Abstract
Sex differences have been identified in many of the behavioral and physiological effects of cannabinoids. While estrogens has been linked to some of these variations, the effects of estrogen on cannabinoid receptor binding have not been characterized within regions of the brain specifically implicated in stress responsivity and emotional behavior. To examine sex differences, and the role of estradiol, in regulation of the cannabinoid receptor, we compared the binding site density of the cannabinoid receptor within the amygdala, hippocampus and hypothalamus in males, cycling females, ovariectomized (OVX) females and estradiol-treated OVX females (OVX + E). Our data reveal that males and OVX females have higher amounts of hypothalamic and lower amounts of amygdalar cannabinoid receptor binding relative to both cycling females and OVX + E females. Within the hippocampus, ovariectomy resulted in an upregulation of cannabinoid receptor binding. These data provide a putative biochemical mechanism mediating the observed behavioral and physiological sex differences in the effects of cannabinoids, particularly with respect to stress and emotional behavior.
Introduction
The neuronal endocannabinoid system, which was first characterized as the neural system mediating the psychoactive effects of cannabis, is a neuromodulatory system which regulates a wide range of physiological and behavioral functions. The neuronal cannabinoid system is comprised of G-protein coupled receptors (CB1 receptors) and the endogenous arachidonate-derived ligands anandamide (AEA) and 2-arachidonoylglycerol (2-AG).
A number of sex differences have been reported in the behavioral and physiological effects of cannabinoids. For example, CB1 receptor agonists are more potent in females than males with respect to antinociception and reduced locomotor activity, but are more potent in males at stimulating food intake and increasing body weight (Diaz et al., 2009; Rubino et al., 2008; Tseng & Craft, 2001). Females have been found to self-administer cannabinoids at higher rates than males (Fattore et al., 2007). In both rodents and humans, females are more sensitive than males to the anxiogenic effects of cannabinoids (Marco et al., 2006; Williamson and Evans, 2000). Consistent with this, females are more sensitive than males to the adverse effects of escalating THC exposure during adolescence on emotional behavior and stress reactivity in adulthood (Rubino et al., 2008). Completely opposite effects have been seen in sexual behavior such that cannabinoids facilitate female but impair male sexual activity (Gorzalka et al., in press).
Differences in the profile of gonadal hormones in males and females could underlie sex differences in the effects of cannabinoids. In particular, the observed sex differences in potency and sensitivity to CB1 receptor agonists could result if steroid hormones differentially regulate cannabinoid receptor expression or sensitivity to ligands. In support of this possibility, several responses to exogenously administered cannabinoids have been found to be dependent on levels of circulating estrogens (Anaraki et al., 2008; Craft & Leitl, 2007). In addition, the higher rate of cannabinoid self administration in females compared to males is reduced following ovariectomy (Fattore et al., 2007). Furthermore, cannabinoid influences on glutamatergic and GABAergic neuronal circuits are highly susceptible to modulation by estradiol (Nguyen & Wagner, 2006) and a recent report has demonstrated that protein levels of the CB1 receptor are significantly lower in females relative to males (Reich et al., 2009).
With respect to the CB1 receptor, CB1 receptor mRNA transcript amounts fluctuate throughout the estrous cycle and respond to estradiol treatment in ovariectomized females (González, et al., 2000) and the estradiol responsiveness of CB1 receptor binding has been examined at the level of the forebrain (Rodriguez de Fonseca et al., 1994; Bonnin et al., 1993). However, the influences of sex and gonadal hormones on the density of cannabinoid receptors within distinct regions in the limbic system involved in emotionality and cognition has not been examined.
Methods
Subjects
Female and male Sprague-Dawley rats (Charles River, Montreal, Canada) weighing between 300 and 400 g were used in this study. All animals were pair housed in standard plastic maternity bins lined with contact bedding and had unrestricted access to tap water and Purina Rat Chow. The animals were kept in colony rooms with a constant temperature of 21±1°C and on a reverse 12:12h light and dark cycle with lights turned off at 0900h. Following arrival, animals undergoing surgery were allowed to acclimate one week prior to surgery. The females were anesthetized with a combination of 75 mg/kg ketamine hydrochloride and 7mg/kg xylazine (both administered intraperitoneally) and were bilaterally or sham ovariectomized using standard surgical procedures. Females were allowed to recover for 2 weeks before estradiol injections began. All experimental procedures were in accordance with the guidelines of the Canadian Council on Animal Care and the Animal Care Committee of the University of British Columbia.
Treatment and Procedure
Ovariectomized females were injected with either vehicle (peanut oil) or 10 μg of estradiol benzoate (EB; Sigma-Aldrich; St. Louis, MO, USA) in 0.1 ml peanut oil. Injections were performed subcutaneously via 26-gauge ½” stainless steel needles. This dose of estradiol was chosen because it exerts reproducible effects on both gene expression and emotional behavior in rats (Hill et al., 2007; Alves et al., 2000).
Forty-eight hours following EB or vehicle injections, when the behavioral effects of EB are maximal, subjects were decapitated, without the use of an anesthetic. Intact males and females not exposed to surgery or injection were sacrificed at a comparable time point following arrival in the laboratory. For all rats, brains were removed immediately and the amygdala, hippocampus and hypothalamus were rapidly dissected out and frozen on dry ice. Brain sections were stored at -80°C until analysis.
Membrane Preparation and Cannabinoid Receptor Binding Assay
Brain sections were homogenized in 10 volumes of TME buffer (50 mM Tris HCl, pH 7.4; 1 mM EDTA and 3 mM MgCl2). Homogenates were centrifuged at 18,000 × g for 20 min and the resulting pellet, which constituted the membrane fraction, was resuspended in 10 volumes of TME buffer. Protein concentrations were determined by the Bradford method (Bio-Rad, Hercules, CA, USA).
CB1 receptor binding assays were performed using a Multiscreen Filtration System with Durapore 1.2-μM filters (Millipore, Bedford, MA). Incubations (total volume = 0.2 mL) were carried out using TME buffer containing 1 mg/mL bovine serum albumin (TME/BSA). Membranes (10 μg protein per incubate) were added to the wells containing 0.25, 0.5, 1.0 or 2.5 nM 3H-CP 55,940, a cannabinoid CB1/CB2 receptor agonist, which also has some affinity for the CB2 receptor. Ten μM Δ9-tetrahydrocannabinol was used to determine non-specific binding. KD and Bmax values were determined by nonlinear curve fitting to the single site binding equation using GraphPad Prism (San Diego, CA, USA). Given the lack of specificity of CP 55,940 for the receptor, all binding data will be referred to as cannabinoid receptor binding.
Statistics
Data comparing males, cycling females, ovariectomized females (OVX) and estradiol-treated, ovariectomized females (OVX+E) were analyzed using one way analyses of variance and post-hoc analyses were performed with Tukey’s tests. All analyses used p<0.05 as an indication of significance.
Results
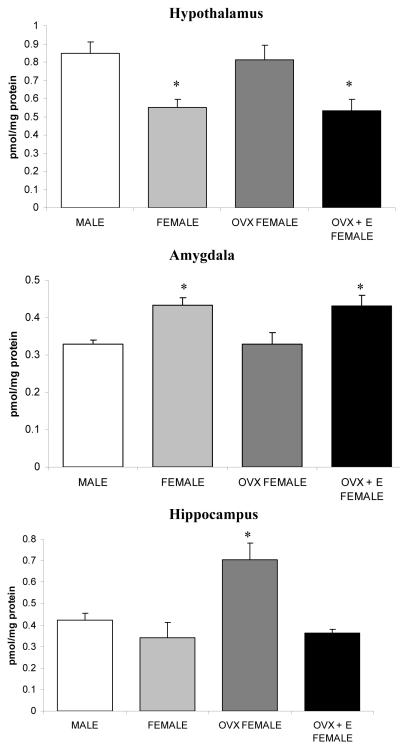
Within the hypothalamus there was a significant effect of treatment on the maximal binding (Bmax) of 3H-CP55940 to the cannabinoid receptor [F (3, 18) = 7.18, p < 0.005: Fig. 1]. Post hoc analysis demonstrated that both males and OVX females exhibited higher levels of cannabinoid receptor binding than either intact females or OVX + E females (all p’s < 0.05 for these comparisons). With respect to binding affinity (Kd) of 3H-CP55940 for the cannabinoid receptor, there was a main effect of treatment [F (3, 18) = 5.37, p < 0.05; Table 1]. Post hoc analysis revealed that both male and OVX female had higher Kd values than the OVX + E females (p < 0.05), but were not different from intact females (p > 0.05).
Figure 1.
Male-female differences in the binding site density of the cannabinoid receptor in limbic forebrain structures: Role of estradiol
The maximal binding site density (Bmax) of the cannabinoid receptor was examined in the hypothalamus, amygdala and hippocampus of males, cycling females (female), ovariectomized females (OVX female) and ovariectomized/estradiol replaced females (OVX + E female). For all treatment conditions, n = 4-5. Data are presented as means +/− SEM. * denotes significant differences (p < .05) relative to unidentified conditions.
Table 1. Male-female differences in the binding affinity of the cannabinoid receptor: Role of estrogen.
Regional differences were found in the effects of sex and estradiol treatment on the binding affinity of the cannabinoid receptor. Within the hippocampus, ovariectomized females (OVX) exhibited a reduced binding affinity (as revealed by an increased concentration of 3H-CP55,940 required to saturate 50% of cannabinoid receptors; Kd) relative to all other groups. In the hypothalamus, males and OVX females exhibited a reduced binding affinity relative to intact females and estrogen-treated OVX females (OVX + E). There were no differences in the Kd of the cannabinoid receptor within the amygdala. For all treatment conditions, n = 4-5. Data are presented as means +/− SEM.
| Binding affinity of 3H-CP55,940 for the cannabinoid receptor (nM) |
|
|---|---|
| Hypothalamus | |
| Male | 1.36 +/− 0.21* |
| Female | 0.92 +/− 0.08 |
| OVX Female | 1.23 +/− 0.12* |
| OVX + E Female | 0.67 +/− 0.07 |
| Amygdala | |
| Male | 0.454 +/− 0.139 |
| Female | 0.680 +/− 0.063 |
| OVX Female | 0.412 +/− 0.032 |
| OVX + E Female | 0.493 +/− 0.129 |
| Hippocampus | |
| Male | 0.424 +/− 0.029 |
| Female | 0.341 +/− 0.071 |
| OVX Female | 0.703 +/− 0.084† |
| OVX + E Female | 0.360 +/− 0.020 |
denotes significant differences (p < .05) relative to OVX + E females
denotes significant differences between OVX females and all other groups.
Within the amygdala, treatment significantly affected the Bmax of 3H-CP55940 binding [F (3, 15) = 6.62, p < 0.01; Fig. 1]. Post hoc analysis revealed that the Bmax of the cannabinoid receptor was significantly lower in both males and OVX females relative to both intact females and OVX + E females (all p’s < 0.05 for these comparisons). There was no effect of treatment on the Kd of 3H-CP55940 binding within the amygdala [F (3, 15) = 1.36, p > 0.05; Table 1].
In the hippocampus, there was an effect of treatment on the Bmax of 3H-CP55940 binding [F (3, 17) = 7.39, p < 0.005: Fig. 1]. Post hoc analysis revealed that Bmax values were significantly higher in hippocampal membranes from OVX females than the other three groups (all p’s < 0.05 for these comparisons). No differences were found among the other three groups. The increase in Bmax in the OVX group was accompanied by a significant effect of treatment on the Kd of the cannabinoid receptor [F (3, 17) = 8.16, p < 0.005: Table 1], with Tukey’s analysis demonstrating that OVX females had a significantly higher Kd for 3H-CP55940 binding than the other three groups (all p’s < 0.05 for these comparisons).
Discussion
Our goal in these studies was to test the global hypothesis that differences in cannabinoid receptor function contribute to sex differences in behavioral and physiological responses to exogenous cannabinoids. We tested the specific hypothesis that estradiol affects the expression and/or binding affinity of the cannabinoid receptor in three brain regions within the limbic circuit. Cannabinoid receptor binding parameters were compared in intact male and female rats; and in female rats following ovariectomy with or without estradiol replacement. We found that cannabinoid receptor binding was affected differentially in the three brain regions examined. In the hypothalamus, cannabinoid receptor binding site density (i.e. Bmax) is lower in females than males, and this difference appears to be estradiol-dependent. In the amygdala, cannabinoid receptor binding site density is higher in females than males, a difference that is also dependent upon the presence of estradiol in the females. In the hippocampus, there was no difference in cannabinoid receptor binding site density between males and females, but OVX females had a significant increase in Bmax compared to both intact females and estradiol-treated OVX females. These data suggest that cannabinoid receptor expression is regulated by estradiol in a brain region-dependent manner. The fact that both increases and decreases in density are observed indicates further that the effect of estradiol is not via a single mechanism.
It is interesting to note that many of the changes in binding site density were accompanied by inverse changes in receptor affinity. It is possible that these changes are mechanistically related. For example, changes in Kd value could be due to desensitization of the receptor; which could be accompanied by or drive changes in receptor binding site density. For example, OVX resulted in an increase in cannabinoid receptor binding site density in the hippocampus, but also resulted in a reduction in the binding affinity of the receptor. Thus, the possibility exists that OVX could result in a desensitization of cannabinoid receptors, which results in a compensatory up-regulation of binding site densities. The relationship between these variables and regulation of cannabinoid receptor expression remains to be determined.
Intact and estradiol-treated OVX females had a higher cannabinoid receptor Bmax in the amygdala compared to males and OVX females, respectively. These data suggest that estradiol exerts a positive effect on the expression of the cannabinoid receptor in the amygdala. These data are consistent with earlier studies demonstrating that ovariectomy decreases cannabinoid receptor binding site density in limbic forebrain (including the amygdala); a change that was reversed with estradiol treatment (Rodríguez de Fonseca et al., 1994). Depending upon the neuronal populations on which the cannabinoid receptors in the amygdalar circuit is modulated, it is also possible that the higher cannabinoid receptor density in the amygdala of females could contribute to the greater sensitivity of females to the anxiogenic effects of the cannabinoids relative to males (Marco et al., 2006; Williamson and Evans, 2000). Future work will seek to determine if this sex difference, and the effects of estradiol, targets a specific neuronal population, such as the GABAergic system, which could account for these effects on anxiety.
On the other hand, cannabinoid receptor binding site density in the hypothalamus is greater in males than intact females. Since OVX females are not different from males and OVX females treated with estradiol are not different from females, these data suggest that circulating estradiol decreases cannabinoid receptor expression in the hypothalamus. Although Rodríguez de Fonseca et al., (1994) also found that females had lower hypothalamic cannabinoid receptor binding site density than males, this difference was insensitive to both estradiol and OVX. It is possible that the difference in findings between that and the present study lies in methodological differences. The earlier study administered a lower dose of estradiol (approximately 0.15 μg/kg vs approximately 30 μg/kg in the present study). The lack of an effect of OVX in that study could have resulted from the use of rats in diestrus as the control; since rats in diestrus already have very low estradiol, OVX might not have produced a large enough change to impact cannabinoid receptor expression. Estradiol has been found to regulate cannabinoid receptor expression on specific neuronal populations within the hypothalamus, such that estradiol appears to decrease cannabinoid receptor function (and possibly expression) at glutamatergic synapses, while increasing its function at GABAergic synapses (Nguyen and Wagner, 2006). Given that endocannabinoid signaling in the hypothalamus is known to regulate neuroendocrine function (Gorzalka et al., in press), these data would suggest that estradiol could exert effects on the ability of cannabinoids to modulate the activation of this neuronal circuit.
In the hippocampus, cannabinoid receptor binding site density did not differ between male and female rats; however, ovariectomized females had significantly higher binding site density that intact females and males. Since the difference between OVX and intact rats was reversed by estradiol replacement, these data suggest that estradiol has an inhibitory effect on cannabinoid receptor density in the hippocampus. Differences in binding between males and OVX females suggest that testosterone also exerts an inhibitory effect on cannabinoid receptor expression in the hippocampus, but further studies are necessary to confirm this.
Our data are in accord with those of Mize & Alper (2000) who reported that estradiol replacement in OVX females decreased CB1 receptor mediated G-protein activation in the hippocampus 2 hr following estradiol administration. Our studies, which were carried out 48 hours after estradiol administration, indicate that estradiol exerts a long-lasting down-regulation of cannabinoid receptor signaling in the hippocampus. Hippocampal CB1 receptors have been linked to memory deficits caused by cannabinoid administration (Wise et al., 2009) and estradiol treatment in OVX rats has been shown to attenuate impaired memory performance caused by cannabinoid treatment (Daniel et al., 2002). Our results indicate that the mechanism behind this phenomenon could be an estradiol-induced down-regulation of cannabinoid receptors in the hippocampus which reduces the amnesic effects of cannabinoids.
We have shown that cannabinoid receptor binding site densities exhibit sex differences and can be modulated by estradiol in several limbic brain regions. These findings may have implications for the sex differences observed with respect to the effects of cannabinoids, particularly the effects of cannabinoids on stress and emotional behavior.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alves SE, McEwen BS, Hayashi S, Korach KS, Pfaff DW, Ogawa S. Estrogen regulated progestin receptors are found in the midbrain raphe but not hippocampus of estrogen receptor alpha (ER alpha) gene-disrupted mice. J. Comp. Neurol. 2000;427:185–195. doi: 10.1002/1096-9861(20001113)427:2<185::aid-cne2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Anakari D, Sianati S, Sadeghi M, Ghasemi M, Javadi P, Mehr S, Dehpour A. Modulation by female sex hormones of the cannabinoid-induced catalepsy and analgesia in ovariectomized rats. Eur. J. Pharmacol. 2008;586:189–196. doi: 10.1016/j.ejphar.2008.02.055. [DOI] [PubMed] [Google Scholar]
- Bonnin A, Fernandez-Ruiz JJ, Martin M, Rodriguez de Fonseca F, Hernandez ML, Ramos JA. delta-9-tetrahydrocannabinol affects mesolimbic dopamineric activity in the female brain: Interaction with estrogens. J. Neural Transm. Gen. Sect. 1993;92:81–95. doi: 10.1007/BF01244868. [DOI] [PubMed] [Google Scholar]
- Craft RM, Leiti MD. Gonadal hormone modulation of the behavioral effects of Δ9-tetrahydrocannabinol in male and female rats. Eur. J. Pharmacol. 2008;578:37–42. doi: 10.1016/j.ejphar.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Winsauer PJ, Brauner IN, Moerschbaecher JM. Estrogen improves response accuracy and attenuates the disruptive effects of Δ9-THC in ovariectomized rats responding under a multiple schedule of repeated acquisition and performance. Behav. Neurosci. 2002;116:989–998. [PubMed] [Google Scholar]
- Diaz S, Farhang B, Hoien J, Stahlman M, Adatia N, Cox JM, Wagner EJ. Sexdifferences in the cannabinoid modulation of appetite, body temperatue and neurotransmission at POMC synapses. Neuroendocrinology. 2009;89:424–440. doi: 10.1159/000191646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Spano MS, Altea S, Angius F, Fadda P, Fratta W. Cannabinoid self-administration in rats: sex differences and the influence of ovarian function. Br. J. Pharmacol. 2007;152:795–804. doi: 10.1038/sj.bjp.0707465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González S, Bisogno T, Wenger T, Manzanares J, Milone A, Berrendero F, Di Marzo V, Ramos JA, Fernández-Ruiz JJ. Sex steroid influences on cannabinoid CB1 receptor mRNA and endocannabinoid levels in the anterior pituitary. Biochem. Bioph. Res. Co. 2000;270:260–266. doi: 10.1006/bbrc.2000.2406. [DOI] [PubMed] [Google Scholar]
- Gorzalka BB, Hill MN, Chang SC. Male-female differences in the effects of cannabinoids on sexual behavior and gonadal hormone function. Horm. Behav. doi: 10.1016/j.yhbeh.2009.08.009. in press. [DOI] [PubMed] [Google Scholar]
- Hill MN, Karacabeyli ES, Gorzalka BB. Estrogen recruits the endocannabinoid system to modulate emotionality. Psychoneuroendocrinol. 2007;32:350–357. doi: 10.1016/j.psyneuen.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Marco EM, Llorente R, Moreno E, Biscaia JM, Guaza C, Viveros MP. Adolescent exposure to nicotine modifies acute functional responses to cannabinoid agonists in rats. Behav. Brain. Res. 2006;172:46–53. doi: 10.1016/j.bbr.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Mize AL, Alper RH. Acute and long-term effects of 17β-estradiol on Gi/o coupled neurotransmitter receptor function in the female rat brain as assessed by agonist-stimulated [35S] GTPγS-binding. Brain. Res. 2000;859:326–333. doi: 10.1016/s0006-8993(00)01998-3. [DOI] [PubMed] [Google Scholar]
- Nguyen QH, Wagner EJ. Estrogen differentially modulates the cannabinoid-induced presynaptic inhibition of amino acid neurotransmission in proopiomelanocortin neurons of the arcuate nucleus. Neuroendocrinology. 2006;84:123–137. doi: 10.1159/000096996. [DOI] [PubMed] [Google Scholar]
- Reich CG, Taylor ME, McCarthy MM. Differential effects of chronic unpredictable stress on hippocampal CB1 receptors in male and female rats. Behav. Brain. Res. 2009;203:264–269. doi: 10.1016/j.bbr.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez de Fonseca A, Cebeira M, Ramos JA, Martín M, Fern’ndez-Ruiz JJ. Cannabinoid receptors in rat brain areas: sexual differences, fluctuations during estrous cycle and changes after gonadectomy and sex steroid replacement. Life Sci. 1994;54:159–170. doi: 10.1016/0024-3205(94)00585-0. [DOI] [PubMed] [Google Scholar]
- Rubino T, Vigano’ D, Realini N, Guidali C, Braida D, Capurro V, Castiglioni C, Cherubino F, Romualdi P, Candeletti S, Sala M, Parolaro D. Chronic delta 9-tetrahydrocannabinol during adolescence provokes sex-dependent changes in the emotional profile in adult rats: behavioral and biochemical correlates. Neuropsychopharmacology. 2008;33:2760–2771. doi: 10.1038/sj.npp.1301664. [DOI] [PubMed] [Google Scholar]
- Tseng AH, Craft RM. Sex differences in antonociceptive and motoric effects of cannabinoids. Eur. J. Pharmacol. 2001;430:41–47. doi: 10.1016/s0014-2999(01)01267-5. [DOI] [PubMed] [Google Scholar]
- Willianson EM, Evans FJ. Cannabinoids in clinical practice. Drugs. 2000;60:1303–1314. doi: 10.2165/00003495-200060060-00005. [DOI] [PubMed] [Google Scholar]
- Wise LE, Thorpe AJ, Lichtman AH. Hippocampal CB1 receptors mediate the memory impairing effects of Δ9-tetrahydrocannabinol. Neuropsychopharmacology. 2009;34:2072–2080. doi: 10.1038/npp.2009.31. [DOI] [PMC free article] [PubMed] [Google Scholar]