Abstract
Pituitary adenylate cyclase-activating polypeptide (PACAP) is an evolutionarily well conserved neuropeptide with multiple functions in the nervous, endocrine, and immune systems. PACAP provides neuroprotection from ischemia and toxin exposure, is anti-inflammatory in gastric inflammatory disease and sepsis, controls proliferative signaling pathways involved in neural cell transformation, and modulates glucohomeostasis. PACAP-based, disease-targeted therapeutics might thus be both effective and benign, enhancing homeostatic responses to behavioral, metabolic, oncogenic, and inflammatory stressors. PACAP signal transduction employs synergistic regulation of calcium and cyclic adenosine monophosphate (cAMP), and noncanonical activation of both calcium- and cAMP-dependent processes. Pharmacological activation of PACAP signaling should consequently have highly specific effects even in vivo. Here, a combined cellular biochemical, pharmacologic, transcriptomic, and bioinformatic approach to understanding PACAP signal transduction by identifying PACAP target genes with oligonucleotide- and cDNA-based microarray is described. Calcium- and cAMP-dependent PACAP signaling pathways for regulation of genes encoding proteins required for neuritogenesis, changes in cell morphology, and cell survival have been traced in PC12 cells. Pharmacological experiments have linked gene expression to cell physiological responses in this system, in which gene silencing can also be employed to confirm the functional significance of induction of specific transcripts. Differential transcriptional responses to metabolic, ischemic, and other stressors in wild type compared to PACAP-deficient mice establish in principle which PACAP-responsive transcripts in culture are PACAP-dependent in vivo. Bioinformatic approaches aid in creating a pipeline for identifying neuropeptide-regulated genes, validating their cellular functions, and defining their expression in the context of neuropeptide signaling physiology, required for discovery of new targets for drug action.
Keywords: bioinformatics, gene discovery, microarray, neuropeptide, neuroprotection, PACAP, PC12, pituitary adenylate cyclase-activating polypeptide, signal transduction, stress response
Introduction
Neuropeptides are slow transmitters that mediate adaptive function and long-term transcriptional encoding of experience. In particular, neuropeptide transmitters in both the central and peripheral nervous systems mediate injury, pain, metabolic, and psychogenic stress responses. Neuropeptides are stored in large dense-core vesicles in neuronal and neuroendocrine cells, from which they are preferentially released by high-frequency neuronal firing. Neuropeptides represent “the language of the stressed nervous system.”1 Changes in gene transcription accompanying neuropeptide-dependent adaptive homeostatic responses are of interest to understand how these informational molecules mediate these stress responses, and to discover potential new targets for drug action. The neuropeptide pituitary adenylate cyclase-activating polypeptide (PACAP) is a paradigm for this process.2,3 PACAP signaling mediates peripheral and central responses to metabolic, ischemic, and environmental stress.4 PACAP has an obligate role in adrenomedullary catecholamine release in glucohomeostasis after profound hypoglycemic stress5 and is required for thermoregulation during development.6 PACAP deficiency affects psychomotor behavior in mice.7 PACAP is required for some aspects of circadian phase-shifting upon altered light input during the sleep-wake cycle in rodents.8 More recently, PACAP has been implicated in modulation of growth factor-induced neuronal proliferation dysregulated in medulloblastic cancer,9 in control of immune regulation in repair following neuronal injury,10 and in human brain function relevant to schizoaffective disorders.11 Finally, PACAP is both cardioprotective and neuroprotective during and following ischemia,12–16 and is neuroprotective against developmental neurotoxicants, such as ethanol.17,18
An mRNA expression profiling approach to understanding neuropeptide function assumes that target gene activation not only occurs but is actually required for neuropeptide action. Application of neuropeptides such as PACAP will invariably result in altered gene expression in responsive cells, that is, those expressing the cognate G protein–coupled receptors, such as the PACAP-responsive PAC1, VPAC1, and VPAC2 receptors. Elevation of intracellular cyclic adenosine monophosphate (cAMP), inositol phosphate, diacylglycerol, or calcium are all known to produce altered gene expression, since the protein kinases they activate (protein kinase A [PKA], PKB, and PKC) in turn activate transcription factors (cyclic AMP response element binding [CREB], activator protein-1 [AP-1], and others) whose cis-active response elements are widely distributed across gene promoters in the mammalian genome.19 The key question, however, is how to identify those transcriptional events required for specific neuropeptide actions in vivo. In the case of PACAP, these actions include neuro-and cardioprotection, behavioral adaptation to various stimuli, anti-inflammatory responses, and metabolic homeostasis. Does neuropeptide receptor activation have a unique transcriptional signature in a particular tissue or physiological context, due to a unique rate, intensity, combinatorial action, or cellular localization of elevation of second messengers? Probing the signaling pathways used to induce neuropeptide-dependent genes, and the consequences of their expression in tissue responses to injury or insult, may identify downstream therapeutic targets. PACAP has both neurotransmitter and trophic actions that are mobilized in immunological, neuronal, glial, and microglial responses to disease. These “emergency response” features of PACAP ameliorate tissue damage, and dysregulation of cellular apoptosis and proliferation, caused by a variety of immunological, inflammatory, and oxidative stressors.2 We summarize here the use of DNA microarray expression profiling with correlative pharmacological, biochemical and gene-silencing approaches, to link PACAP activation of specific signaling pathways to distinct cellular outcomes. These include arrest of cellular proliferation, neurite extension, cell growth, cell survival, and upregulation of genes encoding neuroendocrine-specific proteins. We report a detailed time course for PACAP gene induction during PC12 cell differentiation based on microarray analysis, during which distinct phases of PACAP gene regulation can be discerned. The signaling pathways involved in activation of early, intermediate, and late transcripts by PACAP in PC12 cells provide potential clues for how PACAP may act in vivo, for example, in cerebrocortical neuroprotection. Finally, we suggest that PC12 cells in which PAC1 receptor density and isoform expression is systematically manipulated to mimic PAC1 expression in brain versus peripheral tissues may be utilized to identify novel gene targets for PACAP signaling for neuroprotective or immunomodulatory therapeutics development.
Materials and Methods
In Vivo Studies
In experiments examining the transcriptomes of wild-type versus PACAP-deficient quiescent mice, all animals were males 3–6 months old, from a complete backcross of the PACAP−/− allele5 onto the C57BL/6 background. After cervical dislocation, pairs of adrenal glands were quickly excised and immediately frozen on dry ice. Cerebral cortical samples were obtained by placing whole brains in a rodent brain matrix (RBM-2000C, ASI Instruments, Warren, Michigan), cutting through the optic chiasm and the caudal end of the mammillary bodies, dissecting the lateral and dorsal aspects of the resulting coronal slice, and immediately freezing the tissue on dry ice. RNA was extracted from adrenal glands and cerebral cortex using the RNeasy Mini Kit (Qiagen, Valencia, California) according to standard procedures. Linear amplification of RNA was performed (Amino Allyl MessageAmp II aRNA Amplification Kit, Ambion, Austin, Texas) prior to labeling with Cy3 and Cy5 fluorescent dyes (GE Healthcare UK, Chalfont, Buckinghamshire, England), and microarray hybridization. For adrenal gland, a pool of samples from six wild-type animals was generated, fractions of which were hybridized with samples from individual PACAP-deficient mice. For cerebral cortex, a triplicate “pool versus pool” experiment (n = 6 mice per pool) was run twice, the results of which were subsequently combined. Expression data were filtered in mAdb (http://nciarray.nci.nih.gov) by Loess correction and selection for genes that displayed spot intensity above background in at least 66% of arrays per group. Significance of expression was evaluated using statistical analysis of microarray (SAM)20 at a false discovery rate of ~10%. Resulting gene lists were exported to MS Excel for further manipulation.
In Vitro Studies
PC12 cells (PC12-G clonal line21) were cultured to approximately 80% confluency and treated with 100 nM PACAP-38 (Phoenix Pharmaceuticals, Burlingame, California) for either 10 or 30 min, or 1, 3, 6, 9, 12, 24, or 48 h. RNA was harvested from treated cells and their corresponding time-matched untreated control cells using Trizol (Invitrogen, Carlsbad, California) and isolated using the RNeasy kit (Qiagen). In some studies, PC12 cells were stably transfected with a vector expressing bovine PAC1hop under the control of the cytomegalovirus (CMV) promoter, as described previously,22 and this cell line (PC12-G, clone#9; referred to here as PC12_bPAC1hop) was treated with 100 nM PACAP-38 for 6 h, and harvested for microarray and reverse transcriptase polymerase chain reaction (RT-PCR) analysis, as described earlier.
Microarray and Bioinformatic Analysis Methods for PC12 Cell Time-Course Experiments
Microarray was carried out after RNA amplification and labeling by hybridization to oligonucleotide arrays consisting of the Qiagen mouse MV3 oligo set, with oligonucleotide length generally ~70 nucleotides. General methods for data extraction, normalization, and filtration for quality and dye bias have been summarized in detail previously.23,24 Here, data from 40 microarray experiments is presented (n = 3–4 at nine time points, representing two-color array hybridization of time point labeled cRNA versus pooled all-time point and untreated labeled control cRNA). Self–self hybridization controls consisted in pooled PACAP-treated and vehicle-treated RNA extracts combined and then amplified and labeled in parallel with Cy3 or Cy5 prior to hybridization. Data were analyzed in the NCI mAdb microarray databasing system after standard deArray M-value correction, with allowed quality ratio of 0.3. Data were filtered for (1) values present in >65% of all arrays, and (2) expression ratios >2 in >65% of all arrays. Data meeting these criteria at each time point were exported to MS Excel and means calculated for all data. A record with 917 items was obtained (upregulated transcripts across all time points) and collapsed to a record with 277 items (transcripts upregulated at multiple times from 0 to 48 h). One transcript showing a red (Cy5) dye bias (also elevated in self–self hybridization) was discarded. For multiple items with the same Entrez GeneID (different oligonucleotide targets for the same gene—nine genes in this set) the entry with the highest mean values overall was selected. Finally, transcripts showing two fold or greater upregulation only once between 10 min (0.16 h) and 9 h were discarded on the grounds that upregulation at only one of several closely spaced observations suggests a false-positive observation. This criterion was not applied to single instances of upregulation at widely spaced (12, 24, or 48 h) time points. A total of 178 transcripts were upregulated, with 116 representing mRNAs derived from known (named) genes. Parallel analysis using SAM on the same data set confirmed that upregulation of each of these transcripts was statistically significant. These transcripts were clustered into early, intermediate, and late categories based on the temporal location of the highest mean value for each regulated gene, with highest mean value between 0 and 3 h designated as early, between 3 and 12 h as intermediate, and between 12 and 48 h as late.
Results and Discussion
Overview of a Transcriptomic Approach to Pituitary Adenylate Cyclase-Activating Polypeptide Signaling in PC12 Cells and In Vivo
We have used cell culture models for PACAP function to identify PACAP-responsive genes and the signaling pathways that activate them. These can be compared with PACAP-dependent genes regulated in response to physiological stimuli in wild-type but not in PACAP-deficient mice. Comparing the results of pharmacological treatment on inhibition of PACAP-responsive transcription in cell culture and blockade of PACAP-stimulated cell function provides the opportunity to infer the function of PACAP-dependent genes in mediating neuroprotective, anti-inflammatory, and other effects of PACAP in vivo. Cell culture models for PACAP-dependent gene regulation include neurons cultured from rodent cerebellum, hippocampus, and cerebral cortex; cultured bovine chromaffin cells; and the PC12 pheochromocytoma cell line. Here, we focus on results obtained using the PC12 cell line. PC12 cells can be differentiated by treatment with 1–100 nM PACAP-38, as defined by decreased proliferation and neuritogenesis,25 expression of neuron-specific genes including sodium channel, transin, and others, and abrogation of apoptosis associated with cessation of cell proliferation (Ref. 26 and references therein; and see Fig. 1). Microarray analysis of the PC12 cell transcriptome during PACAP-induced differentiation provides a starting point for the identification of PACAP-responsive genes in neuroendocrine cells (Fig. 2). Some of these may also be PACAP-dependent genes in vivo, which can be investigated as potential therapeutic targets in neurodegenerative, ischemic, or inflammatory conditions in which PACAP has been shown to play a neuroprotective role.
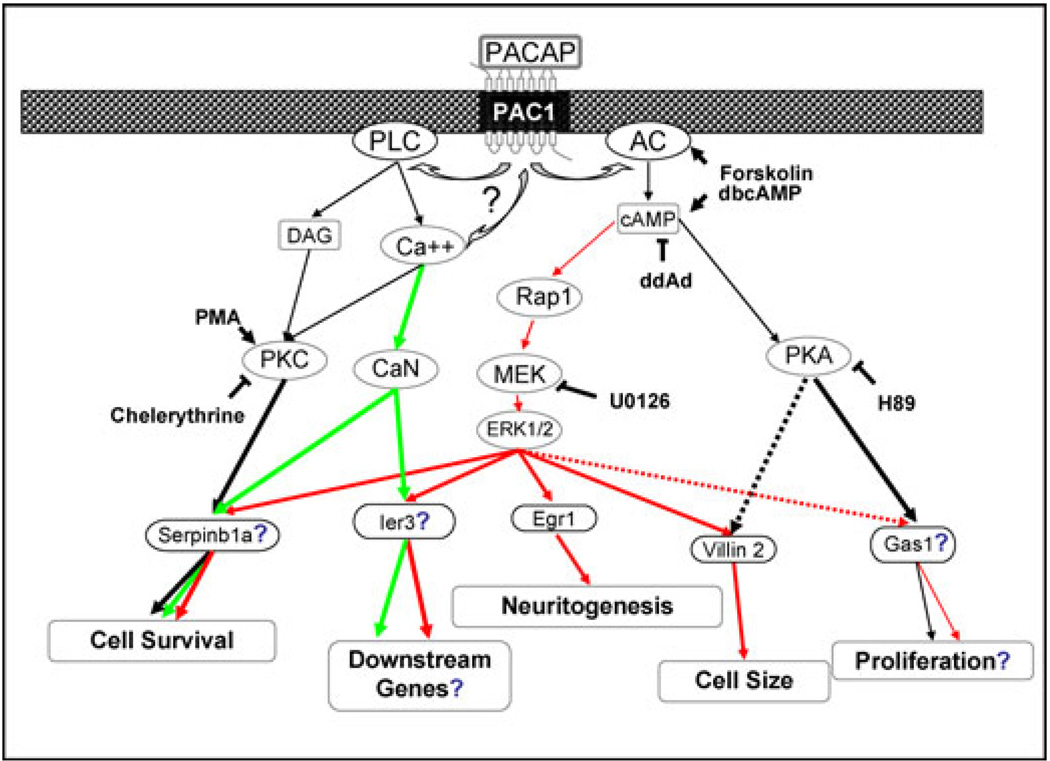
Figure 1.
Establishment of a pituitary adenylate cyclase-activating polypeptide (PACAP) signaling network in PC12 cells mediating multiple aspects of cellular differentiation. Genes induced by PACAP, and the cell readouts survival, neuritogenesis, cell size, and proliferation were measured in the presence and absence of inhibitors of MAP kinase kinase or ERK kinase (MEK), protein kinase C (PKC), calcineurin (CaN), protein kinase A (PKA), adenylate cyclase (AC), and phospholipase C (PLC). Based on microarray capture of PACAP-regulated genes,23,30 correlative pharmacological modulation of gene regulation and cellular outputs,23,34 and siRNA inhibition of cellular responses,34 the pathways and genes regulated by them have been correlated with neuritogenesis, survival, growth arrest, and downstream differentiation in PC12 cells.26 Green arrows indicate calcium-dependent pathways, black arrows indicate PKA- or PKC-dependent pathways, and red arrows indicate extracellular signal-regulated kinase (ERK) -dependent signaling pathways to PACAP-responsive gene activation. Dotted arrows indicate partial contributions to activation. Genes or pathways indicated with ? are hypothesized; those without have been demonstrated using siRNA. Figure summarizes primary data reported previously.23,30,34
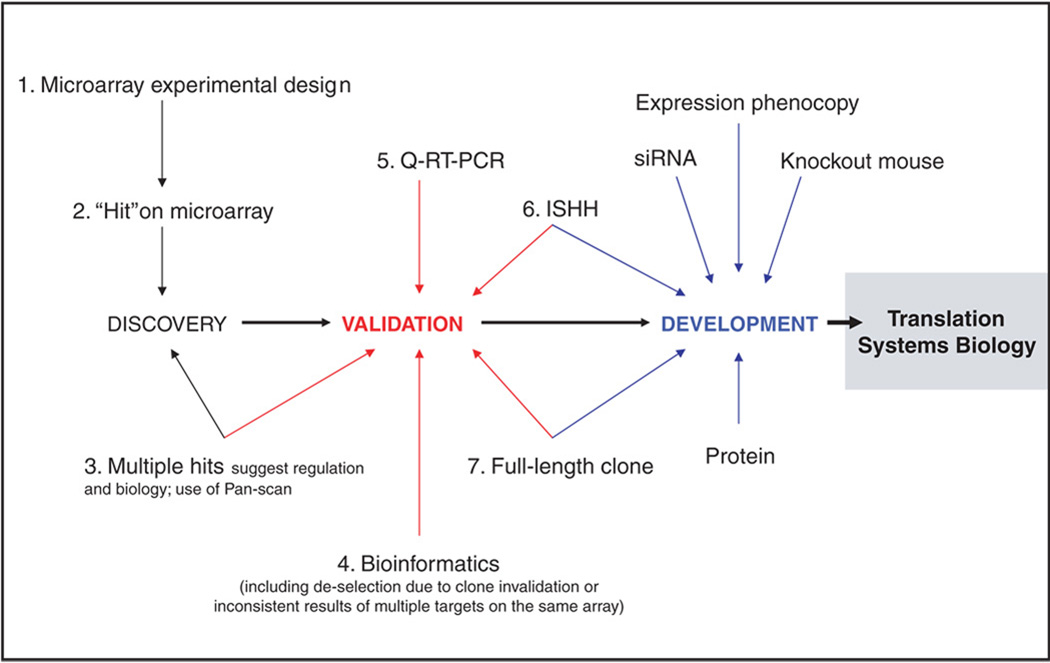
Figure 2.
Microarray target discovery pipeline. Shown are the steps involved in capturing novel transcripts—or known transcripts not previously implicated in specific cellular processes—in cell culture and in vivo, and prioritizing novel genes for further investigation as potential targets for drug development. Abbreviations: ISHH, in situ hybridization histochemistry; Q-RT-PCR, quantitative reverse transcription-polymerase chain reaction.
Microarray analysis is inherently descriptive. However, experimental design can be crucial to final validation of genes and gene products of potential therapeutic interest, and is therefore a critical first step in microarray-based gene discovery. We and others have designed multiple experiments in cell culture to identify transcripts that are PACAP-responsive.23 We have also designed microarray experiments to identify PACAP-dependent transcripts in vivo, that is, under conditions in which endogenous PACAP is required, or exogenous PACAP is effective, for mediating a response to injury.15 Transcripts regulated in both situations likely encode downstream mediators of PACAP action. These may be useful as exogenous pharmacological regulators on their own. They might also be mobilized endogenously if the signaling pathways that induce them can be activated specifically in the whole organism.
Exploring the entire transcriptome space regulated by PACAP in driving cellular processes can be a goal of microarray and post-microarray bioinformatic analysis. Here one asks how the entire transcriptome contributes to a PACAP-induced process such as differentiation. This kind of inquiry is aided by comprehensive identification of PACAP-responsive genes and significantly hampered by underreporting (false-negatives). On the other hand, efficient identification of targets for gene discovery is aided by a low rate of false-positive findings (even at the expense of false-negatives), since target validation can be time-consuming and costly. Therefore step two, obtaining microarray “hits” depends on adjusting the rate of false-positive discovery that represents a tradeoff between systems and discovery approaches. Subsequent steps depicted in Figure 2 emphasize the latter enterprise.
Comparison across multiple studies (meta-analysis) is also a critical part of the downstream process leading to gene discovery (Fig. 2, steps 3 and 4). This is facilitated with a spreadsheet containing results of large numbers of microarray experiments (Pan-scan) with a common theme. A single gene or a batch of genes can be rapidly inspected to help in prioritizing genes for further investigation, based on regulation both in culture and in vivo, that is, by identifying transcripts that are both PACAP-responsive in cell culture and PACAP-dependent in vivo. A critical step in transcript validation is quantitative RT-PCR. While microarray is increasingly accurate as a qualitative tool, the substantial false discovery rate inherent in this high-throughput platform necessitates independent verification before it can be assumed that a given transcript is regulated. This caveat is generalized to virtually all microarray-derived transcriptomic data.27,28 Sometimes overlooked is the importance, for data obtained in vivo, of determining, with in situ hybridization histochemistry, the cell type in which the transcript is expressed. It is also valuable to obtain a full-length clone corresponding to the microarray oligonucleotide hit. This provides a predicted sequence of the protein, the focus of further therapeutics-oriented research, produced by the presumed mRNA identified on microarray.
PC12 Signaling and Cell Processes: The Transitional Transcriptome as a Key to Neuropeptide Actions
Microarray experiments to identify the genes required to program the cellular processes depicted in Figure 1 are actually designed to inventory the transcriptional events required for transition from state A, the undifferentiated cell, to state B, the fully differentiated PC12 cell following approximately 48 h exposure to PACAP. The transitional transcriptome consists of the transcripts present neither at the beginning nor at the end of the process of differentiation, but whose expression is required for differentiation to take place. Initial studies examined the transcriptome of the PACAP-treated PC12 cell after 48 h exposure to PACAP, when differentiation was essentially complete,29 and after 6 hours of PACAP treatment, the minimal time of exposure to PACAP resulting in a full neuritogenic response at 48 h.23 Treatment with cycloheximide at 6 h following PACAP treatment was used to identify, through superinduction, genes transiently expressed during this time period.30 About 170 PACAP-responsive transcripts were identified through these approaches (see Supplementary Table 3 in Samal et al.24). Here, we performed a detailed time course from 10 min after PACAP exposure, to 48 h, in order to gain additional insight into the cascade of transcriptional regulation that enables the several aspects of differentiation initiated by PACAP signaling to unfold.
Temporal Clustering of Gene Regulation during Pituitary Adenylate Cyclase-Activating Polypeptide-Induced PC12 Cell Differentiation
The temporal clustering of the expression profile obtained at 0.16, 0.5, 1, 3, 6, 9, 12, 24, and 48 h after exposure to PACAP is shown in Figure 3. In general, clustering provides two categories of information about gene regulation in a biological process. One is that, empirically, “gene expression clusters tend to be significantly enriched for specific functional categories, which may be used to infer a functional role for unknown genes in the same cluster.”31 A second kind of information that can be derived from clustering in temporally dependent processes is the possibility to identify cis-regulatory elements in promoters of similarly regulated genes.32 In this case, both functions of clustering are relevant. The functional roles available to proteins encoded by PACAP-induced genes depend on the temporal order in which they are expressed. For example, transcripts expressed following induction of PACAP-responsive immediate-early genes (IEGs) may be targets of IEG proteins with activity as transcriptional activators.
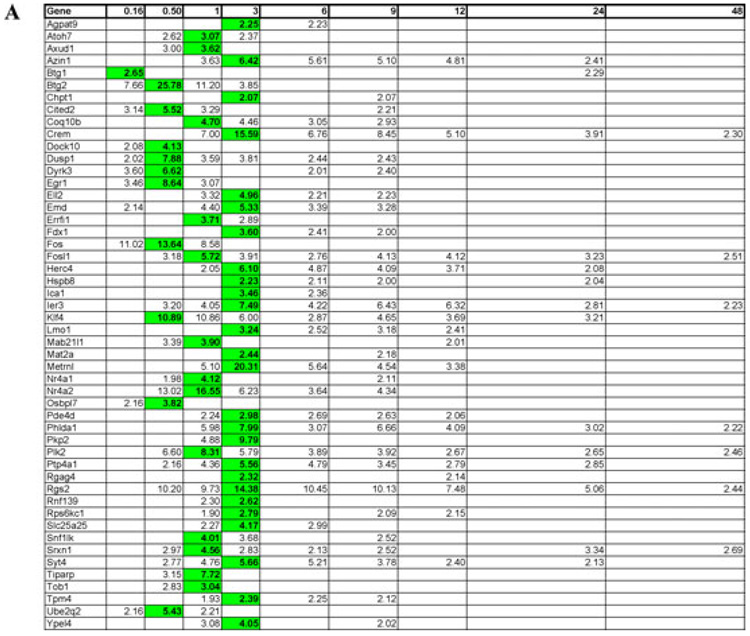
Figure 3.
Time course of induction of pituitary adenylate cyclase-activating polypeptide (PACAP) -responsive transcripts in PC12 cells. Mean upregulation of various mRNA transcripts at 0.16, 0.5, 1, 3, 6, 9, 12, 24, and 48 h after addition of 100 nM PACAP-38. (A) Early regulated transcripts (maximal induction between 0.16–3 h). (B) Intermediate regulated transcripts (maximal induction 6–12 h). (C) Late regulated transcripts (maximal induction 24–48 h). Transcript induction was calculated as described in Materials and Methods. Values shown are mean induction (n = 3–4) compared to control (untreated) PC12 cells pooled from all time points. Mean values for time points at which induction was >two fold in >65% of arrays are shown, with highest mean value attained across the time course (0–48 h) in bold. Further information on genes shown here and their encoded proteins can be accessed on http://www.ncbi.nlm.nih.gov/sites/entrez using the gene symbols listed.
Figure 3A–3C shows the clustering of 116 named transcripts (i.e., transcripts from identified genes) upregulated two fold or more, at various time points after exposure of PC12 cells in culture to 100 nM PACAP-38, into early (0.16–3 h, Fig. 3A), intermediate (6–12 h, Fig. 3B), and late (24–48 h, Fig. 3C) expressed transcripts. Those upregulated transiently between 10 min and 3 h (early transcripts) are likely to contribute to the phenotype of the final differentiated state, even if their cognate proteins are no longer expressed in differentiated PC12 cells. Their roles during the differentiation process could include protection from apoptosis after growth arrest; induction of further downstream but still transitional gene expression; or control of gene expression of the fully differentiated phenotype, including sodium channels, voltage-gated calcium channels, and secretory proteins.29,33
Many of the transcripts associated with the early phase of PACAP treatment have been previously identified in other studies, including Egr1, Ier3, Fos, Fosl1, and Klf4. A role for Egr1 in PACAP-induced neuritogenesis has been established.34 Egr1 transcription is activated by a variety of stimuli in PC12 cells including elevated potassium.35,36 However, continuous overexpression of Egr1 in PC12 cells does not dramatically upregulate many transcripts,37 suggesting that transient or pulsed expression of Egr1 may be required for differentiation. Egr1 is in fact very dynamically regulated by PACAP, and PACAP-induced neuritogenesis is blocked by Egr1 silencing in PC12 cells.34 This suggests that the unique cAMP-dependent, PKA-independent signaling pathway through which PACAP activates Egr134 may reveal intermediate PACAP-dependent genes that are dependent on Egr1 and whose expression is as tightly regulated as that of Egr1 itself in PACAP-mediated processes, including differentiation. With respect to control of apoptosis, it is noteworthy that at least one protein, Ier3 (aka PRG-1, PACAP-regulated gene-1,38) has been reported either to promote or inhibit apoptotic cell death, dependent on cellular context.39–41 Ier3 may function to avert apoptosis during transition from a proliferative to a growth-arrested phase during differentiation, or alternatively may act within the nucleus to control transcription of genes further downstream in differentiation (Fig. 1). Transcripts shown here to be early and robustly upregulated by PACAP include those for proteins associated with transcription (Nr4a2), cellular adhesion (Pkp2, plakophilin 2), periplasmalemmal guanosine triphosphate (GTP) –binding/signaling (Dock10), and adenosine diphosphate (ADP) ribosylation (Tiparp). Thus, multiple modes of signaling appear to be initiated early in PACAP-induced differentiation, and attempts to assign roles in neuritogenesis, abrogation of apoptosis, neuroendocrine-specific protein induction, and cell growth to these proteins is probably merited on the grounds of their early and substantial induction.
The intermediate phase (3–12 h) of PACAP differentiation features fewer transcripts encoding transcriptional regulatory proteins, but rather a complement of induced proteins whose activities are highly variegated, suggesting that key transcription factors induced in the early phase have begun to act on cohorts of receptive downstream genes. SiRNA analysis concentrating on these early proteins, and their consequent differential effects on intermediate and late gene expression, will be informative in linking preexisting signaling pathways required for early gene induction to cellular function. The upregulation of Mapkapk2 and phosphodiesterase 10a in this phase also implies that the signaling pathways capable of conveying PACAP instructions for differentiation to the nucleus are themselves altered during differentiation. The signaling components present in or absent from PACAP-responsive cells in different tissues are thus a critical consideration in applying information obtained from cell culture to signaling in vivo, especially at a systems biological level (see Fig. 2). A striking example is the difference in PACAP signaling dependent on cAMP in cerebellar granule cells and PC12 cells required for survival and differentiation, respectively. Granule cell signaling initiated by PACAP is largely canonical (cAMP- and PKA-dependent), while PC12 cell signaling initiated by PACAP is both canonical and noncanonical (see Science Signaling, http://stke.sciencemag.org/cgi/cm/stkecm; CMP_8038, and http://stke.sciencemag.org/ cgi/cm/stkecm;CMP_11486 and references therein).
Late-phase transcripts induced by PACAP include a number of neuroendocrine-specific genes, such as Kcna2 and Snap25, as predicted for end-stage differentiation. It is noteworthy that fully one-third of the transcripts maximally upregulated at 24 h have returned to baseline expression at 48 h. This demonstrates that the 24-h transcriptome is still in part transitional. The functions of these late-appearing, as well as early transitional, transcripts will need to be unraveled by examining the consequences of silencing these genes on subsequent final differentiation.
Pituitary Adenylate Cyclase-Activating Polypeptide-Dependent Gene Regulation in Adrenal Gland and Central Nervous System In Vivo in Comparison to PC12 Cells
Microarray analysis of the PACAP-deficient cerebrocortical and adrenal gland transcriptomes supports the notion that PACAP is an emergency-response peptide in these two tissues rather than determining the essential characteristics of these parenchymal tissues in the unstressed animal. Using the experimental design described earlier, we found only a relatively small number of transcripts whose expression differed between wild-type and PACAP-deficient animals in cerebral cortex or adrenal gland, and of those transcripts none appeared highly differentially expressed. A grossly dysregulated basal transcriptome in PACAP-deficient mice could complicate the analysis of effects obtained upon physiological stimulation, for example, during hypoglycemia or ischemia. Thus, these results support the validity of the PACAP knockout mouse model in studies involving both central and peripheral stress paradigms, especially in the context of microarray-based experiments designed for discovery of PACAP-dependent/responsive genes. We have previously reported identification of more than 230 transcripts less abundantly expressed in adrenal gland from PACAP knockout mice compared to homozygous +/+ littermates, using a cDNA microarray platform. 24 Since several of the transcripts previously reported to be less abundant in PACAP-deficient mouse adrenal gland are also upregulated by PACAP in PC12 cells, up-regulation of various transcripts in adrenal gland of nominally quiescent wild-type versus PACAP knockout mice may reflect mild handling-induced stress. Validation of lack of sympathoadrenal activation monitored by induction of highly stress-sensitive transcripts, as well as RT-PCR validation of transcripts identified by microarray analysis can control for this variable, which is key to the systems biological interpretation of these data.
Neuropeptide genes are among those most highly regulated by PACAP in bovine chromaffin cells treated with PACAP.42–46 Neuropeptides, including enkephalin, galanin, VIP, substance P, among others, are also consistently upregulated in the adrenal medulla in vivo following splanchnic nerve stimulation. Thus, it was surprising to note the absence of upregulation of neuropeptides by treatment of PC12 cells with PACAP in the time-course study reported here, as well as studies carried out previously. We hypothesized that because the abundance of PAC1 receptor expression in PC12-G cells is too low to support strong calcium influx or secretion of previously accumulated 3H-norepinephrine,22 it might likewise be insufficient to support calcium influx-dependent PACAP induction of neuropeptide genes. Accordingly, we used microarray analysis to investigate genes induced by treatment with 100 nM PACAP-38 for 6 h in PC12_bPAC1hop cells, in which PACAP elicits calcium influx comparable to that seen in mature chromaffin cells of the adrenal medulla. Here, we expected that PACAP stimulation might lead to induction of transcripts associated with the cellular plasticity seen in vivo after prolonged stimulation of the splanchnic nerve. In addition to upregulation of genes such as Ier3, Rgs2, Odc1, Mapkapk2 and others originally reported to be induced by PACAP-38 in wild type PC12 cells,23 the gene encoding substance P (Tac1) was also upregulated in this cell line. Preliminary studies suggest that increased expression of substance P and other neuropeptides in the adrenal gland following reflex splanchnic nerve stimulation47 is also PACAP-dependent.48
A second gene of interest expressed in PC12 cells following PACAP treatment, Ier3, is also up-regulated in a PACAP-dependent manner in the central nervous system following middle cerebral artery occlusion.15 Ier3 is a potential cell survival factor, blocking the generation of reactive oxygen species (ROS) in a number of cell lines, while promoting apoptosis through a nuclear entry mechanism in others.40 It will be of particular interest to determine whether the noncanonical signaling pathways through cAMP and calcium identified as the mode of regulation of Ier3 transcription in PC12 cells49 also regulate expression of Ier3 and other potentially neuroprotective proteins during stroke.
PACAP canonical signaling is likely to be important in constraining hedgehog signaling involved in medulloblastoma generation in cerebellum,50 while noncanonical signaling initiated by PACAP may be more important in neuroendocrine cells, in other parts of the central nervous system, and perhaps even in neural-immune regulation by PACAP in inflammation. Both pathways could be important in the CNS, since PACAP-induced neuroprotection might involve both PKA-dependent glial51 and PKA-dependent and -independent neuronal34 activation.
Summary: Potential for Drug Discovery in the Microarray Pipeline
PACAP signaling in the central and peripheral nervous systems, as in PC12 cells, can occur through a complex network of signaling pathways to affect neuronal survival, differentiation, and likely ongoing neuronal function, including hippocampal-dependent memory and learning,52,53 and cognitive dysfunction in schizoaffective disorders.11 A pipeline can be created using microarray analysis, as exemplified with PACAP-dependent gene regulation in cell culture and in vivo, relevant for three avenues of investigation: target gene discovery, signaling pathway discovery, and gene class discovery. Target discovery can be considered as identification of PACAP-responsive/PACAP-dependent genes, the proteins they encode, and their cellular functions. In this regard, PACAP acting through the PAC1 receptor has characteristics of a master regulator of genes required for neuroprotection. Ier3 is a candidate gene in this category, and other PACAP-responsive genes induced in the PC12_bPAC1hop cell line may be important in PACAPergic cardio- and neuroprotection in vivo. Among the latter is stanniocalcin, highly upregulated by PACAP in PC12_bPAC1hop cells (Mustafa et al., unpublished observations), and known to protect against calcium cytotoxicity in both cardiomyocytes and neurons.54–57
Pathway discovery is of equal interest because of the distinct signaling mechanisms employed by PACAP in different cell types and tissues (see Fig. 4). If PACAP’s long-term effects involve inducing the expression of specific kinase, phosphatase, and phosphodiesterase isoforms that can redirect signaling to newly accessible gene targets, these signaling components might be modulated therapeutically more easily than other PACAP-responsive gene products. PACAP is a major regulator of at least two calcium-modulatory proteins, stanniocalcin (see earlier in the chapter; Mustafa et al., unpublished observations) and selenoprotein T,58 introducing the possibility that an entire class of calcium-handling genes might be regulated by PACAP in response to ischemic challenge and could be identified on the basis of their regulation by PACAP in ischemic disease.
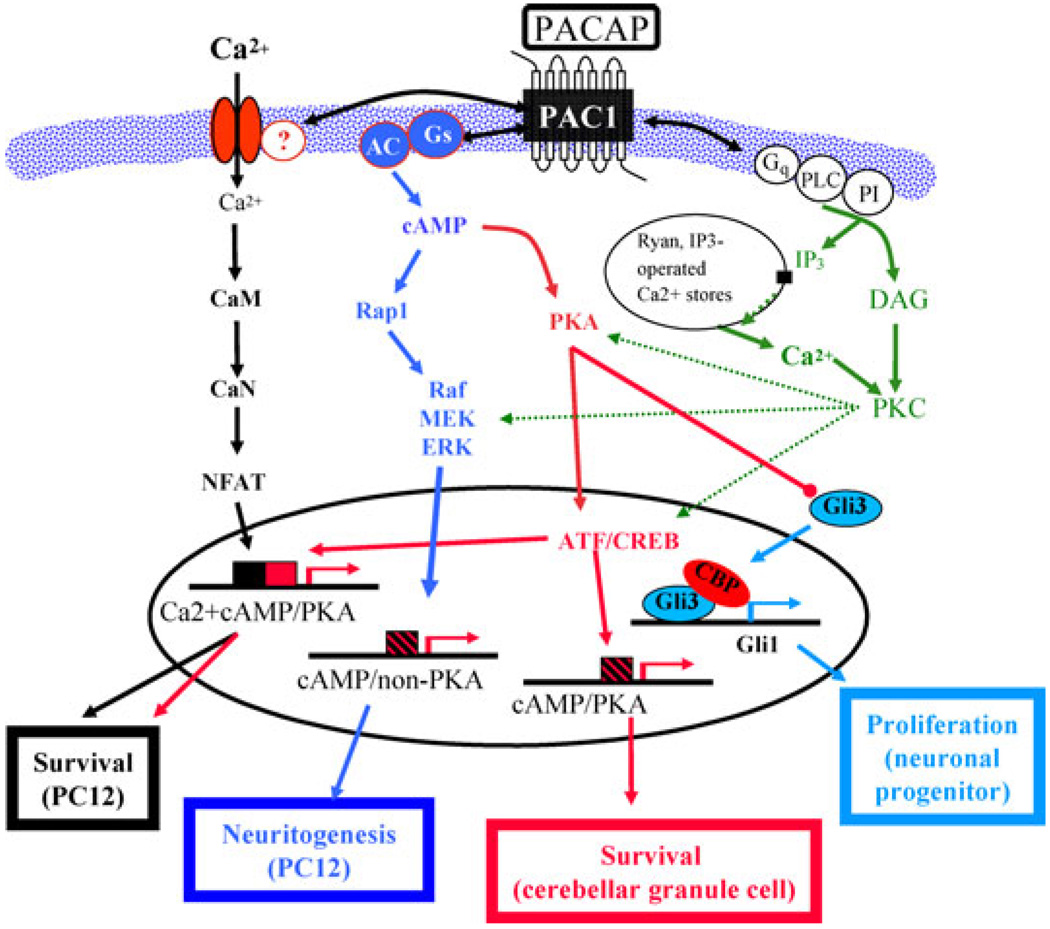
Figure 4.
Implications of PC12 cell microarray/transcriptome analysis for pituitary adenylate cyclase-activating polypeptide (PACAP) actions, and PACAP-activated signaling pathways, in vivo. Depiction of pathways leading to cell survival, neuritogenesis, and proliferation/growth arrest in PC12 cells in vitro and in neurons and neuronal progenitor cells in vitro and in vivo. Only the portion of the sonic hedgehog signaling pathway for cell proliferation through which PACAP modulation is thought to occur50 is shown. Note that both canonical (protein kinase A [PKA] -dependent) and noncanonical (cyclic adenosine monophosphate [cAMP] -dependent, PKA-independent) signaling pathways may exist separately or together, and that either type of calcium-dependent signaling may also coexist with either of these two pathways, depending on cell type. (Adapted from Chen et al.14)
There is significant promise for bridging PACAP gene discovery in easily manipulated cell culture systems such as PC12 cells, and the action of corresponding mechanisms in vivo. Thus, PACAP can modulate dendritic remodeling in hippocampal slices, as well as initiate neuritogenesis in PC12 and other cell types,59 and DISC1 appears to be involved in both PACAP-dependent neuritogenesis in PC12 cells and in hippocampal function and CNS disease.60,61 PAC1-mediated signaling modulates both long-term potentiation in hippocampus and memory in intact animals.52,62
These observations all suggest that microarray-based PACAP-responsive gene discovery in PC12 cells will provide relevant in vivo targets of potential therapeutic importance in maintenance of cognitive function. In addition, the approach described here may also be applicable, with some modification, to identifying other PACAP-responsive genes involved in PACAP’s effects in tumor progression, inflammation, and lymphocyte function reported on by others in this volume.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Hökfelt T, Bartfai T, Bloom F. Neuropeptides: opportunities for drug discovery. Lancet Neurol. 2003;2:463–472. doi: 10.1016/s1474-4422(03)00482-4. [DOI] [PubMed] [Google Scholar]
- 2.Hamelink C, Weihe E, Eiden LE. PACAP: an “emergency response” co-transmitter in the adrenal medulla. In: Vaudry H, Arimura A, editors. Pituitary Adenylate Cyclase-Activating Polypeptide. Norwell, MA: Kluwer-Academic Press; 2003. pp. 227–250. [Google Scholar]
- 3.Sherwood NM, Gray SL, Cummings KJ. Consequences of PACAP gene knockout. In: Vaudry H, Arimura A, editors. Pituitary Adenylate Cyclase-Activating Polypeptide. Norwell, MA: Kluwer Academic Publishers; 2003. pp. 347–360. [Google Scholar]
- 4.Mustafa T, Eiden LE. The secretin super-family: PACAP, VIP and related peptides. In: Lim R, editor. Handbook of Neurochemistry and Molecular Neurobiology: Neuroactive Peptides and Proteins. Vol. XIII. Heidelberg, Germany: Springer; 2006. pp. 1–36. [Google Scholar]
- 5.Hamelink C, et al. Pituitary adenylate cyclase activating polypeptide is a sympathoadrenal neurotransmitter involved in catecholamine regulation and glucohomeostasis. Proc. Natl. Acad. Sci. USA. 2002;99:461–466. doi: 10.1073/pnas.012608999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gray SL, et al. Temperature-sensitive phenotype in mice lacking pituitary adenylate cyclase-activating polypeptide. Endocrinology. 2002;143:3946–3954. doi: 10.1210/en.2002-220401. [DOI] [PubMed] [Google Scholar]
- 7.Hashimoto H, et al. Altered psychomotor behaviors in mice lacking pituitary adenylate cyclase-activating polypeptide (PACAP) Proc. Natl. Acad. Sci. USA. 2001;98:13355–13360. doi: 10.1073/pnas.231094498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colwell CS, et al. Selective deficits in the circadian light response in mice lacking PACAP. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;287:R1194–R1201. doi: 10.1152/ajpregu.00268.2004. [DOI] [PubMed] [Google Scholar]
- 9.Lelievre V, et al. Disruption of the PACAP gene promotes medulloblastoma in ptc1 mutant mice. Dev. Biol. 2008;313:359–370. doi: 10.1016/j.ydbio.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armstrong BD, et al. Impaired nerve regeneration and enhanced neuroinflammatory response in mice lacking pituitary adenylyl cyclase activating peptide. Neuroscience. 2008;151:63–73. doi: 10.1016/j.neuroscience.2007.09.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hashimoto R, et al. Pituitary adenylate cyclase-activating polypeptide is associated with schizophrenia. Mol. Psychiatry. 2007;12:1026–1032. doi: 10.1038/sj.mp.4001982. [DOI] [PubMed] [Google Scholar]
- 12.Reglödi D, et al. Delayed systemic administration of PACAP38 is neuroprotective in transient middle cerebral artery occlusion in the rat. Stroke. 2000;31:1411–1417. doi: 10.1161/01.str.31.6.1411. [DOI] [PubMed] [Google Scholar]
- 13.Sano H, et al. The effect of pituitary adenylate cyclase activating polypeptide on cultured rat cardiocytes as a cardioprotective factor. Regul. Pept. 2002;109:107–113. doi: 10.1016/s0167-0115(02)00193-3. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, et al. Expression profiling of cerebrocortical transcripts during middle cerebral artery occlusion and treatment with pituitary adenylate cyclase-activating polypeptide (PACAP) in the mouse. In: Krieglstein J, Klumpp S, editors. Pharmacology of Cerebral Ischemia. Stuttgart, Germany: Medpharm Scientific Publishers Stuttgart; 2004. pp. 267–277. [Google Scholar]
- 15.Chen Y, et al. Neuroprotection by endogenous and exogenous PACAP following stroke. Regul. Pept. 2006;137:4–19. doi: 10.1016/j.regpep.2006.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohtaki H, et al. Pituitary adenylate cyclase-activating polypeptide (PACAP) decreases ischemic neuronal cell death in association with IL-6. Proc. Natl. Acad. Sci. USA. 2006;103:7488–7493. doi: 10.1073/pnas.0600375103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaudry D, et al. Pituitary adenylate cyclase-activating polypeptide protects rat cerebellar granule neurons against ethanol-induced apoptotic cell death. Proc. Natl. Acad. Sci. USA. 2002;99:6398–6403. doi: 10.1073/pnas.082112699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaudry D, et al. Endogenous PACAP acts as a stress response peptide to protect cerebellar neurons from ethanol or oxidative insult. Peptides. 2005;26:2518–2524. doi: 10.1016/j.peptides.2005.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Impey S, et al. Defining the CREB regulon: a genome-wide analysis of transcription factor regulatory regions. Cell. 2004;119:1041–1054. doi: 10.1016/j.cell.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 20.Zhang S. A comprehensive evaluation of SAM, the SAM R-package and a simple modification to improve its performance. BMC Bioinformatics. 2007;8:230. doi: 10.1186/1471-2105-8-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rausch DM, Iacangelo AL, Eiden LE. Glucocorticoid- and nerve growth factor-induced changes in chromogranin A expression define two different neuronal phenotypes in PC12 cells. Mol. Endocrinol. 1988;2:921–927. doi: 10.1210/mend-2-10-921. [DOI] [PubMed] [Google Scholar]
- 22.Mustafa T, Grimaldi M, Eiden LE. The hop cassette of the PAC1 receptor confers coupling to Ca2+ elevation required for pituitary adenylate cyclase-activating polypeptide-evoked neurosecretion. J. Biol. Chem. 2007;282:8079–8091. doi: 10.1074/jbc.M609638200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaudry D, et al. Analysis of the PC12 cell transcriptome after differentiation with pituitary adenylate cyclase-activating polypeptide (PACAP) J. Neurochem. 2002;83:1272–1284. doi: 10.1046/j.1471-4159.2002.01242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samal B, et al. Meta-analysis of microarray-derived data from PACAP-deficient adrenal gland in vivo and PACAP-treated chromaffin cells identifies distinct classes of PACAP-regulated genes. Peptides. 2007;28:1871–1882. doi: 10.1016/j.peptides.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deutsch PJ, Sun Y. The 38-amino acid form of pituitary adenylate cyclase-activating polypeptide stimulates dual signaling cascades in PC12 cells and promotes neurite outgrowth. J. Biol. Chem. 1992;267:5108–5113. [PubMed] [Google Scholar]
- 26.Ravni A, et al. The neurotrophic effects of PACAP in PC12 cells: control by multiple transduction pathways. J. Neurochem. 2006;98:321–329. doi: 10.1111/j.1471-4159.2006.03884.x. [DOI] [PubMed] [Google Scholar]
- 27.Butte A. The use and analysis of microarray data. Nat. Rev. Drug Discovery. 2002;1:951–960. doi: 10.1038/nrd961. [DOI] [PubMed] [Google Scholar]
- 28.Carmichael ST. Gene expression changes after focal stroke, traumatic brain and spinal cord injuries. Curr. Opin. Neurol. 2003;16:699–704. doi: 10.1097/01.wco.0000102621.38669.77. [DOI] [PubMed] [Google Scholar]
- 29.Grumolato L, et al. Microarray and suppression subtractive hybridization analyses of gene expression in pheochromocytoma cells reveal pleiotropic effects of pituitary adenylate cyclase-activating polypeptide on cell proliferation, survival, and adhesion. Endocrinology. 2003;144:2368–2379. doi: 10.1210/en.2002-0106. [DOI] [PubMed] [Google Scholar]
- 30.Ravni A, et al. Cycloheximide treatment to identify components of the transitional transcriptome in PACAP-induced PC12 cell differentiation. J. Neurochem. 2006;98:1229–1241. doi: 10.1111/j.1471-4159.2006.03962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D’Haeseleer P, et al. How does gene expression clustering work? Nat. Biotechnol. 2005;23:1499–1501. doi: 10.1038/nbt1205-1499. [DOI] [PubMed] [Google Scholar]
- 32.Tavazoie S, et al. Systematic determination of genetic network architecture. Nat. Genet. 1999;22:281–285. doi: 10.1038/10343. [DOI] [PubMed] [Google Scholar]
- 33.Grumolato L, et al. PACAP and NGF regulate common and distinct traits of the sympathoadrenal lineage: effects on electrical properties, gene markers and transcription factors in differentiating PC12 cells. Eur. J. Neurosci. 2003;17:71–82. doi: 10.1046/j.1460-9568.2003.02426.x. [DOI] [PubMed] [Google Scholar]
- 34.Ravni A, et al. A cAMP-dependent, PKA-independent signaling pathway mediating neuritogenesis through Egr1 in PC12 cells. Mol. Pharmacol. 2008;73:1688–1708. doi: 10.1124/mol.107.044792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu W, et al. Expression of depolarization-induced immediate early gene proteins in PC12 cells. J. Neurosci. Res. 2003;72:670–678. doi: 10.1002/jnr.10626. [DOI] [PubMed] [Google Scholar]
- 36.Machado HB, Vician LJ, Herschman HR. The MAPK pathway is required for depolarization-induced “promiscuous” immediate-early gene expression but not for depolarization-restricted immediate-early gene expression in neurons. J. Neurosci. Res. 2008;86:593–602. doi: 10.1002/jnr.21529. [DOI] [PubMed] [Google Scholar]
- 37.James AB, Conway AM, Morris BJ. Genomic profiling of the neuronal target genes of the plasticity-related transcription factor – Zif268. J. Neurochem. 2005;95:796–810. doi: 10.1111/j.1471-4159.2005.03400.x. [DOI] [PubMed] [Google Scholar]
- 38.Schafer H, et al. PRG1: a novel early-response gene transcriptionally induced by pituitary adenylate cyclase activating polypeptide in a pancreatic carcinoma cell line. Cancer Res. 1996;56:2641–2648. [PubMed] [Google Scholar]
- 39.Wu MX. Roles of the stress-induced gene IEX-1 in regulation of cell death and oncogenesis. Apoptosis. 2003;8:11–18. doi: 10.1023/a:1021688600370. [DOI] [PubMed] [Google Scholar]
- 40.Shen L, et al. Distinct domains for anti- and pro-apoptotic activities of IEX-1. J. Biol. Chem. 2006;281:15304–15311. doi: 10.1074/jbc.M600054200. [DOI] [PubMed] [Google Scholar]
- 41.Rocher G, et al. Inhibition of B56-containing protein phosphatase 2As by the early response gene IEX-1 leads to control of Akt activity. J. Biol. Chem. 2007;282:5468–5477. doi: 10.1074/jbc.M609712200. [DOI] [PubMed] [Google Scholar]
- 42.Babinski K, et al. Pituitary adenylate-cyclase activating polypeptide (PACAP) evokes long-lasting secretion and de novo biosynthesis of bovine adrenal medullary neuropeptides. Neuropeptides. 1996;30:572–582. doi: 10.1016/s0143-4179(96)90041-4. [DOI] [PubMed] [Google Scholar]
- 43.Taupenot L, et al. Peptidergic activation of transcription and secretion in chromaffin cells. Cis and trans signaling determinants of pituitary adenylyl cyclase-activating polypeptide (PACAP) J. Clin. Invest. 1998;101:863–876. doi: 10.1172/JCI1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hahm SH, Hsu C-M, Eiden LE. PACAP activates calcium influx-dependent and -independent pathways to couple met-enkephalin secretion and biosynthesis in chromaffin cells. J.Mol. Neurosci. 1998;11:1–15. doi: 10.1385/JMN:11:1:43. [DOI] [PubMed] [Google Scholar]
- 45.Lee H-W, et al. Pituitary adenylate cyclase-activating polypeptide regulation of vasoactive intestinal polypeptide transcription requires Ca2+ influx and activation of the serine/threionine phosphatase calcineurin. J. Neurochem. 1999;73:1769–1772. doi: 10.1046/j.1471-4159.1999.731769.x. [DOI] [PubMed] [Google Scholar]
- 46.Hamelink C, et al. Coincident elevation of cyclic AMP and calcium influx by PACAP-27 synergistically regulates VIP gene transcription through a novel PKA-independent signaling pathway. J. Neurosci. 2002;22:5310–5320. doi: 10.1523/JNEUROSCI.22-13-05310.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fischer-Colbrie R, et al. Transsynaptic regulation of galanin, neurotensin, and substance P in the adrenal medulla: combinatorial control by second-messenger signaling pathways. J. Neurochem. 1992;59:780–783. doi: 10.1111/j.1471-4159.1992.tb09440.x. [DOI] [PubMed] [Google Scholar]
- 48.Stroth N, Hamelink CR, Eiden LE. PACAP-dependent cellular plasticity in the mouse adrenal gland. FASEB J. 2007;21:907.6. [Google Scholar]
- 49.Gerdin MG, Eiden LE. PACAP acts through a cyclic AMP-initiated ERK activation pathway independent of PKA and requiring calcium co-signaling for transcription linked to differentiation in PC12-G cells. FASEB J. 2007;21:A792–A792. [Google Scholar]
- 50.Waschek JA, et al. Hedgehog signaling: new targets for GPCRs coupled to cAMP and protein kinase A. Ann. N.Y. Acad. Sci. 2006;1070:120–128. doi: 10.1196/annals.1317.089. [DOI] [PubMed] [Google Scholar]
- 51.Dasgupta B, Dugan LL, Gutmann DH. The neurofibromatosis 1 gene product neurofibromin regulates pituitary adenylate cyclase-activating polypeptide-mediated signaling in astrocytes. J. Neurosci. 2003;23:8949–8954. doi: 10.1523/JNEUROSCI.23-26-08949.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Otto C, et al. Impairment of mossy fiber long-term potentiation and associative learning in pituitary adenylate cyclase activating polypeptide type I receptor-deficient mice. J. Neurosci. 2001;21:5520–5527. doi: 10.1523/JNEUROSCI.21-15-05520.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matsuyama S, et al. Impaired long-term potentiation in vivo in the dentate gyrus of pituitary adenylate cyclase-activating polypeptide (PACAP) or PACAP type 1 receptor-mutant mice. Neuroreport. 2003;14:2095–2098. doi: 10.1097/00001756-200311140-00017. [DOI] [PubMed] [Google Scholar]
- 54.Zhang K, et al. Stanniocalcin: a molecular guard of neurons during cerebral ischemia. Proc. Natl. Acad. Sci. USA. 2000;97:3637–3642. doi: 10.1073/pnas.070045897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koizumi K, et al. Stanniocalcin 1 prevents cytosolic Ca2+ overload and cell hypercontracture in cardiomyocytes. Circ. J. 2007;71:796–801. doi: 10.1253/circj.71.796. [DOI] [PubMed] [Google Scholar]
- 56.Westberg JA, et al. Hypoxic preconditioning induces elevated expression of stanniocalcin-1 in the heart. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H1766–H1771. doi: 10.1152/ajpheart.00017.2007. [DOI] [PubMed] [Google Scholar]
- 57.Westberg JA, et al. Hypoxic preconditioning induces neuroprotective stanniocalcin-1 in brain via IL-6 signaling. Stroke. 2007;38:1025–1030. doi: 10.1161/01.STR.0000258113.67252.fa. [DOI] [PubMed] [Google Scholar]
- 58.Grumolato L, et al. Selenoprotein T is a PACAP-regulated gene involved in intracellular Ca2 +mobilization and neuroendocrine secretion. FASEB J. 2008;22:1726–1768. doi: 10.1096/fj.06-075820. [DOI] [PubMed] [Google Scholar]
- 59.Henle F, et al. Vasoactive intestinal peptide and PACAP38 control N-methyl-D-aspartic acid-induced dendrite motility by modifying the activities of Rho GTPases and phosphatidylinositol 3-kinases. J. Biol. Chem. 2006;281:24955–24969. doi: 10.1074/jbc.M604114200. [DOI] [PubMed] [Google Scholar]
- 60.Matsuzaki S, Tohyama M. Molecular mechanism of schizophrenia with reference to disrupted-in-schizophrenia 1 (DISC1) Neurochem. Int. 2007;51:165–172. doi: 10.1016/j.neuint.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 61.Hattori T, et al. A novel DISC1-interacting partner DISC1-binding zinc-finger protein: implication in the modulation of DISC1-dependent neurite outgrowth. Mol. Psychiatry. 2007;12:398–407. doi: 10.1038/sj.mp.4001945. [DOI] [PubMed] [Google Scholar]
- 62.Macdonald DS, et al. Modulation of NMDA receptors by pituitary adenylate cyclase activating peptide in CA1 neurons requires G alpha q, protein kinase C, and activation of Src. J. Neurosci. 2005;25:11374–11384. doi: 10.1523/JNEUROSCI.3871-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]