGATGSGYWFDH | 16/16 | VH1 | DP8 | 98.2 | |
| Acute brain plaque/periplaque (donor 95-2) | A | QGYGNLFT | 3/16 | VK1 | DPK1 | 96.0 | |
| CSF (donor 99-1) | B | QQYHSYPWT | 12/17 | VK1 | HK102 | 91.9 | |
| QQSFSTPYT | 3/17 |
Boldface indicates predominant HCDR3 and LCDR3.
We next focused on determining the antigenic targets of the prevalent antibody sequences we had amplified. The dominant antibody heavy-chain sequence rescued from each donor with MS was selected and paired with the most prevalent light-chain sequence recovered from the same sample. Thus, as shown in Table 1, antibody library 95-2 clone A heavy chain was paired with clone A light chain, and from antibody library 99-1, clone B heavy chain was partnered with clone B light chain. The resulting two mAbs, MS-A and MS-B, were engineered into whole human IgG1 antibodies, expressed in Chinese hamster ovary cells, and purified.
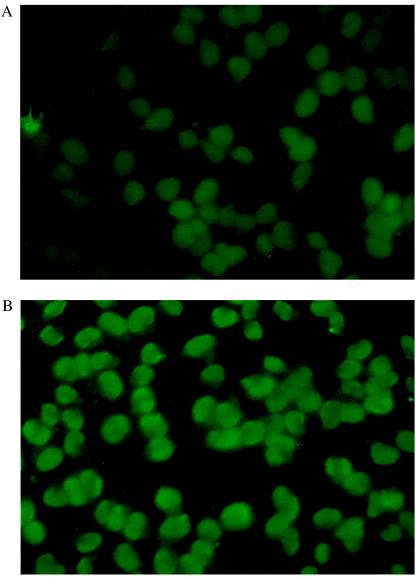
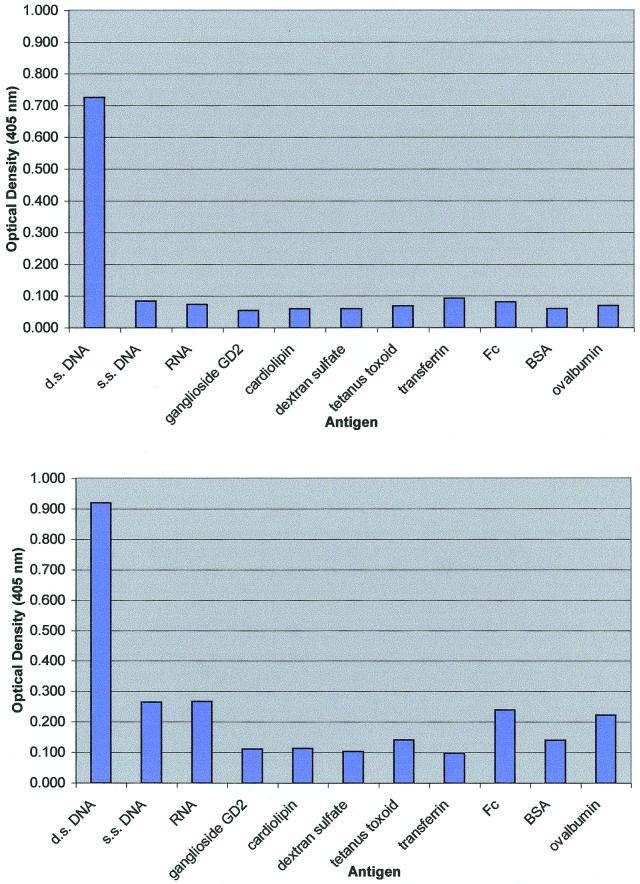
To identify the antigen recognized by each mAb, we probed two λ-phage cDNA expression libraries prepared from active plaque/periplaque regions of different MS brains. Over 2 million clones from each expression library were examined by using mAbs labeled with biotin, but no specific antibody binding was detected. We next determined whether the mAbs were able to recognize antigens present in acetone-fixed monolayers of human oligodendrocyte cells. As illustrated in Fig. 1, both mAbs bound specifically to the nuclei of these cells, suggesting that the antigenic targets of these molecules may be composed of nucleic acid, rather than protein. Subsequently, the antibodies were shown to react strongly and specifically in ELISA with human placental dsDNA, but not with single-stranded DNA, RNA, dextran sulfate, ganglioside GD2, cardiolipin, BSA, ovalbumin, or tetanus toxoid (Fig. 2). The kinetic constants for the binding of the mAbs to dsDNA as a synthetic oligonucleotide or a PCR fragment were then measured by using surface plasmon resonance. As shown in Table 2, both mAbs have affinities for the different presentations of DNA in the range of 1–9 nM.
Figure 1.
Immunofluorescence of human oligodendrocytes with mAbs MS-A (A) and MS-B (B). Bound antibody was detected with a fluorescein isothiocyanate-conjugated goat anti-human IgG.
Figure 2.
ELISA reactivities of purified mAbs MS-A and MS-B against a panel of antigens. s.s., single-stranded.
Table 2.
Kinetic constants for the binding of mAbs MS-A and MS-B to DNA as determined by surface plasmon resonance
| mAb | kon, s−1⋅M−1 | koff, s−1 | Kd, nM |
|---|---|---|---|
| Synthetic oligonucleotide duplex | |||
| MS-A | 1.1 × 105 | 4.0 × 10−4 | 3.6 |
| MS-B | 1.1 × 105 | 1.1 × 10−4 | 1.0 |
| Amplified DNA fragment | |||
| MS-A | 5.8 × 104 | 5.5 × 10−4 | 9.4 |
| MS-B | 6.6 × 104 | 1.9 × 10−4 | 2.9 |
Antibody binding was measured against a short oligonucleotide duplex and against a PCR-amplified DNA fragment of ≈700 bp in length.
Our findings indicate that antibodies binding tightly and specifically to dsDNA were a significant component of intrathecal IgG1 production in the two patients with MS that we investigated. High-affinity antigen-driven DNA-specific IgG responses have long been recognized as a unique serologic hallmark in individuals suffering from systemic lupus erythematosus (SLE), another prevalent chronic inflammatory disorder. In SLE, support for the direct pathogenicity of anti-DNA antibodies derives from the close correlation between disease activity, particularly lupus nephritis, and levels of anti-DNA reactivity in serum, as well as from the ability of anti-DNA antibodies to induce disease in severe combined immunodeficient- and recombination-activating gene-1-deficient mice (38). Intriguingly, an estimated 40–70% of patients with SLE develop CNS involvement, including optic neuritis (39–43). Indeed, the term “lupoid sclerosis” is used to describe a patient presenting with clinical features of both MS and SLE. Furthermore, the incidence of MS and SLE in different members of the same family may indicate shared genetic traits in individuals susceptible to either disease (44, 45).
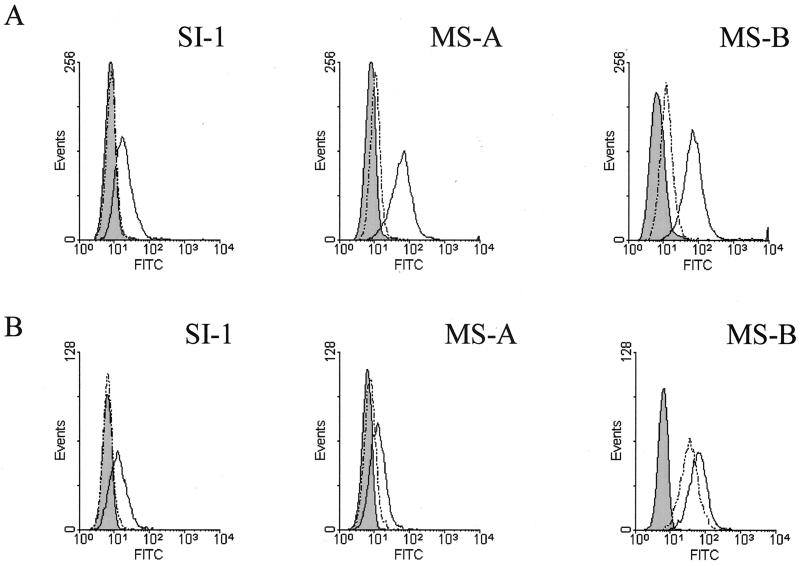
The occurrence of anti-DNA antibodies in MS brain plaques and CSF suggests a possible pathogenic role for these molecules within the CNS. Cell-surface recognition would provide a plausible means by which anti-DNA antibodies could target immune effector functions in vivo against CNS tissues via the activation of complement and by interaction with Fcγ receptors present on local macrophages and microglia. We thus measured the ability of the anti-DNA antibodies we had rescued to bind to the surfaces of neuronal and oligodendrocyte cells in vitro. As illustrated in Fig. 3, the two MS-derived anti-DNA antibodies, together with SI-1, a DNA-specific mAb recovered from an individual suffering from SLE, but not a human antibody (KZ52) recognizing the envelope glycoprotein of Ebola virus, bound specifically to the surface of human oligodendrocyte and rat neuroblastoma cells. To determine whether antibody reactivity with the cell surface was DNA dependent, we pretreated the oligodendrocyte and neuroblastoma cells with DNaseI and again measured antibody binding. We found cell-surface recognition of neuroblastoma cells by each of the antibodies was abolished by DNaseI treatment. In contrast, however, although DNaseI treatment prevented binding of mAbs MS-A and SI-1 with oligodendrocytes, the binding of MS-B with these cells was largely unaffected by DNase treatment. The data indicate that, whereas surface binding of mAbs MS-A and SI-1 to both cell types and MS-B to neuroblastoma cells was DNA dependent, the binding of MS-B to oligodendrocytes seemed to be largely independent of DNA. Thus mAb MS-B, although reacting specifically with dsDNA in ELISA, either may crossreact with a cell-surface antigen devoid of DNA or may recognize surface DNA that is both resistant to enzymatic degradation and inaccessible to the two other mAbs. Our observations also suggest the antigen bound by MS-B may be specific to oligodendrocytes or to human tissues. However, this antibody does not react in ELISA with MBP, MOG, MAG, CNP-1, or PLP (data not shown).
Figure 3.
Reactivity of mAbs MS-A, MS-B, and SI-1 with the surface of rat neuroblastoma (ND-7) (A) and human oligodendrocyte cells (B). Antibody binding to cell populations before and after DNaseI treatment is represented by a solid line (unshaded) and dotted line (unshaded), respectively. Background fluorescence (shaded) was determined in the absence of primary antibody and was in each case equivalent to the fluorescence signal generated when the cells were incubated with mAb KZ52, which is specific for Ebola virus.
Anti-DNA antibodies have been observed previously to bind to the surfaces of certain types of living cells. The antigenic targets of these antibodies have been reported variously as cell surface nucleosomes, as DNA–histone complexes (46–49), or as a series of unidentified membrane proteins with which anti-DNA antibodies crossreact (50). Early reports documented cell membrane-associated DNA as being distinct from the bulk of nuclear DNA, but the function of these molecules remains unknown (51). As described above, our cell-binding studies are supportive of anti-dsDNA antibodies binding effectively to cell-surface components in both a DNA-dependent and -independent manner.
The mechanisms responsible for triggering high-affinity anti-DNA antibody production in susceptible individuals are not known. In both SLE and MS, induction of DNA-specific antibodies may follow the release of large quantities of host DNA from tissues damaged by a primary underlying disease process or infection. However, this argument is at variance with experimental evidence indicating that under normal circumstances, DNA, even in the presence of adjuvant and coupled to a protein carrier, is a poor antigen, typically eliciting no significant antibody production (52). For this reason, high-affinity anti-DNA antibodies are associated closely with the autoimmune state in which the failure of essential immunoregulatory mechanisms, possibly in the form of B cell regulation (53), facilitates the generation of autoantibodies and may precede the development of disease.
Further studies could determine whether the anti-DNA antibodies we have recovered can initiate or exacerbate neurological disease in vivo and may identify molecular mechanisms that might establish common themes between the pathogenesis of MS and SLE. In addition, the frequency and magnitude of the anti-DNA response in MS may be determined by examining the antibody responses in a larger cohort of MS donors. Such studies may prove to be of central importance in establishing how antibodies synthesized in the CNS that specifically recognize DNA or other molecules may, in combination with genetic, environmental, and additional immunological factors, influence the clinical phenotype in MS.
Acknowledgments
We thank Derek Guist, Ann Hessell, and Dawn Slifka for their assistance with this study. This work was supported by a grant from the Donald E. and Delia B. Baxter Foundation (to R.A.W.) and National Institutes of Health Grant NS32623 (to D.H.G.).
Abbreviations
- MS
multiple sclerosis
- CNS
central nervous system
- CSF
cerebrospinal fluid
- SLE
systemic lupus erythematosus
- dsDNA
double-stranded DNA
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.031567598.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.031567598
References
- 1.Kahana E. Biomed Pharmacother. 2000;54:100–102. doi: 10.1016/S0753-3322(00)88859-9. [DOI] [PubMed] [Google Scholar]
- 2.McDonald W I, Ron M A. Philos Trans R Soc London B. 1999;354:1615–1622. doi: 10.1098/rstb.1999.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trapp B D, Peterson J, Ransohoff R M, Rudick R, Mork S, Bo L. N Engl J Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- 4.Archelos J J, Storch M K, Hartung H-P. Ann Neurol. 2000;47:694–706. [PubMed] [Google Scholar]
- 5.Owens G P, Kraus H, Burgoon M P, Smith-Jensen T, Devlin M E, Gilden D H. Ann Neurol. 1998;43:236–243. doi: 10.1002/ana.410430214. [DOI] [PubMed] [Google Scholar]
- 6.Esiri M M. Lancet. 1977;2:478–480. doi: 10.1016/s0140-6736(77)91603-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woyciechowska J L, Brzosko W J. Neurology. 1977;27:620–622. doi: 10.1212/wnl.27.7.620. [DOI] [PubMed] [Google Scholar]
- 8.Lumsden C E. Brain Res. 1971;28:365–390. doi: 10.1016/0006-8993(71)90052-7. [DOI] [PubMed] [Google Scholar]
- 9.Storch M K, Piddlesden S, Haltia M, Iivanainen M, Morgan P, Lassmann H. Ann Neurol. 1998;43:465–471. doi: 10.1002/ana.410430409. [DOI] [PubMed] [Google Scholar]
- 10.Blennow K, Fredman P, Walin A, Gottfries C G, Frey H, Pirttila T, Skoog I, Wikkelso C, Svennerholm L. J Neurol Sci. 1994;121:90–96. doi: 10.1016/0022-510x(94)90161-9. [DOI] [PubMed] [Google Scholar]
- 11.Gilden D H, Devlin M E, Burgoon M P, Owens G P. Mult Scler. 1996;2:179–183. doi: 10.1177/135245859600200403. [DOI] [PubMed] [Google Scholar]
- 12.Vandvik B, Norrby E, Nordal H J, Degre M. Scand J Immunol. 1976;5:979. doi: 10.1111/j.1365-3083.1976.tb03050.x. [DOI] [PubMed] [Google Scholar]
- 13.Mehta P D, Kane A, Thormar H. J Immunol. 1977;118:2254–2261. [PubMed] [Google Scholar]
- 14.Johnson R T. Viral Infections of the Nervous System. Philadelphia: Lippincott–Raven; 1998. [Google Scholar]
- 15.Colombo M, Dono M, Gazzola P, Roncella S, Valetto A, Chiorazzi N, Mancardi G L, Ferrarini M. J Immunol. 2000;164:2782–2789. doi: 10.4049/jimmunol.164.5.2782. [DOI] [PubMed] [Google Scholar]
- 16.Qin Y, Duquette P, Zhang Y, Talbot P, Poole R, Antel J. J Clin Invest. 1998;102:1045–1050. doi: 10.1172/JCI3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baranzini S E, Jeong M C, Butnoi C, Murray R S, Bernard C C A, Oksenberg J R. J Immunol. 1999;163:5133–5144. [PubMed] [Google Scholar]
- 18.'t Hart B A, van Meurs M, Brok H P, Massacesi L, Bauer J, Boon L, Bontrop R E, Laman J D. Immunol Today. 2000;21:290–297. doi: 10.1016/s0167-5699(00)01627-3. [DOI] [PubMed] [Google Scholar]
- 19.Steinman L. Neuron. 1999;24:511–514. doi: 10.1016/s0896-6273(00)81107-1. [DOI] [PubMed] [Google Scholar]
- 20.Compston A, Ebers G, Lassmann H, McDonald I, Matthews B, Wekerle H. McAlpine's Multiple Sclerosis. 3rd Ed. London: Churchill Livingstone; 1998. pp. 409–433. [Google Scholar]
- 21.Martin R, McFarland H F, McFarlin D E. Annu Rev Immunol. 1992;10:153–187. doi: 10.1146/annurev.iy.10.040192.001101. [DOI] [PubMed] [Google Scholar]
- 22.Diaz-Villoslada P, Shih A, Shao L, Genain C P, Hauser S L. J Neuroimmunol. 1999;99:36–43. doi: 10.1016/s0165-5728(99)00099-5. [DOI] [PubMed] [Google Scholar]
- 23.Wucherpfenning K, Catz I, Hausmann S E, Strominger J L, Steinman L, Warren K G. J Clin Invest. 1997;100:1114–1122. doi: 10.1172/JCI119622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raine C S, Cannella B, Hauser S L, Genain C P. Ann Neurol. 1999;46:144–160. doi: 10.1002/1531-8249(199908)46:2<144::aid-ana3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 25.Genain C P, Cannella B, Hauser S L, Raine C S. Nat Med. 1999;5:170–175. doi: 10.1038/5532. [DOI] [PubMed] [Google Scholar]
- 26.Walsh M J, Murray J M. J Clin Invest. 1998;101:1923–1931. doi: 10.1172/JCI1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karni A, Bakimer-Kleiner R, Abramsky O, Ben-Nun A. Arch Neurol. 1999;56:311–315. doi: 10.1001/archneur.56.3.311. [DOI] [PubMed] [Google Scholar]
- 28.Burton D R, Barbas C F, Persson M A A, Koenig S, Chanock R M, Lerner R A. Proc Natl Acad Sci USA. 1991;88:10134–10137. doi: 10.1073/pnas.88.22.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williamson R A, Burioni R, Sanna P P, Partridge L J, Barbas C F, Burton D R. Proc Natl Acad Sci USA. 1993;90:4141–4145. doi: 10.1073/pnas.90.9.4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burton D R, Pyati J, Koduri R, Sharp S J, Thornton G B, Parren P W H I, Sawyer L S W, Hendry R M, Dunlop N, Nara P L, et al. Science. 1994;266:1024–1027. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- 31.Maruyama T, Rodriguez L L, Jahrling P B, Sanchez A, Khan A S, Nichol S T, Peters C J, Parren P W, Burton D R. J Virol. 1999;73:6024–6030. doi: 10.1128/jvi.73.7.6024-6030.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barbas S M, Ditzel H J, Salonen E M, Wei-Ping Y, Silverman G J, Burton D R. Proc Natl Acad Sci USA. 1995;92:2529–2533. doi: 10.1073/pnas.92.7.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barbas C F, Collet T A, Amberg W, Roben P, Binley J M, Hoekstra D, Cababa D, Jones T M, Williamson R A, Pilkington G R, et al. J Mol Biol. 1993;230:812–823. doi: 10.1006/jmbi.1993.1203. [DOI] [PubMed] [Google Scholar]
- 34.Chakrabarti L, Guyader M, Alizon M, Daniel M D, Desrosiers R C, Tiollais P, Sonigo P. Nature (London) 1987;328:543–547. doi: 10.1038/328543a0. [DOI] [PubMed] [Google Scholar]
- 35.Caton A J, Koprowski H. Proc Natl Acad Sci USA. 1990;87:6450–6454. doi: 10.1073/pnas.87.16.6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hexham J M, Partridge L J, Furmaniak J, Petersen V B, Colls J C, Pegg C A S, Smith B R, Burton D R. Autoimmunity. 1994;17:167–179. doi: 10.3109/08916939409010651. [DOI] [PubMed] [Google Scholar]
- 37.Portolano S, Chazenbalk G D, Seto P, Hutchison J S, Rapoport B, McLachlan S M. J Clin Invest. 1992;90:720–726. doi: 10.1172/JCI115943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naparstek Y, Madaio M P. Lupus. 1997;6:307–309. doi: 10.1177/096120339700600318. [DOI] [PubMed] [Google Scholar]
- 39.Crimando J, Hoffman S A. Brain Behav Immun. 1995;9:165–181. doi: 10.1006/brbi.1995.1016. [DOI] [PubMed] [Google Scholar]
- 40.Tola M R, Granieri E, Caniatti L, Paolino E, Monetti C, Dovigo L, Scolozzi R, De Bastiani P, Carreras M. J Neurol. 1992;239:61–64. doi: 10.1007/BF00862972. [DOI] [PubMed] [Google Scholar]
- 41.Moriwaka F, Tashiro K, Fukasawa T, Akino M, Yasuda I, Sagawa A, Hida K. Jpn J Psychiatr Neurol. 1990;44:601–605. doi: 10.1111/j.1440-1819.1990.tb01636.x. [DOI] [PubMed] [Google Scholar]
- 42.Lorcerie B, Marchal G, Borsotti J P, Guard O, Giroud M, Dumas R, Martin F. Rev Med Interne. 1989;10:471–474. doi: 10.1016/s0248-8663(89)80058-x. [DOI] [PubMed] [Google Scholar]
- 43.Kira J, Goto I. J Neurol Neurosurg Psychiatry. 1994;57:1124–1125. doi: 10.1136/jnnp.57.9.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kinnunen E, Juntunen J, Konttinen Y, Kemppinen P, Ketonen L, Kleemola M, Valle M, Koskimies S, Koskenvuo M. Acta Neurol Scand. 1990;81:246–249. doi: 10.1111/j.1600-0404.1990.tb00976.x. [DOI] [PubMed] [Google Scholar]
- 45.McCombe P A, Chalk J B, Pender M P. J Neurol Sci. 1990;97:163–171. doi: 10.1016/0022-510x(90)90215-9. [DOI] [PubMed] [Google Scholar]
- 46.Jacob L, Viard J-P, Allenet B, Anin M F, Slama F B H, Vandekerckhove J, Primo J, Markovitis J, Jacob F, Bach J F, et al. Proc Natl Acad Sci USA. 1989;86:4669–4673. doi: 10.1073/pnas.86.12.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kubota T, Kanai Y, Miyasaka N. Immunol Lett. 1990;23:187–193. doi: 10.1016/0165-2478(90)90190-2. [DOI] [PubMed] [Google Scholar]
- 48.Koutouzov S, Cabrespines A, Amoura Z, Chabre H, Lotton C, Bach J F. Eur J Immunol. 1996;26:472–486. doi: 10.1002/eji.1830260230. [DOI] [PubMed] [Google Scholar]
- 49.Chan T M, Frampton G, Staines N A, Hobby P, Perry G J, Cameron J S. Clin Exp Immunol. 1992;88:68–74. doi: 10.1111/j.1365-2249.1992.tb03041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raz E, Ben-Bassat H, Davidi T, Shlomai Z, Eilat D. Eur J Immunol. 1993;23:383–390. doi: 10.1002/eji.1830230213. [DOI] [PubMed] [Google Scholar]
- 51.Meinke W, Hall M R, Goldstein D A, Kohne D E, Lerner R A. J Mol Biol. 1973;78:43–56. doi: 10.1016/0022-2836(73)90427-0. [DOI] [PubMed] [Google Scholar]
- 52.Madaio M P, Hodder S, Schwartz R S, Stollar B D. J Immunol. 1984;132:872–876. [PubMed] [Google Scholar]
- 53.O'Keefe T L, Williams G T, Batista F D, Neuberger M S. J Exp Med. 1999;189:1307–1313. doi: 10.1084/jem.189.8.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]