Abstract
Catalysis of alternating ROMP with (H2IMes)Cl2Ru=CHPh(OiPr), the second generation Hoveyda-Grubbs catalyst, provided an entirely cyclic alternating polymer. Conditions for the cyclic AROMP were used to prepare a polymer in which one of the repeat units bore a primary alkyl chloride that was used for further elaboration.
Cyclic polymers1 exhibit thermodynamic,1b optical,2 and biophysical3 properties different from those of their linear counterparts. However, the potential applications of cyclic polymers that result from these altered properties have yet to be developed. Particularly intriguing are applications in drug delivery.4
The pharmacokinetics of cyclic polymers favor their use as vehicles for carrying drugs to solid tumors. Presumably because the minimum cross section of a cyclic polymer is larger than that of the corresponding linear polymer, cyclic polymers are filtered more slowly through the pores of the kidney. Relative to similar linear polymers of comparable molecular weights, they have reduced rates of clearance and extended plasma circulation times.5 In addition, and again as a consequence of topology, cyclic polymers exhibit greater tumor accumulations than the related linear polymers; this difference has been attributed to the enhanced permeation and retention (EPR) effect. 6
Cyclic polymers are formed as by-products during the preparation of high molecular weight polymers by intramolecular reactions termed back-biting. In principle, the formation of cyclic polymers can be maximized by long reaction times; however, practical considerations may preclude this as a general strategy.
It is likely that there is much to be learned about the synthesis of cyclic polymers.7,1b Notable recent contributions include strategies based on ring expansions and on “click chemistry.8 The production of cyclic polymers in ROMP experiments has been optimized by the use of custom-synthesized catalysts.9
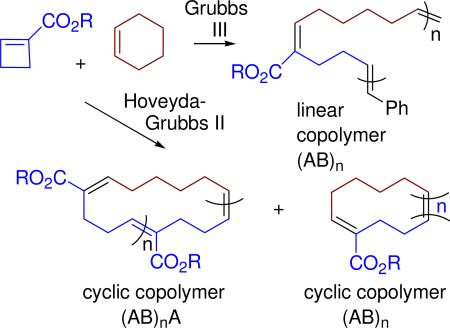
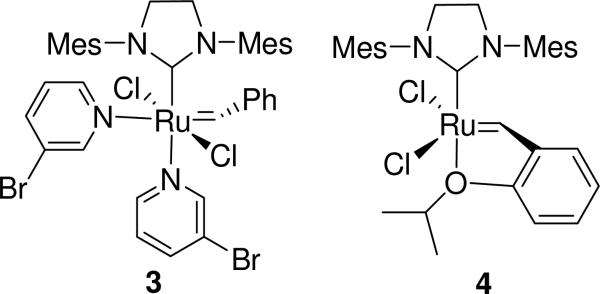
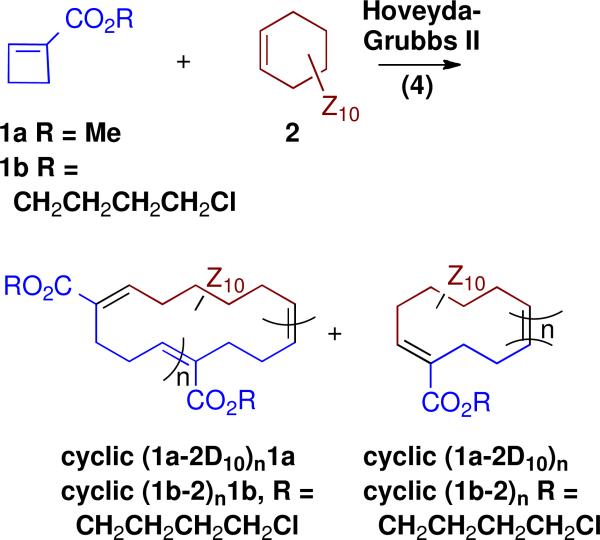
In 2009, we reported the preparation of copolymers of cyclobutene-1-carboxylic acid esters (1, monomer A) and cyclohexenes (2, Z = H or D, monomer B) by alternating ring opening metathesis polymerization (AROMP)10 with the 3rd generation Grubbs catalyst (3) (Figure 1 and Scheme 1).11
Figure 1.
Catalysts used for AROMP and CAROMP.
Scheme 1.
Analysis of the 1H NMR spectrum of the product mixture derived from cyclobutenecarboxylic acid methyl ester (monomer 1a) and cyclohexene D10 (2-D10) led us to conclude that the anticipated alternating linear polymer PhCH=(AB)n=CH2, (1a-2-D10)n was formed, but that it was contaminated with cyclic polymer cyclic (1a-2-D10)n1a in which there was a single AA repeat and cyclic (1a-2-D10)n.
Although we were able to isolate the cyclic AROMP polymers from their linear co-products, this method of preparation was clearly not ideal. Because we recognized the advantages of cyclic polymers for certain applications, we considered modifying the conditions of the AROMP reaction to maximize the back-biting reaction.
Noting the chelated structure of the readily available Hoveyda-Grubbs II catalyst,12,13 we imagined that the transition state for back-biting at the styrene terminus might be stabilized by the ether oxygen-ruthenium interaction (Figure 2). We have therefore tested catalyst 4 for the production of increased amounts of cyclic polymer in the AROMP reaction.
Figure 2.
Proposed stabilization of a conformation disposed to back-biting in Hoveyda-Grubbs II AROMP.
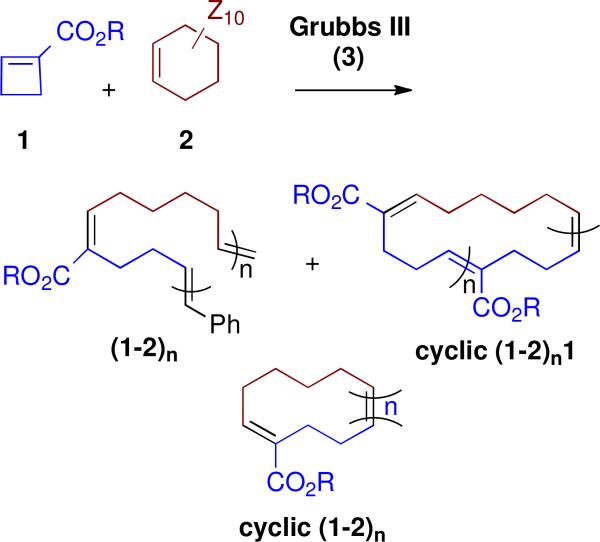
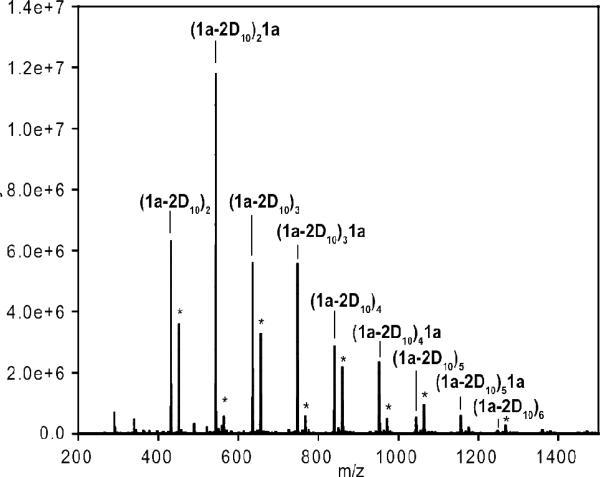
For direct comparison of a Hoveyda-Grubbs II product with the largely linear and easily analyzed Grubbs III AROMP polymer (1a-2-D10)n isolated earlier, we treated a mixture of cyclobutene 1a and cyclohexene D10 (2-D10) with catalyst 4. This reaction provided entirely cyclic copolymer cyclic (1a-2-D10)n1a + cyclic (1a-2-D10)n. (Table S1, Figures S1 and S2). The NMR spectrum showed no styrenyl protons. Furthermore, relative to the three proton methyl ester signal, the signal for the protons on the disubstituted olefin (5.4 ppm) integrated to one and that for the trisubstituted olefinic proton (6.8 ppm) was very small (approximately 0.07 H) These results supported the cyclic alternating polymer hypothesis; the alkene triplet at 6.8 ppm results from formation of an AA repeat, just as in the case of the catalyst 3 polymerization.10 Electrospray mass spectrometry of the cyclic (1a-2-D10)n1a + cyclic (1a-2-D10)n prepared withHoveyda Grubbs II catalyst (4), (Figure 3), showed major peaks for both (AB)n and (AB)nA-type cyclic structures. From the mass spectrum, the number average and weight average molecular weights are 639.54 and 686.09, respectively. The corresponding polydispersity index is 1.07.
Figure 3.
High-resolution LTQ-Orbitrap ESI mass spectroscopic analysis of cyclic (1a-2-D10)1a + cyclic (1a-2-D10)n polymer.1
1MNa+. cyclic (1a-2-D10)2, calcd: 431.3767, found: 431.3778; cyclic (1a-2-D10)21a, calcd: 543.4291, found: 543.4291; cyclic (1a-2-D10)3, calcd: 635.5701, found: 635.5692; cyclic (1a-2-D10)31a, calcd: 747.6226, found: 747.6231; cyclic (1a-2-D10)4, calcd: 839.7636, found: 839.7634; cyclic (1a-2-D10)41a, calcd: 951.8160, found: 951.8163; cyclic (1a-2-D10)5, calcd: 1043.9570, found: 1043.9560; cyclic (1a-2-D10)51a, calcd: 1156.0094, found: 1156.0094; cyclic (1a-2-D10)6, calcd: 1248.1504, found: 1248.1469. *, secondary cross-metathesis products derived from cyclic polymer.
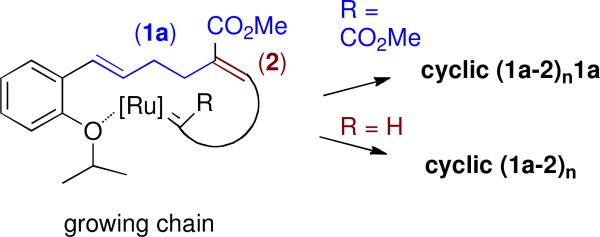
Development of cyclic AROMP polymers for diverse applications is likely to build on experimentation with molecules that bear a variety of functional groups. A library of functionalized cyclic AROMP products could be accessible by ROMPing two libraries of monomers with the Hoveyda-Grubbs II catalyst. Alternatively, a CAROMP library could be obtained by elaboration of polymers derived from a single library of monomers that bear a functional group that can be modified in many ways.
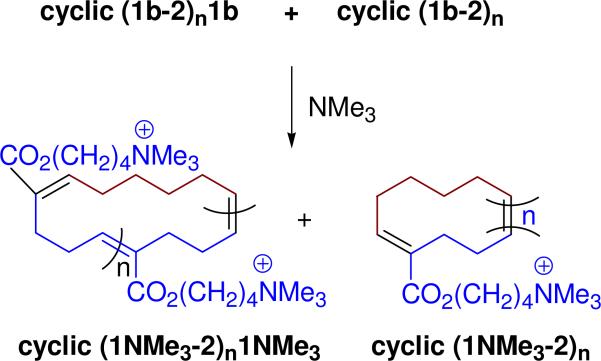
As proof of principle of this latter approach, we have examined the CAROMP of monomer 1b with cyclohexene (2) and demonstrated the efficient alkylation of an amine nucleophile with the resulting chloro-substituted polymer. The Hoveyda-Grubbs II procedure, applied to a solution of these monomers provided a mixture of cyclic (1b-2)n and cyclic (1b-2)n1b that contained no endgroups as indicated by NMR (Table S1 and Figure S3). Its alternating structure, which includes an AA repeat, was confirmed by the the integration of the peaks at 6.8 ppm (trisubstituted olefin protons) and at 5.4 ppm (disubstituted olefin protons) in the 1H-NMR spectrum (Figure S3). From the 1H-NMR spectra, we estimate that the cyclic polymer (some, but not necessarily all of which, contains an AA repeat) is composed of 3-5 AB repeats. This value for n is in good agreement with the number average molecular weight of cyclic (1b-2)n1b + cyclic (1b-2)n determined by gel permeation chromatography using polystyrene as a standard (see SI).
Because we were interested in comparing the physical and biological properties of clean cyclic polymer bearing trimethylammonium substituents with the corresponding polymer derived from the Grubbs III catalyst (mixture of linear and cyclic polymer), we derivatized cyclic (1b-2)n + cyclic (1b-2)n1b by treatment with trimethylamine. This reaction provided the water-soluble polymer cyclic (1NMe3-2)n1NMe3 + cyclic (1NMe3-2)n as indicated by NMR (Figure S4). Thus the CAROMP product from monomer 1b and 2 could be used for the preparation of modified polymers.
In AROMP reactions, the commercially available Hoveyda-Grubbs II catalyst offers easy entry to cyclic polymers uncontaminated by their linear analogs. The use of a monomer that bears a primary alkyl chloride gives polymer that is easily and efficiently modified by nucleophilic substitution.
Expansion of this chemistry should allow cyclic polymers with alternating functionality to be used as templates for the construction of complex macromolecular structures with dense cores. For example, extension of sidechain functional groups with further polymerization chemistry or by attachment of preformed polymers would provide alternating STAR polymers. These novel alternating STAR polymers could be used in a variety of applications that include drug delivery,14 the formation of hydrogels and cell adhesion procedures,15 and electronics.16
Supplementary Material
Scheme 2.
Scheme 3.
Acknowledgment
This research was supported by NIH grants R01GM74776 (K.A.P.), R01HD38519 (N.S.S.), S10RR021008 (N.S.S.), S10RR025072 (Orbitrap), NYSTAR Award (FDPC040076, N.S.S.), and NSF grant CHE0131146 (NMR). We thank Drs. James Marecek and Antonius Köller, Stony Brook University, for their assistance with NMR and mass spectroscopy, respectively.
Footnotes
Supporting Information Available. Detailed descriptions of the experimental procedures and spectra. This material is free of charge via the Internet at http://pubs.acs.org.
References
- 1.a Semlyen JA, editor. Cyclic Polymers. 2nd ed. Kluwer Academic Publishers; Dordrecht: 2000. [Google Scholar]; b Kricheldorf HR. J. Polym. Sci., Part A. 2010;48:251–284. [Google Scholar]
- 2.Han D.i, Tong X, Zhao Y, Galstian T, Zhao Y. Macromolecules. 2010;43:3664–3671. [Google Scholar]
- 3.Chen B, Jerger K, Frechet JMJ, Szoka FC. J. Control. Rel. 2009;140:203–209. doi: 10.1016/j.jconrel.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.a Fox ME, Szoka FC, Frechet JMJ. Acc. Chem. Res. 2009;42:1141–1151. doi: 10.1021/ar900035f. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Grayson SM, Laurent BA, Eugene DM. PMSE Preprints. 2006;95:827–828. [Google Scholar]
- 5.For a rigorous set of experiments, see Nasongkla N, Chen B, Macaraeg N, Fox ME, Frechet JMJ, Szoka FC. J. Am. Chem.. Soc. 2009;131:3842–3843. doi: 10.1021/ja900062u.
- 6.Matsumura Y, Maeda H. Cancer Res. 1986;46:6387–92. [PubMed] [Google Scholar]
- 7.Laurent BA, Grayson SM. Chem. Soc. Rev. 2009;38:2202–2213. doi: 10.1039/b809916m. [DOI] [PubMed] [Google Scholar]
- 8.Laurent BA, Grayson SM. J. Am. Chem. Soc. 2006;128:4238–4239. doi: 10.1021/ja0585836. references therein. [DOI] [PubMed] [Google Scholar]
- 9.a Bielawski CW, Benitez D, Grubbs RH. Science. 2002;297:2041–2044. doi: 10.1126/science.1075401. [DOI] [PubMed] [Google Scholar]; b Bielawski CW, Benitez D, Grubbs RH. J. Am. Chem. Soc. 2003;125:8424–8425. doi: 10.1021/ja034524l. [DOI] [PubMed] [Google Scholar]; c Xia Y, Boydston AJ, Yao Y, Kornfield JA, Gorodetskaya IA, Spiess HW, Grubbs RH. J. Am. Chem. Soc. 2009;131:2670–2677. doi: 10.1021/ja808296a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song A, Parker KA, Sampson NS. J Am Chem Soc. 2009;131:3444–3445. doi: 10.1021/ja809661k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Love JA, Morgan JP, Trnka TM, Grubbs RH. Angew. Chem., Int. Ed. 2002;41:4035–4037. doi: 10.1002/1521-3773(20021104)41:21<4035::AID-ANIE4035>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 12.Garber SB, Kingsbury JS, Gray BL, Hoveyda AH. J. Am. Chem. Soc. 2000;122:8168–8179. erratum 2001, 123, 3186. Kingsbury JS, Harrity JPA, Bonitatebus PJ, Jr., Hoveyda AH. J. Am. Chem. Soc. 1999;121:791–799. See also
- 13.Clark PG, Guidry EN, Chan WY, Steinmetz WE, Grubbs RH. J. Am. Chem. Soc. 2010;132:3405–3412. doi: 10.1021/ja9090337. [DOI] [PubMed] [Google Scholar]
- 14.a Qiu LY, Bae YH. Pharm. Res. 2006;23:1–30. doi: 10.1007/s11095-005-9046-2. [DOI] [PubMed] [Google Scholar]; b Wiltshire JT, Qiao GG. Aust. J. Chem. 2007;60:699–705. [Google Scholar]
- 15.a Hou Y, Schoener CA, Regan KR, Munoz-Pinto D, Hahn MS, Grunlan MA. Biomacromolecules. 2010;11:648–656. doi: 10.1021/bm9012293. [DOI] [PMC free article] [PubMed] [Google Scholar]; b West JL. Scaffolding in Tissue Engineering. Taylor & Francis; Boca Raton: 2006. pp. 275–281. [Google Scholar]
- 16.Kanibolotsky AL, Perepichka IF, Skabara PJ. Chem. Soc. Rev. 2010;39:2695–2728. doi: 10.1039/b918154g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.