Abstract
Aberrant epidermal growth factor receptor (EGFR) signaling is common in cancer. Increased expression of wild type and mutant EGFR is a widespread feature of diverse types of cancer. EGFR signaling in cancer has been the focus of intense investigation for decades primarily for two reasons. First, aberrant EGFR signaling is likely to play an important role in the pathogenesis of cancer, and therefore, the mechanisms of EGFR-mediated oncogenic signaling are of interest. Second, the EGFR signaling system is an attractive target for therapeutic intervention. EGFR gene amplification and overexpression are a particularly striking feature of glioblastoma (GBM), observed in approximately 40% of tumors. GBM is the most common primary malignant tumor of the central nervous system in adults. In approximately 50% of tumors with EGFR amplification, a specific EGFR mutant (EGFRvIII, also known as EGFR type III, de2-7, ΔEGFR) can be detected. This mutant is highly oncogenic and is generated from a deletion of exons 2 to 7 of the EGFR gene, which results in an in-frame deletion of 267 amino acids from the extracellular domain of the receptor. EGFRvIII is unable to bind ligand, and it signals constitutively. Although EGFRvIII has the same signaling domain as the wild type receptor, it seems to generate a distinct set of downstream signals that may contribute to an increased tumorigenicity. In this review, we discuss recent progress in key aspects of EGFR signaling in GBM, focusing on neuropathology, signal transduction, imaging of the EGFR, and the role of the EGFR in mediating resistance to radiation therapy in GBM.
Introduction
The epidermal growth factor receptor (EGFR) induces proliferation and/or has a trophic effect on multiple cell types [1]. The EGFR is expressed at high levels in various types of cancer, suggesting a role in the pathogenesis of multiple cancer types [2]. Furthermore, there is substantial experimental evidence supporting a causal role for aberrant EGFR signaling in cancer pathogenesis and resistance to treatment [3]. EGFR gene amplification and overexpression are a striking feature of glioblastoma (GBM) but are rare in low-grade gliomas, suggesting a causal role for aberrant EGFR signaling in the pathogenesis of GBM. The most common EGFR mutant is named EGFRvIII (EGFR type III, EGFRvIII, de2-7, ΔEGFR) [4,5]. This mutant is generated from a deletion of exons 2 to 7 of the EGFR gene, which results in an in-frame deletion of 267 amino acids from the extracellular domain of the receptor. EGFRvIII is unable to bind ligand, and it signals constitutively. It is important to note that EGFRvIII is usually coexpressed with the wild type (wt) receptor in GBM [4,6]. Coexpression of ligand also has been noted in tumors, suggesting that autocrine or paracrine loops contribute to malignant progression [4,7–9]. There is substantial evidence suggesting that EGFRvIII signaling plays a key role in gliomagenesis [3,10]. A number of studies have demonstrated that the EGFRvIII variant is more tumorigenic than the wt receptor [11-15]. Increased EGFRvIII expression may influence multiple aspects of tumor biology, including survival, proliferation of cells, motility and invasiveness, and resistance to treatment [13,16–19].
The EGFR signaling network thus presents an attractive target for therapeutic intervention, and considerable effort is focused on trying to inhibit the receptor in various types of cancer using antibodies, tyrosine kinase inhibitors (TKIs), or vaccines [20,21]. Anti-EGFR treatment seems to be effective in patients with EGFR tyrosine kinasemutations in lung cancer [22–25]. Cancer cells can become dependent on activated oncogenes for their survival. This phenomenon has been called oncogene addiction. Whereas initial studies showed there is a low rate of response to EGFRvIII inhibitors in GBM overall [26], a subset of patients with coexpression of EGFRvIII and PTEN seemed to be more responsive to anti-EGFR therapy with Erlotinib (Tarceva) in GBM [27,28]. However, a subsequent study reported that the concomitant expression of EGFRvIII with PTEN was not predictive of improved survival in patients treated with Erlotinib [26,29]. These findings suggest that more complex molecular signatures associated with individual tumors may need to be identified for clinically effective targeting of the EGFR system in GBM. Furthermore, certain EGFR mutations, such as tyrosine kinase mutations found in lung cancer, may be more responsive to TKI compared with GBM in which a different spectrum of EGFR mutations is present.
Neuropathological Aspects of EGFR and EGFRvIII in Glioma
Prevalence and Age Distribution
Overall, 36% to 40% of GBMs exhibit EGFR gene amplification [30,31]. In a study of 30 GBMs, EGFR gene amplification was always associated with immunohistochemical EGFR protein overexpression, defined as strong plasma membrane or cytoplasmic immunopositivity in most tumor cells, but 10%of GBMs with EGFR protein overexpression lacked EGFR gene amplification [32,33]. In our own series, 23 (36%) of 64 GBMs had EGFR gene amplification by chromogenic in situ hybridization on a tissue microarray (Table 1). We evaluated EGFR protein expression in these GBMs using the EGFR pharmDx antibody (Dako, Carpinteria, CA) and following the interpretation guidelines provided by the manufacturer. These interpretation guidelines, approved by the US Food and Drug Administration for identifying colorectal cancer patients eligible for treatment with cetuximab, only consider cell membrane staining to represent positive staining. We found a strong, although imperfect correlation between EGFR gene amplification status and EGFR gene expression (Table 1 and Figure 1).
Table 1.
Strong Correlation between EGFR Gene Amplification Status and EGFR Protein Expression at the Cell Membrane in Human GBMs (n = 64).
| EGFR Amplification | EGFR Protein Expression (IHC Score) | ||||
| 0 | 1+ | 2+ | 3+ | Total | |
| No | 14 | 15 | 10 | 2 | 41 |
| Yes, focal | 1 | 1 | 1 | 3 | 6 |
| Yes, diffuse | 0 | 1 | 2 | 14 | 17 |
| Total | 15 | 17 | 13 | 19 | 64 |
Figure 1.
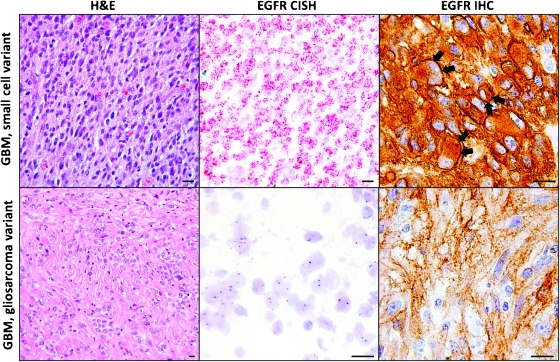
Small cell GBMs are highly cellular tumors composed of monomorphic cells with ovoid nuclei. Most small cell GBMs harbor EGFR gene amplification, shown here by chromogenic in situ hybridization as innumerable red dots corresponding to EGFR gene copies in the tumor cell nuclei. EGFR gene amplification is typically associated with strong cell membrane staining (arrows) for the EGFR protein by IHC, although diffuse cytoplasmic staining is also seen. Gliosarcomas, which are characterized by a collagen-rich stroma, usually do not exhibit EGFR gene amplification. Moderate cytoplasmic EGFR immunopositivity may be seen even in tumors without EGFR gene amplification, but distinct cell membrane staining is rarely, if ever, present.
Most GBMs (54%) overexpress wtEGFR protein and 31% overexpress both wtEGFR and EGFRvIII [34]. By immunohistochemistry (IHC), EGFRvIII is present in 41%of GBMs with EGFR amplification [4]. A small proportion of GBMs (5%) may express EGFRvIII without concomitant EGFR gene amplification, but such tumors, nevertheless, always express high levels of wtEGFR [35]. EGFRvIII expression without EGFR gene amplification is relatively uncommon, suggesting that EGFR gene amplification may precede EGFRvIII mutation.
More than 90% of GBMs are primary GBMs, which arise without evidence of a preceding lower-grade astrocytoma. Secondary GBMs progress from lower-grade astrocytomas and tend to occur in younger patients than primary GBMs (mean age, 45 vs 62 years) [31]. The genetic profiles of primary and secondary GBMs are different and include a much higher prevalence of EGFR gene amplification and EGFR overexpression in primary GBMs compared with secondary GBMs (40% vs 8% and >60% vs <10%, respectively) [31].
The age distribution of patients with EGFR-amplified GBMs reflects that of patients with primary GBMs. EGFR gene amplification is rare in GBMs, occurring in patients younger than age 35. With increasing patient age, EGFR amplification becomes more common. The median age of patients with EGFR-amplified GBM is 62 years [31]. Pediatric GBMs are rare and show genetic differences compared with adult GBMs, including a much lower prevalence of EGFR amplification (0%–5% vs 36%–40%) and EGFR protein overexpression (25% vs >60%) in pediatric GBMs [36,37].
Histopathological Features
As suggested by the old name for GBM “glioblastoma multiforme,” GBMs come in a number of histologic subtypes, including pleomorphic cell GBM(26% of GBMs), gemistocytic GBM(25%) [38], GBM with oligodendroglioma component (15%) [39], small cell GBM (27%) [30], gliosarcoma (2%) [40], and giant cell GBM (1%) [41], as well mixed variants composed of more than one histologic pattern. GBM with oligodendroglioma component includes both astrocytic and oligodendroglial areas and, based on some studies, may be associated with a more favorable prognosis [42]. According to the most recent World Health Organization classification, GBM with oligodendroglioma component is distinguished from anaplastic oligoastrocytoma based on the presence of necrosis only in the former tumor [43]. Small cell GBMs are highly cellular and cytologically monotonous neoplasms with frequent mitoses [30] (Figure 1). Gliosarcomas are biphasic tumors containing areas of malignant mesenchymal tumor (sarcoma) as well as conventional GBM. Giant cell GBMs contain frequent extremely large, multinucleated, and highly pleomorphic cells.
EGFR gene amplification is relatively common in small cell GBMs (69%) [30,44] but rare in gliosarcomas (0%) [45] and giant cell GBMs (6%) [46]. Similarly, wtEGFR and EGFRvIII expression is more common in small cell GBMs compared with non-small cell GBMs (83% vs 35% and 50% vs 21%, respectively) [44] (Figure 1). A subset of small cell GBMs with EGFR amplification shows areas with oligodendroglial histologic features, including round nuclei with perinuclear halos [44]. Given the evidence that the clinical behavior of such GBMs is similar to conventional GBMs [44], we do not use the designation “GBMwith oligodendroglioma component” for EGFR-amplified GBMs with oligodendroglial features.
Distribution of Tumor Cells with EGFR Amplification
In some GBMs, almost all of the tumor cells have EGFR amplification, but in other GBMs, the proportion of tumor cells with EGFR amplification can vary from 10% to 60% in different areas of the same tumor [47,48]. According to one study, EGFR amplified cells were more frequent near the edge of the tumor compared with the center [47], but no such correlation was found in another study [48]. In our own series, EGFR gene amplification was diffuse in 27%and focal in 9% of GBMs (Table 1). Of the GBMs with strong (3+) EGFR protein expression at the cell membrane, 74% showed diffuse EGFR gene amplification (affecting most of the tumor cells), and an additional 16% had focal EGFR gene amplification. Of the GBMs with no EGFR protein expression at the cell membrane (IHC score 0), none had diffuse EGFR gene amplification and 7% had focal EGFR gene amplification.
Effect on Prognosis
Among GBMs (grade IVastrocytomas), EGFRvIII expression has no effect on survival [35]. The prevalence of EGFRvIII expression in anaplastic astrocytomas (grade III astrocytomas) is much lower than in GBMs (9% vs 31%) [35]. Like EGFR amplification, EGFRvIII expression in tumors diagnosed as anaplastic astrocytomas is predictive of GBM-like clinical behavior [35,44]. Many of such anaplastic astrocytomas likely represent undersampled GBMs. Thus, EGFR gene amplification is one of the genetic hallmarks of GBM. In diagnostic neuropathology practice, identification of neoplastic astrocytes with EGFR amplification by fluorescent or chromogenic in situ hybridization constitutes strong evidence that the tumor is a GBM, or at least should be treated like a GBM, even when the histologic criteria for GBM are not met because of the absence of necrosis and microvascular proliferation in the biopsy. Several studies have shown that although EGFR amplification and EGFR protein overexpression have no effect on prognosis when patients of all ages are analyzed together, EGFR amplification is associated with a worse prognosis among younger patients and with a more favorable prognosis among older patients (aged >45, >55, or >60 years, depending on study) [47,49–52]. EGFR amplification does not preclude an unusually long survival, as 26% of GBM patients surviving longer than 3 years have GBMs with EGFR amplification [53].
Imaging of EGFR and Tumor Response to Anti-EGFR Treatment
Given the importance of the EGFR signaling pathway in tumor aggressiveness, treatment resistance, and poor prognosis in various tumor types, in the past several years, a great deal of effort has been made in developing molecular imaging approaches to noninvasively evaluate EGFR status and therapeutic response to EGFR targeting agents. Specific EGFR targeting imaging probes have been developed for multiple imaging modalities including positron emission tomography (PET), single photon emission computed tomography (SPECT), optical imaging, and magnetic resonance imaging (MRI). The ultimate goal of in vivo imaging of EGFR is to enable clinicians to stratify the patients who are likely to benefit from EGFR targeting therapeutics and monitor treatment efficacy.
Radiopharmaceuticals (PET and SPECT) for Imaging EGFR
Both PET and SPECT are nuclear medicine imaging techniques involving introduction of radioactive tracer material into subjects and detection of gamma rays emitted directly or indirectly from the tracer. Because of the superb sensitivity and clinical applicability of PET and SPECT imaging, development of radiotracers for imaging EGFR has attracted intense interest. Two classes of PET or SPECT probes have been developed by radiolabeling either ligands or antibodies against extracellular EGFR binding domain or small molecule TKIs targeting intracellular receptor tyrosine kinase domain [54–56]. Among the first class, usage of the natural EGFR ligand, EGF [57], or the antibodies including the intact monoclonal antibody such as cetuximab [58] or fragments of the antibodies such as F(ab′)2 or Affibody [59,60] has been investigated. Attempts to develop small molecule reversible or irreversible TKI probes have been mainly focused on the 4-anilinoquinazoline class of compounds that had been originally developed for therapy [54]. Pal et al. [61] showed that 124I radiolabeled TKI that irreversibly binds to the phosphorylated EGFR may be a surrogate marker for EGFR activation. A wide variety of isotopes ranging from short half-lives 11C and 18F to longer ones like 177Lu have been used to label such anti-EGFR molecules. The rationale for choosing an isotope relies mostly on its characteristics and pharmacokinetics, which should typically have a half-life, ideally matching the biological half-life of the end compound to maximize signal-to-noise ratio. For instance, longer half-life radiotracers such as 177Lu (∼6 days) are useful in labeling a whole antibody of EGFR that has longer clearance time, whereas the shorter half-life ones like 11C (∼20 min) and 18F (∼2 h) are used in labeling fragments of EGFR antibody, namely, Diabody or Affibody. Comprehensive information about radiopharmaceuticals for imaging EGFR can be found in the excellent review articles by Mishani et al. [54] and Cai et al. [56]. In addition to EGFR overexpression, EGFR mutations have been found in considerable numbers of cancer patients. As discussed in this review, EGFRvIII is the most common and highly oncogenic EGFR mutant in GBM, and imaging the status of EGFRvIII could be of great value in stratifying patients and monitoring treatment in GBM. Takasu et al. [62] showed that 3C10 monoclonal antibody, specifically recognizing EGFRvIII, labeled by technetium Tc 99m (99mTc) significantly accumulated in the EGFRvIII-expressing intracranial glioma xenografts in nude mice. Extensive preclinical work on animal tumor models has been conducted to detect in vivo EGFR. Most recently, Liu et al. [63] reported the first application of PET EGFR imaging on humans, suggesting PD153035, a small molecule TKI labeled with 11C, as a promising PET tracer.
Optical Imaging of EGFR Expression
The cheap, high-throughput optical imaging has been rapidly adapted to cancer research [64]. In vivo fluorescence imaging depends on fluorescence probes that emit light, which can be captured by a charge-coupled device camera. Fluorescence imaging by using conjugates of the EGFR ligand, EGF, or anti-EGFR antibodies with fluorophores or Quantum dots (QDs) has been investigated in vivo in the detection of EGFR in various tumors of animal models [65–68]. Ke et al. [65] applied EGF-Cy5.5 to assess EGFR expression and showed that significantly higher fluorescent light intensity was detected in EGFR+ than in EGFR- mammary tumors. In another study, Hama et al. described a two-step activation process to visualize EGFR by administering biotinylated cetuximab followed by neutravidin-fluorescent conjugate. An ∼10-fold amplification of the optical fluorescence signal was achieved in the EGFR+ tumors [66]. Long-wavelength fluorophores such as near-infrared fluorescence has several advantages over short-wavelength visible lights including deeper penetration owing to less tissue absorption and scattering of light and minimal autofluorescence. Thus, using near-infrared fluorescence enables to image deepseated orthotopic tumors in small animal models. In the clinical setting, optical imaging has recently emerged as an attractive approach to facilitate identification of infiltrative tumors and sentinel lymph node metastases through endoscopy or during intraoperative visualization [69,70].
MRI Monitoring of Anti-EGFR Treatment
In contrast to more emphases on developing imaging probes to visualize EGFR expression by PET or optical imaging, MRI has been mostly used to evaluate EGFR or anti-EGFR treatment response based on conventional MRI parameters, namely, changes in T2-weighted or T1-weighted contrast-enhanced signal intensity, which are believed to reflect pathophysiological characteristics. However, a few studies have demonstrated MRI's capability of imaging EGFR expression based on MRI contrast agents conjugated with anti-EGFR monoclonal antibody or ScFvEGFR [67,71]. Applying MRI parameters to predict EGFR amplification in GBM of patients was reported by Aghi et al. [72]. Significantly higher T2/T1 ratio and T2 border sharpness coefficients was found in the cohort of GBMwith amplified EGFR, which may correlate with increased angiogenesis, edema, and invasion. A more recent study by Batchelor et al. [73] showed that functional MRI parameters such as vascular permeability based on dynamic contrast-enhanced MRI and apparent diffusion coefficients determined by diffusion-weighted MRI are useful in evaluating early response of GBM to a pan TKI, AZD2171.
Other than the imaging modalities mentioned above, power Doppler ultrasound has also been applied to monitoring tumor vascular changes induced by anti-EGFR erlotinib [74]. Recognizing the complexity and the importance of the EGFR downstream signaling pathways, possibly contributing to the mechanism of resistance to anti-EGFR, the capability of imaging not only EGFR itself but, more importantly, the interplay of EGFR and the downstream factors, will be the most challenging task.
Signal Transduction
What makes EGFRvIII more tumorigenic than the wt receptor? The cytoplasmic (signaling) domain is the same for the wtEGFR and EGFRvIII, and it has been proposed that altered kinetics of signaling may explain the differences in oncogenic potential between the wt and mutant EGFR [75,76]. Binding of ligand to the wtEGFR results in rapid internalization of the receptor, followed by dephosphorylation and degradation or recycling of the receptor [77]. Expression of EGFRvIII results in a constitutive tyrosine phosphorylation of EGFRvIII. Because EGFRvIII does not bind EGF, its internalization is slowed, promoting a state of low-level continuous signaling from activated receptors at the cell membrane [75]. Increased membrane persistence of activated receptors is known to favor mitogenic signaling [78]. A mechanistic explanation for failure of EGFRvIII internalization and down-regulation was provided by a study demonstrating that the reduced signal intensity associated with EGFRvIII resulted in failure of EGFRvIII to form complexes with Cbls, SETA or endophilin A1, which leads to decreased dinternalization [76]. The altered kinetics of EGFRvIII activation could result in a distinct set of downstream signals compared with the wtEGFR, and a number of studies have investigated signal transduction by the mutant EGFR. There are reports of constitutive activation of the phosphatidylinositol 3-kinase/Akt pathway in cells expressing EGFRvIII [79,80] leading to a down-regulation of p27 [81]. In addition, EGFRvIII-mediated activation of Ras [82] and extracellular signal-regulated kinases [83] has also been reported. Another study has shown that SHP2 activates EGFRvIII but not wtEGFR through the activation of mitogen-activated protein kinase (MAPK) [84]. The expression of EGFRvIII in U87MG cells leads to an increase in Bcl-XL and resistance to apoptotic cell death in response to chemotherapy [85]. Other studies have reported an important role for JNK activation in EGFRvIII signaling [86]. A recent study has proposed that myristoylated alanine-rich protein kinase C substrate contributes to EGFRvIII-mediated invasiveness [87].
Although the wtEGFR also activates these signals in response to ligand, it does not seem to do so constitutively even when overexpressed. It has been proposed that signals generated by the wt receptor are terminated more efficiently because ligand binding is an important mechanism of receptor internalization and signal termination [88]. However, a number of studies have shown that increased expression of the wt receptor also overwhelms mechanisms for receptor internalization, dephosphorylation, and degradation and can result in persistent activation of wt receptors [89,90]. Thus, questions about how the altered kinetics of EGFRvIII translates into more oncogenic signals compared with the wt receptor persist. In our previous study, wtEGFR and EGFRvIII were inducibly expressed at similar levels in glioma cell lines followed by gene expression profiling [18]. It was found that the expression of wtEGFR in glioma cells resulted in increased expression of a wide spectrum of genes, including genes involved not only in proliferation but also in growth suppression, immune modulation, metabolism, and transcription. Increased expression of the wt receptor without EGF stimulation results in up-regulation of a total of 93 genes, suggesting that the wt receptor also signals constitutively. If EGF is added to cells expressing high levels of wtEGFR, there is a further increase in the number of genes expressed to 159 [18]. As previous studies have suggested, gene induction by wtEGFR was more robust compared with EGFRvIII, which generated weak induction of a small group of genes.
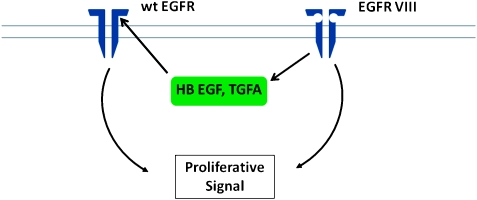
The frequent coexpression of wtEGFR with EGFRvIII in GBM raises the question of an interaction between the two. EGFRvIII may heterodimerize with wtEGFR and coexpression of the two receptors enhances cell proliferation and survival [91]. An additional mechanism was suggested by the observation that EGFRvIII induces expression of heparin binding epidermal growth factor (HB-EGF) and transforming growth factor α (TGFα), ligands for the wtEGFR. Because EGFRvIII does not bind ligand, this suggests that EGFRvIII generates an autocrine/paracrine loop using the wtEGFR in glioma cells [18]. Autocrine loops, in which both the receptor and the ligand are produced by the same tumor cells, may be an important contributor to the growth autonomy of cancer cells [92]. Coexpression of EGFR and TGFα is well documented in EGFR amplification-positive glioma as is the coexpression of EGFR and HB-EGF [8,9]. The major experimental support for the significance of autocrine loops is derived from studies showing that, whereas expression of the receptor alone (EGFR) has a weak transforming effect on cells, coexpression of ligand (TGFα) results in a robust increase in transformation [93]. In addition, strategies aimed at neutralizing ligands such as TGFα have been shown to decrease growth of cells harboring such loops [8,94]. The experimental support for the biological significance of this EGFRvIII-mediated autocrine loop was provided by demonstrating that antibodies to HB-EGF (but not TGFα) resulted in inhibition of EGFRvIII mediated glioma cell proliferation (Figure 2). Furthermore, EGFRvIII expression correlated with HB-EGF expression in GBM [18].
Figure 2.
EGFRvIII-HBEGF-wtEGFR autocrine loop generated by EGFRvIII and mediated by HB-EGF. In this model, the combined action of EGFRvIII plus wtEGFR generates the biological response.
In addition to HB-EGF and TGFα, we found that EGFRvIII expression results in expression of EPH receptor A2 (EphA2), IL-8, mitogen-activated protein kinase kinase kinase kinase 4 (MAP4K4), FOSL1, epithelial membrane protein 1 (EMP1), and DUSP6, all of which are known to influence oncogenic signaling pathways [18]. Previous studies have established that EphA2 [18,95–99], FosL1-Fra1 [99,100], IL8/CXCL8 [101,102], and EMP1 [103] have been detected in glioma or glioma cell lines. IL-8/CXCL8 is a chemotactic factor that may play an important role in angiogenesis and tumor development [104,105] and is upregulated in PTEN-negative glioma [106]. The EphA2 is a receptor tyrosine kinase that is frequently overexpressed in cancer and may play an important role in glioma [18,95–98,107, 108]. MAP4K4 (hepatocyte progenitor kinase-like/germinal center kinase-like kinase) is broadly expressed in cancer and can modulate cellular transformation, invasion, and Ras-mediated transformation [109,110]. Another gene, DUSP6 (MKP3), is upregulated by Ras signaling [111,112] and by mutant EGFR in glioma and lung cancer [18,113]. EMP1 is increased in leiyomyoma [114] and may play a role in glioma [103]. FOS-like antigen 1 (FosL1, Fra1) is linked to erlotinib sensitivity in glioma [115] and is induced in response to the activation of Ras or β-catenin pathways [116]. This molecular signature may hold important clues to EGFRvIII mediated tumorigencity [18], and further studies are needed to elucidate the biological significance of EGFRvIII-mediated gene induction in glioma.
EGFRvIII and DNA Double-Strand Break Repair
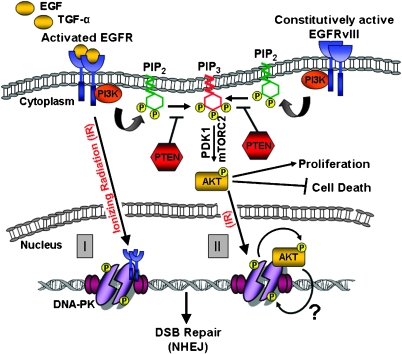
Whereas the activation of proproliferative and antiapoptotic pathways by EGFR is an established concept, recent reports reveal a novel link between EGFR signaling and the repair of radiation-induced DNA double-strand breaks (DSBs). It has been clear for quite some time now that EGFR expression or activation correlates with the radioresistance of cells and tumors [117]. Monoclonal antibodies or small molecule inhibitors targeting EGFR can increase sensitivity to ionizing radiation (IR) [19,118–120]. A direct correlation has been shown to exist between EGFR expression levels and radiation resistance in cells; these results have subsequently been extended to human head and neck carcinoma samples where a direct correlation has been demonstrated between EGFR expression levels and poor prognosis [121–124]. That EGFR may respond to radiation-induced DNA damage in a direct manner was first indicated by reports showing that IR could induce rapid and transient phosphorylation of EGFR [125–128]. The ramifications of radiation-induced EGFR activation remained unknown until Dittmann et al. [129] demonstrated that IR induces the nuclear translocation of EGFR (in addition to its activation; Figure 3). While in the nucleus, EGFR interacts with and stimulates the kinase activity of the 5DNA-dependent protein kinase catalytic subunit (DNA-PKcs) [130]. DNA-PKcs is a key enzyme in the nonhomologous end joining (NHEJ) pathway of DSB repair [131]; hence, the activation of DNA-PKcs by EGFR results in proficient repair of DSBs and provides an explanation for the increase in radioresistance conferred by EGFR. More importantly, the anti-EGFR antibody, cetuximab, can inhibit EGFR nuclear transport and interaction with DNA-PKcs, resulting in a radiosensizing effect [132], thereby validating the possibility of using anti- EGFR therapy for radiosensitizing purposes. In retrospect, a physical association between EGFR and DNA-PKcs had been demonstrated by Bandyopadhyay et al. [133]more than 10 years back who postulated that EGFR may be important for the maintenance of DNA-PKcs in the nucleus. In the past couple of years, a number of reports have reconfirmed this link between EGFR signaling and DSB repair; many of these reports indicate that signaling through the phosphatidylinositol 3-kinase (PI3K)-Akt or MAPK pathways might also impinge on DNA-PK activation rather than the direct interaction between EGFR and DNA-PK initially proposed by the group of Rodemann [19,119,120,134–137]. Contrary to the effect of wtEGFR, EGFR with mutations in the tyrosine kinase domain seem to have an inhibitory effect on DSB repair in lung cancer cells, possibly because of a deficit in nuclear translocation of these mutant forms of EGFR [119,120]. The most common deletion mutant, EGFRvIII, occurs commonly in GBMs [138]. EGFRvIII lacks the extracellular, ligand-binding domain but is constitutively active and resistant to receptor internalization and attenuation. EGFRvIII is also phosphorylated in response to IR and actually displays even higher levels of IR-induced activation compared with the wt receptor [139]. We find that EGFRvIII contributes to the radioresistance of glioma-relevant cells and tumors by promoting the repair of DSBs vis-à-vis hyperactivation of DNA-PKcs [19], similar to that reported for the wt receptor in lung cancer cells. However, unlike observations in lung cancer cells, in the context of murine astrocytes, human glioma cells, or orthotopic brain tumors, we fail to observe any nuclear relocalization of EGFRvIII on radiation [19] nor is there any evidence of nuclear localization of EGFR in tissue arrays of human GBM samples (our unpublished data). It is therefore plausible that, in the context of gliomas, specific signal cascades emanating from the EGFRvIII receptor [138], rather than an actual physical association between EGFRvIII and DNA-PK, might promote efficient DSB repair. Several lines of evidence indicate that whereas ligand-activated EGFR stimulates both the RAS-RAF-MAPK and PI3KAkt pathways [138], EGFRvIII may preferentially activate the PI3K-Akt pathway [140–142], and activation of the PI3K-Akt pathway by EGFRvIII may be more robust than by wtEGFR [139]. We therefore speculate that the increase in radioresistance conferred by the EGFRvIII may be executed through the PI3-Akt pathway. Activated Akt prevents apoptosis by inhibiting proapoptotic factors such as BAD (BCL2 antagonist of cell death) and procaspase-9 and stimulates cell proliferation [143] by activating mammalian target of rapamycin [144]. The following reports postulate that activated Akt may also play a role in the repair of DSBs. IR stimulates the activation of Akt and its phosphorylation at threonine 308 and serine 473 [125,145]. Dampening of PI3K-Akt signaling using small molecule inhibitors impairs DSB repair in GBM [135] and breast cancer cells [146], whereas siRNA-mediated Akt knockdown impairs DSB repair in cancer cells [137], and this results in radiation sensitivity. Nevertheless, hyperactivation of the PIK-Akt pathway due to deletion of PTEN promotes DSB repair and radiation survival [135]. We, too, find that the PI3K inhibitor LY294002 can abrogate the proficient DSB repair conferred by EGFRvIII overexpression and that expression of a constitutively active version of Akt can mimic the effects of EGFRvIII overexpression on DSB repair in astrocytes [19]. Significantly, a recent report demonstrated that Akt translocates to the nucleus on irradiation and associates with DNA-PK at the sites of DSBs [147–149]. Activation of DNA-PKcs involves its phosphorylation on serine/threonine residues [130,131]. As Akt is a serine/threonine kinase, it is possible that hyperactivation of DNA-PK by EGFRvIII might be mediated by Akt (Figure 3). The putative connection among EGFRvIII, Akt, and DNA-PKcs is worthy of detailed investigation in the future.
Figure 3.
EGFR signaling and NHEJ. The binding of EGF or TGFα to EGFR activates the following pathways: (1) PI3K-Akt-1 pathway, (2) Ras/RAF/MAPK/extracellular signal-regulated (ERK) pathway, and (3) signal transducer and activation of transcription (STAT) pathway (only the PI3K-Akt-1 pathway is shown for simplicity). EGFRvIII, a common deletion mutant that lacks the ligand-binding extracellular domain, is constitutively active and signals preferentially through the PI3K-Akt-1 pathway. In this pathway, activated PI3K phosphorylates phosphatidylinositol-4,5-biphosphate (PIP2) generating phosphatidylinositol-3,4,5-triphosphate (PIP3). PIP3 anchors Akt-1 to the plasma membrane, where it is phosphorylated by mammalian target of rapamycin complex 2 (mTORC2) and 3-phosphoinositide-dependent kinase 1 (PDK1). Activated Akt-1 phosphorylates a variety of downstream targets that enhance proliferation and inhibit cell death. The PTEN tumor suppressor negatively regulates PI3K-Akt-1 signaling by reversing PIP3 back to PIP2. Two models have been proposed to explain the connection between EGFR and NHEJ. In one scenario, wtEGFR translocates into the nucleus in response to IR, interacts with DNA-PKcs (DNA-dependent protein kinase, catalytic subunit), and stimulates its DNA repair activity (I). In another scenario, Akt-1 translocates into the nucleus in response to IR and interacts with DNA-PKcs (II). Phosphorylation of Akt-1 by DNA-PKcs promotes survival (curved arrow) and we hypothesize that reciprocal phosphorylation of DNA-PKcs by Akt-1 might promote DSB repair through NHEJ.
Concluding Comments
The identification of EGFR amplification and mutation in GBM has led to important advances in demonstrating that the EGFR (in combination with other genetic alterations) is likely to play an important role in the pathogenesis of this disease. GBMs are highly resistance to treatment with radiation and chemotherapy and aberrant EGFR signaling contributes to this resistance. Thus, the EGFR remains an attractive target for therapeutic intervention in GBM, although initial attempts to target the EGFR have not been effective in GBM. Furthermore, important progress has been made in detection of aberrant EGFR signaling with improved neuropathological tools and advances in imaging. However, a number of important questions remain unanswered, including some fundamental questions. Activation of the EGFR seems to trigger a diverse array of signals within the cell and identifying the key downstream signals that mediate specific biological responses such as cell proliferation or motility remains a challenge. The increased oncogenic potential of specific EGFR mutants such as EGFRvIII remains incompletely understood, because even the wtEGFR has been shown to signal constitutively when overexpressed. Another question that deserves further study is the effect of EGFR overexpression on downstream signal transduction. Previous studies have suggested that increased expression or activation of receptor tyrosine kinase signaling systems does not necessarily lead to a simple amplification of downstream signals. Thus, dose-dependent changes in both oncogene-induced downstream signal transduction as well as biological responses have been reported [150,151], and an improved understanding of downstream signaling is likely to improve EGFR targeting in GBM.
Acknowledgments
The authors thank Sandili Chauncey and Cristel V. Camacho for generating illustrations.
Footnotes
A.H. is supported by National Institutes of Health grant R01NS062080-01A2 and D.Z. is supported by National Institutes of Health grant 1R21 CA141348-01A1. S.B is supported by grants from National Aeronautics and Space Administration (NNA05CS97G and NNX10AE08G) and from the Cancer Prevention and Research Institute of Texas (RP100644).
References
- 1.Carpenter G, Cohen S. Epidermal growth factor. J Biol Chem. 1990;265:7709–7712. [PubMed] [Google Scholar]
- 2.Gullick WJ. Prevalence of aberrant expression of the epidermal growth factor receptor in human cancers. Br Med Bull. 1991;47:87–98. doi: 10.1093/oxfordjournals.bmb.a072464. [DOI] [PubMed] [Google Scholar]
- 3.Huang PH, Xu AM, White FM. Oncogenic EGFR signaling networks in glioma. Sci Signal. 2009;2:re6–re6. doi: 10.1126/scisignal.287re6. [DOI] [PubMed] [Google Scholar]
- 4.Ekstrand AJ, James CD, Cavenee WK, Seliger B, Pettersson RF, Collins VP. Genes for epidermal growth factor receptor, transforming growth factor α, and epidermal growth factor and their expression in human gliomas in vivo. Cancer Res. 1991;51:2164–2172. [PubMed] [Google Scholar]
- 5.Wong AJ, Ruppert JM, Bigner SH, Grzeschik CH, Humphrey PA, Bigner DS, Vogelstein B. Structural alterations of the epidermal growth factor receptor gene in human gliomas. Proc Natl Acad Sci USA. 1992;89:2965–2969. doi: 10.1073/pnas.89.7.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biernat W, Huang H, Yokoo H, Kleihues P, Ohgaki H. Predominant expression of mutant EGFR (EGFRvIII) is rare in primary glioblastomas. Brain Pathol. 2004;14:131–136. doi: 10.1111/j.1750-3639.2004.tb00045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aaronson SA. Growth factors and cancer. Science. 1991;254:1146–1153. doi: 10.1126/science.1659742. [DOI] [PubMed] [Google Scholar]
- 8.Tang P, Steck PA, Yung WK. The autocrine loop of TGF-α/EGFR and brain tumors. J Neurooncol. 1997;35:303–314. doi: 10.1023/a:1005824802617. [DOI] [PubMed] [Google Scholar]
- 9.Mishima K, Higashiyama S, Asai A, Yamaoka K, Nagashima Y, Taniguchi N, Kitanaka C, Kirino T, Kuchino Y. Heparin-binding epidermal growth factor-like growth factor stimulates mitogenic signaling and is highly expressed in human malignant gliomas. Acta Neuropathol (Berl) 1998;96:322–328. doi: 10.1007/s004010050901. [DOI] [PubMed] [Google Scholar]
- 10.Nicholas MK, Lukas RV, Jafri NF, Faoro L, Salgia R. Epidermal growth factor receptor-mediated signal transduction in the development and therapy of gliomas. Clin Cancer Res. 2006;12:7261–7270. doi: 10.1158/1078-0432.CCR-06-0874. [DOI] [PubMed] [Google Scholar]
- 11.Batra SK, Castelino-Prabhu S, Wikstrand CJ, Zhu X, Humphrey PA, Friedman HS, Bigner DD. Epidermal growth factor ligand-independent, unregulated, cell-transforming potential of a naturally occurring human mutant EGFRvIII gene. Cell Growth Differ. 1995;6:1251–1259. [PubMed] [Google Scholar]
- 12.Moscatello DK, Montgomery RB, Sundareshan P, McDanel H, Wong MY, Wong AJ. Transformational and altered signal transduction by a naturally occurring mutant EGF receptor. Oncogene. 1996;13:85–96. [PubMed] [Google Scholar]
- 13.Nagane M, Coufal F, Lin H, Bogler O, Cavenee WK, Huang HJ. A common mutant epidermal growth factor receptor confers enhanced tumorigenicity on human glioblastoma cells by increasing proliferation and reducing apoptosis. Cancer Res. 1996;56:5079–5086. [PubMed] [Google Scholar]
- 14.Nishikawa R, Ji XD, Harmon RC, Lazar CS, Gill GN, Cavenee WK, Huang HJ. A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorigenicity. Proc Natl Acad Sci USA. 1994;91:7727–7731. doi: 10.1073/pnas.91.16.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holland EC, Hively WP, DePinho RA, Varmus HE. A constitutively active epidermal growth factor receptor cooperates with disruption of G1 cell-cycle arrest pathways to induce glioma-like lesions in mice. Genes Dev. 1998;12:3675–3685. doi: 10.1101/gad.12.23.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lal A, Glazer CA, Martinson HM, Friedman HS, Archer GE, Sampson JH, Riggins GJ. Mutant epidermal growth factor receptor up-regulates molecular effectors of tumor invasion. Cancer Res. 2002;62:3335–3339. [PubMed] [Google Scholar]
- 17.Boockvar JA, Kapitonov D, Kapoor G, Schouten J, Counelis GJ, Bogler O, Snyder EY, McIntosh TK, O'Rourke DM. Constitutive EGFR signaling confers a motile phenotype to neural stem cells. Mol Cell Neurosci. 2003;24:1116–1130. doi: 10.1016/j.mcn.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Ramnarain DB, Park S, Lee DY, Hatanpaa KJ, Scoggin SO, Otu H, Libermann TA, Raisanen JM, Ashfaq R, Wong ET, et al. Differential gene expression analysis reveals generation of an autocrine loop by a mutant epidermal growth factor receptor in glioma cells. Cancer Res. 2006;66:867–874. doi: 10.1158/0008-5472.CAN-05-2753. [DOI] [PubMed] [Google Scholar]
- 19.Mukherjee B, McEllin B, Camacho CV, Tomimatsu N, Sirasanagandala S, Nannepaga S, Hatanpaa KJ, Mickey B, Madden C, Maher E, et al. EGFRvIII and DNA double-strand break repair: a molecular mechanism for radioresistance in glioblastoma. Cancer Res. 2009;69:4252–4259. doi: 10.1158/0008-5472.CAN-08-4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heimberger AB, Crotty LE, Archer GE, Hess KR, Wikstrand CJ, Friedman AH, Friedman HS, Bigner DD, Sampson JH. Epidermal growth factor receptor VIII peptide vaccination is efficacious against established intracerebral tumors. Clin Cancer Res. 2003;9:4247–4254. [PubMed] [Google Scholar]
- 21.Mendelsohn J, Baselga J. The EGF receptor family as targets for cancer therapy. Oncogene. 2000;19:6550–6565. doi: 10.1038/sj.onc.1204082. [DOI] [PubMed] [Google Scholar]
- 22.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 23.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 24.Dowell JE, Minna JD. EGFR mutations and molecularly targeted therapy: a new era in the treatment of lung cancer. Nat Clin Pract Oncol. 2006;3:170–171. doi: 10.1038/ncponc0476. [DOI] [PubMed] [Google Scholar]
- 25.Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, Majem M, Lopez-Vivanco G, Isla D, Provencio M, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 26.Karpel-Massler G, Schmidt U, Unterberg A, Halatsch ME. Therapeutic inhibition of the epidermal growth factor receptor in high-grade gliomas: where do we stand? Mol Cancer Res. 2009;7:1000–1012. doi: 10.1158/1541-7786.MCR-08-0479. [DOI] [PubMed] [Google Scholar]
- 27.Mellinghoff IK, Wang MY, Vivanco I, Haas-Kogan DA, Zhu S, Dia EQ, Lu KV, Yoshimoto K, Huang JH, Chute DJ, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353:2012–2024. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- 28.Friedman HS, Bigner DD. Glioblastoma multiforme and the epidermal growth factor receptor. N Engl J Med. 2005;353:1997–1999. doi: 10.1056/NEJMp058186. [DOI] [PubMed] [Google Scholar]
- 29.Brown PD, Krishnan S, Sarkaria JN, Wu W, Jaeckle KA, Uhm JH, Geoffroy FJ, Arusell R, Kitange G, Jenkins RB, et al. Phase I/II trial of erlotinib and temozolomide with radiation therapy in the treatment of newly diagnosed glioblastoma multiforme: North Central Cancer Treatment Group Study N0177. J Clin Oncol. 2008;26:5603–5609. doi: 10.1200/JCO.2008.18.0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burger PC, Pearl DK, Aldape K, Yates AJ, Scheithauer BW, Passe SM, Jenkins RB, James CD. Small cell architecture—a histological equivalent of EGFR amplification in glioblastoma multiforme? J Neuropathol Exp Neurol. 2001;60:1099–1104. doi: 10.1093/jnen/60.11.1099. [DOI] [PubMed] [Google Scholar]
- 31.Ohgaki H, Kleihues P. Genetic pathways to primary and secondary glioblastoma. Am J Pathol. 2007;170:1445–1453. doi: 10.2353/ajpath.2007.070011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tohma Y, Gratas C, Biernat W, Peraud A, Fukuda M, Yonekawa Y, Kleihues P, Ohgaki H. PTEN (MMAC1) mutations are frequent in primary glioblastomas (de novo) but not in secondary glioblastomas. J Neuropathol Exp Neurol. 1998;57:684–689. doi: 10.1097/00005072-199807000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe K, Tachibana O, Sata K, Yonekawa Y, Kleihues P, Ohgaki H. Overexpression of the EGF receptor and p53 mutations are mutually exclusive in the evolution of primary and secondary glioblastomas. Brain Pathol. 1996;6:217–223. doi: 10.1111/j.1750-3639.1996.tb00848.x. discussion 223-214. [DOI] [PubMed] [Google Scholar]
- 34.Heimberger AB, Hlatky R, Suki D, Yang D, Weinberg J, Gilbert M, Sawaya R, Aldape K. Prognostic effect of epidermal growth factor receptor and EGFRvIII in glioblastoma multiforme patients. Clin Cancer Res. 2005;11:1462–1466. doi: 10.1158/1078-0432.CCR-04-1737. [DOI] [PubMed] [Google Scholar]
- 35.Aldape KD, Ballman K, Furth A, Buckner JC, Giannini C, Burger PC, Scheithauer BW, Jenkins RB, James CD. Immunohistochemical detection of EGFRvIII in high malignancy grade astrocytomas and evaluation of prognostic significance. J Neuropathol Exp Neurol. 2004;63:700–707. doi: 10.1093/jnen/63.7.700. [DOI] [PubMed] [Google Scholar]
- 36.Suri V, Das P, Jain A, Sharma MC, Borkar SA, Suri A, Gupta D, Sarkar C. Pediatric glioblastomas: a histopathological and molecular genetic study. Neuro Oncol. 2009;11:274–280. doi: 10.1215/15228517-2008-092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sung T, Miller DC, Hayes RL, Alonso M, Yee H, Newcomb EW. Preferential inactivation of the p53 tumor suppressor pathway and lack of EGFR amplification distinguish de novo high grade pediatric astrocytomas from de novo adult astrocytomas. Brain Pathol. 2000;10:249–259. doi: 10.1111/j.1750-3639.2000.tb00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Korshunov A, Golanov A, Sycheva R. Immunohistochemical markers for prognosis of cerebral glioblastomas. J Neurooncol. 2002;58:217–236. doi: 10.1023/a:1016218117251. [DOI] [PubMed] [Google Scholar]
- 39.He J, Mokhtari K, Sanson M, Marie Y, Kujas M, Huguet S, Leuraud P, Capelle L, Delattre JY, Poirier J, et al. Glioblastomas with an oligodendroglial component: a pathological and molecular study. J Neuropathol Exp Neurol. 2001;60:863–871. doi: 10.1093/jnen/60.9.863. [DOI] [PubMed] [Google Scholar]
- 40.Meis JM, Martz KL, Nelson JS. Mixed glioblastoma multiforme and sarcoma. A clinicopathologic study of 26 radiation therapy oncology group cases. Cancer. 1991;67:2342–2349. doi: 10.1002/1097-0142(19910501)67:9<2342::aid-cncr2820670922>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 41.Kozak KR, Moody JS. Giant cell glioblastoma: a glioblastoma subtype with distinct epidemiology and superior prognosis. Neuro Oncol. 2009;11:833–841. doi: 10.1215/15228517-2008-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller CR, Dunham CP, Scheithauer BW, Perry A. Significance of necrosis in grading of oligodendroglial neoplasms: a clinicopathologic and genetic study of newly diagnosed high-grade gliomas. J Clin Oncol. 2006;24:5419–5426. doi: 10.1200/JCO.2006.08.1497. [DOI] [PubMed] [Google Scholar]
- 43.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. WHO Classification of Tumours of the Central Nervous System. Lyon, France: 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perry A, Aldape KD, George DH, Burger PC. Small cell astrocytoma: an aggressive variant that is clinicopathologically and genetically distinct from anaplastic oligodendroglioma. Cancer. 2004;101:2318–2326. doi: 10.1002/cncr.20625. [DOI] [PubMed] [Google Scholar]
- 45.Reis RM, Konu-Lebleblicioglu D, Lopes JM, Kleihues P, Ohgaki H. Genetic profile of gliosarcomas. Am J Pathol. 2000;156:425–432. doi: 10.1016/S0002-9440(10)64746-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peraud A, Watanabe K, Schwechheimer K, Yonekawa Y, Kleihues P, Ohgaki H. Genetic profile of the giant cell glioblastoma. Lab Invest. 1999;79:123–129. [PubMed] [Google Scholar]
- 47.Kleinschmidt-DeMasters BK, Lillehei KO, Varella-Garcia M. Glioblastomas in the older old. Arch Pathol Lab Med. 2005;129:624–631. doi: 10.5858/2005-129-0624-GITOO. [DOI] [PubMed] [Google Scholar]
- 48.Okada Y, Hurwitz EE, Esposito JM, Brower MA, Nutt CL, Louis DN. Selection pressures of TP53 mutation and microenvironmental location influence epidermal growth factor receptor gene amplification in human glioblastomas. Cancer Res. 2003;63:413–416. [PubMed] [Google Scholar]
- 49.Simmons ML, Lamborn KR, Takahashi M, Chen P, Israel MA, Berger MS, Godfrey T, Nigro J, Prados M, Chang S, et al. Analysis of complex relationships between age, p53, epidermal growth factor receptor, and survival in glioblastoma patients. Cancer Res. 2001;61:1122–1128. [PubMed] [Google Scholar]
- 50.Smith JS, Tachibana I, Passe SM, Huntley BK, Borell TJ, Iturria N, O'Fallon JR, Schaefer PL, Scheithauer BW, James CD, et al. PTEN mutation, EGFR amplification, and outcome in patients with anaplastic astrocytoma and glioblastoma multiforme. J Natl Cancer Inst. 2001;93:1246–1256. doi: 10.1093/jnci/93.16.1246. [DOI] [PubMed] [Google Scholar]
- 51.Shinojima N, Tada K, Shiraishi S, Kamiryo T, Kochi M, Nakamura H, Makino K, Saya H, Hirano H, Kuratsu J, et al. Prognostic value of epidermal growth factor receptor in patients with glioblastoma multiforme. Cancer Res. 2003;63:6962–6970. [PubMed] [Google Scholar]
- 52.Batchelor TT, Betensky RA, Esposito JM, Pham LD, Dorfman MV, Piscatelli N, Jhung S, Rhee D, Louis DN. Age-dependent prognostic effects of genetic alterations in glioblastoma. Clin Cancer Res. 2004;10:228–233. doi: 10.1158/1078-0432.ccr-0841-3. [DOI] [PubMed] [Google Scholar]
- 53.Krex D, Klink B, Hartmann C, von Deimling A, Pietsch T, Simon M, Sabel M, Steinbach JP, Heese O, Reifenberger G, et al. Long-term survival with glioblastoma multiforme. Brain. 2007;130:2596–2606. doi: 10.1093/brain/awm204. [DOI] [PubMed] [Google Scholar]
- 54.Mishani E, Abourbeh G, Eiblmaier M, Anderson CJ. Imaging of EGFR and EGFR tyrosine kinase overexpression in tumors by nuclear medicine modalities. Curr Pharm Des. 2008;14:2983–2998. doi: 10.2174/138161208786404326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pantaleo MA, Nannini M, Maleddu A, Fanti S, Nanni C, Boschi S, Lodi F, Nicoletti G, Landuzzi L, Lollini PL, et al. Experimental results and related clinical implications of PET detection of epidermal growth factor receptor (EGFr) in cancer. Ann Oncol. 2009;20:213–226. doi: 10.1093/annonc/mdn625. [DOI] [PubMed] [Google Scholar]
- 56.Cai W, Niu G, Chen X. Multimodality imaging of the HER-kinase axis in cancer. Eur J Nucl Med Mol Imaging. 2008;35:186–208. doi: 10.1007/s00259-007-0560-9. [DOI] [PubMed] [Google Scholar]
- 57.Velikyan I, Sundberg AL, Lindhe O, Hoglund AU, Eriksson O, Werner E, Carlsson J, Bergstrom M, Langstrom B, Tolmachev V. Preparation and evaluation of (68)Ga-DOTA-hEGF for visualization of EGFR expression in malignant tumors. J Nucl Med. 2005;46:1881–1888. [PubMed] [Google Scholar]
- 58.Cai W, Chen K, He L, Cao Q, Koong A, Chen X. Quantitative PETof EGFR expression in xenograft-bearing mice using 64Cu-labeled cetuximab, a chimeric anti-EGFR monoclonal antibody. Eur J Nucl Med Mol Imaging. 2007;34:850–858. doi: 10.1007/s00259-006-0361-6. [DOI] [PubMed] [Google Scholar]
- 59.Tolmachev V, Friedman M, Sandstrom M, Eriksson TL, Rosik D, Hodik M, Stahl S, Frejd FY, Orlova A. Affibody molecules for epidermal growth factor receptor targeting in vivo: aspects of dimerization and labeling chemistry. J Nucl Med. 2009;50:274–283. doi: 10.2967/jnumed.108.055525. [DOI] [PubMed] [Google Scholar]
- 60.Takahashi H, Herlyn D, Atkinson B, Powe J, Rodeck U, Alavi A, Bruce DA, Koprowski H. Radioimmunodetection of human glioma xenografts by monoclonal antibody to epidermal growth factor receptor. Cancer Res. 1987;47:3847–3850. [PubMed] [Google Scholar]
- 61.Pal A, Glekas A, Doubrovin M, Balatoni J, Namavari M, Beresten T, Maxwell D, Soghomonyan S, Shavrin A, Ageyeva L, et al. Molecular imaging of EGFR kinase activity in tumors with 124I-labeled small molecular tracer and positron emission tomography. Mol Imaging Biol. 2006;8:262–277. doi: 10.1007/s11307-006-0049-0. [DOI] [PubMed] [Google Scholar]
- 62.Takasu S, Takahashi T, Okamoto S, Oriuchi N, Nakayashiki N, Okamoto K, Muramatsu H, Hayashi T, Nakahara N, Mizuno M, et al. Radioimmunoscintigraphy of intracranial glioma xenograft with a technetium-99m-labeled mouse monoclonal antibody specifically recognizing type III mutant epidermal growth factor receptor. J Neurooncol. 2003;63:247–256. doi: 10.1023/a:1024320516341. [DOI] [PubMed] [Google Scholar]
- 63.Liu N, Li M, Li X, Meng X, Yang G, Zhao S, Yang Y, Ma L, Fu Z, Yu J. PET-based biodistribution and radiation dosimetry of epidermal growth factor receptor-selective tracer 11C-PD153035 in humans. J Nucl Med. 2009;50:303–308. doi: 10.2967/jnumed.108.056556. [DOI] [PubMed] [Google Scholar]
- 64.Shu X, Royant A, Lin MZ, Aguilera TA, Lev-Ram V, Steinbach PA, Tsien RY. Mammalian expression of infrared fluorescent proteins engineered from a bacterial phytochrome. Science. 2009;324:804–807. doi: 10.1126/science.1168683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ke S, Wen X, Gurfinkel M, Charnsangavej C, Wallace S, Sevick-Muraca EM, Li C. Near-infrared optical imaging of epidermal growth factor receptor in breast cancer xenografts. Cancer Res. 2003;63:7870–7875. [PubMed] [Google Scholar]
- 66.Hama Y, Urano Y, Koyama Y, Choyke PL, Kobayashi H. Activatable fluorescent molecular imaging of peritoneal metastases following pretargeting with a biotinylated monoclonal antibody. Cancer Res. 2007;67:3809–3817. doi: 10.1158/0008-5472.CAN-06-3794. [DOI] [PubMed] [Google Scholar]
- 67.Yang L, Mao H, Wang YA, Cao Z, Peng X, Wang X, Duan H, Ni C, Yuan Q, Adams G, et al. Single chain epidermal growth factor receptor antibody conjugated nanoparticles for in vivo tumor targeting and imaging. Small. 2009;5:235–243. doi: 10.1002/smll.200800714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Adams KE, Ke S, Kwon S, Liang F, Fan Z, Lu Y, Hirschi K, Mawad ME, Barry MA, Sevick-Muraca EM. Comparison of visible and near-infrared wavelength-excitable fluorescent dyes for molecular imaging of cancer. J Biomed Opt. 2007;12:024017. doi: 10.1117/1.2717137. [DOI] [PubMed] [Google Scholar]
- 69.Barker FG, II, Chang SM. Improving resection of malignant glioma. Lancet Oncol. 2006;7:359–360. doi: 10.1016/S1470-2045(06)70669-6. [DOI] [PubMed] [Google Scholar]
- 70.Jaffer FA, Weissleder R. Molecular imaging in the clinical arena. JAMA. 2005;293:855–862. doi: 10.1001/jama.293.7.855. [DOI] [PubMed] [Google Scholar]
- 71.Suwa T, Ozawa S, Ueda M, Ando N, Kitajima M. Magnetic resonance imaging of esophageal squamous cell carcinoma using magnetite particles coated with anti-epidermal growth factor receptor antibody. Int J Cancer. 1998;75:626–634. doi: 10.1002/(sici)1097-0215(19980209)75:4<626::aid-ijc22>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 72.Aghi M, Gaviani P, Henson JW, Batchelor TT, Louis DN, Barker FG., II Magnetic resonance imaging characteristics predict epidermal growth factor receptor amplification status in glioblastoma. Clin Cancer Res. 2005;11:8600–8605. doi: 10.1158/1078-0432.CCR-05-0713. [DOI] [PubMed] [Google Scholar]
- 73.Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, Cohen KS, Kozak KR, Cahill DP, Chen PJ, Zhu M, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cerniglia GJ, Pore N, Tsai JH, Schultz S, Mick R, Choe R, Xing X, Durduran T, Yodh AG, Evans SM, et al. Epidermal growth factor receptor inhibition modulates the microenvironment by vascular normalization to improve chemotherapy and radiotherapy efficacy. PLoS One. 2009;4:e6539–e6539. doi: 10.1371/journal.pone.0006539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang HS, Nagane M, Klingbeil CK, Lin H, Nishikawa R, Ji XD, Huang CM, Gill GN, Wiley HS, Cavenee WK. The enhanced tumorigenic activity of a mutant epidermal growth factor receptor common in human cancers is mediated by threshold levels of constitutive tyrosine phosphorylation and unattenuated signaling. J Biol Chem. 1997;272:2927–2935. doi: 10.1074/jbc.272.5.2927. [DOI] [PubMed] [Google Scholar]
- 76.Schmidt MH, Furnari FB, Cavenee WK, Bogler O. Epidermal growth factor receptor signaling intensity determines intracellular protein interactions, ubiquitination, and internalization. Proc Natl Acad Sci USA. 2003;100:6505–6510. doi: 10.1073/pnas.1031790100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wiley HS. Trafficking of the ErbB receptors and its influence on signaling. Exp Cell Res. 2003;284:78–88. doi: 10.1016/s0014-4827(03)00002-8. [DOI] [PubMed] [Google Scholar]
- 78.Di Fiore PP, Gill GN. Endocytosis and mitogenic signaling. Curr Opin Cell Biol. 1999;11:483–488. doi: 10.1016/s0955-0674(99)80069-6. [DOI] [PubMed] [Google Scholar]
- 79.Choe G, Horvath S, Cloughesy TF, Crosby K, Seligson D, Palotie A, Inge L, Smith BL, Sawyers CL, Mischel PS. Analysis of the phosphatidylinositol 3′-kinase signaling pathway in glioblastoma patients in vivo. Cancer Res. 2003;63:2742–2746. [PubMed] [Google Scholar]
- 80.Moscatello DK, Holgado-Madruga M, Emlet DR, Montgomery RB, Wong AJ. Constitutive activation of phosphatidylinositol 3-kinase by a naturally occurring mutant epidermal growth factor receptor. J Biol Chem. 1998;273:200–206. doi: 10.1074/jbc.273.1.200. [DOI] [PubMed] [Google Scholar]
- 81.Narita Y, Nagane M, Mishima K, Huang HJ, Furnari FB, Cavenee WK. Mutant epidermal growth factor receptor signaling down-regulates p27 through activation of the phosphatidylinositol 3-kinase/Akt pathway in glioblastomas. Cancer Res. 2002;62:6764–6769. [PubMed] [Google Scholar]
- 82.Prigent SA, Nagane M, Lin H, Huvar I, Boss GR, Feramisco JR, Cavenee WK, Huang HS. Enhanced tumorigenic behavior of glioblastoma cells expressing a truncated epidermal growth factor receptor is mediated through the Ras-Shc-Grb2 pathway. J Biol Chem. 1996;271:25639–25645. doi: 10.1074/jbc.271.41.25639. [DOI] [PubMed] [Google Scholar]
- 83.Lorimer IA, Lavictoire SJ. Activation of extracellular-regulated kinases by normal and mutant EGF receptors. Biochim Biophys Acta. 2001;1538:1–9. doi: 10.1016/s0167-4889(00)00129-4. [DOI] [PubMed] [Google Scholar]
- 84.Zhan Y, O'Rourke DM. SHP-2-dependent mitogen-activated protein kinase activation regulates EGFRvIII but not wild-type epidermal growth factor receptor phosphorylation and glioblastoma cell survival. Cancer Res. 2004;64:8292–8298. doi: 10.1158/0008-5472.CAN-03-3143. [DOI] [PubMed] [Google Scholar]
- 85.Nagane M, Levitzki A, Gazit A, Cavenee WK, Huang HJ. Drug resistance of human glioblastoma cells conferred by a tumor-specific mutant epidermal growth factor receptor through modulation of Bcl-XL and caspase-3-like proteases. Proc Natl Acad Sci USA. 1998;95:5724–5729. doi: 10.1073/pnas.95.10.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Antonyak MA, Kenyon LC, Godwin AK, James DC, Emlet DR, Okamoto I, Tnani M, Holgado-Madruga M, Moscatello DK, Wong AJ. Elevated JNK activation contributes to the pathogenesis of human brain tumors. Oncogene. 2002;21:5038–5046. doi: 10.1038/sj.onc.1205593. [DOI] [PubMed] [Google Scholar]
- 87.Micallef J, Taccone M, Mukherjee J, Croul S, Busby J, Moran MF, Guha A. Epidermal growth factor receptor variant III-induced glioma invasion is mediated through myristoylated alanine-rich protein kinase C substrate overexpression. Cancer Res. 2009;69:7548–7556. doi: 10.1158/0008-5472.CAN-08-4783. [DOI] [PubMed] [Google Scholar]
- 88.Waterman H, Yarden Y. Molecular mechanisms underlying endocytosis and sorting of ErbB receptor tyrosine kinases. FEBS Lett. 2001;490:142–152. doi: 10.1016/s0014-5793(01)02117-2. [DOI] [PubMed] [Google Scholar]
- 89.Wiley HS. Anomalous binding of epidermal growth factor to A431 cells is due to the effect of high receptor densities and a saturable endocytic system. J Cell Biol. 1988;107:801–810. doi: 10.1083/jcb.107.2.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lund KA, Opresko LK, Starbuck C, Walsh BJ, Wiley HS. Quantitative analysis of the endocytic system involved in hormone-induced receptor internalization. J Biol Chem. 1990;265:15713–15723. [PubMed] [Google Scholar]
- 91.Luwor RB, Zhu HJ, Walker F, Vitali AA, Perera RM, Burgess AW, Scott AM, Johns TG. The tumor-specific de2-7 epidermal growth factor receptor (EGFR) promotes cells survival and heterodimerizes with the wild-type EGFR. Oncogene. 2004;23:6095–6104. doi: 10.1038/sj.onc.1207870. [DOI] [PubMed] [Google Scholar]
- 92.Sporn MB, Todaro GJ. Autocrine secretion and malignant transformation of cells. N Engl J Med. 1980;303:878–880. doi: 10.1056/NEJM198010093031511. [DOI] [PubMed] [Google Scholar]
- 93.Di Marco E, Pierce JH, Fleming TP, Kraus MH, Molloy CJ, Aaronson SA, Di Fiore PP. Autocrine interaction between TGF α and the EGF-receptor: quantitative requirements for induction of the malignant phenotype. Oncogene. 1989;4:831–838. [PubMed] [Google Scholar]
- 94.Filmus J, Shi W, Spencer T. Role of transforming growth factor α (TGF-α) in the transformation of ras-transfected rat intestinal epithelial cells. Oncogene. 1993;8:1017–1022. [PubMed] [Google Scholar]
- 95.Hatano M, Eguchi J, Tatsumi T, Kuwashima N, Dusak JE, Kinch MS, Pollack IF, Hamilton RL, Storkus WJ, Okada H. EphA2 as a glioma-associated antigen: a novel target for glioma vaccines. Neoplasia. 2005;7:717–722. doi: 10.1593/neo.05277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wykosky J, Gibo DM, Stanton C, Debinski W. EphA2 as a novel molecular marker and target in glioblastoma multiforme. Mol Cancer Res. 2005;3:541–551. doi: 10.1158/1541-7786.MCR-05-0056. [DOI] [PubMed] [Google Scholar]
- 97.Miao H, Li DQ, Mukherjee A, Guo H, Petty A, Cutter J, Basilion JP, Sedor J, Wu J, Danielpour D, et al. EphA2 mediates ligand-dependent inhibition and ligand-independent promotion of cell migration and invasion via a reciprocal regulatory loop with Akt. Cancer Cell. 2009;16:9–20. doi: 10.1016/j.ccr.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu F, Park PJ, Lai W, Maher E, Chakravarti A, Durso L, Jiang X, Yu Y, Brosius A, Thomas M, et al. A genome-wide screen reveals functional gene clusters in the cancer genome and identifies EphA2 as a mitogen in glioblastoma. Cancer Res. 2006;66:10815–10823. doi: 10.1158/0008-5472.CAN-06-1408. [DOI] [PubMed] [Google Scholar]
- 99.Wykosky J, Gibo DM, Stanton C, Debinski W. Interleukin-13 receptor α 2, EphA2, and Fos-related antigen 1 as molecular denominators of high-grade astrocytomas and specific targets for combinatorial therapy. Clin Cancer Res. 2008;14:199–208. doi: 10.1158/1078-0432.CCR-07-1990. [DOI] [PubMed] [Google Scholar]
- 100.Debinski W, Gibo DM. Fos-related antigen 1 modulates malignant features of glioma cells. Mol Cancer Res. 2005;3:237–249. doi: 10.1158/1541-7786.MCR-05-0004. [DOI] [PubMed] [Google Scholar]
- 101.Tatenhorst L, Senner V, Puttmann S, Paulus W. Regulators of G-protein signaling 3 and 4 (RGS3, RGS4) are associated with glioma cell motility. J Neuropathol Exp Neurol. 2004;63:210–222. doi: 10.1093/jnen/63.3.210. [DOI] [PubMed] [Google Scholar]
- 102.Brat DJ, Bellail AC, Van Meir EG. The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro-oncol. 2005;7:122–133. doi: 10.1215/S1152851704001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bredel M, Bredel C, Juric D, Harsh GR, Vogel H, Recht LD, Sikic BI. Functional network analysis reveals extended gliomagenesis pathway maps and three novelMYC-interacting genes in human gliomas. Cancer Res. 2005;65:8679–8689. doi: 10.1158/0008-5472.CAN-05-1204. [DOI] [PubMed] [Google Scholar]
- 104.Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell. 2004;6:447–458. doi: 10.1016/j.ccr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 105.Wen XF, Yang G, Mao W, Thornton A, Liu J, Bast RC, Le XF. HER2 signaling modulates the equilibrium between pro- and antiangiogenic factors via distinct pathways: implications for HER2-targeted antibody therapy. Oncogene. 2006;25:6986–6996. doi: 10.1038/sj.onc.1209685. [DOI] [PubMed] [Google Scholar]
- 106.de la Iglesia N, Konopka G, Lim KL, Nutt CL, Bromberg JF, Frank DA, Mischel PS, Louis DN, Bonni A. Deregulation of a STAT3-interleukin 8 signaling pathway promotes human glioblastoma cell proliferation and invasiveness. J Neurosci. 2008;28:5870–5878. doi: 10.1523/JNEUROSCI.5385-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zelinski DP, Zantek ND, Stewart JC, Irizarry AR, Kinch MS. EphA2 overexpression causes tumorigenesis of mammary epithelial cells. Cancer Res. 2001;61:2301–2306. [PubMed] [Google Scholar]
- 108.Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE. EphA2: a determinant of malignant cellular behavior and a potential therapeutic target in pancreatic adenocarcinoma. Oncogene. 2004;23:1448–1456. doi: 10.1038/sj.onc.1207247. [DOI] [PubMed] [Google Scholar]
- 109.Wright JH, Wang X, Manning G, LaMere BJ, Le P, Zhu S, Khatry D, Flanagan PM, Buckley SD, Whyte DB, et al. The STE20 kinase HGK is broadly expressed in human tumor cells and can modulate cellular transformation, invasion, and adhesion. Mol Cell Biol. 2003;23:2068–2082. doi: 10.1128/MCB.23.6.2068-2082.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Collins CS, Hong J, Sapinoso L, Zhou Y, Liu Z, Micklash K, Schultz PG, Hampton GM. A small interfering RNA screen for modulators of tumor cell motility identifies MAP4K4 as a promigratory kinase. Proc Natl Acad Sci USA. 2006;103:3775–3780. doi: 10.1073/pnas.0600040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Warmka JK, Mauro LJ, Wattenberg EV. Mitogen-activated protein kinase phosphatase-3 is a tumor promoter target in initiated cells that express oncogenic Ras. J Biol Chem. 2004;279:33085–33092. doi: 10.1074/jbc.M403120200. [DOI] [PubMed] [Google Scholar]
- 112.Bloethner S, Chen B, Hemminki K, Muller-Berghaus J, Ugurel S, Schadendorf D, Kumar R. Effect of common B-RAF and N-RAS mutations on global gene expression in melanoma cell lines. Carcinogenesis. 2005;26:1224–1232. doi: 10.1093/carcin/bgi066. [DOI] [PubMed] [Google Scholar]
- 113.Sato M, Vaughan MB, Girard L, Peyton M, Lee W, Shames DS, Ramirez RD, Sunaga N, Gazdar AF, Shay JW, et al. Multiple oncogenic changes (K-RAS (V12), p53 knockdown, mutant EGFRs, p16 bypass, telomerase) are not sufficient to confer a full malignant phenotype on human bronchial epithelial cells. Cancer Res. 2006;66:2116–2128. doi: 10.1158/0008-5472.CAN-05-2521. [DOI] [PubMed] [Google Scholar]
- 114.Arslan AA, Gold LI, Mittal K, Suen TC, Belitskaya-Levy I, Tang MS, Toniolo P. Gene expression studies provide clues to the pathogenesis of uterine leiomyoma: new evidence and a systematic review. Hum Reprod. 2005;20:852–863. doi: 10.1093/humrep/deh698. [DOI] [PubMed] [Google Scholar]
- 115.Halatsch ME, Low S, Mursch K, Hielscher T, Schmidt U, Unterberg A, Vougioukas VI, Feuerhake F. Candidate genes for sensitivity and resistance of human glioblastoma multiforme cell lines to erlotinib. Laboratory investigation. J Neurosurg. 2009;111:211–218. doi: 10.3171/2008.9.JNS08551. [DOI] [PubMed] [Google Scholar]
- 116.Giancotti V. Breast cancer markers. Cancer Lett. 2006;243:145–159. doi: 10.1016/j.canlet.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 117.Nyati MK, Morgan MA, Feng FY, Lawrence TS. Integration of EGFR inhibitors with radiochemotherapy. Nat Rev Cancer. 2006;6:876–885. doi: 10.1038/nrc1953. [DOI] [PubMed] [Google Scholar]
- 118.Balaban N, Moni J, Shannon M, Dang L, Murphy E, Goldkorn T. The effect of ionizing radiation on signal transduction: antibodies to EGF receptor sensitize A431 cells to radiation. Biochim Biophys Acta. 1996;1314:147–156. doi: 10.1016/s0167-4889(96)00068-7. [DOI] [PubMed] [Google Scholar]
- 119.Das AK, Chen BP, Story MD, Sato M, Minna JD, Chen DJ, Nirodi CS. Somatic mutations in the tyrosine kinase domain of epidermal growth factor receptor (EGFR) abrogate EGFR-mediated radioprotection in non-small cell lung carcinoma. Cancer Res. 2007;67:5267–5274. doi: 10.1158/0008-5472.CAN-07-0242. [DOI] [PubMed] [Google Scholar]
- 120.Das AK, Sato M, Story MD, Peyton M, Graves R, Redpath S, Girard L, Gazdar AF, Shay JW, Minna JD, et al. Non-small-cell lung cancers with kinase domain mutations in the epidermal growth factor receptor are sensitive to ionizing radiation. Cancer Res. 2006;66:9601–9608. doi: 10.1158/0008-5472.CAN-06-2627. [DOI] [PubMed] [Google Scholar]
- 121.Akimoto T, Hunter NR, Buchmiller L, Mason K, Ang KK, Milas L. Inverse relationship between epidermal growth factor receptor expression and radiocurability of murine carcinomas. Clin Cancer Res. 1999;5:2884–2890. [PubMed] [Google Scholar]
- 122.Ang KK, Berkey BA, Tu X, Zhang HZ, Katz R, Hammond EH, Fu KK, Milas L. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002;62:7350–7356. [PubMed] [Google Scholar]
- 123.Milas L, Fan Z, Andratschke NH, Ang KK. Epidermal growth factor receptor and tumor response to radiation: in vivo preclinical studies. Int J Radiat Oncol Biol Phys. 2004;58:966–971. doi: 10.1016/j.ijrobp.2003.08.035. [DOI] [PubMed] [Google Scholar]
- 124.Sheridan MT, O'Dwyer T, Seymour CB, Mothersill CE. Potential indicators of radiosensitivity in squamous cell carcinoma of the head and neck. Radiat Oncol Investig. 1997;5:180–186. doi: 10.1002/(SICI)1520-6823(1997)5:4<180::AID-ROI3>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 125.Contessa JN, Hampton J, Lammering G, Mikkelsen RB, Dent P, Valerie K, Schmidt-Ullrich RK. Ionizing radiation activates Erb-B receptor dependent Akt and p70 S6 kinase signaling in carcinoma cells. Oncogene. 2002;21:4032–4041. doi: 10.1038/sj.onc.1205500. [DOI] [PubMed] [Google Scholar]
- 126.Dent P, Reardon DB, Park JS, Bowers G, Logsdon C, Valerie K, Schmidt-Ullrich R. Radiation-induced release of transforming growth factor α activates the epidermal growth factor receptor and mitogen-activated protein kinase pathway in carcinoma cells, leading to increased proliferation and protection from radiation-induced cell death. Mol Biol Cell. 1999;10:2493–2506. doi: 10.1091/mbc.10.8.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schmidt-Ullrich RK, Mikkelsen RB, Dent P, Todd DG, Valerie K, Kavanagh BD, Contessa JN, Rorrer WK, Chen PB. Radiation-induced proliferation of the human A431 squamous carcinoma cells is dependent on EGFR tyrosine phosphorylation. Oncogene. 1997;15:1191–1197. doi: 10.1038/sj.onc.1201275. [DOI] [PubMed] [Google Scholar]
- 128.Schmidt-Ullrich RK, Valerie K, Fogleman PB, Walters J. Radiationinduced autophosphorylation of epidermal growth factor receptor in human malignant mammary and squamous epithelial cells. Radiat Res. 1996;145:81–85. [PubMed] [Google Scholar]
- 129.Dittmann K, Mayer C, Fehrenbacher B, Schaller M, Raju U, Milas L, Chen DJ, Kehlbach R, Rodemann HP. Radiation-induced epidermal growth factor receptor nuclear import is linked to activation of DNA-dependent protein kinase. J Biol Chem. 2005;280:31182–31189. doi: 10.1074/jbc.M506591200. [DOI] [PubMed] [Google Scholar]
- 130.Burma S, Chen DJ. Role of DNA-PK in the cellular response to DNA double-strand breaks. DNA Repair (Amst) 2004;3:909–918. doi: 10.1016/j.dnarep.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 131.Burma S, Chen BP, Chen DJ. Role of non-homologous end joining (NHEJ) in maintaining genomic integrity. DNA Repair (Amst) 2006;5:1042–1048. doi: 10.1016/j.dnarep.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 132.Dittmann K, Mayer C, Rodemann HP. Inhibition of radiation-induced EGFR nuclear import by C225 (Cetuximab) suppresses DNA-PK activity. Radiother Oncol. 2005;76:157–161. doi: 10.1016/j.radonc.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 133.Bandyopadhyay D, Mandal M, Adam L, Mendelsohn J, Kumar R. Physical interaction between epidermal growth factor receptor and DNA-dependent protein kinase in mammalian cells. J Biol Chem. 1998;273:1568–1573. doi: 10.1074/jbc.273.3.1568. [DOI] [PubMed] [Google Scholar]
- 134.Golding SE, Morgan RN, Adams BR, Hawkins AJ, Povirk LF, Valerie K. Pro-survival AKTand ERK signaling from EGFR and mutant EGFRvIII enhances DNA double-strand break repair in human glioma cells. Cancer Biol Ther. 2009;8:730–738. doi: 10.4161/cbt.8.8.7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kao GD, Jiang Z, Fernandes AM, Gupta AK, Maity A. Inhibition of phosphatidylinositol-3-OH kinase/Akt signaling impairs DNA repair in glioblastoma cells following ionizing radiation. J Biol Chem. 2007;282:21206–21212. doi: 10.1074/jbc.M703042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rodemann HP, Dittmann K, Toulany M. Radiation-induced EGFR-signaling and control of DNA-damage repair. Int J Radiat Biol. 2007;83:781–791. doi: 10.1080/09553000701769970. [DOI] [PubMed] [Google Scholar]
- 137.Toulany M, Kehlbach R, Florczak U, Sak A, Wang S, Chen J, Lobrich M, Rodemann HP. Targeting of AKT1 enhances radiation toxicity of human tumor cells by inhibiting DNA-PKcs-dependent DNA double-strand break repair. Mol Cancer Ther. 2008;7:1772–1781. doi: 10.1158/1535-7163.MCT-07-2200. [DOI] [PubMed] [Google Scholar]
- 138.McLendon RE, Turner K, Perkinson K, Rich J. Second messenger systems in human gliomas. Arch Pathol Lab Med. 2007;131:1585–1590. doi: 10.5858/2007-131-1585-SMSIHG. [DOI] [PubMed] [Google Scholar]
- 139.Lammering G, Hewit TH, Valerie K, Contessa JN, Amorino GP, Dent P, Schmidt-Ullrich RK. EGFRvIII-mediated radioresistance through a strong cytoprotective response. Oncogene. 2003;22:5545–5553. doi: 10.1038/sj.onc.1206788. [DOI] [PubMed] [Google Scholar]
- 140.Chu CT, Everiss KD, Wikstrand CJ, Batra SK, Kung HJ, Bigner DD. Receptor dimerization is not a factor in the signalling activity of a transforming variant epidermal growth factor receptor (EGFRvIII) Biochem J. 1997;324(pt 3):855–861. doi: 10.1042/bj3240855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Learn CA, Hartzell TL, Wikstrand CJ, Archer GE, Rich JN, Friedman AH, Friedman HS, Bigner DD, Sampson JH. Resistance to tyrosine kinase inhibition by mutant epidermal growth factor receptor variant III contributes to the neoplastic phenotype of glioblastoma multiforme. Clin Cancer Res. 2004;10:3216–3224. doi: 10.1158/1078-0432.ccr-03-0521. [DOI] [PubMed] [Google Scholar]
- 142.Li B, Yuan M, Kim IA, Chang CM, Bernhard EJ, Shu HK. Mutant epidermal growth factor receptor displays increased signaling through the phosphatidylinositol-3 kinase/AKT pathway and promotes radioresistance in cells of astrocytic origin. Oncogene. 2004;23:4594–4602. doi: 10.1038/sj.onc.1207602. [DOI] [PubMed] [Google Scholar]
- 143.Friedmann BJ, Caplin M, Savic B, Shah T, Lord CJ, Ashworth A, Hartley JA, Hochhauser D. Interaction of the epidermal growth factor receptor and the DNA-dependent protein kinase pathway following gefitinib treatment. Mol Cancer Ther. 2006;5:209–218. doi: 10.1158/1535-7163.MCT-05-0239. [DOI] [PubMed] [Google Scholar]
- 144.Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24:7455–7464. doi: 10.1038/sj.onc.1209085. [DOI] [PubMed] [Google Scholar]
- 145.Edwards E, Geng L, Tan J, Onishko H, Donnelly E, Hallahan DE. Phosphatidylinositol 3-kinase/Akt signaling in the response of vascular endothelium to ionizing radiation. Cancer Res. 2002;62:4671–4677. [PubMed] [Google Scholar]
- 146.Friedmann B, Caplin M, Hartley JA, Hochhauser D. Modulation of DNA repair in vitro after treatment with chemotherapeutic agents by the epidermal growth factor receptor inhibitor gefitinib (ZD1839) Clin Cancer Res. 2004;10:6476–6486. doi: 10.1158/1078-0432.CCR-04-0586. [DOI] [PubMed] [Google Scholar]
- 147.Bozulic L, Surucu B, Hynx D, Hemmings BA. PKBα/Akt1 acts downstreamofDNA-PK in theDNA double-strand break response and promotes survival. Mol Cell. 2008;30:203–213. doi: 10.1016/j.molcel.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 148.Lees-Miller SP. PIKK-ing a new partner: a new role for PKB in the DNA damage response. Cancer Cell. 2008;13:379–380. doi: 10.1016/j.ccr.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 149.Boehme KA, Kulikov R, Blattner C. p53 stabilization in response to DNA damage requires Akt/PKB and DNA-PK. Proc Natl Acad Sci USA. 2008;105:7785–7790. doi: 10.1073/pnas.0703423105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Habib AA, Chun SJ, Neel BG, Vartanian T. Increased expression of epidermal growth factor receptor induces sequestration of extracellular signal-related kinases and selective attenuation of specific epidermal growth factor-mediated signal transduction pathways. Mol Cancer Res. 2003;1:219–233. [PubMed] [Google Scholar]
- 151.Sarkisian CJ, Keister BA, Stairs DB, Boxer RB, Moody SE, Chodosh LA. Dose-dependent oncogene-induced senescence in vivo and its evasion during mammary tumorigenesis. Nat Cell Biol. 2007;9:493–505. doi: 10.1038/ncb1567. [DOI] [PubMed] [Google Scholar]